Highlights
-
•
The cationic alpha-helical amphibian AMPs (CαAMPs) from different amphibian species have been classified into five categories from net charge +1 to +5, and the antibacterial activity of each category has been summarized using MIC values.
-
•
Changing the charge of the peptides in an optimum way will provide CαAMPs with the highest antibacterial and lowest hemolytic activity.
-
•
Establishing a validated standard approach to assess AMPs' antibacterial and hemolytic activities is essential.
Keywords: Antimicrobial peptide, Alpha-helical, Amphibian, Cationic, Charge
Abstract
Antibiotic resistance is a severe threat to the world's public health, which has increased the need to discover novel antibacterial molecules. In this context, an emerging class of naturally occurring short peptide molecules called antimicrobial peptides (AMPs) has been considered potent antibacterial agents. Amphibians are one of the significant sources of AMPs, which have been extensively studied for the last few decades. Most amphibian AMPs are cationic, and several of these cationic AMPs adopt a well-defined alpha-helical structure in the presence of bacterial membranes. These cationic alpha-helical amphibian AMPs (CαAMPs) can selectively and preferentially bind with the negatively charged surfaces of Gram-positive and Gram-negative bacteria through electrostatic interaction, considered the main reason for their antibacterial activities. Here, we categorized these CαAMPs according to their charge, and to calculate the charge density; we divided the charge of each peptide by its corresponding length. To investigate the effect of charge among these categories, charge or charge density under each charge category was plotted against their corresponding minimum inhibitory concentration (MIC). Moreover, the effect of charge modification of some CαAMPs under specific charge categories in the context of MIC and hemolysis was also discussed. The information in this review will help us understand the antibacterial activity of accessible CαAMPs depending on each charge category across species. Additionally, this study suggests that designing novel functional antibacterial agents requires charge modification optimally.
Graphical abstract

1. Introduction
With the increasing usage and administration of antibiotics, the efficacy of existing antimicrobial agents is becoming resisted by most pathogens at an alarming rate (Michael et al., 2014). Besides, mutation (Read and Woods, 2014) and the impetuous evolving nature of the bacterial genome (San Millan, 2018; Rozwandowicz et al., 2018) and gaining the antibacterial resistant genome via lateral gene transfer (Sun et al., 2019) are also prominent factors of antibacterial resistance. Scientists and researchers are trying to find alternative options, especially broad-spectrum antibiotics, with complete devotion and sincerity to overcome this threatening situation (Levy and Marshall, 2004). Antimicrobial peptides (AMPs) can be an excellent option as effective antimicrobial agents against resistant variants.
AMPs are a varied class of molecules consisting of short polypeptide sequences that are synthesized naturally in almost every multicellular organism as their first line of defense (Zasloff, 2002). They are the immunoregulatory molecules of the organisms, regulated and produced for their inherited immunity (Zhang, 2016). Despite their differences in structural properties, AMPs share several qualities. Because most AMPs are cationic, with a net positive charge range from +3 to +11, they can preferentially connect to the negatively charged surface of bacteria via electrostatic binding (Hancock and Diamond, 2000). Gram-negative bacteria (e.g., Escherichia coli, Pseudomonas aeruginosa) have an extra outer membrane enriched with anionic lipopolysaccharide, whereas Gram-positive bacteria (e.g., Staphylococcus aureus, Streptococcus pneumoniae, and Bacillus subtilis) have a thick peptidoglycan layer enriched with anionic teichoic acids surrounding their cytoplasmic membrane. Furthermore, both Gram-positive and Gram-negative bacteria have the anionic cytoplasmic membranes due to the presence of phosphatidylglycerol (PG) and cardiolipin. Unlike bacteria, human RBCs have a cell membrane of neutral phospholipids such as phosphatidylcholine, sphingomyelin, and phosphatidylethanolamine (Otvos, 2005). As a result, these peptides have weak interaction with this neutral membrane. Due to these structural differences, they have lesser toxicity to mammalian cells (lower hemolytic activity) but higher toxicity to both Gram-positive and Gram-negative bacteria. The peptides' hydrophobic residues cause the molecule to bind tightly to the hydrophobic portion of lipid membranes, while their cationic residues are responsible for electrostatic interactions with negatively charged bacterial surfaces (Ebenhan et al., 2014). These electrostatic and hydrophobic interactions disrupt the bacterial membrane. Several models have been proposed to explain the mechanism of disruption, such as the barrel-stave, toroidal pore, and carpet model. The structural features of the peptide and the lipid membrane affect the mode of membrane disruption (Sani and Separovic, 2016).
