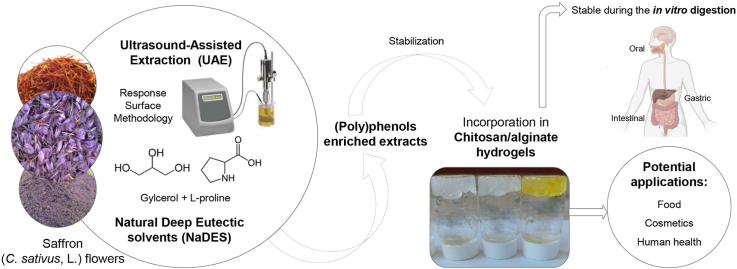
Abstract
The current saffron production system is generating several hundreds of tons of tepal waste, because only stigmas are used for food. Consequently, the valorization of saffron floral by-products by developing stable functional ingredients could lead to the environmental impact minimization. Thus, the main aim of this study was to develop innovative green extraction processes from saffron floral by-products by using Natural Deep Eutectic Solvents (NaDES) and ultrasound-assisted extraction (UAE) as ecological extraction method. Response surface methodology was used to optimize process parameters. To improve the stability of the optimal extracts, they were incorporated into chitosan/alginate hydrogels, studying their water-uptake and water retention capacity and the total phenolic content (TPC) during the in vitro digestion. The results indicated that the optimal extraction, regarding total phenolic and flavonoid content, was achieved in 20 min, using 180 W ultrasound power and 90% of NaDES. The results of the DPPH assay revealed the potent antioxidant activity of saffron floral by-products. The chitosan/alginate hydrogels incorporating the as-obtained NaDES extracts showed favorable properties whereas the TPC remained stable under intestinal conditions. Therefore, NaDES combined with UAE was an efficient technique to isolate high added-value compounds from saffron flowers, succeeding also the valorization of discarded waste by using green and low-cost strategies. Furthermore, these novel hydrogels could be used as promising candidates for food or cosmetic applications.
Keywords: NaDES, Sustainability, Crocus sativus L., Antioxidant capacity, Bioactive compounds, Environmental impact
Graphical abstract
Highlights
-
•
C. sativus floral by-products are sustainable materials to develop new ingredients.
-
•
NaDES with UAE efficiently extract bioactive compounds from saffron flowers.
-
•
Saffron flowers are good natural sources of antioxidant compounds as (poly)phenols.
-
•
Chitosan/alginate hydrogels present favorable properties for potential applications.
-
•
After the in vitro digestion, TPC values remain stable into the hydrogel's matrices.
1. Introduction
Saffron, the most expensive spice in the world, is widely cultivated in Iran, India, Spain, Greece, Italy and Turkey. Apart from its applications in food, it has been used in medicine due to its bioactive compounds content (Melnyk et al., 2010). It is produced from the red dried stigmas of Crocus sativus L. flowers, but the rest of the flower, mostly composed of six purple tepals and three yellow stamens, is considered as waste material (Fig. 1) (Mathew, 1977). Nevertheless, recent research showed that saffron processing waste are promising natural sources of bioactive compounds, such as polyphenols including flavonol glycosides, anthocyanins and phenolic acids (Table 1) (Cerdá-Bernad et al., 2022a, Cerdá-Bernad et al., 2022b, Cerdá-Bernad et al., 2022c, Cerdá-Bernad et al., 2022d; Da Porto and Natolino, 2018; Lakka et al., 2019). Then, one of the greatest interests of saffron bioactive compounds is because of their benefits on the human health through their high antioxidant capacity with potential applications in food, cosmetic and pharmaceutical industries (Cerdá-Bernad et al., 2022a, Cerdá-Bernad et al., 2022b, Cerdá-Bernad et al., 2022c, Cerdá-Bernad et al., 2022d). In the post-pandemic food sector, the valorization of a wide range of antioxidant bioresources (such as spices, medicinal plants, cereal processing by-products, edible and wild flowers, among others) through their use as functional food ingredients is one of the innovations with the greatest potential, since their bioactive composition content may play an important role in human health, highlighting even antiviral activity (Galanakis, 2022; Galanakis et al., 2020, 2021). Moreover, the valorization of saffron bio-residues could improve the sustainability and profitability of saffron industry since to produce 1 kg of saffron are necessary around 350 kg of tepals (Sánchez-Vioque et al., 2012).
Fig. 1.
Parts of Crocus sativus L. flower.
Table 1.
Summary of the main bioactive compounds presented in saffron stigmas and saffron floral by-products (tepals and stamens).
| Bioactive Compounds | Saffron stigmas | Saffron floral by-products | |
|---|---|---|---|
| (Poly)phenols | Flavonoids |
Flavonols: kaempferol, quercetin and isorhamnetin glycosides Flavanols: epicatechin |
Flavonols: kaempferol, quercetin, myricetin and isorhamnetin glycosides Anthocyanins: delphinidin, petunidin and malvidin glycosides |
| Phenolic acids | Gallic acid, hydroxybenzoic acid, coumaric acid, rosmarinic acid, vanillic acid, caffeic acid | Gallic acid, hydroxybenzoic acid, coumaric acid, syringic acid, hydroxycinnamic acids | |
| Carotenoid-related | Crocetin, crocin, β-carotene, zeaxanthin |
Lutein diesters with lauric, myristic, palmitic and stearic acids | |
| Terpenoids | Picrocrocin, safranal | – | |
The extraction process of bioactive compounds from saffron floral bio-residues has been done through conventional extraction methods (maceration, distillation, Soxhlet extraction) (Ozkan et al., 2021). However, to minimize the environmental impact of common volatile organic solvents and to improve the extraction efficiency there is a need of new green and energy-saving technologies that can be transferred to industrial scale such as ultrasound-assisted extraction, and to use environmentally friendly solvents such as NaDES to obtain phenolic-enriched extracts. Ultrasound-assisted extraction process presents several advantages in contrast to conventional technologies: higher extraction efficiency, lower energy consumption and cost, as well as shorter extraction time and rapid temperature rise leading to a lower thermal degradation of compounds of interest (Nagarajan et al., 2019; Sarfarazi et al., 2020).
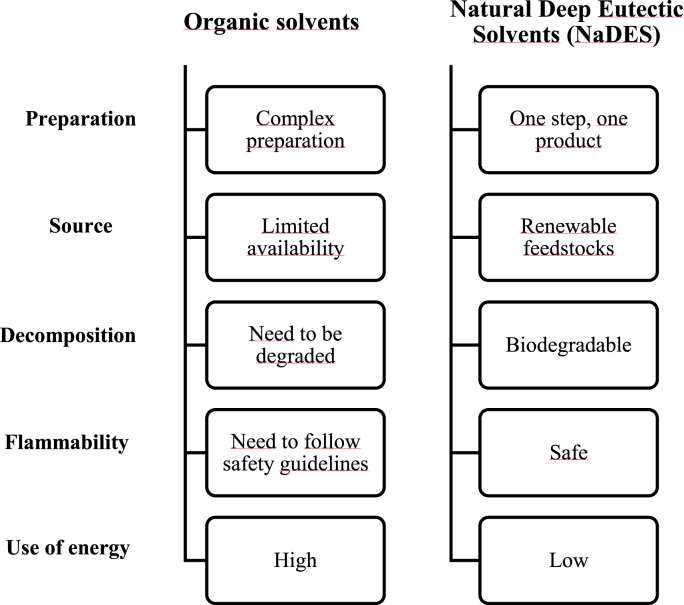
NaDES, a novel category of green and sustainable solvents, are mixtures of two or more naturally occurring compounds, a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) (e.g., organic acids, amino acids, sugars), in specific molar ratios (Choi et al., 2011). These mixtures can form intermolecular hydrogen bonds and van der Waals interactions that cause a considerable reduction of the melting point compared to that of each initial compound. NaDES have exceptional properties (Fig. 2): biodegradability, non-volatility, non-flammability, ability to dissolve several compounds, lack of toxicity and low cost, and have the striking ability to act as effective extraction media and protective carriers of the extracted bioactive phytochemicals (Koutsoukos et al., 2019; Skarpalezos and Detsi, 2019; Tzani et al., 2021).
