Abstract
Enteropathogenic Escherichia coli (EPEC) and Shiga toxin-producing E. coli (STEC) induce cytoskeletal changes in infected epithelial cells. To further characterize host cytosolic responses to infection, a series of specific cell-signaling inhibitors were employed. Initial bacterial adhesion to HEp-2 epithelial cells was not reduced, whereas α-actinin accumulation in infected cells was blocked by a phosphoinositide-specific phospholipase C inhibitor (ET-18-OCH3), phosphoinositide 3-kinase inhibitors (wortmannin and LY294002), and a 5-lipoxygenase inhibitor, nordihydroguaretic acid. A cyclooxygenase-2 inhibitor (NS-398), however, did not block α-actinin reorganization in response to EPEC and STEC infections. Understanding signal transduction responses to enteric pathogens could provide the basis for the development of novel therapeutic strategies.
Enteropathogenic Escherichia coli (EPEC) is an enteric pathogen colonizing the small intestine and colon of infants with persistent watery diarrhea (28). Shiga toxin-producing E. coli (STEC), also variously referred to as enterohemorrhagic E. coli and verocytotoxin-producing E. coli, is a large-bowel pathogen causing both outbreaks and sporadic cases of hemorrhagic colitis and hemolytic-uremic syndrome (28). Both EPEC and STEC induce biochemical and morphological changes in infected eukaryotic cells. The cytoskeletal effects resulting in the attaching and effacing (A/E) lesion are characterized by intimate adherence of bacteria to epithelial cells with localized destruction and vesiculation of brush-border microvilli and rearrangement beneath adherent organisms of host cell cytoskeletal proteins, including filamentous actin, α-actinin, talin, ezrin, and myosin light chain (8).
Induction of A/E lesion formation by EPEC and STEC is associated with a number of nuclear signaling events (30), as well as inducing changes in the cytosol of the infected host cell, including increased levels of inositol-1,4,5-trisphosphate (5, 11), cytosolic free calcium, and protein phosphorylation (1). Multiple signal transduction pathways converge to induce rearrangements of the actin cytoskeleton. The aim of this study was to further delineate responses in the host cell cytosol to EPEC and STEC infection by using a series of highly specific inhibitors against signaling pathways mediated by the enzymes phosphoinositide-specific phospholipase C, phosphoinositide 3-kinase, 5-lipoxygenase, and cyclooxygenase.
EPEC strain E2348/69 (serotype O127:H6) was kindly provided by E. Boedeker (University of Maryland, Baltimore). STEC strain CL56 (O157:H7) was donated by M. Karmali (Hospital for Sick Children, Toronto, Ontario, Canada). UMD864, kindly supplied by J. B. Kaper (University of Maryland), is an espB deletion mutant of E2348/69. It was used as a negative control because of its inability to trigger host signal transduction events (15). Bacteria were grown for 3 h in static, nonaerated Penassay broth (Difco, Detroit, Mich.) at 37°C to provide a mid-logarithmic-phase-growth culture, since bacteria at this stage induce more rapid formation of A/E lesions than do organisms grown to stationary phase (29).
The human laryngeal epithelial cell line HEp-2 (American Type Culture Collection, Manassas, Va.) was cultured in minimal essential medium (Gibco Laboratories, Grand Island, N.Y.) supplemented with 15% heat-inactivated fetal calf serum (Cansera International, Inc., Rexdale, Ontario, Canada), 0.5% glutamine (Gibco), 0.1% sodium bicarbonate (Gibco), and 2% penicillin-streptomycin (Gibco) in 25-cm2 tissue culture flasks (Corning Glass Works, Corning, N.Y.) at 37°C in 5% CO2.
