Keywords: carbon nanotube fibers, electromyography, motor systems, muscles, neurophysiology
Abstract
Executing complex behaviors requires precise control of muscle activity. Our understanding of how the nervous system learns and controls motor skills relies on recording electromyographic (EMG) signals from multiple muscles that are engaged in the motor task. Despite recent advances in tools for monitoring and manipulating neural activity, methods for recording in situ spiking activity in muscle fibers have changed little in recent decades. Here, we introduce a novel experimental approach to recording high-resolution EMG signals using parylene-coated carbon nanotube fibers (CNTFs). These fibers are fabricated via a wet spinning process and twisted together to create a bipolar electrode. Single CNTFs are strong, extremely flexible, small in diameter (14–24 µm), and have low interface impedance. We present two designs to build bipolar electrode arrays that, due to the small size of CNTF, lead to high spatial resolution EMG recordings. To test the EMG arrays, we recorded the activity of small (4 mm length) vocal muscles in songbirds in an acute setting. CNTF arrays were more flexible and yielded multiunit/bulk EMG recordings with higher SNR compared with stainless steel wire electrodes. Furthermore, we were able to record single-unit recordings not previously reported in these small muscles. CNTF electrodes are therefore well-suited for high-resolution EMG recording in acute settings, and we present both opportunities and challenges for their application in long-term chronic recordings.
NEW & NOTEWORTHY We introduce a novel approach to record high-resolution EMG signals in small muscles using extremely strong and flexible carbon nanotube fibers (CNTFs). We test their functionality in songbird vocal muscles. Acute EMG recordings successfully yielded multiunit recordings with high SNR. Furthermore, they successfully isolated single-unit spike trains from CNTF recordings. CNTF electrodes have great potential for chronic EMG studies of small, deep muscles that demand high electrode flexibility and strength.
INTRODUCTION
Acquiring a skilled behavior requires the development of precise coordination across neurons and muscles. It is unknown how the brain generates, refines, and controls precise patterns of activity during motor skill learning. To examine this fundamental question in motor control, it is necessary to record electromyographic (EMG) activity from the muscles (Fig. 1A) that control the pertinent behavior. Existing methods used to record EMG activity rely on surface electrodes or intramuscular electrodes comprising fine wires or rigid bipolar needles (1–6). Surface electrodes can include two contacts (7) or larger number of electrodes and are noninvasively placed on the subject’s skin (8). The location of surface electrodes makes them incapable of recording EMG activity from small or deep internal muscles (8–10). Fine-wire electrodes are placed in the muscle with a needle or by applying pressure to the electrode to penetrate the muscle. Rigid needle electrodes are similarly inserted directly into the muscle.
Figure 1.
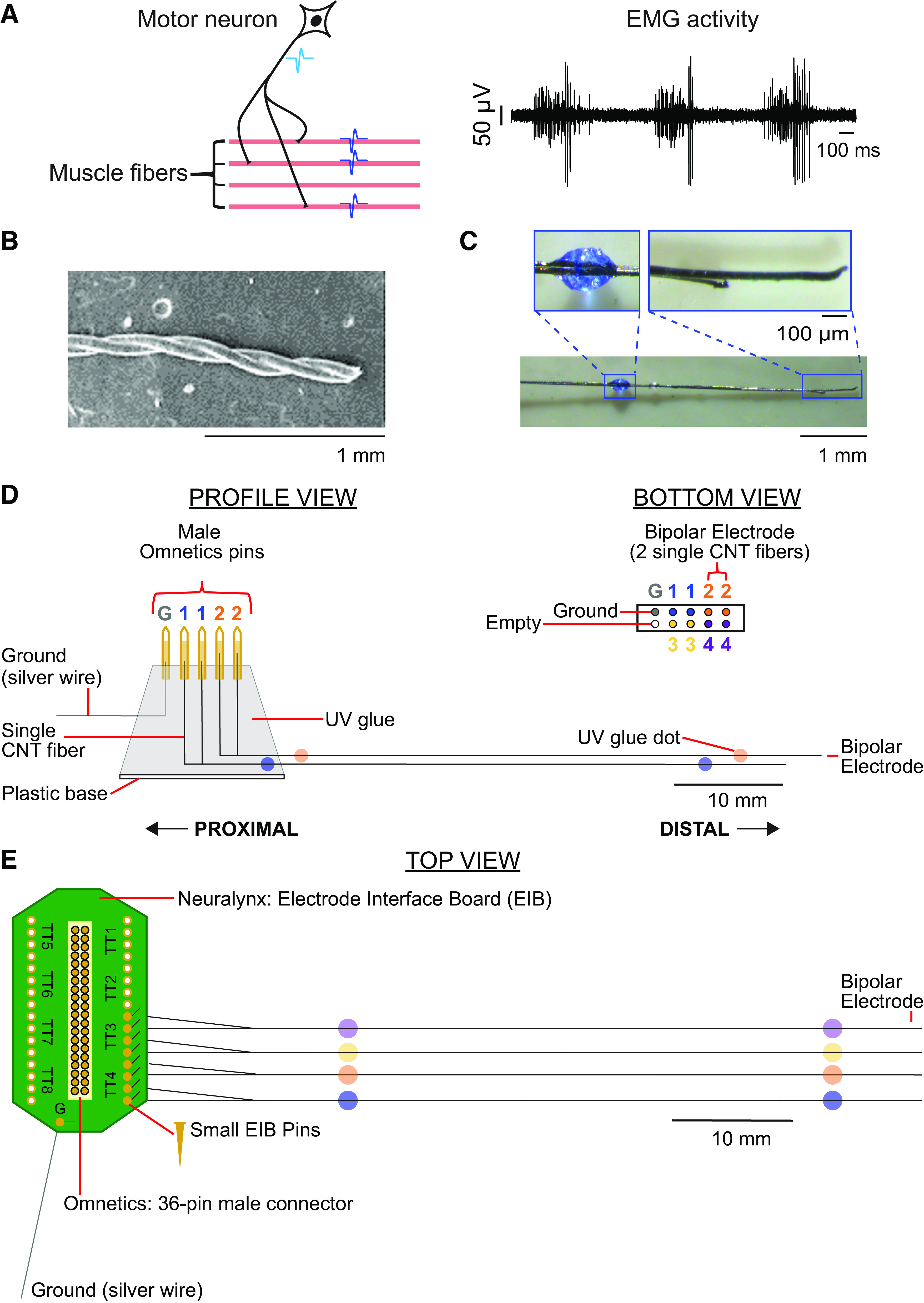
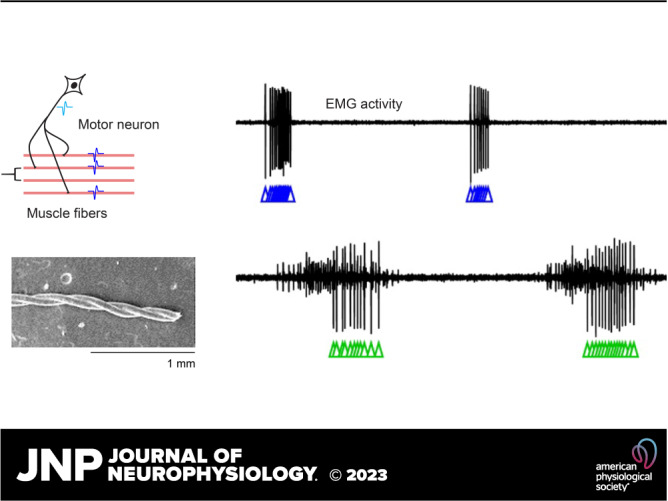
Motor units, EMG, and carbon nanotube fibers (CNTFs) array construction. A, left: schematic of a motor unit, which consists of all muscle fibers (shown in pink) innervated by a single-motor neuron (shown in black). When a motor neuron fires an action potential (light blue waveform), the action potential moves down the axon to the muscle fibers it innervates, causing the innervated muscle fibers to fire a near-synchronous volley of action potentials (dark blue waveforms). Right: example recordings of multiunit EMG activity. B: scanning electron microscopy image of two parylene-coated carbon nanotube fibers twisted together, creating a bipolar electrode. C: each bipolar electrode was labeled with colored dots of ultraviolet (UV) glue. Each electrode array consisted of four CNTF bipolar electrodes and one ground wire. D: CNTF array schematic for design 1. Each proximal end of four CNTF bipolar electrodes and one ground wire were secured into male Omnetics pins with carbon glue, creating a two by five electrode array. E: CNTF array schematic for design 2. Each proximal end of a CNTF bipolar electrode was inserted into side-by-side gold-plated holes on a Neuralynx electrode board interface and secured with a gold attachment pin.
