Abstract
Microbial biostimulants (MBs) promote plant growth and stress tolerance in a sustainable manner. However, precise field trials of MBs are required in natural setting with a range of crop varieties to harness the benefits of biostimulants on crop yield improvement. This study investigated the effects of two MBs, Trichoderma album and Bacillus megaterium, on an onion cultivar's growth, nutritional qualities, antioxidant properties, and yield potentials under field conditions for two successive years. Before transplantation, onion bulbs were gelatin-coated with 2.0 and 4.0 g L−1 of each of the MB. Results revealed that MBs-pretreated onion plants exhibited better growth indices, photosynthetic pigment contents, and yield-attributing features like bulb weight than control plants. Nutraceutical analysis demonstrated that T. album-pretreated (by 2.0 g L−1) onion cultivar enhanced the level of K+ (by 105.79%), Ca2+ (by 37.77%), proline (by 34.21%), and total free amino acids (by 144.58%) in bulb tissues over the control plants. Intriguingly, the pretreatment with both T. album and B. megaterium (by 2.0 g L−1) increased the levels of total soluble carbohydrates (by 19.10 and 84.02%), as well as antioxidant properties, including increased activities of superoxide dismutase (by 58.52 and 31.34%), catalase (by 164.71 and 232%), ascorbate peroxidase (by 175.35 and 212.69%), and glutathione-S-transferase (by 31.99 and 9.34%) and improved the contents of ascorbic acid (by 19.1 and 44.05%), glutathione (by 6.22 and 33.82%), and total flavonoids (by 171.98 and 56.24%, respectively) in the bulb tissues than control plants. Although both MBs promoted the growth and nutraceutical qualities of onion bulbs, T. album pretreatment showed better effects than that of B. megaterium in the field settings. Based on the morphophysiological attributes and biochemical properties, a low dose (2.0 g L−1) was more effective than a high dose (4.0 g L−1) of T. album in promoting onion growth. Overall, the current research findings imply that T. album might be a potential MB in improving growth and quality attributes, and hence the productivity of onion cultivars under field circumstances.
Keywords: Antioxidant properties, Bacillus megaterium, Microbial biostimulants, Nutrition, Onion, Trichoderma album, Yield
1. Introduction
The global agricultural production system has been challenged with multiple issues, such as high food demand for a growing population, escalation of poverty and malnutrition, and mismanagement of natural resources [1,2]. The unprecedented consequences of global climate change, the shrinking trend of cultivable areas, and the intensification of farming practices have further hindered sustainable food production worldwide [3]. Thus, an environment-friendly, sustainable farming approach is a demand of the current agricultural system for ensuring global food supply [3]. In this context, application of biostimulants is considered as an eco-friendly, efficient, and widely acceptable green technology to enhance plant productivity while maintaining ecological balance and safe foods for humans [4]. The biostimulants are increasingly being used in agricultural sectors, as indicated by their market value of US$ 3.2 billion in 2021, which is predicted to climb to US$ 5.6 billion by 2026 (https://www.marketsandmarkets.com/Market-Reports).
Biostimulants are a group of compounds that can increase plant nutrient usage efficacy, accelerate growth and productivity, promote biotic and abiotic stress acclimation, and change the rhizosphere in a beneficial way [[5], [6], [7], [8]]. Biostimulants can broadly be categorized into (i) microbial and (ii) non-microbial groups depending on their origin [9]. Among the microbial biostimulants (MBs), Trichoderma album and Bacillus megaterium are well-known plant growth-promoting rhizobacteria (PGPR) and plant growth-promoting fungi (PGPF), respectively. MBs are widely recognized for their effectiveness in small doses, and once they enter plant tissues, they can activate signaling networks, biosynthetic pathways, and hormonal interactions, all of which drive plant growth and development, as well as stress acclimation responses [10,11]. To improve crop growth and productivity, MBs play various positive roles associated with (i) solubilization, uptake, and translocation of macro- and micro-nutrients, (ii) primary and secondary metabolism, which induce phytochemical accumulation, (iii) development of robust root system for foraging surrounding soils to improve nutrient use efficiency, (iv) improvement of photosynthetic activity to promote growth, and (v) stimulation of antioxidant defense systems to reduce oxidative stress burden [5,[12], [13], [14], [15], [16]]. Despite having various positive functions, how biostimulants trigger key metabolic processes to regulate plant growth is yet to be elucidated in detail [12]. Most biostimulant-related research has concentrated on their positive effects in countering various biotic and abiotic challenges, whereas little attention has been paid to the growth-promoting impact of MBs on economically significant crops under field circumstances.
Onion (Allium cepa) is one of the world's oldest cultivated vegetables and the third most important horticultural spice with a high economic value [17,18]. In addition to its culinary use, onion has long been accepted by many aboriginal societies as a therapeutic resource to reduce the risk of cancer, diabetes, inflammation, and cardiovascular diseases [[18], [19], [20], [21]]. Egypt is the fourth largest onion producer globally and ranked sixth among the major onion exporter countries in terms of export (https://www.worldstopexports.com/onions-exports-by-country/; accessed on 6 February 2021). Onion is considered a prime cash crop in Egypt; hence the country emphasizes the improvement of the production and quality properties of onion while ensuring the maximum use of its limited land area. Given the biostimulating functions of T. album and B. megaterium, the current study aimed at checking how these MBs boost growth, nutritional quality, and yield attributes of onion in the field settings by evaluating various physiological and biochemical mechanisms underlying the growth-promoting effects of both MBs.
2. Materials and methods
2.1. Study location, weather, soil qualities, and plant materials
This study was conducted using a high-yielding onion cultivar Giza 6. The experimental field was rented from the research farm (longitude 31°11′21.42″E and latitude 27°10′48.48″N) of the Faculty of Agriculture, Al-Azhar University, Assiut, Egypt. For two years in a row (2016/2017 and 2017/2018), the field research was conducted from November through April. Temperatures in the research farm area varied from 10 to 28 °C (in 2016) to 11–27 °C (in 2017). Before the experiment was set, soil samples were taken from 0 to 30 cm depth of the experimental field, and various physio-chemical features of the soil were measured following the procedures of Webster [22] (Table S1).
