Abstract
Introduction:
Little is known about the interplay between neutrophil heterogeneity in neonates in health and disease states. Olfactomedin-4 (OLFM4) marks a subset of neutrophils that has been described in adults and pediatric patients but not neonates, and this subset is thought to play a role in modulating the host inflammatory response.
Methods:
This is a prospective cohort of neonates who were born between June 2020 and December 2021 at the University of Cincinnati Medical Center NICU. Olfactomedin-4 positive (OLFM4+) neutrophils were identified in the peripheral blood using flow cytometry.
Results:
OLFM4+ neutrophil percentage was not correlated with gestational age or developmental age. Neonates with sepsis had higher percentage than those without the condition, 66.9% (IQR 24.3-76.9%) vs. 21.5% (IQR 10.6-34.7%) respectively, p= 0.0003. At birth, high percentage of OLFM4+ neutrophil percentage was associated with severe chorioamnionitis at 49.1% (IQR 28.2-61.5%) compared to those without it at 13.7% (IQR 7.7-26.3%), p < 0.0001. Amongst neonates without sepsis, the percentages of OLFM4+ neutrophils were lower in the BPD/early death group compared to those without BPD, 11.8% (IQR 6.3-29.0%) vs. 32.5% (IQR 18.5-46.1%), p= 0.003, and this retained significance in a multiple logistic regression model that included gestational age, birthweight, and race.
Conclusion:
This is the first study describing OLFM4+ neutrophils in neonates and it shows that this neutrophil subpopulation is not influenced by gestational age but is elevated in inflammatory conditions such as sepsis and severe chorioamnionitis, and lower percentage at birth is associated with developing bronchopulmonary dysplasia.
Keywords: Prematurity, Sepsis, Chorioamnionitis, Bronchopulmonary dysplasia, Neutrophil Subset, Olfactomedin-4
Introduction
Neutrophils play a major role in the pathogenesis of many relevant neonatal pathologies affecting their outcomes: neutrophils function differently in neonates, which may explain the high mortality and morbidity rate in neonatal sepsis [1, 2]; histological chorioamnionitis is mainly driven by neutrophils [3], is implicated in preterm birth occurring more frequently as gestational age at birth decreases [4], and leads to systemic inflammation in the neonate that has negative impact on outcomes [5-7]; high neutrophil to lymphocyte ratio early in life has been associated with developing bronchopulmonary dysplasia [8].
Neutrophil heterogeneity is gaining interest in recent decades [9]; however, this phenomenon is not well described in neonates in health and disease states. A subset of neutrophils marked by olfactomedin-4 (OLFM4) in its specific granules has been described recently by several groups in humans and mice [10-12]. About 25% of circulating neutrophils in the peripheral blood of healthy adults are OLFM4 positive (OLFM4+) [12]. Although this subpopulation’s role is not yet fully understood, human neutrophils release OLFM4 during NETosis, and this leads to two distinct OLFM-4-defined NETs that are speculated to have different immunological effects [13]. Animal studies have shown that OLFM4 attenuates inflammation in the presence of bacterial pathogens by inhibiting cytokine production [14]. Another study showed that OLFM4+ neutrophils are increased following major organ injury, suggesting a possible role in organ repair and systemic inflammatory response [15]. Although this subset has not been described before in neonates, a study showed that neonates born before 32 weeks who developed BPD upregulated the OLFM4 gene in whole blood samples compared to those who did not in the first month of life [16].
Given what is known about OLFM4+ neutrophils and the lack of any knowledge about this subset in neonates, we conducted this prospective study to characterize this subset in neonates and its association with age, sepsis and severe chorioamnionitis, and the development of BPD.
Material and Methods
All study procedures were approved by the Institutional Review Board committee at Cincinnati Children's Hospital Medical Center and University of Cincinnati.
Study Population and Enrollment
This is a prospective cohort of neonates who were admitted to the University of Cincinnati Medical Center NICU between June 2020 and December 2021. Neonates who had a complete blood count obtained and had at least 50 μL of blood residual sample in the laboratory were enrolled. If the neonate did not have sufficient residual sample, they were excluded. For comparisons between developmental age and percentage of OLFM4 positive neutrophils, control pediatric and adult data came from previously reported datasets [10, 15].