For small muscles in small experimental animals, such as songbirds, the stiffness of traditional fine-wire metal electrodes creates a number of difficulties. Songbird vocal muscles are located deeper in the body than, for example, the muscles of the rodent forelimb, and are thus more prone to muscle injury at the implant site (6). The muscle fibers in the songbird vocal organ produce 50–100 times lower stress (11) and are smaller in diameter (12) than skeletal muscles, and thus are impeded more than skeletal muscles by stiff wire implants when contracting. Furthermore, it is important for the lead-out wires to be very flexible to prevent strain on the vocal organ, which could interfere with natural singing behavior, a behavior much more sensitive and easily restricted than e.g., locomotion. The lack of flexibility in fine-wire electrodes also limits the number of channels that can be implanted simultaneously. Recording with four fine-wire bipolar channels is extremely challenging (13), thus songbird vocal muscles provide a good test case for more flexible EMG electrode designs.
To address these obstacles, we developed a novel electrode technology that uses carbon nanotube fibers (CNTFs) to create bipolar electrodes (Fig. 1). Single CNTFs are five times stronger than graphene fibers and are the best conductive polymer (14), have a flexural rigidity about four times that of platinum-iridium (Pt-Ir) fine-wire electrodes (15), and can be produced as small as ∼14–24 µm diameter threads (Fig. 1B). Electrochemically, CNTFs have 15–20 times lower interface impedance than Pt-Ir fine-wire electrodes of the same diameter (16). Compared with Pt-Ir, CNTFs are more biocompatible, producing a reduced inflammatory response (16–18). In addition, CNTFs have been successfully used to record neural activity from mice cortex and thalamic reticular nucleus in acute brain slices (19), chronically stimulate neurons and record single-unit neural activity in rats (20), and acutely stimulate and record from nerves in small animal models (21). These properties make CNTFs ideal candidates for constructing multichannel electrode arrays for EMG recordings of small muscles, allowing EMG recordings in muscles that were previously not possible. However, we currently do not know whether CNTF bipolar electrodes could be used to record high-amplitude EMG activity in such muscles.
In this paper, we introduce two designs to build bipolar electrode arrays with the CNTF technology, where each array consisted of four CNTF bipolar electrodes and one ground wire (Fig. 1, D and E). We measured the bending stiffness of these arrays to be eight times lower than the same array made of stainless steel wire (SSW) electrodes. We furthermore present a procedure to record EMG activity from small muscles in an acute experimental paradigm. Our results show that CNTF electrodes can attain high spatial resolution, recording both bulk (multiunit) and single-unit EMG activity across multiple small muscles. In acute recording situations, we observed signal-to-noise ratios (SNRs) in the CNTF bipolar electrodes between 8 and 44 for multiunit activity, which is up to 7.1 times higher than SSW electrodes, and between 39 and 62 for single-unit activity, which we never observed using traditional SSW electrodes. The SNR of CNTF remained consistent over several hours, demonstrating the stability of the EMG recordings within and across multiple muscles.
METHODS
Animals
Fourteen adult (>90 days post hatch) songbirds were used in this study. Eight birds were used for acute EMG recordings with CNTF bipolar electrodes. Two female Bengalese finches (Lonchura striata domestica; Table 1, birds 1 and 2) were used to record EMG activity from vocal muscles of the songbird vocal organ, the syrinx: musculus syringealis ventralis (ventral syringeal; VS) and m. tracheobronchialis ventralis superficialis (superficial ventral tracheobronchial; sVTB; Fig. 2). Three female Bengalese finches (birds 3, 4, and 5) were used to record EMG activity from one of the respiratory muscles [expiratory muscle group (Exp); Fig. 2]. Bengalese finches were bought from a supplier or bred in our laboratory at Emory University, United States, and singly housed in sound-attenuating chambers with food and water. In addition, two female and one male zebra finch (Taeniopygia guttata; birds 6, 7, and 8) were used to record EMG activity from VS and sVTB. Six additional male zebra finches (birds 9–14) were used for acute EMG recordings with SSW bipolar electrodes. All zebra finches were from a breeding colony housed in indoor aviaries at the University of Southern Denmark (SDU), Denmark. Sound chambers and aviaries were kept on a 12:12-h light/dark cycle, and ambient temperature was maintained between 24°C to 28°C. Bengalese finch experiments were conducted at Emory University, and the zebra finch experiments were conducted at SDU. All experimental protocols were approved by Emory University and SDU Institutional Animal Care and Use Committees.
Table 1.
Summary of data used in analysis
| Animal ID | Electrode Type | Recording # | Electrode # | Electrode Placement |
Recording Duration, min |
# Breath Cycles |
# Single Units Extracted |
Mean Single-Unit Spike Rate, Hz | Within-Burst Single-Unit Spike Rate, Hz |
|---|---|---|---|---|---|---|---|---|---|
| Bird 1 (F-BF) | CNTF | 1 | 1 | R. VS | 191 | 29,255 | 1 | 8.83 | 27.80 |
| CNTF | 1 | 2 | L. VS | 137* | 21,540 | 1 | 10.29 | 39.63 | |
| CNTF | 1 | 3 | L. sVTB | 191 | 29,311 | 0 | |||
| Bird 2 (F-BF) | CNTF | 1 | 1 | L. VS | 8 | 861 | 1 | 8.98 | 60.44 |
| CNTF | 2 | 2 | L. VS | 70* | 9,372 | 0 | |||
| CNTF | 2 | 3 | L. VS | 109 | 14,290 | 0 | |||
| Bird 3 (F-BF) | CNTF | 1 | 1 | R. Exp | 29 | 3,467 | 0 | ||
| CNTF | 1 | 2 | R. Exp | 29 | 2,551 | 1 | 2.83 | 93.49 | |
| CNTF | 1 | 3 | R. Exp | 29 | 2,585 | 1 | 4.75 | 111.56 | |
| Bird 4 (F-BF) | CNTF | 1 | 1 | R. Exp | 151 | 14,543 | 1 | 8.48 | 46.30 |
| CNTF | 1 | 2 | R. Exp | 151 | 14,557 | 0 | |||
| Bird 5 (F-BF) | CNTF | 1 | 1 | L. Exp | 249 | 16,300 | 1 | 10.43 | 81.02 |
| CNTF | 1 | 2 | L. Exp | 249 | 17,360 | 1 | 31.51 | 75.90 | |
| CNTF | 1 | 3 | L. Exp | 181* | 11,677 | 1 | 5.42 | 32.93 | |
| Bird 6 (F-ZF) | CNTF | 1 | 1 | L. sVTB | 11 | 1,560 | 1 | 13.46 | 75.03 |
| CNTF | 1 | 2 | L. sVTB | 11 | 1,509 | 0 | |||
| CNTF | 2 | 1 | L. sVTB | 3 | 328 | 1 | 15.76 | 106.08 | |
| CNTF | 2 | 2 | L. sVTB | 3 | 353 | 0 | |||
| CNTF | 2 | 3 | L. VS | 3 | 354 | 0 | |||
| CNTF | 3 | 1 | R. sVTB | 4 | 398 | 0 | |||
| Bird 7 (F-ZF) | CNTF | 1 | 1 | L. VS | 12 | 1,231 | 1 | 15.76 | 142.15 |
| CNTF | 2 | 2 | L. VS | 10 | 718 | 0 | |||
| Bird 8 (M-ZF) | CNTF | 1 | 1 | L. VS | 13 | 1,184 | 0 | ||
| CNTF | 1 | 2 | L. sVTB | 13 | 1,192 | 0 | |||
| Bird 9 (M-ZF) | SSW | 1 | 1 | sVTB | 6 | 749 | 0 | ||
| Bird 10 (M-ZF) | SSW | 1 | 1 | L. sVTB | 3 | 435 | 0 | ||
| Bird 11 (M-ZF) | SSW | 1 | 1 | VS | 3 | 334 | 0 | ||
| SSW | 2 | 1 | sVTB | 5 | 252 | 0 | |||
| Bird 12 (M-ZF) | SSW | 1 | 1 | sVTB | 3 | N/A | 0 | ||
| Bird 13 (M-ZF) | SSW | 1 | 1 | L. sVTB | 5 | N/A | 0 | ||
| SSW | 1 | 3 | L. VS | 5 | N/A | 0 | |||
| SSW | 2 | 2 | L. sVTB | 4 | N/A | 0 | |||
| Bird 14 (M-ZF) | SSW | 1 | 1 | L. sVTB | 5 | N/A | 0 | ||
| SSW | 1 | 2 | L. VS | 5 | N/A | 0 | |||
| SSW | 1 | 3 | R. VS | 5 | N/A | 0 |
As described in methods, we quantified whether each recording from both CNTF and SSW electrodes included one or more isolatable single motor unit (“# single units extracted”). In cases where we were able to isolate a single motor unit, we quantified both the single unit’s mean firing rate over time (mean rate across bursting and silent periods) as well as its mean firing rate within each burst (mean rate within bursts, excluding interspike intervals that separate spikes from consecutive respiratory bursts). Note that because different motor units fire different number of spikes per burst, a unit with a low mean firing rate can have a high within-burst firing rate (if the average burst contains few spikes but those spikes occur with very short interspike intervals) and vice versa. As described in the text, all CNTF and SSW electrodes were placed on the muscle surface. BF, Bengalese finch; CNTF, carbon nanotube fiber bipolar electrodes; Exp, expiratory muscle group; F, female; L, left; M, male; R, right; SSW, stainless steel fine-wire bipolar electrodes; sVTB, superficial ventral tracheobronchial; VS, ventral syringeal; ZF, zebra finch. *Recordings that were terminated due to signal loss. All other recordings were terminated by experimenter when the signal SNR was still robust because sufficient data had been collected.