Localization of α-actinin was detected in infected epithelial cells, as described previously (12). Briefly, HEp-2 cells were seeded onto two-well chamber slides (Nunc, Inc., Naperville, Ill.) and were cultured overnight to obtain a subconfluent growth. Before bacterial infection the tissue culture medium was replaced with medium without antibiotics. Monolayers were then infected with 109 E. coli bacteria at a multiplicity of infection of 100:1 at 37°C in 5% CO2. After 3 h, nonadherent organisms were removed by washing the tissue culture cells six times with sterile, phosphate-buffered saline at pH 7.4. The monolayers were then fixed in 100% cold methanol at room temperature for 10 min. After being washed three times with phosphate-buffered saline, fixed cells were incubated with a 1-in-100 dilution of murine monoclonal immunoglobulin G anti-α-actinin (Sigma, Oakville, Ontario, Canada) as primary antibody for 1 h at 37°C with gentle shaking. Rewashed monolayers were then incubated with a 1-in-100 dilution fluorescein isothiocyanate-conjugated rabbit anti-murine immunoglobulin G (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) as secondary antibody with protection from light for 1 h at 37°C with continuous gentle agitation. Slides were then mounted with SlowFade Antifade Kits (Molecular Probes, Eugene, Oreg.) and examined by alternating phase-contrast and fluorescence microscopy (Leitz Dialux 22; Leica Canada, Inc., Willowdale, Ontario, Canada). Phase-contrast microscopy was used to quantitate the number of adherent bacteria on 100 randomly selected HEp-2 cells. The number of A/E lesions, as measured by the number of foci of α-actinin accumulation in the same epithelial cells, was determined by immunofluorescence microscopy by three independent observers.
Signal transduction inhibitors were purchased from Calbiochem (San Diego, Calif.). HEp-2 cells were preincubated with the phosphoinositide-specific phospholipase C inhibitor 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3) (26), wortmannin or 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) as a phosphoinositide 3-kinase inhibitor (35, 36), the 5-lipoxygenase inhibitor nordihydroguaretic acid (NDGA) (21), or a cyclooxygenase-2 inhibitor, N-(2-cyclohexyloxy-4-nitrophenyl)-methanesulfonamide (NS-398) (7), for 30 min to 3 h, at dosages recommended in previous studies. After removal of the inhibitor, tissue culture cells were rinsed thoroughly with minimal essential medium prior to infection with 109 bacteria for 3 h at 37°C. The inhibitory effects of cell-signaling inhibitors were determined by their ability to disrupt formation of A/E lesions, as indicated by the number of foci of α-actinin accumulation in infected epithelial cells.
Synthesis of leukotriene B4 in HEp-2 cells with and without exposure to a lipoxygenase inhibitor was measured in both culture supernatants and cell sonicates by immunoassay, as described previously (2). Samples were stored at −70°C and were assayed at 2 weeks using a commercial enzyme immunoassay (Cayman Chemical Co., Ann Arbor, Mich.). Results are expressed as means ± standard deviation. Analysis of variance (ANOVA) was used to test differences between multiple groups.
ET-18-OCH3 inhibits α-actinin accumulation in EPEC- and STEC-infected cells.
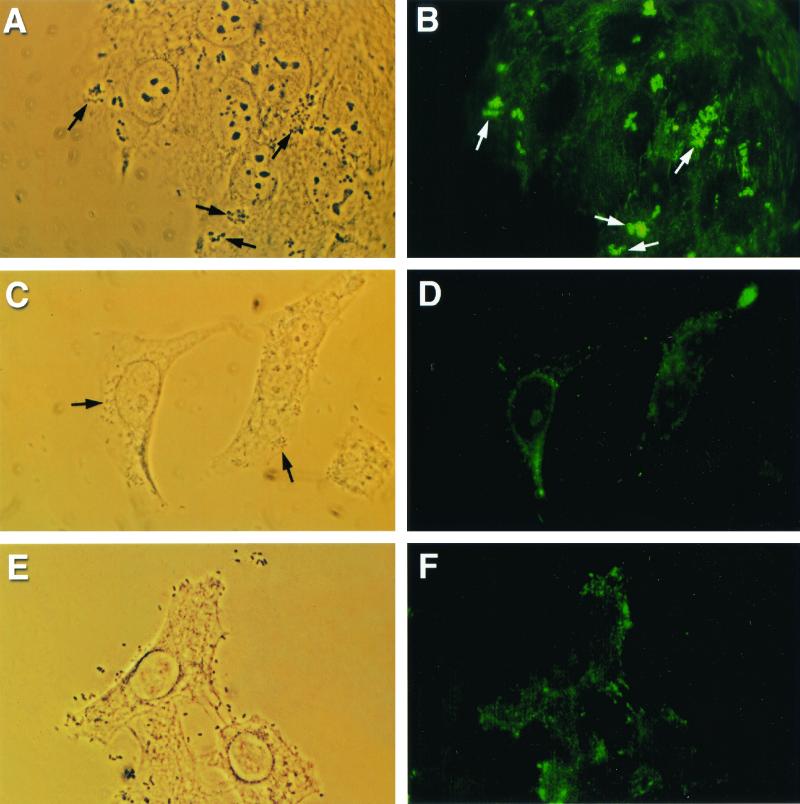
