Abstract
The Min proteins are involved in determining cell division sites in bacteria and have been studied extensively in rod-shaped bacteria. We have recently shown that the gram-negative coccus Neisseria gonorrhoeae contains a min operon, and the present study investigates the role of minD from this operon. A gonococcal minD insertional mutant, CJSD1, was constructed and exhibited both grossly abnormal cell division and morphology as well as altered cell viability. Western blot analysis verified the absence of MinD from N. gonorrhoeae (MinDNg) in this mutant. Hence, MinDNg is required for maintaining proper cell division and growth in N. gonorrhoeae. Immunoblotting of soluble and insoluble gonococcal cell fractions revealed that MinDNg is both cytosolic and associated with the insoluble membrane fraction. The joint overexpression of MinCNg and MinDNg from a shuttle vector resulted in a significant enlargement of gonococcal cells, while cells transformed with plasmids encoding either MinCNg or MinDNg alone did not display noticeable morphological changes. These studies suggest that MinDNg is involved in inhibiting gonococcal cell division, likely in conjunction with MinCNg. The alignment of MinD sequences from various bacteria showed that the proteins are highly conserved and share several regions of identity, including a conserved ATP-binding cassette. The overexpression of MinDNg in wild-type Escherichia coli led to cell filamentation, while overexpression in an E. coli minD mutant restored a wild-type morphology to the majority of cells; therefore, gonococcal MinD is functional across species. Yeast two-hybrid studies and gel-filtration and sedimentation equilibrium analyses of purified His-tagged MinDNg revealed a novel MinDNg self-interaction. We have also shown by yeast two-hybrid analysis that MinD from E. coli interacts with itself and with MinDNg. These results indicate that MinDNg is required for maintaining proper cell division and growth in N. gonorrhoeae and suggests that the self-interaction of MinD may be important for cell division site selection across species.
Present knowledge of bacterial cell division has largely been acquired from the study of rod-shaped bacteria. In Escherichia coli, normal cell division occurs with the formation of a midcell septum that ensures two equally sized daughter cells (25). Improper division site selection can lead to septation at the cell poles, resulting in a minicell phenotype that is characterized by round, anucleate minicells and short filaments (1, 9).
In E. coli, division at the midcell is maintained by a set of proteins, MinCEc, MinDEc, and MinEEc, encoded by the minB locus (7, 9). MinCEc acts as a general division inhibitor that is capable of blocking cell division when expressed at high levels. However, the presence of MinDEc greatly reduces the amount of MinCEc required to induce cell filamentation; hence, MinDEc has been termed an activator of MinCEc (10). In contrast, MinDEc does not block E. coli cell division in the absence of MinCEc (10). Studies with green fluorescent protein (GFP) fusions to either MinCEc or MinDEc have shown that both proteins exhibit rapid pole-to-pole oscillations (13, 32, 33, 35). The oscillation of MinCEc is dependent upon MinDEc (33), and an interaction between the two has been shown using yeast two-hybrid assays (15). Presumably, MinDEc concentrates MinCEc at polar regions to inhibit cell division at these locations (13, 33). The target of the MinCDEc inhibitor is believed to be the essential cell division protein FtsZ, which assembles early at division sites (3, 4, 12, 14); however, a recent study suggested that another early-acting cell division protein, FtsA, may be targeted as well (18). MinEEc provides topological specificity to the MinCDEc complex by restricting the activity of the division inhibitor complex to polar regions. Studies with MinEEc-GFP indicated that the protein formed a ring at midcell that could counteract MinCDEc function (31). More recent studies revealed that MinEEc also oscillates from pole to pole, suggesting that it acts by moving the division inhibitor away from the midcell and to the poles (11).
Of the three Min proteins, MinD is the most ubiquitously distributed (30). This protein has a highly conserved nucleotide-binding site and shares homology with several proteins, such as RepA and ParA, that are involved in the maintenance and partitioning of certain plasmids (8). MinDEc has been shown to bind ATP and to possess ATPase activity. The precise role of ATP-binding and hydrolysis remains unclear; however, the mutation of a highly conserved lysine residue in the ATP-binding site abolished the ability of the protein to activate MinCEc-mediated division inhibition (8). MinDEc seems to sensitize MinCEc to the effects of MinEEc. In the absence of MinDEc, the overexpression of MinCEc can still inhibit cell division despite an overexpression of MinEEc, which presumably would prevent division inhibition at all potential division sites (10). Furthermore, the localization of MinEEc at midcell, as well as its intracellular oscillation, is dependent upon MinDEc; therefore, MinDEc may promote the association of MinEEc to the inner membrane (11, 31).
In comparison to bacilli, little is known about how cocci select a midcell division site. We are using the gram-negative coccus Neisseria gonorrhoeae as a model organism to investigate Min protein function in round cells. Division in N. gonorrhoeae occurs along perpendicularly alternating planes and results in a tetrad of daughter cells (43). We have recently shown that both N. gonorrhoeae and Neisseria meningitidis possess minC, minD, and minE homologues encoded as part of a large 17-kb gene cluster (30). Deletion of minCNg in N. gonorrhoeae led to abnormal cell division and cell lysis (30). MinCNg, which can complement an E. coli minC mutant, is a division inhibitor whose overexpression causes filamentation in wild-type E. coli (30).
This is the first study to investigate the role of MinD in a coccal organism, and our evidence indicates that minDNg is required for maintaining proper cell division in N. gonorrhoeae. In the present study, an N. gonorrhoeae minD insertional mutant was generated, and it displayed aberrant cell division and morphology, accompanied by altered cell viability. Gonococcal expression of MinCNg and MinDNg together from a shuttle vector led to significant cell enlargement, indicative of cell division inhibition. These results indicate that MinDNg is required for maintaining proper cell division and growth in N. gonorrhoeae, likely in conjunction with MinCNg. MinDNg is also functional across species, since its overexpression in wild-type E. coli led to cell filamentation, while its overexpression in an E. coli minD mutant restored a wild-type morphology to most of the cells. Yeast two-hybrid studies and gel-filtration and sedimentation equilibrium analyses of purified His-tagged MinDNg showed that MinDNg could interact with itself. The interaction of E. coli MinD with itself and with gonococcal MinD was also detected by the yeast two-hybrid system. MinD-MinD interaction in bacteria of differing morphologies may reflect an importance of this event in maintaining proper cell division site selection across genera.
MATERIALS AND METHODS
Strains and growth conditions.
The strains used in this study are presented in Table 1. E. coli DH5α was used as a host to clone gonococcal min genes. E. coli PB103 was used to provide chromosomal DNA for PCR amplification of minDEc. E. coli strains DH5α, DR105 (minC), PB104 (minD), and PB114 (ΔminCDE) were used in expression studies. E. coli BL21(DE3) was used as an expression strain for the purification of His-tagged MinDNg (His-MinDNg). E. coli GM48 (dam) (23) was used to prepare unmethylated pSR1 (Table 2) for construction of the minDNg insertional mutant. E. coli strains were grown at 37°C in Luria-Bertani (LB) medium (Difco). Streptomycin-resistant (Strr) N. gonorrhoeae CH811 (27) was used to generate the minDNg insertional mutant, N. gonorrhoeae CJSD1. N. gonorrhoeae F62 (42), a reference strain in our laboratory, was used as a host for studies on the overexpression of MinCNg and MinDNg using a modified shuttle vector (26). N. gonorrhoeae strains were grown on GC medium base (Difco) supplemented with Kellogg's defined supplement (GCMBK) for 18 to 24 h at 35°C in a humid, 5% CO2 environment (19, 26). Liquid cultures were grown in GCMBK containing 0.04% NaHCO3, incubated at 35°C with 5% CO2, with shaking at 200 rpm. When required, antibiotics were added to media in the following concentrations: 100 μg of ampicillin/ml, 25 μg of chloramphenicol/ml, and 100 μg of kanamycin/ml for E. coli and 1 mg of streptomycin/ml, 5 μg of chloramphenicol/ml, and 75 μg of kanamycin/ml for N. gonorrhoeae. Saccharomyces cerevisiae SFY526 was used in yeast two-hybrid assays to study Min protein interactions. Yeast were grown at 30°C on yeast extract-peptone-adenine-dextrose (YPAD) medium or on the appropriate synthetic dropout media as described by the Clontech Yeast Two-Hybrid Manual.
