Abstract
Conventional antibiotics are associated with various side-effects. Therefore, there is need of using plant-derived antibiotics with fewer side-effects. Grewia tembensis and Xerophyta spekei, which have been extensively utilized in the Mbeere community, were studied to support their folkloric use and demonstrate their antibacterial capabilities. Salmonella Typhi ATCC 1408, Bacillus subtilis ATCC 21332, Staphylococcus aureus ATCC 25923, and Escherichia coli ATCC 25922 were all used in this study. As a standard reference, Ciprofloxacin (100 μg/ml) was employed, and 5% DMSO was used as a negative reference. Tests for antibacterial activities included disc diffusion, minimum inhibitory concentrations, and bactericidal concentrations. G. tembensis exhibited effects on S. aureus only with Mean Zone Inhibition (MZI) of 07.07 ± 0.07 to 12.33 ± 0.33 mm and 08.33 ± 0.33 to 11.67 ± 0.33 mm for stem bark and leaf extracts respectively. While X. spekei extract had effects on S. aureus with MZI of 07.67 ± 0.33 to 14.67 ± 0.33 mm and B. subtilis with MZI of 09.67 ± 0.33 to 14.33 ± 0.33 mm. Ciprofloxacin demonstrated significantly higher activities as compared to the plant extracts in all the concentrations (p < 0.05), while 5% DMSO had no activity. GC-MS analysis demonstrated the availability of compounds with known antibacterial effects. Therefore, the current study recommends ethnomedicinal and therapeutic use of G. tembensis and X. spekei as antibacterial agents.
Keywords: Antibacterials, Grewia tembensis, Xerophyta spekei, Infectious diseases, Ethyl acetate
1. Introduction
The spread of infectious diseases has reportedly been facilitated by increased human population in major cities, environmental degradation, and lack of proper health care services [1]. Some conditions that lead to the increased number of individuals with compromised immunity have also led to a large number of people being subjected to bacterial infections [2]. There has been a rise in the use of counterfeit antibacterial agents due to the increased expense of medication, especially in underdeveloped countries, some of which are of sub-standard quality. Their use has led to a loss of lives and resulted in antibacterial drug-resistant pathogens [3].
Antibacterial resistance to commonly used antibiotics is currently increasing at an alarming rate, which has complicated the management of bacterial infections [4]. Some of the elements that cause this phenomenon include misuse of over-the-counter antibiotics, noncompliance to antibiotic dosage due to their adverse side effects, and use of under-dose due to the high expense of medication [4,5]. Therefore, there is a critical need for alternative and complementary antibacterial therapies which are affordable and have fewer side effects.
Since time immemorial, medicinal plants have been used as therapeutic drugs to prevent or heal diseases as they are associated with fewer side effects and are affordable [6]. Natural antibacterial components have been discovered in medicinal plants. However, only a few have been scientifically evaluated and validated [7]. The two plants used in the current study; Xerophyta spekei and Grewia tembensis are used by herbalists as medicinal herbs in the Mbeere community in Embu County [8]. However, their ethyl acetate antibacterial activities have yet to be shown scientifically. Therefore, this experiment was executed to explore, establish and confirm the two medicinal plant extracts’ antibacterial capabilities in vitro.
X. spekei, also called Vellozia spekei, is a member of the Velloziaceae family. It's common in Kenya, Zambia, Tanzania, Zimbabwe, and Ethiopia. It is a shrub of about 2–5 m tall with 6–12 cm thick branched stems. The leaves are congregated on one edge of the branch [9]. The flowers appear 1–4 in a leaf axial at the stem apex [9]. In the Embu community, it is known as Kianduri and is used to treat dog bites and diabetes [8]. The Mbeere community uses it to treat dog bites and diabetes [8]. Traditional herbalists in South Africa use it as an analgesic and an anti-inflammatory [10].
G. tembensis is a small multi-stemmed perennial shrub about 4 m high with long, narrow, smooth, gray stems belonging to the family Tiliaceae. The leaves are green, thinly hairy, and slightly rough above with jagged margins. Flowers are white to pink, and the fruits are usually 2–4 lobed, green when young but turn orange when mature. It grows in moderately dry areas in Eastern Africa [11]. The Kipsigis call it Chesarebut, the Mbeere call it Muruba [12]. G. tembensis is used in Djibouti to treat microbial infections like abscesses and furuncle [13]. The Turkana community uses G. tembensis to treat coughs and eat its fruits as food [14]. The Kamba community calls it Muvindaviti or Mutuva and uses its roots to treat typhoid [12].
Ethyl acetate was chosen as the extraction solvent in the current study because it has been demonstrated to be a medium polar solvent with the capacity to extract molecules with less polarity as well as slightly polar compounds [15]. Prior research has also demonstrated that ethyl acetate-based extraction provided excellent secondary metabolite yields, which in turn led to excellent antibacterial outcomes [15]. According to past literature, use of ethyl acetate solvent extraction has shown better antibacterial efficience in comparison to other organic solvents [16,17].
2. Materials and methods
2.1. Plant materials collection and preparation
In May 2021, a practicing village traditional herbalist assisted in the collection of fresh X. spekei (whole plant without roots) and G. tembensis (leaves and stem barks) parts from Gikuyari village in Embu County, Kenya. The plants were brought to the university, where they were confirmed by a qualified taxonomist and a sample of each plant was deposited in the Kenya National Museum (KNM) herbarium for future reference. For X. spekei and G. tembensis, respectively, voucher numbers for the specimens were assigned as PN/002/27698/2018 and PN/003/27698/2018. The plants were cleaned thoroughly with running tap water, rinsed with distilled water (DH2O), and then cut into little pieces. Following a 28-day period of shade drying, the plants were ground into fine powder and stored at room temperature in tightly sealed containers awaiting the extraction process.
2.2. Extraction procedure
G. tembensis stem bark (400 g) powder was soaked in 1200 ml of ethyl acetate solvent. In addition, 800 g of X. spekei whole plant dry powder and 300 g of G. tembensis dry leaves powder were separately soaked in 2.4 L and 0.9 L of the solvent, respectively. All the plants were soaked for 72 h. To guarantee complete dissolution, the solutions were occasionally whirled. The solutions were then decanted and vacuum filtered using Whatman's filter paper No. 1 in a Buchner funnel. The filtrates were thereafter separately concentrated with the help of a rotary evaporator at 90 rpm at 60 °C under vacuum to evaporate the solvent. The resultant extracts were stored at 4 °C in clean, sterile glasses awaiting the bioassay experiments.
2.3. Experimental design
This particular study was carried out using a completely randomized study design.
2.4. Bacterial test organisms and controls
B. subtilis ATCC 21332, S. aureus ATCC 25923, E. coli ATCC 25922, as well as and S. Typhi ATCC 1408 bacterial isolates were obtained from Kenyatta University's Microbiology Laboratory. Ciprofloxacin was used as a positive reference antibiotic, while 5% DMSO was used as a negative reference.
2.4.1. Maintenance of bacterial stock cultures
To get fresh colonies, the stock bacterial pathogens were streaked on Mueller Hinton Agar followed by 24-h incubation at 37 °C. To obtain fresh bacterial growth suspensions, a sterile wire loop was used to pick three to four colonies and mix with 10 ml of sterile Mueller Hinton Broth in sterile glass tubes, the tubes were then incubated at 37°C for 24-h. The freshly obtained bacterial suspensions were maintained at 4°C [18].
2.5. Sterile paper discs preparation
Whatman's filter papers No 1 were punched with the aid of a paper punch to prepare 6 mm diameter paper discs. Prior to sterilization, they were placed in bijou bottles and autoclaved at 121 °C for 15 min. After which, they were stored in a dry clean place until use.
