Abstract
Background Platelet-rich plasma (PRP) has local anti-inflammatory actions, which is being used as a treatment in various tendinopathies.
Purpose The aim of the study is to compare the clinical results of PRP injection and corticosteroid injection in the management of de Quervain's tenosynovitis (DQTSV).
Patients and Methods In this prospective study, 60 patients of DQTSV, fulfilling the predefined inclusion and exclusion criteria, were randomised into two groups. In group 1 ( n = 30), patients received a single injection of autologous PRP and in group 2 ( n = 30) they received a single injection of corticosteroid (methylprednisolone). All patients were followed up at 1 month, 3 months, 6 months, and 1 year for evaluation by Finkelstein test, visual analogue scale (VAS), DASH (Disabilities of the Arm, Shoulder and Hand) score, and Modified Mayo Wrist score (MMWS).
Results In both the groups improvement occurred in Finkelstein test, VAS score, DASH score, and MMWS which were found to be statistically significant at all points of follow-ups when compared to the pre-intervention values. Comparison of scores between the two groups did not show any statistical significance. No complications were reported in PRP group. Statistically significant complications ( p -value = 0.026) like subcutaneous fat atrophy, depigmentation, and temporary increase in pain were seen in eight patients in the corticosteroid group with an overall complication rate of 26.67%.
Conclusion Both the modalities are equally effective in the management of DQTSV remittance. PRP is equally effective as corticosteroid in reducing symptoms of first dorsal compartment stenosing tenosynovitis. PRP may have a lower complication profile, however, this benefit should be weighed against the slight increase in cost and time of PRP preparation and injection.
Level of Evidence Level 2, prospective comparative study.
Keywords: de Quervain's tenosynovitis, PRP, corticosteroid, Finkelstein test, DASH, Modified Mayo wrist score
De Quervain's disease is a stenosing tenosynovitis of the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB) tendons which pass through the first dorsal compartment at the level of radial styloid process. 1 It affects women up to six times more often than men and is associated with the dominant hand during middle age. 2 3 The onset is gradual with chronic pain in the wrist and radial styloid which may refer up to the arm and also distally to the thumb. Swelling with or without tenderness may be present over the styloid process. Abduction of the thumb is restricted and forced adduction is painful. The diagnosis is made by history and physical examination. Finkelstein's test 3 (deviating the wrist to the ulnar side while grasping the thumb, results in pain) is positive in typical cases. De Quervain's disease must be differentiated from the intersection syndrome (in which pain, swelling, and in severe cases, crepitus is found 4 cm proximal to the wrist) and arthritis of the first carpometacarpal and/or scaphotrapezial-trapezoid joints. 4
Conservative therapy is the traditional way of treatment which includes activity modifications, rest, ice, non-steroidal anti-inflammatory drugs, therapeutic exercises, and splinting. Corticosteroid (CS) injection has been the mainstay of treatment for those patients who do not respond to the above. 5 Other described treatments include acupuncture, 6 ozone oxygen and hyaluronic acid injections, 7 ultrasound-guided percutaneous needle tenotomy and platelet-rich plasma (PRP) injection, 8 and prolotherapy. 9 After failure of all the conservative efforts, surgical release of the first dorsal compartment and decompression of the stenosed APL and EPB tendons are often considered. CS like methylprednisolone, a potent anti-inflammatory, acts locally by inhibiting the early phenomena of the inflammatory process (edema, fibrin deposition, capillary dilation, migration of phagocytes into the inflamed areas and phagocytic activity), and later manifestations (capillary proliferation, fibroblast proliferation, and deposition of collagen) and still later cicatrisation. 10 Complications like local infection, post injection steroid flare (temporary worsening of pain in the first 24–36 hours after injection), atrophy of subcutaneous fat, and local depigmentation of skin and tendon rupture are often noted. 11 Similar anti-inflammatory action can be achieved by PRP. PRP can be defined as the volume of plasma fraction from autologous blood with the platelet concentration above baseline count (150,000 platelets/mm 3 ). The ideal platelet concentrate is 1.5 million/μL (5–7× baseline) and could be as high as 3 million/μL (10× baseline). In the last 10 years there has been an increased use of PRP for a myriad of enthesopathies. The rationale for the use of PRP is to stimulate the natural healing cascade and tissue regeneration by a “supraphysiologic” release of platelet derived factors directly at the site of injection. Studies have shown that the side-effects like local infection, tendon rupture, and local depigmentation are very rare with PRP injections as compared to CS injections. 8 10 12 13 14 15 16
Our aim of this study was to evaluate the efficacy of PRP as compared to CS for the management of de Quervain's disease.
