Keywords: hepatic lobule, heterogeneity, liver cancer, liver zonation
Abstract
Tumor heterogeneity is a hallmark of cancer but a challenging problem to dissect mechanistically. Less recognized is that cells within normal tissues are also remarkably diverse. Hepatocytes are a great example because their spatial positioning and the local microenvironment govern their genetic heterogeneity. Recent studies show that primary liver tumors display heterogeneity similar to that observed in the normal tissue providing clues to the cellular precursor of the tumor and how variations in the lobule microenvironment support tumor formation and aggressiveness. Identifying the principles that control cellular diversity in a healthy liver may highlight potential mechanisms driving hepatic tumor heterogeneity.
CELLULAR HETEROGENEITY IN NORMAL HEPATIC PHYSIOLOGY
The complex three-dimensional anatomy of the liver consists of hundreds of thousands of hexagonal lobules. At the periphery of these lobules, blood rich in oxygen, nutrients, and hormones enters through portal vessels (periportal region), flowing directionally toward a single central vein (pericentral region; Fig. 1). Consumption and degradation of these factors by hepatocytes create a gradient along the porto-central axis. In addition, endothelial and immune cells’ nonuniform secretion of morphogens and chemokines likewise contributes to the highly variable environment across the lobule. In turn, these gradients drive differential gene expression and segregation of functional processes in multiple cell types, known as liver zonation (1, 2).
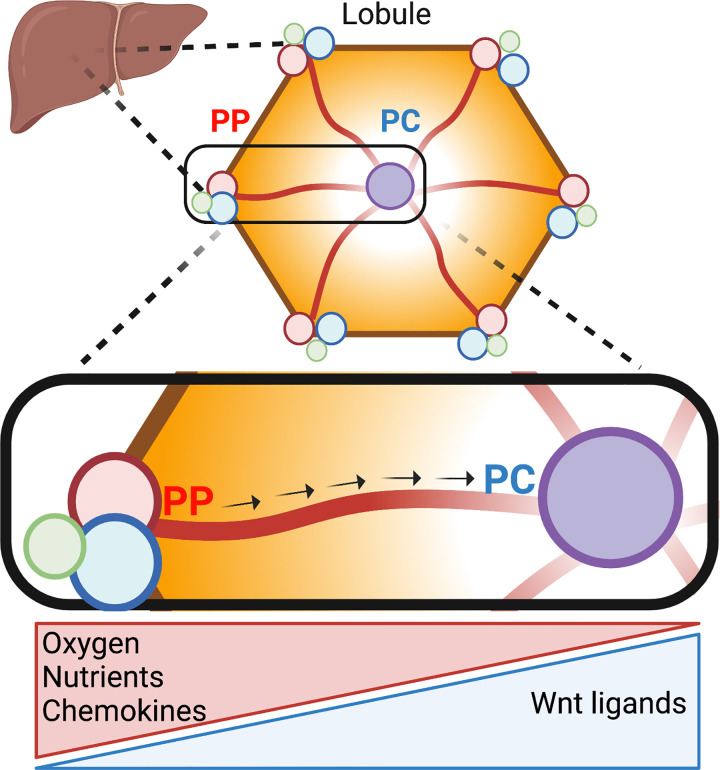
Figure 1.
Liver lobule anatomy and microenvironment. Hepatocytes in the liver are organized in hexagonal units called lobules. The portal triad in the corner of the lobule consists of the portal vein (blue), portal artery (red), and bile duct (green). Blood rich in oxygen and nutrients flows from the periportal toward the pericentral region (arrows). Hepatocytes remove and secrete factors into the bloodstream, thus shaping gradients along the periportal (PP)-pericentral (PC) axis. In addition, nonparenchymal cells, such as immune and endothelial cells, secrete nonuniformly chemokines and Wnt ligands, respectively. As a result, hepatocytes in different parts of the lobule are exposed to distinct microenvironments that determine their gene expression and function. Illustration created using BioRender.
