Abstract
This study evaluated the impacts of the probiotic, Lactobacillus sakei (L. sakei), and the extract of hawthorn, Crataegus elbursensis, on growth and immunity of the common carp exposed to acetamiprid. Fish (mean ± SE: 11.48 ± 0.1 g) feeding was done with formulated diets (T1 (control): no supplementation, T2: 1 × 106 CFU/g LS (Lactobacillus sakei), T3: 1 × 108 CFU/g LS, T4: 0.5% hawthorn extract (HWE), and T5: 1% HWE) for 60 days and then exposed to acetamiprid for 14 days. The growth performance improved in the fish fed LS at dietary level of 1 × 108 CFU/g, even after exposure to acetamiprid (P < 0.05). Intestinal Lactobacillus sakei (CFU/g) load increased (P < 0.05), following supplementation with the probiotic-enriched diet. The LS-treated fish had increases in the activity of digestive enzymes (P < 0.05). Both LS and HWE stimulated antioxidant enzymes and immune system components in serum and mucus (alkaline phosphatase (ALP), protease, total Ig, and lysozyme) (P < 0.05). However, the changes were different depending on the kind of the supplement. The malondialdehyde (MDA) levels decreased in HWE-treated fish after acetamiprid exposure (P < 0.05). Both LS and HWE reduced the liver metabolic enzymes (LDH, ALP, AST, ALT, and LDH) in serum both before and after exposure to the pesticide (P < 0.05). However, each enzyme exhibited a different change trend depending on the type of the supplement. HWE showed a stress-ameliorating effect, as glucose and cortisol levels declined in the HWE-treated fish (P < 0.05). This study indicated the immunomodulatory impacts of LS (1 × 108 CFU/g) and HWE (at dietary levels of 0.5–1%). The probiotic showed more performance compared to HWE. However, the HWE mitigated oxidative stress more efficiently than the probiotic.
1. Introduction
Today, the development of agriculture has been with widespread use of pesticides (PCs) to control pests [1]. PCs enter aquatic ecosystems through various ways, including field drainage and runoff from land, and adversely affect aquatic life including fish [2]. Since the use of PCs is an unavoidable issue, we must look for solutions to minimize the harmful impacts of these chemicals on aquatic organisms. Probiotics are applied in fish culture as dietary supplements for various purposes. It is a real fact that probiotics improve growth, digestibility, immune system, and resistance to diseases in fish [3–5]. Furthermore, it is reported that probiotics may be useful in ameliorating the toxicity induced by PCs [6–8]. However, this role is rarely studied in fish [9].
In addition to probiotics, herbal supplements and their compounds are known to enhance fish immunity [10–12] and reduce the toxic effects of PCs ([13, 14][15, 16]). However, these studies are few and we need to develop our knowledge about herbs and their role as toxin-ameliorating agent in fish. The immune and antioxidant-stimulating properties of medicinal herbs mainly return to a group of compounds such as flavonoids, carotenoids, alkaloids, tannins, lectins, terpenoids, and polyphenols in their biochemical composition [17–19].
The hawthorn is a species of the family Rosaceae that is used in traditional medicine for a long time [20]. In traditional medicine, hawthorn is used to treat digestive disruptions, blood stasis, hypertension, hyperlipidemia, amenorrhea, insomnia, arthritis, and muscle pains [21]. In addition, many studies have reported a variety of functions for hawthorn including antimicrobial, antioxidant, anti-inflammatory, and liver protective activities [22–24]. The biochemical composition of hawthorn fruit includes various phytochemicals such as phenolic acids, flavonoids, proanthocyanins, essential oils, and aromatic amines [23]. In fish, a few studies have used hawthorn in diet to improve the cellular and humoral immunity. In challenge with Vibrio harveyi, better immunity is obtained in hawthorn-treated golden pompano, Trachinotus ovatus [21]. Hawthorn, Crataegus mexicana, improved antioxidant and immune system in Longfin yellowtail and Seriola rivoliana [25]. Literatures have showed no study about protective effects of hawthorn against pesticides. Acetamiprid is a neonicotinoid insecticide, which is widely applied in agriculture for control of pests throughout the world [26, 27]. The aim of the present study was to examine the potentials of the hawthorn extract and the probiotic bacteria Lactobacillus sakei on growth performance, immune and antioxidant potentials, and resistance to acetamiprid toxicity in the common carp, Cyprinus carpio.
2. Materials and Methods
2.1. Probiotic Preparations
The probiotic Lactobacillus sakei subsp. sakei 15521 was prepared from the Iranian Research Organization for Science and Technology in Tehran, Iran, as lyophilized form and incubated in Rogosa and Sharpe agar (MRS) culture medium at 38°C for 48 h. Then, the medium was centrifuged for at 4°C (×4000g) 4 min and the supernatant discarded. The pellets were washed three times using phosphate buffer, bacteria added to the phosphate buffer again, and the experimental concentrations were determined at 600 nm by a spectrophotometer. Finally, the experimental concentrations of bacteria were added to the basic food [28].
2.2. Hawthorn Extract
The fruits of hawthorn were provided from Shast Kalate forest, Gorgan, Iran. The fruits were dried at 40°C in an oven. 200 g of dried hawthorn powder was added to 80% ethanol and stirred in an incubator with shaking for 24 h. After that, the suspended particles were removed using Whatman No. 1 paper. The extract was concentrated by a rotary evaporator at 40°C, pulverized by freeze drying, and stored at −18°C until use.
2.3. Antioxidant Power of the Extract
The antioxidant power of the extract was evaluated by four methods as follows and the results presented in Table 1.
Table 1.
Evaluation of the antioxidant power of hawthorn extract using different methods.
| Assay methods | |
|---|---|
| Total phenolics (mg GAE/g) | 65.2 ± 1.10 |
| Total flavonoids (mg QE/g) | 2.10 ± 0.11 |
| DPPH % inhibition percentage | 64.77 ± 2.20 |
| Total antioxidant capacity (μg/ml) | 0.52 ± 0.08 |
2.3.1. Antioxidant Activity by Free Radical Scavenging (DPPH) Method
100 μl of the extract was mixed with 0.2 ml of 0.1 M 2,2-diphenyl-1-picrylhydrazyl (DPPH) in 150 μM methanol, incubated at 22°C for 35 min, and then its absorbance read at 520 nm. Antioxidant activity was finally calculated using the following formula [29]:
| (1) |
2.3.2. Total Phenol Assay
Total phenolic evaluation was performed using the Folin–Ciocalteu method. Briefly, 0.12 ml of the extract was mixed with 0.05 ml of 10% Folin–Ciocalteu reagent. 30 μl of 20% saturated sodium carbonate solution was added to the solution, incubated for 1 h at 37°C, and absorbance was read at 735 nm after 10 min of storage under dark condition [30].
2.3.3. Total Flavonoid Assay
The total flavonoid content of the hawthorn extract was assayed at 510 nm using aluminum chloride method [31]. A mixture of 250 μl of the extract, 1250 μl of distilled water, and 75 μl of sodium nitrate solution (5%) was prepared, and then, aluminum chloride (10%) was added upon 5 min incubation at 23°C. After incubation, a solution of sodium hydroxide (500 μl) and distilled water (775 μl) was prepared and added to the solution and homogenized, and the adsorption spectrum was read.
2.3.4. Evaluation of Total Antioxidant Capacity (TAC)
To assay TAC, the extract solution (0.1 ml) and 1 ml of the reagent (0.6 M sulfuric acid + 28 mM sodium phosphate + 4 mM ammonium moly date) were poured into a tube and sealed for 1.5 h in water bath at 95°C. After 5 min, the absorbance was read at 695 nm [32].
2.4. Experimental Diets
To prepare experimental diets, firstly a commercial feed (protein: 34%, fat: 6%, fiber: 5%, moisture: 8%, ash: 9%, and phosphorus: 1%) was purchased as basal diet from Faradaneh company, Iran, which did not contain any supplements. The basal diet was thoroughly ground, mixed with some water to make dough, pelleted by grinder, and dried at 35°C. The experimental diets were prepared by adding Lactobacillus sakei at concentrations 1 × 106 and 1 × 108 CFU/g feed and hawthorn extract at levels of 0.5 and 1% according to Tan et al. [21]. Doses were selected based on positive results from previous reports on the growth and health of other aquatic animals [21, 33, 34].
2.5. Fish and Experimental Procedure
650 common carps (8.2 ± 0.24 g; mean ± SE) were provided from a local farm in Khuzestan province (Shushtar city, Iran) and transferred to a local farm in Tehran. The specimens were stocked in 1000 l tanks and acclimatized for 2 weeks with culture condition (temperature: 25 ± 0.6°C, dissolved oxygen: 6.8 ± 0.5 mg/l, pH: 7.4 ± 0.3, nonionized ammonia: 0.04 ± 0.03). During acclimation period, fish were fed basal diet 3 times a day (2.5% of body weight). After acclimation period, fish (n = 600) (11.48 ± 0.1 g; mean ± SE) were distributed into 15 tanks (40 fish/tank) as four experimental treatments (T2: fish fed diet containing 1 × 106 probiotic, T3: fish fed diet containing 1 × 108 probiotic, T4: fish fed diet containing 0.5% hawthorn extract, T5: fish fed diet containing 1% hawthorn extract) and one control group (T1 (control): nonsupplemented fish) in three replicates. Fish were fed experimental diets at a feeding rate of 2.5% of body weight for 60 days [35].