Fig. 2.
Comparison of organic solvents vs NaDES.
NaDES extractions are usually combined with high-energy non-conventional extraction techniques like ultrasound-assisted extraction to improve the extraction yield and to reduce the extraction time, the degradation of bioactives and any adverse ecological effects. The application of ultrasonic energy causes the formation and collapse of cavitation bubbles, and the generation of high pressure and temperature that accelerates the disruption of cell walls of plants, promoting the release of their bioactive content and improving the mass transfer (Wen et al., 2018).
To improve the stabilization of the bioactive ingredients, gel-like structures are used for their encapsulation. Hydrogels are three-dimensional network structures obtained by polymers that can absorb large amounts of water. Recently hydrogels are being studied as delivery systems of different active ingredients with potential application in the food industry (Ćorković et al., 2021). Chitosan is a cationic biopolymer, considered as GRAS (Generally Recognized as Safe) with good properties such as low toxicity, biocompatibility, biodegradability, and the ability, under acidic conditions, to be manufactured into various forms such as hydrogels, films, emulsions, among others (Detsi et al., 2020). Sodium alginate is an anionic biopolymer, commonly used as encapsulation matrix showing simple gelation with divalent cations such as Ca2+, and it is cheap, available, non-toxic, biocompatible, biodegradable and can be processed easily in hydrophilic solvents like water. Thus, the combination of alginate, using CaCl2 as ionic cross-linker with chitosan developing chitosan/alginate hydrogels could have a potential use as new drug delivery systems.
The main aim of this research was to evaluate innovative eco-friendly extraction processes as UAE process efficiency, at laboratory scale, to obtain high-added value compounds from saffron floral by-products, using a central composite design to optimize UAE process conditions (extraction time, power, and NaDES:water ratio). Extracts were characterized regarding total phenolic and flavonoids content (TFC) and antioxidant activity by DPPH assay. The best NaDES-extract in terms of bioactive content was incorporated into chitosan/alginate hydrogels to obtain new stabilized bioactive ingredients to examine their potential for practical applications. Therefore, this research contributes to the valorization of a currently unexploited biomass which could improve the sustainability of the saffron spice production and profitability of this industrial sector, optimizing the environmental impact and developing new high added-value ingredients.
2. Material and methods
2.1. Plant material and reagents
Saffron floral by-products were obtained from Spain (Castilla-La Mancha region, 2020 harvest season), and composed mainly of tepals and stamens. Stigmas were already detached following traditional procedures (DOCM, 1999). All fresh flowers were frozen in liquid nitrogen and kept at −80 °C until freeze-dried during 48 h (Christ Alpha 2–4, B. Braun Biotech International, Melsungen; Germany). Freeze-dried flowers were crushed and sieved (500 μm mesh size), and kept at −20 °C until further analysis.
Saffron stigmas were supplied by the Spanish company Verdú Cantó Saffron Spain, S.L and were from Greek (Kozani area) cultivation, being the moisture lower than 11%. Saffron threads were crushed and sieved (500 μm mesh size), and stored at 4 °C until further analysis.
Reagents: L-proline (Panreac, Barcelona, Spain), D,L-lactic acid (LabKem, Barcelona, Spain, 80% aq. Sol.), glycerol anhydrous (Penta, Katovice, Czech Republic, 99.9%), anhydrous betaine (Alfa Aesar, Ward Hill, MA, USA), anhydrous citric acid (Fluka, Charlotte, NC, USA), anhydrous D(+)-Glucose (Sigma Aldrich, St. Louis, MO, USA), Folin-Ciocalteu reagent (Merck Millipore, Darmstadt, Germany), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Catechin (Sigma Aldrich, St. Louis, MO, USA), alpha-amylase, bile salts and pancreatin (Sigma Aldrich, St. Louis, MO, USA), phosphate-buffered saline (PBS) and pepsin (Thermo Fisher Scientific, Waltham, MA, USA), hydrochloric acid and sodium bicarbonate (Panreac, Barcelona, Spain).
2.2. NaDES
The synthesis of NaDES was done using the heating method as described by Tzani et al. (2021) with slight modifications, mixing appropriate amounts of starting materials under continuous and vigorous stirring for 1–4 h at temperatures between 50 and 80 °C under an inert atmosphere in a round-bottom flask (Table 2). The homogenous mixtures were transferred into glass vials, hermetically closed and stored in dark at room temperature until their further use. The pH of the prepared NaDES was measured by a 744 pH Meter Metrohm at 25 ± 2 °C. The NADESs viscosities were measured in triplicate at 25 °C by the Brookfield DV1 Viscometer using the spindle SC4-18. The temperature of the samples was maintained using a Julabo F12 thermostat bath (± 0.01 °C). Each measurement was carried out at a constant time of about 7 min and the viscosities were measured with an accuracy of ± 2%.
Table 2.
Synthesized NaDES.
| No. | NaDES | HBA | HBD | Molar ratio | Conditions |
|---|---|---|---|---|---|
| 1 | Bet/LA | Betaine | D,L-Lactic acida | 1:2:2.5 | 50 °C, 1 h |
| 2 | Glu/LA | D-(+)-Glucose | D,L-Lactic acida | 1:5:6.2 | 65 °C, 2 h |
| 3 | Bet/Gly/Water | Betaine | Glycerol | 1:3:1 | 70 °C, 3 h |
| 4 | Pro/CA/Water | L-proline | Citric acid | 2:1:3 | 80 °C, 4 h |
| 5 | Pro/Gly | L-proline | Glycerol | 1:2 | 65 °C, 3 h |
Lactic acid is 80% aqueous solution, the composition of water is considered as it could participate in the hydrogen bonding of the NaDES.
2.3. Box–Behnken experimental Design—Response Surface Methodology
Response Surface Methodology was employed to optimize the extraction process and predict the responses which are affected by experimental variables. Using Design-Expert® software version 12.0 (trial version) (Stat-Ease, Inc., Minneapolis, USA), a Box–Behnken design (BBD) with three independent variables was applied to evaluate the effect of time (A), power (B) and % NaDES (w/w) in the NaDES:H2O mixture (C) on the bioactive content (total phenolic content and total flavonoid content) and antioxidant activity of saffron flowers. The coded variable levels are summarized in Table 3. The experimental data were fitted to a third-order polynomial, which correlated each response to the factors.
Table 3.