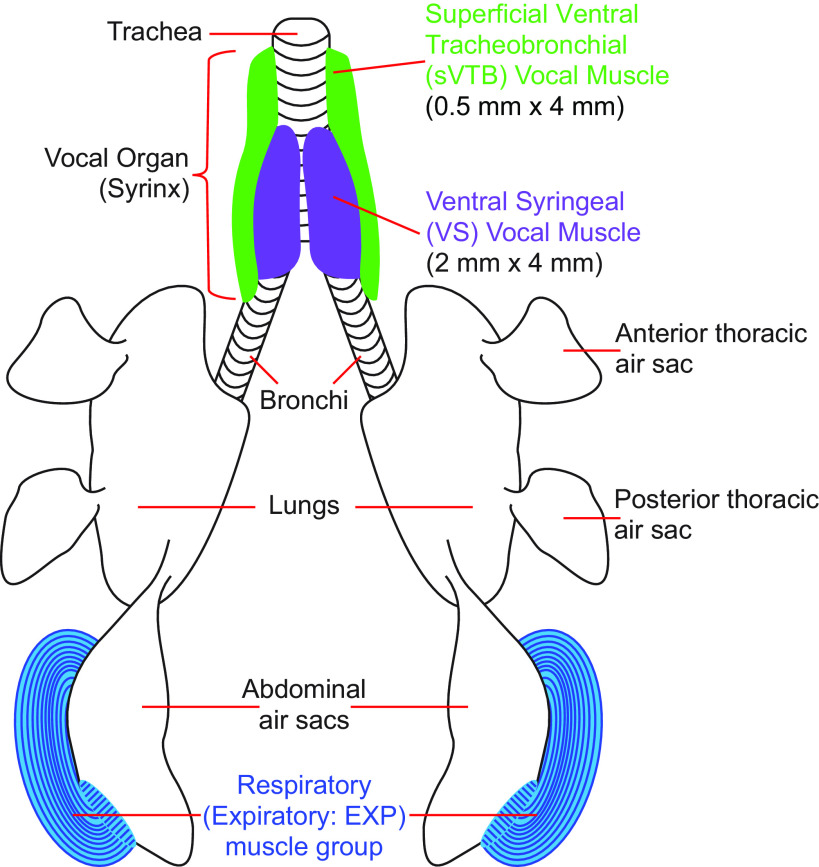
Figure 2.
Illustration of the respiratory and vocal system of songbirds. We measured EMG activity in either the right or left expiratory (Exp) muscle group or the right and/or left ventral syringeal (VS) muscles and/or superficial ventral tracheobronchial (sVTB) muscles. VS and sVTB muscles are part of the songbird vocal organ, syrinx.
Carbon Nanotube Fiber Fabrication
Purified carbon nanotubes (CNTs) were dissolved in chlorosulfonic acid at 2 weight % and wet-spun into carbon nanotube fibers (CNTFs, single-fiber diameter = 22.1 ± 1.1 µm) as previously described (14, 17, 18). CNTFs fabricated using this method are strong, flexible, light-weight, conductive, and possess significantly lower interface impedance than metal electrodes (14, 16–18). Next, 2.5 µm of Parylene C were deposited onto CNTF for insulation using SCS Labcoter 2 vacuum deposition system, leaving only the ends exposed. High-resolution scanning electron microscopy was used to measure single-fiber diameters (16), which range from 14 to 24 µm.
Carbon Nanotube Fibers Array Construction
CNTF bipolar electrodes.
Two coated CNTFs were twisted around each other at ∼20 turns/cm using a cord maker. This bipolar electrode pair was given another layer of coating (∼2 µm Parylene C) to hold the twists in place (Fig. 1B). It is near impossible to precisely cut the CNTF with standard scissors because of their combined strength and flexibility. We used a new #11 scalpel blade on a glass plate under a stereomicroscope to trim the bipolar CNTF electrode to ≈6.5 cm. To prepare the electrodes for insertion into muscles, the two CNTF of each bipolar electrode were separated 4–6 mm on the distal (recording) end. The two separated CNTFs were then cut so one tip was shorter than the other, creating an offset between the tips that varied between 0.2 and 1 mm (Fig. 1C). The tips were pushed back together with forceps and then secured to each other with ultraviolet (UV) glue (Solarez UV-cure thin, hard formula; Solarez, Minneapolis, MN) up until the offset of the tips. A dot of colored UV glue (a mixture of colored acrylic paint and UV glue) was placed ∼1 cm from the offset of the tips on the distal end (Fig. 1C) and ∼2 cm from the proximal end (Fig. 1, D and E) of the bipolar electrode. The colored glue bead helped to identify the electrodes and corresponding recording channels after muscle implantation, as well as to help place the electrode on the muscle.
CNTF electrode array.
We used two different designs to construct the CNTF electrode array. For design 1, 10 male pins (Omnetics, Minneapolis, MN) were placed in two rows of five pins in a 36-channel female Omnetics connector (NSD 36 VV GS 4; Omnetics, Minneapolis, MN). The two by five male pin configuration created an electrode array consisting of four bipolar CNTF electrodes and one ground wire (Fig. 1D). The proximal end of two CNTFs of a prepared bipolar electrode was separated 1.5 cm. Each separated CNTF proximal end was placed into individual Omnetics male connector pins side-by-side (Fig. 1D, bottom view). The pin and proximal end of the electrode were covered with carbon glue (Wire Glue; Anders product, Andover, MA), making an electrical connection between the CNTF and the Omnetics male connector pin. These steps were repeated for the other three bipolar electrodes, securing one more bipolar electrode on the bottom row and two bipolar electrodes on the top row. A 15 mm × 139.7 µm diameter perfluoroalkoxy (PFA)-coated silver wire (Cat. No. 785500; A-M Systems, Sequim, WA) was used for the ground wire. The PFA coating on the silver wire was stripped on both ends, and one end was secured to the male pin with carbon glue. After the carbon glue dried (∼8–12 h), the proximal end of the electrode array (excluding the tips of the Omnetics male connector pins) was covered with UV glue. Then, a thin 10 × 15 mm plastic square was secured to the base of the Omnetics male connector pins (opposite of the recording end) with UV glue (Fig. 1D, profile view). The plastic square was used to create a base to handle and secure the CNTF electrode array. The electrode array was then removed from the 36-channel female Omnetics connector with forceps.
Although design 1 was lightweight and small (10 mm × 14 mm), the design presented a few disadvantages including the average amount of time to build the electrode array (approximately 8 h over 2 days) and the fragility of the structure. To address these challenges, we created an alternative design that was easier and faster to build (Fig. 1E). For design 2, the ends of two CNTFs of a prepared bipolar electrode were separated 1.5–2 cm on the proximal end. Each proximal end was inserted into side-by-side gold-plated holes from the bottom of an electrode interface board (EIB) (EIB-36-PTB; Neuralynx, Bozeman, MT). The output of the Omnetics end is a 36-pin male Omnetics connector (NPD-36-AA-GS 4 Guide Posts; Omnetics, Minneapolis, MN). A gold electrode attachment pin (Small HEAD PLATE Pins; Neuralynx, Bozeman, MT) was then placed in the hole from the top of the head plate, catching the CNTF. Pliers with offset tips were used to push the pin further into the hole and pinch the CNTF. This action stripped off a small section of Parylene C coating, making an electrical connection between the CNTF and gold on the EIB, securing the CNTF into place. We continued attaching the CNTF for the remainder of the CNTF electrodes (maximum of 15 bipolar electrodes and ground). A 15-mm PFA-coated silver wire was used for the ground wire. Both ends of the coating were stripped off the wire, and one end of the wire was soldered to the ground connection on the EIB. In addition to being faster to assemble, the larger base (20 mm × 11 mm) of the EIB makes design 2 easier for the experimenter to grasp and manipulate during the experiment. Although both designs are reusable, the use of the attachment pin in design 2 to secure the CNTF in place (compared with UV glue in design 1) allows for the experimenter to remove and replace the used CNTF with unused CNTF by using the offset tip pliers to push the attachment pin from the underside of the 36-pin male Omnetics connector, removing the pin.