2.6. Preparation of extracts dilutions and impregnation of discs
One hundred mg of G. tembensis and X. spekei extracts were weighed then placed in sterile 2 ml micro-centrifuge tubes. A 100 mg/ml stock concentration was prepared by adding 1 ml of 5% DMSO to the weighed extracts, the mixture was then properly vortexed followed by sonication to enable complete dissolution. Concentrations from 3.125 mg/ml to 50 mg/ml were prepared by serial dilutions which were made by mixing 500 μL of the extracts’ stock solution with 500 μL of 5% DMSO. Fifteen μL of the serially diluted extracts were used to impregnate sterile paper discs. Prior to being place on the surface of the inoculated media, the impregnated discs were left to air dry for about 20 min in a biosafety cabinet. As a positive antibiotic reference, 100 μg of ciprofloxacin powder dissolved in 1000 μL of sterile normal saline was used, while 5% DMSO was used as a negative reference.
2.7. Antibacterial activity test
Disc diffusion technique done in triplicates as previously explained by Hudzicki [19] was used to determine the extracts antibacterial effectiveness. Using sterile cotton swabs, bacterial inocula were evenly streaked on the surface of already prepared Mueller Hinton Agar media. After which, the inoculated culture plates were left in the biosafety cabinet to dry prior to placing the impregnated discs. Impregnated discs containing different dilutions of the extracts, negative control as well as positive control were then placed on the surface of the agar surface, one at a time using a sterile pair of forceps. To facilitate proper infiltration of the extracts into the media, the plates were left for 15 min in sterile environment [20] followed by 37 °C incubation for 24-h [21]. Clear zones of inhibition around the discs were then measured in millimeters (mm) using a ruler and documented in spreadsheets.
2.8. Minimum inhibitory concentrations (MICs)
Minimum inhibitory concentration was done in triplicates following the procedure explained by Nikolic et al. [22]. Different concentrations ranging from 100 mg/ml to 1.5625 mg/ml of the extracts were prepared by adding equal volumes (100 μL) of the extracts to Mueller Hinton Broth in different sterile 96-well plates. Thereafter, 20 μL of each bacterial suspension (0.5 McFarland turbidity), was added to each well then incubated for 24-h at 37 °C. This was followed by addition of 50 μL of 1% resazurin solution indicator to each well. The sterile plates were re-incubated for 30 min at 37 °C [23] and the lowest concentration that prevented visible blue to pink resazurin color change was considered the MIC [23]. Similar dilutions were done for Ciprofloxacin (positive antibacterial reference drug) powder, whereas 5% DMSO was utilized as the negative reference.
2.9. Minimum bactericidal concentrations (MBCs)
To determine the MBC, 10 μL of the antibacterial agents from every well with concentrations at and above the MIC were streaked over the surface of Mueller Hinton Agar using a sterile cotton swab [18], followed by 37 °C incubation of the agar plates for 24-h. The least concentration with no visible bacterial growth on the Mueller Hinton Agar was considered the MBC [24]. Notable bacterial growth on the surface of Mueller Hinton Agar plates was documented as bacteriostatic effects of the antibacterial agent, whereas lack of visible bacterial growth on the Mueller Hinton Agar plates surface was considered as bactericidal activity of the tested antibacterial agent. Each experiment was done in triplicates.
2.10. Quantitative phytochemical screening
Phytochemical screening in the current study was done using GC-MS to determine and quantify phytochemicals available in the ethyl acetate extracts of X. spekei and G. tembensis. A 7890 A Gas-Chromatograph attached to a 5975C mass selective sensor consisting of an HP5 MS low bleed capillary column was used. The mass spectrometer's operating specifications comprised a relative detector gain mode, a 70eV ionization energy, a 3.3 min' filament delay time, a 1666μ/sec scan speed, a scan range of 40–550 m/z, a 230 °C ion source temperature, as well as a 180 °C quadrupole temperature. A carrier gas of 99.9% helium was used, flowing at a constant rate of 1.25 ml per minute. Mass transfer temperature was set at 200 °C while the injector line transfer temperature set at 250 °C, with an injection volume of 1 μL. Oven temperature was set at 35 °C for 5 min and increased to 280 °C for 24.5 min at a rate of 10 °C per minute. The temperature was then raised to 285 °C for 20.5 min at the rate of 50 °C per minute to a total of 50 min run time.
2.11. Data management and statistical analysis
The data in this particular study were tabulated in Microsoft excel spreadsheet before being imported into Minitab software version 17.00, where descriptive statistical values were expressed as mean ± SEM. One-way ANOVA for inferential statistics and Tukey's post hoc test for pairwise comparison and separation of means were used. A p value of <0.05 was considered statistically significant. The findings were presented in tables and graphs. Comparison of the obtained data was matched with mass-spectral library search data from the National Institute of Standards and Technology 08 and 11 to assist in the identification of the phytochemicals found in each extract, where each unique peak represented a particular chemical substance.
3. Results
3.1. Antibacterial properties
3.1.1. Antibacterial effects of ethyl acetate extracts of X. spekei and G. tembensis (Fresen)
Antibacterial activities of G. tembensis ethyl acetate stem bark extract were tested at different concentrations against bacterial pathogens S. aureus, B. subtilis, E. coli, as well as S. Typhi in comparison with the standard antibiotic, Ciprofloxacin, and the diluent, DMSO. The extract exhibited antibacterial activities on S. aureus only with MZI ranging from 07.07 mm to 12.33 mm in diameter (Table 1).
Table 1.
Antibacterial properties of ethyl acetate extracts of X. spekei and G. tembensis (Fresen).
| Treatment | Mean zones of inhibition (mm) |
|||
|---|---|---|---|---|
|
X. spekeiextract |
G. tembensis stem bark extract |
Grewia tembensis leaf extract |
||
| S. aureus | B. subtilis | S. aureus | S. aureus | |
| 5% DMSO | 06.00 ± 0.00f | 06.00 ± 0.00d | 06.00 ± 0.00e | 06.00 ± 0.00e |
| Ciprofloxacin (100 μg/ml) | 26.33 ± 0.33a | 29.67 ± 0.33a | 26.33 ± 0.33a | 26.33 ± 0.33a |
| Extracts (mg/ml) | ||||
| 100 | 14.67 ± 0.33b | 14.33 ± 0.33b | 12.33 ± 0.33b | 11.67 ± 0.33b |
| 50 | 13.33 ± 0.33b | 13.33 ± 0.33b | 10.33 ± 0.33c | 10.00 ± 0.58bc |
| 25 | 11.33 ± 0.33c | 11.00 ± 0.58c | 09.33 ± 0.33c | 08.33 ± 0.33cd |
| 12.5 | 09.67 ± 0.33cd | 09.67 ± 0.33c | 07.67 ± 0.33d | 06.00 ± 0.00e |
| 6.25 | 08.33 ± 0.67de | 06.00 ± 0.00d | 07.07 ± 0.07de | 06.00 ± 0.00e |
| 3.125 | 07.67 ± 0.33ef | 06.00 ± 0.00d | 06.00 ± 0.00e | 06.00 ± 0.00e |
Values of Mean Zones of Inhibition are expressed as Mean ± SEM. Values with the same superscript letter within the same column are not significantly different (p > 0.05) after one-way ANOVA followed by Tukey's post hoc test.
There was no notable effect against B. subtilis (Gram-positive) and Gram-negative (S. Typhi, and E. coli) on all the extract concentrations (Table 1). The ethyl acetate stem bark extract of G. tembensis at concentration 100 mg/ml, recorded an MZI >12 mm against S. aureus which was significantly different from concentrations ranging from 50 mg/ml to 3.125 mg/ml (p < 0.05; Table 1). There was statistical similarity in the antibacterial effects of extract concentrations 50 mg/ml as well as 25 mg/ml on S. aureus (p > 0.05; Table 1). Likewise, there was statistical similarity in the effects of extract concentrations 6.25 mg/ml and 12.5 mg/ml and extract concentrations 6.25 mg/ml and 3.125 mg/ml (p > 0.05; Table 1), although there was no activity at concentration 3.125 mg/ml. As the extracts concentrations increased, the inhibition zones against S. aureus also increased (Table 1). Ciprofloxacin produced significantly higher inhibitory zones (MZI>25 mm) than all the extract dilutions and DMSO (p < 0.05; Table 1), although DMSO exhibited no activity on all the tested bacterial pathogens (Table 1).