2.2. Biostimulant formulations
This study used Bacillus megaterium (BM, 10 × 106 cell g−1) and Trichoderma album (TA, 10 × 106 spores g−1) as MBs. The tested compounds were obtained from the Botany Department, Al-Azhar University, Assiut, Egypt. Two doses of TA (TA1 and TA2) and two doses of BM (BM1 and BM2) (1.0 L for each case) were prepared by adding the required amount of corresponding spores equivalent to 2.0 and 4.0 g, respectively. Following that preparation, 2.0 g gelatin was added to each solution, followed by heating at 35 ± 2 °C with continuous stirring until uniformly mixed.
2.3. Preparation of field and experimental layout
Before sowing, seeds were pretreated with Vitavax (Bayer Crop Science, St. Louis, USA) (5.0 g kg−1) to prevent spoilage due to seed-borne diseases. Pretreated seeds were sown in a seedbed of 3 m in length, 1.2 m in width, and with a height between 10 and 15 cm to raise the seedlings. Before sowing, soils of the seedbeds were mixed with chlorpyriphos (Bayer Crop Science, St. Louis, USA) (2 mL L−1) to eradicate soil-borne insects and pests. Six times ploughing followed by 3-times laddering were done to the whole field to obtain proper tilth and labeled soil. In order to ensure adequate nitrogen supply, 40 kg ammonium sulfate (21%) ha−1 was applied at three stages, including 7 days prior to transplantation of the seedlings, 30 days after transplantation (DAT), and 90 DAT, during the whole experimental period. Thirty kg triple superphosphate (TSP) as a source of phosphorus (P), 80 kg potassium sulfate as a source of potassium (K), and 110 kg gypsum were added to each ha of the land as a basal fertilizer. To ensure the supply of essential nutrients, 25 tons of farmyard manure was spread over each hectare of the experimental land. To prepare 50-day-old onion bulbs for transplantation, a rigorous and precise coating process was employed that involved immersing the bulbs in a potent gelatin solution of B. megaterium and T. album at a concentration of 2.0 and 4.0 g per L solution for 30 min. The onion bulbs in the control group were subjected to a gelatin coating that was devoid of any beneficial biostimulants.
The whole experiment was divided into five treatments, including (i) biostimulant-free control plants (control), (ii) 2.0 g T. album L−1 pretreated plants (TA1), (iii) 4.0 g T. album L−1 pretreated plants (TA2), (iv) 2.0 g B. megaterium L−1 pretreated plants (BM1), and (v) 4.0 g B. megaterium L−1 pretreated plants (BM2). Seedlings were transplanted following randomized complete block design (RCBD) with three replications in 3.5 m × 3.0 m sized plots. The experimental plots were demarcated by narrow canals measuring 45 cm in width, and the individual plants were spaced at a distance of 10 cm from each other. To promote optimal bulb development and increase overall yield and quality, the onion fields were subjected to weekly light irrigation to ensure adequate moisture levels in the field. To promote desiccation of the outermost scale and increase the onion's shelf life, irrigation was discontinued 20 days prior to harvest. To prevent the growth and spread of weeds, regular weeding was carried out throughout the experimental period. Leaf and bulb samples were harvested at 90 DAT in the year 2016 to determine various biochemical attributes. Morphological features were recorded in both seasons (2016/2017 and 2017/2018).
2.4. Quantification of vegetative parameters and photosynthetic pigments
Plant height was measured using a measuring scale, while the number of leaves was determined by simple counting at 120 DAT. The levels of different photosynthetic pigments like chlorophylls (Chls) (Chl a, Chl b, and total Chls) and carotenoids in onion fresh leaves tissues under different treatments were measured following the spectrophotometric method [23]. Anthocyanin content was also quantified in fresh leaf samples of onions [24].
2.5. Quantification of different mineral nutrient contents
The onion bulb was sliced and kept in paper sacs prepared for drying purposes and then the slices were dried in an aerated oven (40 °C) until the bulbs reached a constant weight. Afterward, following the method of Williams and Twine [25], onion dried bulb samples were digested and the contents of K+, Ca2+, and Mg2+ were quantified. The SO42− level was quantified from the aqueous extract of dried onion bulbs using the turbidimetric system [26].
2.6. Measurement of hydrogen peroxide, superoxide, and malondialdehyde contents
Malondialdehyde (MDA) level in fresh bulb tissues was determined according to the method of Heath and Packer [27]. Hydrogen peroxide (H2O2) and superoxide (O2•−) contents were quantified from fresh onion bulb tissues according to published procedures [28,29], respectively.
2.7. Extraction and assessment of enzyme activities
The protocol reported by Dawood and Azooz [30] was followed to prepare enzyme extract from fresh onion bulb samples. The activity of superoxide dismutase (SOD) (EC: 1.15.1.1), ascorbate peroxidase (APX) (EC: 1.11.1.11), catalase, (CAT) (EC: 1.11.1.6), glutathione peroxidase (GPX) (EC: 1.11.1.9), and glutathione S-transferase (GST) (EC: 2.5.1.18) was estimated using the methods of Misra and Fridovich [31], Silva et al. [32], Noctor et al. [33], Flohé and Günzler [34], and Ghelfi et al. [35], respectively. The total antioxidant capacity in the freshly harvested onion bulbs was determined using a previously published protocol [36].
2.8. Determination of the contents of proline, water-soluble proteins, total free amino acids, and soluble carbohydrates
The levels of proline (Pro), water-soluble proteins, total free amino acids, and total soluble carbohydrates in the fresh bulbs of onion were estimated following the procedures of Zhang and Huang [37], Bradford [38], Lee and Takahashi [39], and Ci et al. [40], respectively.
2.9. Assessment of the levels of ascorbic acid, glutathione, pyruvic acid, phenolics, and flavonoids
Ascorbic acid (AsA), glutathione (GSH), pyruvic acid, total phenolics, and total flavonoids contents were measured from fresh onion bulbs according to the methods outlined by Jagota and Dani [41], Ellman [42], Randle and Bussard [43], Aery [44], and Zou et al. [45], respectively.
2.10. Yield and yield-attributing properties
After harvesting at 180 DAT, the onion bulb length, weight, and width were determined with the aid of a slide caliper. Fresh weight (FW) of the onions bulbs was taken using a digital balance. Finally, the total fresh production of onion bulbs was calculated and reported in tons per hectare. Yield-attributing properties were recorded in the year of 2016/2017 and 2017/2018.