Patients and Methods
In this prospective randomized comparative study, two groups of patients with de Quervain's tenosynovitis (DQTSV) receiving different lines of treatment were followed up for a period of 1 year and evaluated using multiple parameters in our tertiary care institution. Institutional ethical clearance was obtained prior to study. A total of 60 patients with 30 patients in each group were included in this study. The inclusion criterion was a clinical diagnosis of DQTSV, which was made with Finkelstein's test. 3 Patients with a previous history of wrist trauma, first CMC joint arthritis, Dupuytren's disease, history of CS injection, uncontrolled diabetes mellitus, pregnancy, myxedema, rheumatoid arthritis, or gout were excluded from the study. The patients were then randomly allocated a group according to computer generated randomization sequence. In group 1 ( n = 30) patients received a single injection of autologous PRP and in group 2 ( n = 30) the patients received a single injection of CS (methylprednisolone). An informed written consent for participation in the study was taken and the patient information sheet was provided to the patient along with detailed explanation of both the procedures. Along with the patients' medical history, demographics, vital signs, general physical examination, the following assessments were performed and results collected: Finkelstein test, 3 visual analogue scale (VAS) score, 17 Modified Mayo wrist score (MMWS), 18 and DASH (Disabilities of the Arm, Shoulder and Hand) score. 19
Group-1 (PRP Group): Patients in this group received PRP injection prepared by using patient's own blood. Each patient received single injection dose. The PRP was obtained under strict aseptic precautions.
Pre-injection Requisites
No CS injections should have been given within 6 months prior to the PRP injection.
All the anti-inflammatory should have been discontinued at least 1 week prior to the injection.
PRP Preparation and Injection
Under all aseptic conditions, 11 mL of patient's blood was collected in a sterile syringe using 18-gauge needle and then transferred to BD Vacutainers containing buffered sodium citrate 3.2% as anticoagulant. This was further processed through centrifugation at a frequency of 800 Hz for 8 minutes. The centrifuged product then gets separated into a basal layer containing RBCs, a buffy coat containing leukocytes at the junction, platelets just above it, and platelet poor plasma from downward above ( Fig. 1 ). PRP was extracted from the interface of the two layers using sterile syringe and cannula. A fraction of PRP sample, along with patient's unprocessed blood sample was analyzed for platelet count assessment.
Fig. 1.

BD vacutainers containing centrifuged product-basal layer containing RBCs, a buffy coat containing leucocytes, platelets rich plasma just above it, and platelet poor plasma from below upward. RBC, red blood cell.
APL and EPB tendons were identified by asking the patient to extend the thumb. Under all aseptic precautions and with sterile technique, 22 to 24-gauge needle was used to inject the 3 mL PRP solution. Needle was held at an angle of 45 degrees in line with the two tendons, and then advanced until it strike the tendons, then withdrawn slightly and solution was injected. This maneuver was done to ensure that the solution was injected in the tendon sheath and not in tendon tissue. Injection was also given at the most tender point/radial styloid ( Fig. 2 ). Following injection, a compression bandage was applied. NSAIDs were not prescribed for pain relief. Patients were instructed to elevate the limb, apply ice to the thumb and provide rest for at least a period of 3 days. They were followed up at day 1, day 3, and 2 weeks for inspection of the injection area. For an evaluation of symptoms and functional scores, they were again followed up at 1 month, 3 months, 6 months, and 1 year.
Fig. 2.