2.6. Acetamiprid and Exposure Trial
Before exposure test, the lethal and acute dosages of acetamiprid for the fish were determined to select experimental concentrations. To estimate lethal range of acetamiprid, fish (n = 30, 10/tank) were exposed to dosages of 0, 6, 8, 10, 12, 14, 16, and 18 mg/l of acetamiprid for 96 h to estimate the LC50 [36]. The mortality of the fish was recorded upon exposure with time at 24, 48, 72, and 96 h. The probit statistical analysis was used to estimate the lethal concentrations (Table 2) inducing 10% (LC10), 30% (LC30), 50% (LC50), and 70% (LC70) mortality.
Table 2.
Lethal concentrations (LC10-90) of acetamiprid (Mospilan) over time (24-96 h) for Cyprinus carpio.
| Point | Concentration (mg/l) | |||||||
|---|---|---|---|---|---|---|---|---|
| LC50 24 h |
Upper bound Lower bound |
LC50 48 h |
Upper bound Lower bound |
LC50 72 h |
Upper bound Lower bound |
LC50 96 h |
Upper bound Lower bound |
|
| LC10 | 9.87 | 10.76 8.58 |
8.64 | 9.65 7.18 |
7.75 | 8.78 6.27 |
6.02 | 7.11 4.40 |
| LC30 | 12.18 | 12.87 11.37 |
11.30 | 12.04 10.39 |
10.34 | 11.09 9.41 |
8.49 | 9.28 7.45 |
| LC50 | 13.78 |
14.52
13.10 |
13.14 |
13.92
12.40 |
12.13 |
12.87
11.40 |
10.21 |
10.92
9.44 |
| LC70 | 15.39 | 16.37 14.64 |
14.98 | 16.04 14.17 |
13.92 | 14.88 13.17 |
11.92 | 12.76 11.21 |
| LC90 | 17.70 | 19.24 16.67 |
17.63 | 19.32 16.49 |
16.51 | 18.02 15.47 |
14.40 | 15.73 13.47 |
After feeding period, fish were exposed to acetamiprid at a concentration of 25% of LC50 for 14 days [16]. The static renewal design was used with the daily water change of 70%.
2.7. Growth Indices
After the feeding experiment, feeding was stopped for 24 h and fish were anesthetized using 100 mg/l eugenol. Growth and nutrition indices were calculated by sampling all fish per tank using the following formulas [35]:
| (2) |
where Fiw is the final weight and Iwi is the initial weight.
| (3) |
2.8. Sampling
The blood and mucus samples were taken after feeding period and after 14 days of exposure to acetamiprid.
2.9. Digestive Enzyme Activity
To determine digestive enzyme activities, fish (n = 3/tank) were randomly sampled, euthanized using high dosage of eugenol, dissected, and after the intestine tissue separated. The intestine was emptied and weighed and then homogenized mechanically using Tris buffer (Heidolph® SilentCrusher-M, Heidolph, Nuremberg, Germany) [37]. The supernatant was obtained by centrifugation at 4°C (×6000g for 10 min) and stored at −80°C. Amylase was estimated at 600 nm upon reaction of the enzyme with 2% starch as substrate [38]. Lipase was assayed at 405 nm upon the action of the enzyme on polyphenol myristate, as target [39]. Protease enzyme was measured at 440 nm by García-Carreño [40] method. Azo-casein was used as target for the enzyme.
2.10. Intestinal Microbial Population
After disinfecting of the skin by 70% ethanol, the fish abdominal cavity was dissected and the intestine separated, washed, and homogenized in phosphate buffer (PBS, pH = 7.2) using a tissue homogenizer. The homogenized solution was diluted in phosphate buffer. The bacterial colonies were grown on MRS (Merck, Germany) and TSA medium at 30°C for 48 h to assay lactic acid bacteria (LAB) and total intestinal bacteria (TBC), respectively [41].
2.11. Immunological Assays
To determine immune parameters of serum, fish (n = 3/tank) were anesthetized by eugenol (90 mg/l), and blood was taken from caudal vein using a 1.5 ml syringe, stored in heparinized tube, left at 23°C for 90 min h, and centrifuged at 4°C (3500 × g, 8 min) to collect serum. The serum was stored at −75°C for further assays. Mucus sampling was done by putting fish in polyethylene bags containing saline solution. The supernatant was separated after 3 min through centrifuging of the mucus at 4°C (2650 × g for 12 min) [42].
Serum and mucosal lysozyme activity was measured at 550 nm according to Mirghaed et al. [43] method based on the ability of serum or mucus in lysis of Micrococcus luteus.
Complement activity was measured using sheep red blood cells [44]. 500 μl of serum sample was diluted sequentially (pH = 7) using veronal buffer (EGTA + gelatin + magnesium, pH = 7). 200 μl of red blood cell suspension was added to each tube. The tubes were incubated for 15 min at 15°C. Hemolysis was stopped by adding 10 mmol gelatin veronal buffer−EDTA. After centrifugation, the amount of hemolysis was measured in supernatant at 414 nm.
Total Ig content was calculated based on Siwicki [45] method through calculating the difference between protein content of serum and mucus before and after precipitating by 12% polyethylene glycol. The activity of myeloperoxidase (MPO) was estimated at 450 nm by a microplate reader upon reaction of the enzyme with tetramethylbenzidine hydrochloride as target [46]. Nitroblue tetrazolium (NBT) reduction was assayed at 540 nm upon reaction of the samples with N,N-dimethylformamide [47]. The protease activity was assayed at 450 nm upon reaction of the enzyme with azo-casein (100 mM) as target at 30°C for 20 h [48].
2.12. Biochemical and Enzymatic Assays
The antioxidant potentials were evaluated by estimating glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) using assay kits (Zellbio, Berlin, Germany) and manufacturers' instructions. SOD was assayed upon reduction of cytochrome C [49]. CAT was estimated upon decomposition rate of hydrogen peroxide [50]. Malondialdehyde (MDA) as an indicator of lipid peroxidation was measured based on its reaction with thiobarbituric acid (TBARS) [51].
Liver enzymes were assayed using commercial kits (Pars Azmun Co., Tehran, Iran) for ALP, AST, and ALT according to the manufacturer's protocol [52].
An ELISA method was applied to assay cortisol levels using an assay kit (IBL Co., Germany). Glucose changes were also measured by Pars Azmun commercial kit, Iran [52]. Total protein in serum was measured by the Bradford [53] method. Also, the albumin concentration was estimated by colorimetric method using the Nicholson method at 620 nm [54]. Globulin was assayed by calculation of the difference of protein and albumin content in blood. The activity of alkaline phosphatase activity in mucus was measured by the Pars Azmun commercial kit, Iran, at 405 nm according to manufacturer's instructions [55].
2.13. Data Analysis
The data (mean ± SE) was analysed by version 16 of SPSS software. After normality test by the Kolmogorov-Smirnov test, the differences among the means were evaluated by one-way analysis of variance, followed by the comparison of the means by Tukey test.
3. Results
3.1. Growth Parameters
The results of fish growth are presented in Table 3. The final weight (FW) and WG increased (P < 0.05) in fish of 1 × 106 (T2) and 1 × 108 (T3) in comparison with nontreated fish. SGR had similar values (P > 0.05) among nontreated fish, 1 × 106 probiotic and hawthorn extract treatments. The FCR values with the lowest value in T3 were lower in T2 and T3 than in nontreated fish and other treatments (P < 0.05). FCR values had no differences between fish 0.5% and 1% hawthorn extract (P > 0.05). SR values exhibited no differences among control and other experimental groups (P > 0.05).
Table 3.