G. tembensis leaf extract antibacterial potential was tested at different concentrations against selected bacterial pathogens S. aureus, B. subtilis, E. coli, as well as S. Typhi in comparison with the standard antibiotic, Ciprofloxacin, and the diluent, DMSO. The extract exhibited antibacterial activities on S. aureus only with MZI ranging from 08.33 mm to 11.67 mm in diameter (Table 1). The extract and DMSO were inactive on E. coli, B. subtilis, as well as S. Typhi in all the extract concentrations (Table 1). Similarly, the extract also showed no activity on S. aureus at concentrations ranges of 3.125 mg/ml to 12.5 mg/ml (Table 1). There was statistical similarity in the effect of the extract concentration 100 mg/ml as well as 50 mg/ml (p > 0.05; Table 1). Same statistical similarity was noted in the extract concentration 25 mg/ml and 50 mg/ml (p > 0.05). G. tembensis leaf extract's antibacterial effect increased with an increase in concentration (Table 2), with Ciprofloxacin producing significantly larger inhibition zones than all the extract concentrations and DMSO (p < 0.05; Table 1).
Table 2.
Minimum inhibitory and bactericidal concentrations of ciprofloxacin, X. spekei and G. tembensis extracts.
| MBC |
MIC |
|||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/ml) |
Concentration (mg/ml) |
|||||||
| Bacterial strain | X. spekei extract | G. tembensis stem bark extract | G. tembensis leaf extract | Ciprofloxacin (μg/ml) | X. spekei extract | G. tembensis stem bark extract | G. tembensis leaf extract | Ciprofloxacin (μg/ml) |
| S. aureus | 33.33 ± 8.33b | 100.00 ± 0.00a | 100.00 ± 0.00a | 1.30 ± 0.26a | 5.21 ± 1.04b | 25.00 ± 0.00a | 33.33 ± 8.33a | 0.16 ± 0.03a |
| B. subtilis | 100.00 ± 0.00a | NA | NA | 0.65 ± 0.13ab | 25.00 ± 0.00a | NA | NA | 0.16 ± 0.03a |
| S. Typhi | NA | NA | NA | 0.78 ± 0.00ab | NA | NA | NA | 0.13 ± 0.03a |
| E. coli | NA | NA | NA | 0.26 ± 0.06b | NA | NA | NA | 0.05 ± 0.00a |
Values were conveyed as mean ± std error of mean. Values having similar superscript letters within a particular column are insignificantly distinct after one way Analysis of Variance and Tukey's post hoc (p > 0.05) NA stands for not active.
Ethyl acetate extract of X. spekei showed notable antibacterial activities against tested Gram-positive microbes, whereas there was no notable effect against tested Gram-negative microbes (Table 1). X. spekei extract showed high effects on B. subtilis and S. aureus with recorded MZI of >12 mm at extract concentrations between 50 mg/ml and 100 mg/ml (Table 1), although there was statistical similarity in the effects of both extract concentrations (p > 0.05). There was no activity against B. subtilis at concentrations 3.125 mg/ml and 6.25 mg/ml. However, X. spekei extract showed activity against S. aureus in all the tested concentrations (Table 1). Additionally, at 12.5 mg/ml and 25 mg/ml concentrations, there was statistical similarity in antibacterial effects on B. subtilis as well as S. aureus (p > 0.05; Table 1). Concentrations 6.25 mg/ml and 12.5 mg/ml and concentrations 3.125 mg/ml and 6.25 mg/ml also showed statistical similarities in their antibacterial effects against S. aureus (p > 0.05; Table 1). The antibacterial property of X. spekei extract on the tested Gram-positive bacteria was concentration-dependent (as concentration increased, extract's activity also increased) (Table 1).
The reference drug, Ciprofloxacin, exhibited a significantly greater effect against all tested microbes than the extract and DMSO (p < 0.05; Table 1). The diluent (5% DMSO) demonstrated no activity on all the tested bacterial pathogens (Table 1).
3.2. Comparison of antibacterial effects of ethyl acetate extracts of X. spekei, and G. tembensis against S. aureus
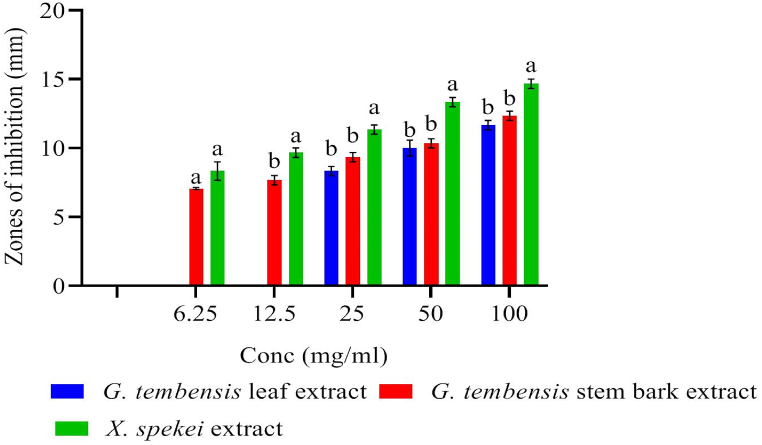
Antibacterial effects of X. spekei and G. tembensis (leaf and stem bark) extracts were tested against S. aureus and compared using one-way ANOVA. G. tembensis leaf and stem bark extracts demonstrated statistically similar effects from 25 mg/ml to 100 mg/ml concentrations against S. aureus (p > 0.05; Fig. 1). The ethyl acetate extracts of X. spekei demonstrated significantly different antibacterial effects from G. tembensis leaf and stem bark extracts against S. aureus, at 12.5 mg/ml to 100 mg/ml concentration ranges (p < 0.05; Fig. 1). At concentration 6.25 mg/ml, X. spekei extract exhibited statistically similar effects with G. tembensis stem bark extract against S. aureus (p > 0.05; Fig. 1). Comparative activities of G. tembensis leaf extract at concentrations range of 3.125 mg/ml to 12.5 mg/ml and G. tembensis stem bark extract at concentration 3.125 mg/ml were not done as there were no extract activities at these concentrations (Fig. 1).
Fig. 1.
Comparison of antibacterial properties of ethyl acetate extracts of X. spekei and G. tembensis against S. aureus. Bar graphs having distinct letters within a given concentration are significantly insignificant (p < 0.05).
3.3. Minimum inhibitory concentrations
The test extracts showed varied bacterial growth inhibitions and were thus subjected to minimum inhibitory and bactericidal concentrations depending on the pathogens they had effects on. The mean MIC means ranged from 5.21 ± 1.04 to 25.00 ± 0.00 mg/ml, 25.00 ± 0.00 mg/ml, and 33.33 ± 8.33 mg/ml for ethyl acetate extract of X. spekei, stem bark, and leaf extracts of G. tembensis, respectively (Table 2). X. spekei extract showed significantly different inhibition effects on B. subtilis as well as S. aureus (p < 0.05; Table 2), while the reference drug, Ciprofloxacin exhibited statistically similar inhibitory effects against all the tested pathogens (p > 0.05; Table 2).