Within hepatocytes, aerobic and catabolic processes such as oxidative phosphorylation, fatty acid oxidation, and gluconeogenesis are predominantly localized to the oxygen-rich periportal hepatocytes, whereas more anaerobic and anabolic processes such as glycolysis and lipogenesis are in pericentral regions. Despite liver zonation being identified over a century ago, factors that govern this spatial heterogeneity are not fully understood. The impact of liver zonation on liver disease and cancer is even less explored. The importance of spatial heterogeneity in the context of cancer biology has drawn recent attention (3). Particularly concerning the breakdown of normal tissue organization and the subsequent alteration in the microenvironment that can promote cancer progression. Here, we highlight the similarities between normal liver cellular heterogeneity and liver tumor subtypes. We then outline the known pathways that regulate cellular diversity in the hepatic lobule and propose that these drivers can explain the observed heterogeneity in primary tumors and degrees of aggressiveness. In addition to providing invaluable insights into tumor biology, empirical experimentation of these similarities will deepen our understanding of pathways, ensuring the maintenance of cellular variations in physiology and providing a new perspective into the transformation process.
CLASSIFICATION OF HEPATOCELLULAR CARCINOMA
Primary liver cancer is the only neoplasm that has increased in mortality in the United States in the past two decades (4). Liver cancer is composed of a group of heterogeneous tumors; hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA), a mix of both HCC and iCCA, as well as fibrolamellar HCC and pediatric hepatoblastoma (5). The current approach to classifying liver tumors is based on distinct molecular and clinical features between the different types of liver tumors. More recently, technological advancements have significantly improved the ability to identify further subtypes within each liver tumor class, particularly hepatocellular carcinoma (HCC), given that it represents 90% of all cases and is the most diverse (5). These advances include single nucleotide polymorphism arrays, transcriptomics, and genome sequencing to allow the characterization of different HCC tumors based on their molecular signatures. Improvements in classification lead to improved precision during treatment. The subtypes of these different molecular signatures can predict patient outcomes (5, 6), highlighting the importance of HCC classification in the treatment process. Mainly since cellular diversity in HCC is the leading cause of drug resistance and mortality (7, 8). The ability to classify tumors based on their molecular features has improved treatment specificity in multiple malignancies (4). However, HCC classification is based solely on genetic heterogeneity and is not used to guide therapy. Defining HCC subtypes will ultimately allow specific and effective treatments for patients—a vital consideration because therapy resistance is primarily driven by the vast diversity of HCC tumors (7, 9).
CELLULAR DIVERSITY IN HEPATOCELLULAR CARCINOMA AND INTRALOBULE HETEROGENEITY
Hepatocellular carcinoma, like the liver lobule, consists of a mixture of cells. This mixed assortment is driven by intrinsic tumor factors, including genetic and epigenetic drivers, and host features such as age and sex, environmental influences, lifestyle, and selection pressures from various treatments (7, 10). In addition, the tumor microenvironment also contributes to the tremendous diversity in hepatocellular carcinoma (HCC; 7, 8). As a result, tumor heterogeneity is manifested as intertumor, where tumors from different patients display diversity, or intratumor heterogeneity, representing the divergence in phenotypes within a single tumor. Another type of heterogeneity that is less appreciated is intralobule heterogeneity, where tumors exhibit molecular signatures associated with lobular zonation (Fig. 2). This level of heterogeneity may be driven by the local tumor environment providing an opportunity for additional subclassification.
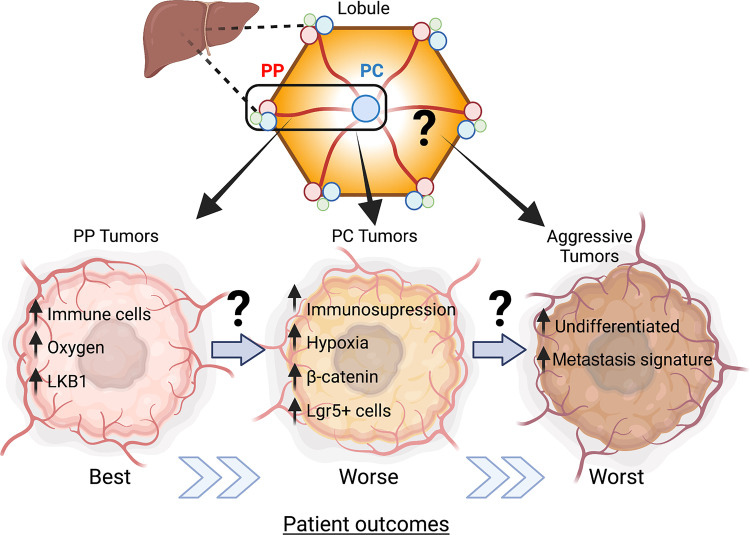
Figure 2.