Flow cytometry Studies
Flow cytometry was done according to our lab protocol [10]. 50 μL of blood was processed within 72 hours of acquisition. Sample preparation is discussed in the supplementary materials. Flow cytometry analysis was done on BD FACSCanto II (BD, Franklin Lakes, NJ USA), and flow cytometry data was analyzed using FlowJo (BD, Franklin Lakes, NJ USA). The following antibodies were used: Anti-CD66b (1:200 BioLegend, clone G10FP) and anti-CD16 (1:200 BD Biosciences, clone 368). Neutrophils were identified by forward and side scatter then by expression of CD66b and CD16 (shown in supplementary Fig. 1)
Definitions
Gestational age at birth was obtained from the documented gestational age at birth in the admission history and physical examination note in the electronic medical chart. Bronchopulmonary dysplasia was defined as the need of respiratory support at 36 weeks corrected gestational age. Stages of Bronchopulmonary dysplasia were defined as follows [17]: stage I: the need of non-invasive support that is equal or less than 2 lpm; stage II: the need for non-invasive support that is greater than 2 lpm or positive pressure ventilation; and stage III: the need for invasive positive pressure ventilation.
Early Death from an Apparent Respiratory Origin: Since we did not have access to autopsy reports when performed, we defined early respiratory death clinically as follows: death despite maximum invasive respiratory support (100% FiO2, invasive ventilation) with radiographic evidence of respiratory distress syndrome or pulmonary interstitial emphysema within the first 7 days of life without evidence of infection.
Severe Chorioamnionitis: The diagnosis of severe chorioamnionitis was made based on placental pathology report stating there were findings consistent with stage III chorioamnionitis [18].
Sepsis: Positive blood culture with evidence of systemic inflammatory response and continuation of antibiotics for at least 7 days. We further subclassified sepsis as follows: sepsis: sepsis without shock or evidence of 2 or more organ dysfunctions; severe sepsis: sepsis with two or more organ dysfunctions; septic shock: sepsis with cardiogenic dysfunction [19].
Statistical Analysis
Categorical variables were described as number of total and/or percentages and compared using the Fisher’s exact test. Non-normally distributed continuous variables were described using the median and 25th-75th interquartile ranges. Two groups were compared using the Mann-Whitney test. Multiple group comparisons were done using the Kruskal-Wallis test with Dunn’s multiple comparison test. Correlation between two continuous variables was done by calculating the Spearman coefficient. Statistical comparisons were done using Prism 9 (GraphPad Software, San Diego, CA USA). Multiple logistic regression analysis was done in R studio (Integrated Development Environment for R. RStudio, PBC, Boston, MA USA). p-value less or equal than 0.05 was considered significant.
Results
Study Cohort
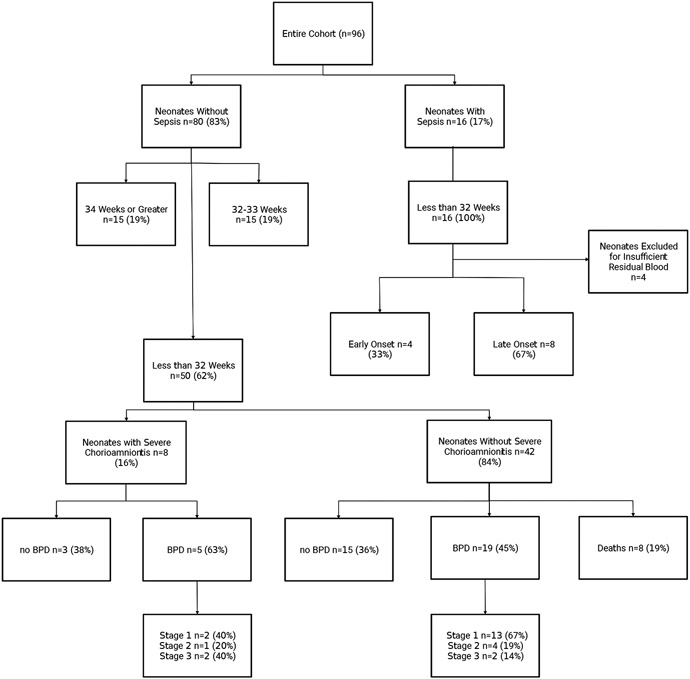
The cohort included a total of 96 neonates. The study flow chart (shown in Fig. 1) shows the distribution of neonates according to gestational age at birth, diagnosis, and outcome. All neonates with sepsis were born before 32 weeks gestation. 4 neonates with sepsis were excluded due to insufficient residual blood.