In comparison, X. spekei extract exhibited inhibitory effects at lower concentrations against S. aureus than the other two extracts (Table 2), although its mean inhibitory concentration was statistically similar to that of Ciprofloxacin against S. aureus (p > 0.05; Table 2). Similarly, the inhibitory potentials of both stem bark and leaf extracts of G. tembensis on S. aureus had a statistical similarity (p > 0.05; Table 2). X. spekei extract's mean inhibitory concentration value was significantly greater than that of Ciprofloxacin on B. subtilis (p <0.05; Table 2).
3.4. Minimum bactericidal concentrations
Generally, the tested extracts had significantly greater MBC values than MIC values against each of the tested bacterial pathogens (Table 2). Mean MBC were from 33.33 ± 8.33 to 100.00 ± 0.00 mg/ml, 100.00 ± 0.00 mg/ml and 100.00 ± 0.00 mg/ml for X. spekei and G. tembensis (stem bark and leaf) extracts respectively (Table 2). X. spekei extract showed significantly higher bactericidal effect against S. aureus than B. subtilis (p < 0.05; Table 2). Additionally, X. spekei extract's bactericidal effect against S. aureus was at lower concentration than the other two extracts (Table 2). Ciprofloxacin's bactericidal effects were statistically similar against S. aureus, S. Typhi as well as B. subtilis (p > 0.05; Table 2).
In comparison to Ciprofloxacin, the bactericidal effect of X. spekei extract against S. aureus, was at a significantly higher concentration than Ciprofloxacin (p < 0.05) but at a significantly lower concentration than the other two extracts (p < 0.05; Table 2). Both leaf and stem bark extracts of G. tembensis demonstrated statistically similar bactericidal effects against S. aureus (p > 0.05). In addition, Ciprofloxacin had bactericidal effects at significantly lower concentrations than all the extracts (p < 0.05; Table 2).
3.5. Quantitative phytochemical composition of ethyl acetate extracts of G. tembensis stem bark, G. tembensis leaf, and X. spekei
Phytochemical screening results for stem bark extract of G. tembensis as presented in Table 3, showed n-Hexadecanoic acid, a fatty acid derivative, had the highest concentration at 88.06 ± 3.04 μg/g while the compound 2-Pentanone a ketone had the lowest concentration at 0.40 ± 0.00 μg/g. The extract comprised 38.1% fatty acids, 30.6% terpenoids, 11.6% hydrocarbons, 10.2% steroids, 4.0% phenolic compounds, 2.3% aldehyde compounds, 1.4% ketones, 0.6% tocopherol, 0.6% benzene derivatives, 0.4% methoxybenzoic acid compounds and 0.2% aromatic amine compounds.
Table 3.
Quantitative phytochemical Compounds analysis in ethyl acetate stem bark extract of G. tembensis.
| Chemical Class | Compound | % abundance | MF | MW (g/mol) | Conc (μg/g) |
|---|---|---|---|---|---|
| Ketone | 2-Pentanone | 0.1 | C5H10O | 86.13 | 0.40 ± 0.00 |
| 5-Decanone | 1.3 | C10H20O | 156.26 | 6.93 ± 0.24 | |
| Terpenoid | AR- Curcumene | 0.3 | C15H22 | 202.33 | 1.83 ± 0.07 |
| 2(4H)- Benzofuranone, 5,6,7,7a- tetrahydro- 4,4,7a-trimethyl- | 0.4 | C11H16O2 | 180.243 | 2.03 ± 0.07 | |
| Phytol acetate<E−> | 0.6 | C22H42O2 | 338.6 | 3.02 ± 0.11 | |
| 2- Pentadecanone,6,10,14- trimethyl- | 0.8 | C18H36O | 268.477 | 4.21 ± 0.15 | |
| Phytol, acetate | 0.5 | C22H42O2 | 338.6 | 2.60 ± 0.09 | |
| 4,8,12,16-Tetramethylheptadecan-4-olide | 2.6 | C21H40O2 | 324.5 | 14.05 ± 0.49 | |
| Squalene | 2.1 | C30H50 | 410.7 | 11.31 ± 0.39 | |
| trans-Geranylgeraniol | 0.8 | C20H34O | 290.5 | 4.13 ± 0.14 | |
| 2,6,10, 14- Hexadecatetraen-1- ol,3,7,11,15- tetramethyl-, acetate, (E,E,E)- | 0.7 | C22H36O2 | 332.5 | 3.79 ± 0.13 | |
| Ursa- 9(11),12- dien-3-ol | 2.4 | C30H48O | 424.7 | 12.97 ± 0.45 | |
| .alpha.-Amyrin | 1.4 | C30H50O | 426.7 | 7.81 ± 0.27 | |
| .beta.-Amyrin | 0.9 | C30H50O | 426.7 | 4.78 ± 0.17 | |
| Lup-20(29)-en-3-one | 3.6 | C30H48O | 424.7 | 19.67 ± 0.68 | |
| Taraxasterol | 3.2 | C30H50O | 426.7 | 17.59 ± 0.61 | |
| 2,2,4a,6a,8a,9,12 b,14a- Octamethyl- 1,2,3,4,4a,5,6,6a, 6 b,7,8,8a, 9,12,12a,12 b,13,14,14a, 14 b- eicosahydropicene | 1.8 | C30H50 | 410.7 | 9.77 ± 0.34 | |
| Lupan-3-ol, acetate | 3.0 | C32H54O2 | 470.8 | 16.36 ± 0.57 | |
| Friedelan-3-one | 4.3 | C30H50O | 426.7 | 23.17 ± 0.80 | |
| Fatty acid and derivatives | Adipic acid, 2-ethylhexyl isobutyl ester | 2.5 | C18H34O4 | 314.5 | 13.76 ± 0.48 |
| Methyl 18-methylnonadecanoate | 1.2 | C21H42O2 | 326.6 | 6.67 ± 0.23 | |
| Tetracosanoic acid, methyl ester | 1.2 | C25H50O2 | 382.7 | 6.56 ± 0.23 | |
| Heneicosyl acetate | 1.4 | C23H46O2 | 354.6 | 7.42 ± 0.26 | |
| Hexacosyl acetate | 1.0 | C28H56O2 | 424.7 | 5.32 ± 0.18 | |
| Tetracosyl acetate | 0.9 | C26H52O2 | 396.7 | 5.00 ± 0.17 | |
| Hydrocarbon | Octacosane | 0.3 | C28H58 | 394.8 | 1.53 ± 0.05 |
| Nonadecane | 0.3 | C19H40 | 268.5 | 1.79 ± 0.06 | |
| Tricosane | 1.8 | C23H48 | 324.6 | 9.91 ± 0.34 | |
| Tetracosane | 1.8 | C24H50 | 338.7 | 9.93 ± 0.34 | |
| Pentacosane | 1.4 | C25H52 | 352.7 | 7.56 ± 0.26 | |
| Hexacosane | 1.3 | C26H54 | 366.7 | 7.26 ± 0.25 | |
| Tritriacontane | 2.7 | C33H68 | 464.9 | 14.61 ± 0.50 | |
| Octacosane | 1.8 | C28H58 | 394.8 | 9.63 ± 0.33 | |
| Aldehyde | Tridecanedial | 1.4 | C13H24O2 | 212.33 | 7.85 ± 0.27 |
| Octadecanal | 0.9 | C18H36O | 268.5 | 4.71 ± 0.16 | |
| Phenolic compound | Phenol, 2,5-dimethyl-, acetate | 2.1 | C10H12O2 | 164.2 | 11.27 ± 0.39 |
| Phenol, 2,4- bis (1- methyl-1- phenylethyl)- | 1.9 | C24H26O | 330.5 | 10.3 ± 0.36 | |
| Tocopherol | Vitamin E | 0.6 | C29H50O2 | 430.7 | 3.09 ± 0.11 |
| Phytosterols | Campesterol | 3.2 | C28H48O | 400.7 | 17.39 ± 0.60 |
| Stigmasterol | 3.8 | C29H48O | 412.7 | 20.54 ± 0.71 | |
| .gamma.-Sitosterol | 1.3 | C29H50O | 414.7 | 7.14 ± 0.25 | |
| Stigmast-4-en-3-one | 1.9 | C29H48O | 412.7 | 10.58 ± 0.37 | |
Key: Conc = Concentration, Mins = Minutes, MF = Molecular formula, RT = Retention time, MW = Molecular weight.