Intralobule heterogeneity and hepatocellular carcinoma (HCC). Molecular classification of human-derived HCC tumors revealed subclasses that preserved normal periportal (PP) and pericentral (PC) gene expression. These tumors also correlated with worsening patient outcomes. We propose that factors in the normal microenvironment may provide a fertile ground for cellular transformation and tumor aggressiveness, thus contributing to intralobule heterogeneity. Illustration created using BioRender.
In the case of HCC, genomic profiling methods allowed molecular subtyping of tumors revealing two significant subclasses, proliferative and nonproliferative (5, 11). Tumors in the proliferative group are highly heterogeneous with enrichment of signaling pathways involved in cell proliferation. On the other hand, nonproliferative tumors maintain certain features of normal hepatocytes, with a subset of these tumors containing mutations in the CTNNB1 gene leading to activation of canonical Wnt signaling. However, this subclass is considered less aggressive (5).
The molecular signature of HCC tumors is a crucial indicator of patient outcome. A recent study specifically dissected the molecular features of the nonproliferative HCC (6). They found that tumors that resembled the gene signature of periportal hepatocytes had the lowest potential for early recurrence (6). These tumors retained periportal processes such as gluconeogenesis and amino acid catabolism. Conversely, another subclass of tumors displayed a pericentral-like phenotype, with higher rates of the β-catenin gene mutations and fatty acid and bile salt synthesis. Periportal-like tumors were less aggressive than their pericentral-like counterparts and had better clinical outcomes. However, both tumors maintained metabolic zonation signatures like normal tissue and were classified as nonproliferative with favorable survival. Notably, in this study, periportal and pericentral-like tumors were identified in different patients. Whether these tumors originate from distinct liver regions and whether this spatial knowledge can guide treatment is yet to be determined (Fig. 2).
In the same study, another subclass of HCCs was identified. These tumors displayed signatures of extracellular-matrix remodeling and stem cell reprogramming. This subclass was undifferentiated, more aggressive, and had a poorer prognosis than the periportal and pericentral-like tumors, which maintained a higher degree of differentiation and metabolic zonation. This suggests that tumors preserve certain aspects of normal liver zonation, which may be linked to clinical outcomes. Based on the genetic signature of the tumors, it seems that periportal and pericentral tumors arise from periportal and pericentral hepatocytes, respectively. However, currently, there is no direct evidence for that.
Furthermore, it is not known whether hepatic tumors develop on a continuum with less aggressive periportal-like tumors progressing to pericentral-like, which later become undifferentiated and aggressive, leading to worse patient outcomes (Fig. 2). This raises the fascinating question, does location in the lobule matter for tumor aggressiveness? And if so, how does the unique microenvironment contribute to the malignant process?
HCC CELL-OF-ORIGIN EVIDENCED BY INTRALOBULE HETEROGENEITY
Intralobular tumor heterogeneity might indicate the tumor’s spatial cellular origin. In other words, could periportal and pericentral-like tumors merely express signature genes associated with a location, or are they indicative of the spatial cellular origin of the tumor? Multiple studies suggest that adult hepatocytes are the primary source of liver cancer (5). In a landmark study, Lgr5+ pericentral hepatocytes were found to be the primary cells of origin for DEN-induced HCC (12). These pericentrally located, chemically induced tumors had reduced glutamine synthetase (GS) expression, a pericentral protein, as opposed to oncogene-induced tumors, which maintained pericentral-like GS expression. This suggests that different transforming agents have different outcomes in tumors growing in the exact location. However, it is undetermined which hepatocyte population undergoes transformation. Defining the source of cancer-initiating cells in the various oncogenic contexts is the first essential step in understanding the source of intralobular heterogeneity.