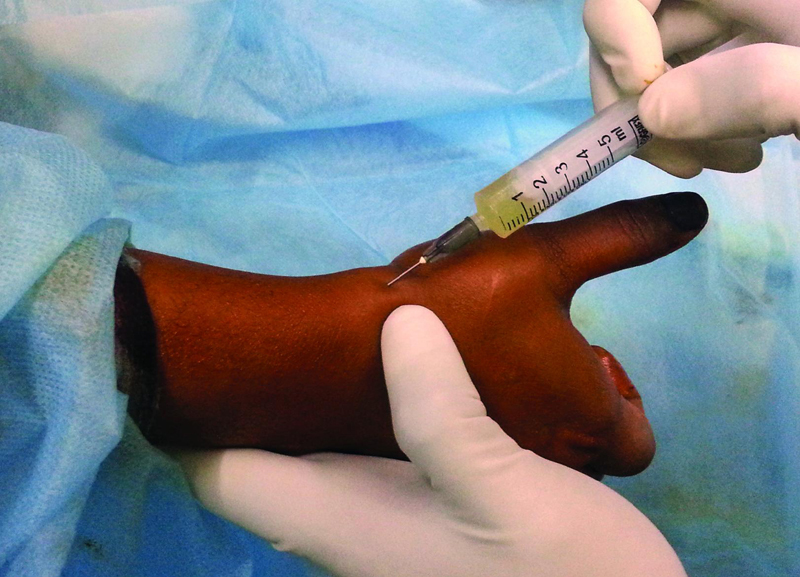
Technique of injecting PRP into the first dorsal compartment. PRP, platelet rich plasma.
Group 2 (CS GROUP): Patients in this group were treated with an injection of 1 mL (40 mg) methylprednisolone acetate with a fine gauge (25–27) needle.
Pre Injection Requisites
No previous CS injections should have been given within 6 months period.
All anti-inflammatory medications should have been discontinued at least 1 week prior to injection.
Corticosteroid Preparation and Injection
A solution of 40 mg (1 mL) methylprednisolone was taken into syringe with a fine needle (25–27 gauge). CS injection was injected in a similar manner as PRP. Similar post-injection and follow-up instructions were given.
No patients were lost to follow-up. Clinical assessment at all follow-up visits were done by:
Finkelstein test.
Visual analogue scale score.
The DASH score: The score ranges from 0 (no disability) to 100 (most severe disability). 19
MMWS: The total score ranges from 0 to 100 points with higher scores indicating a better result. An excellent result is defined as 90 to 100 points, good is 80 to 89, fair is 65 to 79 points, and poor is less than 65 points. 18
Statistical analysis: The data was managed using Microsoft Excel spreadsheet. Statistical testing was conducted with the Statistical Package for The Social Science System (SPSS Ver 21.0). Continuous variables were presented as mean ± SD, and categorical variables were presented as absolute numbers and percentage. The comparison of normally distributed continuous variables between the groups was performed using Student's t -test. Comparison of variables pre- and post-intervention within the groups was performed using Paired t -test. Nominal categorical data between the groups was compared using Chi-square test or Fisher's exact test as appropriate. The level of clinical significance was set at p = 0.05 with a confidence interval of 95% for all tests. p <0.05 was considered statistically significant.
Results
Mean age of patients in group 1 (PRP) was 35.83 ± 8.48 years while the mean age of patients in group 2 (CS) was 37.80 ± 6.44 years. There were 73.3% females in PRP group and 66.7% females in CS group. In both the groups right side was more often involved side but in CS group it was 76.7% involved while in PRP group it was 56.7% involved. Dominant hand was involved in 24 cases (80%) in group 1 (PRP) and in 21 cases (70%) in group 2 (CS). The groups were homogenous and comparable with respect to age ( p = 0.316), gender distribution ( p = 0.573), right side involvement ( p = 0.100), and hand dominance ( p = 0.371). All 60 patients were thoroughly evaluated at baseline (day 0) and then at each subsequent visits on the basis of four parameters (Finkelstein Test, VAS, DASH score, and MMWS).
Finkelstein test: In both the groups, the differences between pre-intervention and post-intervention values at all visits were statistically significant ( p <0.001) ( Tables 1 and 2 ). Finkelstein test between two groups showed statistically insignificant results at 1 month ( p = 0.795) and subsequent follow-up visits ( Table 3 ).
Mean VAS score: In both the groups, it was noted that there was a significant reduction in VAS score from pre-injection level to each point of follow-up with p -value <0.001 ( Tables 1 and 2 ). The baseline VAS of both groups was comparable with mean values of 6.73 ± 1.44 in group 1 and 6.53 ± 1.48 in group 2. At all the points of further follow-up visits, the differences of mean VAS between two groups were found to be statistically insignificant ( Table 3 ).