TABLE 1.
Bacterial and yeast strains used
| Strain | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| E. coli DH5α | supE44 ΔlacU169 (80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Gibco |
| E. coli GM48 | thr-1 ara-14 leuB6 fhuA31 lacY1 tsx-78 supE44 galT22 galK2 λ−dcm-1 dam-3 thi-1 | 23; E. coli Genetic Stock Center |
| E. coli BL21(DE3) | F− dcm ompT hsdSB(r−B m−B) gal λ (DE3) | Stratagene |
| E. coli PB103 | dadR1 trpE61 trpA62 tna-5 purB+ | 9 |
| E. coli DR105 | zcf-117::Tn10/dadR1 trpE61 trpA62 tna-5 minC3 tetR | P. de Boer, Case Western Reserve University |
| E. coli PB104 | dadR1 trpE61 trpA62 tna-5 purB+ λ−minD1 | 9 |
| E. coli PB114 | dadR1 trpE61 trpA62 tna-5 purB+ λ− ΔminCDE aph (Kmr) | 7 |
| N. gonorrhoeae CH811 | Auxotype (A)/serotype (S)/plasmid content (P) class: nonrequiring/IB-2/plasmid-free, Strr | 27 |
| N. gonorrhoeae CJSD1 | CH811 StrrminD::Cm | This study |
| N. gonorrhoeae F62 | A/S/P class: proline/IB-7/2.6 | 42 |
| S. cerevisiae SFY526 | MATa ura3-52 his3-200 ade2-101 lys2-801 trp 1-901 leu2-3 112 canr gal4-542 gal80-538 URA3::GAL1UAS-GAL1TATA-lacZ | Clontech |
TABLE 2.
Plasmids used
| Plasmid | Characteristic(s) | Source or reference |
|---|---|---|
| pUC18 | Cloning vector (Apr) | Amersham Pharmacia |
| pSR1 | pBluescript KS II(+) with 2,810-bp amplicon containing minCDENg | 30 |
| pSR3 | pUC18 containing minDNg under lac promoter | This study |
| pJSDcat | pSR1 with cat inserted into minDNg | This study |
| pET30a | Cloning vector (Kmr) to produce N-terminal fusions to 6×-His tag | Novagen |
| pJSHD2 | pET30a containing 6×-His-minDNg | This study |
| pFP10 | Gonococcal shuttle vector (Cmr) | 26 |
| pFP20 | pFP10 modified with kanamycin resistance cassette and additional restriction sites | This study |
| pFP22 | pFP20 containing minCNg and 503-bp upstream region of the minCNg start codon | This study |
| pFP23 | pFP20 containing minDNg and 714-bp upstream region of the minDNg start codon | This study |
| pFP25 | pFP20 containing minCDNg and 503-bp upstream region of the minCNg start codon | This study |
| pGBT9 | Yeast two-hybrid plasmid encoding GAL4 DNA-BD (Amr) | Clontech |
| pGBT9minD | pGBT9 encoding GAL4 DNA-BD fused to MinDNg | This study |
| pGAD424 | Yeast two-hybrid plasmid encoding GAL4-AD (Amr) | Clontech |
| pGADminD | pGAD424 encoding GAL4-AD fused to MinDNg | This study |
| pSRBD-D | pGBT9 encoding GAL4 DNA-BD fused to MinDEc | This study |
| pSRAD-D | pGAD424 encoding GAL4-AD fused to MinDEc | This study |
| pVA3 | Yeast two-hybrid plasmid encoding murine p53, used for positive control interaction with pTD1 | Clontech |
| pLAM5′ | Yeast two-hybrid plasmid encoding human laminC, used for negative control interaction with pTD1 | Clontech |
| pTD1 | Yeast two-hybrid plasmid encoding SV40 large T antigen, used for positive control interaction with pVA3 | Clontech |
Oligonucleotide primers and PCR.
The oligonucleotide primers used for PCR amplification of the min genes were designed using Primer Designer Software (Scientific and Education Software) and were synthesized by the University of Ottawa Biotechnology Research Institute. Whole-cell suspensions of N. gonorrhoeae CH811 or E. coli PB103 were used to provide chromosomal DNA template for PCR by diluting cells from overnight cultures grown on GCMBK or LB, respectively, in double-distilled H2O. Cell concentrations were adjusted to 0.5 McFarland Equivalence Turbidity Standard (Remel). PCRs were carried out in a Perkin Elmer Gene Amp PCR System 9600 Thermocycler as follows: 3 min at 94°C, 30 cycles of 15 s at 94°C, annealing for 15 s at temperatures dependent upon the primer pair used, extension at 72°C for times dependent on expected product size, and a final 5-min extension at 72°C. Reactions were carried out in a final volume of 100 μl containing 1× PCR buffer with 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 0.2 μg of each primer, and 2.5 U of Taq DNA polymerase (Boehringer Mannheim Canada).
Identification and cloning of minDNg and cloning of minDEc.
We previously identified the gonococcal minCDE cluster by analysis of the raw DNA sequence data from the N. gonorrhoeae FA1090 genome project (34) and subsequently amplified, cloned, and sequenced this cluster from N. gonorrhoeae strain CH811 (GenBank accession number AF345908) (30). The minDNg gene was PCR amplified using the 5′ primer MinD1 (GCGCGAATTCGTGGCAAAAATTATTGTAG) and the 3′ primer MinD2 (GCGCGGATCCACCTTATCCTCCGAACAGA), incorporating EcoRI and BamHI restriction sites (underlined). The amplicon was ligated into EcoRI/BamHI-digested pUC18 (Amersham Pharmacia Biotech) to form pSR3 (Table 2). For yeast two-hybrid assays, minDNg amplified with primers MinD1 and MinD2 was ligated in frame to both the GAL4 DNA binding domain (BD) of pGBT9 and the GAL4 activation domain (AD) of pGAD424 (Clontech). The plasmids obtained were pGBT9minD (BD-MinDNg) and pGADminD (AD-MinDNg), respectively (Table 2). E. coli minD was amplified using the 5′ primer EcMinDup (GCGCGAATTCATGGCACGCATTATTGTTGT) and the 3′ primer EcMinDdown (GCGCGGATCCTTATCCTCCGAACAAGCGTT), incorporating EcoRI and BamHI restriction sites (underlined), respectively. This amplicon was ligated into pGBT9 and pGAD424 as above to generate pSRBD-D and pSRAD-D for yeast two-hybrid studies. To purify MinDNg, the coding region of minDNg was cloned in frame with the N-terminal 6×-His tag (His-MinDNg) of pET30a (Novagen) using the 5′ primer MinD7pet30 (CATGCCATGGTGGTGGCAAAAATTATTGTA) and the 3′ primer MinD2 (see above), incorporating NcoI and BamHI sites, respectively. This clone was named pJSHD2 (Table 2). Transformation of all plasmids was carried out in E. coli DH5α, as described previously (36). Gene fusions were confirmed by DNA sequencing performed at the University of Ottawa Biotechnology Research Institute.
Modification of shuttle vector and overexpression studies of MinDNg and MinCNg in N. gonorrhoeae.
The E. coli-N. gonorrhoeae shuttle vector pFP20 (Table 2) was constructed by replacing the chloramphenicol acetyltransferase (cat) cassette from pFP10 (Table 2) (26) with a kanamycin resistance cassette and by the insertion of extra restriction sites as follows. The kanamycin resistance cassette from pET30a (Novagen) was PCR amplified using the 5′ primer CJ1 (GCGCAAGCTTGCCGTCTGAACTTTCTCATAGCTCACGCTG) and the 3′ primer DJ26 (GCGCCTGCAGGGTACCATGGAATTCATATG GCCGTCTGAAATTCTTAGAAAAACTCATCG), each containing a gonococcal uptake sequence (bold). CJ1 also incorporated a HindIII site, while DJ26 contained restriction sites for PstI, KpnI, NcoI, EcoRI, and NdeI (underlined regions). The amplicon was digested with PstI and HindIII prior to ligation. pFP10 was digested with PstI and HindIII to eliminate the cat cassette, and the desired vector fragment was isolated from 1% agarose gels using the QIAquik gel extraction kit (Qiagen). The two fragments were ligated to generate pFP20.