REGULATORS OF HEPATOCYTE ZONATION AND THEIR POTENTIAL IMPACT ON INTRALOBULE HETEROGENEITY IN CANCER
The distinct microenvironments across the porto-central axis in the lobule drive differential gene expression in hepatocytes (2; Fig. 1) and may create a more conducive region for tumor initiation and growth. Tumors activated by a single oncogene may develop different molecular signatures shaped by inflammation and microenvironment (5). This strongly suggests that spatial variations in environmental factors can explain underlying mechanisms of intralobular heterogeneity and that studying healthy liver zonation can provide insights into this phenomenon.
Wnt ligands secreted from pericentral endothelial cells are a significant factor in shaping liver zonation (13). Consequently, β-catenin and its negative regulator, adenomatous polyposis coli (APC), regulate pericentral and periportal gene expression, respectively (14, 15). Désert et al. (6) proposed that the periportal-like phenotype demonstrates the lowest aggressiveness of HCCs, and that β-catenin mutations and activation drive the periportal-like phenotype toward an aggressive pericentral one. Indeed, dysregulated β-catenin signaling can be found in up to 50% of HCC tumors and is correlated with tumor progression and worse clinical outcomes (16). Furthermore, activation of β-catenin leads to upregulation of pericentral genes and HCC development (17). Despite the importance of β-catenin for maintaining normal liver zonation, its dysregulation may be disruptive and exacerbate tumorigenesis. Whether genetic expression of β-catenin and overall tumor molecular signature truly reflect the spatial position in the lobule is unknown and warrants investigation.
Lower oxygen availability in pericentral regions of the lobule results in hypoxia-induced factor 1α (HIF1α)-induced pathways such as glycolysis. Similarly, activation of HIF1α in HCC increased glycolysis and lactate production (18). These tumors displayed an aggressive phenotype, supporting the observation that pericentral-like HCCs have worse clinical outcomes and may be governed by oxygen availability.
Another factor that may shape intralobular heterogeneity is the zonation of immune cells across the lobule. Detection of gut-derived bacteria by liver sinusoidal endothelial cells creates a chemokine gradient across the lobule, which concentrates immune cells in periportal regions (1). This zonation of immune cells helps to protect unique cell populations surrounding the central vein, thereby maintaining liver zonation. This nonuniform distribution of immune cells may explain the better prognosis of periportal tumors, as they are in a region of heightened immunity. Conversely, pericentral regions high in Wnt/β-catenin signaling may offer a more immune-suppressive environment, given that β-catenin activation in HCC promotes immune evasion and therapy resistance (19). Recently, the immune microenvironment of HCC was classified into three distinct immune subtypes: Immune-high, Immune-mid, and Immune-low. Within this classification, HCC tumors categorized as immunosuppressive also displayed elevated Wnt/β-catenin signaling (10). Additional evidence of pericentral regions being immunosuppressive environment comes from a study showing that hypoxia-induced vascular endothelial growth factor signaling drove T cell suppression in patients with HCC (7).
Furthermore, high lactate levels, typical in the lobule’s pericentral regions, are known to have immunosuppressive effects in tumors (20). Recently, a survey of the immune environment was performed at a single-cell resolution in human primary liver tumors. They identified five subtypes with distinct immune features in which neutrophils have emerged as promoting tumor progression (21). Collectively, these data highlight environmental factors leading to elevated immunity in periportal areas of the lobule and more immunosuppressive in pericentral regions. Understanding the factors regulating the positioning and function of immune cells in the lobule of healthy tissue is crucial for developing approaches to improve immune responses to HCC.