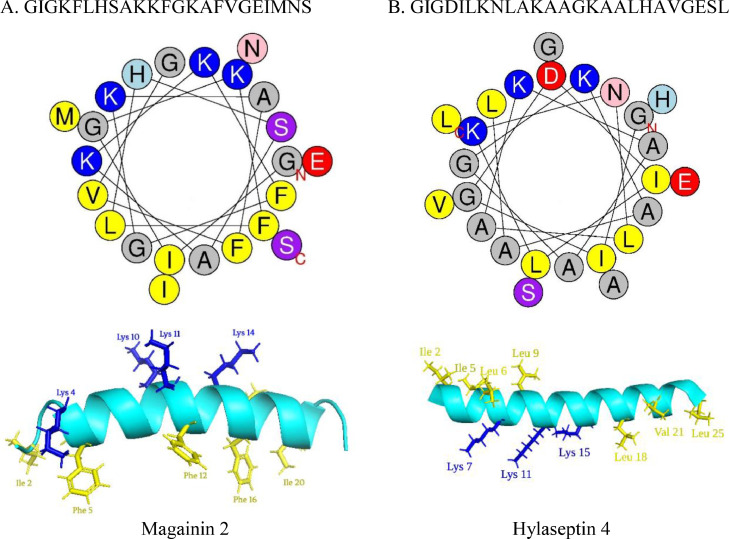
Cationic AMPs are obtained from various sources, and amphibians are one of the major sources of AMPs. Many amphibian, cationic AMPs remain unstructured in an aqueous solvent. However, in the presence of a lipid membrane, they are converted into a well-defined secondary structure (e.g., alpha-helix or beta-sheet), which plays an important role in antibacterial activity (Takahashi et al., 2010; Hasan et al., 2022). These amphibian, cationic AMPs having alpha-helical structures can be called as CαAMPs. In the alpha-helical structure, a rod-like structure is formed by a tight right-handed twist in the amino acid chain. This shape is caused by hydrogen bonding between the hydrogen in an amino group and the oxygen in a carboxyl group. Most alpha helices are amphiphilic, which means they contain both hydrophilic and hydrophobic regions. Usually, the hydrophilic residues are located opposite to the hydrophobic residues. The secondary structure and helical wheel projection of two well-studied CαAMPs are shown in Fig. 1.
Fig. 1.
Helical wheel representation and alpha-helix structure of Magainin 2 (A) and Hylaseptin 4 (B). The helical wheel representations were obtained from https://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py. The alpha-helix structure was drawn by PyMOL (version 2.4.1.). In both A & B, cationic and hydrophobic residues are represented by blue and yellow color, respectively. The letters 'N' and 'C' stand for the N- and C-terminal, respectively.
These CαAMPs have received much attention in recent years due to their powerful broad-spectrum antibacterial properties, and some notable CαAMPs are summarized in Table 1. Here, we classified these CαAMPs based on their charge, and to calculate the charge density, we divided the charge of each peptide by its length. To investigate the effect of charge among these categories, charge or charge density under each charge category was plotted against the corresponding minimum inhibitory concentration (MIC). Furthermore, the effect of charge modification of some CαAMPs in specific charge categories on MIC and hemolysis has been discussed. It is challenging to compare the antibacterial activities of these CαAMPs because numerous researchers employed various microbial strains to investigate the MIC values to assess the antimicrobial activity. For ease of comparison, we have selected a particular strain for a single plot or graph from different literature. The information in this review will be helpful for understanding the antibacterial activity of accessible CαAMPs depending on each charge category across species. In addition, this study suggests that designing novel functional antibacterial agents requires charge modification in an optimal way.
Table 1.
Some notable amphibian alpha-helical peptides with their sequences, sources, and major bioactivities.