Gonococcal minC and minD were cloned separately and together into pFP20. Gonococcal minC and the 503-bp upstream region shown to possess promoter activity (29) were PCR amplified using the 5′ primer CJ3 (GCGCGAATTCTAATGGCAATCGCAAATTA) and the 3′ primer JSC2 (GCGCCTGCAGCAAACAATTACTCTGAGCC), which incorporated EcoRI and PstI sites (underlined), respectively. Gonococcal minDNg was PCR amplified using the 5′ primer Min12 (GCGCGCGAATTCATGATGGTTTATATAAT) and the 3′ primer JSD2 (GCGCCTGCAGACCTTATCCTCCGAACAGA), incorporating EcoRI and PstI sites, respectively. This amplicon contained minDNg and an upstream region consisting of the coding region of minCNg which has been shown to have minDNg promoter activity (29). Finally, minCNg and minDNg were amplified together using primers CJ3 and JSD2. All amplicons were digested with EcoRI and PstI and ligated into pFP20, which was previously digested with the same restriction enzymes. The resulting clones were named pFP22 (minCNg), pFP23 (minDNg), and pFP25 (minCDNg) and were transformed into N. gonorrhoeae F62 as outlined previously (37). The stability of each plasmid within the transformants was verified by plasmid isolation and DNA gel electrophoresis.
Comparison of MinD sequences.
The minD gene sequences from various bacteria were obtained at the WIT web site (http://wit.mcs.anl.gov/WIT2/). BLAST searches for minD homologues in other organisms were carried out in their respective sequence databases, provided by the Institute for Genomic Research (http://www.tigr.org/tdb/mdb/mdb.html). Translation of minD gene sequences was done using PCGene Software (Intelligenetics Inc.). Protein alignments were performed using ClustalW Version 1.8 software (http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html), and shading was done using BOXSHADE Version 3.21 (http://www.ch.embnet.org/software/BOX_form.html).
Separation of soluble and insoluble gonococcal cell fractions.
N. gonorrhoeae CH811 cells were collected from solid GCMBK and resuspended in 2 ml of phosphate-buffered saline (PBS) (pH 7.4). Cells were lysed by sonication (Fisher Scientific 60 Sonic Dismembrator) using 3 cycles of four 5-s bursts, with a 1-min time interval on ice between cycles. The cell extract was spun at 100,000 × g for 1 h at 4°C (Beckman TL-100 ultracentrifuge), and the soluble supernatant was kept. The insoluble pellet was washed twice with 2 ml of PBS and resuspended in 2 ml of PBS. Protein concentrations for both fractions were determined using the Bio-Rad protein assay kit, and similar amounts of protein were used for Western blotting.
Construction of N. gonorrhoeae minD knockout, CJSD1.
Plasmid pSR1 (Table 2) was transformed into E. coli GM48 (dam) and isolated from this strain using the Qiagen Miniprep Kit. pSR1 was digested at its unique BclI site near the 5′ end of minDNg, 179 bp downstream from the translational start site of minDNg. The cat cassette from pACYC184 (New England Biolabs) was PCR amplified using the 5′ primer HMcat1 (TGCTCACTTTGTGCTGGTGTCCCTGTTGATA) and the 3′ primer HMcat2 (TCGTCACTTTGTGTTCTGCCATTCATCC). Both the cat cassette and BclI-digested pSR1 were blunt-ended with T4 DNA polymerase (New England Biolabs), ligated together, and transformed into E. coli DH5α to produce pJSDcat (Table 2). The presence and orientation of the cat insert in minDNg was confirmed by PCR analysis, followed by DNA sequencing performed at the University of Ottawa Biotechnology Research Institute. N. gonorrhoeae CH811 cells were transformed with pJSDcat to allow for insertional inactivation of minDNg by homologous recombination as described previously (16). The insertion of cat into the chromosomal minDNg was confirmed by PCR analysis.
Analysis of N. gonorrhoeae and E. coli cells by phase and electron microscopy.
N. gonorrhoeae CH811, CJSD1, and F62 (transformed with various plasmids) and E. coli DH5α and PB104 (transformed with pUC18 or pSR3) were fixed with 0.2% glutaraldehyde and 6% formaldehyde prior to adhesion to coverslips precoated with 0.01% polylysine. Coverslips were then placed onto a slide containing a drop of 50% glycerol and were sealed to the slide. Samples were analyzed by phase-contrast microscopy using a Zeiss Axioskop microscope. Transmission electron microscopy was performed on N. gonorrhoeae strains CH811 and CJSD1 as previously described (2). N. gonorrhoeae F62 transformed with either pFP20 or pFP25 were fixed in 1.6% glutaraldehyde and examined by transmission electron microscopy at the Laboratory Pathology facilities, Civic Campus, Ottawa Hospital, Ottawa, Ontario, Canada. The diameters of 40 cells from pFP20 and pFP25 transformants were measured from electron micrographs taken at the same magnification, and average diameters were calculated for both samples. Statistical analysis using the unpaired Student's t test was applied to the averages, and differences were considered significant for P values of <0.001.
Growth studies.
To ensure that equivalent numbers of N. gonorrhoeae CH811 and CJSD1 cells would be used in growth studies, the inocula were standardized as follows. Each strain was diluted 25-fold from overnight liquid GCMBK cultures to obtain an optical density at 550 nm (OD550) of ∼0.05. Tenfold serial dilutions were performed to obtain the number of CFU/milliliter that were present at that OD. For growth studies, overnight cultures of N. gonorrhoeae CH811 and CJSD1 cells were diluted as above to inoculate 25 ml of liquid GCMBK with ∼1 × 106 cells for growth studies spanning 13 h. One milliliter of culture was removed at fixed times to measure the OD550, and it was serially diluted in liquid GCMBK. One hundred microliters from selected dilutions were plated on GCMBK agar media supplemented with streptomycin for N. gonorrhoeae CH811 or with streptomycin and chloramphenicol for N. gonorrhoeae CJSD1. The plates were incubated for 24 h prior to counting or for an additional 24 h if the colonies were too small to be effectively counted. Growth studies were performed twice for each strain.
Purification of His-MinDNg.
A 250-ml log-phase culture of E. coli BL21(DE3) carrying pJSHD2 was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 37°C and shaking at 250 rpm. Protein purification using His-Bind resin (Novagen) was carried out as previously described (30) using the following solutions described by the manufacturer, with the indicated changes in imidazole concentration: 30 ml of binding buffer (5 mM imidazole), 21 ml of wash buffer 1 (20 mM imidazole), 18 ml of wash buffer 2 (30 mM imidazole), 9 ml of wash buffer 3 (60 mM imidazole), and 12 ml of elution buffer (250 mM imidazole). Protein identity was confirmed by P. Thibault, Institute of Biological Sciences, National Research Council of Canada, using mass spectrometry.
Protein analysis and Western blots.
Polyclonal anti-MinCNg and anti-MinDNg antisera were previously produced (30). The antisera was affinity purified by being bound to immobilized His-MinCNg or His-MinDNg on nitrocellulose membranes, as outlined previously (41), prior to Western blotting. Cell extracts from N. gonorrhoeae and E. coli were prepared by resuspending cells recovered from log phase in 750 μl of PBS (pH 7.4). Cell suspensions were lysed by sonication (Sonifier cell disruptor 350) and then centrifuged at 4°C for 30 min at 10,000 × g in a Sorvall RC 5C Plus centrifuge. The supernatant fractions were recovered and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (36). Protein concentrations were standardized for each sample, as determined by densitometric analysis using the Alpha Imager 1220 v5.04 (AlphaEase version 5.00; Alpha Innotech Corp.). SDS-PAGE-resolved proteins were transferred to Immobilon-P membranes (Millipore Corporation) and blocked as described previously (30). Blots were probed for 1 h at room temperature with anti-gonococcal MinD antisera diluted 1:500 to 1:750 or with anti-MinCNg antisera diluted 1:100 in TTBS (0.05% Tween-20 in Tris-buffered saline), followed by incubation with goat anti-rabbit secondary antibody conjugated to alkaline phosphatase (1:3,000; Bio-Rad). Blots were developed using the Atto Phos Plus kit (JBL Scientific Inc.). Densitometric analysis was performed to compare protein levels.