Fine-wire electrode array.
To compare the performance of the CNTF electrode arrays and standard fine-wire electrode arrays, we built the same arrays as described in detail above with 25-µm diameter, Teflon-insulated SSW electrodes (California Fine Wire Company, CA).
Mechanical Testing of Array Flexibility
We compared the mechanical resistance to bending of an intact CNTF versus SSW electrode arrays by measuring the force needed to bend the combined electrodes of assembled arrays (22). We used four-channel assembled arrays and placed them horizontally on a raised aluminum platform. The electrodes were clamped between two 1-mm thick, 20-mm wide glass microscope slides to prevent them from moving. The array connector was placed on one end of the glass and the bundle consisting of four free electrodes exited on the other side. At 1.0 mm from the exit point, we depressed the bundle with a dual-mode ergometer (model 300 C, Aurora Scientific, Canada) that measured force and displacement. All signals were digitized at 6 kHz and 16 bit (USB-6259, National Instruments). All control and analysis software were written in Matlab.
We applied 20 sinusoidal cycles with 1.0-mm peak-to-peak amplitude. Force signals were lowpass filtered at 1 kHz (2nd order Butterworth filter with zero-phase shift). The loading curves of cycles 5–20 were isolated and a linear regression curve was fitted to each cycle. We report the mean slope of the 15 cycles per array.
EMG Data Collection
Before surgery, arrays were connected to a 36-pin female Omnetics connector that interfaced with the Intan digital 16-channel bipolar recording headstage (RHD2216, Intan technologies, Los Angeles, CA). A flexible clamp was used to hold the plastic base (design 1) or the Omnetics EIB (design 2) of the CNTF or fine-wire array during acute recordings. The EMG signals were digitized at 30 kHz at the headstage and delivered to a computer via the RHD recording controller (Intan technologies). All recordings were terminated by experimenter when the signal SNR was still robust because sufficient data had been collected, except for three recordings, which were terminated due to signal loss.
Acute EMG Surgery
Birds were water- and food-deprived an hour before the start of surgery. Each bird was anesthetized initially with ketamine (40 mg/kg) and midazolam (3 mg/kg) intramuscularly. Anesthesia was maintained throughout the experiment using 0%–3% (vol/vol) isoflurane in oxygen gas and/or injections of midazolam as needed. Breathing rate was monitored throughout the surgery. All birds participated in acute experiments to either record EMG activity from the vocal muscles or respiratory muscles.
Syrinx exposure and electrode implant.
Following anesthesia, the bird was placed on its back and secured to the surgery table. A vertical 2–3-mm incision was made in the skin from the furcula to the base of the trachea. Any fat was carefully separated with blunt forceps and retractors, revealing the interclavicular air sac. An 8-0 suture was loosely tied midway down the trachea. The bipolar electrodes were routed through the suture tied around the trachea to secure the electrodes in place while recording EMG activity. This positioned the electrodes to be parallel to the long axis of the trachea, and the distal (recording) end of the electrodes was located where the trachea and air sac connect.
Before opening the air sac, isoflurane was reduced to 0.5% followed by an intramuscular injection of midazolam (3 mg/kg). Approximately 5 min after injection, the interclavicular air sac was cut, the syrinx was exposed, and the isoflurane was turned off. Thereafter, intramuscular injections of midazolam were used as needed for anesthesia maintenance. We placed individual CNTF bipolar electrodes or SSW bipolar electrodes on top of vocal muscles without removing fascia surrounding the targeted muscle. The exposed distal tip of the ground wire was placed subcutaneously near by the implanted muscles. Muscle activity was recorded from Musculus syringealis ventralis (VS) and/or M. tracheobronchialis ventralis superficialis (sVTB). VS is located on the ventral portion of the syrinx near the midline, and sVTB is located directly lateral to VS (Fig. 2).
Expiratory muscle group exposure and electrode implant.
Following anesthesia, the bird was placed on either its right or left side and secured to the surgery table. A 10–12-mm incision was made rostral to the pubic bone and dorsal to the femoral joint, exposing the expiratory muscle group (Fig. 2) in the same manner as previous studies (23). Forceps were used to place individual CNTF bipolar electrodes or SSW bipolar electrodes on top left or right expiratory muscle group without removing fascia. The exposed distal tip of the ground wire was placed subcutaneously near the implanted muscles.
EMG Data Analysis
In songbirds, the expiratory muscle group (m. obliquus externus abdominis, m. obliquus internus, and m. transversus abdominis) controls ventilation in the exhalation phase of the breath cycle, where one breath cycle is one inhalation and one exhalation. This group of muscles contracts around the abdominal air sacs during expiration (Fig. 2), controlling pressure through the air sac system that drives sound production and surrounds the vocal organ (13, 23–27). The expiratory muscle group and vocal muscles are typically rhythmically active when the animal is fully anesthetized (23, 27).
Defining signal and noise regions in EMG data.
The rhythmic activity of the muscles allowed us to track the amplitude of segments of signal and noise within a breathing cycle. The recorded EMG signals were band-pass filtered between 350 and 7,500 Hz, rectified by taking the absolute value of the signal, and then smoothed with a square filter of width 17.5 ms (smoothing window). We refer to the periods were the expiratory muscle group and the vocal muscles are active as the “signal.” We fitted a Gaussian mixture model to the distribution of voltage amplitude, using either three or four Gaussian functions, to fit the bimodal distribution. The intersection of the two Gaussians directly after the first peak in the histogram was used to set the threshold value between signal and “noise” (i.e., low threshold). All data greater than the low threshold were considered signal. This produced region boundaries for each defined signal phase for every recorded breath cycle in the filtered data. The noise floor (i.e., noise phase) was determined by taking the data points between each defined signal phase for every recorded breath cycle. To make sure that no noise data points were included in the signal phase and vice versa, we adjusted the region boundaries for each signal and noise phase by ± 3*smoothing windows (3*17.5 = 52.5 ms), defining the signal regions and noise regions for each breath cycle used to calculate SNR.
Signal-to-noise ratio: multiunit activity.
To determine the SNR for multiunit activity, we calculated the root-mean-square (rms) value of the
amplitude for the signal region and the rms of the amplitude for the corresponding noise region. To find the rms of a set of values (a), take the square root of the arithmetic mean of the squared values. We then divided the rms of the signal region of one breath cycle (Sn) by the rms of the corresponding noise region (Nn).
One SNR value was computed for each individual breath cycle. We were unable to differentiate the signal region from the noise region in the SSW bipolar electrodes of three birds (Table 1; birds 12–14), who were omitted from further analysis.
Signal-to-noise ratio: single-unit activity.
We identified single-motor units in the recorded EMG data with a previously published spike-sorting algorithm (28). Briefly, we performed principal components analysis (PCA) on EMG voltage waveforms and examined their projections along the first two principal components and used k-means clustering to identify clusters of waveforms that corresponded to individual motor units or undifferentiated, multiunit background signals. We then quantified the extent of overlap between clusters. Waveform clusters with overlaps of less than 1% were classified as single units, and recordings with larger overlaps were classified as multiunit recordings. If one (or more than one) cluster of waveforms has less than 1% overlap with the other clusters, we classify the waveforms belonging to that cluster as coming from one (or more than one) well-isolated single unit (23, 28). If no cluster of waveforms has less than 1% overlap, then we note the relevant recording as containing zero well-isolated units (see Table 1). This technique yielded quantitative estimates of motor unit isolation that agreed well with both qualitative assessments of unit isolation and estimates based on spike refractory periods. After isolating the spikes from single motor units, we then computed the SNR of each spike by dividing the peak spike amplitude (i.e., absolute value of the largest peak of the identified single unit) by the rms of voltage in the adjacent noise region, where the latter quantity is computed as described in Signal-to-noise ratio: multiunit activity. All values presented are mean ± standard deviation.