Treatment of cell monolayers with various concentrations of the phosphoinositide-specific phospholipase C inhibitor ET-18-OCH3, along its ethanol vehicle, did not affect the ability of EPEC strain E2348/69, STEC strain CL56, or the signaling-deficient mutant UMD864 to adhere to HEp-2 monolayers. The number of adherent bacteria on epithelial cells remained comparable under each of the different treatment conditions (data not shown). Furthermore, microcolony formation and localized adherence of EPEC were still detectable (Fig. 1A). The morphology of the bacterial microcolonies was not altered. When HEp-2 cells were infected with E2348/69 for 3 h at 37°C, the accumulation of α-actinin, demonstrated by bright foci of fluorescence, corresponded to sites of bacterial adhesion (Fig. 1B). However, when tissue culture cells were pretreated with 80 μM ET-18-OCH3 before infection, α-actinin accumulation was reduced (Fig. 1C and D). The vehicle alone did not affect the α-actinin reorganization. Similar inhibitory effects, following preincubation of host cells with ET-18-OCH3, were observed during infection with STEC O157:H7 strain CL56 (data not shown). No α-actinin response was detected using UMD864 as a negative control (Fig. 1E and F). Table 1 summarizes the results of semiquantitation of the effects of the phosphoinositide-specific phospholipase C inhibitor ET-18-OCH3 on α-actinin accumulation in both EPEC- and STEC-infected cells. A dose-dependent inhibition on the formation of A/E lesions on infected epithelial cells induced by these pathogenic bacteria was demonstrated (ANOVA, P < 0.05).
FIG. 1.
Reduced A/E lesion formation with phospholipase C inhibitor ET-18-OCH3 (approximate magnification, ×1,250). (A) Phase-contrast micrograph demonstrating adherent EPEC strain E2348/69 (arrows) on HEp-2 monolayers after coincubation for 3 h at 37°C. (B) Corresponding fluorescence micrograph showing α-actinin accumulation, demonstrated by bright foci of fluorescence (arrows), underneath adherent microcolonies of bacteria. (C) Phase-contrast micrograph showing adherent E2348/69 on ET-18-OCH3-pretreated epithelial cells (arrows). (D) α-Actinin accumulation under adherent EPEC was not observed in the corresponding fluorescence micrograph. (E) Phase-contrast micrograph demonstrating UMD864 adherent to tissue culture cells. (F) A negative α-actinin response was detected when HEp-2 cells were infected with a signaling-deficient mutant.
TABLE 1.
Semiquantitation of the effects of inhibitors on aggregates of α-actinin accumulation in cells infected with EPEC and STEC strains
| Inhibitor | Concn of inhibitor | Effect of inhibitors on different strains (no. of α-actinin foci/100 HEp-2 cells)
|
||
|---|---|---|---|---|
| E2348/69 | CL56 | UMD864 | ||
| ET-18-OCH3 | 0 μM | 92 ± 5 | 60 ± 3 | 10 ± 4 |
| Ethanol alone | 90 ± 5 | 62 ± 7 | 10 ± 2 | |
| 50 μM | 20 ± 7a | 10 ± 5a | 5 ± 4 | |
| 80 μM | 8 ± 4a | 3 ± 3a | 4 ± 5 | |
| Wortmannin | 0 nM | 85 ± 5 | 70 ± 3 | 8 ± 4 |
| Water alone | 85 ± 1 | 73 ± 4 | 8 ± 1 | |
| 10 nM | 44 ± 2a | 42 ± 3a | 6 ± 1 | |
| LY294002 | 0 μM | 90 ± 2 | 55 ± 6 | 10 ± 6 |
| DMSO alone | 87 ± 7 | 61 ± 5 | 4 ± 4 | |
| 30 μM | 75 ± 6 | 54 ± 2 | 4 ± 3 | |
| 100 μM | 55 ± 2a | 30 ± 2a | 8 ± 1 | |
| 150 μM | 50 ± 7a | 35 ± 5a | 6 ± 1 | |
| NDGA | 0 μM | 92 ± 2 | 80 ± 3 | 8 ± 2 |
| DMSO alone | 90 ± 2 | 78 ± 6 | 7 ± 2 | |
| 30 μM | 94 ± 3 | 73 ± 9 | 7 ± 4 | |
| 75 μM | 17 ± 7a | 10 ± 1a | 6 ± 4 | |
| NS-398 | 0 μM | 90 ± 3 | 65 ± 6 | 6 ± 3 |
| Ethanol alone | 90 ± 3 | 68 ± 5 | 9 ± 3 | |
| 10 μM | 92 ± 5 | 68 ± 2 | 8 ± 1 | |
| 20 μM | 88 ± 2 | 73 ± 4 | 8 ± 1 | |
| 50 μM | 84 ± 8 | 68 ± 6 | 6 ± 4 | |
ANOVA, P < 0.05 (n = 4 to 6 separate experiments).