Yeast two-hybrid assays.
Plasmids pGBT9, pGAD424, pGBT9minD, pGADminD, pSRBD-D, pSRAD-D, pVA3, pLAM5′, and pTD1 (Table 2) were transformed singly or in combination into S. cerevisiae SFY526 using the lithium acetate method (Clontech Yeast Two-Hybrid Manual), and transformants were plated on the appropriate synthetic dropout media. Yeast colonies were screened for β-galactosidase activity using the colony-lift and liquid assays (using o-nitrophenyl-d-galactopyranoside [ONPG] as a substrate) described by Clontech. Liquid assays were performed twice for each interaction.
Gel-filtration chromatography.
Prior to gel-filtration chromatography, purified His-MinDNg was dialyzed overnight at 4°C against PBS (pH 7.4) and 0.2% Tween-20. The protein was applied to a Sephadex 200 column (Amersham Pharmacia Biotech) equilibrated with PBS (pH 7.4) and a flow rate of 0.5 ml/min. Protein was monitored by UV absorbance at 280 nm, and 0.5-ml fractions were collected and analyzed by SDS-PAGE.
Sedimentation equilibrium analysis.
Prior to analytical ultracentrifugation, purified His-MinDNg was dialyzed overnight at 4°C against Buffer A (50 mM Tris, 20 mM NaCl, 3 mM β-mercaptoethanol, 1 mM EDTA; pH 7.5) prior to analysis. Sedimentation equilibrium experiments were carried out at 20°C in a Beckman XLI analytical ultracentrifuge using Absorbance optics following the procedures previously described (22). Aliquots (110 μl) of sample solution were loaded into 6-sector charcoal-filled epon sample cells, allowing 3 concentrations of sample to be run simultaneously. Runs were performed at a minimum of two different speeds, and each speed was maintained until there was no significant difference in radial distance2/2 versus absorbance scans taken 2 h apart to ensure that equilibrium was achieved.
The sedimentation equilibrium data was evaluated using the NONLIN program, which incorporates a nonlinear least-squares curve-fitting algorithm (17). This program allows the analysis of both single and multiple data files. Data can be fit to either a single ideal species model or models containing up to four associating species, depending on which parameters are permitted to vary during the fitting routine. The partial specific volume of the protein and the buffer solution density were estimated using the SEDNTERP program, which incorporates calculations detailed previously (21).
RESULTS
The gonococcal minD knockout mutant, CJSD1, exhibits aberrant cell morphology and decreased viability.
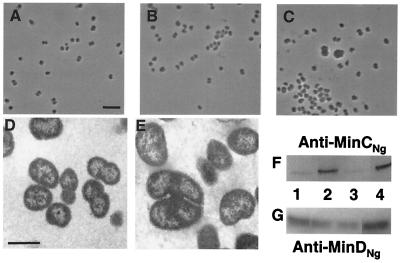
An N. gonorrhoeae minD insertional mutant, CJSD1, was constructed by disrupting minDNg with a cat cassette. PCR analysis and DNA sequencing showed that the cat insert was in the opposite orientation of transcription of minDNg. Western blot analyses on soluble cell extracts from wild-type N. gonorrhoeae CH811 and the minD insertional mutant CJSD1 using affinity-purified anti-MinDNg polyclonal antisera showed no MinDNg expression in the latter strain (Fig. 1E, lane 2). N. gonorrhoeae CJSD1 exhibited cells of various sizes which were often clustered together and showed signs of lysis (Fig. 1B), while wild-type N. gonorrhoeae CH811 cells displayed uniform size and shape, as observed by phase-contrast microscopy (Fig. 1A). Analysis of N. gonorrhoeae CJSD1 by electron microscopy revealed grossly abnormal cell morphologies, characterized by irregularly shaped cells and the presence of ghost cells devoid of intracellular contents (Fig. 1D). Furthermore, septum formation resulted in unequally sized cells. These observations contrast the typical diplococcal morphology of wild-type N. gonorrhoeae CH811 (Fig. 1C).
FIG. 1.
Effects of insertional inactivation of minD on the cell morphology of N. gonorrhoeae CH811. Phase-contrast micrographs of wild-type N. gonorrhoeae CH811 (A) and an N. gonorrhoeae minD knockout mutant, CJSD1 (B). Both panels are at the same magnification, and the scale bar in panel A represents 5 μm. (C) Electron microscopy shows the typical diplococcal morphology of N. gonorrhoeae CH811. (D) Electron micrograph of N. gonorrhoeae CJSD1 showing aberrant cell morphologies, including cells of various sizes and cells devoid of intracellular contents. The scale bars in panels C and D represent ∼1 μm. (E) Western blot analysis of cell extracts using affinity-purified anti-MinDNg antisera show the expression of MinDNg in N. gonorrhoeae CH811 (lane 1) but not in N. gonorrhoeae CJSD1 (lane 2).
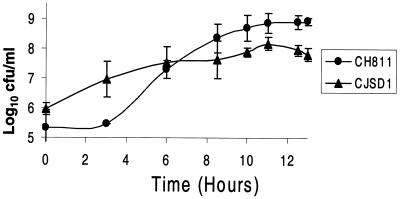
The growth of N. gonorrhoeae CJSD1 was compared to that of its parental wild-type strain in liquid cultures. The number of CFU of N. gonorrhoeae CJSD1/milliliter was higher than that of wild-type N. gonorrhoeae CH811 for approximately the first 6 h but subsequently became significantly less than that of the wild-type for the following 7 h (Fig. 2). In addition, the mutant strain appeared to reach stationary phase after about 6 h, about 4 to 5 h sooner than the wild type, as assessed by viable counts (Fig. 2) and absorbance readings (data not shown). Wild-type colonies could be visualized and counted after the normal incubation time of 18 to 24 h; however, N. gonorrhoeae CJSD1 colonies did not appear until 42 to 48 h. Furthermore, once CJSD1 colonies did appear they were always variable in size, especially with the appearance of many tiny colonies. This is in contrast to the near uniformity and larger size of control N. gonorrhoeae CH811 colonies (data not shown).
FIG. 2.
Comparison of the viability of wild-type N. gonorrhoeae CH811 (circles) and minD knockout CJSD1 (triangles). N. gonorrhoeae CH811 colonies appeared 18 to 24 h after plating, while CJSD1 colonies did not appear until 42 to 48 h after plating.
Overexpression of MinDNg and MinCNg together in N. gonorrhoeae causes cell enlargement.
N. gonorrhoeae F62 cells were transformed with the shuttle vectors pFP20, pFP22 (minCNg), pFP23 (minDNg), or pFP25 (minCDNg) (Table 2), and cell morphologies of each transformant were examined by phase-contrast microscopy. Gonococci transformed with the unmodified pFP20 vector exhibited a typical, uniform, round shape and characteristic diplococcal morphology (Fig. 3A). Cells transformed with pFP22 or pFP23, carrying minCNg or minDNg, respectively, did not exhibit any noticeable morphological changes from the control, as assessed by phase-contrast microscopy (Fig. 3B). However, transformants carrying pFP25, carrying both minCNg and minDNg, exhibited obvious cell enlargement, with some cells having twice the normal diameter (Fig. 3C).
FIG. 3.