How do hepatocytes across the lobule integrate the multitude of signals governing cellular heterogeneity and zonated metabolism? One potential mediator is the nutrient sensor and AMPK activator, liver kinase B1 (LKB1). Our group has recently demonstrated that LKB1 acts as a critical brake for glucagon-induced fasting to prevent unchecked catabolism (22). We identified LKB1 as a regulator of liver zonation, with loss of hepatic LKB1 causing increased periportal and decreased pericentral gene expression in the lobule. Interestingly, LKB1 also acts as a tumor suppressor gene, with lower LKB1 expression found in patient-derived HCC and cell lines and associated with worse clinical outcomes (23). Furthermore, reduced LKB1 expression was positively correlated with lower E-cadherin, a periportal marker. This suggests that eliminating periportal regions may result in HCC progression and supports the observation that periportal-like tumors have less aggressive phenotype (6).
Tumors must metabolically adapt to nutrient availability, and this flexibility has become evident in HCC development. Strong evidence suggests that HCC tumor growth relies on lipids to supply energy and cellular building blocks (9, 24). This increase in lipid availability stems from an elevation in fatty acid synthesis, which is elevated in HCC (25) and associated with poorer patient outcomes (26). Since pericentral cells have elevated fatty acid synthesis compared with periportal cells in the liver lobule (27), this may explain the more aggressive nature of pericentral-like tumors. If cells that are metabolically predisposed to synthesize lipids become cancerous, then this appetite for lipid synthesis may exacerbate and drive tumor growth, leading to worse clinical outcomes. This reiterates that location within the tissue shapes intrinsic heterogeneity in tumors and the healthy liver lobule.
CONCLUSIONS AND FUTURE DIRECTIONS
Advances in microscopy and various omics approaches have enhanced our knowledge of heterogeneity in normal tissues and cancer over the past decade. The spatial positioning of cells within healthy tissues impacts their exposure to essential factors and interactions with other cells, which, in turn, determines their genetic profile and metabolic activities. Our fundamental understanding of how liver zonation is regulated in normal physiology is still in its infancy. Furthermore, there is evidence that normal heterogeneity shares similarities with subtypes of liver tumors. Defining the regulatory pathways of liver zonation in health will illuminate potential mechanisms for HCC tumor initiation and heterogeneity. These mechanisms may improve the ability to classify tumor subtypes and thus guide the development of spatially relevant therapies based on the tumor’s spatial location and the distinct microenvironment it is exposed to. The spatial positioning of cells within tissues may be a central driver of genetic and functional diversity in other organs and their respective tumors.
GRANTS
This paper was supported by National Cancer Institute Grant 1ZIABC011828-02 (to N. Porat-Shliom).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.P.-S. conceived and designed research; R.P.C. and N.P.-S. prepared figures; R.P.C. and N.P.-S. drafted manuscript; R.P.C., S.W.S.K., and N.P.-S. edited and revised manuscript; N.P.-S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Porat-Shliom lab, Drs. Win Arias, Snorri Thorgeirsson, and Larry Samelson for critical reading of the manuscript.
REFERENCES
- 1. Gola A, Dorrington MG, Speranza E, Sala C, Shih RM, Radtke AJ, Wong HS, Baptista AP, Hernandez JM, Castellani G, Fraser IDC, Germain RN. Commensal-driven immune zonation of the liver promotes host defence. Nature 589: 131–136, 2021. doi: 10.1038/s41586-020-2977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunningham RP, Porat-Shliom N. Liver zonation—revisiting old questions with new technologies. Front Physiol 12: 732929, 2021. doi: 10.3389/fphys.2021.732929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seferbekova Z, Lomakin A, Yates LR, Gerstung M. Spatial biology of cancer evolution. Nat Rev Genet. In press. doi: 10.1038/s41576-022-00553-x. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 12: 436, 2015. doi: 10.1038/nrclinonc.2015.121. [DOI] [PubMed] [Google Scholar]
