Introduction
Lichen spinulosus (LS) is a follicular keratotic disorder and a variant of keratosis pilaris. LS usually shows a localized distribution, but a rare, generalized variant exists in the setting of chronic diseases such as HIV and Crohn’s disease.1,2
Follicular mucinosis (FM) can occur in a primary idiopathic form, or as a secondary phenomenon due to inflammatory skin conditions such as eczema or lymphoma-associated follicular mucinosis (LAFM).3 In this case report, we describe a healthy young adult who presented with generalized LS and associated incidental, secondary FM.
Case report
A 21-year-old female was referred with xerosis, generalized spiny skin lesions, and a provisional diagnosis of eczema that did not improve on a medium-strength topical corticosteroid. She did not report other medical ailments, medication, atopy, or allergies, and a personal or family history of similar skin lesions was absent.
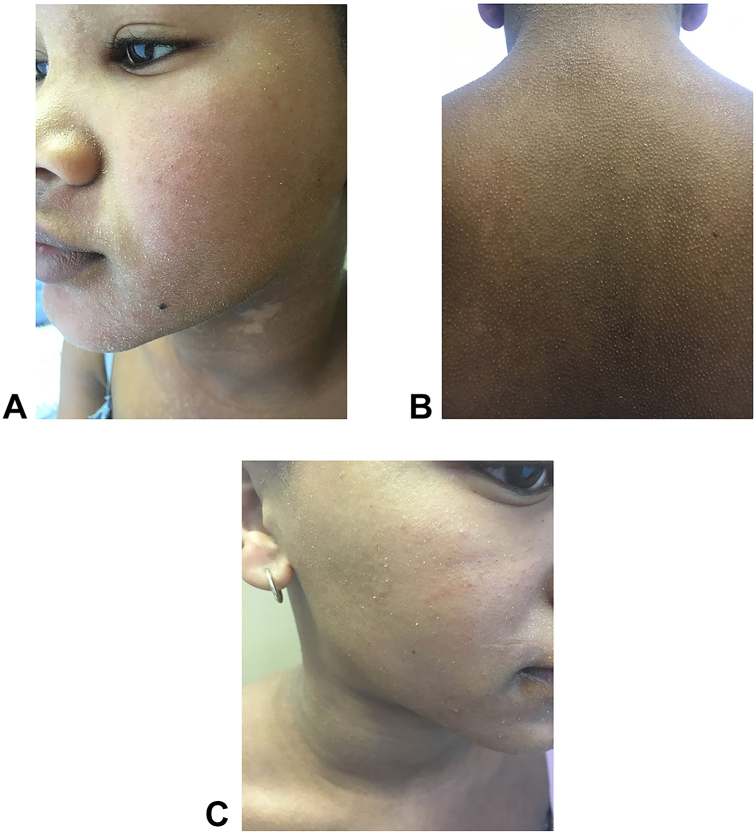
Clinical examination revealed multiple minute digitate hyperkeratosis which were folliculocentric. The distribution symmetrically involved the face, neck, trunk, and upper limbs (Fig 1, A and B). Perilesional erythema, palmoplantar keratoderma, or signs of a nutritional deficiency were absent.
Fig 1.
A, Multiple minute digitate hyperkeratosis, folliculocentric, of the left cheek and nose. Note: Dennie-Morgan lines and postinflammatory hypopigmentation on the neck. B, Multiple minute digitate hyperkeratosis, folliculocentric, symmetrically distributed, on the back. C, Drastically reduced lesions on the right heek following treatment with a systemic retinoid.
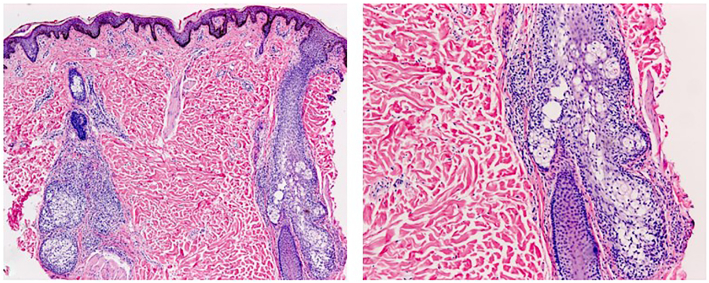
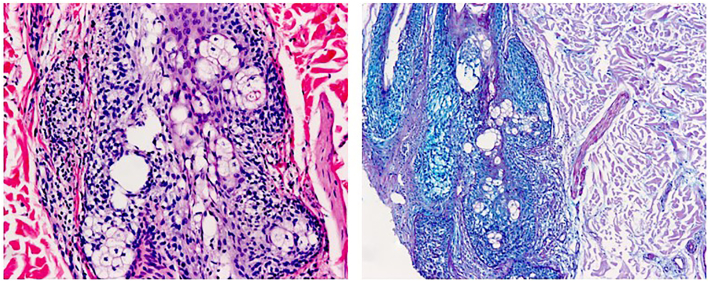
Histopathological assessment of hematoxylin and eosin stained sections from a 3-mm punch biopsy obtained from her back showed keratin plugs within hair follicle infundibula, sparse perifollicular lymphocytes, and cystic spaces in the outer root sheath of hair follicles and sebaceous glands (Fig 2). An Alcian blue-periodic acid–Schiff stain confirmed mucin within the spaces (Fig 3). Immunohistochemistry revealed cluster of differentiation (CD)3- and predominantly CD4-positive small T lymphocytes. CD8 positive T lymphocytes comprised a minority of the cells, while CD20 positive small B lymphocytes and CD30 positive cells were inconspicuous. T-cell receptor gene rearrangement testing failed for technical reasons and was not repeated.