| Name | Length | Sequence | Source | Major bioactivities | Reference |
|---|---|---|---|---|---|
| Aurein 2.5 | 16 | GLFDIVKKVVGAFGSL | Ranoidea aurea | Antibacterial, antifungal, anticancer | (Rozek, 2000) |
| Brevinin-1E | 24 | FLPLLAGLAANFLPKIFCKITRKC | Rana esculenta | Antibacterial | (Simmaco et al., 1994) |
| Caerin-1.1 | 25 | GLLSVLGSVAKHVLPHVVPVIAEHL | Litoria splendida | Antibacterial, antiviral | (Wong et al., 1997) |
| Citropin-1.1 | 16 | GLFDVIKKVASVIGGL | Litoria citropa | Antibacterial | (Wegener et al., 1999) |
| Distinctin | 25 | NLVSGLIEARKYLEQLHRKLKNCKV | Phyllomedusa distincta | Antibacterial | (Batista et al., 2001) |
| Esculentin-1 | 46 | GIFSKLGRKKIKNLLISGLKNVGKEVGMDVVRTGIDIAGCKIKGEC | Rana esculenta | Antibacterial | (Rozek, 2000) |
| Gaegurin-1 | 33 | SLFSLIKAGAKFLGKNLLKQGACYAACKASKQC | Rana rugosa | Antibacterial, antifungal, antiprotozoal | (Park et al., 1994) |
| Hylaseptin P1 | 14 | GILDAIKAIAKAAG | Hyla punctata | Antibacterial | (Prates et al., 2004a) |
| Kassinatuerin-1 | 21 | GFMKYIGPLIPHAVKAISDLI | Kassina senegalensis | Antibacterial, antifungal | (Mattute et al., 2000) |
| Magainin 1 | 23 | GIGKFLHSAGKFGKAFVGEIMKS | Xenopus laevis | Antibacterial, antifungal, antiviral, antiprotozoal | (Zasloff, 1987) |
| Magainin 2 | 23 | GIGKFLHSAKKFGKAFVGEIMNS | Xenopus laevis | Antibacterial, antifungal, antiviral, antiprotozoal | (Zasloff, 1987) |
| Nigrocin-1 | 33 | GLLDSIKGMAISAGKGALQNLLKVASCKLDKTC | Rana nigromaculata | Antibacterial, antifungal | (Park et al., 2001) |
| Ocellatin-1 | 25 | GVVDILKGAGKDLLAHLVGKISEKV | Leptodactylus ocellatus | Antibacterial | (Nascimento et al., 2004) |
| PGLa | 21 | GMASKAGAIAGKIAKVALKAL | Xenopus laevis | Antibacterial, antifungal | (Andreu et al., 1985) |
| Phylloseptin-1 | 19 | FLSLIPHIVSGVASIAKHF | Phyllomedusa sauvagei | Antibacterial, antifungal | (Zhang et al., 2010) |
| Pleurain-A1 | 26 | SIITMTKEAKLPQLWKQIACRLYNTC | Babina pleuraden | Antibacterial, antifungal | (Wang et al., 2007) |
| Ranatuerin-1 | 25 | SMLSVLKNLGKVGLGFVACKINKQC | Rana catesbeiana | Antibacterial, antifungal | (Goraya et al., 1998) |
| Rugosin-A | 33 | GLLNTFKDWAISIAKGAGKGVLTTLSCKLDKSC | Rana rugosa | Antibacterial | (Suzuki et al., 1995) |
| Temporin-A | 13 | FLPLIGRVLSGIL | Rana temporaria | Antibacterial, antifungal, antiviral | (Simmaco et al., 1996) |
2. Effect of charge on CαAMPs from different sources
The net charge of CαAMPs is one of the most critical parameters for antibacterial activities. However, other structural parameters such as peptide length, charge, amino acid composition, helicity, amphiphilicity, etc., also contribute in their activities. Investigating the effects of charge or charge density on the antibacterial activity of these CαAMPs derived from various amphibian sources is crucial.
The net charge of a peptide can be defined as the sum of all the charges of its polar groups of the peptide, which is calculated by the following expression:
Here, ƩQ−, the sum of the negative charge for functional groups (e.g., -SH, -PhOH, -COOH), and ƩQ+, the sum of the positive charge for functional groups (e.g., -NH3+, =NH2+, NH+) of the certain peptide (Lee et al., 2016). For example, magainin 2 has the sequence of GIGKFLHSAKKFGKAFVGEIMNS and contains 4 Lys-residues, each having +1 charge and 1 Glu-residue having −1 charge. As a result, Magainin 2 has a net charge of +3. Then, the charge density of each CαAMP is calculated by net charge divided by the AMP length (i.e., the total number of amino acids in the sequence). As magainin 2 contains 23 amino acids, the charge density of this CαAMP is 0.1304.
At first, the CαAMPs from different amphibian species have been classified into five categories from net charge +1 to +5. Table 2 summarizes the length, charge density, MIC, and source of several CαAMPs of these five categories. For ease of comparison, we used the MIC values obtained from E. coli ATCC 25922.
Table 2.