RESULTS
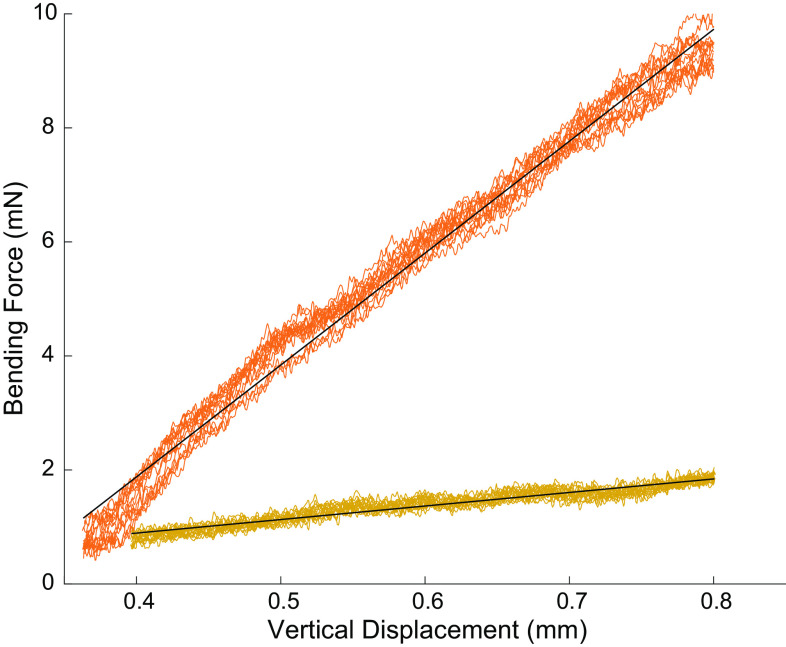
We build four-channel bipolar CNTF electrode arrays and compared their mechanical bending stiffness to SSW arrays. We found that the bending stiffness of CNTFs were 8.3 times lower compared with SSW arrays (Fig. 3).
Figure 3.
The bending stiffness of carbon nanotube fiber (CNTF) arrays is over eight times lower than stainless steel fine wire. The bending stiffness of a four-channel array electrode bundle (black lines) was 2.4 ± 0.1 mN/mm for CNTF (yellow, P ≪ 0.01, R2 = 0.91) and 19.6 ± 0.5 mN/mm for SSW arrays (orange, P ≪ 0.01, R2 = 0.99). The CNTF arrays thus required 8.3 times less force to bend the same amount compared with a SSW array. SSW, stainless-steel wire.
To determine whether bipolar CNTF electrode arrays can record multi- and single-unit motor activity from multiple small muscles, we recorded acute EMG in songbird vocal and respiratory muscles. We tested 24 CNTF electrodes in six muscles (left and right VS, sVTB, and expiratory muscle group) across 8 birds (Table 1). In addition, we tested 11 SSW electrodes in four muscles (left and right VS and sVTB) across six animals. Acute recordings lasted between 3–249 min, and in most cases were terminated by the experimenter while signal amplitude was still high, rather than ending due to a loss of EMG signal (see methods).
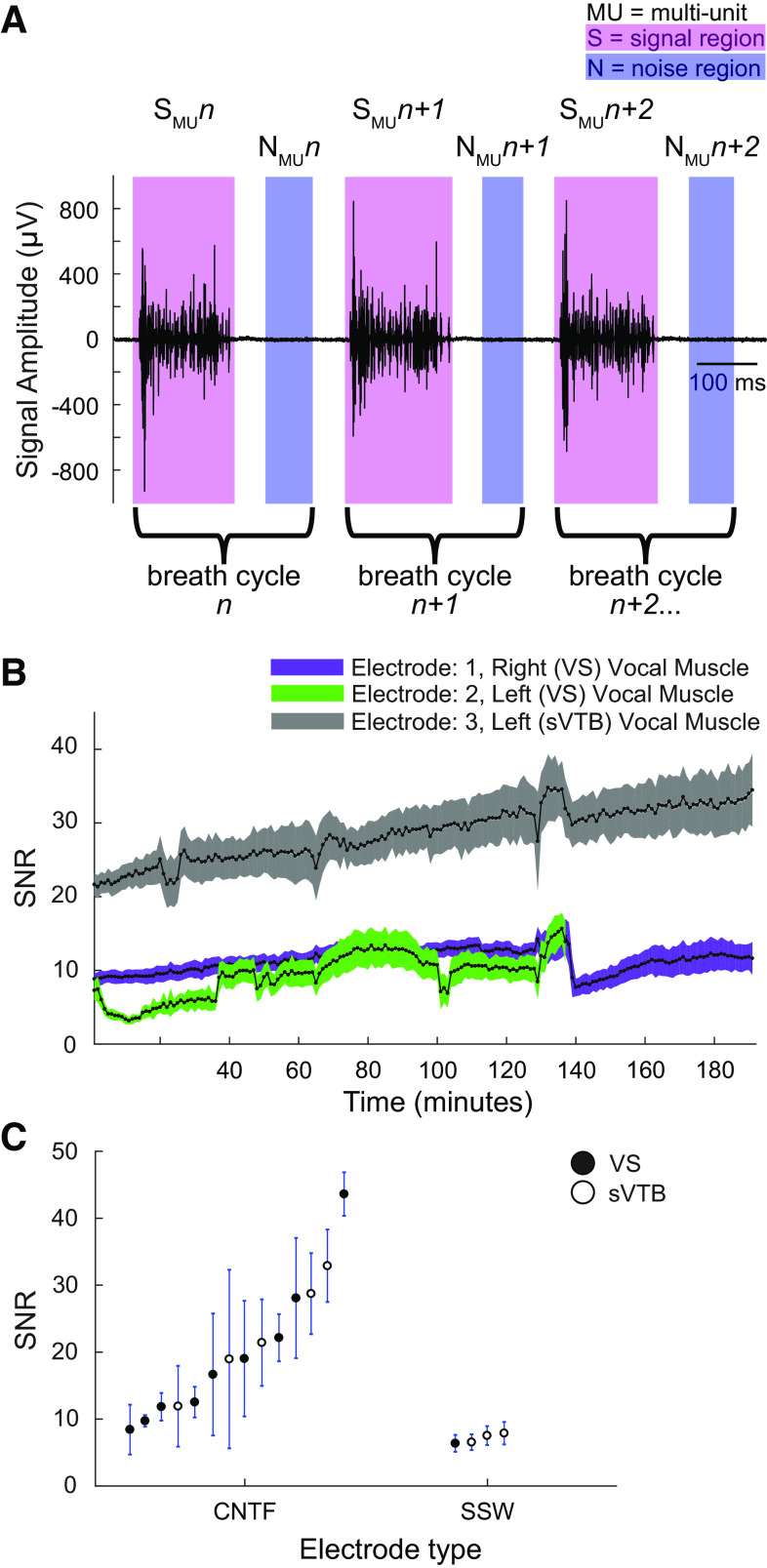
The CNTF bipolar electrodes can record multiunit motor activity within and across multiple small vocal muscles simultaneously (Figs. 4 and 5). We were able to record not only from three bipolar electrodes in two vocal muscles but also different ensembles of activity from the same muscle while the recording sites were less than 2 mm from one another. As illustrated by the multiunit recording shown in Fig. 5A (top, blue inset boxes), two bipolar electrodes onto sVTB recorded different ensembles of motor units, as illustrated by the different waveforms in the highlighted channels. The SNR of the CNTF electrodes remained stable or increased over several hours in acute recordings. Figure 4B shows the SNR for three electrodes recording muscle activity on a different vocal muscle simultaneously within the same bird (Table 1; bird 1) over an extended (>3 h) period (electrode 1: n = 29,255 breath cycles, electrode 2: n = 21,540 breath cycles, electrode 3: n = 29,311 breath cycles). Taking the longest recording of eight individuals, their multiunit SNR for all the electrodes ranged from 8 to 44 (Fig. 4C). These recordings demonstrate that the CNTFs can record discrete motor unit populations in multiple small bird muscles.
Figure 4.
Multiunit data analysis and results. A: an example recording (Table 1: bird 1, recording 1, electrode 3) of multiunit EMG activity from VS muscle. The signal (pink shaded regions) and noise (blue shaded regions) regions were used to calculate the SNR (see methods). B: example recording (Table 1: bird 1, recording 1, electrodes 1, 2, and 3) of the multiunit SNR values for an extended continuous recording in three vocal muscles. All SNR values for each complete breath cycle were averaged across 1 min. C: the SNR of CNTFs (birds 1–8) in vocal muscles ranged from 8.4 ± 3.7 to 43.6 ± 3.3 and was significantly higher (one-way ANOVA, P = 0.032) compared with SSW (birds 9–11) with SNR of 6.2 ± 1.2 to 7.9 ± 1.7. CNTF, carbon nanotube fiber; SNR, signal-to-noise ratio; SSW, stainless-steel wire; VS, ventral syringeal.