The BBD matrix of UAE of the experimental design.
| Experiments | Independent variables: UAE conditions |
||
|---|---|---|---|
| Time (min) (A) | Power (Watt) (B) | NaDES %(w/w) (C) | |
| 1 | 35 (+1) | 180 (+1) | 60 (0) |
| 2 | 20 (0) | 60 (−1) | 30 (−1) |
| 3 | 20 (0) | 120 (0) | 60 (0) |
| 4 | 20 (0) | 120 (0) | 60 (0) |
| 5 | 35 (+1) | 60 (−1) | 60 (0) |
| 6 | 20 (0) | 180 (+1) | 30 (−1) |
| 7 | 5 (−1) | 60 (−1) | 60 (0) |
| 8 | 20 (0) | 60 (−1) | 90 (+1) |
| 9 | 20 (0) | 180 (+1) | 90 (+1) |
| 10 | 35 (+1) | 120 (0) | 90 (+1) |
| 11 | 20 (0) | 120 (0) | 60 (0) |
| 12 | 20 (0) | 120 (0) | 60 (0) |
| 13 | 35 (+1) | 120 (0) | 30 (−1) |
| 14 | 5 (−1) | 120 (0) | 90 (+1) |
| 15 | 5 (−1) | 180 (+1) | 60 (0) |
| 16 | 5 (−1) | 120 (0) | 30 (−1) |
2.4. NaDES—Ultrasound assisted extraction process
Extractions of freeze-dried saffron flowers were carried out using a Vibra-Cell VCX 400 (400 W) Ultrasonics High Intensity Processor (Sonics and Materials Inc., Newtown, USA), equipped with a piezoelectric converter and a 13 mm diameter titanium alloy (Ti–6Al–4V) probe. The variable parameters included time (5−35 min), power (60−180 W) and NaDES concentration (30–90%). The mass:solvent ratio was set at 1:20 (w/v), and the samples were kept in an ice bath during the UAE to avoid overheating. The pulse sequence was set at 6 s on – 2 s off. Sixteen extractions were carried out, following the designed conditions presented in Table 3. Once the extraction was completed, by centrifugation at 10000 rpm for 10 min and by filtration under vacuum, the supernatant was recovered from the solid material. All the extracts were stored in dark at 4 °C until further analysis.
Once the extracts were characterized in terms of bioactive compounds content, the best parameters UAE conditions (20 min, 180 W, 90% NaDES (Pro/Gly)) were used for Greek saffron stigma extraction, following the same procedure explained. The extractions were performed in triplicate.
Initially, a screening was carried out to select the optimal NaDES using UAE. The five synthesized NaDES (Table 2) were studied, using as UAE variables: 15 min, 160 W and 65% w/w of NaDES in the NaDES-water system. The mass:solvent ratio was set at 1:20 (w/v), following the same procedure as above. The extracts were characterized in terms of TPC and TFC, and the optimal NaDES selected was Pro/Gly (1:2) which was employed for the 16 experiments.
2.5. Extract characterization
2.5.1. Total phenolic content
TPC was determined using the Folin Ciocalteau colorimetric method as described by Tzani et al. (2021), with slight modifications. Briefly, 50 μL of the saffron floral extracts were mixed with 3 mL of milli-Q water and 250 μL of Folin-Ciocalteu's reagent and the solutions were stirred in dark for 2 min. Then, 750 μL of saturated aqueous Na2CO3 solution were added and were stirred again and the final volume of the solution was adjusted to 5 mL with milli-Q water. Protected from light, samples were stirred and incubated at room temperature during 1 h. Gallic acid (1 mg/mL) was used as a reference standard (7.5–240 mg/L) and absorbance was measured at 755 nm using a Jasco V-770 UV–Vis/NIR spectrophotometer. The experiments were performed in triplicate and the results were expressed as mg gallic acid equivalent (mg GAE) per gram of dry weight (dw).
2.5.2. Total flavonoid content
TFC was determined as described by Çam and Hışıl (2010) with some modifications. Briefly, 0.2 mL of sample were mixed with 0.8 mL of water and 60 μL of sodium nitrite (5%) solution. After 5 min, 60 μL of aluminum trichloride (10%) solution were added. After 6 min, 0.4 mL of sodium hydroxide (1 M) solution were added and the volume was adjusted to 2 mL with water. Catechin (1 mg/mL) was used for quantification (20–100 mg/L) and absorbance was measured at 510 nm using a Jasco V-770 UV–Vis/NIR spectrophotometer. The experiments were performed in triplicate and the results were expressed as mg of catechin equivalents (mg CE) per gram of dw.
2.5.3. DPPH radical scavenging ability
The antioxidant capacity by the radical scavenging capacity measuring method by the 2-2,diphenyl-1-picryl-hydrazide (DPPH) stable radical, were carried out in the optimal extracts in terms of their bioactive content, following the method described by Boly et al. (2016). Briefly, 5 mg of DPPH were dissolved in ethanol with a final volume of 50 mL. In a 96-well plate, 100 μL of DPPH solution were added to 100 μL of saffron extracts diluted to 2% v/v in ethanol (initial concentration C) and then the same procedure was followed for samples diluted in ethanol, with concentrations 0.8C, 0.6C, 0.4C and 0.2C. The samples were incubated for 30 min in dark at room temperature and then the absorbance was measured at 515 nm using a Molecular Devices SpectraMax 250 Microplate UV/Vis Reader. The percentage of inhibition was calculated using the following Eq. (1):
| (1) |
where Ablank is the absorbance of the blank samples containing ethanol/DPPH and Asample is the absorbance of each sample containing extract. A curve of % inhibition against samples was plotted and the concentration of the sample required for 50% inhibition of DPPH radicals was determined (IC50), expressed as mg of saffron flower extracts per mL of sample. The experiments were performed in triplicate.
2.6. Chitosan/alginate hydrogels synthesis
A solution of chitosan (0.2% w/v) in aqueous lactic acid (3% v/v) was prepared. To this solution, the NADES extract was added in order to prepare a solution of final concentration 2% v/v (solution A). Next, a 3% w/v aqueous solution of sodium alginate (SA) was prepared by adding 3 g of SA powder to 100 mL of distilled water and stirring at 70 °C until the mixture became homogeneous (solution B). Then, 2 mL of solution A and 5 mL of solution B were mixed and an aqueous solution of calcium chloride (1% w/v), used to crosslink the alginate and chitosan, were added dropwise to the mixture. After 24 h, the excess of CaCl2 was drained out and hydrogels were obtained. These hydrogels were washed 3 times with distilled water and the water remaining on the surface was subsequently removed. The hydrogels were lyophilized using a freeze drier (ModulyoD Thermo Fisher Scientific, Waltham, MA, USA) for 48 h.
2.7. Structure of hydrogels
The shape and surface of the hydrogels was observed with a stereomicroscope Leica MZ95 (Leica, Spain).
2.8. Water-uptake and water-retention capacity
The water-uptake capacity of the hydrogel after drying was measured by a conventional gravimetric method. The weight of the dried hydrogel sample was accurately weighed, and then the dry samples were soaked in deionized water. The water-uptake capacity of the prepared Chit/Alg/extract/NaDES hydrogels was measured in PBS solution (pH = 7.4), at room temperature, and calculated by the following Eq. (2):
| (2) |
where m0 is the initial weight of dry sample and m1 is the mass swollen weight of the hydrogel at time t. After the predetermined time points the samples were extracted from the solution and weighted quickly.
The Chit/Alg/extract/NaDES hydrogels after reaching its maximum water-uptake capacity (equilibrium) at pH = 7.4, 37 °C it was used in the following water retention experiments. When water-uptake capacity (%) is the maximum, we assume that the water-retention (%) of the hydrogel is 100%. Thus, we calculate the water-retention with the aid of the following Eq. (3):
| (3) |
where m0 is the initial weight of dry sample, m1 is the mass swollen weight of the hydrogel at time t, and m2 is the weight of the hydrogel at the time of maximum water-uptake capacity (equilibrium). All the experiments were performed in triplicate.
2.9. In vitro digestion of hydrogels
The in vitro digestion was performed according to Gawlik-Dziki et al. (2009) and Cerdá-Bernad et al. (2021) with slight modifications. Briefly, the hydrogels were homogenized in a stomacher laboratory blender for 30 s to simulate mastication with the presence of 5 mL of PBS (hydrogel:PBS 1:50 w/v). The solution was adjusted to pH 6.75 and alpha-amylase (E.C. 3.2.1.1.) was added to obtain 100 U per mL of enzyme activity. Then, the samples were subjected to simulated gastric digestion for 60 min at 37 °C under stirring, adjusting the solution to pH 3 with 1 M HCl, and pepsin was added to reach a concentration of 3 g/L. For intestinal simulation, the pH was increased to 7 with 1 M NaHCO3 and 4.5 g/L of bile salts and 1 g/L of pancreatin were added. The samples were incubated during 120 min at 37 °C. Samples were taken at the different stages of in vitro digestion and filtered for TPC analysis: after oral digestion, after 60 min of gastric digestion, and after 60 min and 120 min of intestinal digestion.