The major class of secondary metabolites identified in the leaf extract of G. tembensis were 59.33% of terpenoids, 16.17% fatty acids, 15.08% hydrocarbons, 6.58% phenolic compounds, 1.79% ester compounds, 0.79% steroids, 0.19% naphthalene compounds, 0.04% aldehyde, 0.03% toloudine derivative (Table 4). Further, phytochemical investigations of this plant extract showed the presence of 40 compounds with trans-3-Penten-2-ol, an alkenol compound, having the lowest concentration at 0.01 ± 0.00 μg/g while Squalene, a triterpenoid, having the highest concentration at 14.17 ± 0.24 μg/g.
Table 4.
Quantitative phytochemical compounds’ analysis of ethyl acetate leaf extract of G. tembensis.
| Chemical Class | Compound | %abundance | MF | MW (g/mol) | Conc(μg/g) |
|---|---|---|---|---|---|
| Alkenol | trans-3-Penten-2-ol | 0.02 | C5H10O | 86.13 | 0.01 ± 0.00 |
| Aldehyde | Pivalaldehyde, semicarbazone | 0.04 | C6H13N3O | 143.19 | 0.03 ± 0.00 |
| Toluidine derivative | Prilocaine | 0.03 | C13H20N2O | 220.31 | 0.02 ± 0.00 |
| Terpenoids | Limonene | 0.22 | C10H16 | 136.23 | 0.16 ± 0.00 |
| 2 (4H)- Benzofuranone,5,6,7,7a- tetrahydro- 4,4, 7a-trimethyl- | 1.34 | C11H16O2 | 180.24 | 1.01 ± 0.02 | |
| Bicyclo [3.1.1] heptane, 2,6, 6-trimethyl-, (1.α., 2.β, 5.α.)- | 3.92 | C10H18 | 138.25 | 2.95 ± 0.05 | |
| 2-Pentadecanone, 6,10, 14-trimethyl- | 1.14 | C18H36O | 268.50 | 0.86 ± 0.01 | |
| 3,7,11, 15-Tetramethyl-2- hexadecen-1-ol | 1.22 | C20H40O | 296.50 | 0.92 ± 0.02 | |
| Phytol, acetate | 2.06 | C22H42O2 | 338.60 | 1.55 ± 0.03 | |
| 5,9,13- Pentadecatrien-2-one, 6,10,14-trimethyl -,(E,E)- | 2.88 | C18H30O | 262.40 | 2.17 ± 0.04 | |
| trans-Geranylgeraniol | 2.14 | C20H34O | 290.50 | 1.62 ± 0.03 | |
| Phytol | 4.60 | C20H40O | 296.50 | 3.47 ± 0.06 | |
| Phytol acetate<E−> | 2.92 | C22H42O2 | 338.60 | 2.20 ± 0.04 | |
| Squalene | 18.78 | C30H50 | 410.70 | 14.17 ± 0.2 | |
| .beta.-Amyrin | 9.03 | C30H50O | 426.70 | 6.81 ± 0.12 | |
| Lup-20(29)-en-3-one | 3.65 | C30H48O | 424.70 | 2.75 ± 0.05 | |
| Taraxasterol | 2.31 | C30H50O | 426.70 | 1.75 ± 0.03 | |
| Friedelan-3-one | 0.42 | C30H50O | 426.70 | 0.32 ± 0.01 | |
| Nerolidol 1 | 1.40 | C15H26O | 222.37 | 1.05 ± 0.02 | |
| 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 1.30 | C10H16O | 152.23 | 0.98 ± 0.02 | |
| Fatty acid and derivatives | Trans-13- Octadecenoic acid, methyl ester | 1.39 | C19H36O2 | 296.50 | 1.05 ± 0.02 |
| 9,12,15- Octadecatrienoic acid, ethyl ester,(Z,Z, Z)- | 5.52 | C20H34O2 | 306.50 | 4.17 ± 0.07 | |
| Carbonic acid, pentadecyl 2,2,2-trichloroethyl ester | 1.14 | C18H33Cl3O3 | 403.80 | 0.86 ± 0.01 | |
| Adipic acid,.beta.-citronellyl tetradecyl ester | 0.68 | C28H52O4 | 452.71 | 0.51 ± 0.01 | |
| 13-Tetradecen-1-ol acetate | 1.79 | C16H30O2 | 254.41 | 1.35 ± 0.02 | |
| Hydrocarbon | Tricosane | 3.10 | C23H48 | 324.60 | 2.34 ± 0.04 |
| Eicosane | 1.52 | C20H42 | 282.50 | 1.14 ± 0.02 | |
| Tetracosane | 3.77 | C24H50 | 338.70 | 2.85 ± 0.05 | |
| Octadecane | 2.50 | C18H38 | 254.50 | 1.89 ± 0.03 | |
| Hexacosane | 1.70 | C26H54 | 366.70 | 1.28 ± 0.02 | |
| Phenolic compound | Phenol, 3,5-dimethyl- | 1.58 | C8H10O | 122.16 | 1.19 ± 0.02 |
| Phenol, 2,4- bis (1-methyl-1- phenylethyl)- | 3.34 | C24H26O | 330.50 | 2.52 ± 0.04 | |
| dl-.alpha.-Tocopherol | 1.66 | C29H50O2 | 430.70 | 1.25 ± 0.02 | |
| Phytosterols | Stigmasterol | 0.16 | C29H48O | 412.70 | 0.12 ± 0.00 |
| Phytosterols | .gamma.-Sitosterol | 0.63 | C29H50O | 414.70 | 0.47 ± 0.01 |
Key: Conc = Concentration, Mins = Minutes, MF = Molecular formula, MW = Molecular weight.
Phytochemical compounds of X. spekei extract, showed several secondary metabolites in different concentrations with Ursa-9(11),12-dien-3-one, a triterpenoid, having the highest concentration at 38.39 ± 2.40 μg/g whereas 1,16 Cyclocorynan 17-oic acid, 19, 20- didehydro-, methyl ester, (16 S, 19 E)-, an alkaloid, having the lowest concentration at 0.04 ± 0.00 μg/g (Table 5). Additionally, phytochemical screening of ethyl acetate of X. spekei extract, revealed 33.34% terpenoids, 27.90% fatty acids, 23.93% steroids, 9.76% hydrocarbons, 1.92% phenolic compounds, 1.02% tocopherols, 0.83% tetracarboxylic acids, 0.97% benzene derivatives, 0.20% ketones, 0.11% dialkylaminodiphenylbutanol ester and 0.02% alkaloid.
Table 5.