- 5. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 152: 745–761, 2017. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 6. Désert R, Rohart F, Canal F, Sicard M, Desille M, Renaud S, Turlin B, Bellaud P, Perret C, Clément B, Lê Cao K-A, Musso O. Human hepatocellular carcinomas with a periportal phenotype have the lowest potential for early recurrence after curative resection. Hepatology 66: 1502–1518, 2017. doi: 10.1002/hep.2925. [DOI] [PubMed] [Google Scholar]
- 7. Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, Rae Z, Hernandez JM, Davis JL, Martin SP, Kleiner DE, Hewitt SM, Ylaya K, Wood BJ, Greten TF, Wang XW. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36: 418–430.e6, 2019. doi: 10.1016/j.ccell.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang XW, Thorgeirsson SS. The biological and clinical challenge of liver cancer heterogeneity. Hepat Oncol 1: 349–353, 2014. doi: 10.2217/Hep.14.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang M, Han J, Xing H, Zhang H, Li Z, Liang L, Li C, Dai S, Wu M, Shen F, Yang T. Dysregulated fatty acid metabolism in hepatocellular carcinoma. Hepat Oncol 3: 241–251, 2016. doi: 10.2217/hep-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 68: 1025–1041, 2018. doi: 10.1002/hep.29904. [DOI] [PubMed] [Google Scholar]
- 11. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149: 1226–1239.e4, 2015. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 12. Ang CH, Hsu SH, Guo F, Tan CT, Yu VC, Visvader JE, Chow PKH, Fu NY. Lgr5(+) pericentral hepatocytes are self-maintained in normal liver regeneration and susceptible to hepatocarcinogenesis. Proc Natl Acad Sci USA 116: 19530–19540, 2019. doi: 10.1073/pnas.1908099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: a sequel to the Wnt-Wnt situation. Hepatol Commun 2: 845–860, 2018. doi: 10.1002/hep4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell 10: 759–770, 2006. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 15. Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 43: 817–825, 2006. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 16. Vilchez V, Turcios L, Marti F, Gedaly R. Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol 22: 823–832, 2016. doi: 10.3748/wjg.v22.i2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, Perret C. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA 101: 17216–17221, 2004. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamaguchi T, Iizuka N, Tsunedomi R, Hamamoto Y, Miyamoto T, Iida M, Tokuhisa Y, Sakamoto K, Takashima M, Tamesa T, Oka M. Glycolysis module activated by hypoxia-inducible factor 1alpha is related to the aggressive phenotype of hepatocellular carcinoma. Int J Oncol 33: 725–731, 2008. doi: 10.3892/ijo_00000058. [DOI] [PubMed] [Google Scholar]
- 19. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A. β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov 9: 1124–1141, 2019. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark 19: 353–363, 2017. doi: 10.3233/CBM-160336. [DOI] [PubMed] [Google Scholar]
- 21. Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, Feng M, Wang F, Cheng J, Li Z, Zhan Q, Deng M, Zhu J, Zhang Z, Zhang N. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612: 141–147, 2022. doi: 10.1038/s41586-022-05400-x. [DOI] [PubMed] [Google Scholar]
- 22. Acevedo-Acevedo S, Stefkovich ML, Kang SWS, Cunningham RP, Cultraro CM, Porat-Shliom N. LKB1 acts as a critical brake for the glucagon-mediated fasting response. Hepatol Commun 6: 1949–1961, 2022. doi: 10.1002/hep4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sha L, Lian F, Li K, Chen C, Zhao Y, He J, Huang S, Wu G. Under-expression of LKB1 is associated with enhanced p38-MAPK signaling in human hepatocellular carcinoma. Int J Clin Exp Pathol 11: 5525–5535, 2018. [PMC free article] [PubMed] [Google Scholar]
- 24. Berardi DE, Bock-Hughes A, Terry AR, Drake LE, Bozek G, Macleod KF. Lipid droplet turnover at the lysosome inhibits growth of hepatocellular carcinoma in a BNIP3-dependent manner. Sci Adv 8: eabo2510, 2022. doi: 10.1126/sciadv.abo2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, Sekiya M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Nagai R, Ishibashi S, Kadowaki T, Makuuchi M, Ohnishi S, Osuga J-I, Yamada N. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer 41: 1316–1322, 2005. doi: 10.1016/j.ejca.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 26. Yamashita T, Honda M, Takatori H, Nishino R, Minato H, Takamura H, Ohta T, Kaneko S. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol 50: 100–110, 2009. doi: 10.1016/j.jhep.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 27. Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther 53: 275–354, 1992. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]