Mean DASH score: In group 1, the mean DASH score at day 0 was 27.53 ± 6.46 as compared to group 2 (26.98 ± 7.02). During subsequent follow-up visits, it exhibited gradual improvement with statistically significant difference ( p< 0.001) in both groups ( Table 1 ). The differences between the mean DASH scores of the two groups before intervention ( p = 0.753) and subsequent follow-ups after intervention were found comparable and statistically insignificant ( Table 4 ).
Mean MMWS: In both the groups, the mean MMWS showed statistically significant improvement between day 0 and during every subsequent follow-up visits ( Tables 1 and 2 ). But the differences of mean MMWS between the two groups at pre-intervention and subsequent follow-ups were statistically insignificant ( Table 4 ).
Complications: In group 1, the complications like local depigmentation, subcutaneous fat atrophy, temporary pain, and tendon rupture, etc. were not seen. In group 2, local depigmentation was seen in four cases (13.33%), subcutaneous fat atrophy in three cases (10%), and temporary increase in pain in one case (3.33%). Total complication rate was 26.67%. The difference between groups was statistically significant with p -value <0.05 ( Table 5 ).
In majority of our samples the platelet amplification was between 2.5 and 3.4. The mean platelet amplification was found to be 3.05 ± 0.314. This implies that the platelet count in PRP was on an average 3.05 times more than the platelet count in blood.
Table 1. Functional assessment of Group-1 (PRP) by Finkelstein test, VAS score, DASH score and MMWS.
| Group-1 (PRP) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finkelstein test | VAS score | DASH score | MMWS | |||||||||
| Frequency | % | p -Value (Pre–post intervention) | Mean ± SD | Mean difference from pre-intervention ± SD | p -Value | Mean ± SD | Mean difference from pre intervention ± SD | p -Value | Mean ± SD | Mean difference from pre intervention ± SD | p -Value | |
| Pre- intervention (Day 0) |
30 | 100.0% | – | 6.73 ± 1.44 | – | – | 27.53 ± 6.46 | – | – | 64.00 ± 6.22 | – | – |
| Post –intervention (1 mo) |
17 | 56.7% | <0.001 | 3.67 ± 2.60 | (−)3.07 ± 2.27 | <0.001 | 11.37 ± 9.46 | (−)16.16 ± 7.83 | <0.001 | 75.50 ± 11.32 | 11.50 ± 9.21 | <0.001 |
| Post- intervention (3 mo) |
10 | 33.3% | <0.001 | 1.87 ± 1.78 | (−)4.87 ± 1.76 | <0.001 | 5.88 ± 6.56 | (−)21.65 ± 7.23 | <0.001 | 82.83 ± 8.68 | 18.83 ± 8.38 | <0.001 |
| Post- intervention (6 mo) |
5 | 16.7% | <0.001 | 0.83 ± 0.99 | (−)5.90 ± 1.54 | <0.001 | 2.38 ± 3.87 | (−)25.15 ± 6.25 | <0.001 | 88.83 ± 6.91 | 24.83 ± 7.82 | <0.001 |
| Post- intervention (1 y) |
2 | 6.7% | <0.001 | 0.40 ± 0.62 | (−)6.33 ± 1.45 | <0.001 | 0.49 ± 0.85 | (−)27.04 ± 6.25 | <0.001 | 92.50 ± 4.10 | 28.50 ± 6.04 | <0.001 |
Abbreviations: %, percentage; SD, standard deviation.
Table 2. Functional assessment of Group-2 (CORTICOSTEROID) by Finkelstein test, VAS score, DASH score, and MMWS.