Wortmannin inhibits aggregation of α-actinin in EPEC- and STEC-infected cells.
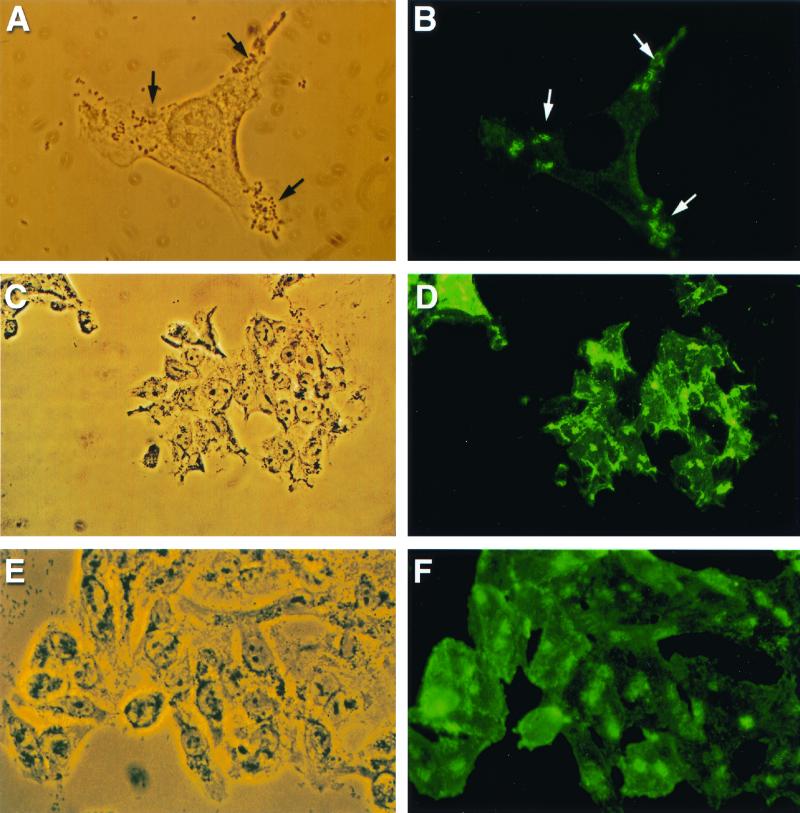
Wortmannin suspended in distilled water had no effect on initial bacterial adherence to HEp-2 epithelial cells. In the absence of the phosphoinositide 3-kinase inhibitor, formation of A/E lesions demonstrated by α-actinin accumulation was observed (Fig. 2A and B). By contrast, after wortmannin treatment, fewer and less intense foci of α-actinin localization were detected in EPEC strain E2348/69-infected tissue culture cells (Fig. 2C and D). Preincubation of HEp-2 cells with wortmannin before infection with STEC strain CL56 resulted in a similar change in the α-actinin response (data not shown). The presence of 10 nM wortmannin led to 45 and 40% reductions in α-actinin accumulation beneath adherent E2348/69 and CL56, respectively (ANOVA, P < 0.05). A dose-dependent effect of wortmannin was not tested because of its nonspecific inhibitory effects at concentrations higher than those employed in this study (6). More prolonged incubation (6 h) resulted in toxicity to the host epithelial cells.
FIG. 2.
Reduced A/E lesion formation with phosphoinositide 3-kinase inhibitor wortmannin (10 nM) (approximate magnification, ×1,250). (A) EPEC strain E2348/69 showing initial adherence to tissue culture HEp-2 cells by phase-contrast microscopy (arrows). (B) Intense foci of fluorescence demonstrate reaggregation of α-actinin (arrows) corresponding to areas of bacterial attachment. (C) Phase-contrast micrograph depicting adherent E2348/69 on wortmannin-pretreated HEp-2 cells (arrows). (D) Fewer foci of α-actinin accumulation (arrows) under attaching bacteria were detected in the corresponding fluorescence micrograph.
LY294002 inhibits α-actinin reorganization in EPEC- and STEC-infected epithelia.