2.11. Statistical analysis
One-way analysis of variance (ANOVA) of the collected data and mean comparison test by least significant difference (LSD) test at P < 0.05 were carried out using Statistix 10 software. Different alphabetical letters were assigned to symbolize the significant variations among the different treatments. Data were presented in the figures and tables as means ± standard errors (SEs) of three independent replicates for each treatment.
3. Results
3.1. Effect of T. album and B. megaterium on the vegetative parameters of onions
In 2016, the plant height was increased by 29.83 and 26.00% in TA1-and TA2-treated plants, whereas 25.83 and 27.83% increments were observed for BM1-and BM2-treated plants, respectively, as compared with the control plants (Table 1). On the other hand, the leaf number of plants treated with both MBs did not differ significantly relative to control plants. In 2017, an increase in plant height by 35.00 and 30.85% and leaf number by 30.00 and 19.17%, were found in TA1-and TA2-treated plants, respectively, in comparison with control plants (Table 1). The application of BM1 and BM2 to onion plants also increased plant height by 30.85 and 32.94% and leaf number by 33.75 and 18.33%, respectively, relative to control plants (Table 1).
Table 1.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on the growth and yield-related properties of Giza 6 cultivar after 120 days of transplantation.
| Treatments |
Plant height (cm) |
Leaf number |
Bulb length (cm) |
Bulb width (cm) |
Bulb weight (g) |
Total onion yield (t ha─1) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Control | 50.00 ± 2.32b | 49.17 ± 0.82b | 11.00 ± 0.14a | 10.00 ± 0.19b | 5.80 ± 0.38b | 5.83 ± 0.23c | 6.13 ± 0.03c | 6.60 ± 0.44b | 148.00 ± 15.50b | 181.00 ± 13.05b | 50.00 ± 7.57b | 48.08 ± 1.34c |
| TA1 | 64.92 ± 0.79a | 66.38 ± 2.55a | 12.67 ± 1.01a | 13.00 ± 0.29a | 7.37 ± 0.20a | 7.57 ± 0.26ab | 8.10 ± 0.36b | 8.33 ± 0.43a | 262.67 ± 20.33a | 264.00 ± 21.70a | 67.62 ± 5.24a | 67.60 ± 1.01a |
| TA2 | 63.00 ± 0.50a | 64.33 ± 0.36a | 11.42 ± 0.22a | 11.92 ± 0.46a | 7.23 ± 0.17a | 7.73 ± 0.12a | 8.97 ± 0.12a | 9.13 ± 0.12a | 244.67 ± 8.51a | 235.67 ± 11.47a | 62.86 ± 2.18ab | 63.53 ± 3.39ab |
| BM1 | 62.92 ± 1.37a | 64.33 ± 1.45a | 12.17 ± 0.46a | 13.38 ± 0.10a | 7.47 ± 0.13a | 7.13 ± 0.12b | 8.90 ± 0.12a | 8.83 ± 0.47a | 250.00 ± 16.17a | 241.00 ± 15.50a | 64.29 ± 4.13ab | 65.57 ± 1.04ab |
| BM2 | 63.92 ± 1.88a | 65.36 ± 1.66a | 11.50 ± 0.38a | 11.83 ± 0.35a | 7.47 ± 0.03a | 7.07 ± 0.15b | 8.80 ± 0.06a | 8.93 ± 0.19a | 237.00 ± 30.01a | 236.00 ± 5.51a | 60.95 ± 7.74ab | 61.40 ± 0.43b |
Data presented are means with standard errors (n = 3 individual replicates). A least significant difference test was used to compare the treatments, and statistically significant differences among them were indicated by different alphabetic letters in the column (P < 0.05). BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively; ha, hectare.
3.2. Effect of T. album and B. megaterium on photosynthetic pigments of onion leaves
In comparison with control plants, TA1 plants displayed significantly higher levels of Chl a (by 17%), Chl b (by 137.34%), and total Chls (by 34.34%), as well as lower levels of carotenoids (by 8.34%) (Fig. 1a–d). TA2 plants had significantly lower amount of Chl a (by 4.90%) and a higher amount of Chl b (by 15.95%) than that of control plants, while the contents of total Chls and carotenoids remained comparable between TA2 and control plants (Fig. 1a–d). A significant improvement in the contents of Chl b (by 47.97%), total Chls (by 3.36%), and carotenoids (by 8.71%), and a decrease in the level of Chl a (by 4.38%) were observed in BM1 plants when contrasted with control plants (Fig. 1a–d). Compared with the control plants, BM2 plants exhibited significantly increased level of carotenoids (by 13.50%) and decreased level of Chl a (by 4.21%); however, Chl b and total Chl levels remained comparable between BM2 and control plants (Fig. 1a–d).
Fig. 1.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on photosynthetic pigments in the leaves of Giza 6. (a) Chlorophyll (Chl) a, (b) Chl b, (c) total Chls, and (d) carotenoids contents were determined after 90 days of transplantation. Data presented are means with standard errors (n = 3 individual replicates). Statistically significant differences among the treatments were indicated by different alphabetical letters on the bars, as determined by a least significant difference test (P < 0.05). BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively; FW, fresh weight.
3.3. Effect of T. album and B. megaterium on the ROS levels and antioxidant enzyme activities in onion bulbs
In comparison with the corresponding values of control plants, a significant reduction in the contents of O2•− (by 33.93 and 32.74%), H2O2 (by 55.55 and 41.74%), and MDA (by 21.84 and 21.45%) were found in TA1 and TA2 plants, respectively (Fig. 2a–c). Similarly, BM1 and BM2 plants displayed significantly lower levels of O2•− (by 24.21% only in BM1 plants), H2O2 (by 27.40 and 16.20%), and MDA (by 31.38 and 33.52%, respectively) when equated with those of control plants (Fig. 2a–c). In comparison with control plants, a significant improvement in the activities of SOD (by 58.52%, only in TA1 plants), CAT (by 164.71 and 85.51%), APX (by 175.35 and 81.08%), GPX (by 139.24 and 94.85%), and GST (by31.99 and 16.72%) were observed in TA1 and TA2 plants, respectively; and the SOD activity between TA2 and control plants remained comparable (Fig. 2d–h). Likewise, a significant enhancement in the activity of SOD (by 31.34%, only in BM1 plants), CAT (by 232 and 28.99%), APX (by 212.69 and 45.64%), and GST (by 9.34 and 5.22%) in BM1 and BM2 plants, respectively, relative to control plants; nonetheless, GPX activity in control plants did not differ significantly with that of BM1 and BM2 plants (Fig. 2d–h).