The growth performance and survival rate in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets.
| Status | Parameters | T 1 (control) | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|---|
| Before challenge | IW (g) | 11.53 ± 0.26 | 11.46 ± 0.29 | 11.36 ± 0.18 | 11.36 ± 0.26 | 11.66 ± 0.24 |
| FW (g) | 42.70 ± 1.04c | 48.00 ± 0.45ab | 51.23 ± 0.88a | 45.23 ± 0.67bc | 43.46 ± 0.78c | |
| WG (g) | 31.16 ± 0.90c | 36.53 ± 0.74ab | 39.86 ± 0.89a | 33.86 ± 0.93bc | 31.80 ± 0.70c | |
| FCR | 1.98 ± 0.08a | 1.70 ± 0.05bc | 1.48 ± 0.04c | 1.79 ± 0.03ab | 1.93 ± 0.05ab | |
| SGR (%d−1) | 2.18 ± 0.03b | 2.43 ± 0.10ab | 2.58 ± 0.10a | 2.30 ± 0.06ab | 2.19 ± 0.03b | |
| SR (%) | 95.33 ± 2.33 | 96.66 ± 2.02 | 97.66 ± 2.33 | 96.66 ± 1.66 | 95.00 ± 1.15 | |
|
| ||||||
| After challenge | IW (g) | 42.70 ± 1.15c | 47.56 ± 0.77ab | 51.10 ± 0.98a | 45.23 ± 0.67bc | 43.46 ± 1.06bc |
| FW (g) | 48.33 ± 1.25d | 56.73 ± 0.66ab | 60.93 ± 1.50a | 53.36 ± 0.75bc | 51.26 ± 0.63cd | |
| WG (g) | 5.63 ± 0.31b | 9.16 ± 0.81 | 9.83 ± 0.70 | 8.13 ± 0.66ab | 7.80 ± 0.79ab | |
| FCR | 2.58 ± 0.12a | 1.63 ± 0.11b | 1.52 ± 0.07b | 1.76 ± 0.12b | 1.90 ± 0.12b | |
| SGR (%d−1) | 0.88 ± 0.04 | 1.25 ± 0.11 | 1.25 ± 0.07 | 1.18 ± 0.09 | 1.18 ± 0.13 | |
| SR (%) | 93.33 ± 1.66a | 93.33 ± 1.66a | 93.33 ± 3.33a | 95.00 ± 2.88a | 93.33 ± 1.66a | |
IW: initial weight; FW: final weight; WG: weight gain; FCR: feed conversion ratio; SGR: specific growth rate; SR: survival rate. T1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
After exposure to acetamiprid, FW values of T2, T3, and T4 had higher values in comparison with nontreated fish (P < 0.05). No differences were found in FW between control and T5 (P > 0.05). WG values in fish of 1 × 106 and 1 × 108 probiotic were higher than those in control (P < 0.05). FCR values with the lowest value in T3 were lower in fish of 1 × 106 and 1 × 108 probiotic than in control and other treatments (P < 0.05). FCR values had clear decreases in all supplemented treatments in comparison with control (P < 0.05). Treatment T3 had lower FCR in comparison with others (P < 0.05). SR values had no differences (P > 0.05) in all experimental groups.
3.2. Digestive Enzymes
Digestive enzyme activity of the groups is presented in Table 4. Protease and amylase activities in the probiotic treatments increased in comparison with control (P < 0.05). Similar values were found in protease and amylase activities of control and fish of 0.5% and 1% hawthorn extract (P > 0.05). Lipase activity had no differences (P > 0.05) among all experimental groups after the feeding period.
Table 4.
The activity of digestive enzymes in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets.
| Parameters | T 1 (control) | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|
| Amylase (U/mg protein) | 1.10 ± 0.20c | 2.50 ± 0.23ab | 3.00 ± 0.17a | 1.60 ± 0.22bc | 1.53 ± 0.20bc |
| Protease (U/mg protein) | 5.23 ± 0.50c | 8.53 ± 0.50a | 8.16 ± 0.61ab | 7.03 ± 0.43abc | 5.86 ± 0.52bc |
| Lipase (U/mg protein) | 11.23 ± 0.67 | 11.90 ± 0.60 | 12.03 ± 0.57 | 11.33 ± 0.81 | 10.33 ± 0.52 |
T 1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
3.3. Bacterial Load of the Intestine
The concentration of Lactobacillus sakei (CFU/g) in intestine tissue considerably increased in T2 and T3 in comparison with control and T4 and T5 (supplementary file (available here), P < 0.05). Bacterial concentration of control had no differences with those in fish of 0.5% and 1% hawthorn extract (supplementary file, P > 0.05). Total bacterial concentration (TBC) in the intestine also exhibited no differences (P < 0.05) in all groups (supplementary file).
3.4. Serum Immune Parameters
The results of the immune parameters are presented in Table 5. The lysozyme activity elevated in the supplemented groups than in control (P < 0.05). Lysozyme activity had similar values (P > 0.05) among all supplemented groups. The Ig levels and ACH50 activity had no differences (P > 0.05) in all groups. MPO activity increased (P < 0.05) in probiotic treatments and 0.5% hawthorn extract in comparison with control. The maximum MPO was observed in T3 (P < 0.05). NBT activity increased in the probiotic supplemented fish in comparison with control (P < 0.05). Protease activity showed significant increases (P < 0.05) in T5 in comparison with control, while the enzyme activity of control had no differences with other treatments (P > 0.05).
Table 5.
The serum immune parameters in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets.
| Status | Parameters | T 1 (control) | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|---|
| Before challenge | Lysozyme (U/ml) | 27.33 ± 1.01c | 36.26 ± 1.39ab | 40.06 ± 0.97a | 34.60 ± 1.15b | 35.76 ± 0.86ab |
| ACH50 (U/ml) | 114.83 ± 3.44 | 115.56 ± 3.90 | 116.00 ± 3.32 | 123.03 ± 2.58 | 118.00 ± 3.78 | |
| Total Ig (mg/dl) | 18.90 ± 1.09 | 22.83 ± 1.01 | 21.86 ± 1.07 | 21.86 ± 1.04 | 22.90 ± 1.02 | |
| NBT (OD at 540) | 0.21 ± 0.01b | 0.39 ± 0.03a | 0.42 ± 0.03a | 0.33 ± 0.04ab | 0.34 ± 0.03ab | |
| MPO (OD at 450) | 1.23 ± 0.28c | 3.06 ± 0.26ab | 3.50 ± 0.32a | 2.56 ± 0.29ab | 2.10 ± 0.20bc | |
| Protease (%) | 4.43 ± 0.34b | 4.73 ± 0.29ab | 5.36 ± 0.40ab | 5.53 ± 0.26ab | 6.00 ± 0.28a | |
|
| ||||||
| After challenge | Lysozyme (U/ml) | 20.23 ± 0.95b | 25.40 ± 1.06a | 26.26 ± 0.72a | 28.03 ± 0.83a | 28.90 ± 0.97a |
| ACH50 (U/ml) | 104.50 ± 2.36c | 107.66 ± 2.04bc | 109.90 ± 1.59abc | 118.70 ± 1.64a | 115.00 ± 2.88ab | |
| Total Ig (mg/dl) | 14.56 ± 0.82b | 18.16 ± 0.72ab | 18.60 ± 0.87a | 18.20 ± 0.75ab | 18.53 ± 0.72a | |
| NBT (OD at 540) | 0.16 ± 0.01b | 0.23 ± 0.00ab | 0.25 ± 0.02a | 0.21 ± 0.01ab | 0.22 ± 0.01ab | |
| MPO (OD at 450) | 1.03 ± 0.14b | 1.60 ± 0.20ab | 2.50 ± 0.34a | 2.20 ± 0.17a | 1.60 ± 0.20ab | |
| Protease (%) | 2.36 ± 0.34 | 3.43 ± 0.40 | 3.65 ± 0.45 | 3.43 ± 0.47 | 3.40 ± 0.45 | |
Total Ig: total immunoglobulin; ACH50: alternative complement activity; NBT: nitroblue tetrazolium; MPO: myeloperoxidase activity. T1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
After exposure to acetamiprid, the lysozyme exhibited more activity (P < 0.05) in all treatments than in control. Lysozyme showed similar values (P > 0.05) in supplemented fish. ACH50 elevated (P < 0.05) in T4 and T5 in comparison with control. ACH50 activity had no differences (P > 0.05) among nontreated and probiotic-treated fish. The Ig was higher (P < 0.05) in fish of 1 × 108 probiotic and 5% hawthorn extract than in nontreated fish. MPO activity raised in fish of 1 × 108 probiotic and 1% hawthorn extract (P < 0.05), while it had no differences in supplemented fish (P > 0.05). NBT raised in T3 in comparison with control (P < 0.05).
3.5. Immune Parameters of Mucus
The mucosal lysozyme elevated in T3 and T5 in comparison with control after the feeding period (Figure 1(a), P < 0.05). Similar activity was observed for lysozyme in all supplemented groups (Figure 1(a), P > 0.05). The Ig levels with maximum levels in T5 elevated in all supplemented fish (Figure 1(b), P > 0.05), while it showed similar values (P > 0.05) among fish of 1 × 108 probiotic and hawthorn extract treatments (Figure 1(b)). ALP (Figure 1(c)) increased (P < 0.05) in the probiotic and 1% hawthorn extract treatment in comparison with control. ALP had similar values in all supplemented fish (Figure 1(c), P > 0.05). Protease raised in T3 compared to control (Figure 1(d), P < 0.05). Protease exhibited similar activity in others (Figure 1(d), P > 0.05).
Figure 1.