Fig 1. Study Flowchart.
A Study flowchart showing the distribution of neonates according to gestational age, sepsis, severe chorioamnionitis, and BPD outcome.
Olfactomedin-4 Positive Neutrophils Do Not Correlate with Gestational Age at Birth or Developmental Age
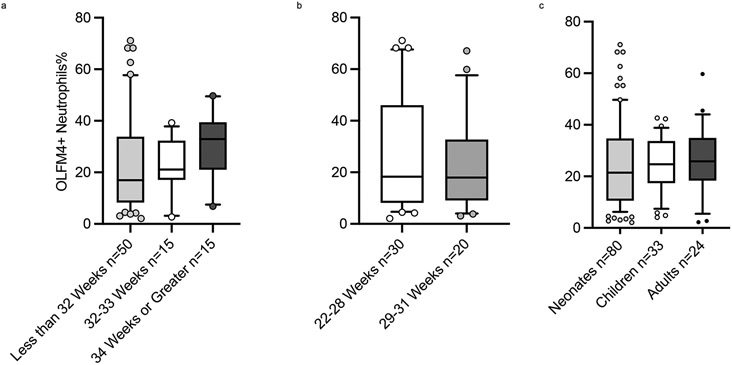
There appears to be no association between OLFM4+ neutrophils and gestational age at birth, Spearman r= 0.18()()(), p= 0.11. Furthermore, there was no difference between the percentages according to gestational age groups (shown in Fig. 2. a and b). When comparing our neonatal cohort with children and adult percentages, there was no statistically significant difference between the groups, neonates 21.5% (IQR 10.6-34.7%) vs. children 24.7% (IQR 17.4-33.7%) vs. adults 25.9% (IQR 18.3), p= 0.97 (shown in Fig. 2. c). The percentage of OLFM4+ neutrophils was weakly correlated with the total white blood cell count, Spearman r =0.28, p= 0.01, and absolute neutrophil count, Spearman r= 0.37, p= 0.001 (Shown in supplementary Fig. 2).
Fig 2. Olfactomedin-4 Positive Neutrophils and Age.
Boxes and line represent the 25th-75th percentiles and median, whiskers represent 10th-90th percentiles, and dots represent values out of 10th-90th percentiles range. a. The percentage of OLFM4+ neutrophils is comparable between gestational age groups, less than 32 weeks (n=50) (light grey bar) 17.0%, 32-33 weeks (n=15) (white bar) 21.1%, 34+ weeks (n=15) (dark grey bar) 32.9%, p 0.14 by the Kruskal-Wallis test. b. The percentage of OLFM-4 positive neutrophils is not influenced by gestational age in those born less than 32 weeks, 22-28 weeks (n=30) (white bar) 18.3% and 29-31 weeks (n=20) (grey bar) 18.0%, p= 0.70 by the Mann-Whitney test The percentage of OLFM4+ neutrophils is comparable between developmental age groups, neonates without sepsis (n=80) (light grey bar) 21.5%, children (n=33) (white bar) 24.7%, adults (n=24) (dark grey bar) 25.9%, p-value 0.78 by the Kruskal-Wallis test.