Bacterial adherence to tissue culture cells was not affected by pretreatment of the epithelial cells with the phosphoinositide 3-kinase inhibitor LY294002 carried in dimethyl sulfoxide (DMSO) as the vehicle. Infection of HEp-2 cells with STEC strain CL56 resulted in recruitment of α-actinin protein at the site of bacterial attachment. In contrast, LY294002 inhibited α-actinin accumulation in CL56-infected cells, whereas DMSO alone did not affect the α-actinin response. Similar inhibitory effects on α-actinin localization were detected during infection with EPEC strain E2348/69. The inhibitory effect of LY294002 on EPEC- and STEC-induced A/E lesions was dose dependent (Table 1).
NDGA inhibits α-actinin rearrangement in EPEC- and STEC-infected cells.
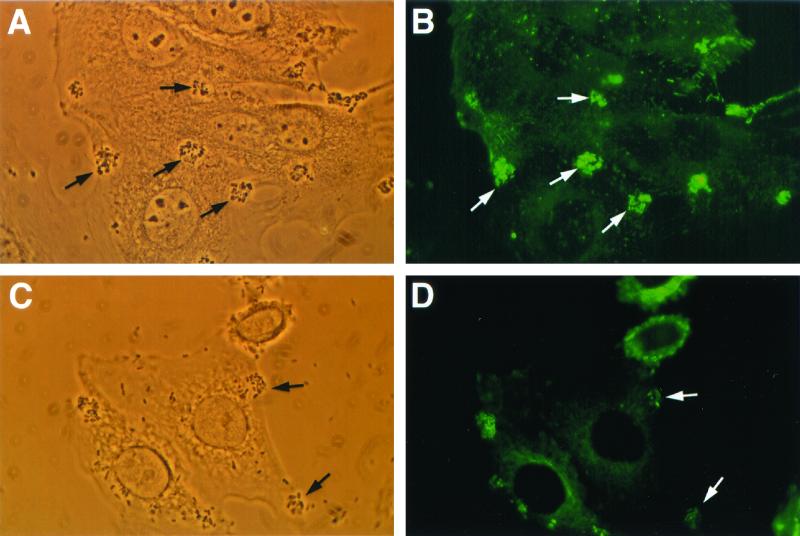
NDGA pretreatment of tissue culture cells did not reduce the ability of bacteria to adhere to HEp-2 monolayers (data not shown). STEC strain CL56 caused the formation of A/E lesions in the absence of the leukotriene inhibitor NDGA (Fig. 3A and B). By contrast, fewer adherent bacteria were accompanied by foci of α-actinin accumulation when HEp-2 tissue culture cells were preincubated with the cell-signaling inhibitor (Fig. 3C and D). Similar inhibitory effects on α-actinin rearrangement were observed during EPEC infection (Fig. 3E and F). As summarized in Table 1, NDGA inhibited the recruitment of α-actinin in both EPEC- and STEC-infected HEp-2 cells in a dose-dependent pattern.
FIG. 3.
Reduced A/E lesion formation with 5-lipoxygenase inhibitor NDGA (approximate magnification, ×1,250). (A) Phase-contrast micrograph showing initial adherence of STEC O157:H7 strain CL56 to HEp-2 cells (arrows) following infection for 3 h at 37°C. (B) Bright foci of α-actinin fluorescence were detected in infected HEp-2 cells in regions subjacent to areas of bacterial adhesion (arrows). (C) Phase-contrast micrograph shows that CL56 adhered to NDGA-pretreated epithelial cells. (D) A negative α-actinin response was detected in the corresponding fluorescence micrograph. (E) Phase-contrast micrograph of EPEC strain E2348/69 (O127:H6) binding to epithelial cells in tissue culture. (F) Few well-defined foci of α-actinin fluorescence in HEp-2 cells below adherent EPEC were observed.
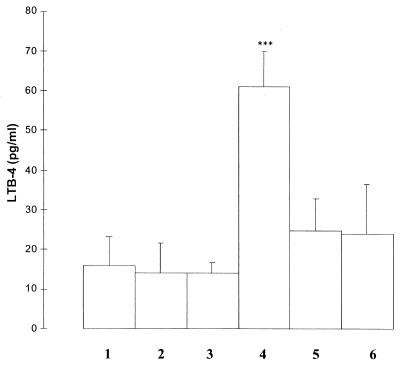
As shown in Fig. 4, EPEC infection of tissue culture cells stimulated the production of lipoxygenase products of arachidonic acid metabolism as measured by the stable leukotriene B4. As expected, preincubation of tissue culture cells with the lipoxygenase inhibitor NDGA significantly inhibited the production of leukotriene B4 (Fig. 4).