Quantitative analysis of phytochemical compounds in ethyl acetate X. spekei extract.
| Chemical Class | Compound | %abundance | MF | MW (g/mol) | Conc(μg/g) |
|---|---|---|---|---|---|
| Benzene derivative | 1-tert-Butyl-3-nitrobenzene | 0.37 | C10H13NO2 | 179.22 | 1.01 ± 0.06 |
| Benzoic acid, 2, 4-dihydroxy-3, 6-dimethyl-, methyl ester | 0.60 | C10H12O4 | 196.19 | 1.64 ± 0.10 | |
| Hydrocarbon | Heneicosane, 10-methyl- | 1.09 | C22H46 | 310.60 | 2.96 ± 0.19 |
| 1-Docosene | 2.53 | C22H44 | 308.60 | 6.88 ± 0.43 | |
| Octacosane | 0.44 | C28H58 | 394.80 | 1.19 ± 0.07 | |
| Hexadecane | 0.96 | C16H34 | 226.44 | 2.59 ± 0.16 | |
| Hexadecene<1-> | 0.53 | C16H32 | 224.42 | 1.43 ± 0.09 | |
| 50.47 Heneicosane | 2.27 | C21H44 | 296.60 | 6.18 ± 0.39 | |
| Hexacosane | 1.71 | C26H54 | 366.70 | 4.65 ± 0.29 | |
| Heneicosane | 0.23 | C21H44 | 296.60 | 0.63 ± 0.04 | |
| Dialkylaminodiphenylbutanol ester | Pyrroliphene | 0.11 | C23H29NO2 | 351.50 | 0.29 ± 0.02 |
| Fatty acid and derivatives | Tetradecanoic acid | 0.44 | C14H28O2 | 228.37 | 1.19 ± 0.07 |
| Methyl stearate | 0.49 | C19H38O2 | 298.50 | 1.33 ± 0.08 | |
| n-Hexadecanoic acid | 8.99 | C16H32O2 | 256.42 | 24.41 ± 1.53 | |
| Pentadecanol<n-> | 0.57 | C15H32O | 228.41 | 1.56 ± 0.10 | |
| Ethyl hexadecanoate | 1.29 | C18H36O2 | 284.50 | 3.51 ± 0.22 | |
| Linoleic acid ethyl ester | 4.68 | C20H36O2 | 308.50 | 12.7 ± 0.79 | |
| 9-Octadecenoic acid, methyl ester, (E)- | 0.69 | C19H36O2 | 296.50 | 1.87 ± 0.12 | |
| Palmitoleic acid | 0.37 | C16H30O2 | 254.41 | 1.00 ± 0.06 | |
| Methyl hexadecanoate | 0.27 | C17H34O2 | 270.50 | 0.73 ± 0.05 | |
| Ketone | Z-11-Pentadecenol | 0.20 | C15H30O | 226.40 | 0.55 ± 0.03 |
| Phenolic compound | Benzenethiol, 2,4,6-tris(1-methylethyl)- | 0.24 | C15H24S | 236.40 | 0.64 ± 0.04 |
| Phenol, 2,4-bis(1-methyl-1- phenylethyl)- | 1.47 | C24H26O | 330.50 | 3.99 ± 0.25 | |
| Phenol, 3,5-bis(1, 1-dimethylethyl)- | 0.21 | C14H22O | 206.32 | 0.56 ± 0.03 | |
| Alkaloid | 1,16- Cyclocorynan −17-oic acid, 19, 20- didehydro-, methyl ester, (16 S, 19 E)- | 0.02 | C20H22N2O2 | 322.40 | 0.04 ± 0.00 |
| Terpenoids | 4,4, 6a,6 b,8a,11,12, 14 b-Octamethyl-1,4,4a,5,6,6a,6 b,7,8,8a,9,10,11,12,12a,14,14a, 14 b-octadecahydro- 2H-picen-3-one | 6.63 | C30H48O | 424.70 | 18.01 ± 1.13 |
| Squalene | 1.46 | C30H50 | 410.70 | 3.97 ± 0.25 | |
| Phytol | 0.58 | C20H40O | 296.50 | 1.58 ± 0.10 | |
| 9-Undecen-2-one, 6,10-dimethyl- | 0.95 | C13H24O | 196.33 | 2.59 ± 0.16 | |
| Phytol, acetate | 3.52 | C22H42O2 | 338.60 | 9.57 ± 0.60 | |
| Tributyl acetylcitrate | 0.83 | C20H34O8 | 402.50 | 2.25 ± 0.14 | |
| Ursa-9(11),12-dien-3-one | 14.14 | C30H46O | 422.70 | 38.39 ± 2.40 | |
| 4,8,12,16-Tetramethylheptadecan-4-olide | 2.96 | C21H40O2 | 324.50 | 8.04 ± 0.50 | |
| Lup-20(29)-en-3-one | 1.48 | C30H48O | 424.70 | 4.03 ± 0.25 | |
| Widdrol | 0.49 | C15H26O | 222.37 | 1.34 ± 0.08 | |
| Lanost-8-en-3-one | 0.82 | C30H50O | 426.70 | 2.21 ± 0.14 | |
| 2-Pentadecanone, 6,10,14-trimethyl- | 0.31 | C18H36O | 268.50 | 0.84 ± 0.05 | |
| Tocopherol | 2H-1-Benzopyran-6-ol, 3,4-dihydro- 2,8-dimethyl-2- (4,8,12 -trimethyltridecyl)-, [2 R-[2 R*(4R*,8R*)]]- | 1.02 | C27H46O2 | 402.65 | 2.76 ± 0.17 |
| Phytosterols | 9,19- Cyclo-25, 26-epoxyergostan-3-ol, 4,4, 14- trimethyl-, acetate | 1.21 | C33H54O3 | 498.78 | 3.28 ± 0.21 |
| Stigmast-4-en-3-one | 5.96 | C29H48O | 412.70 | 16.19 ± 1.01 | |
| 17- (1,5-Dimethylhexyl)-10,13- dimethyl-2,3,4,7,8,9,10,11, 12,13,14,15,16, 17-tetradecahydro-1h-cyclopenta[a]Phenanthren-3-ol | 2.18 | C27H48O | 386.65 | 5.93 ± 0.37 | |
| Stigmasterol | 0.87 | C29H48O | 412.70 | 2.36 ± 0.15 | |
| 4,22-Stigmastadiene-3-one | 1.02 | C29H46O | 410.70 | 2.78 ± 0.17 | |
| .gamma.-Sitosterol | 12.69 | C29H50O | 414.70 | 34.46 ± 2.15 |
Key: Conc = Concentration, Mins = Minutes, MF = Molecular formula, RT = Retention time, MW = Molecular weight.
4. Discussion
Herbal plants are known to produce secondary metabolites with known effects against bacterial pathogens and less adverse effects in comparison to conventional antibacterial agents [25]. Various plants have been used traditionally as antibacterial agents however, they lack scientific validation and documentation on their usage. This current study determined the in vitro antibacterial effects of the ethyl acetate extracts of X. spekei and G. tembensis stem bark and leaves against E. coli, B. subtilis, S. Typhi, as well as S. aureus.
This is considerably the initial report about the antibacterial effects of ethyl acetate stem bark extracts of G. tembensis. However, previous studies have shown antibacterial capabilities of stem bark extracts of other plants of the genus Grewia. For instance, the ethanol stem bark extracts of Grewia mollis have exhibited antibacterial potentials on E. coli, S. aureus as well as Streptococcus sp [26]. The ethyl acetate stem bark extracts of G. tembensis exhibited notable antibacterial effects against S. aureus only with MZI ranges of 07.07 ± 0.07 to 12.33 ± 0.33 mm. This concurs with a report by Akwu et al. [27], which confirmed that lupeol compounds from the stem bark of Grewia lasiocarpa were inactive on S. Typhi and E. coli.
Similarly, this is considerably the initial study about the antibacterial potential of ethyl acetate leaf extracts of G. tembensis. However, previous experiments have demonstrated that the leaves of other Grewia genus plants have antibacterial activities. The methanol, n-hexane, and ethyl acetate leaf extracts of Grewia pubescens have been shown to possess antibacterial properties [28]. Ethyl acetate leaf extracts of G. tembensis exhibited antibacterial capability against only S. aureus in a dilution-dependent trend with MZI ranging from 08.33 ± 0.33 to 11.67 ± 0.33 mm. These findings agrees with a study which indicated that the ethyl acetate leaf extracts of G. plagiophylla displayed no antibacterial potential against E. coli and S. Typhi but had activity against S. aureus [29]. This was also in agreement with a study that found no activity against B. subtilis when the methanol and ethanol fruit extracts of Phoenix dactylifera and ethanol and methanol seed extracts of Clitoria ternatea were tested on B. subtilis, S. Typhi, B. cereus, and E. coli [30].