Olfactomedin-4 Positive Neutrophil Percentage Is Increased in Sepsis and in Neonates with Severe Chorioamnionitis
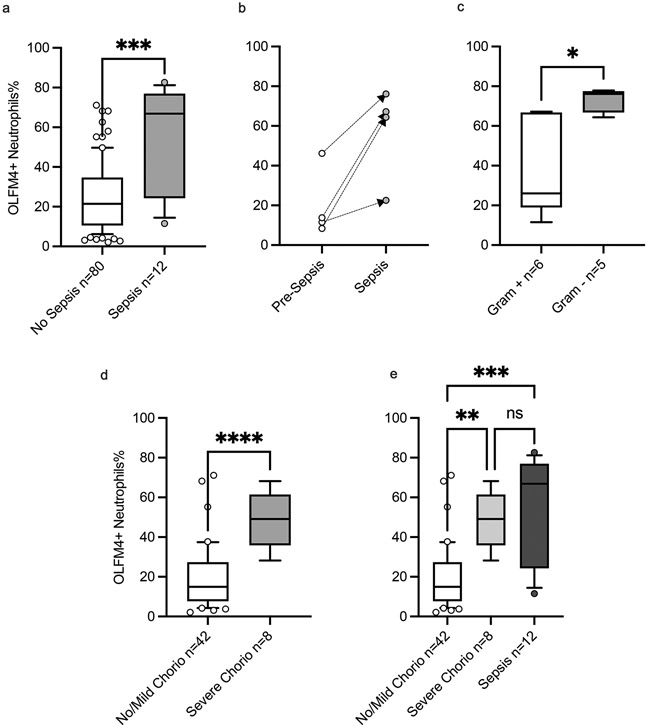
OLFM4+ neutrophil percentages were higher in neonates who were diagnosed with sepsis compared to those who were not, 66.9% (IQR 24.3-76.9%) vs. 21.5% (IQR 10.6-34.7%), p= 0.0003 (shown in Fig. 3. a). There were four neonates that had flow cytometry done before sepsis and during sepsis and in those, we saw elevation in the OLFM4+ neutrophil percentages in sepsis compared to their pre-sepsis level (Shown in Fig. 3. b). Although there was no difference in the percentages according to sepsis timing (early- vs. late-onset), severity, or mortality (Shown in Supplementary Fig. 3), neonates who had sepsis due to gram negative organisms had a higher percentage of OLFM-4 positive neutrophils compared to those who had it due to gram positive organisms, 76.1% (IQR 66.8-77.6%) vs. 26.1% (IQR 18.9-66.8%), p= 0.01 (Shown in Fig. 3. c).
Fig 3. Olfactomedin-4 Positive Neutrophils and Inflammation in Preterm Neonates.
Boxes and line represent the 25th-75th percentiles and median, whiskers represent 10th-90th percentiles, and dots represent values out of 10th-90th percentiles range. a. The percentage of OLFM4+ neutrophils is higher in neonates with sepsis (n=12) (grey bar) 66.9% compared to those without sepsis (n=80) (white bar) 21.5%, p= 0.0003 by the Mann-Whitney test. b. The percentage of OLFM-4 positive neutrophils is increased in sepsis compared to prior sepsis in 4 neonates who had studies done before (white circles) and during sepsis (grey circles); 12.7% vs. 65.8%, p = 0.055 by the Mann-Whitney test. c. Neonates with gram negative sepsis had higher percentage of OLFM-4 positive neutrophils compared to those with gram positive sepsis, 76.7% (n=6) (grey bar) vs. 26.1% (n=6) (white bar), p= 0.009 by the Mann-Whitney test. d. The percentage of OLFM4+ neutrophils is higher in neonates with severe chorioamnionitis at birth (n=8) (grey bar) 49.1% compared to those without it (n=42) (white bar) 15.0%, p< 0.0001 by the Mann-Whitney test. e. Neonates with sepsis (n=12) (dark grey bar) 66.9% had comparable percentage of OLFM-4 positive neutrophils to neonates born with severe chorioamnionitis (n=8) (light grey bar) 49.1%, p> 0.99 by the Dunn’s multiple comparison test.
Among neonates without sepsis, OLFM4+ neutrophils were higher in neonates who were born with severe chorioamnionitis compared to those who were not, ()49.1% (IQR 28.2-61.5% vs. 13.7% (IQR 7.7-26.3%) respectively, p < 0.0001 (Shown in Fig. 3. d). Interestingly, the percentage of OLFM4+ neutrophils were comparable between those with sepsis and severe chorioamnionitis (shown in Fig. 3. e).