| Group-2 (Corticosteroid) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finkelstein test | VAS score | DASH score | MMWS score | |||||||||
| Frequency | % | p -Value (Pre–post intervention) | Mean ± SD | Mean difference from pre-intervention ± SD | p -Value | Mean ± SD | Mean difference from pre-intervention ± SD | p -Value | Mean ± SD | Mean difference from pre-intervention ± SD | p -Value | |
| Pre-intervention (Day 0) |
30 | 100.0% | – | 6.53 ± 1.48 | – | – | 26.98 ± 7.02 | – | – | 64.83 ± 5.65 | – | – |
| Post-intervention (1 mo) |
16 | 53.3% | <0.001 | 3.27 ± 2.33 | (−)3.27 ± 1.68 | <0.001 | 10.84 ± 9.41 | (−)6.14 ± 7.35 | <0.001 | 75.67 ± 11.20 | 10.83 ± 8.42 | <0.001 |
| Post-intervention (3 mo) |
11 | 36.7% | <0.001 | 2.30 ± 2.32 | (−)4.23 ± 1.83 | <0.001 | 6.82 ± 8.70 | (−)20.16 ± 6.86 | <0.001 | 82.00 ± 9.34 | 17.17 ± 7.27 | <0.001 |
| Post-intervention (6 mo) |
5 | 16.7% | <0.001 | 1.23 ± 1.61 | (−)5.30 ± 1.64 | <0.001 | 3.02 ± 5.13 | (−)23.96 ± 6.37 | <0.001 | 86.83 ± 7.13 | 22.00 ± 6.90 | <0.001 |
| Post-intervention (1 y) |
3 | 10.0% | <0.001 | 0.47 ± 0.78 | (−)6.07 ± 1.34 | <0.001 | 1.21 ± 2.83 | (−)25.77 ± 6.57 | <0.001 | 90.83 ± 5.88 | 26.00 ± 6.35 | <0.001 |
Abbreviations: %, percentage; SD, standard deviation.
Table 3. Comparison of Finkelstein test and VAS score between Group-1 and Group-2.
| Finkelstein test | VAS score | |||||||
|---|---|---|---|---|---|---|---|---|
| Group-1 (PRP) | Group-2 (Corticosteroid) |
p -Value | Group-1 (PRP) |
Group-2 (Corticosteroid) |
p -Value | |||
| Frequency | % | Frequency | % | Mean ± SD | Mean ± SD | |||
| Pre-intervention Day 0 |
30 | 100.0% | 30 | 100.0% | – | 6.73 ± 1.44 | 6.53 ± 1.48 | 0.597 |
| 1 mo | 17 | 56.7% | 16 | 53.3% | 0.795 | 3.67 ± 2.60 | 3.27 ± 2.33 | 0.533 |
| 3 mo | 10 | 33.3% | 11 | 36.7% | 0.787 | 1.87 ± 1.78 | 2.30 ± 2.32 | 0.420 |
| 6 mo | 5 | 16.7% | 5 | 16.7% | 1.000 | 0.83 ± 0.99 | 1.23 ± 1.61 | 0.251 |
| 1 y | 2 | 6.7% | 3 | 10.0% | 1.000 | 0.40 ± 0.62 | 0.47 ± 0.78 | 0.715 |
Abbreviations: %, percentage; SD, standard deviation.
Table 4. Comparison of DASH score and MMWS between Group 1 and Group 2.
| DASH score | MMWS | |||||
|---|---|---|---|---|---|---|
| Group-1 (PRP) | Group-2 (Corticosteroid) |
p -Value | Group-1 (PRP) |
Group-2 (Corticosteroid) |
p -Value | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Pre-intervention day 0 |
27.53 ± 6.46 | 26.98 ± 7.02 | 0.753 | 64.00 ± 6.22 | 64.83 ± 5.65 | 0.589 |
| 1 mo | 11.37 ± 9.46 | 10.84 ± 9.41 | 0.829 | 75.50 ± 11.32 | 75.67 ± 11.20 | 0.954 |
| 3 mo | 5.88 ± 6.56 | 6.82 ± 8.70 | 0.633 | 82.83 ± 8.68 | 82.00 ± 9.34 | 0.722 |
| 6 mo | 2.38 ± 3.87 | 3.02 ± 5.13 | 0.587 | 88.83 ± 6.91 | 86.83 ± 7.13 | 0.274 |
| 1 y | 0.49 ± 0.85 | 1.21 ± 2.83 | 0.183 | 92.50 ± 4.10 | 90.83 ± 5.88 | 0.208 |
Abbreviations: %, percentage; SD, standard deviation.