Fig. 2.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on the accumulation of reactive oxygen species and, malondialdehyde (MDA), and antioxidant enzyme activities in the bulbs of Giza 6. (a) The contents of (a) superoxide (O2•−), (b) hydrogen peroxide (H2O2), and (c) MDA, and the activities of (d) superoxide dismutase (SOD), (e) catalase (CAT), (f) ascorbate peroxidase (APX), (g) glutathione peroxidase (GPX), and (h) glutathione S-transferase (GST) were determined after 90 days of transplantation. Data presented are means with standard errors (n = 3 individual replicates). Statistically significant differences among the treatments were indicated by different alphabetical letters on the bars, as determined by a least significant difference test (P < 0.05). BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively; FW, fresh weight.
3.4. Effect of T. album and B. megaterium on onions’ bulb non-enzymatic antioxidant properties and pyruvic acid content
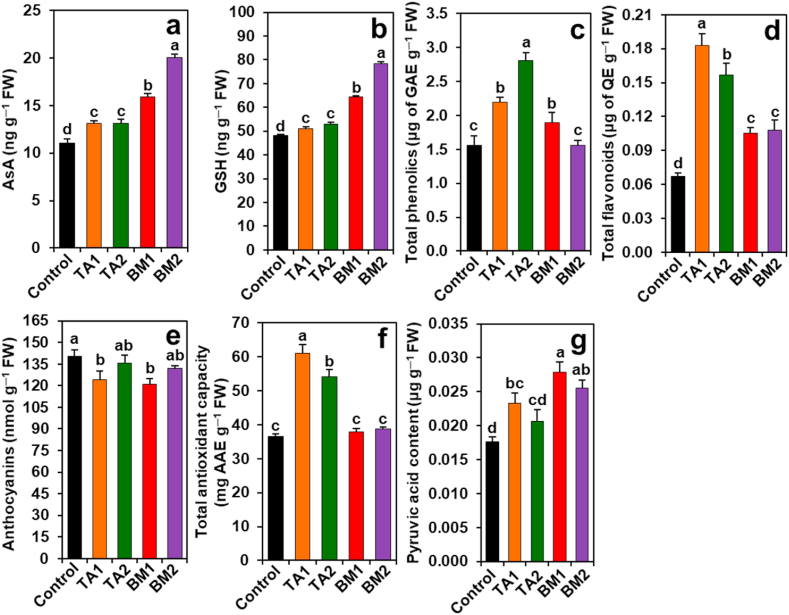
Compared with the corresponding values of control plants, TA1 and TA2 plants exhibited significant enhancement in the contents of AsA (by 19.1 and 18.74%), GSH (by 6.22 and 9.80%), total phenolics (by 40.43 and 80.20%), total flavonoids (by 171.98 and 133.37%), pyruvic acid (by 32.30%, only in TA1 plants), and total antioxidant capacity (by 66.84 and 48.05%, respectively); but a significant decrease in anthocyanin content by 11.53% in TA1 plants (Fig. 3a–g). Similarly, a significant increase in the levels of AsA (by 44.05 and 81.53%), GSH (by 33.82 and 63.01%), total phenolics (by 21.69%, only in BM1 plants), total flavonoids (by 56.24 and 60.08%), pyruvic acid (by 58.33 and 54.62%), and decrease in the content of anthocyanin (by 13.81% only in BM1 plants) were also observed in BM1 and BM2 plants, respectively, when equated with the corresponding values of control plants. The total antioxidant capacity of control plants remained comparable with BM1 and BM2 plants (Fig. 3a–g).
Fig. 3.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on non-enzymatic antioxidant properties and pyruvic acid in the bulbs of Giza 6. The levels of (a) ascorbic acid (AsA), (b) glutathione (GSH), (c) total phenolics, (d) total flavonoids, (e) anthocyanin, (f) total antioxidant capacity, and (g) pyruvic acid were determined after 90 days of transplantation. Statistically significant differences among the treatments were indicated by different alphabetical letters on the bars, as determined by a least significant difference test (P < 0.05). BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively; AAE, l-ascorbic acid equivalent; FW, fresh weight; GAE, gallic acid equivalent; QE, quercetin equivalent.
3.5. Effect of T. album and B. megaterium on the mineral contents of onion bulbs
Significantly higher levels of K+ (by 105.79 and 76.85%) and Ca2+ (by 37.77 and 26.73%) were recorded in TA1 and TA2 plants, respectively, when compared with control plants (Fig. 4a and b). Likewise, in BM1 and BM2 plants, K+ contents increased significantly by 35.05 and 67.20%, respectively, relative to control plants (Fig. 4a). No significant variation was recorded for Ca2+ levels between the control and BM1 and BM2 plants (Fig. 4b). TA1, TA2, and BM1 plants displayed a significantly reduced level of Mg2+ (by 15.38, 11.54, and 11.53%, respectively) compared with the control plants; but the Mg2+ level remained comparable between control and BM2 plants (Fig. 4c). Similarly, relative to control plants, significantly lower levels of SO42− (by 19.68 and 33.97%) was found in TA1 and TA2 plants, respectively; however, SO42− level in control plants did not differ significantly with BM1 and BM2 plants (Fig. 4d).
Fig. 4.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on different mineral levels in the bulbs of Giza 6. (a) Potassium (K+), (b) calcium (Ca2+), (c) magnesium (Mg2+), and (d) sulfate (SO42−) contents were quantified after 90 days of transplantation. Data presented are means and standard errors (n = 3 individual replicates). Statistically significant differences among the treatments were indicated by different alphabetical letters on the bars, as determined by a least significant difference test (P < 0.05). BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively; DW, dry weight.