Olfactomedin-4 Positive Neutrophil at Birth Correlates with Development of Bronchopulmonary Dysplasia
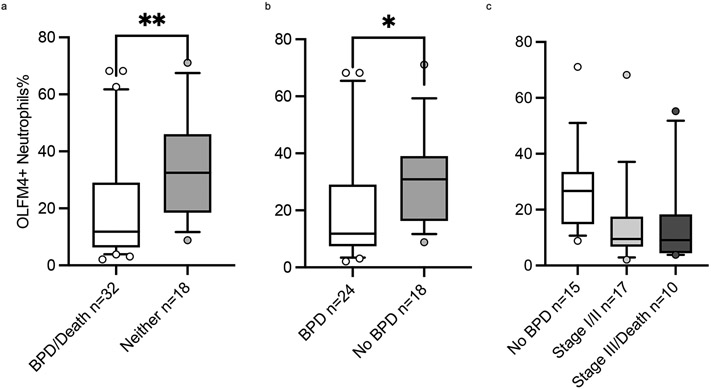
To better examine the association between this neutrophil subset and respiratory outcomes, we looked at preterm neonates born at less than 32 who did not have sepsis. In this group (n=50), the rate of BPD or early death was 64% (n=32) and they were distributed as follows: stage I 47% (n=15); stage II 15% (n=5); stage III 13% (n=4); Death 25% (n=8). When comparing the demographics of the groups, there were several variables that were different (Shown in Table. 1), but white blood cell indices were comparable (shown in supplementary Table. 1). The percentages of OLFM4+ neutrophils were lower in the BPD/early death group compared to those without BPD, 11.8% (IQR 6.3-29.0%) vs. 32.5% (IQR 18.5-46.1%), p= 0.003 (Shown in Fig. 4. a), and this difference remained significant when comparing only those who survived (shown in Fig When we conducted a multiple logistic regression analysis that included the significant different variables between the groups, OLFM4+ neutrophil percentage remained significantly different between the two groups (Shown in Table. 1). Furthermore, there was a non-statistically significant trend of lower OLFM4+ neutrophils in those with more severe phenotype, (Shown in Fig. 4. c). Finally, when we compared those in the lowest percentiles (less than 25th percentile) according to their percentage of OLFM4+ neutrophils at birth, they were much more likely to have stage III BPD/early death than rest of the cohort, OR 6.2 (95% CI 1.4-27.0), p= 0.02, and this was nearing significance in a multiple logistic regression model that included gestational age at birth, birthweight, and race. When comparing survivors only, those in the lowest percentiles compared to the rest of cohort were more likely to have stage II or III BPD, OR 5.8 (95% CI 1.3-27.5), p= 0.049 and this retained significance in our logistic regression modeling (shown in Table. 2).
Table 1:
Characteristics of Neonates less than 32 weeks gestation and BPD/Early Death
| Characteristic | BPD/Early Death n=32 |
No BPD n=18 | p-value | Adjusted p-value e |
|---|---|---|---|---|
| Olfactomedin-4+ Neutrophil % [IQR] | 11.8% [6.3–29.0%] | 32.5% [18.5–46.1%] | 0.003b | 0.02 |
| Gestational Age weeks [IQR] | 27 [24-29] | 29 [27-31] | 0.01b | 0.01 |
| Born at 28 Weeks or Less n % | 24 (75%) | 8 (44%) | 0.04c | - |
| Birth Weight gram [IQR] | 949 [734-1319] | 1268 [1020-1561] | 0.04b | 0.38 |
| Small for Gestational Age n % | 4 (13%) | 1 (6%) | 0.64c | - |
| Sex: Female n % | 13 (41%) | 10 (56%) | 0.38c | - |
| Male n % | 19 (59%) | 8 (44%) | ||
| Race: Black n % | 4 (13%) | 9 (50%) | ||
| Other n % | 3 (10%) | 3 (17%) | 0.007d | 0.01 |
| White n % | 25 (77%) | 6 (33%) | ||
| Multiple Gestation n % | 12 (38%) | 4 (22%) | 0.35c | - |
| Antenatal Steroids n % | 27 (84%) | 16 (89%) | >0.99c | - |
| 5 Minute APGAR Score [IQR] | 8 [4-9] | 8 [8-9] | 0.20c | - |
| Receiving Treatment for PDAa n % | 1 (4%) | 0 (0%) | >0.99c | - |
| Sever Chorioamnionitis n % | 5 (16%) | 3 (17%) | 0.92c | - |
Included only infants who survived beyond 7 days (n=42)
p-value calculated using the Mann-Whitney test
p-value calculated using the Fisher’s exact test
p-value calculated using the Fisher’s exact test comparing neonates who are black to those who are not
adjusted p-value calculated using a multiple logistic regressions model including OLFM4+ neutrophils percentage, gestational age, birth weight, and race
Fig 4. Ofactomedin-4 Positive Neutrophils at Birth and Bronchopulmonary Dysplasia.