Table 5. Comparison between Group 1 and Group 2 in terms of complications.
| Complications | Group 1 | Group 2 | p -Value | ||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Nil | 30 | 100.0% | 22 | 73.33% | 0.026 |
| Local depigmentation | 0 | 0.0% | 4 | 13.33% | |
| Subcutaneous fat atrophy | 0 | 0.0% | 3 | 10% | |
| Temporary increase in pain | 0 | 0.0% | 1 | 3.33% | |
| Total | 30 | 100% | 30 | 100% | |
Discussion
When the conservative treatment for the DQTSV fails, surgical release of the first dorsal compartment and decompression of the APL and EPB tendons can be considered. But due to complications associated with surgery like painful neuroma, 20 superficial radial nerve neuropathies and entrapment, 20 scar adherence to underlying tendons, 21 residual pain, and wound infections, treatment like local CS injection is the mainstay for DQTSV treatment. CS like methylprednisolone acetate have promising effects in DQTSV but have some side effects limiting its use in this disease. These are tendon rupture, depigmentation, subcutaneous atrophy, and temporary increase in pain. 22 Therefore, there has been a search for a different modality which should have same or better effects than CS but without their known complications. In recent era PRP has emerged as a good treatment option for the management of this condition. Various studies have shown PRP injections as a promising treatment modality in various tendinopathies with minimal risks. 12 13 14 15 Although many studies have shown CS to be effective for de Quervain's disease, very limited literature is available to support the use of PRP in DQTSV and their comparison in treating this disease. 8 10 16 Considering the above, this study was designed to evaluate as well as compare the efficacy of autologous PRP injection with CS injection in the management of DQTSV.
In our study 60 patients who fulfilled the predefined inclusion and exclusion criteria were randomised equally into two groups. Group 1 received single dose of PRP injection while group 2 received single dose of CS injection. Regular follow-ups were done for a period of 1 year. The baseline characteristics of patients in both the groups were found to be homogenous and comparable with no statistical difference in terms of age distribution, gender, side involved, and hand dominance. The PRP prepared in our study had a platelet amplification of approximately three times the baseline count which was reported as two times by Martinell et al. 23 We used single spin method of PRP generation which is in accordance with other studies. 16 24 We used the citrate dextrose solution as an anticoagulant for PRP preparation and did not use any exogenous activator as was done in some previous studies. 8 16 23 24 Peck and Ely 8 used 3 mL of PRP to inject the tendon sheath of abductor pollicis longus and EPB tendons. We also used the same quantity of PRP. In both the groups, only one injection was given. Clinical and functional evaluations at follow-ups were done by Finkelstein test, VAS, DASH, and MMWS.
In PRP group, Finkelstein test showed statistically significant improvement at each follow-up after 1 month of treatment. It was positive only in two cases, i.e., 6.7% of cases after the end of 1 year which was statistically significant. In our study mean VAS score also showed statistically significant improvement at 1 year following intervention. In the study by Peck and Ely 8 VAS reduced 63% after 6 months (14 of 100 or 1.4 of 10). A different study mentioned that the initial VAS score was 9.42, post-procedural 6 months VAS was 3.92, a statistically significant improvement was observed in terms of pain relief after a single PRP injection. 25 In our study, mean VAS decreased to 0.83 ± 0.99 at 6 months. The Mean DASH score in our study improved from 27.53 ± 6.46 to 0.49 ± 0.85 following 1 year after intervention, this was found to be statistically significant ( p <0.001). Mean MMWS also improved from 64 ± 6.22 (pre-intervention) to 92.50 ± 4.10 (1-year post-intervention) showing statistically significant improvement.
In CS group; Finkelstein test exhibited statistically significant improvement at each follow-up and after 1 year of injection (remained positive in only three cases, i.e., 10.0% of cases after the end of 1 year). A study carried out by Shivanna et al 26 concluded that after the end of treatment, only two out of 60 patients had positive test which is comparable to our study. In our study, mean VAS score also showed statistically significant improvement (from 6.53 ± 1.48 to 0.47 ± 0.78) after 1 year. In the study by McDermott et al, 27 VAS after the end of treatment was 2.2. The Mean DASH score in our study improved from 26.98 ± 7.02 to 1.21 ± 2.83 after 1 year post-intervention which was found to be statistically significant. This is comparable to study done by Mehdinasab and Alemohammad. 28 In study done by McDermott et al, 27 DASH decreased to a value of 18.39. The mean MMWS in our study improved from 64.83 ± 5.65 to 90.83 ± 5.88 after 1 year and this change was statistically significant.
To the best of our knowledge. there are no similar studies available in the literature to which our results could be compared side by side.