Effects of overexpressing minCNg and/or minDNg in gonococcal cells using the shuttle vector pFP20. (A) Phase-contrast micrograph of N. gonorrhoeae F62 cells transformed with control shuttle vector pFP20 exhibit characteristic diplococcal morphology. (B) N. gonorrhoeae F62 cells transformed with pFP23 (minDNg) do not show altered morphologies by phase-contrast microscopy. Gonococci transformed with pFP22 (minCNg) had a similar morphology to the cells shown in panel B. (C) N. gonorrhoeae F62 cells transformed with pFP25 (minCDNg) exhibit noticeable cell enlargement, including cells up to twice the normal diameter. Panels A through C are all at the same magnification, and the scale bar in panel A represents 5 μm. (D) Electron micrograph of N. gonorrhoeae F62 cells transformed with control vector pFP20 display normal diplococcal morphology. (E) Electron micrograph of N. gonorrhoeae F62 cells transformed with pFP25 (minCDNg) showing enlarged cells. Panels D and E are at the same magnification, and the scale bar in panel D represents ∼1 μm. Western blot to determine MinCNg (F) and MinDNg (G) expression levels in N. gonorrhoeae F62 transformed with shuttle vectors. Soluble fractions of N. gonorrhoeae F62 cells transformed with control pFP20 (lane 1), pFP22 (minCNg) (lane 2), pFP23 (minDNg) (lane 3), and pFP25 (minCDNg) (lane 4) were probed with affinity-purified anti-MinCNg (F) or anti-MinDNg (G) antisera.
Electron microscopy was used to further examine the morphology of N. gonorrhoeae F62 cells transformed with either the control pFP20 or pFP25 (minCDNg). Cells transformed with pFP25 (minCDNg) (Fig. 3E) were larger than those transformed with pFP20 (Fig. 3D), yet they still retained diplococcal morphology. Out of 40 randomly measured cells containing pFP25 (minCDNg), 38 had diameters (ranging from ∼0.95 to 1.50 μm) which were larger than the average diameter of the control transformants (∼0.90 μm). The average diameter of pFP25 transformants was calculated to be ∼28% greater than that of pFP20 transformants, and statistical analysis confirmed that this difference was significant (P < 0.001).
Western blotting using affinity-purified anti-MinCNg antisera showed that gonococcal cells transformed with pFP22 (minCNg) and pFP25 (minCDNg) had ∼1.4 and ∼1.5 times, respectively, the level of MinCNg (Fig. 3F, lanes 2 and 4) as cells transformed with control pFP20 (Fig. 3F, lane 1). When extracts were probed with anti-MinDNg antisera, cells transformed with pFP25 (minCDNg) had ∼1.2 times the MinDNg level detected in cells containing pFP20 (Fig. 3G, lanes 1 and 4). Curiously, the levels of MinDNg in gonococci transformed with pFP22 (minCNg) or pFP23 (minDNg) (Fig. 3G, lanes 2 and 3) were consistently lower (∼0.9 times) than MinDNg levels in cells transformed with the control vector (Fig. 3G, lane 1).
MinD is located in cytosolic and membrane fractions of gonococcal cell extracts.
Soluble and insoluble wild-type N. gonorrhoeae CH811 cell fractions were separated by high-speed centrifugation and probed with affinity-purified anti-MinDNg polyclonal antisera. Densitometric analysis revealed that approximately 40% of MinDNg was associated with the insoluble membrane fraction (Fig. 4, lane 1), while 60% of the protein was detected in the cytosol (Fig. 4, lane 2).
FIG. 4.

Detection of MinD in gonococcal cell fractions. Western blot using anti-MinDNg antisera to probe insoluble membrane fraction (lane 1) and soluble cytosolic fraction (lane 2) of N. gonorrhoeae CH811 cell extracts.
MinD proteins from various bacteria are highly conserved.
The alignment of the deduced amino acid sequences of MinD proteins from several bacteria, as well as from Archaea and a chloroplast, shows regions of extensive conservation distributed throughout MinD, particularly at the N terminus (Fig. 5). All the MinD proteins analyzed have a conserved ATP-binding site (39) that is similar to amino acid residues 10 to 17 (GKGGVGKT) of MinDNg (GenBank accession number AF345908). In addition, several amino acids appear to be strictly conserved in MinD across all species and correspond to the MinDNg residues Ser9, Lys11, Gly12, Gly13, Lys16, Leu27, Asn45, Gly51, Asp122, Pro124, Ala138, Asp139, Pro147, Glu148, and Pro227.
FIG. 5.
Alignment of MinD proteins from various species. Abbreviations: Ng, N. gonorrhoeae; Nm, N. meningitidis; Ec, E. coli; St, S. enterica serovar Typhimurium; Yp, Yersinia pestis; Vc, Vibrio cholerae; Hp, Helicobacter pylori; Sy, Synechochistis sp.; Gt, Guillardia theta (chloroplast); Dr, Deinococcus radiodurans; Aa, Aquifex aeolicus; Bs, Bacillus subtilis; Tm, Thermotoga maritima; Mj, Methanococcus janaaschii (Archaea); and Ct, Chlamydia trachomatis (Ct). Black boxes indicate amino acid residues that are identical to the consensus at their respective positions. Gray boxes indicate amino acids that are similar to the consensus.
Overexpression of MinDNg in rod-shaped E. coli shows that gonococcal MinD is active across species.
To determine the effects of gonococcal minD expression in rod-shaped bacteria, E. coli DH5α cells were transformed with plasmid pSR3 encoding minDNg (Table 2). Cells transformed with the control pUC18 vector exhibited normal rod-shaped morphology (Fig. 6A), while the same strain transformed with pSR3 exhibited a mixed phenotype of long filaments and short rods in the absence of IPTG induction (Fig. 6B). Western blot analysis indicated that MinDNg was overexpressed in E. coli DH5α transformed with pSR3 (Fig. 6C, lane 2). The control showed only a faint signal corresponding to the resident MinD from E. coli (Fig. 6C, lane 1).
FIG. 6.
Overexpression of MinDNg in E. coli DH5α. (A) E. coli DH5α cells transformed with pUC18 control have a typical wild-type rod morphology. (B) E. coli DH5α cells transformed with pSR3 (minDNg) exhibit a filamentous phenotype. The scale bar in panel A is 5 μm, and both figures are at the same magnification. (C) Western blot using anti-MinDNg antisera to probe soluble fractions of E. coli DH5α (pUC18) (lane 1) and E. coli DH5α (pSR3) (lane 2).
Plasmid pSR3 was transformed into E. coli PB104 (Table 1) to determine whether minDNg could complement the minDEc mutation in this strain. E. coli PB104 has a MinD protein with an amino acid substitution (G262A) that results in an inactive protein and a classical minicell phenotype characterized by short filaments and minicells (Fig. 7A) (9, 20). When transformed with pSR3, the majority of E. coli PB104 cells exhibited a short-rod morphology with very few filaments and minicells (Fig. 7B), indicating that partial complementation of the minDEc mutation was achieved. Western blot analysis shows the overexpression of gonococcal MinDNg in PB104 (pSR3) (Fig. 7C, lane 2) compared to the level of expression of the inactive mutant MinDEc in PB104 (pUC18) (Fig. 7C, lane 1). When pSR3 was transformed into an E. coli minC mutant (DR105) and an E. coli strain lacking all three min genes (PB114), both strains retained their typical minicell phenotype (data not shown). This suggested that the complementation in strain PB104 by MinDNg was MinCEc-dependent.
FIG. 7.
Complementation of minD mutation in E. coli PB104 expressing gonococcal MinD from pSR3. (A) E. coli P104 cells transformed with pUC18 display a typical minicell phenotype. (B) E. coli PB104 cells transformed with pSR3 (minDNg) are mostly short rods, with few longer filaments and minicells. (C) Western blot using anti-MinDNg antisera to probe soluble fractions of E. coli PB104 (pUC18) (lane 1) and E. coli PB104 (pSR3) (lane 2).
Interaction of MinD with itself.