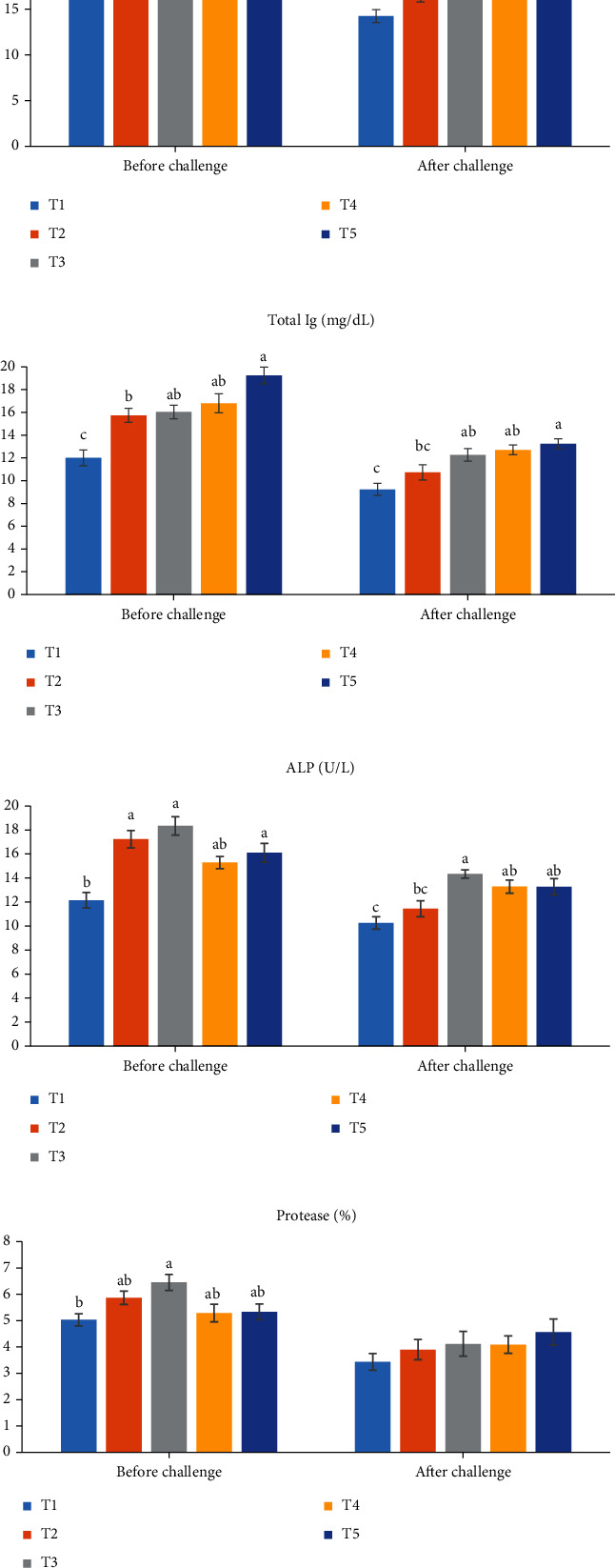
The mucus immune parameters in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets: T1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Total Ig: total immunoglobulin; ALP: alkaline phosphatase activity. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
After exposure to acetamiprid, the lysozyme (Figure 1(a)) and ALP (Figure 1(c)) activities and Ig (Figure 1(b)) levels elevated (P < 0.05) in T3, T4, and T5 in comparison with control, while those had similar values (P > 0.05) in control and T2 (Figures 1(a)–1(c)). The enzyme protease activity was similar (P > 0.05) for all groups (Figure 1(d)).
3.6. Blood Biochemicals
The results of the liver metabolic enzymes are presented in Table 6. Liver metabolic enzymes showed significant changes in serum in the treatments after feeding period (P < 0.05). AST and ALT activities declined in T5 in comparison with control (P < 0.05). In addition, AST and ALT showed similar activities among all supplemented groups (P > 0.05). ALP activity had no differences among all treatments (P > 0.05). LDH activity decreased (P < 0.05) in T3, T4, and T5 in comparison with control and T2. The lowest LDH activity (P < 0.05) was related to T5.
Table 6.
The liver metabolic enzymes in blood in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets.
| Status | Parameters | T 1 (control) | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|---|
| Before challenge | ALT (U/l) | 24.83 ± 1.87a | 23.16 ± 1.58ab | 21.00 ± 1.73ab | 18.50 ± 1.44ab | 16.66 ± 1.20b |
| AST (U/l) | 93.33 ± 4.37a | 88.33 ± 5.81ab | 78.00 ± 4.93ab | 75.66 ± 6.35ab | 64.66 ± 5.20b | |
| ALP (U/l) | 135.00 ± 8.66 | 127.66 ± 6.48 | 121.00 ± 7.81 | 117.00 ± 6.08 | 107.66 ± 5.36 | |
| LDH (U/l) | 586.00 ± 10.21a | 553.33 ± 14.74ab | 521.00 ± 11.59b | 462.33 ± 10.39c | 446.33 ± 14.83c | |
|
| ||||||
| After challenge | ALT (U/l) | 27.83 ± 1.87a | 25.83 ± 1.64ab | 24.33 ± 1.45ab | 20.63 ± 2.58ab | 17.66 ± 1.20b |
| AST (U/l) | 119.66 ± 4.09a | 108.33 ± 3.75ab | 97.33 ± 4.66bc | 84.66 ± 4.80cd | 73.33 ± 6.06d | |
| ALP (U/l) | 160.66 ± 5.81a | 144.33 ± 5.36ab | 132.33 ± 7.21bc | 127.00 ± 5.50bc | 110.66 ± 4.97c | |
| LDH (U/l) | 735.00 ± 13.22a | 661.66 ± 17.05b | 622.66 ± 11.85b | 483.33 ± 41.52c | 471.00 ± 18.00c | |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; LDH: lactate dehydrogenase. T1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
The cortisol levels (Table 7, P < 0.05) declined in T4 and T5 in comparison with nontreated fish. Cortisol levels in control showed similar levels with fish of 1 × 106 and 1 × 108 probiotic (P > 0.05). Glucose had similar values (P > 0.05) among experimental groups (Table 7). The globulin, total protein, and albumin levels increased (P < 0.05) in T4 and T5 in comparison with control (Table 7). Total protein, albumin, and globulin content of control had no differences (P < 0.05) with T2 and T3 (Table 7, P > 0.05). The highest levels of total protein, globulin, and albumin were found in T4 (Table 7).
Table 7.
The serum biochemical profile in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets.
| Status | Parameters | T 1 (control) | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|---|
| Before challenge | Cortisol (ng/ml) | 187.16 ± 3.94a | 172.00 ± 4.04ab | 168.66 ± 5.20ab | 157.66 ± 4.33b | 153.00 ± 5.29b |
| Glucose (mg/dl) | 79.00 ± 3.21 | 75.50 ± 3.17 | 71.33 ± 2.60 | 77.00 ± 3.78 | 71.33 ± 2.40 | |
| TP (g/dl) | 2.95 ± 0.13b | 3.33 ± 0.09ab | 3.32 ± 0.14ab | 3.89 ± 0.22a | 3.82 ± 0.14a | |
| Globulin (g/dl) | 1.65 ± 0.13b | 2.00 ± 0.05ab | 1.94 ± 0.04ab | 2.21 ± 0.14a | 2.16 ± 0.12a | |
| Albumin (g/dl) | 1.30 ± 0.05c | 1.33 ± 0.04bc | 1.38 ± 0.10abc | 1.68 ± 0.09a | 1.66 ± 0.03ab | |
|
| ||||||
| After challenge | Cortisol (ng/ml) | 207.66 ± 4.05a | 193.66 ± 4.40ab | 185.66 ± 4.63b | 165.00 ± 2.88c | 157.00 ± 3.60c |
| Glucose (mg/dl) | 88.33 ± 2.60a | 82.66 ± 2.90ab | 84.66 ± 2.72ab | 80.00 ± 1.73ab | 75.66 ± 2.33b | |
| TP (g/dl) | 2.77 ± 0.13b | 3.03 ± 0.09ab | 3.19 ± 0.11ab | 3.53 ± 0.19a | 3.47 ± 0.16a | |
| Globulin (g/dl) | 1.57 ± 0.12 | 1.77 ± 0.06 | 1.68 ± 0.06 | 1.93 ± 0.06 | 1.98 ± 0.10 | |
| Albumin (g/dl) | 1.20 ± 0.05 | 1.26 ± 0.05 | 1.50 ± 0.16 | 1.59 ± 0.15 | 1.49 ± 0.05 | |
TP: total protein. T1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
The MDA (Table 8) levels and CAT activity (Table 8) showed no changes among all groups (P > 0.05). SOD activity (Table 8) raised (P < 0.05) in T3, T4, and T5 in comparison with control. Maximum SOD (P < 0.05) was observed in T5. GPx (Table 8) was higher in T5 than in control (P < 0.05).
Table 8.