On comparing the two groups, the differences in all of the above scores were statistically not significant and none of the modality came out to be superior in terms of pain relief and functional improvement. El Sheikh et al 16 showed significant improvements in VAS and quick DASH scores after 6 months of treatment and they reported that the CS group had better pain relief, hand function tests, and ultrasonographic findings at 1 month, but at 6 months follow-up, the PRP group had statistically significant better pain relief, hand function tests, and ultrasonographic findings than CS group. To the best of our knowledge no other comparative study has been done between PRP and CS injections in DQTSV. Our study showed both PRP and CS injections as equally beneficial modality for de Quervain's disease but none of them came out to be superior. To arrive at a definitive conclusion, a greater number of studies need to be performed. While we did not use ultrasound guidance for the injection, we accept arguably that this may allow for a more accurate placement of the PRP and it could be considered. This may be perceived as a shortcoming of our study.
No studies have reported any complication of PRP except very small risk of infection. 12 13 22 We also noticed this procedure to be well tolerated by the patients with no or a minimal risk of complication. On the other hand, in CS group, four cases of local depigmentation, three cases of subcutaneous fat atrophy, and one case of temporary increase in pain were reported which was statistically significant and these changes were reversed in 3 to 4 months period. Total complications reported were 26.67% which was slightly higher than the study done by Shivanna et al. 26 There was no incidence of nerve injury, tendon rupture, or infection.
The pain at donor site for PRP preparation was insignificant as it did not demand an analgesic and subsided in a day. The total time taken for PRP injection from its preparation to injection was about 40 minutes as compared to steroid injection which was about 25 minutes. Both the groups were treated on outpatient basis. Our hospital being a tertiary centre with a free of cost availability of consumables and blood bank for the preparation of PRP as well as CS injection, we did not incur any cost for both these procedures. Hence both the procedures were comparable in terms of cost. Even if it is not available at other centers the cost of above consumables is very nominal but slightly more in case of PRP due to equipment (Centrifuge and Sodium Citrate Vacutainer) involved in the preparation.
Considering the ease of preparation and administration, and cost effectiveness, we conclude that both autologous PRP and CS can become extremely useful modalities which can curtail the need for more invasive surgery in DQTSV. Based on our results and complications encountered with CS use, it is concluded that PRP is an equally efficacious but a safer alternative when compared to CS for the treatment of DQTSV.
Limitations of our study were small sample size, absence of control group, very basic injection technique without the use of ultrasonographic guidance, and scanty literature to compare the results. Effective and further long-term double blinded studies are necessary to conclusively document their effectiveness and long-term safety. Furthermore, the method of PRP preparation needs to be standardized and exact parameters related to its properties need to be defined.
Funding Statement
Funding None.
Conflict of Interest None declared.
Statement of Human and Animal Rights-
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed Consent
All patients enrolled in this study gave informed consent to participate and no identifying factors were used.
Availability of Data and Material
Data was collected from the patients reported in our hospital.
Code Availability
Not applicable.
References
- 1.Viikari-Juntura E.Tenosynovitis, peritendinitis and the tennis elbow syndrome Scand J Work Environ Health 198410(Spec no. 6):443–449. [DOI] [PubMed] [Google Scholar]
- 2.de Quervain F. On a form of chronic tendovaginitis by Dr. Fritz de Quervain in la Chaux-de-Fonds. 1895. Am J Orthop. 1997;26(09):641–644. [PubMed] [Google Scholar]
- 3.Finkelstein H. Stenosing tendovaginitis at the radial styloid process. J Bone Joint Surg Am. 1930;12:509–540. [Google Scholar]
- 4.Zingas C, Failla J M, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain's tendinitis. J Hand Surg Am. 1998;23(01):89–96. doi: 10.1016/S0363-5023(98)80095-6. [DOI] [PubMed] [Google Scholar]