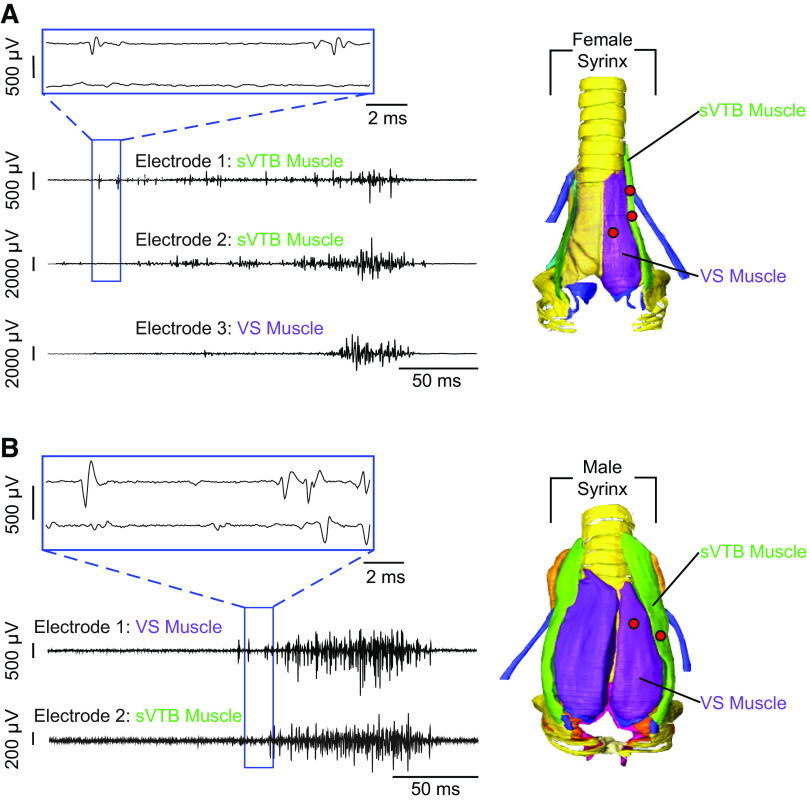
Figure 5.
Recording distinct motor unit populations within and across muscles. A: left is an example recording (Table 1: bird 6, recording 2, electrodes 1, 2, and 3) of different multiunit EMG activity within (electrodes 1 and 2) and across (electrode 3) multiple vocal muscles simultaneously in a female ZF bird. B: left is an example recording (Table 1: bird 8, recording 1, electrodes 1 and 2) of different multiunit EMG activity across two vocal muscles simultaneously (VS and sVTB) in a male ZF bird. In both examples, all electrodes are picking up different motor units in the identified muscle. The right panels in both A and B are ventral views of the female and male syrinx (adapted from Ref. 23), respectively, and the corresponding muscles shown in the example recordings. The female and male syrinx have uniform muscle definitions and muscle attachment sites. The red dots on the syrinx represent the location of the recording electrodes. sVTB, superficial ventral tracheobronchial; VS, ventral syringeal; ZF, zebra finch.
To compare the recording quality of the CNTFs to commonly used SSW electrodes, we quantified and compared SNR values of acute EMG recording between CNTFs (Table 1: birds 1–8) and SSW bipolar electrodes of the same diameter (Table 1; birds 9–11). There was no difference in SNR distributions between muscle groups (i.e., VS and sVTB; Kolmogorov–Smirnov test, P = 0.985), allowing us to combine muscle groups when comparing SNR values between CNTFs and SSW electrodes. The SNR of CNTF recordings was significantly and up to 7.1 times higher compared with SSW (one-way ANOVA, P = 0.03; Fig. 4C).
The high spatial and temporal resolution and small size of the CNTF bipolar electrodes not only allowed us to record discrete motor unit populations within one muscle but also we were also to isolate single-unit activity (Fig. 6). As described in methods, we used a well-established spike-sorting algorithm (28) to isolate the voltage waveforms of single units in songbird vocal muscles. Briefly, this algorithm performs PCA on each voltage waveform and represents the population of recorded waveforms as points in the space of the first two principal components (PC 1 and PC 2), and then asks whether a “cluster” of similarly shaped waveforms (spikes from a single motor unit, magenta dots in Fig. 6A, top right plot) are significantly different from the remainder of the waveforms represented in the PC1-PC2 coordinates. Figure 6A shows an example of this spike-sorting method used to extract a single motor unit from one subject (Table 1; bird 3, recording 1, electrode 3). In total, we recorded with 24 CNTF electrodes in eight animals (“Electrode type” CNTF in Table 1). Of these 24 CNTF recordings, 12 yielded well-isolated single units (Table 1). Of the animals implanted with CNTF electrodes, we recorded at least one single motor unit in 7/8 subjects (Table 1). Figure 6B depicts the SNR for one single-unit recording from the expiratory muscle group (bird 3, electrode 3; n = 2,585 breath cycles). The SNR ranged from 39.3 ± 5.8 to 61.9 ± 5.2. Other examples of single-unit recordings are shown in Fig. 6C (Table 1; bird 7, recording 1 and bird 5, recording 1, electrode 3; top and bottom, respectively). As described in prior work (23, 26, 27, 29, 30), motor unit activity in the expiratory muscle occurs in phasic bursts, which are locked to the respiratory rhythm.
Figure 6.
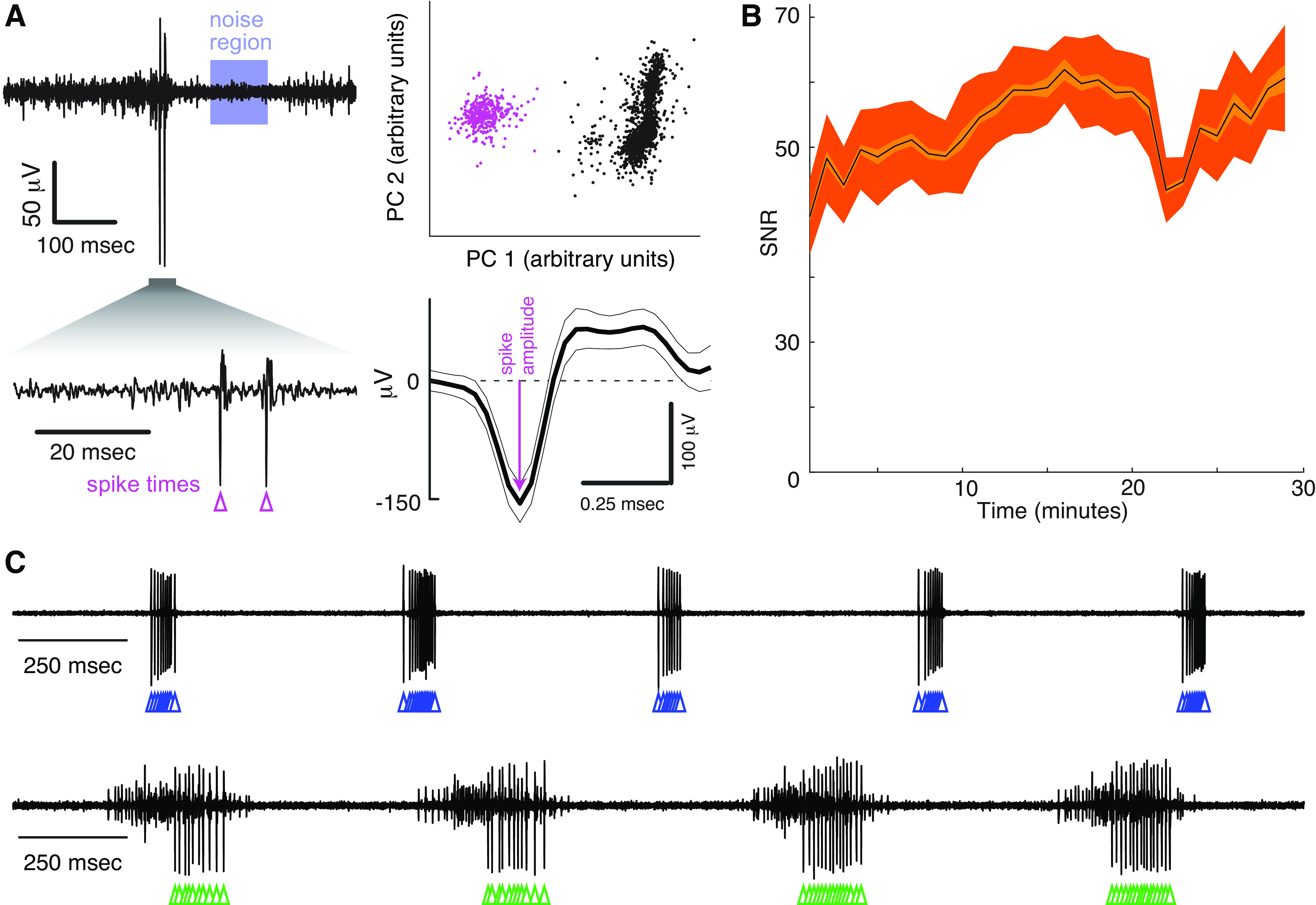
CNTF electrodes successfully record the activity of single-motor units in small muscles. A, top left: example recording (Table 1: bird 3, recording 1, electrode 3) of single-unit EMG activity from expiratory muscle. We used a previously published spike-sorting algorithm (27) that uses principal component analysis to distinguish voltage waveforms belonging to a single motor unit (magenta dots, top right) from background noise (black dots, top right). The peak amplitude of each single unit waveform and the corresponding noise region (blue shaded region) were used to calculate the SNR, as explained in methods. B: example recording (Table 1: bird 3, recording 1, electrode 3) of the SNR values in a 29-min continuous recording in the right expiratory muscle (n = 2,585 breath cycles). All SNR values for each complete breath cycle were averaged across 1 min. C: additional examples of single motor unit recordings (note that top and bottom traces come from different subjects but are plotted on the same time scale; Table 1: bird 7, recording 1 and bird 5, recording 1, electrode 3; top and bottom, respectively). Colored triangles in A and C indicate the spike times of single motor units as determined by our spike-sorting algorithm (see methods). CNTF, carbon nanotube fiber; SNR, signal-to-noise ratio.