3.6. Effect of T. album and B. megaterium on the levels of osmoprotectants in onion bulbs
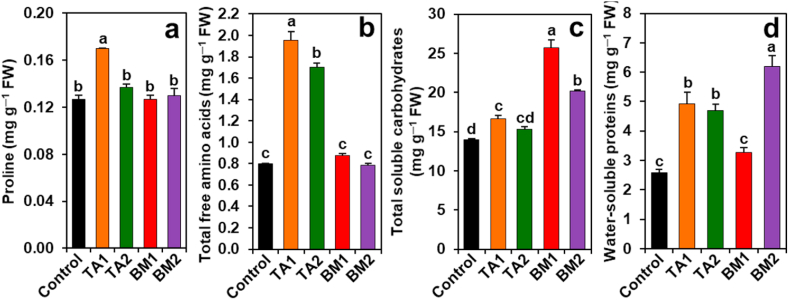
In comparison with control plants, TA1 and TA2 plants displayed significantly increased levels of total free amino acids (by 144.58and 112.92%) and water-soluble proteins (by 90.46 and 81.70%, respectively) (Fig. 5b, d). TA1 plants showed a significant enhancement in the contents of Pro and total soluble carbohydrates by 34.21 and 19.10%, respectively, than that of control plants, but the levels of Pro and total soluble carbohydrates did not alter significantly between control and TA2 plants (Fig. 5a, c). The contents of total soluble carbohydrates (by 84.02 and 44.05%) and water-soluble proteins (by 139.56%, only in BM2 plants) were significantly increased in BM1 and BM2 plants, respectively, relative to the corresponding values of control plants (Fig. 5c and d). However, compared with the control plants, no significant variations were detected for Pro and total free amino acids in BM1 and BM2 plants (Fig. 5a and b).
Fig. 5.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on osmoprotectant levels in the bulbs of Giza 6. The levels of (a) proline, (b) total free amino acids, (c) total soluble carbohydrates, and (d) water-soluble proteins were determined after 90 days of transplantation. Statistically significant differences among the treatments were indicated by different alphabetical letters on the bars, as determined by a least significant difference test (P < 0.05). BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively; FW, fresh weight.
3.7. Effect of T. album and B. megaterium on yield and yield-associated attributes of onions
In 2016, a significant increase in bulb length (by 27.01 and 24.72%), bulb width (by 32.07 and 46.20%), bulb weight (by 77.48 and 65.32%), and total onion yield (by 35.24 and 25.71%) were recorded in TA1 and TA2 plants, respectively, as compared with the values observed in control plants (Table 1). BM1 and BM2 plants also exhibited significant increases in bulb length (by 28.74 and 28.74%), bulb width (by 45.11 and 43.48%), bulb weight (by 68.92 and 60.14%), and total onion yield (by 28.57 and 21.91%, respectively) than that of control plants (Table 1).
In 2017, a significant increase in bulb length (by 29.71 and 32.51%), bulb width (by 26.26 and 38.38%), bulb weight (by 45.86 and 30.20%), and total onion yield (by 40.61 and 32.15%) were recorded in TA1 and TA2 plants, respectively, in comparison with control plants (Table 1). Relative to control plants, BM1 and BM2 plants also displayed a significant increase in bulb length (by 22.29 and 21.14%), bulb width (by 33.84 and 35.35%), bulb weight (by 33.15 and 30.39%), and total onion yield (by 36.38 and 27.71%, respectively) (Table 1).
4. Discussion
Application of MBs has been recognized as an excellent technique to boost the growth and yield potential of many plants, including onions [[46], [47], [48]]. The results of this study demonstrate that pretreating onion bulbs with T. album and B. megaterium led to significant improvements in several plant growth parameters, including plant height and leaf number (Table 1), which were accompanied by high levels of photosynthetic pigments (Fig. 1a–d). In the current study, TA1 plants displayed significantly higher amounts of Chl a, Chl b, and total Chls compared with control plants (Fig. 1a–c), implying that Trichoderma at low concentration (2 g L−1) boosted photosynthetic pigment levels to maintain better growth of onion under field conditions. Similarly, BM1 plants exhibited significantly higher levels of Chl b, total Chls, and carotenoids (Fig. 1b–d), which also indicates lower concentrations (2 g L−1) of B. megaterium has a more prominent role in improving the plant photosynthetic pigment levels. Photosynthetic pigments, including Chls and carotenoids are the integral components of photosynthetic machinery that drive plant's photosynthetic activity and consequently the accumulation of plant biomass [49]. Carotenoids not only enhance the nutraceutical properties of food [50], but they also play a crucial role in preventing various chronic human illness, including cancer, skin disease, cardiovascular disease, eye disease, and some neurological disorders [51].
Plants growing in the field conditions often suffer from various climate change-induced abnormalities, which can lead to an accumulation of reactive oxygen species (ROS). The present study suggested that plants that were not treated with biostimulants significantly increased the amount of ROS products, such as O2•− and H2O2, as well as the MDA level in their bulb tissues, which might indicate that plants encounter to some extent, oxidative stress under field circumstances (Fig. 2a–c). By contrast, onion plants pretreated with MBs (T. album and B. megaterium) effectively reduced the accumulations of O2•−, H2O2, and MDA in their bulbs, implying that MBs protect the onion plants from oxidative burden in field environments (Fig. 2a–c). The detoxification of ROS is intricately associated with a vibrant antioxidant defense system [52]. In the current study, a positive correlation between the inductions of the antioxidant defense system with the decreased level of ROS in MBs-treated field-grown onion bulbs were observed. MBs-treated onion bulbs maintained an increased activity of CAT, APX, and SOD, which presumably contributed to the detoxification of O2•− and H2O2 (Fig. 2a, b, d–f). The increased activity of GPX and GST, as well as the contents of AsA and GSH in the bulb tissues of MBs-pretreated onion plants, also confirmed the H2O2 removal process involving the ascorbate-glutathione dependent mechanism (Fig. 2, Fig. 3a, b).