FIG. 4.
Leukotriene B4 (LTB-4) production is enhanced in EPEC-infected cells and inhibited by lipoxygenase inhibitor NDGA. Lane 1, lysates of HEp-2 cells incubated with DMSO (1 μl/ml); lane 2, lysate derived from cells incubated for 1 h with NDGA (35 μM); lane 3, cell lysates following incubation with NDGA (75 μM) for 1 h; lane 4, lysates obtained from cells incubated with EPEC strain E2348/69 (108) for 3 h; lane 5, cell lysates from HEp-2 cells preincubated for 1 h with NDGA (35 μM) and then infected with EPEC; lane 6, lysates from cells preincubated with NDGA (75 μM) for 1 h and then infected with EPEC. Results are the mean values of four separate experiments each run in duplicate. ∗∗∗, P < 0.01 (ANOVA).
NS-398 does not inhibit α-actinin reorganization in EPEC- and STEC-infected HEp-2 monolayers.
Pretreatment of tissue culture cells with NS-398 did not reduce initial bacterial attachment. The cyclooxygenase-2 inhibitor NS-398 did not affect the α-actinin rearrangement during EPEC and STEC infection, even at concentrations of up to 50 μM, despite the 50% inhibitory concentration of NS-398 of 3.8 μM (7) (Table 1).
EPEC and STEC are both enteric pathogens that constitute a major risk to human health. While it is known that both EPEC and STEC are capable of inducing cytoskeletal rearrangements leading to the formation of A/E lesions in host epithelial cells, the underlying mechanisms remain unclear. Human laryngeal epithelial cells are employed as a model system in many of these studies since they mimic changes observed in intestinal epithelia during infection in vivo (1).
Indirect immunofluorescence was employed as the technique to detect aggregates of α-actinin in host epithelial cells following infection with both EPEC and STEC. Previous studies have shown that this experimental technique compares favorably with both the fluorescent-actin test, using phalloidin conjugated to fluorescein to detect foci of polymerized actin, and transmission electron microscopy, used to identify the morphological features of the A/E lesion (12). An advantage of fluorescence microscopy is that it affords an opportunity to scan a large number of infected host cells for evidence of changes in the cytoskeleton following bacterial infection (14).
Four potential signal transduction pathways were investigated in this study. First, elevations in inositol-1,4,5-trisphosphate are present within the cytoplasm of both EPEC- and STEC-infected cells (5, 11). Since inositol-1,4,5-trisphosphate is one of the end products in the hydrolysis of phosphatidylinositol-4,5-bisphosphate by the enzyme phospholipase C, our hypothesis was that phospholipase C could play a central role in mediating the activation of the signal transduction pathway leading to both A/E lesion formation and diarrhea. To test the hypothesis, the phospholipase C inhibitor ET-18-OCH3 was initially employed.
The ether lipid analogue ET-18-OCH3 inhibits phosphoinositide-specific phospholipase C, although the precise mechanism of inhibition remains unknown (26). Since ET-18-OCH3 blocked α-actinin accumulation in infected HEp-2 cells in a dose-dependent manner, the present study demonstrates that phospholipase C is likely to be involved in the signal transduction pathway, leading to the formation of A/E lesions in response to both EPEC and STEC infections.
Phosphoinositides are important regulators and signaling molecules of a variety of proteins linked to the actin cytoskeleton (17, 31). For instance, phosphoinositide 3-kinase is involved in the regulation of actin polymerization (16). As a result, the phosphoinositide 3-kinase inhibitors wortmannin and LY294002 were utilized to determine if phosphoinositide 3-kinase is a component of the pathway that leads to A/E lesion formation following EPEC and STEC infections. The fungal metabolite wortmannin inhibits multiple signaling enzymes at higher concentrations (6). However, at nanomolar concentrations wortmannin selectively targets phosphoinositide 3-kinase by irreversibly inhibiting the catalytic subunit of phosphoinositide 3-kinase (36). The inhibition of α-actinin aggregation below adherent EPEC and STEC by wortmannin indicates that phosphoinositide 3-kinase is also involved in the generation of A/E lesion formation. As a complementary assay, LY294002, a specific inhibitor of phosphoinositide 3-kinase which reversibly inhibits phosphoinositide 3-kinase by competing with ATP for its substrate binding site (35), was also shown to inhibit the formation of A/E lesions. Taken together, these findings demonstrate that phosphoinositide 3-kinase plays a role in the formation of A/E lesions following infection of epithelial cells with EPEC and STEC.