Fig 2.
Two folliculo-sebaceous structures are depicted, surrounded by lymphocytes. Lymphocytes are also present within the follicular epithelium. Note tiny spaces within the epithelium. (Hematoxylin and eosin stain ×40 and ×100)
Fig 3.
Mucin demonstrated within the spaces and within the follicular keratinocytes. (Alcian Blue, ×100). The apparently empty spaces contain wisps of mucin, implying the loss of mucin during processing of the specimen.
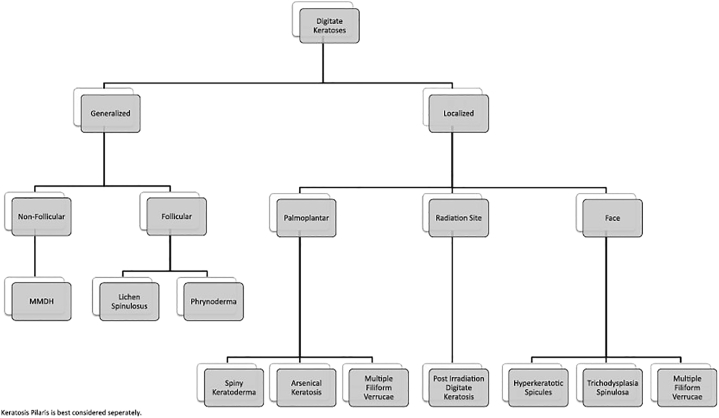
The preferred diagnosis of LS and secondary FM was made using a diagnostic algorithm (Fig 4) from the article titled “Multiple minute digitate hyperkeratosis (MMDH): A proposed algorithm for the digitate keratoses”.4 Vitamin A levels were normal.
Fig 4.
Diagnostic algorithm for digitate keratoses.4MMDH, Multiple minute digitate hyperkeratosis.
Permission granted from Elsevier for reuse of the algorithm.
She was started on isotretinoin 0.5 mg/kg and topical emollients. After 3 weeks of isotretinoin therapy, there was a marked improvement in the texture of her skin, and there were fewer hyperkeratotic spines (Fig 1, C). She continued to use isotretinoin for a 7-month course, and thereafter topical emollients were prescribed. Her condition spontaneously resolved 9 months after presenting to the dermatology clinic.
Discussion
In 1883, Crocker described a disorder he named “lichen pilaris seu spinulosus.” The currently accepted term is LS, and it predominantly occurs in the second decade of life.5
The aetiology of LS is unknown, but it has been postulated that there is a genetic predisposition or a follicular reaction pattern. The lesions appear suddenly and persist for weeks to months, followed by spontaneous disappearance with rare exceptions of persistent lesions.2,5
The pathogenesis of LS involves a follicular hyperkeratotic plug that blocks the hair follicle.2 The histopathology of LS, as in our case, is non-specific, showing a dilated hair follicle with keratin plugging and a perifollicular lymphocytic infiltrate. The FM was an unexpected find.
Given the generalized distribution and folliculocentricity of the skin lesions in the current patient, the differential diagnosis of LS includes phrynoderma and keratosis pilaris atrophicans. However, the lack of clinical signs of a vitamin deficiency and normal vitamin A levels militate against phrynoderma. Keratosis pilaris atrophicans was excluded as there was no secondary scarring or alopecia in affected areas. Non-follicular and localized forms of multiple minute digitate hyperkeratosis were also excluded.