Boxes and line represent the 25th-75th percentiles and median, whiskers represent 10th-90th percentiles, and dots represent values out of 10th-90th percentiles range. a. The percentage of OLFM-4 positive neutrophils were lower at birth in neonates with BPD or death (n=32) (white bar) 11.8% compared to neonates who experienced neither (n=15) (grey bar) 32.5%, p= 0.003 by the Mann-Whitney test. b. Amongst survivors (n=42), the percentage of OLFM-4 positive neutrophils were lower in those who experienced BPD (n=24) (white bar) 11.8% compared to those who did not (n=18) (grey bar) 30.9%, p= 0,01 by the Mann-Whitney test. c. Amongst neonates born without severe chorioamnionitis, there was a non-statistically significant trend of lower percentage of OLFM-4 positive neutrophils in those with more severe phenotype; No BPD (n=15) (white bar) 26.7%; BPD stage I or II (n=17) (light grey bar) 9.5%; BPD stage III or death (n= (dark grey bar) 9.1%.
Table 2.
Odds Ratio of Outcome if OLFM4+ Neutrophils Percentage Is Lower Than The 25th Percentile (< 8.3%)
| Outcome | Odds Ratio | Adjusted Odds Ratio a | Adjusted p-value a |
|---|---|---|---|
| Stage III BPD or Death | 6.2 | 20.0 | 0.07 |
| Stage II or III BPD in survivors only | 5.8 | 130 | 0.01 |
Calculated using a multiple logistic regressions model including OLFM4+ Neutrophils percentage (Lower than the 25th percentile or greater), gestational age, birth weight, and race
Discussion
Because of the critical role neutrophils play in neonatal immune response, here we begin to explore neutrophil heterogeneity in neonates by describing a subset of neutrophils that express OLFM4 and its association with relevant neonatal morbidities. Given the influence of gestational age on the development of the immune system [20], we assessed for association between the percentage of OLFM4+ neutrophils at birth relative to gestational age, and we found that there was no correlation between gestational age and the percentage of OLFM4+ neutrophils. We also found no difference when we compared the neonatal group with other developmental age groups. These data suggest that gestational age or developmental age do not significantly influence the percentage of OLFM4+ neutrophils. Although there were statistically significant correlations between the percentages of OLFM4+ neutrophils and total white blood cell counts and absolute neutrophil counts (ANCs), these associations were weak, and the white counts and ANCs were comparable between neonates who had BPD/early death and those who did not.
Previous work has shown high OLFM4 expression and increased percentage of OLFM4+ neutrophils are both associated with a severe phenotype in various infectious diseases [21, 22]. Consistent with prior studies, we found that neonates with sepsis, and particularly gram-negative sepsis, had higher percentages of OLFM4+ neutrophils compared to those who did not. Like sepsis, neonates born with severe chorioamnionitis also had a higher percentage of OLFM4+ neutrophils compared to those who did not. This further supports that this subset is increased in inflammatory conditions. To date, no data exists to suggest the signals necessary for regulation of the percentage of OLFM4+ neutrophils, but it appears that humans with severe inflammatory phenotypes upregulate this subset.