The current study noted that there is lack of published report available on antibacterial capabilities of ethyl acetate extracts of X. spekei. Xerophyta retinervis which belongs to the same genus as X. spekei has illustrated antimicrobial, antiulcer as well as antiinflammatory potentials. In South Africa, X. retinervis is used by traditional herbalists to cure rhinitis and headache [31]. The whole plant of X. retinervis is traditionally used in the Pretoria region of South Africa as a therapy for asthma and nose bleeding [32]. The ethyl acetate extract of X. spekei produced notable inhibition zones beginning from 07.67 ± 0.33 to 14.67 ± 0.33 mm against S. aureus and inhibition zones beginning from 09.67 ± 0.33 to 14.33 ± 0.33 mm against B. subtilis but had no antibacterial activities on the tested Gram-negative microbes. This was in consensus with Sapkota et al. [33], who showed that ethanol leaf extracts of Artimisia vulgaris and Eupharbia hirta had an antibacterial impact on B. subtilis and S. aureus but were inactive on K. pneumoniae, E. coli, S. dysenteriae, as well as S. Typhi. Additionally, this also agrees with the findings of a study by Seaman [34] that illustrated that ethyl acetate extracts of X. retinervis had antibacterial activity on S. aureus.
The antibacterial effects of the tested plant extracts in the current study are attributed to the presence of various secondary metabolites. Compounds like terpenoids have been known to exhibit antibacterial effects by interfering with bacterial oxygen uptake and oxidative phosphorylation which are two important essential processes in microorganisms [5]. Phytol, a diterpenoid which was found in all the studied extracts, has known antibacterial activity [35]. Additionally, Lup-20(29)-en-3-one, a triterpenoid, which was also present in X. spekei and G. tembensis extracts, has also been known to have antibacterial effects [36].
Alkanes act by interfering with bacterial cell membrane integrity causing bacterial cell death [37]. Octacosane, a straight-chain alkane, which was found in G. tembensis stem bark and X. spekei extracts, has known antibacterial properties [38]. Another hydrocarbon, Heneicosane, which was found in both X. spekei and G. tembensis leaf extracts, has previously shown antibacterial activities [39].
Fatty acids act by inhibiting bacterial enzyme activity, and direct bacterial cell lysis [40]. Fatty acid like n-Hexadecanoic acid (palmitic acid), found in all the tested extracts, has been shown to possess antimicrobial effects [41]. Another fatty acid, Tetradecanoic acid, that was present in both X. spekei and G. tembensis stem bark extracts, has previously been shown to have antibacterial effects [42].
Phenolic compounds, are known to cause bacterial cell lysis in addition to membrane-disturbing properties as their mode of antibacterial efficacy [43]. Phenol, 3,5-bis (1, 1-dimethylethyl)-, which was present in X. spekei extract has been shown to have antibacterial activities [44]. Phenol, 2,5-dimethyl-, acetate which was found in G. tembensis stem bark extract has displayed antimicrobial activities [45].
Stigmasterol, a phytosterol which was present in X. spekei, G. tembensis stem bark, and leaf extracts, has been previously shown to have antibacterial activities [46]. Stigmast-4-en-3-one, also a phytosterol, present in both X. spekei and G. tembensis stem bark extracts, has been shown to have antibacterial effects [47].
This study had some limitations. The first limitation was lack of previous published data which led to limited access to information on antibacterial activities of the studied plant extracts leading to limited reference and comparison to the activity of these studied plants. The second limitation was limited availability of resources which limited our capability in conducting more biological assays.
5. Conclusions
The current experiment aimed at confirming the traditional use of X. spekei and G. tembensis medicinal plants against E. coli, B. subtilis, S. Typhi, and S. aureus by investigating their in vitro antibacterial potential and the presence of phytochemicals associated with antibacterial properties. The ethyl acetate extracts of the studied plant extracts demonstrated varied antibacterial potentials with X. spekei extract, exhibiting activity on S. aureus and B. subtilis only while both the stem bark and leaf extracts of G. tembensis exhibited activity on S. aureus alone. Overall, our current findings indicate that the studied extracts can be potential candidates to extract therapeutic antibacterial agents for managing and treating bacterial illnesses.
Author contribution statement
Paul Ochieng Nyalo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. George Isanda Omwenga, Mathew Piero Ngugi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Ethical approval
The experimental procedures and protocols in this study were approved by Kenyatta University Graduate School approval committee.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Kenyatta University's Biochemistry, Microbiology and Biotechnology department for giving them an opportunity to carry out this study in the laboraties. We also appreciate the technical support of Mr. Daniel Gitonga, Mr. Kimani James, as well as Mr. Ibrahim Waweru.
References
- 1.Noah D., Fidas G. National Intelligence Council; Washington DC: 2000. The Global Infectious Disease Threat and its Implications for the United States. [PubMed] [Google Scholar]
- 2.Ford N., Shubber Z., Meintjes G., Grinsztejn B., Eholie S., Mills E.J., et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2(10):e438–e444. doi: 10.1016/S2352-3018(15)00137-X. [DOI] [PubMed] [Google Scholar]
- 3.Kelesidis T., Kelesidis I., Rafailidis P.I., Falagas M.E. Counterfeit or substandard antimicrobial drugs: a review of the scientific evidence. J. Antimicrob. Chemother. 2007;60(2):214–236. doi: 10.1093/jac/dkm109. [DOI] [PubMed] [Google Scholar]
- 4.Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6(1):1–8. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochieng Nyalo P., Isanda Omwenga G., Piero Ngugi M. GC-MS analysis, antibacterial and antioxidant potential of ethyl acetate leaf extract of Senna singueana (delile) grown in Kenya. Evid Based Complement Alternat Med. 2022:2022. doi: 10.1155/2022/5436476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., Patil S.S., Pendharkar N. Antimicrobial and anti-inflammatory activity of aqueous extract of Carica papaya. J HerbMed Pharmacol. 2017;6(4):148–152. [Google Scholar]
- 7.Imanirampa L., Alele P.E. Antifungal activity of Cleome gynandra L. aerial parts for topical treatment of Tinea capitis: an in vitro evaluation. BMC Compl. Alternative Med. 2016;16(1):1–8. doi: 10.1186/s12906-016-1187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kareru P.G., Kenji G.M., Gachanja A.N., Keriko J.M., Mungai G. Traditional medicines among the Embu and Mbeere peoples of Kenya. Afr. J. Tradit., Complementary Altern. Med. 2007;4(1):75–86. doi: 10.4314/ajtcam.v4i1.31193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanga V.O., Dong X., Oulo M.A., Munyao J.N., Mkala E.M., Kirika P.…Hu G.W., Wanga V.O., Dong X., Oulo M.A., Munyao J.N., Mkala E.M., et al. The complete chloroplast genome sequence of Xerophyta spekei (Velloziaceae) Mitochondrial DNA Part B Resour. 2020;5(1):100–101. doi: 10.1080/23802359.2019.1698365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellermann T. Warburgia Salutaris; 2010. A Pharmacological Investigations of South Africa Lichens, Dessication-Tolerant Plants and Medicinal Tree. [Google Scholar]