Using the yeast two-hybrid system, a novel gonococcal MinD self-interaction was detected. Yeast colonies transformed with pGBT9minD and pGADminD (Table 2), encoding fusions of MinDNg to GAL4 DNA binding and activation domains, respectively, appeared blue in color. Yeast transformed singly with either one of the plasmids appeared white, indicating that the fusion proteins themselves were incapable of activating the yeast lacZ reporter gene. Liquid β-galactosidase assays performed on the yeast transformants also verified the MinDNg-MinDNg interaction, giving values of 12.3 ± 3.4 Miller units relative to the 27.2 ± 6.0 Miller units recorded for the positive control interaction of simian virus 40 (SV40) large T antigen and p53 provided (Table 3). An interaction of E. coli MinD with itself was also detected, although it was not as strong as that observed for gonococcal MinD (Table 3). Interestingly, MinDNg and MinDEc could strongly interact with each other in either orientation tested (Table 3). An interaction between MinCNg and MinDNg was not detected; however, an interaction between gonococcal MinD and MinC of E. coli was observed using the yeast two-hybrid system (data not shown). In addition, the interaction between E. coli MinC and MinD was confirmed using the yeast two-hybrid system (data not shown), which was previously described for MinC and MinD from both E. coli and Bacillus subtilis (15, 24).
TABLE 3.
Yeast two-hybrid analysis of MinD self-interactions
| Fusion to GAL4 DNA-BD | Fusion to GAL4-AD | Interaction detected | β-Galactosidase activity (Miller units)a |
|---|---|---|---|
| MinDNg | MinDNg | Yes | 12.3 ± 3.4 |
| MinDEc | MinDEc | Yes | 4.4 ± 0.9 |
| MinDNg | MinDEc | Yes | 14.3 ± 0.6 |
| MinDEc | MinDNg | Yes | 28.8 ± 7.8 |
| p53b | SV40 large T antigenb | Yes | 27.2 ± 6.0 |
| LaminCc | SV40 large T antigenc | No | 0.1 ± 0.1 |
One unit equals the amount of β-galactosidase which hydrolyzes 1 μmol of ONPG per min per cell.
Positive control interaction.
Negative control.
To confirm that MinDNg can interact with itself, His-tagged MinDNg (His-MinDNg) was purified for gel-filtration studies. The functionality of His-MinDNg was suggested by the extensive filamentation observed in E. coli BL21(DE3) cells expressing the fusion protein from pJSHD2 (Table 2) (data not shown), similar to E. coli DH5α expressing untagged MinDNg from pSR3 (Fig. 6B). The functionality of the fusion protein was further verified by expressing His-MinDNg in E. coli minC and minD mutants. These strains lack the T7 RNA polymerase needed for His-MinDNg expression from pJSHD2; hence, the gene encoding the fusion protein was cloned into pUC18. E. coli DR105 (minC) expressing His-MinDNg retained its minicell phenotype, while E. coli PB104 (minD) became filamentous, indicative of cell division arrest. Expression levels of His-MinDNg were likely not ideal to correct the minicell phenotype as observed with untagged MinDNg. Together these observations show that His-MinDNg is functional and requires the presence of MinC to inhibit cell division.
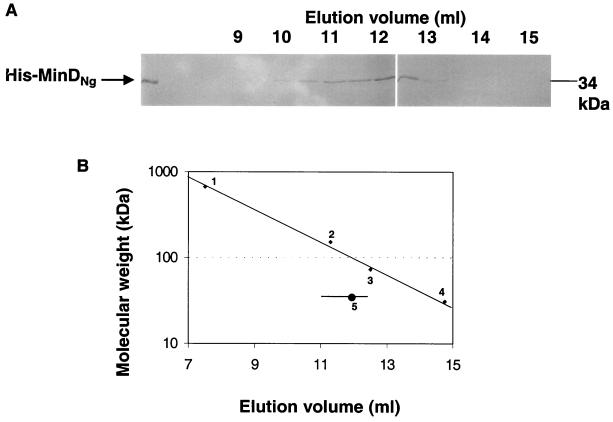
Purified His-MinDNg was applied to a Superdex 200 column, and column samples were collected and analyzed by SDS-PAGE. In comparison with the elution of control proteins, the majority of His-MinDNg eluted at an apparent molecular size of at least twice its monomeric molecular size of 34 kDa (Fig. 8A and B). This indicated that the protein passed through the column as a dimer and possibly as higher homomeric forms, in support of the yeast two-hybrid data.
FIG. 8.
Gel-filtration chromatography of purified His-MinDNg. Purified protein was applied to a Superdex 200 column equilibrated with PBS (pH 7.4) at a flow rate of 0.5 ml/min. (A) Eluted fractions analyzed by SDS-PAGE. (B) Elution of His-MinDNg relative to the following protein standards: (1) thyroglobulin (∼669 kDa), (2) goat anti-human immunoglobulin G (∼150 kDa), (3) bovine prothrombin (72 kDa), and (4) carbonic anhydrase (31 kDa). His-MinDNg eluted between 11 and 12.5 ml (horizontal line), with the majority eluting at ∼12 ml (circle).
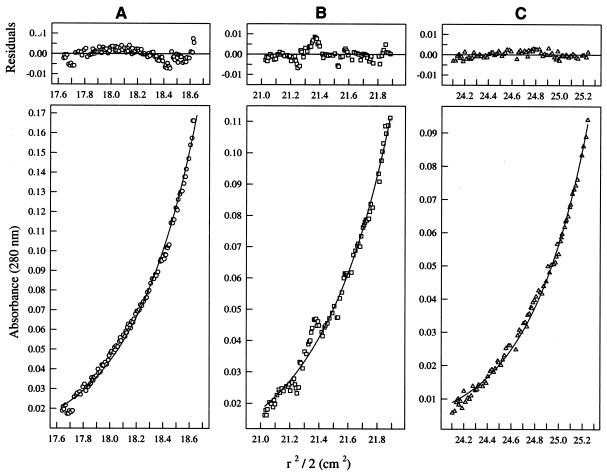
The self-interaction of His-MinDNg was also confirmed by sedimentation equilibrium analysis. The data points from three different loading concentrations of His-MinDNg, depicting both the concentration and migration of the protein, matched well with the theoretical fit lines of a dimer (Fig. 9A through C, lower panels). Further analysis showed that these data points had little deviation from their respective theoretical fit lines (Fig. 9A through C, upper panels). The protein was shown to have an average apparent molecular weight of 67,955, supporting a single-species model of a dimer, which would have an expected molecular weight of 69,364. Degradation of the protein sample was observed over time when sedimentation analyses were run at rotor speeds greater than 16,000 rpm; therefore, data from higher speed sets were not available.
FIG. 9.
Plots illustrating sedimentation equilibrium data from the following three loading concentrations of His-MinDNg fit globally to a single species model: (A) 0.39 mg/ml; (B) 0.27 mg/ml; (C) 0.18 mg/ml. The run was performed at 20°C and 16,000 rpm in a Beckman XL1 analytical ultracentrifuge. Lower panels display radial distance2/2 (r2/2) versus absorbance plots. Symbols represent measured data points and solid lines represent theoretical fit lines. Upper panels display the random deviation of the data points from the fit lines, indicating a good fit to the dimeric model.
DISCUSSION
Since cocci lack obvious cell poles, it has been suggested that they do not contain any min genes (40). However, we have recently shown that the gram-negative coccus N. gonorrhoeae contains a min operon with minC, minD, and minE genes (30). Although cocci do not have cell poles, the disruption of cell division site selection machinery should elicit some morphological changes. The N. gonorrhoeae minD insertional mutant, CJSD1, exhibited a grossly aberrant phenotype characterized by daughter cells of differing sizes and shapes, consistent with improper division planes being initiated. A similar phenotype has been observed in a gonococcal minC insertional mutant that displayed atypical division (30). Increased cell lysis of strain CJSD1 was observed by phase-contrast and electron microscopy and was suggested by absorbance readings of N. gonorrhoeae CJSD1 liquid cultures. Hence, the disruption of minDNg may have affected cell wall integrity, although less severely than that observed in an N. gonorrhoeae minC mutant (30).
The loss of MinDNg production altered the viability of N. gonorrhoeae CJSD1, leading to a decrease in cell viability during the latter half of the growth curve. In rod-shaped bacteria, polar divisions due to min mutations produce nonviable, anucleate minicells (7, 9). It is possible that improper cell division in strain CJSD1 would also result in daughter cells with decreased and/or lost viability, and this was observed. For each successive division a portion of the cells would become nonviable, and this may explain the delayed appearance of N. gonorrhoeae CJSD1 colonies on solid media as well. Furthermore, increased cell lysis likely contributed to the lower viability of the gonococcal minD mutant. Curiously, we observed a higher viability of this mutant than of wild-type cells early in the growth experiments (<6 h). It is possible that mutant cells may have become multinucleated at the onset of growth, resulting in a higher number of viable nucleated cells than that of the control. As more undesirable divisions occur, a corresponding increase in anucleate and nonviable mutant cells may account for the decreased viability of strain CJSD1 at later growth stages.