Perhaps most notably, when evaluating neonates without sepsis, we found that low percentage of OLFM4+ neutrophils at birth was associated with the occurrence of early death from a respiratory cause or BPD. It is possible that the OLFM4+ neutrophils could attenuate acute inflammation, which is integral in the pathophysiology of BPD [23]. Although the underpinning of this mechanism is not yet known, there are two plausible pathways by which OLFM4+ neutrophils could confer anti-inflammatory effects. First, assuming there are pathogens implicated in the disease process, OLFM4 may work by inhibiting LPS-induced p65 phosphorylation or NOD1/NOD2 mediated NF-Kb activation [14, 24]. Second, assuming there are no pathogens involved, OLFM4 has been shown to down regulate sonic hedgehog signaling [25], which is important in the process of pulmonary fibrosis and scaring [26]. Therefore, OLFM4 could play an integral role in the development of BPD. Due to the limited blood sampling in the unit where this study was conducted, we could not assess whether those who developed the disease changed the percentage of OLFM4+ neutrophils overtime. The role of OLFM4 and BPD merits further investigation, this is supported by our finding that low percentage at birth of OLFM4+ neutrophils is associated with BPD and prior transcriptional studies have shown that increased expression of OLFM4 is among the highest differentially expressed genes in the blood of those patients who go on to develop BPD [16].
The immune system is complex and integral in the pathogenesis of many conditions facing the preterm neonate. It is interesting that the percentages of OLFM4+ neutrophils were low in neonates who had BPD/early death and high in in neonates with severe chorioamnionitis, which has been implicated in increasing the risk of developing BPD [5]. This phenomenon needs further studying, but it is possible that the overwhelming inflammation in severe chorioamnionitis overwhelms the influence of OLFM4 on the development of the neonatal lungs.
There are limitations to this study. First, the presented work was done in a single center with a limited number of neonates. Second, there some neonates who did not have a complete blood count on admission and therefore, they were missed in our study as our study protocol did not allow for acquisition of umbilical cord blood. Finally, our findings are associative in nature only, however there is increasingly strong support for the role of OLFM4+ neutrophils in human pathology. Repeating this study in a cohort where all neonates are sampled at different time points will be necessary to validate these findings, and mechanistic studies in animal models of sepsis, chorioamnionitis, and BPD are needed to understand these associations.
Conclusion
This is the first study describing OLFM4+ neutrophils in neonates and it shows that this neutrophil subpopulation is not influenced by gestational age, elevated in inflammatory conditions such as sepsis and severe chorioamnionitis, and may play a role in the pathogenesis of developing bronchopulmonary dysplasia.
Supplementary Material
Acknowledgment
This work is dedicated to Hector Wong. He was a wonderful mentor that greatly influenced this work. We hope his legacy will continue in the work of his many mentees.
Funding Sources
This study was supported by Dr. Alder’s K08GM124298-04 grant from the National Institute of Health.
Footnotes
Statement of Ethics
This study was reviewed and approved by the institutional review board committee at Cincinnati Children's Hospital Medical Center and the University of Cincinnati, approval number 2020-0235.
Waiver of informed consent was granted by the institutional review board committee at Cincinnati Children's Hospital Medical Center and the University of Cincinnati to the study given the minimal risk posed on study subjects.
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Data Availability Statement
The flow cytometry data that support the findings of this study are not publicly available due to containing information that could compromise research participants but are available from the corresponding author, Faris Al-Gharaibeh, upon reasonable request.