The serum antioxidant enzymes in common carp, Cyprinus carpio, after 60 days of supplementation with experimental diets.
| Status | Parameters | T 1 (control) | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|---|
| Before challenge | MDA (μmol/l) | 50.23 ± 2.22 | 48.56 ± 1.84 | 48.30 ± 2.04 | 45.43 ± 2.52 | 42.23 ± 1.30 |
| SOD (U/ml) | 30.43 ± 0.80b | 32.40 ± 0.94ab | 34.36 ± 0.74a | 35.96 ± 0.72a | 36.13 ± 0.80a | |
| CAT (U/ml) | 112.16 ± 3.60a | 113.16 ± 2.89a | 115.00 ± 2.68a | 114.33 ± 4.33a | 115.66 ± 3.32a | |
| GPx (U/ml) | 150.03 ± 2.56b | 155.00 ± 2.88ab | 157.16 ± 3.60ab | 160.00 ± 2.02ab | 165.00 ± 1.73a | |
|
| ||||||
| After challenge | MDA (μmol/l) | 61.23 ± 2.91a | 56.16 ± 2.36ab | 56.23 ± 2.40ab | 49.60 ± 2.26bc | 43.90 ± 1.37c |
| SOD (U/ml) | 29.70 ± 0.95c | 32.90 ± 1.06bc | 34.53 ± 0.89ab | 36.80 ± 1.04ab | 37.50 ± 0.68a | |
| CAT (U/ml) | 110.26 ± 3.42 | 110.50 ± 2.59 | 113.06 ± 2.73 | 113.16 ± 4.18 | 116.80 ± 3.34 | |
| GPx (U/ml) | 145.83 ± 2.51c | 152.43 ± 2.69bc | 155.23 ± 3.03abc | 160.00 ± 3.88ab | 167.33 ± 1.76a | |
MDA: malondialdehyde activity; SOD: superoxide dismutase activity; CAT: catalase activity; GPx: glutathione peroxidase activity. T1 (control): nonsupplemented fish; T2: fish fed diet containing 1 × 106 probiotic; T3: fish fed diet containing 1 × 108 probiotic; T4: fish fed diet containing 0.5% hawthorn extract; T5: fish fed diet containing 1% hawthorn extract. Data represented as mean ± SE. Different letters in the same row indicate significant differences (P < 0.05).
After exposure to acetamiprid (Table 6), the ALT activity decreased in T5 in comparison with control (P < 0.05). ALT activity exhibited similar values (P > 0.05) among all supplemented groups. ALP and AST activities decreased in T3, T4, and T5 in comparison with control (P < 0.05). The lowest ALP and AST activities were found in T5 (Table 6, P < 0.05). There were no differences in ALP and AST activities between control and T2 (P > 0.05). The LDH activity of all treatments decreased in comparison with control (P < 0.05). The lowest LDH activity (P < 0.05) was related to T4 and T5.
Cortisol (Table 7) decreased in T3, T4, and T5 in comparison with nontreated fish (P < 0.05). Cortisol concentrations had similar values in control and T2 (P > 0.05). Glucose (Table 7) declined in T5 in comparison with nontreated fish (P < 0.05). Total protein (Table 7) increased in T4 and T5 in comparison with nontreated fish (P < 0.05). Albumin and globulin (Table 7) content in control had similar values (P > 0.05) with the supplemented fish.
The MDA (Table 8) levels reduced (P < 0.05) in T4 and T5 in comparison with control. SOD activity packed (P < 0.05) in fish of 1 × 108 probiotic and 0.5-1% hawthorn extract in comparison with control, with maximum activity in T5. GPx activity raised (P < 0.05) in hawthorn extract treatments in comparison with control. GPx in nontreated fish was similar to fish of 1 × 106 and 1 × 108 probiotic (P > 0.05). The CAT activity in treated fish showed no changes (P > 0.05) after exposure to acetamiprid.
4. Discussion
The application of probiotics and herbs has increased in aquaculture to improve fish growth and immunity. We investigated the prompting impacts of the probiotic, Lactobacillus sakei, and a medicinal plant, hawthorn extract (HWE), on growth, immunity, and the toxin resistance ability in the common carp. The growth results showed that the probiotic at high dietary level (1 × 108 CFU/g) can effectively improve the growth performance (i.e., FW, WG, SGR, and FCR), even after exposure to acetamiprid, as the highest growth efficiency was related 1 × 108 CFU/g probiotic. In addition, we observed increases in the intestinal load of lactic acid bacteria in the probiotic-supplemented fish in comparison with control, indicating the efficient modulation of intestinal bacterial flora by the dietary probiotic. By contrast, such effects were not observed in fish fed HWE (i.e., T4: 0.5% HWE and T5: 1% HWE). Therefore, these results indicate that the probiotic has more improving effect on growth compared to the plant extract. The enhancing effect of L. sakei on growth may return to the general role of probiotics in improving digestion and absorption of the nutrients, digestive enzyme activities, and production of growth-stimulant metabolites and in excluding the pathogenic bacteria in the gut, as previously shown in many researches [56, 57]. In this regard, we observed elevations in the activity of amylase and protease in response to dietary L. sakei. As the results showed, the use of the probiotic improved growth indices, even after exposure of the fish to acetamiprid. Although the role of probiotics in reducing the pesticide toxicity has been experimentally reported [6, 7, 58, 59], it is rarely studied in fish [9, 60]. The probiotic bacteria may break down pesticides over bioremediation process using the enzymes including phosphotriesterases, phosphatases, carboxylesterases, and organophosphate hydrolases to meet their needs to nitrogen, carbon, and energy [61, 62]. In addition, fermentation is another process that probiotic bacteria may use to metabolize pesticides [6]. Similarly, a combination of the probiotics (Bacillus subtilis + Lactococcus lactis and L. lactis + Saccharomyces cerevisiae) in diet of Indian carp Labeo rohita mitigated the retarded growth in fenvalerate-exposed fish, which was related to the prompting effects of the probiotic on food consumption [60]. In Java barb, Barbonymus gonionotus, the use of Lactobacillus spp. in diet reasonably restored the reduced growth in fish exposed to the pesticide Sumithion [9].
Many researches have shown the role of probiotics and herbs in improving the fish immune system [63–66]. The immune-stimulating properties of Lactobacillus sakei is also reported in fish [33, 34, 67]. The results of this study confirmed this immunogenic role, because we observed increases in immune components and also antioxidant enzymes in the supplemented fish; however, the change trends were different depending on the kind of the supplement. In this regard, fish fed diet containing 1 × 108 probiotic and 0.5 and 1% hawthorn extract showed higher values of antioxidant and immune components in almost all treatments both before and after exposure to acetamiprid. However, hawthorn treatments seem to be more effective in improving the immune and antioxidant system than the probiotic treatments. Although the stimulating properties of probiotics on fish immunity are widely reported, there is little data about this function with pesticides [68]. Probiotics improve the immune system in a variety of ways, such as modulating of intestinal bacterial flora, competing with and eliminating pathogenic bacteria in the gut, stimulating the activity of innate immune system components such as lysozyme, complement, and immunoglobulin, and upregulation of immune-related gene expressions [65, 69–71]. Also, as mentioned earlier, probiotics can biodegradate and fermentate the pesticides in the gastrointestinal tract, which may reduce their immunotoxic impacts [6, 61, 62]. Although the role of probiotics as antioxidants and also their inducing effect on enzymatic antioxidant enzyme system are reported, its mechanism is still unknown. Antioxidant function of probiotics in fish is attributed to their prompting impacts on modulation of antioxidant genes. For example, in the gilthead seabream Sparus aurata, the expression of SOD and GPx in the mucus is upregulated in Shewanella putrefaciens- and Bacillus-treated fish [72]. Similarly, the SOD and GPx values were stimulated in response to dietary B. licheniformis in O. mossambicus [73]. In Indian carp, Labeo rohita, the diet containing Bacillus subtilis + Lactococcus lactis and L. lactis + Saccharomyces cerevisiae mitigated the toxic effects of the insecticide fenvalerate. In their study, the probiotic-containing diets had sparing effects on SOD and CAT activities. In addition, the probiotic improved NBT, total protein, and albumin values in blood of fenvalerate-exposed fish [60].
There were many studies reporting increases in MDA levels as the main marker of oxidative stress, following exposure to pesticides in fish [74, 75]. MDA levels declined in hawthorn extract-supplemented fish, which may suggest an ameliorating effect for the supplement on the oxidative stress. The mitigating role of medicinal plants on pesticide-induced oxidative stress has been also reported in other studies, which is usually attributed to their stimulating effects on antioxidant enzymes and the presence of some compounds such as phenolic compounds and flavonoids in their biochemical composition [16, 76, 77].
In blood, increased concentration of hepatic enzymes (ME) may indirectly reflect liver dysfunctions and damages, although ME are not generally specific [78]. The use of the probiotic and hawthorn reduced LME levels both before and after exposure to the pesticide. However, each enzyme showed different change trends depending on the type of the supplement. Decreased levels of LME may demonstrate a protective effect for the supplements on the liver [79]. In this regard, it seems that the use of 1% hawthorn has more performance than other supplements, because LME decreased in this treatment. Similarly, the reducing effects of probiotics [80] and herbs [77, 81, 82] on LME are previously reported in pesticide-treated fish.
As the main stress hormone, cortisol is released in blood after exposure to variety of stressors. Cortisol breaks down hepatic glycogen stores to release glucose into the bloodstream to meet energetic costs of the stress [83, 84]. In this study, hawthorn extract showed a stress-ameliorating effect, because cortisol and glucose levels reduced in HWE-treated fish both before and after exposure to pesticide. Pesticide-induced stress in fish and following increases in cortisol and glucose have been reported in many studies [85–87]. The current results were in line with previous studies that have reported the mitigating effect of herbs on stress caused by pesticides [13, 16, 88, 89]. In probiotic treatments, the cortisol levels decreased only in the treatment 1 × 108 CFC/g probiotic after exposure to the pesticide, which may return to the mitigating impacts of probiotics on stress [90, 91]. In this study, although fish growth and immunity improved in the supplemented fish, the survival rate was not affected over exposure to the pesticide.