2.10. Statistical analysis
Results were expressed as the mean ± standard deviation. The mean comparisons were carried out using an analysis of variance (ANOVA) and by the Tukey multiple range test, using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, USA). The significant differences were stablished as (p < 0.05).
3. Results and discussion
3.1. Preliminary extraction experiments
In this study, the influence of solvent, pH of the extraction solvent systems and viscosity in the recovery of phenolics and flavonoids from saffron floral by-products was examined (Table 4).
Table 4.
Total phenolic content (TPC) and total flavonoid content (TFC) obtained from preliminary NaDES-UAE (extraction conditions: 15min, 160W, NaDES/water = 65:35 w/w) and pH of the extraction solvent systems at 25 °Ca.
| No. | NaDES | pH (NaDES/water = 65:35 w/w) | Viscosity (mPa·s) | TPC (mg GAE/g dw) | TFC (mg CE/g dw) |
|---|---|---|---|---|---|
| 1 | Bet/LA/Water | 2.85 | 139.8 | 18.90 ± 0.30b | 1.30 ± 0.05c |
| 2 | Glu/LA/Water | 1.32 | 411.1 | 21.88 ± 0.58b | 1.16 ± 0.08c |
| 3 | Bet/Gly/Water | 6.36 | 519.0 | 31.73 ± 0.08a | 5.85 ± 0.17b |
| 4 | Pro/CA/Water | n.mb | n.mb | 33.56 ± 0.45a | 8.47 ± 0.10a |
| 5 | Pro/Gly | 7.59 | 5064.0 | 35.15 ± 1.99a | 8.04 ± 0.49a |
Means ± standard deviation in the same column followed by different lowercase letters indicate statistically significant differences at (p ≤ 0.05) for each sample (n = 3).
n.m.: not measured due to the very high viscosity.
Bet/Gly/Water (1:3:1) and Pro/Gly (1:2) were some of the optimal solvents to isolate phenolic and flavonoids compounds by UAE from saffron flowers. Then, the pH could strongly influence the recovery of these antioxidant compounds, since using acidic solvents, TPC and TFC values were lower than those obtained using solvents with a neutral pH. A plausible explanation is that the low pH of some NaDES may affect the chemical composition of extracts by release of other bioactive compounds or cause structural changes (Inada et al., 2015). However, using the acid-containing NaDES solvent Pro/CA/Water (2:1:3) a high TPC and TFC values were obtained, so other factors such as viscosity may have influenced the extraction process of bioactive compounds.
The NaDES Pro/Gly (1:2) was selected as a promising green solvent to implement the extraction process and its optimization through the experimental design.
3.2. Experimental design
To optimize the extraction of secondary metabolites from saffron flowers through innovative eco-friendly extraction processes and by using ecological extraction media to obtain high-value bioactive compounds, the extraction method should be considered, as well as the conditions of the extraction process, since they have a strong influence on the extraction yield.
A design of experiments (DOE) was carried out to isolate bioactive compounds from Crocus sativus L. flowers. To optimize the extraction procedure and to evaluate UAE as a green extraction method and NaDES as green extraction media, the effect of process variables (TPC and TFC, and the antioxidant capacity by DPPH assay) were evaluated on a BBD with three independent variables: time (A), power (B) and NaDES:water solvent ratios (C).
3.2.1. Bioactive content
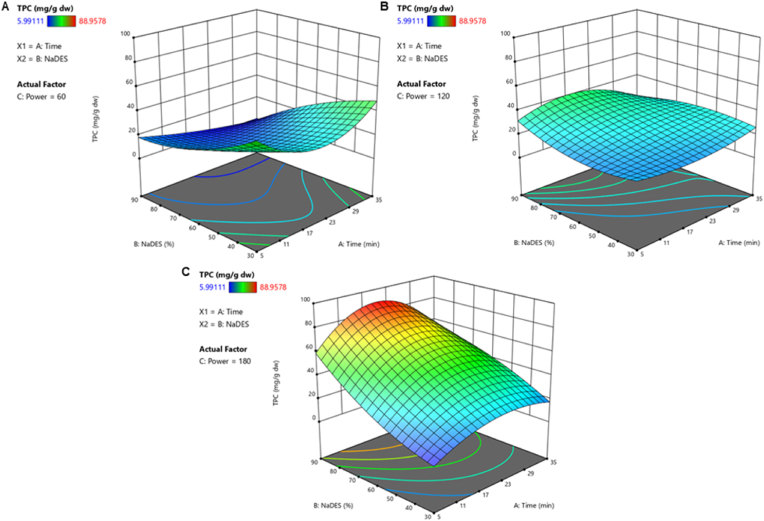
The results of the effects of the three independent variables studied in UAE on phenolics compounds extraction are shown in Fig. 3. As it can be seen in the 3D surface response plots which depict the correlation between the factors of Time (A) and % NaDES (B) in stable Power (C) conditions each time at 60 W, 120 W and 180 W which are the 3 levels of factor C. Then, the study of the interaction between time and NaDES solvent ratio keeping constant the power allows to adjust the solvent polarity to increase the affinity and specificity of the UAE. The values of TPC were lower using as extraction factors shorter time, 30% of NaDES solvent ratio and 60 or 120 W of power (blue surfaces in Fig. 3A and 3B). Nevertheless, the values were higher using 20 or 35 min, 60 or 90% of NaDES solvent and 180 W of power during the UAE (red surfaces in Fig. 3C). The optimal extraction regarding TPC was using 20 min, 180 W and 90% of NaDES (88.96 ± 1.08 mg GAE/g dw), indicating that increasing the extraction time and NaDES solvent ratio while keeping constant the power at 180 W, lead to an increase in the extraction yield of these bioactive compounds. This fact may be due to the significant effect of power on extracting bioactive compounds from saffron flowers by some interconnected mechanisms: accelerating the breakdown of plant cells due to a fast-localized increase in temperature and pressure, increasing the permeability and release of intracellular compounds and improving the mass transfer and diffusion (González-Centeno et al., 2015). Besides, a higher ratio of NaDES solvent than water could improve the solubilization of non-water-soluble metabolites of plant cells.
Fig. 3.
Response surface plot representing the effects of time, temperature and NaDES solvent ratio on Total Phenolic Content (TPC) from saffron floral by-products. (A) Power was kept constant at 60 W. (B) Power was kept constant at 120 W. (C) Power was kept constant at 180 W. Lower values are represented in blue and higher values in red. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The experimental values of the TPC obtained for the UAE of bioactive compounds are showed in Table 5. The statistical analysis of the 16 runs revealed that the TPC is best described by the reduced cubic model, being the independent variables: time (A), power (B) and NaDES:water solvent ratios (C):
Table 5.