The metabolism of arachidonic acid by the enzymes lipoxygenase and cyclooxygenase results in a wide range of oxidized products with potent biological activities (34). Both lipoxygenase and cyclooxygenase products of arachidonate are abundant in the human gut (23, 32). Biological effects include modulation of fluid secretion, electrolyte secretion, and remodeling of cytoplasmic actin filaments (22, 27). NDGA inhibits the activity of 5-lipoxygenase (21), an enzyme that generates leukotrienes from arachidonic acid. The concentration-dependent inhibition of α-actinin accumulation observed following EPEC and STEC infection of HEp-2 cells in the presence of NDGA indicates that leukotrienes are involved in the signal transduction cascade, leading to cytoskeletal reorganization in infected eukaryotic cells. In contrast, whereas cyclooxygenase-2 plays a role in mediating host inflammatory responses (13), the enzyme appears not to be involved in the formation of A/E lesions in response to EPEC and STEC infections.
Initial bacterial adherence to host cells is necessary for subsequent signal transduction and intimate attachment (10). The number of bacteria adherent to HEp-2 cells did not differ markedly following pretreatment of cells with each of the inhibitors of cell signaling. This finding indicates that neither the inhibitors nor the vehicles in which they were suspended disrupted the first stage of initial bacterial attachment to host cells following EPEC and STEC infection. Therefore, the inhibitory effects of the signaling inhibitors on their protein targets are responsible for the observed reduction in formation of A/E lesions. Toxicity of each inhibitor, along with its vehicle, was evaluated to ensure the viability of tissue culture cells. The observation that neither HEp-2 cell numbers nor morphology was affected by preincubation with any of the inhibitors or with the vehicle alone supports the specificity of the observed responses.
Based on the findings reported in this study, multiple signal transduction events likely are involved in the A/E lesions formed in response to EPEC and STEC infections. Comparable involvement of multiple cytosolic second messengers also has been observed in epithelial cells in response to Salmonella infection (20). Although the mechanisms by which EPEC and STEC produce diarrhea are not completely defined, an increase in gut permeability as a result of changes in the intercellular tight junction has been postulated (33). Indeed, the tight junction-associated protein zonula occludens-1 is disrupted following both EPEC (25) and STEC infections (24). Recent evidence indicates that a bacterial protein. EspF, mediates the damage to intercellular tight junction integrity (3, 18). α-Actinin serves as a bridge between the translocated intimin receptor, Tir, and the reorganized cytoskeleton (9). α-Actinin also provides a link between the C terminus of zonula occludens-1 in the tight junction and filamentous actin of the cell cytoskeleton (4). Signaling molecules, such as protein kinase C and myosin light-chain kinase, are second messengers in the cytosol and lead to an increase in transepithelial permeability (24). Therefore, future studies should use these enzyme inhibitors to examine the role of phospholipase C, phosphoinositide 3-kinase, and 5-lipoxygenase as mediators of EPEC- and STEC-induced changes in tight junction permeability.
Acknowledgments
G.K.P.W. was the recipient of an Ontario Graduate Scholarship and a Restracomp Studentship from the Hospital for Sick Children. N.S.B. was the recipient of a Summer Student Scholarship from the Crohn's and Colitis Foundation of Canada. This work was supported by operating grants from the Canadian Institutes of Health Research. P.M.S. is the recipient of a Canada Research Chair in Gastrointestinal Disease.