In rods, the overexpression of division inhibitors such as MinC or MinD either singly or in combination leads to cell filamentation (7, 8). Inhibition of cell division in cocci should result in enlarged cells. Studies have shown that penicillin G induces cell enlargement in N. gonorrhoeae (43). Round rodA mutants of Salmonella enterica serovar Typhimurium grown under conditions that prevented cell division were also reported to have become significantly larger in size and accompanied by cell lysis (6). Furthermore, we have observed significant cell enlargement of round E. coli rodA cells overexpressing either MinCNg or MinDNg (unpublished data). The use of a stable shuttle vector has allowed gene overexpression studies to be conducted in gonococci that were previously not possible due to a lack of suitable genetic tools. N. gonorrhoeae F62 cells became noticeably enlarged only when both MinCNg and MinDNg were overexpressed from the same plasmid, pFP25 (minCDNg), indicative of a synergistic relationship between the two proteins that inhibits division.
Whether the overexpression of MinCNg and MinDNg together is an absolute requirement for gonococcal cell enlargement is not known. In E. coli, the overexpression of either MinCEc or MinDEc in wild-type cells can inhibit cell division (7, 8). Hence, it is possible that the gonococcal overexpression of either MinCNg or MinDNg to levels higher than that achieved in the present study may affect normal cell division. Although MinCNg levels were increased in gonococci transformed with pFP22 (minCNg), there was no increase in MinDNg levels in cells carrying pFP23 (minDNg); hence, the consequences of overexpressing MinDNg alone in N. gonorrhoeae are presently unknown. Studies in our laboratory indicate that the minDNg upstream region included in pFP23 possesses promoter activity (29); however, it is possible that flanking chromosomal DNA sequences that normally influence minDNg promoter activity are disrupted upon cloning minDNg into a plasmid. Alternatively, differences in the ability of Min proteins to affect cell division in rods and cocci may reflect differences in the sensitivity of each model system to these proteins.
There are several conserved regions distributed throughout MinD, including a highly conserved ATP-binding site which has been reported in E. coli MinD and other nucleotide binding proteins (8, 39). The wide distribution of MinD homologues, ranging from gram-negative and gram-positive bacteria to Archaea and chloroplasts, suggests an important role for this protein in cell processes. The protein from Chlamydia trachomatis is quite divergent from the others and may have another function in this organism. The 15 completely conserved amino acids identified from our alignments may be important for MinD function. The minD1 mutation in E. coli has been characterized as a Gly-to-Ala substitution at amino acid 262, and this mutation is enough to generate an inactive protein (9, 20). From the MinD alignments, we note that the E. coli Gly262 residue (Fig. 5; 9 amino acids from the C terminus) is not fully conserved among bacteria, including N. gonorrhoeae. Therefore, this residue may be specific for proper MinD function in E. coli and related species alone. Despite this, the high identity (73%) between MinD proteins from N. gonorrhoeae and E. coli and the ability of both proteins to interact with each other likely allowed for MinDNg functionality across both species. Overexpression of gonococcal MinD in rod-shaped E. coli led to extensive filamentation, further verifying the ability of MinDNg to affect cell division. Furthermore, the expression of gonococcal MinD in an E. coli minD mutant, PB104, resulted in a partial complementation of the typical minicell phenotype; hence, in E. coli the gonococcal MinD protein retained some of the properties inherent to MinDEc. Excessive production of gonococcal MinD may have prevented the full complementation of the E. coli minD mutant. It is believed that an increased MinD/MinE ratio will affect MinD oscillation frequency in E. coli and result in a small number of minicells (32), which was observed in E. coli PB104 expressing gonococcal MinD. The ability of gonococcal MinD to cause filamentation in E. coli is also dependent upon MinC, since MinDNg was unable to alter the minicell phenotype in strains lacking minCEc. These observations, as well as the interaction of MinDNg with MinCEc and the enlargement of gonococcal cells overexpressing both MinCNg and MinDNg, support the role of MinDNg as an activator of MinC in either N. gonorrhoeae or E. coli.
Although our evidence suggests that MinCNg and MinDNg are both required to inhibit cell division in the gonococcus, we did not detect a MinCNg-MinDNg interaction using yeast two-hybrid assays, which have been reported for the homologues of E. coli and B. subtilis (15, 24). It is possible that the gonococcal MinC fusion was unstable or poorly expressed in the yeast reporter strain. Furthermore, different yeast two-hybrid assays may detect different Min protein interactions, likely due to steric considerations, protein expression, and protein stability within yeast. An example includes the E. coli MinE self-interaction which was detected by one group using yeast two-hybrid methods (28), but the same interaction was not detected by another group using a different yeast two-hybrid system (15). Using a dot blot procedure, we have observed an interaction between purified His-tagged MinCNg and His-MinDNg; therefore, it is possible that the two proteins may interact, and we are investigating this further.
We have identified the self-interaction of MinDNg, as well as of MinDEc, by using yeast two-hybrid assays and have verified the MinDNg interaction using gel-filtration and sedimentation equilibrium techniques. It has been suggested that, in E. coli, oscillating MinDEc may self-assemble into a transient tube-like lattice structure at the cell pole following an initial interaction with a polar nucleation site(s) (35, 38). Our observations of an E. coli MinD self-interaction supports this theory. Furthermore, crystal structure analysis of MinD from Archaeoglobus fulgidus revealed that the ATPase sites are formed across two monomers, suggesting a role for dimerization during cell division (5). Since it is difficult to define a midcell in a coccus, especially one that divides along more than one plane, the interaction of MinDNg with itself may form a lattice that spans the inner membrane of the gonococcus to support cell division inhibition at all possible division sites except at key locations that define characteristic gonococcal septation planes. Although it is unknown whether gonococcal MinD or MinC exhibit intracellular movement, the formation of a stable internal lattice may preclude the need for MinDNg to oscillate in order to exert its effects. We note, however, that MinDNg is found in both soluble and insoluble gonococcal cell fractions; hence, the possibility of intracellular MinDNg trafficking remains. Localization studies on MinDNg within the gonococcus should provide more insight into this; however, methods other than visualizing GFP fusions may have to be used due to the small size of these cells.
The present study is the first to investigate the role of MinD from a coccus. We have shown that MinDNg is required for maintaining proper cell division, morphology, and viability in the gonococcus. Through the use of a convenient stable shuttle vector in N. gonorrhoeae, we were able to show that MinDNg can act with MinCNg to cause significant cell enlargement in gonococci. In addition, MinDNg is functional in E. coli, likely due to its conservation across species. The self-interaction of MinD proteins from either cocci or rods suggests that this interaction may be important for controlling cell division site selection in both cell types despite their morphological differences.
ACKNOWLEDGMENTS
This project was partially funded by grants from the Canadian Bacterial Diseases Network (Centers of Excellence) and the Canadian Institutes of Health Research (formerly Medical Research Council of Canada) to J.R.D. We thank T. J. Beveridge and D. Moyles, University of Guelph, for their electron microscopy work performed at the Guelph Regional SDM facility, which is partially funded by a Major Facilities Access Grant from NSERC (Natural Sciences and Engineering Research Council).
We thank P. Rippstein for electron microscopy performed at the Laboratory Pathology facilities, Ottawa Hospital, Civic Campus. We thank P. Anderson, University of Ottawa, for access to gel filtration chromatography equipment; P. Thibault, Institute of Biological Sciences, National Research Council of Canada, for mass spectrometry determinations; and P. de Boer, Case Western Reserve University School of Medicine, for providing E. coli strains PB103, DR105, PB104, and PB114. We also thank Mylène Thériault for assistance in cloning and light microscopy studies.