Experimental results of total phenolic content (TPC), total flavonoid content (TFC) and DPPH assay for the ultrasound-assisted extraction of bioactive compounds from Spanish saffron floral-by products, and for Greek saffron stigmas extracted with the optimal UAE conditionsa.
| Extraction | UAE Conditions |
Responses |
|||||
|---|---|---|---|---|---|---|---|
| Time (min) | Power (W) | NaDES (Pro/Gly 1:2) (%) | TPC (mg GAE/g dw) | TFC (mg CE/g dw) | DPPH Scavenging Ability, IC50 (mg of extract/mL) | ||
| Saffron floral by-products | 1 | 35 | 180 | 60 | 42.27 ± 2.55b | 3.60 ± 0.32cde | 6.64 ± 0.24b |
| 2 | 20 | 60 | 30 | 27.89 ± 0.61cde | 3.10 ± 0.07e | – | |
| 3 | 20 | 120 | 60 | 27.10 ± 0.83cde | 3.67 ± 0.08cde | – | |
| 4 | 20 | 120 | 60 | 28.21 ± 2.41cd | 4.60 ± 0.04abc | – | |
| 5 | 35 | 60 | 60 | 29.38 ± 2.13c | 4.93 ± 0.25 ab | – | |
| 6 | 20 | 180 | 30 | 22.15 ± 0.02efg | 3.15 ± 0.08e | – | |
| 7 | 5 | 60 | 60 | 26.66 ± 0.45cdef | 4.54 ± 0.03abcd | – | |
| 8 | 20 | 60 | 90 | 5.99 ± 0.15h | 1.30 ± 0.06f | – | |
| 9 | 20 | 180 | 90 | 88.96 ± 1.08a | 4.36 ± 0.48bcd | 2.06 ± 0.15a | |
| 10 | 35 | 120 | 90 | 20.17 ± 0.67g | 0.60 ± 0.11f | – | |
| 11 | 20 | 120 | 60 | 29.26 ± 0.58c | 4.77 ± 0.14 ab | – | |
| 12 | 20 | 120 | 60 | 27.49 ± 2.23cde | 5.49 ± 0.05a | – | |
| 13 | 35 | 120 | 30 | 26.01 ± 2.57cdefg | 3.02 ± 0.13e | – | |
| 14 | 5 | 120 | 90 | 31.56 ± 0.09c | 2.73 ± 0.24e | 6.36 ± 0.51b | |
| 15 | 5 | 180 | 60 | 23.24 ± 1.68defg | 3.59 ± 0.42de | – | |
| 16 | 5 | 120 | 30 | 21.17 ± 0.78 fg | 2.75 ± 0.56e | – | |
| Saffron stigmas | 20 | 180 | 90 | 95.66 ± 9.34 | 9.56 ± 0.60 | 2.74 ± 0.47 | |
Means ± standard deviation in the same column followed by different lowercase letters indicate statistically significant differences at (p ≤ 0.05) for each sample (n = 3).
TPC = 28.01 + 5.44 A + 11.23 B + 19.31 C - 4.06 AB + 4.08 AC + 22.18 BC - 4.57 A2 + 1.29 B2 + 6.95 C2 - 10.09 A2B - 16.94 A2C - 7.07 AB2
The proposed model was significant with the F-value equal to 417.92 and the p-value less than 0.05. The coefficient R2 was 0.9994, indicating accuracy and a good fit of the experimental data with the calculated ones, and the adjusted coefficient R2 0.9970 which verifies the adequacy of the model.
According to the results, NaDES:water solvent ratios (C) is the factor that contributes the most to the extraction of phenolic content. Based on the TPC results (Table 5), the values were higher than those reported in previous research using dried saffron tepals from Italy extracted by UAE during 15 min (23 kHz) using water as extracting agent (1150.63 ± 11.23 mg GAE/100 g dw) (Stelluti et al., 2021) or Iranian saffron petals extracted by UAE during 40.61 min (135.5 W), using water as extracting agent (863 mg/100 g) (Hashemi Gahruie et al., 2020). It should be noted that there is also an improvement of TPC extraction respect to conventional extraction methods as sonication, in Spanish saffron floral by-products (32.82 ± 2.23 mg GAE/g dw) (Cerdá-Bernad et al., 2022a, Cerdá-Bernad et al., 2022b, Cerdá-Bernad et al., 2022c, Cerdá-Bernad et al., 2022d).
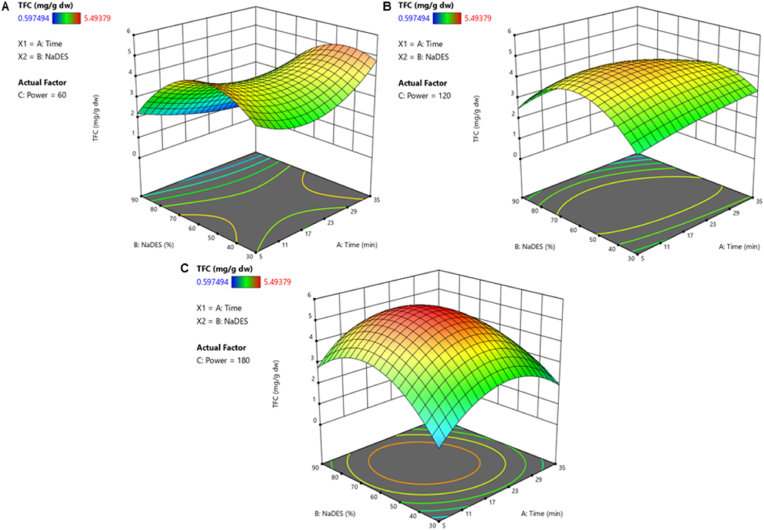
Regarding TFC, the results of the interaction effects of the three independent variables studied in UAE on flavonoids content are shown in Fig. 4. This figure illustrates the interaction between time and NaDES solvent ratio, keeping each time the power during the extraction process constant in the 60 W, 120 W and 180 W which are the 3 levels set in the Power factor. The flavonoids concentration in the extract was positively affected by temperature and solvent ratio. As it can be seen in the response surface plot, the shape of the Fig. 4C indicated that, keeping the power constant at 180 W, flavonoids extraction was enhanced (red surface) with 20 min of extraction time and 60% of NaDES solvent.
Fig. 4.
Response surface plot representing the effects of time, temperature and NaDES solvent ratio on Total Flavonoid Content (TFC) from saffron floral by-products. (A) Power was kept constant at 60 W. (B) Power was kept constant at 120 W. (C) Power was kept constant at 180 W. Lower values are represented in blue and higher values in red. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The experimental values of the TFC obtained by UAE from saffron flowers are showed in Table 5. The statistical analysis of the 16 runs revealed that the TFC is best described by the reduced cubic model, being the independent variables: time (A), power (B) and NaDES:water solvent ratios (C):
| TFC = 4.69–0.1819 A - 0.1469 B + 0.7783 C - 0.6010 AB - 0.0956 AC + 0.7501 BC - 0.5864 A2 - 1.77 B2 - 0.4628 A2B - 1.35 A2C |
The proposed model was significant with the F-value equal to 4.86 and the p-value less than 0.05. The coefficient R2 was 0.9068, and the adjusted coefficient R2 0.7203.
According to the TFC results (Table 5), the values were higher than other studies that showed TFC values for 130 mg/100 g of Iranian saffron petals extracted by UAE (Hashemi Gahruie et al., 2020). Furthermore, TFC results were similar to those reported by that study Spanish saffron floral by-products extracts obtained by MAE using ethanol as extracting agent (Cerdá-Bernad,et al., 2022).
Regarding Greek saffron stigmas, they were extracted following the UAE conditions of extraction number 9 (Table 5), since it was the optimum extraction considering the simultaneous maximization of the TFC and TPC values for saffron floral by-products (20 min, 90% of NaDES and 180 W). The results showed that high content of total phenols (95.66 ± 9.34 mg GAE/g dw) and flavonoids (9.56 ± 0.60 mg CE/g dw) were obtained from saffron stigmas. The TPC obtained is higher than the ones reported by other studies, in which Greek saffron stigmas extracts had values of 34.00 ± 3.22 mg GAE/g dw, extracted by usual methods as sonication, but TFC values were lower (17.39 ± 4.14 mg CE/g dw) (Cerdá-Bernad et al., 2022a, Cerdá-Bernad et al., 2022b, Cerdá-Bernad et al., 2022c, Cerdá-Bernad et al., 2022d). However, TFC values obtained for saffron stigmas are higher than those presented by Karimi et al. (2010) for saffron stigmas methanol extracts from Iran (5.8 ± 0.12 mg rutin equivalents/g dw).