For comparison, we collected 11 recordings with paired stainless steel wires in a total of six animals (“Electrode type” SSW in Table 1). Using the same spike-sorting algorithm and settings, we were not able to isolate single motor units in any of these 11 recordings (Table 1). Moreover, a prior study that implanted 20 songbirds with intramuscular fine-wire electrodes for chronic recording similarly did not produce any isolatable motor units (27). These attempts do not exclude the possibility of isolating single units from SSW electrodes in this system, for example, with more sensitive spike sorters. It does however show that with the same algorithm, recordings with CNT electrodes yielded more single units than those with SSW electrodes. The CNTF electrodes, therefore, exceed the performance of fine wires for isolating individual motor units.
DISCUSSION
We showed that CNTFs can be used for acute EMG recordings. CNTF have many characteristics that make them an ideal material to record EMG activity within and across multiple small muscles. First, the high flexibility and high mechanical strength of CNTFs allow to record from locations where electrode stiffness needs to be very low to avoid animal discomfort. In addition, they allow the experimenter to easily handle the electrode without damaging its integrity. Second, because the CNTF bipolar electrodes have a diameter of only ∼50 µm, we were able to record different ensembles of motor units from two different bipolar electrodes within the same small vocal muscle (Fig. 5A). Third, the small diameter of the electrodes and low interface impedance allows for high resolution and quality recordings that result in large SNR (Figs. 3, B and C, and 6B). Together, these properties yield high-resolution EMG recordings superior to those obtained by the traditional wire electrodes we tested, including providing, to our best knowledge, the first record of single motor unit spike trains in the muscles of the songbird vocal organ.
For a given electrode design, lower electrode impedances provide higher-fidelity recordings of neural and muscular activity. Although we did not systematically measure the impedance of the CNTF electrodes during the acute recordings, prior work has compared electrical properties of CNTF to other electrode materials in vitro (16). The electrochemical impedance for CNTF measured 20.4 ± 8.2 kΩ, which is 2.6–6 times lower than tungsten wires with a similar diameter (16, 31). The low impedance generated by the CNTF compared with other electrode materials suggests that the CNTF bipolar electrodes can produce a higher SNR. Indeed, our data confirm that the CNTFs’ SNR is up to 7.1 times higher compared with SSW electrodes of similar diameter (Fig. 4C). The SNR in SSW is comparable to our lowest reported multiunit SNR value of 3.1 ± 0.5 (bird 1, electrode 2) and far below our mean multiunit SNR of 15.5. It is consistent with data from a prior study reporting SNR of fine-wire electrodes in reinnervated muscle grafts of 3.8–4.6 (32). The CNTF electrodes thus compare favorably with traditional fine-wire EMG.
This paper establishes CNTF electrodes as tools for recording EMG activity in acute settings. In addition to the applications shown above, this technology could be expanded by greatly increasing the number of bipolar electrodes placed on the surface of an individual muscle. Increasing the electrode count per muscle would sample data from different subpopulations of motor units. Combining our experimental tools with multichannel spike-sorting algorithms (33) would allow us to isolate the waveforms of individual motor units. Moreover, CNTF arrays can easily be used in a wide range of species and muscle groups. Overall, CNTFs allow for EMG recordings in muscles where EMG was previously unattainable. In addition, CNTFs may reduce animal discomfort when implanting muscles for chronic data collection, interfering less with natural behaviors.
The flexibility of CNTFs makes them an excellent material to build bipolar electrodes for chronic recordings. In chronic recordings, two ways to attach electrodes to a muscle are typically used: on the surface of the muscle or intramuscularly. Using CNTFs in a chronic study would require a biologically compliant tissue adhesive to secure the CNTF bipolar electrode on the surface of the muscle. However, the internal use of tissue adhesives, such as cyanoacrylates (specifically short-chain forms), risks disrupting muscle function due to toxicity from formaldehyde release (34, 35). Alternatively, the CNTF electrodes can be implanted intramuscularly. However, because CNTF electrodes lack the stiffness and a sharp tip to penetrate through muscle tissue, they would need a rigid shuttle to push the CNTF through the muscle fascia and into the tissue, such as PEG (16), or could be sutured directly into the muscle. Once the limitation of securing the CNTF bipolar electrode to the targeted muscle is solved, CNTFs could be used in the future for chronic studies that require EMG recording within and across multiple small and deep muscles.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This research was supported by the National Science Foundation (grant GRFP DGE-1444932 to A.R.P.), NIH (R01NS099375, R01NS084844, and R01NS109237 to S.J.S., 5T90DA032466 and T32 HD071845 to A.R.P.), US Air Force Office of Scientific Research (grant FA9550-15-1-0370 to M.P.), and the Robert A. Welch Foundation (grant C-16 to M.P.) and NovoNordisk Foundation, Denmark (grant NNF17OC0028928 to C.P.H.E.).
DISCLOSURES
M.P. has a financial interest in DexMat, Inc., which is commercializing CNT fibers and threads. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.R.P., M.P., S.J.S., and C.P.H.E. conceived and designed research; A.R.P., J.S.Y., M.P., and C.P.H.E. performed experiments; A.R.P., S.J.S., and C.P.E. analyzed data; A.R.P., S.J.S., and C.P.H.E. interpreted results of experiments; A.R.P., S.J.S., and C.P.H.E. prepared figures; A.R.P., S.J.S., and C.P.H.E. drafted manuscript; A.R.P., S.J.S., and C.P.H.E. edited and revised manuscript; A.R.P., M.P., S.J.S., and C.P.H.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Maria Anthonsen, Julia A. Coco, Oliver S. Dewey, and Lauren W. Taylor for technical support.