- 5.Ilyas A M. Nonsurgical treatment for de Quervain's tenosynovitis. J Hand Surg Am. 2009;34(05):928–929. doi: 10.1016/j.jhsa.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 6.da Silva J BG, Batigália F. Acupuncture in De Quervain's disease: a treatment proposal. Acupunct Med. 2014;32(01):70–72. doi: 10.1136/acupmed-2013-010486. [DOI] [PubMed] [Google Scholar]
- 7.Moretti M. Effectiveness of oxygen-ozone and hyaluronic acid injections in De Quervain's syndrome. Int J Ozone Ther. 2012;11(01):31–33. [Google Scholar]
- 8.Peck E, Ely E. Successful treatment of de Quervain tenosynovitis with ultrasound-guided percutaneous needle tenotomy and platelet-rich plasma injection: a case presentation. PM R. 2013;5(05):438–441. doi: 10.1016/j.pmrj.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Tseng V, Ibrahim V, Yokel N R. Prolotherapy for dequervain's tenosynovitis/tendonosis: a case report. PM R. 2012;4(10):S275. [Google Scholar]
- 10.Peters-Veluthamaningal C, Winters J C, Groenier K H, Meyboom-DeJong B. Randomised controlled trial of local corticosteroid injections for de Quervain's tenosynovitis in general practice. BMC Musculoskelet Disord. 2009;10:131. doi: 10.1186/1471-2474-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardone D A, Tallia A F. Joint and soft tissue injection. Am Fam Physician. 2002;66(02):283–288. [PubMed] [Google Scholar]
- 12.Gosens T, Peerbooms J C, van Laar W, den Oudsten B L. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(06):1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 13.Soomekh D J. Current concepts for the use of platelet-rich plasma in the foot and ankle. Clin Podiatr Med Surg. 2011;28(01):155–170. doi: 10.1016/j.cpm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Sampson S, Gerhardt M, Mandelbaum B.Platelet rich plasma injection grafts for musculoskeletal injuries: a review Curr Rev Musculoskelet Med 20081(3-4):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rha D W, Park G Y, Kim Y K, Kim M T, Lee S C. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27(02):113–122. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 16.El Sheikh A, Mahmoud S A, El-Rahim M Abd. The role of platelet rich plasma in comparison with corticosteroids in the treatment of De Quervain tenosynovitis. Med J Cairo Univ. 2020;88:141–148. [Google Scholar]
- 17.Chaffee A, Yakuboff M, Tanabe T. Responsiveness of the VAS and McGill pain questionnaire in measuring changes in musculoskeletal pain. J Sport Rehabil. 2011;20(02):250–255. doi: 10.1123/jsr.20.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Cooney W P, Bussey R, Dobyns J H, Linscheid R L. Difficult wrist fractures. Perilunate fracture-dislocations of the wrist. Clin Orthop Relat Res. 1987;(214):136–147. [PubMed] [Google Scholar]
- 19.The Upper Extremity Collaborative Group (UECG) . Hudak P L, Amadio P C, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected] Am J Ind Med. 1996;29(06):602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe S W, Hotchkiss R N, Pederson W C, Kozin S H. 6th ed. Philadelphia, PA: Elsevier; 2010. Green's Operative Hand Surgery. Vol. 2; pp. 2067–2088. [Google Scholar]
- 21.Harvey F J, Harvey P M, Horsley M W. De Quervain's disease: surgical or nonsurgical treatment. J Hand Surg Am. 1990;15(01):83–87. doi: 10.1016/s0363-5023(09)91110-8. [DOI] [PubMed] [Google Scholar]
- 22.Dean B J, Lostis E, Oakley T, Rombach I, Morrey M E, Carr A J. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014;43(04):570–576. doi: 10.1016/j.semarthrit.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Martinelli N, Marinozzi A, Carnì S, Trovato U, Bianchi A, Denaro V. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop. 2013;37(05):839–842. doi: 10.1007/s00264-012-1741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim E, Lee J H. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R. 2014;6(02):152–158. doi: 10.1016/j.pmrj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Jeyaraman M. The prospective study on efficacy and functional outcome of autologous platelet rich plasma injection in musculoskeletal disorders. EC Orthopaedics. 2018;9:849–863. [Google Scholar]
- 26.Shivanna D, Manjunath D, Holagundi L, Kumar H VM. Outcome of intra-sheath steroid injection for De Quervain's tenosynovitis. The Internet Journal of Hand Surgery. 2014;6(01):1–5. [Google Scholar]
- 27.McDermott J D, Ilyas A M, Nazarian L N, Leinberry C F. Ultrasound-guided injections for de Quervain's tenosynovitis. Clin Orthop Relat Res. 2012;470(07):1925–1931. doi: 10.1007/s11999-012-2369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehdinasab S A, Alemohammad S A. Methylprednisolone acetate injection plus casting versus casting alone for the treatment of de Quervain's tenosynovitis. Arch Iran Med. 2010;13(04):270–274. [PubMed] [Google Scholar]