Therefore, NaDES combined with UAE efficiently extracted bioactive compounds from saffron floral by-products, providing new information about the optimal factors of UAE at laboratory scale. Moreover, UAE is a method that could be easier scaled up at an industrial scale comparing to other methods, such as microwave extraction.
3.2.2. In vitro antioxidant properties
As showed in Table 5, DPPH assay results revealed the antioxidant activities of saffron floral by-products of the optimal extracts, in terms of phenolic and flavonoid content. All the studied extracts showed remarkably high antioxidant power by DPPH assay which evaluates the in vitro radical scavenging ability of saffron extracts. The best DPPH radical scavenging ability was shown by extract 9 for saffron floral by-products (IC50 = 2.06 ± 0.15 mg/mL). Besides, Greek saffron stigmas extracts showed a good antioxidant activity (IC50 = 2.74 ± 0.47 mg/mL) that could be linked to their high bioactive content, such as total phenolic compounds and flavonoids.
Therefore, saffron flowers extracts presented a potential antioxidant activity that may be related to their bioactive content and could be used as natural antioxidant compounds to protect against the effects of oxidative stress, contributing to prevent several diseases. Furthermore, due to their high antioxidant power, these phenolic-enriched extracts from saffron stigmas and saffron floral by-products may be employed as ingredients to fortify food products, improving their functional properties, as well as to increase the preservation as natural additives, or having potential applications in the cosmetic industry, since previous studies have reported the potentiality of using phenols from oil mill wastewater as UV booster (Galanakis, 2018; Galanakis et al., 2018).
3.3. Chitosan/alginate hydrogels
Currently, chitosan/alginate hydrogels are novel economical candidates to use as delivery systems of different bioactive ingredients, since are safe for consumption, biocompatible, biodegradable and made with low-cost natural polymers (Ćorković et al., 2021; Detsi et al., 2020; Shewan and Stokes, 2013).
The optimal NaDES-extracts (extract 9 for saffron floral by-products, and saffron stigmas extracts), which were a natural rich source of bioactive ingredients with high antioxidant capacity, were incorporated into chitosan/alginate hydrogels to improve their stability and in order to study their potential use as a formulation for the development of functional food products or as efficient delivery systems of phenolic compounds.
In the Fig. S1, the surface of the different freeze-dried chitosan/alginate hydrogels incorporating saffron floral by-products or stigmas extracts is shown at different magnifications. The figure indicates the fibrous structure of chitosan/alginate hydrogels, and also a porous structure, as a result of the pores formed during the process of phase separation in the freeze-drying treatment (Baysal et al., 2013; Ehterami et al., 2019). Besides, in the Fig. S1B, saffron stigmas extracts incorporated into the structure of chitosan/alginate hydrogels can be distinguish due to their orange-red color.
3.3.1. Water-uptake and water-retention capacity
Water-uptake capacity and structural stability of hydrogels are important factors for their practical use in food products or for other applications such as hydrogel-based delivery systems, since a direct relationship could be between swelling properties of hydrogels and permeability of solute (Gehrke et al., 2006).
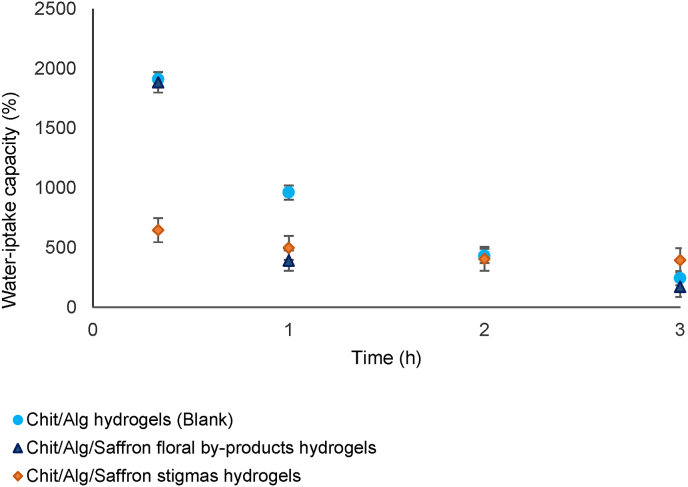
The data of the water-uptake capacity showed that all chitosan/alginate hydrogels had a maximum water-uptake at 20 min, which decreased by the time, especially for chitosan/alginate hydrogels incorporating the as-obtained NaDES-saffron floral by-products extracts, having a similar behavior with the blank samples (Fig. 5). However, chitosan/alginate hydrogels with NaDES-saffron stigmas extracts showed a different water-uptake capacity which could be owed to a different interaction between the functional groups of extracts with gel matrices (Bera and Dutta, 2017).
Fig. 5.
Water-uptake capacity of chitosan/alginate hydrogels over time. The error bars represent the standard deviation (n = 3).
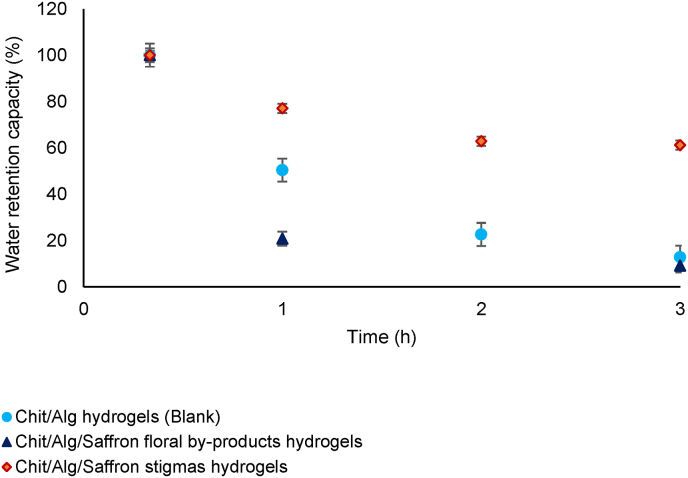
In view of practical applications, water retention capacity is also an important parameter to develop hydrogels with a strong water absorption capacity, endowing them widespread applications such as drug-delivery systems, among others (Lv et al., 2019).
Regarding the data of water retention capacity measured when hydrogels reached the maximum water-uptake capacity (equilibrium) (Fig. 6), chitosan/alginate hydrogels containing the as-obtained NADES- saffron stigmas extracts presented the highest water retention capacity being around 80% after 1 h, compared with chitosan/alginate hydrogels containing the NaDES-saffron floral by-products extracts (20%). Besides, as it was expected, the water retention decreased by the time in the blank and chitosan/alginate hydrogels with NaDES-saffron floral by-products extract, being lower than 20% after 3 h. Nevertheless, the water retention capacity of chitosan/alginate hydrogels with saffron stigmas remained stable from 2 h, being around 60%, showing adequate properties to conserve large amounts of water over time.
Fig. 6.
Water-retention capacity of chitosan/alginate hydrogels over time. The error bars represent the standard deviation (n = 3).
3.3.2. In vitro digestion
Currently, several studies are focused into the development of hydrogels as a tool for the delivery of plant-derived phenolic compounds, since they present biocompatible properties, low toxicity and exceptional biological properties (Micale et al., 2020).
Then, chitosan/alginate hydrogels could be promising candidates to retain and preserve high-value compounds. The impact of the in vitro digestion in the stability of the phenolic compounds of the saffron extracts incorporated in the hydrogels was evaluated.