REFERENCES
- 1.Baldwin T J, Brooks S F, Knutton S, Manjarrez Hernandez H A, Aitken A, Williams P H. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1990;58:761–765. doi: 10.1128/iai.58.3.761-765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell C J, Elliot E J, Wallace J L, Redmond D M, Payne J, Li Z, O'Loughlin E V. Do eicosanoids cause colonic dysfunction in experimental E. coli O157:H7 (EHEC) infection? Gut. 2000;46:806–812. doi: 10.1136/gut.46.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnenberg M S, Whittam T S. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Investig. 2001;107:539–548. doi: 10.1172/JCI12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanning A S, Jameson B J, Jesaitis L A, Anderson J M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 5.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 7.Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 8.Goosney D L, DeVinney R, Finlay B B. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infect Immun. 2001;69:3315–3322. doi: 10.1128/IAI.69.5.3315-3322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goosney D L, DeVinney R, Pfuetzner R A, Frey E A, Strynadka N C, Finlay B B. Enteropathogenic E. coli translocated intimin receptor, Tir, interacts directly with alpha-actinin. Curr Biol. 2000;10:735–738. doi: 10.1016/s0960-9822(00)00543-1. [DOI] [PubMed] [Google Scholar]
- 10.Hicks S, Frankel G, Kaper J B, Dougan G, Phillips A D. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect Immun. 1998;66:1570–1578. doi: 10.1128/iai.66.4.1570-1578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismaili A, Philpott D J, Dytoc M T, Soni R, Ratnam S, Sherman P M. α-actinin accumulation in epithelial cells infected with attaching and effacing gastrointestinal pathogens. J Infect Dis. 1995;172:1393–1396. doi: 10.1093/infdis/172.5.1393. [DOI] [PubMed] [Google Scholar]
- 13.Kawai S. Cyclooxygenase selectivity and the risk of gastro-intestinal complications of various non-steroidal anti-inflammatory drugs: a clinical consideration. Inflamm Res. 1998;47:S102–S106. doi: 10.1007/s000110050291. [DOI] [PubMed] [Google Scholar]
- 14.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai L C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma A D, Metjian A, Bagrodia S, Taylor S, Abrams C S. Cytoskeleton reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase γ, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin T F J. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- 18.McNamara B P, Koutsouris A, O'Connell C B, Nougayrede J P, Donnenberg M S, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Investig. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mecsas J, Raupach B, Falkow S. The Yersinia Yops inhibit invasion of Listeria, Shigella, and Edwardsiella but not Salmonella into epithelial cells. Mol Microbiol. 1998;28:1269–1281. doi: 10.1046/j.1365-2958.1998.00891.x. [DOI] [PubMed] [Google Scholar]
- 20.Pace J, Hayman M J, Galan J E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 21.Peppelenbosch M P, Qiu R G, de Vries-Smits A M M, Tertoolen L G J, de Laat S W, McCormick F, Hall A, Symons M H, Bos J L. Rac mediates growth factor-induced arachidonic acid release. Cell. 1995;81:849–856. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 22.Peppelenbosch M P, Tertoolen L G J, Hage W J, de Laat S W. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- 23.Peterson J W, Finkelstein R A, Cantu J, Gessell D L, Chopra A K. Cholera toxin B subunit activates arachidonic acid metabolism. Infect Immun. 1999;67:794–799. doi: 10.1128/iai.67.2.794-799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philpott D J, McKay D M, Mak W, Perdue M H, Sherman P M. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect Immun. 1998;66:1680–1687. doi: 10.1128/iai.66.4.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 26.Powis G, Seewald M J, Gratas C, Melder D, Riebow J, Modest E J. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992;52:2835–2840. [PubMed] [Google Scholar]
- 27.Rask-Madsen J. Eicosanoids and their role in the pathogenesis of diarrhoeal diseases. Clin Gastroenterol. 1986;15:545–566. [PubMed] [Google Scholar]
- 28.Roe A J, Gally D L. Enteropathogenic and enterohaemorrhagic Escherichia coli and diarrhoea. Curr Opin Infect Dis. 2000;13:511–517. doi: 10.1097/00001432-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Rosenshine I, Ruschkowski S, Finlay B B. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect Immun. 1996;64:966–973. doi: 10.1128/iai.64.3.966-973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-kappa B in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A, Hall M N. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz H, Fromm M, Bode H, Scholz P, Riecken E O, Schulzke J D. Tumor necrosis factor-alpha induces C1− and K+ secretion in human distal colon driven by prostaglandin E2. Am J Physiol. 1996;271:G669–G674. doi: 10.1152/ajpgi.1996.271.4.G669. [DOI] [PubMed] [Google Scholar]
- 33.Sears C L. Molecular physiology and pathophysiology of tight junctions. V. Assault of the tight junction by enteric pathogens. Am J Physiol. 2000;279:G1129–G1134. doi: 10.1152/ajpgi.2000.279.6.G1129. [DOI] [PubMed] [Google Scholar]
- 34.Sigal E. The molecular biology of mammalian arachidonic acid metabolism. Am J Physiol. 1991;260:L13–L28. doi: 10.1152/ajplung.1991.260.2.L13. [DOI] [PubMed] [Google Scholar]
- 35.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 36.Wymann M P, Bulgarelli-Leva G, Zvelebil M J, Pirola L, Vanhaesebroeck B, Waterfield M D, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]