REFERENCES
- 1. Basmajian JV, Luca CJ. Muscles Alive: Their Functions Revealed by Electromyography. Baltimore, MD: Williams & Wilkins, 1985. [Google Scholar]
- 2. Bogey RA, Perry J, Bontrager EL, Gronley JK. Comparison of across-subject EMG profiles using surface and multiple indwelling wire electrodes during gait. J Electromyogr Kinesiol 10: 255–259, 2000. doi: 10.1016/S1050-6411(00)00015-8. [DOI] [PubMed] [Google Scholar]
- 3. Farina D, Negro F. Accessing the neural drive to muscle and translation to neurorehabilitation technologies. IEEE Rev Biomed Eng 5: 3–14, 2012. doi: 10.1109/RBME.2012.2183586. [DOI] [PubMed] [Google Scholar]
- 4. Farrell TR, Weir RFF. A comparison of the effects of electrode implantation and targeting on pattern classification accuracy for prosthesis control. IEEE Trans Biomed Eng 55: 2198–2211, 2008. doi: 10.1109/TBME.2008.923917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raez MB, Hussain MCS, Mohd-Yasin F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol Proced Online 8: 11–35, 2006. [Erratum in Biol Proced Online 8: 163, 2006]. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turker KS. Electromyography: some methodological problems and issues. Phys Ther 73: 698–710, 1993. doi: 10.1093/ptj/73.10.698. [DOI] [PubMed] [Google Scholar]
- 7. Loeb GE, Gans C. Electromyography for Experimentalists. Chicago, IL: University of Chicago Press, 1986. [Google Scholar]
- 8. Cram J, Kasman GS, Holtz J. Introduction to Surface Electromyography. Gaithersburg, MD: Aspen Publishers, 1998. [Google Scholar]
- 9. De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech 13: 135–163, 1997. doi: 10.1123/jab.13.2.135. [DOI] [Google Scholar]
- 10. Perry J, Easterday CS, Antonelli DJ. Surface versus intramuscular electrodes for electromyography of superficial and deep muscles. Phys Ther 61: 7–15, 1981. doi: 10.1093/ptj/61.1.7. [DOI] [PubMed] [Google Scholar]
- 11. Adam I, Maxwell A, Rößler H, Hansen EB, Vellema M, Brewer J, Elemans CPH. One-to-one innervation of vocal muscles allows precise control of birdsong. Curr Biol 31: 3115–3124.e5, 2021. doi: 10.1016/j.cub.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mead AF, Osinalde N, Ortenblad N, Nielsen J, Brewer J, Vellema M, Adam I, Scharff C, Song Y, Frandsen U, Blagoev B, Kratchmarova I, Elemans CP. Fundamental constraints in synchronous muscle limit superfast motor control in vertebrates. eLife 6: e29425, 2017. doi: 10.7554/eLife.29425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goller F, Cooper BG. Peripheral motor dynamics of song production in the zebra finch. Ann N Y Acad Sci 1016: 130–152, 2004. doi: 10.1196/annals.1298.009. [DOI] [PubMed] [Google Scholar]
- 14. Yan JS, Orecchioni M, Vitale F, Coco JA, Duret G, Antonucci S, Pamulapati SS, Taylor LW, Dewey OS, Sante MD, Segura AM, Gurcan C, Lisa FD, Yilmazer A, McCauley MD, Robinson JT, Razavi M, Ley K, Delogu LG, Pasquali M. Biocompatibility studies of macroscopic fibers made from carbon nanotubes: implications for carbon nanotube macrostructures in biomedical applications. Carbon 173: 462–476, 2021. doi: 10.1016/j.carbon.2020.10.077. [DOI] [Google Scholar]
- 15. Zhu F, Zhu J, Zhang X, Wang Y, Su J, McCallum GA, Zhang X, Sui X, Durand DM. Flexural characterization of carbon nanotube (CNT) yarn neural electrodes. Mater Res Express 6: 045402, 2019. doi: 10.1088/2053-1591/aafbf7. [DOI] [Google Scholar]
- 16. Vitale F, Summerson SR, Aazhang B, Kemere C, Pasquali M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 9: 4465–4474, 2015. doi: 10.1021/acsnano.5b01060. [DOI] [PubMed] [Google Scholar]
- 17. Behabtu N, Young CC, Tsentalovich DE, Kleinerman O, Wang X, Ma AW, Bengio EA, ter Waarbeek RF, de Jong JJ, Hoogerwerf RE, Fairchild SB, Ferguson JB, Maruyama B, Kono J, Talmon Y, Cohen Y, Otto MJ, Pasquali M. Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 339: 182–186, 2013. doi: 10.1126/science.1228061. [DOI] [PubMed] [Google Scholar]
- 18. Tsentalovich DE, Headrick RJ, Mirri F, Hao J, Behabtu N, Young CC, Pasquali M. Influence of carbon nanotube characteristics on macroscopic fiber properties. ACS Appl Mater Interfaces 9: 36189–36198, 2017. doi: 10.1021/acsami.7b10968. [DOI] [PubMed] [Google Scholar]
- 19. Vitale F, Vercosa DG, Rodriguez AV, Pamulapati SS, Seibt F, Lewis E, Yan JS, Badhiwala K, Adnan M, Royer-Carfagni G, Beierlein M, Kemere C, Pasquali M, Robinson JT. Fluidic microactuation of flexible electrodes for neural recording. Nano Lett 18: 326–335, 2018. doi: 10.1021/acs.nanolett.7b04184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez NT, Buschbeck E, Miller S, Le AD, Gupta VK, Ruhunage C, Vilinsky I, Ma Y. Carbon nanotube fibers for neural recording and stimulation. ACS Appl Bio Mater 3: 6478–6487, 2020. doi: 10.1021/acsabm.0c00861. [DOI] [PubMed] [Google Scholar]
- 21. Lissandrello CA, Gillis WF, Shen J, Pearre BW, Vitale F, Pasquali M, Holinski BJ, Chew DJ, White AE, Gardner TJ. A micro-scale printable nanoclip for electrical stimulation and recording in small nerves. J Neural Eng 14: 036006, 2017. doi: 10.1088/1741-2552/aa5a5b. [DOI] [PubMed] [Google Scholar]
- 22. Fischer WJ, Hirn U, Bauer W, Schennach R. Testing of individual fiber-fiber joints under biaxial load and simultaneous analysis of deformation. Nordic Pulp Paper Res J 27: 237–244, 2012. doi: 10.3183/npprj-2012-27-02-p237-244. [DOI] [Google Scholar]
- 23. Srivastava KH, Holmes CM, Vellema M, Pack AR, Elemans CP, Nemenman I, Sober SJ. Motor control by precisely timed spike patterns. Proc Natl Acad Sci USA 114: 1171–1176, 2017. doi: 10.1073/pnas.1611734114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. During DN, Ziegler A, Thompson CK, Ziegler A, Faber C, Muller J, Scharff C, Elemans CPH. The songbird syrinx morphome: a three-dimensional, high-resolution, interactive morphological map of the zebra finch vocal organ. BMC Biol 11: 1, 2013. doi: 10.1186/1741-7007-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hartley RS. Expiratory muscle activity during song production in the canary. Respir Physiol 81: 177–187, 1990. doi: 10.1016/0034-5687(90)90044-y. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt MF, Martin Wild J. The respiratory-vocal system of songbirds: anatomy, physiology, and neural control. Prog Brain Res 212: 297–335, 2014. doi: 10.1016/B978-0-444-63488-7.00015-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srivastava KH, Elemans CP, Sober SJ. Multifunctional and context-dependent control of vocal acoustics by individual muscles. J Neurosci 35: 14183–14194, 2015. doi: 10.1523/JNEUROSCI.3610-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sober SJ, Wohlgemuth MJ, Brainard MS. Central contributions to acoustic variation in birdsong. J Neurosci 28: 10370–10379, 2008. doi: 10.1523/JNEUROSCI.2448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Philos Trans R Soc Lond B Biol Sci 354: 927–939, 1999. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wild JM, Goller F, Suthers RA. Inspiratory muscle activity during bird song. J Neurobiol 36: 441–453, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 31. Prasad A, Sanchez JC. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J Neural Eng 9: 026028, 2012. doi: 10.1088/1741-2560/9/2/026028. [DOI] [PubMed] [Google Scholar]
- 32. Vu PP, Vaskov AK, Irwin ZT, Henning PT, Lueders DR, Laidlaw AT, Davis AJ, Nu CS, Gates DH, Gillespie RB, Kemp SWP, Kung TA, Chestek CA, Cederna PS. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci Transl Med 12: eaay2857, 2020. doi: 10.1126/scitranslmed.aay2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pachitariu M, Steinmetz NA, Kadir SN, Carandini M, Harris KD. Fast and accurate spike sorting of high-channel count probes with KiloSort. In: Advances in Neural Information Processing Systems 29: Annual Conference on Neural Information Processing Systems 2016, December 5–10, 2016, Barcelona, Spain, edited by Lee D, Sugiyama M, Luxburg U, Guyon I, Garnett R. Red Hook, NY: Curran Associates, Inc., 2016. [Google Scholar]
- 34. Ayyildiz SN, Ayyildiz A. Cyanoacrylic tissue glues: biochemical properties and their usage in urology. Turk J Urol 43: 14–24, 2017. doi: 10.5152/tud.2017.09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia Cerda D, Ballester AM, Aliena-Valero A, Caraben-Redano A, Lloris JM. Use of cyanoacrylate adhesives in general surgery. Surg Today 45: 939–956, 2015. doi: 10.1007/s00595-014-1056-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.