REFERENCES
- 1.Adler H I, Fisher W D, Cohen A, Hardigree A A. Miniature Escherichia coli cells deficient in DNA. Proc Natl Acad Sci USA. 1967;57:321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beveridge T J, Popkin T J, Cole R M. Electron Microscopy. In: Gerhardt P, Murray R G E, Woods W A, Krieg N R, editors. Methods for general and molecular microbiology. Washington, D.C.: ASM Press; 1994. pp. 44–70. [Google Scholar]
- 3.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 4.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordell S C, Löwe J. Crystal structure of the bacterial cell division regulator MinD. FEBS Lett. 2001;492:160–165. doi: 10.1016/s0014-5793(01)02216-5. [DOI] [PubMed] [Google Scholar]
- 6.Costa C S, Antòn D N. Conditional lethality of cell shape mutations of Salmonella typhimurium: rodA and mre mutants are lethal on solid but not liquid medium. Curr Microbiol. 1999;38:137–142. doi: 10.1007/pl00006777. [DOI] [PubMed] [Google Scholar]
- 7.de Boer P A J, Crossley R E. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in Escherichia coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 8.de Boer P A J, Crossley R E, Hand A R, Rothfield L I. The MinD protein is a membrane ATPase required for the correct placement of the Escherichia coli division site. EMBO J. 1991;10:4371–4380. doi: 10.1002/j.1460-2075.1991.tb05015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer P A J, Crossley R E, Rothfield L I. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J Bacteriol. 1988;170:2106–2112. doi: 10.1128/jb.170.5.2106-2112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer P A J, Crossley R E, Rothfield L I. Roles of MinC and MinD in the site-specific septation block mediated by the MinCDE system of Escherichia coli. J Bacteriol. 1992;174:63–70. doi: 10.1128/jb.174.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu X, Shih Y-L, Zhang Y, Rothfield L I. The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc Natl Acad Sci USA. 2001;98:980–985. doi: 10.1073/pnas.031549298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci USA. 1999;96:14819–14824. doi: 10.1073/pnas.96.26.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Lutkenhaus J. Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol. 1999;34:82–90. doi: 10.1046/j.1365-2958.1999.01575.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Lutkenhaus J. Analysis of MinC reveals two independent domains involved in interaction with MinD and FtsZ. J Bacteriol. 2000;182:3965–3971. doi: 10.1128/jb.182.14.3965-3971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Cao C, Lutkenhaus J. Interaction between FtsZ and inhibitors of cell division. J Bacteriol. 1996;178:5080–5085. doi: 10.1128/jb.178.17.5080-5085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janik A, Juni E, Heym G A. Genetic transformation as a tool for detection of Neisseria gonorrhoeae. J Clin Microbiol. 1976;4:71–81. doi: 10.1128/jcm.4.1.71-81.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson M L, Correia J J, Yphantis D A, Halvorson H R. Analysis of data from the analytical ultracentrifuge by non-linear least-squares techniques. Biophys J. 1981;36:575–588. doi: 10.1016/S0006-3495(81)84753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justice S S, Garcia-Lara J, Rothfield L I. Cell division inhibitors SulA and MinC/MinD block septum formation at different steps in the assembly of the Escherichia coli division machinery. Mol Microbiol. 2000;37:410–423. doi: 10.1046/j.1365-2958.2000.02007.x. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg D S, Jr, Peacock J R, Deacon W F, Brown L, Pirkle C I. Neisseria gonorrhoeae I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labie C, Bouché F, Bouché J-P. Minicell-forming mutants of Esherichia coli: suppression of both DicB- and MinD-dependent division inhibition by inactivation of the minC gene product. J Bacteriol. 1990;172:5852–5855. doi: 10.1128/jb.172.10.5852-5855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laue T M, Shah B D, Ridgeway T M, Pelletier S L. Computer-aidedinterpretation of analytical sedimentation data for proteins. In: Harding S E, Rowe A J, Horton J C, editors. Analytical ultracentrifugation in biochemistry and polymer science. Cambridge, United Kingdom: Royal Society of Chemistry; 1991. pp. 90–125. [Google Scholar]
- 22.Laue T M, Stafford W F., III Modern applications of analytical ultracentrifugation. Annu Rev Biophys Biomol Struct. 1999;28:75–100. doi: 10.1146/annurev.biophys.28.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Marinus M G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973;127:47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- 24.Marston A L, Errington J. Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol Microbiol. 1999;33:84–96. doi: 10.1046/j.1365-2958.1999.01450.x. [DOI] [PubMed] [Google Scholar]
- 25.Nanninga N. Morphogenesis of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:110–129. doi: 10.1128/mmbr.62.1.110-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagotto F, Salimnia H, Totten P A, Dillon J R. Development of a stable shuttle vector for Neisseria gonorrhoeae, Haemophilus sp. and other bacteria. Gene. 2000;244:13–19. doi: 10.1016/s0378-1119(99)00557-0. [DOI] [PubMed] [Google Scholar]
- 27.Picard F J, Dillon J R. Biochemical and genetic studies with arginine and proline auxotypes of Neisseria gonorrhoeae. Can J Microbiol. 1989;35:1069–1075. doi: 10.1139/m89-179. [DOI] [PubMed] [Google Scholar]
- 28.Pichoff S, Vollrath B, Touriol C, Bouche J. Deletion analysis of gene minE which encodes the topological specificity factor of cell division in Escherichia coli. Mol Microbiol. 1995;18:321–329. doi: 10.1111/j.1365-2958.1995.mmi_18020321.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez-Arcos, S., H. Salimnia, I. Bergevin, M. Paradis, and J. R. Dillon. Expression of Neisseria gonorrhoeae cell division genes ftsZ, ftsE, and minD is influenced by environmental conditions. Res. Microbiol., in press. [DOI] [PubMed]
- 30.Ramirez-Arcos S, Szeto J, Beveridge T J, Victor C, Francis F, Dillon J R. Deletion of the cell division inhibitor MinC results in lysis of Neisseria gonorrhoeae. Microbiology. 2001;147:225–237. doi: 10.1099/00221287-147-1-225. [DOI] [PubMed] [Google Scholar]
- 31.Raskin D M, de Boer P A J. The MinE ring: an FtsZ-independent cell structure required for selection of the correct division site in E. coli. Cell. 1997;91:685–694. doi: 10.1016/s0092-8674(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 32.Raskin D M, de Boer P A J. Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raskin D M, de Boer P A J. MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol. 1999;181:6419–6424. doi: 10.1128/jb.181.20.6419-6424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe B A, Lin S P, Song L, Yuan X, Clifton S, Ducey T, Lewis L, Dyer D W. Gonococcal genome sequencing project (funded by USPHS/NIH grant no. AI38399). Norman: University of Oklahoma; 2001. [Google Scholar]
- 35.Rowland S L, Fu X, Sayed M A, Zhang Y, Cook W R, Rothfield L I. Membrane redistribution of the Escherichia coli MinD protein induced by MinE. J Bacteriol. 2000;182:613–619. doi: 10.1128/jb.182.3.613-619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 37.Seifert H S, So M. Genetic systems in pathogenic neisseria. Methods Enzymol. 1991;204:342–357. doi: 10.1016/0076-6879(91)04017-i. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan S M, Maddock J R. Bacterial division: finding the dividing line. Curr Biol. 2000;10:249–252. doi: 10.1016/s0960-9822(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 39.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver K E. Enterococcal genetics. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 2000. pp. 259–271. [Google Scholar]
- 41.Weiss D S, Pogliano K, Carson M, Guzman L-M, Fraipont C, Nguyen-Distèche M, Losick R, Beckwith J. Localization of the Escherichia coli cell division protein FtsI (PBP3) to the division site and cell pole. Mol Microbiol. 1997;25:671–681. doi: 10.1046/j.1365-2958.1997.5041869.x. [DOI] [PubMed] [Google Scholar]
- 42.West S E, Clark V L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989;2:S92–S103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westling-Häggström B, Elmros T, Normark S, Winblad B. Growth pattern and cell division in Neisseria gonorrhoeae. J Bacteriol. 1977;29:333–342. doi: 10.1128/jb.129.1.333-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]