The outputs of this study revealed the immunomodulatory properties of LS (at dietary levels of 1 × 108 CFU/g) and HWE (at dietary levels of 0.5-1%) in the common carp. The probiotic exhibited more growth-prompting effect compared to HWE. However, the HWE mitigated oxidative stress more efficiently compared to the probiotic.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experiments were performed following the protocol approved by the committee of ethics of the Baharavaran Nastaran Agricultural Applied Scientific Training Center, Applied Scientific University, Qom, Iran (1063; 2021).
Conflicts of Interest
The authors have no conflict of interest.
Authors' Contributions
Abdul-Hassan Mahdi Salih was responsible for conceptualization; Sahar Golgouneh and Safoura Abarghouei were responsible for methodology; Ramaswamy Sivaraman was responsible for software; Rahim Alhamzawi and Kakhor M. Khalikov were responsible for validation; Rahim Alhamzawi and Zahraa Haleem Al-qaim were responsible for data curation; Sahar Golgouneh, Abdul-Hassan Mahdi Salih, and Mohammed Abed Jawad were responsible for writing—original draft preparation; Ali Hussein Adhab and Andrés Leonardo Vázquez-Cárdenas were responsible for writing—review and editing; Abdul-Hassan Mahdi Salih and Sahar Golgouneh were responsible for supervision; Abdul-Hassan Mahdi Salih was responsible for project administration. All authors have read and agreed to the published version of the manuscript.
Supplementary Materials
The file contains Supplementary Figure 2 with legends.
References
- 1.Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Frontiers in Public Health . 2016;4:p. 148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mojiri A., Zhou J. L., Robinson B., et al. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere . 2020;253, article 126646 doi: 10.1016/j.chemosphere.2020.126646. [DOI] [PubMed] [Google Scholar]
- 3.Akhter N., Wu B., Memon A. M., Mohsin M. Probiotics and prebiotics associated with aquaculture: a review. Fish & Shellfish Immunology . 2015;45(2):733–741. doi: 10.1016/j.fsi.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Hai N. V. The use of probiotics in aquaculture. Journal of Applied Microbiology . 2015;119(4):917–935. doi: 10.1111/jam.12886. [DOI] [PubMed] [Google Scholar]
- 5.Sahu M. K., Swarnakumar N. S., Sivakumar K., Thangaradjou T., Kannan L. Probiotics in aquaculture: importance and future perspectives. Indian Journal of Microbiology . 2008;48(3):299–308. doi: 10.1007/s12088-008-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadi M., Shadnoush M., Sohrabvandi S., Yousefi M., Khorshidian N., Mortazavian A. M. Probiotics as potential detoxification tools for mitigation of pesticides: a mini review. International Journal of Food Science & Technology . 2021;56(5):2078–2087. doi: 10.1111/ijfs.14880. [DOI] [Google Scholar]
- 7.Trinder M., Bisanz J. E., Burton J. P., Reid G. Probiotic lactobacilli: a potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Beneficial Microbes . 2015;6(6):841–847. doi: 10.3920/BM2015.0022. [DOI] [PubMed] [Google Scholar]
- 8.Trinder M., McDowell T. W., Daisley B. A., et al. Probiotic Lactobacillus rhamnosus reduces organophosphate pesticide absorption and toxicity to Drosophila melanogaster. Applied and Environmental Microbiology . 2016;82(20):6204–6213. doi: 10.1128/AEM.01510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kole K., Islam M. R., Mrong C. E., et al. Toxicological effect of sumithion pesticide on the hematological parameters and its recovery pattern using probiotic in Barbonymus gonionotus. Toxicology Reports . 2022;9:230–237. doi: 10.1016/j.toxrep.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elumalai P., Kurian A., Lakshmi S., Faggio C., Esteban M. A., Ringø E. Herbal immunomodulators in aquaculture. Reviews in Fisheries Science & Aquaculture . 2021;29(1):33–57. doi: 10.1080/23308249.2020.1779651. [DOI] [Google Scholar]
- 11.Galina J., Yin G., Ardo L., Jeney Z. The use of immunostimulating herbs in fish. An overview of research. Fish Physiology and Biochemistry . 2009;35(4):669–676. doi: 10.1007/s10695-009-9304-z. [DOI] [PubMed] [Google Scholar]
- 12.Ghafarifarsani H., Hoseinifar S. H., Aftabgard M., Van Doan H. The improving role of savory (Satureja hortensis) essential oil for Caspian roach (Rutilus caspicus) fry: growth, haematological, immunological, and antioxidant parameters and resistance to salinity stress. Aquaculture . 2022;548, article 737653 doi: 10.1016/j.aquaculture.2021.737653. [DOI] [Google Scholar]
- 13.Hajirezaee S., Rafieepour A., Shafiei S., Rahimi R. Immunostimulating effects of Ginkgo biloba extract against toxicity induced by organophosphate pesticide, diazinon in rainbow trout, Oncorhynchus mykiss: innate immunity components and immune-related genes. Environmental Science and Pollution Research . 2019;26(9):8798–8807. doi: 10.1007/s11356-019-04327-7. [DOI] [PubMed] [Google Scholar]
- 14.Rabie M., Asri Y., Ahmadi K. Effect of Milk thistle plant, Vitis vinifera extract on immune system of rainbow trout (Oncorhynchus mykiss) challenge by diazinon. International Journal of Aquatic Biology . 2016;4(3):208–214. [Google Scholar]
- 15.Al-Shawi S. G., Al-Younis Z. K., Yousif A. Y., Shichiyakh R. A., Zekiy A. O., Naserabad S. S. Dietary silymarin, Silybum marianum extract ameliorates cadmium chloride toxicity in common carp, Cyprinus carpio. Annals of Animal Science . 2022;22(2):741–750. doi: 10.2478/aoas-2021-0065. [DOI] [Google Scholar]
- 16.Hajirezaee S., Rafieepour A., Rahimi R., Shafiei S. Effects of gingko,Ginkgo bilobaextract on metabolic hormones, liver histology, and growth parameters of rainbow trout,Oncorhynchus mykissexposed to diazinon. Toxin Reviews . 2021;40(4):632–644. doi: 10.1080/15569543.2019.1616209. [DOI] [Google Scholar]
- 17.Abdel-Latif H. M., Abdel-Daim M. M., Shukry M., Nowosad J., Kucharczyk D. Benefits and applications of Moringa oleifera as a plant protein source in aquafeed: a review. Aquaculture . 2022;547, article 737369 doi: 10.1016/j.aquaculture.2021.737369. [DOI] [Google Scholar]
- 18.Kuralkar P., Kuralkar S. V. Role of herbal products in animal production - an updated review. Journal of Ethnopharmacology . 2021;278, article 114246 doi: 10.1016/j.jep.2021.114246. [DOI] [PubMed] [Google Scholar]
- 19.Rex J. R. S., Muthukumar N. M. S. A., Selvakumar P. M. Phytochemicals as a potential source for anti-microbial, anti-oxidant and wound healing-a review. MOJ Bioorganic & Organic Chemistry . 2018;2(2):61–70. [Google Scholar]
- 20.Nazhand A., Lucarini M., Durazzo A., et al. Hawthorn (Crataegus spp.): an updated overview on its beneficial properties. Forests . 2020;11(5):p. 564. doi: 10.3390/f11050564. [DOI] [Google Scholar]
- 21.Tan X., Sun Z., Huang Z., et al. Effects of dietary hawthorn extract on growth performance, immune responses, growth- and immune-related genes expression of juvenile golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish & Shellfish Immunology . 2017;70:656–664. doi: 10.1016/j.fsi.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Jia L., Popovich D. G., Hao J. Hawthorn flavonoid extract: antioxidant activity and growth inhibition effect on cancer cells. Food Science . 2010;31:220–223. [Google Scholar]
- 23.Keser S., Celik S., Turkoglu S., Yilmaz Ö., Turkoglu I. The investigation of some bioactive compounds and antioxidant properties of hawthorn (Crataegus monogyna subsp. monogyna jacq.) Journal of Intercultural Ethnopharmacology . 2014;3(2):51–55. doi: 10.5455/jice.20140120103320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadić V. M., Dobrić S., Marković G. M., et al. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. Journal of Agricultural and Food Chemistry . 2008;56(17):7700–7709. doi: 10.1021/jf801668c. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Becerril M., Martínez-Preciado A., Guluarte C., et al. Phytochemical composition and immunobiological activity of Hawthorn Crataegus mexicana nanoencapsulated in Longfin yellowtail Seriola rivoliana leukocytes. Fish & Shellfish Immunology . 2019;92:308–314. doi: 10.1016/j.fsi.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Mohammad S., Ahmed S. M. Adsorptive removal of acetamiprid pesticide from aqueous solution using environmentally friendly natural and agricultural wastes. Desalination Water Treat . 2019;145:280–290. doi: 10.5004/dwt.2019.23644. [DOI] [Google Scholar]