References
- 1.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. 2010;29(3):315–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai S, Thompson DK, Anderson PJ, Yang JY. Short- and Long-Term Neurodevelopmental Outcomes of Very Preterm Infants with Neonatal Sepsis: A Systematic Review and Meta-Analysis. Children (Basel). 2019;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, et al. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92(2):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medicine Io. Preterm Birth: Causes, Consequences, and Prevention. Behrman RE, Butler AS, editors. Washington, DC: The National Academies Press; 2007. 790 p. [PubMed] [Google Scholar]
- 5.Villamor-Martinez E, Alvarez-Fuente M, Ghazi AMT, Degraeuwe P, Zimmermann LJI, Kramer BW, et al. Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw Open. 2019;2(11):e1914611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, Passera S, Cavallaro G, Degraeuwe P, et al. Chorioamnionitis Is a Risk Factor for Intraventricular Hemorrhage in Preterm Infants: A Systematic Review and Meta-Analysis. Front Physiol. 2018;9:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soraisham AS, Trevenen C, Wood S, Singhal N, Sauve R. Histological chorioamnionitis and neurodevelopmental outcome in preterm infants. J Perinatol. 2013;33(1):70–5. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Chen C, Zhang X, Weng X, Sheng A, Zhu Y, et al. High Neutrophil-to-Lymphocyte Ratio Is an Early Predictor of Bronchopulmonary Dysplasia. Front Pediatr.2019;7:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019;40(7):565–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alder MN, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin-4 Is a Candidate Marker for a Pathogenic Neutrophil Subset in Septic Shock. Crit Care Med. 2017;45(4):e426–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Yan M, Liu Y, McLeish KR, Coleman WG Jr., Rodgers GP. Olfactomedin 4 inhibits cathepsin C-mediated protease activities, thereby modulating neutrophil killing of Staphylococcus aureus and Escherichia coli in mice. J Immunol. 2012;189(5):2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemmensen SN, Bohr CT, Rorvig S, Glenthoj A, Mora-Jensen H, Cramer EP, et al. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol. 2012;91(3):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welin A, Amirbeagi F, Christenson K, Bjorkman L, Bjornsdottir H, Forsman H, et al. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS One. 2013;8(7):e69575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Yan M, Liu Y, Wang R, Li C, Deng C, et al. Olfactomedin 4 down-regulates innate immunity against Helicobacter pylori infection. Proc Natl Acad Sci U S A. 2010;107(24):11056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassam AF, Levinsky NC, Mallela JP, Angel K, Opoka A, Lahni P, et al. Olfactomedin 4-Positive Neutrophils Are Upregulated after Hemorrhagic Shock. Am J Respir Cell Mol Biol. 2021;64(2):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrzyk JJ, Kwinta P, Wollen EJ, Bik-Multanowski M, Madetko-Talowska A, Gunther CC, et al. Gene expression profiling in preterm infants: new aspects of bronchopulmonary dysplasia development. PLoS One. 2013;8(10):e78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med. 2019;200(6):751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–48. [DOI] [PubMed] [Google Scholar]
- 19.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37(2):439–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson LS, Hedou J, Ganio EA, Stelzer IA, Feyaerts D, Harbert E, et al. Single-Cell Analysis of the Neonatal Immune System Across the Gestational Age Continuum. Front Immunol. 2021;12:714090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Rodgers GP. Olfactomedin 4 Is a Biomarker for the Severity of Infectious Diseases. Open Forum Infect Dis. 2022;9(4):ofac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kangelaris KN, Clemens R, Fang X, Jauregui A, Liu T, Vessel K, et al. A neutrophil subset defined by intracellular olfactomedin 4 is associated with mortality in sepsis. Am J Physiol Lung Cell Mol Physiol. 2021;320(5):L892–L902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11(5):354–62. [DOI] [PubMed] [Google Scholar]
- 24.Gong F, Li R, Zheng X, Chen W, Zheng Y, Yang Z, et al. OLFM4 Regulates Lung Epithelial Cell Function in Sepsis-Associated ARDS/ALI via LDHA-Mediated NF-kappaB Signaling. J Inflamm Res. 2021;14:7035–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Liu W, Chen W, Zhu J, Deng CX, Rodgers GP. Olfactomedin 4 deficiency promotes prostate neoplastic progression and is associated with upregulation of the hedgehog-signaling pathway. Sci Rep. 2015;5:16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou J, Ji J, Chen X, Cao H, Tan Y, Cui Y, et al. Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J. 2021;288(11):3530–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The flow cytometry data that support the findings of this study are not publicly available due to containing information that could compromise research participants but are available from the corresponding author, Faris Al-Gharaibeh, upon reasonable request.