FM is a rare tissue reaction showing localized accumulation of mucin in sebaceous glands, and the outer root sheath of hair follicles.6 The aetiology and pathogenesis of FM are unknown. It has been postulated that mucin production may be due to the subsequent stimulation of follicular keratinocytes by surrounding T-helper lymphocytes.7
Primary FM has a benign, usually self-limiting course. It presents most commonly as a solitary lesion in the head and neck distribution of children and young adults. Another clinical variant of primary FM occurs in older patients and shows more widespread lesions that can last indefinitely.8
Secondary FM has been associated with inflammatory disorders (eg, atopic dermatitis, discoid lupus erythematosus), drug eruptions (eg, imatinib), and numerous other disorders.7,9 LAFM and especially follicular mycosis fungoides should be actively excluded.10 Infrequently, cutaneous T-cell lymphoma other than mycosis fungoides may be associated with FM.10 The distinction between FM as a reactive process vs FM associated with cutaneous T-cell lymphoma is often subjective. LAFM usually occurs in older male patients with multiple, more widely distributed lesions compared to other forms of FM.3,9 Regarding histopathology, LAFM shows more inflammatory cells and more pronounced epidermotropism with atypical lymphocytes.3,11 T-cell receptor gene rearrangement typically shows monoclonality in LAFM.11 The CD4:CD8 ratio is raised in LAFM compared to other forms of FM.12 An accurate diagnosis depends on the careful clinicopathological correlation of all the criteria. Every case of FM requires an individualized approach. Given the constellation of clinicopathological findings and the initial good response to treatment, followed by resolution of the skin lesions strongly militates against the possibility of an underlying cutaneous T-cell lymphoma in our patient.
In conclusion, here is no specific treatment for LS or FM. Due to the generalized and symmetric distribution of this patient’s hyperkeratotic lesions, the decision was made to use a systemic keratolytic agent such as low-dose (0.5 mg/kg) isotretinoin. The patient responded, and the lesions started improving after 3 weeks of treatment. As mentioned above, the risk assessment for T-cell lymphoma has limitations, and close follow-up of the patient will be performed with serial skin biopsies if the clinical picture recurs.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Stellenbosch University Human Research Ethics Committee (HREC) approval. HREC reference no: C22/03/006.
Patient consent: Written consent was obtained from the participant and consent forms are on file. Consent was obtained for the publication of medical information and patient photographs in print and online by the authors. The patient was aware that the information may be made publicly available.
References
- 1.Venkatesh A., Dupuis E., Prajapati V., Rao J. Generalized lichen spinulosus in a 4-year-old boy without systemic disease. Arch Dermatol. 2012;148(7):865–866. doi: 10.1001/archdermatol.2012.188. [DOI] [PubMed] [Google Scholar]
- 2.Hawsawi K.A., Almehmadi K., Alraddadi B., Aljuhani O. Lichen spinulosus: case report and review of literatures. J Heal Sci. 2015;5(3A):20–22. [Google Scholar]
- 3.Hooper K.K., Smoller B.R., Brown J.A. Idiopathic follicular mucinosis or mycosis fungoides? Classification and diagnostic challenges. Cutis. 2015;95(6):E9–E14. [PubMed] [Google Scholar]
- 4.Caccetta T.P., Dessauvagie B., McCallum D., Kumarasinghe S.P. Multiple minute digitate hyperkeratosis: a proposed algorithm for the digitate keratoses. J Am Acad Dermatol. 2012;67(1):e49–e55. doi: 10.1016/j.jaad.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Friedman S.J. Lichen spinulosus. Clinicopathologic review of thirty-five cases. J Am Acad Dermatol. 1990;22(2):261–264. doi: 10.1016/0190-9622(90)70035-G. [DOI] [PubMed] [Google Scholar]
- 6.Cömert A., Akin O., Demirkesen C. Follicular mucinosis mimicking lichen spinulosus in an 11-year-old boy. Eur J Dermatol. 2007;17(6):544–545. doi: 10.1684/ejd.2007.0278. [DOI] [PubMed] [Google Scholar]
- 7.Arca E., Köse O., Taştan H.B., Gür A.R., Safali M. Follicular mucinosis responding to isotretinoin treatment. J Dermatolog Treat. 2004;15(6):391–395. doi: 10.1080/09546630410023575. [DOI] [PubMed] [Google Scholar]
- 8.Lewars M., Levin J., Purcell S. Follicular mucinosis. Indian Dermatol Online J. 2013;4(4):333–335. doi: 10.4103/2229-5178.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mir-Bonafé J.M., Cañueto J., Fernández-López E., Santos-Briz A. Follicular mucinosis associated with Nonlymphoid skin conditions. Am J Dermatopathol. 2014;36(9):705–709. doi: 10.1097/01.dad.0000451564.29918.31. [DOI] [PubMed] [Google Scholar]
- 10.Heymann W.R. Predicting the nature of follicular mucinosis: still a sticky situation. J Am Acad Dermatol [Internet] 2019;80(6):1524–1525. doi: 10.1016/j.jaad.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Rongioletti F., Rebora A. Cutaneous Mucinoses Microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23(3):257–267. doi: 10.1097/00000372-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Rongioletti F., De Lucchi S., Meyes D., et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37(1):15–19. doi: 10.1111/j.1600-0560.2009.01338.x. [DOI] [PubMed] [Google Scholar]