- 27.Yu X. Y., Mu C. L., Gu C., Liu C., Liu X. J. Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils. Chemosphere . 2011;85(8):1284–1289. doi: 10.1016/j.chemosphere.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Rasouli Y., Moradi M., Tajik H., Molaei R. Fabrication of anti-Listeria film based on bacterial cellulose and Lactobacillus sakei -derived bioactive metabolites; application in meat packaging. Food Bioscience . 2021;42, article 101218 doi: 10.1016/j.fbio.2021.101218. [DOI] [Google Scholar]
- 29.Fukumoto L. R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural and Food Chemistry . 2000;48(8):3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 30.da Silva C. P., Soares-Freitas R. A. M., Sampaio G. R., et al. Identification and action of phenolic compounds of Jatoba-do-cerrado (Hymenaea stignocarpa Mart.) on α-amylase and α-glucosidase activities and flour effect on glycemic response and nutritional quality of breads. Food Research International . 2019;116:1076–1083. doi: 10.1016/j.foodres.2018.09.050. [DOI] [PubMed] [Google Scholar]
- 31.Scapin G., Schmidt M. M., Prestes R. C., Rosa C. S. Phenolics compounds, flavonoids and antioxidant activity of chia seed extracts (Salvia hispanica) obtained by different extraction conditions. International Food Research Journal . 2016;23(6):2341–2346. [Google Scholar]
- 32.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry . 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 33.Harikrishnan R., Balasundaram C., Heo M. S. Lactobacillus sakei BK19 enriched diet enhances the immunity status and disease resistance to streptococcosis infection in kelp grouper, Epinephelus bruneus. Fish & shellfish immunology . 2010;29(6):1037–1043. doi: 10.1016/j.fsi.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Reyes-Becerril M., Angulo C., Estrada N., Murillo Y., Ascencio-Valle F. Dietary administration of microalgae alone or supplemented with Lactobacillus sakei affects immune response and intestinal morphology of Pacific red snapper (Lutjanus peru) Fish & Shellfish Immunology . 2014;40(1):208–216. doi: 10.1016/j.fsi.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Yousefi M., Adineh H., Reverter M., et al. Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish & Shellfish Immunology . 2021;109:12–19. doi: 10.1016/j.fsi.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 36.OECD. OECD Guidelines for the Testing of Chemicals . Organization for Economic; 1994. [Google Scholar]
- 37.Gisbert E., Mozanzadeh M. T., Kotzamanis Y., Estevez A. Weaning wild flathead grey mullet (Mugil cephalus) fry with diets with different levels of fish meal substitution. Aquaculture . 2016;462:92–100. doi: 10.1016/j.aquaculture.2016.04.035. [DOI] [Google Scholar]
- 38.Robyt J. F., Whelan W. J. The β-amylases. Starch and its derivates . London: Academic press; 1968. [Google Scholar]
- 39.Iijima N., Tanaka S., Ota Y. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiology and Biochemistry . 1998;18(1):59–69. doi: 10.1023/A:1007725513389. [DOI] [Google Scholar]
- 40.García-Carreño F. L. Protease inhibition in theory and practice. Biotechnology Education . 1992;3(4):145–150. [Google Scholar]
- 41.Merrifield D. L., Dimitroglou A., Bradley G., Baker R. T. M., Davies S. J. Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria. Aquaculture Nutrition . 2010;16(5):504–510. doi: 10.1111/j.1365-2095.2009.00689.x. [DOI] [Google Scholar]
- 42.Ross N. W., Firth K. J., Wang A., Burka J. F., Johnson S. C. Changes in hydrolytic enzyme activities of naïve Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Diseases of Aquatic Organisms . 2000;41(1):43–51. doi: 10.3354/dao041043. [DOI] [PubMed] [Google Scholar]
- 43.Mirghaed A. T., Yarahmadi P., Hosseinifar S. H., Tahmasebi D., Gheisvandi N., Ghaedi A. The effects singular or combined administration of fermentable fiber and probiotic on mucosal immune parameters, digestive enzyme activity, gut microbiota and growth performance of Caspian white fish (Rutilus frisii kutum) fingerlings. Fish & Shellfish Immunology . 2018;77:194–199. doi: 10.1016/j.fsi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Yano T. Assays of hemolytic complement activity. Techniques in Fish Immunology . 1992;2:131–141. [Google Scholar]
- 45.Siwicki A. Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozymeactivity in serum and organs and total immunoglobulin level in serum. In: Siwicki A. K., Anderson D. P., Walvga J., editors. Disease Diagnosis and Prevention Methods . Olsztyn, Poland: FAO publisher; 1993. pp. 105–112. [Google Scholar]
- 46.Quade M. J., Roth J. A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Veterinary Immunology and Immunopathology . 1997;58(3-4):239–248. doi: 10.1016/S0165-2427(97)00048-2. [DOI] [PubMed] [Google Scholar]
- 47.Anderson D. P., Siwicki A. K. Duration of protection againstAeromonas salmonicidain brook trout immunostimulated with glucan or chitosan by injection or immersion. The Progressive Fish-Culturist . 1994;56(4):258–261. doi: 10.1577/1548-8640(1994)056<0258:DOPAAS>2.3.CO;2. [DOI] [Google Scholar]
- 48.Hoseinifar S. H., Zoheiri F., Lazado C. C. Dietary phytoimmunostimulant Persian hogweed (Heracleum persicum) has more remarkable impacts on skin mucus than on serum in common carp (Cyprinus carpio) Fish & Shellfish Immunology . 2016;59:77–82. doi: 10.1016/j.fsi.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Hosseini M. J., Shaki F., Ghazi-Khansari M., Pourahmad J. Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochemistry and Biophysics . 2014;70(1):367–381. doi: 10.1007/s12013-014-9922-7. [DOI] [PubMed] [Google Scholar]
- 50.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta . 1991;196(2-3):143–151. doi: 10.1016/0009-8981(91)90067-M. [DOI] [PubMed] [Google Scholar]
- 51.Rajabiesterabadi H., Yousefi M., Hoseini S. M. Enhanced haematological and immune responses in common carp Cyprinus carpio fed with olive leaf extract-supplemented diets and subjected to ambient ammonia. Aquaculture Nutrition . 2020;26(3):763–771. doi: 10.1111/anu.13035. [DOI] [Google Scholar]
- 52.Adineh H., Naderi M., Yousefi M., Khademi Hamidi M., Ahmadifar E., Hoseini S. M. Dietary licorice (Glycyrrhiza glabra) improves growth, lipid metabolism, antioxidant and immune responses, and resistance to crowding stress in common carp, Cyprinus carpio. Aquaculture Nutrition . 2021;27(2):417–426. doi: 10.1111/anu.13194. [DOI] [Google Scholar]
- 53.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry . 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Nicholson J., Wolmarans M. R., Park G. R. The role of albumin in critical illness. British Journal of Anaesthesia . 2000;85(4):599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 55.Oroji E., Mehrgan M. S., Islami H. R., Sharifpour I. Dietary effect of Ziziphora clinopodioides extract on zootechnical performance, immune response, and disease resistance against Yersinia ruckeri in Oncorhynchus mykiss. Aquaculture Reports . 2021;21, article 100827 doi: 10.1016/j.aqrep.2021.100827. [DOI] [Google Scholar]
- 56.Assan D., Kuebutornye F. K. A., Hlordzi V., et al. Effects of probiotics on digestive enzymes of fish (finfish and shellfish); status and prospects: a mini review. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology . 2022;257, article 110653 doi: 10.1016/j.cbpb.2021.110653. [DOI] [PubMed] [Google Scholar]
- 57.Balcázar J. L., De Blas I., Ruiz-Zarzuela I., Cunningham D., Vendrell D., Múzquiz J. L. The role of probiotics in aquaculture. Veterinary Microbiology . 2006;114(3-4):173–186. doi: 10.1016/j.vetmic.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Feng P., Ye Z., Kakade A., Virk A. K., Li X., Liu P. A review on gut remediation of selected environmental contaminants: possible roles of probiotics and gut microbiota. Nutrients . 2019;11(1):p. 22. doi: 10.3390/nu11010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarlak Z., Khosravi-Darani K., Rouhi M., Garavand F., Mohammadi R., Sobhiyeh M. R. Bioremediation of organophosphorus pesticides in contaminated foodstuffs using probiotics. Food Control . 2021;126, article 108006 doi: 10.1016/j.foodcont.2021.108006. [DOI] [Google Scholar]
- 60.Mohapatra S., Chakraborty T., Prusty A. K., Kumar K., Prasad K. P., Mohanta K. N. Fenvalerate induced stress mitigation by dietary supplementation of multispecies probiotic mixture in a tropical freshwater fish, Labeo rohita (Hamilton) Pesticide Biochemistry and Physiology . 2012;104(1):28–37. doi: 10.1016/j.pestbp.2012.06.006. [DOI] [Google Scholar]