- 11.Gebreyohannes D.T. University of Nairobi; 2013. Ecology of Medicinal Plants and Their Integration into Primary Healthcare in Kajiado County, Kenya. [Google Scholar]
- 12.Kokwaro J.O. University of Nairobi press; 2009. Medicinal Plants of East Africa. [Google Scholar]
- 13.Hassan-Abdallah A., Merito A., Hassan S., Aboubaker D., Djama M., Asfaw Z., et al. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013;148(2):701–713. doi: 10.1016/j.jep.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Mutie F.M., Gao L.L., Kathambi V., Rono P.C., Musili P.M., Ngugi G., et al. An ethnobotanical survey of a dryland botanical garden and its environs in Kenya: the mutomo hill plant sanctuary. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/1543831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jibril S., Zakari A., Kendeson C.A., Abdullahi I., Idris M.M., Sirat H.M. Phytochemical and antibacterial screening of leaf extracts from Cassia singueana Del.(Fabaceae) Bayero J Pure Appl Sci. 2021;13(1):108–112. [Google Scholar]
- 16.Praveena B., Pradeep S.N. Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus) Food Nutr. Sci. 2012;2012 [Google Scholar]
- 17.Arora D.S., Sood H. In vitro antimicrobial potential of extracts and phytoconstituents from Gymnema sylvestre R. Br. leaves and their biosafety evaluation. Amb. Express. 2017;7(1):1–13. doi: 10.1186/s13568-017-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieberi B.M., Omwenga G.I., Wambua R.K., Samoei J.C., Ngugi M.P. Screening of the dichloromethane: methanolic extract of Centella asiatica for antibacterial activities against Salmonella typhi, Escherichia coli, Shigella sonnei, Bacillus subtilis, and Staphylococcus aureus. Sci. World J. 2020:2020. doi: 10.1155/2020/6378712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009:1–13. December 2009. [Google Scholar]
- 20.Manandhar S., Luitel S., Dahal R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019;2019 doi: 10.1155/2019/1895340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mwitari P.G., Ayeka P.A., Ondicho J., Matu E.N., Bii C.C. Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolić M., Vasić S., Đurđević J., Stefanović O., Čomić L., Nikoli M., et al. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujev J Sci. 2014;36(36):129–136. [Google Scholar]
- 23.Elshikh M., Ahmed S., Funston S., Dunlop P., McGaw M., Marchant R., et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016;38(6):1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang C.G., Hah D.S., Kim C.H., Kim Y.H., Kim E., Kim J.S. Evaluation of antimicrobial activity of the methanol extracts from 8 traditional medicinal plants. Toxicol. Res. 2011;27(1):31–36. doi: 10.5487/TR.2011.27.1.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyaya S., Yadav D., Chandra R., Arora N. Evaluation of antibacterial and phytochemical properties of different spice extracts. Afr. J. Microbiol. Res. 2018;12(2):27–37. [Google Scholar]
- 26.Shagal M.H., Kubmarawa D., Idi Z. Phytochemical screening and antimicrobial activity of roots, stem-bark and leave extracts of Grewia mollis. Afr. J. Biotechnol. 2012;11(51):11350–11353. [Google Scholar]
- 27.Akwu N., Naidoo Y., Singh M., Thimmegowda S.C., Nundkumar N., Lin J. Isolation of lupeol from Grewia lasiocarpa stem bark: antibacterial, antioxidant, and cytotoxicity activities. Biodiversitas J Biol Divers. 2020;21(12) [Google Scholar]
- 28.Hamid A.A., Oguntoye S.O., Alli S.O., Akomolafe G.A., Aderinto A., Otitigbe A., et al. Chemical composition, antimicrobial and free radical scavenging activities of Grewia pubescens. Chem. Int. 2016;2(4):254–261. [Google Scholar]
- 29.Douglas K., Gitonga A. Antimicrobial activity of Bridelia micrantha and Grewia plagiophylla leaf extracts. J Pharm Res Int. 2016:1–7. [Google Scholar]
- 30.Rahman M.M., Shahriar M.R., Meghla N.S., Ishika T., Roy P.C., Kamruzzaman M. Antimicrobial activity of some medicinal plant extracts against Gram positive and Gram negative bacteria in Bangladesh. Asian J Med Biol Res. 2017;3(4):405–411. [Google Scholar]
- 31.Semenya S.S., Maroyi A. Ethnobotanical study of curative plants used by traditional healers to treat rhinitis in the Limpopo Province, South Africa. Afr. Health Sci. 2018;18(4):1076–1087. doi: 10.4314/ahs.v18i4.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motlhatlego K.E. 2014. Evaluation of Plants Used in African Traditional Medicine for Asthma and Related Conditions. [Google Scholar]
- 33.Sapkota P., Bhattarai S., Bajracharya A.M., Lakhe P.B., Shrestha N. Antimicrobial screening of some medicinal plants against selected bacterial species. Sci. World. 2020;13(13):20–23. [Google Scholar]
- 34.Seaman T. 2005. The Antimicrobial and Antimycobacterial Activity of Plants Used for the Treatment of Respiratory Ailments in Southern Africa and the Isolation of Anacardic Acid from Ozoroa Paniculosa. [Google Scholar]
- 35.Rukshana M.S., Doss A., Kumari P.R. Phytochemical screening and GC-MS analysis of leaf extract of Pergularia daemia (Forssk) Chiov. Asian J. Plant Sci. Res. 2017;7(1):9–15. [Google Scholar]
- 36.Nimbeshaho F., Mwangi C., Orina F., Chacha M., Adipo N., Moody J., et al. Antimycobacterial activities, cytotoxicity and phytochemical screening of extracts for three medicinal plants growing in Kenya. J. Med. Plants Res. 2020;14(4):129–143. [Google Scholar]
- 37.Kang M.K., Nielsen J. Biobased production of alkanes and alkenes through metabolic engineering of microorganisms. J. Ind. Microbiol. Biotechnol. 2017;44(4–5):613–622. doi: 10.1007/s10295-016-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karthikeyan K., Dhanapal C.K., Gopalakrishnan G. GC-MS analysis of petroleum ether extract of Alysicarpus monilifer - whole plant. 2016;8(3):94–99. [Google Scholar]
- 39.Biswas S.M., Chakraborty N., Bhowmik P.C. Cuticular wax of tectona grandis L. Leaves–A resist marker. Plant Pathog Biochem Anal Biochem. 2017;6(330):1009–2161. [Google Scholar]
- 40.Desbois A.P., Smith V.J. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010;85(6):1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 41.Awa E.P., Ibrahim S., Ameh D.A. GC/MS analysis and antimicrobial activity of diethyl ether fraction of methanolic extract from the stem bark of Annona senegalensis Pers. Int J Pharm Sci Res. 2012;3(11):4213. [Google Scholar]
- 42.Jumina J., Mutmainah M., Purwono B., Kurniawan Y.S., Syah Y.M. Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules. 2019;24(20):3692. doi: 10.3390/molecules24203692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reichling J., Suschke U., Schneele J., Geiss H.K. Antibacterial activity and irritation potential of selected essential oil components–structure-activity relationship. Nat. Prod. Commun. 2006;1(11) 1934578X0600101116. [Google Scholar]
- 44.Rai D.K., Sharma V., Pal K., Gupta R.K. Comparative phytochemical analysis of Cuscuta reflexa Roxb. Parasite grown on north India by GC-MS. Trop Plant Res. 2016;3(2):428–443. [Google Scholar]
- 45.Mukhtar M., Adamu H.M., Falalu M.Y. GC-MS analysis and identification of constituents present in the root extract of Mitragyna inermis. J. Pharmacogn. Phytochem. 2016;5(6):17. [Google Scholar]
- 46.Mailafiya M.M., Yusuf A.J., Abdullahi M.I., Aleku G.A., Ibrahim I.A.A., Yahaya M., et al. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J Med Plants Econ Dev. 2018;2(1):1–5. [Google Scholar]
- 47.Ragasa C.Y., Tepora M.M., Rideout J.A. Antimicrobial activities of sterol from Pycnarrhena manillensis. ACGC Chemmical Res Comm. 2009;23:31–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.