Non-enzymatic antioxidants, such as total phenolics and total flavonoids, also increased upon MBs pretreatment to field-grown onion bulbs (Fig. 3c and d). Phenolics and flavonoids are well-known for protecting cell membrane integrity from oxidative damage by quenching ROS in the face of various climate change-induced anomalies [53,54]. These findings suggested that, pretreatment with MBs helped onion bulbs to maintain a better status of phenolics and flavonoids in conferring protection against unanticipated climate change-induced oxidative damage. Apart from ROS detoxification, improvement in the levels of antioxidants also heightened the nutraceutical properties of onion that play a fundamental role in human health. For example, GSH and AsA are essential in the synthesis of neurotransmitters and collagen and the prevention of Parkinson's and liver illnesses [54,55]. Phenolics and flavonoids provide numerous health benefits, including immuno-regulatory, anti-atherogenic, anti-thrombotic, anti-inflammatory, anti-allergic, cardioprotective antioxidants, as well as anti-cancer and anti-diabetic activities [56,57]. Collectively, MBs pretreatment increased both enzymatic and non-enzymatic components of the antioxidant defense system, triggering efficient ROS detoxification and reducing cellular damage, resulting in improved onion bulb growth in field circumstances.
The current study observed that onion plants pretreated with MBs, particularly with T. album, increased the content of K+ and Ca2+ in their bulb tissues, compared with MBs-untreated plants (Fig. 4a and b). Trichoderma spp. are well-recognized as a potent natural decomposing agent, which might help in improving nutrient availability in soils [58]. Trichoderma pretreatment significantly reduced the content of SO42− (Fig. 4d), which plausibly suggested the role of Trichoderma in the synthesis of different, sulfur-rich compounds such as GSH (Fig. 3b) in field circumstances to preserve and improve the quality of onion cultivar [16].
In the current study, TA1 plants exhibited significantly higher levels of Pro than control plants, implying that under field conditions, a lower dosage of T. album contributed to improved osmotic adjustment and helped to conserve the better water status of the onion bulbs (Fig. 5a). Compared with control plants, free amino acid levels in the bulb tissues were significantly increased in TA1 and TA2 plants (Fig. 5b). Free amino acids are well-recognized as valuable metabolites transferred from senescence leaves to onion bulb tissues during the maturation process [59]. This suggests that the increased leaf number in T. album-pretreated onion plants might play a role in transporting amino acids to onion bulbs during the process of maturation [16, 60]; [Table 1]. Carbohydrate content is one of the most important ingredients of onion bulb quality, as about 80% of onion bulb dry weight is composed of carbohydrates [61]. Soluble carbohydrate content was notably increased in both T. album and B. megaterium treated onion plants compared with the control plants (Fig. 5c). MBs pretreatment increased the source-to-sink transfer of sugar molecules at the time of onion bulb swelling, resulting in increased carbohydrate levels in the bulb tissues [16,62]. Consistent with findings of the current work, Mei et al. [63] and Pandey et al. [64] also reported Trichoderma spp. and Bacillus spp. increased the content of total carbohydrates in pretreated Cucumis sativus and Amaranthus hypochondriacus plants.
Increased level of water-soluble protein in the bulb tissues of T. album (both TA1 and TA2 plants) and B. megaterium (only in BM2 plants)-pretreated onion plants also indicated amino acid metabolism and nitrogen assimilation (Fig. 5d). The onion bulb, which has a strong pungent flavor, has recently attracted the attention of manufacturers for use in sauces, canned soups, and extracts, as well as solid in the form of onion powder and onion salt [65]. Pyruvic acid content has been widely accepted to quantify the pungency index in onions [66]. The findings of the study demonstrated that MBs pretreatment considerably enhanced pyruvic acid contents in TA1, BM1, and BM2 plants in comparison with that in control plants (Fig. 3g). This suggests that onion plants pretreated with MBs could be an effective strategy for increasing the pungency of onion in order to improve their acceptability to food manufacturers.
As a reflection of the overall up-regulations of various growth-related parameters induced by T. album and B. megaterium, the yield attributes were significantly enhanced in onion plants, especially in T. album-pretreated plants. Thus, it could be postulated that the photo-assimilates formed in the arial part translocate from source to sink during the onion bulb's maturation period, resulting in increased length, diameter, and weight of onion bulbs (Fig. 6) [67]. Overall, the results showed that pretreatment with both MBs had a substantial influence on increasing onion growth in field conditions. Importantly, T. album at low concentration (2 g L−1) showed better potential in promoting onion growth and yield performance by increasing photosynthetic pigment levels in leaves, as well as mineral contents, antioxidant properties, and nutritional values of onion bulbs. The current study inferred that unknown climate change-related factors may reduce onion quality and production in natural settings, which must be addressed to meet consumer demand for onions. Thus, it can be proposed that, T. album and B. megaterium may be used as potential MBs to increase onion growth and yield in an environmentally sustainable manner to keep up with the rising onion supply for large populations.
Fig. 6.
Effects of Trichoderma album (TA) and Bacillus megaterium (BM) on bulb growth of Giza 6 onion cultivar. The photographs were captured after 90 days of transplantation in the year of 2017. BM1 and BM2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 BM-coated bulbs, respectively; TA1 and TA2, onion plants emerged from 2.0 g L−1 and 4.0 g L−1 TA-coated bulbs, respectively.
Author contribution statement
Nabil A. Younes: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Touhidur Rahman Anik: Analyzed and interpreted the data; Wrote the paper.
Md. Mezanur Rahman and Mohammad Golam Mostofa: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ahmed A. Wardany and Mona F.A. Dawood: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Lam-Son Phan Tran: Conceived and designed the experiments; Wrote the paper.
A.A.H. Abdel Latef: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14203.
Contributor Information
Lam-Son Phan Tran, Email: son.tran@ttu.edu.
A.A.H. Abdel Latef, Email: moawad76@gmail.com.