- 61.Barman D. N., Haque M. A., Islam S. M. A., Yun H. D., Kim M. K. Cloning and expression of oph B gene encoding organophosphorus hydrolase from endophytic Pseudomonas sp. BF1-3 degrades organophosphorus pesticide chlorpyrifos. Ecotoxicology and Environmental Safety . 2014;108:135–141. doi: 10.1016/j.ecoenv.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Yuan Y., Yang C., Song C., Jiang H., Mulchandani A., Qiao C. Anchorage of GFP fusion on the cell surface of Pseudomonas putida. Biodegradation . 2011;22(1):51–61. doi: 10.1007/s10532-010-9375-7. [DOI] [PubMed] [Google Scholar]
- 63.Awad E., Awaad A. Role of medicinal plants on growth performance and immune status in fish. Fish & Shellfish Immunology . 2017;67:40–54. doi: 10.1016/j.fsi.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Lazado C. C., Caipang C. M. A. Mucosal immunity and probiotics in fish. Fish & Shellfish Immunology . 2014;39(1):78–89. doi: 10.1016/j.fsi.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 65.Nayak S. K. Probiotics and immunity: a fish perspective. Fish & Shellfish Immunology . 2010;29(1):2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Rudiansyah M., Abdelbasset W. K., Jasim S. A., et al. Beneficial alterations in growth performance, blood biochemicals, immune responses, and antioxidant capacity of common carp (Cyprinus carpio) fed a blend of Thymus vulgaris, Origanum majorana, and Satureja hortensis extracts. Aquaculture . 2022;555, article 738254 doi: 10.1016/j.aquaculture.2022.738254. [DOI] [Google Scholar]
- 67.Reyes-Becerril M., Ascencio F., Gracia-Lopez V., Macias M., Roa M. C., Esteban M. Á. Single or combined effects of Lactobacillus sakei and inulin on growth, non-specific immunity and IgM expression in leopard grouper (Mycteroperca rosacea) Fish Physiology and Biochemistry . 2014;40(4):1169–1180. doi: 10.1007/s10695-014-9913-z. [DOI] [PubMed] [Google Scholar]
- 68.Mohapatra S., Chakraborty T., Prusty A. K., PaniPrasad K., Mohanta K. N. Beneficial effects of dietary probiotics mixture on hemato-immunology and cell apoptosis of Labeo rohita fingerlings reared at higher water temperatures. PLoS One . 2014;9(6, article e100929) doi: 10.1371/journal.pone.0100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aguirre-Guzman G., Lara-Flores M., Sánchez-Martínez J. G., Campa-Córdova A. I., Luna-González A. The use of probiotics in aquatic organisms: a review. African Journal of Microbiology Research . 2012;6(23):4845–4857. [Google Scholar]
- 70.Denev S., Beev G., Staykov Y., Moutafchieva R. Microbial ecology of the gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture. International Aquatic Research . 2009;1(1):p. 1. [Google Scholar]
- 71.Gómez G. D., Balcázar J. L. A review on the interactions between gut microbiota and innate immunity of fish: Table 1. FEMS Immunology & Medical Microbiology . 2008;52(2):145–154. doi: 10.1111/j.1574-695X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 72.Esteban M. A., Cordero H., Martínez-Tomé M., Jiménez-Monreal A. M., Bakhrouf A., Mahdhi A. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.) Fish & Shellfish Immunology . 2014;39(2):532–540. doi: 10.1016/j.fsi.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Giri S. S., Sukumaran V., Oviya M. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish & Shellfish Immunology . 2013;34(2):660–666. doi: 10.1016/j.fsi.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Dragun Z., Filipović Marijić V., Krasnići N., et al. Malondialdehyde concentrations in the intestine and gills of Vardar chub (Squalius vardarensis Karaman) as indicator of lipid peroxidation. Environmental Science and Pollution Research . 2017;24(20):16917–16926. doi: 10.1007/s11356-017-9305-x. [DOI] [PubMed] [Google Scholar]
- 75.Oropesa A. L., García-Cambero J. P., Soler F. Glutathione and malondialdehyde levels in common carp after exposure to simazine. Environmental Toxicology and Pharmacology . 2009;27(1):30–38. doi: 10.1016/j.etap.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Khalil S. R., Abd Elhakim Y., Abd El-fattah A. H., Farag M. R., Abd El-Hameed N. E., Abd Elhakeem E. M. Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): stress and immune encoding gene expression. Fish & Shellfish Immunology . 2020;100:208–218. doi: 10.1016/j.fsi.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Rafieepour A., Hajirezaee S., Rahimi R. Dietary oregano extract (Origanum vulgare L.) enhances the antioxidant defence in rainbow trout, Oncorhynchus mykiss against toxicity induced by organophosphorus pesticide, diazinon. Toxin Reviews . 2019;39(4):397–407. doi: 10.1080/15569543.2018.1550092. [DOI] [Google Scholar]
- 78.Ghelichpour M., Mirghaed A. T., Hoseini S. M., Jimenez A. P. Plasma antioxidant and hepatic enzymes activity, thyroid hormones alterations and health status of liver tissue in common carp (Cyprinus carpio) exposed to lufenuron. Aquaculture . 2020;516, article 734634 doi: 10.1016/j.aquaculture.2019.734634. [DOI] [Google Scholar]
- 79.Sun Z., Tan X., Wei Z., et al. Effects of dietary dandelion extract on the growth performance, serum biochemical parameters, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) at different feeding period. Fish & Shellfish Immunology . 2022;120:280–286. doi: 10.1016/j.fsi.2021.11.034. [DOI] [PubMed] [Google Scholar]
- 80.Dawood M. A., Moustafa E. M., Gewaily M. S., et al. Ameliorative effects of Lactobacillus plantarum L-137 on Nile tilapia (Oreochromis niloticus) exposed to deltamethrin toxicity in rearing water. Aquatic Toxicology . 2020;219, article 105377 doi: 10.1016/j.aquatox.2019.105377. [DOI] [PubMed] [Google Scholar]
- 81.Banaei M., Sureda A., Shahaf S., Fazilat N. Protective effects of silymarin extract on malthion-induced zebra cichlid (Cichlasoma nigrofasciatum) hepatotoxicity. Iranian Journal of Toxicology . 2015.
- 82.Nimavathi V., Jayanthi J., Ragunathan M. Ameliorative action of Tribulus terrestris on atrazine exposed fresh water fish Oreochromis Mossambicus (Wkh Peters, 1852) Uttar Pradesh Journal of Zoology . 2021;42(19):11–16. [Google Scholar]
- 83.Ellis T., Yildiz H. Y., López-Olmeda J., et al. Cortisol and finfish welfare. Fish Physiology and Biochemistry . 2012;38(1):163–188. doi: 10.1007/s10695-011-9568-y. [DOI] [PubMed] [Google Scholar]
- 84.Vijayan M. M., Aluru N., Leatherland J. F. Stress response and the role of cortisol. Fish diseases and Disorders . 2010;2:182–201. doi: 10.1079/9781845935535.0182. [DOI] [Google Scholar]
- 85.Fırat Ö., Cogun H. Y., Yüzereroğlu T. A., et al. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiology and Biochemistry . 2011;37(3):657–666. doi: 10.1007/s10695-011-9466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghasemzadeh J., Sinaei M., Bolouki M. Biochemical and histological changes in fish, spotted scat (Scatophagus argus) exposed to diazinon. Bulletin of Environmental Contamination and Toxicology . 2015;94(2):164–170. doi: 10.1007/s00128-014-1454-8. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X., Zhong Y., Tian H., Wang W., Ru S. Impairment of the cortisol stress response mediated by the hypothalamus- pituitary-interrenal (HPI) axis in zebrafish (Danio rerio) exposed to monocrotophos pesticide. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology . 2015;176-177:10–16. doi: 10.1016/j.cbpc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Amiri Resketi M., Yeganeh S., Jani Khalili K. Dietary sour lemon (Citrus limon) peel essential oil supplementation for reduction of deltamethrin-induced stress in rainbow trout (Oncorhynchus mykiss) Journal of the World Aquaculture Society . 2021;52(1):105–123. doi: 10.1111/jwas.12749. [DOI] [Google Scholar]
- 89.Gupta S. K., Pal A. K., Sahu N. P., et al. Dietary microbial levan ameliorates stress and augments immunity in Cyprinus carpio fry (Linnaeus, 1758) exposed to sublethal toxicity of fipronil. Aquaculture Research . 2014;45(5):893–906. doi: 10.1111/are.12030. [DOI] [Google Scholar]
- 90.Carnevali O., de Vivo L., Sulpizio R., et al. Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture . 2006;258(1-4):430–438. doi: 10.1016/j.aquaculture.2006.04.025. [DOI] [Google Scholar]
- 91.Taoka Y., Maeda H., Jo J. Y., et al. Growth, stress tolerance and non-specific immune response of Japanese flounder Paralichthys olivaceus to probiotics in a closed recirculating system. Fisheries Science . 2006;72(2):310–321. doi: 10.1111/j.1444-2906.2006.01152.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The file contains Supplementary Figure 2 with legends.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