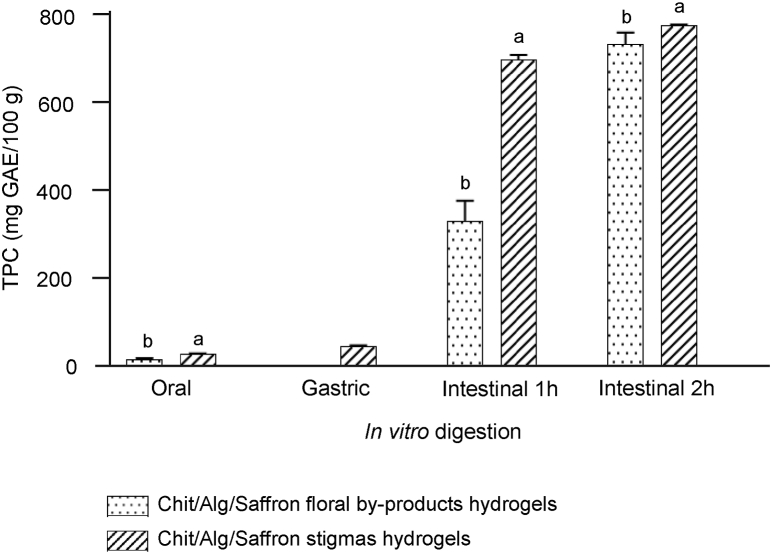
In Fig. 7 is shown the TPC of the different hydrogels during the oral, gastric and intestinal (1 h and 2 h) in vitro digestion. The results showed that there is an increase of TPC after 1 h of intestinal digestion, which, for the Chit/Alg/Saffron stigmas hydrogels, remained practically unchanged during the 2 h of intestinal digestion. This fact could be due to intestinal conditions (neutral pH, presence of pancreatic enzymes and bile salts) that would lead to an improvement in the release of bioactive compounds (Ketnawa et al., 2022). In the case of Chit/Alg/Saffron floral by-products hydrogels a significant increase of TPC was observed from 1 h to 2 h, which can be attributed to the different phenolic compounds that are present in the NaDES extract of saffron floral by-products. Furthermore, the TPC released after 180 min of oral and gastrointestinal in vitro digestion, for Chit/Alg/Saffron stigmas and Chi/Alg/Saffron floral by-products hydrogels, was around 56–58%.
Fig. 7.
Total phenolic content (TPC) of chitosan/alginate hydrogels during the oral and gastrointestinal in vitro digestion. The error bars represent the standard deviation and different lowercase letters indicate statistically significant differences (p ≤ 0.05) for each sample in each phase (n = 3).
Under gastric conditions, no phenolic content could be detected for chitosan/alginate hydrogels with NaDES-saffron floral by-products extracts and the TPC for the hydrogels with NaDES-saffron stigmas extracts was very low, although it was higher than the TPC of the oral phase. This gradual increase may be due to the release of some phenolic compounds bound to carbohydrates in conditions of low pH and pepsin action (Cunha et al., 2018).
Therefore, after the in vitro gastrointestinal digestion, TPC values were very high, remaining stable into the hydrogel's matrices, but further research is necessary to identify these phenolic compounds and to study if these bioactive compounds were absorbed in the colon to exert their beneficial effects on human health.
4. Conclusions
This study evaluated the implementation and optimization of UAE using NaDES as green extracting solvents to obtain valuable compounds from saffron flowers, providing new information of an efficient environmental-friendly process. This process is characterized by the ease of NaDES preparation, the availability and low cost of the NaDES components as well as the scalability of the NaDES preparation. Taking everything into account one can envisage that this extraction process could be industrially exploited. Moreover, this method also implemented the valorization of saffron floral by-products, a high-value biomass that is currently unexploited, contributing to the improvement of the sustainability of the saffron spice production and profitability of this industrial sector. According to the findings of this research, the novel chitosan/alginate hydrogels showed favorable properties and were suitable matrices to incorporate bioactive extracts which may be used as promising candidates for various applications like food or cosmetic, among others.
Funding
This article is supported by the Partnership for Research and Innovation in the Mediterranean Area (PRIMA) program, project SAFFROMFOOD. The PRIMA program is supported by the European Union. The research was also supported by the regional agency “Diputación de Alicante”, Alicante, Spain.
CRediT authorship contribution statement
Débora Cerdá-Bernad: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Ioanna Pitterou: Methodology. Andromachi Tzani: Conceptualization, Writing – review & editing, Supervision. Anastasia Detsi: Conceptualization, Writing – review & editing, Supervision. María José Frutos: Writing – review & editing, Supervision, Project administration, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments:
Thanks to Green Chemical Engineering Network towards upscaling sustainable processes (CA18224)—GREENERING funded by COST (European Cooperation in Science and Technology) for the STSM grant and thanks to the Spanish-Ministry of Education, Culture and Sport for the FPU-PhD fellowship (FPU18/02225).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100469.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Baysal K., Aroguz A.Z., Adiguzel Z., Baysal B.M. Chitosan/alginate crosslinked hydrogels: preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013;59:342–348. doi: 10.1016/j.ijbiomac.2013.04.073. [DOI] [PubMed] [Google Scholar]
- Bera S., Dutta D. Encapsulation and release of a bacterial carotenoid from hydrogel matrix: characterization, kinetics and antioxidant study. Eng. Life Sci. 2017;17(7):739–748. doi: 10.1002/elsc.201600238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly R., Lamkami T., Lompo M., Dubois J., Guissou I.P. 2016. DPPH Free Radical Scavenging Activity of Two Extracts from Agelanthus Dodoneifolius (Loranthaceae) Leaves. [Google Scholar]
- Çam M., Hışıl Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010;123(3):878–885. doi: 10.1016/j.foodchem.2010.05.011. [DOI] [Google Scholar]
- Cerdá-Bernad D., Baixinho J.P., Fernández N., Frutos M.J. Evaluation of microwave-assisted extraction as a potential green technology for the isolation of bioactive compounds from saffron (Crocus sativus L.) floral by-products. Foods. 2022;11(15):2335. doi: 10.3390/foods11152335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Bernad D., Clemente-Villalba J., Valero-Cases E., Pastor J.-J., Frutos M.-J. Novel insight into the volatile profile and antioxidant properties of Crocus sativus L. flowers. Antioxidants. 2022;11(9):1650. doi: 10.3390/antiox11091650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Bernad D., Costa L., Serra A.T., Bronze M.R., Valero-Cases E., Pérez-Llamas F., Candela M.E., Arnao M.B., Barberán F.T., Villalba R.G., García-Conesa M.-T., Frutos M.-J. Saffron against neuro-cognitive disorders: an overview of its main bioactive compounds, their metabolic fate and potential mechanisms of neurological protection. Nutrients. 2022;14(24) doi: 10.3390/nu14245368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Bernad D., Valero-Cases E., Pastor J.-J., Frutos M.J. Saffron bioactives crocin, crocetin and safranal: effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022;62(12):3232–3249. doi: 10.1080/10408398.2020.1864279. [DOI] [PubMed] [Google Scholar]
- Cerdá-Bernad D., Valero-Cases E., Pastor J.J., Frutos M.J., Perez-Llamas F. Probiotic red quinoa drinks for celiacs and lactose intolerant people: study of functional, physicochemical and probiotic properties during fermentation and gastrointestinal digestion. Int. J. Food Sci. Nutr. 2021:1–11. doi: 10.1080/09637486.2021.1921707. [DOI] [PubMed] [Google Scholar]
- Choi Y.H., van Spronsen J., Dai Y., Verberne M., Hollmann F., Arends I.W.C.E., Witkamp G.-J., Verpoorte R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011;156(4):1701–1705. doi: 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćorković I., Pichler A., Šimunović J., Kopjar M. Hydrogels: characteristics and application as delivery systems of phenolic and aroma compounds. Foods. 2021;10(6):1252. doi: 10.3390/foods10061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha I., Melo D., Verruck S., Maran B., Prudencio E., Amante E. Bioaccessibility of phenolic compounds of Araucaria angustifolia from seed water extracts during in vitro simulated gastrointestinal conditions. Food Nutr. Sci. 2018;9:1137–1146. doi: 10.4236/fns.2018.910082. [DOI] [Google Scholar]