Mohammad Golam Mostofa, Email: mostofam@msu.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rouphael Y., Colla G. Editorial: biostimulants in agriculture. Front. Plant Sci. 2020;11:40. doi: 10.3389/fpls.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia S.N., Osburn B.I., Jay-Russell M.T. One health for food safety, food security, and sustainable food production. Front. Sustain. Food Syst. 2020;4:1. [Google Scholar]
- 3.Mishenin Y., Yarova I., Koblianska I. In: Ecological Intensification of Natural Resources for Sustainable Agriculture. Jhariya M.K., et al., editors. Springer; Singapore: 2021. Ecologically harmonized agricultural management for global food security; pp. 29–76. [Google Scholar]
- 4.Rouphael Y., Spíchal L., Panzarová K., Casa R., Colla G. High-throughput plant phenotyping for developing novel biostimulants: from lab to field or from field to lab? Front. Plant Sci. 2018;9:1197. doi: 10.3389/fpls.2018.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouphael Y., Colla G. Toward a sustainable agriculture through plant biostimulants: from experimental data to practical applications. Agronomy. 2020;10:1461. [Google Scholar]
- 6.Zargar Shooshtari F., Souri M.K., Hasandokht M.R., Jari S.K. Glycine mitigates fertilizer requirements of agricultural crops: case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020;7(1):1–10. [Google Scholar]
- 7.Noroozlo Y.A., Souri M.K., Delshad M. Effects of foliar application of glycine and glutamine amino acids on growth and quality of sweet basil. Adv. Hortic. Sci. 2019;33(4) [Google Scholar]
- 8.Souri M.K., Bakhtiarizade M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019;243:472–476. [Google Scholar]
- 9.Colla G., Rouphael Y. Biostimulants in horticulture. Sci. Hortic. 2015;196:1–34. [Google Scholar]
- 10.Couto C.A., Peixoto C.P., Vieira E.L., Carvalho E.V., Peixoto V.A.B. Action of cinetina, butyric acid and gibberellic acid on the emergency of sunflower under aluminum stress. Comun. Sci. 2012;3:206–210. [Google Scholar]
- 11.Malik A., Mor V.S., Tokas J., Punia H., Malik S., Malik K., Sangwan S., Tomar S., Singh P., Singh N., Singh G. Biostimulant-treated seedlings under sustainable agriculture: a global perspective facing climate change. Agronomy. 2021;11:14. [Google Scholar]
- 12.Rouphael Y., Franken P., Schneider C., Schwarz D., Giovannetti M., Agnolucci M., De Pascale S., Bonini P., Colla G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015;196:91–108. [Google Scholar]
- 13.Elansary H.O., Mahmoud E.A., El-Ansary D.O., Mattar M.A. Effects of water stress and modern biostimulants on growth and quality characteristics of mint. Agronomy. 2019;10:6. [Google Scholar]
- 14.Francesca S., Arena C., Hay Mele B., Schettini C., Ambrosino P., Barone A., Rigano M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy. 2020;3:363. [Google Scholar]
- 15.Petropoulos S.A., Fernandes Â., Plexida S., Chrysargyris A., Tzortzakis N., Barreira J.C., Barros L., Ferreira I.C. Biostimulants application alleviates water stress effects on yield and chemical composition of greenhouse green bean (Phaseolus vulgaris L.) Agronomy. 2020;10:181. [Google Scholar]
- 16.Younes N.A., Rahman M., Wardany A.A., Dawood M.F., Mostofa M.G., Keya S.S., Abdel Latef A.A.H., Tran L.S.P. Antioxidants and bioactive compounds in licorice root extract potentially contribute to improving growth, bulb quality and yield of onion (Allium cepa) Molecules. 2021;26:2633. doi: 10.3390/molecules26092633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidhu J.S., Ali M., Al-Rashdan A., Ahmed N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019;56:1811–1819. doi: 10.1007/s13197-019-03625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teshika J.D., Zakariyyah A.M., Zaynab T., Zengin G., Rengasamy K.R., Pandian S.K., Fawzi M.M. Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. Crit. Rev. Food Sci. Nutr. 2019;59(sup1):S39–S70. doi: 10.1080/10408398.2018.1499074. [DOI] [PubMed] [Google Scholar]
- 19.Asemani Y., Zamani N., Bayat M., Amirghofran Z. Allium vegetables for possible future of cancer treatment. Phyther. Res. 2019;33:3019–3039. doi: 10.1002/ptr.6490. [DOI] [PubMed] [Google Scholar]
- 20.Janabi A.H.W., Kamboh A.A., Saeed M., Xiaoyu L., BiBi J., Majeed F., Naveed M., Mughal M.J., Korejo N.A., Kamboh R., Alagawany M. Flavonoid-rich foods (FRF): a promising nutraceutical approach against lifespan-shortening diseases. Iran J. Basic Med. Sci. 2020;23:140. doi: 10.22038/IJBMS.2019.35125.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kothari D., Lee W., Do Kim S.K. Allium flavonols: health benefits, molecular targets, and bioavailability. Antioxidants. 2020;9:888. doi: 10.3390/antiox9090888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster R. Soil sampling and methods of analysis. Eur. J. Soil Sci. 2008;59:1010–1011. [Google Scholar]
- 23.Lichtenthaler H.K., Wellburn A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;11:591–592. [Google Scholar]
- 24.Krizek D.T., Kramer G.F., Upadhyaya A., Mirecki R.M. UV‐B response of cucumber seedlings grown under metal halide and high-pressure sodium/deluxe lamps. Physiol. Plantarum. 1993;88:350–358. [Google Scholar]
- 25.Williams V., Twine S. In: Modern Methods of Plant Analysis. Peach K., Tracey M.V., editors. Springer; Berlin, Germany: 1960. Flame photometric method for sodium, potassium and calcium; pp. 3–5. [Google Scholar]
- 26.Bardsley C.E., Lancaster J.D. Methods of Soil Analysis. John Wiley & Sons, Ltd; 1965. Sulfur; pp. 1102–1116. [Google Scholar]
- 27.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 28.Yu C.W., Murphy T.M., Lin C.H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003;30:955–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- 29.Yang H., Wu F., Cheng J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011;127:1237–1242. doi: 10.1016/j.foodchem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Dawood M.F.A., Azooz M.M. Concentration-dependent effects of tungstate on germination, growth, lignification-related enzymes, antioxidants, and reactive oxygen species in broccoli (Brassica oleracea var. italica L.) Environ. Sci. Pollut. Res. 2019;26:36441–36457. doi: 10.1007/s11356-019-06603-y. [DOI] [PubMed] [Google Scholar]