- Da Porto C., Natolino A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: comparison of extraction methods. Food Chem. 2018;258:137–143. doi: 10.1016/j.foodchem.2018.03.059. [DOI] [PubMed] [Google Scholar]
- Detsi A., Kavetsou E., Kostopoulou I., Pitterou I., Pontillo A.R.N., Tzani A., Christodoulou P., Siliachli A., Zoumpoulakis P. Nanosystems for the encapsulation of natural products: the case of chitosan biopolymer as a matrix. Pharmaceutics. 2020;12(7):669. doi: 10.3390/pharmaceutics12070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCM Specifications for the saffrons protected by this Denomination of Origin, which includes those saffrons produced in a defined geographical area of the Autonomous Community of Castilla-La Mancha. Official Gazette Castilla-La Mancha. 1999;19(10):1098–1112. [Google Scholar]
- Ehterami A., Salehi M., Farzamfar S., Samadian H., Vaez A., Ghorbani S., Ai J., Sahrapeyma H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019;51:204–213. doi: 10.1016/j.jddst.2019.02.032. [DOI] [Google Scholar]
- Galanakis C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018;79:98–105. doi: 10.1016/j.tifs.2018.07.010. [DOI] [Google Scholar]
- Galanakis C.M. Sustainable applications for the valorization of cereal processing by-products. Foods. 2022;11(2) doi: 10.3390/foods11020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis C.M., Aldawoud T.M.S., Rizou M., Rowan N.J., Ibrahim S.A. Food ingredients and active compounds against the Coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods. 2020;9(11) doi: 10.3390/foods9111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis C.M., Rizou M., Aldawoud T.M.S., Ucak I., Rowan N.J. Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci. Technol. 2021;110:193–200. doi: 10.1016/j.tifs.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis C.M., Tsatalas P., Galanakis I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crop. Prod. 2018;111:30–37. doi: 10.1016/j.indcrop.2017.09.058. [DOI] [Google Scholar]
- Gawlik-Dziki U., Dziki D., Baraniak B., Lin R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2009;42(1):137–143. doi: 10.1016/j.lwt.2008.06.009. [DOI] [Google Scholar]
- Gehrke S.H., Fisher J.P., Palasis M., Lund M.E. Factors determining hydrogel permeability. Ann. N. Y. Acad. Sci. 2006;831(1):179–184. doi: 10.1111/j.1749-6632.1997.tb52194.x. [DOI] [PubMed] [Google Scholar]
- González-Centeno M.R., Comas-Serra F., Femenia A., Rosselló C., Simal S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): experimental kinetics and modeling. Ultrason. Sonochem. 2015;22:506–514. doi: 10.1016/j.ultsonch.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Hashemi Gahruie H., Parastouei K., Mokhtarian M., Rostami H., Niakousari M., Mohsenpour Z. Application of innovative processing methods for the extraction of bioactive compounds from saffron (Crocus sativus) petals. J. Appl. Res. Med. Aromatic Plant. 2020;19 doi: 10.1016/j.jarmap.2020.100264. [DOI] [Google Scholar]
- Inada K.O.P., Oliveira A.A., Revorêdo T.B., Martins A.B.N., Lacerda E.C.Q., Freire A.S., Braz B.F., Santelli R.E., Torres A.G., Perrone D., Monteiro M.C. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct.Foods. 2015;17:422–433. doi: 10.1016/j.jff.2015.06.002. [DOI] [Google Scholar]
- Karimi E., Oskoueian E., Hendra R., Jaafar H.Z.E. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15(9):6244–6256. doi: 10.3390/molecules15096244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketnawa S., Reginio F.C., Jr., Thuengtung S., Ogawa Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: a review. Crit. Rev. Food Sci. Nutr. 2022;62(17):4684–4705. doi: 10.1080/10408398.2021.1878100. [DOI] [PubMed] [Google Scholar]
- Koutsoukos S., Tsiaka T., Tzani A., Zoumpoulakis P., Detsi A. Choline chloride and tartaric acid, a Natural Deep Eutectic Solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019;241 doi: 10.1016/j.jclepro.2019.118384. [DOI] [Google Scholar]
- Lakka A., Grigorakis S., Karageorgou I., Batra G., Kaltsa O., Bozinou E., Lalas S., Makris D.P. Saffron processing wastes as a bioresource of high-value added compounds: development of a green extraction process for polyphenol recovery using a Natural Deep Eutectic Solvent. Antioxidants. 2019;8(12):586. doi: 10.3390/antiox8120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q., Wu M., Shen Y. Enhanced swelling ratio and water retention capacity for novel super-absorbent hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2019;583 doi: 10.1016/j.colsurfa.2019.123972. [DOI] [Google Scholar]
- Mathew B. Crocus sativus and its allies (Iridaceae) Plant Systemat. Evol. 1977;128(1):89–103. doi: 10.1007/BF00985174. [DOI] [Google Scholar]
- Melnyk J.P., Wang S., Marcone M.F. Chemical and biological properties of the world's most expensive spice: saffron. Food Res. Int. 2010;43(8):1981–1989. doi: 10.1016/j.foodres.2010.07.033. [DOI] [Google Scholar]
- Micale N., Citarella A., Molonia M.S., Speciale A., Cimino F., Saija A., Cristani M. Hydrogels for the delivery of plant-derived (poly)phenols. Molecules. 2020;25(14):3254. doi: 10.3390/molecules25143254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan J., Krishnamurthy N.P., Nagasundara Ramanan R., Raghunandan M.E., Galanakis C.M., Ooi C.W. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: extraction, characterization and kinetic studies. Food Chem. 2019;296:47–55. doi: 10.1016/j.foodchem.2019.05.135. [DOI] [PubMed] [Google Scholar]
- Ozkan K., Bayram Y., Karasu S., Karadag A., Sagdic O. In: Saffron. Galanakis C.M., editor. Academic Press; 2021. Chapter 3 - extraction of bioactive compounds from saffron species; pp. 99–141. [DOI] [Google Scholar]
- Sánchez-Vioque R., Rodríguez-Conde M.F., Reina-Ureña J.V., Escolano-Tercero M.A., Herraiz-Peñalver D., Santana-Méridas O. In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.) Ind. Crop. Prod. 2012;39:149–153. doi: 10.1016/j.indcrop.2012.02.028. [DOI] [Google Scholar]
- Sarfarazi M., Jafari S.M., Rajabzadeh G., Galanakis C.M. Evaluation of microwave-assisted extraction technology for separation of bioactive components of saffron (Crocus sativus L.) Ind. Crop. Prod. 2020;145 doi: 10.1016/j.indcrop.2019.111978. [DOI] [Google Scholar]
- Shewan H.M., Stokes J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013;119(4):781–792. doi: 10.1016/j.jfoodeng.2013.06.046. [DOI] [Google Scholar]
- Skarpalezos D., Detsi A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019;9(19):4169. doi: 10.3390/app9194169. [DOI] [Google Scholar]
- Stelluti S., Caser M., Demasi S., Scariot V. Sustainable processing of floral bio-residues of saffron (Crocus sativus L.) for valuable biorefinery products. Plants. 2021;10(3):523. doi: 10.3390/plants10030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzani A., Kalafateli S., Tatsis G., Bairaktari M., Kostopoulou I., Pontillo A.R.N., Detsi A. Natural deep eutectic solvents (NaDESs) as alternative green extraction media for ginger (Zingiber officinale roscoe) Sustain. Chem. 2021;2(4):576–598. doi: 10.3390/suschem2040032. [DOI] [Google Scholar]
- Wen C., Zhang J., Zhang H., Dzah C.S., Zandile M., Duan Y., Ma H., Luo X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops – a review. Ultrason. Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.