- 31.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 32.Silva E.N., Silveira J.A., Aragão R.M., Vieira C.F., Carvalho F.E. Photosynthesis impairment and oxidative stress in Jatropha curcas exposed to drought are partially dependent on decreased catalase activity. Acta Physiol. Plant. 2019;41:1–12. [Google Scholar]
- 33.Noctor G., Mhamdi A., Foyer C.H. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ. 2016;39:1140–1160. doi: 10.1111/pce.12726. [DOI] [PubMed] [Google Scholar]
- 34.Flohé L., Günzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 35.Ghelfi A., Gaziola S.A., Cia M.C., Chabregas S.M., Falco M.C., Kuser-Falcão P.R., Azevedo R.A. Cloning, expression, molecular modelling and docking analysis of glutathione transferase from Saccharum officinarum. Ann. Appl. Biol. 2011;159:267–280. [Google Scholar]
- 36.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z., Huang R. Analysis of malondialdehyde, chlorophyll proline, soluble sugar, and glutathione content in Arabidopsis seedling. Bio-protocol. 2013;3:e817. [Google Scholar]
- 38.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y.P., Takahashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966;14:71–77. [Google Scholar]
- 40.Ci D., Jiang D., Dai T., Jing Q., Cao W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere. 2009;77:1620–1625. doi: 10.1016/j.chemosphere.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 41.Jagota S.K., Dani H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982;127:178–182. doi: 10.1016/0003-2697(82)90162-2. [DOI] [PubMed] [Google Scholar]
- 42.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 43.Randle W.M., Bussard M.L. Streamlining onion pungency analyses. Hortic. Sci. (HORTSCI) 2019;28:60. [Google Scholar]
- 44.Aery N.C. CRC Press; Boca Raton, FL, USA: 2010. Manual of Environmental Analysis. [Google Scholar]
- 45.Zou Y., Lu Y., Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004;52:5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]
- 46.Mógor A.F., Amatussi J.O., Mógor G., Gemin L.G. Biostimulant action of Lithothamnium sp. promoting growth, yield, and biochemical and chemical changes on onion. J. Appl. Phycol. 2021;33:1905–1913. [Google Scholar]
- 47.Amiri Forotaghe Z., Souri M.K., Ghanbari Jahromi M., Mohammadi Torkashvand A. Influence of humic acid application on onion growth characteristics under water deficit conditions. J. Plant Nutr. 2022;45(7):1030–1040. [Google Scholar]
- 48.Forotaghe Z.A., Souri M.K., Jahromi M.G., Torkashvand A.M. Physiological and biochemical responses of onion plants to deficit irrigation and humic acid application. Open Agric. 2021;6(1):728–737. [Google Scholar]
- 49.Sharma A., Kumar V., Shahzad B., Ramakrishnan M., Singh Sidhu G.P., Bali A.S., Handa N., Kapoor D., Yadav P., Khanna K., Bakshi P. Photosynthetic response of plants under different abiotic stresses: a review. J. Plant Growth Regul. 2020;39:509–531. [Google Scholar]
- 50.Honda M. Pigments from Microalgae Handbook. 2020. Nutraceutical and pharmaceutical applications of carotenoids; pp. 449–469. [Google Scholar]
- 51.Manfred E., Adrian W. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Rezayian M., Niknam V., Ebrahimzadeh H. Oxidative damage and antioxidative system in algae. Toxicol Rep. 2019;6:1309–1313. doi: 10.1016/j.toxrep.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amist N., Bano C., Singh N.B. Molecular Plant Abiotic Stress. John Wiley & Sons, Ltd; 2019. Antioxidative machinery for redox homeostasis during abiotic stress; pp. 65–90. [Google Scholar]
- 54.Ali S.S., Ahsan H., Zia M.K., Siddiqui T., Khan F.H. Understanding oxidants and antioxidants: classical team with new players. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- 55.Fang T., Zhen Q., Liao L., Owiti A., Zhao L., Korban S.S., Han Y. Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem. 2017;225:132–137. doi: 10.1016/j.foodchem.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Vizcaino F., Fraga C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018;646:107–112. doi: 10.1016/j.abb.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Siddiquee S., Shafawati S.N., Naher L. Effective composting of empty fruit bunches using potential Trichoderma strains. Biotechnol. Rep. 2017;13:1–7. doi: 10.1016/j.btre.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu P., Weng R., Xu Y., Feng Y., He L., Qian Y., Qiu J. Metabolic changes in different tissues of garlic plant during growth. J. Agric. Food Chem. 2020;68:12467–12475. doi: 10.1021/acs.jafc.0c04178. [DOI] [PubMed] [Google Scholar]
- 60.de Bang T.C., Husted S., Laursen K.H., Persson D.P., Schjoerring J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021;229:2446–2469. doi: 10.1111/nph.17074. [DOI] [PubMed] [Google Scholar]
- 61.Krähmer A., Böttcher C., Gudi G., Stürtz M., Schulz H. Application of ATR-FTIR spectroscopy for profiling of non-structural carbohydrates in onion (Allium cepa L.) bulbs. Food Chem. 2021;360 doi: 10.1016/j.foodchem.2021.129978. [DOI] [PubMed] [Google Scholar]
- 62.Abeed A.H., Ali M., Ali E.F., Majrashi A., Eissa M.A. Induction of Catharanthus roseus secondary metabolites when Calotropis procera was used as Bio-stimulant. Plants. 2021;10:1623. doi: 10.3390/plants10081623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mei L.I., Hua L.I.A.N., Su X.L., Ying T.I.A.N., Huang W.K., Jie M.E.I., Jiang X.L. The effects of Trichoderma on preventing cucumber fusarium wilt and regulating cucumber physiology. J. Integr. Agric. 2019;18:607–617. [Google Scholar]
- 64.Pandey C., Bajpai V.K., Negi Y.K., Rather I.A., Maheshwari D.K. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi J. Biol. Sci. 2018;25:1066–1071. doi: 10.1016/j.sjbs.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galmarini C.R., Goldman I.L., Havey M.J. Genetic analyses of correlated solids, flavor, and health-enhancing traits in onion (Allium cepa L.) Mol. Gen. Genet. 2001;265:543–551. doi: 10.1007/s004380100445. [DOI] [PubMed] [Google Scholar]
- 66.Clark C.J., Shaw M.L., Wright K.M., McCallum J.A. Quantification of free sugars, fructan, pungency and sweetness indices in onion populations by FT-MIR spectroscopy. J. Sci. Food Agric. 2018;98:5525–5533. doi: 10.1002/jsfa.9099. [DOI] [PubMed] [Google Scholar]
- 67.Majumder S., Yadav P. Variation in concentration of Shallot virus X in shallot plants at different developmental stages and its implication in diagnosis. Arch. Phytopathol. Plant Protect. 2021;54:2130–2140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.