Charge density and minimum inhibitory concentration of several AMPs against Escherichia coli ATCC 25922.
| Peptide | Sequence | Length | Charge density | MIC (μM) | Species | Reference |
|---|---|---|---|---|---|---|
| Charge +1 | ||||||
| Aurein-1.2 | GLFDIIKKIAESF | 13 | 0.076 | 16 | Litoria aurea, Litoria raniformis | (Rozek et al., 2000) |
| Caerin-1.1 | GLLSVLGSVAKHVLPHVVPVIAEHL | 25 | 0.04 | 8.45 | Litoria splendida | (Wong et al., 1997) |
| Nigrocin-2GRa | GLLSGILGAGKHIVCGLSGLC | 21 | 0.047 | 25 | Rana grahami | (Conlon et al., 2006) |
| Ocellatin-4 | GLLDFVTGVGKDIFAQLIKQI | 21 | 0.047 | 64 | Leptodactylus ocellatus | (Nascimento et al., 2007) |
| Phylloseptin-2 | FLSLIPHAINAVSAIAKHS | 19 | 0.052 | 15 | Phyllomedusa genus | (Leite et al., 2005) |
| Charge +2 | ||||||
| Citropin-1.1 | GLFDVIKKVASVIGGL | 16 | 0.125 | 10 | Litoria citropa | (Wegener et al., 1999) |
| Hylaseptin-P1, HSP1 | GILDAIKAIAKAAG | 14 | 0.142 | 24.4 | Hypsiboas punctatus | (Prates et al., 2004b) |
| Japonicin-1 | FFPIGVFCKIFKTC | 14 | 0.142 | 30 | Rana japonica | (Isaacson et al., 2002) |
| Kassinatuerin-1 | GFMKYIGPLIPHAVKAISDLI | 21 | 0.095 | 6.25 | Kassina senegalensis | (Conlon et al., 2005b) |
| Temporin-A | FLPLIGRVLSGIL | 13 | 0.153 | 100 | Rana temporaria | (Simmaco et al., 1996) |
| Charge +3 | ||||||
| Alyteserin-1c | GLKEIFKAGLGSLVKGIAAHVAS | 23 | 0.13 | 25 | Alytes obstetricans | (Michael Conlon et al., 2009) |
| Brevinin-1SPb | FLPIIAGMAAKVICAITKKC | 20 | 0.15 | 50 | Rana septentrionalis | (Bevier et al., 2004) |
| Brevinin-2LTa | GAFGDLLKGVAKEAGMKLLNMAQCKLSGKC | 30 | 0.1 | 32.5 | Hylarana latouchii | (Wang et al., 2012) |
| Hylin a1 | IFGAILPLALGALKNLIK | 18 | 0.166 | 32 | Hypsiboas albopunctatus | (Castro et al., 2009) |
| Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | 23 | 0.13 | 12.5 | Xenopus laevis | (Zasloff, 1987) |
| Ocellatin-F1 | GVVDILKGAAKDIAGHLASKVMNKL | 25 | 0.12 | 100 | Leptodactylus fallax | (Rollins-Smith et al., 2005) |
| Palustrin-2AJ1 | GFMDTAKNVAKNVAVTLIDKLRCKVTGGC | 29 | 0.103 | 13 | Amolops jingdongensis | (Chen et al., 2012b) |
| Palustrin-2c | GFLSTVKNLATNVAGTVIDTLKCKVTGGCRS | 31 | 0.096 | 120 | Rana palustris | (Basir et al., 2000) |
| Pseudin-2 | GLNALKKVFQGIHEAIKLINNHVQ | 24 | 0.124 | 8 | Pseudis paradoxa | (Park et al., 2011) |
| Ranalexin-1Ca | FLGGLMKAFPALICAVTKKC | 20 | 0.15 | 4 | Rana clamitans | (Halverson et al., 2000) |
| Raniseptin-1 | AWLDKLKSLGKVVGKVALGVAQNYLNPQQ | 29 | 0.103 | 5 | Hypsiboas raniceps | (Magalhães et al., 2008) |
| XPF-St1 | GVWSTVLGGLKKFAKGGLEAIVNPK | 25 | 0.12 | 8 | Xenopus tropicalis | (Ali et al., 2001) |
| Charge +4 | ||||||
| Ascaphin-1 | GFRDVLKGAAKAFVKTVAGHIAN | 23 | 0.173 | 3 | Ascaphus truei | (Conlon et al., 2004) |
| Brevinin-1AUa | FLPILAGLAAKLVPKVFCSITKKC | 24 | 0.166 | 13 | Rana aurora | (Conlon et al., 2005a) |
| Brevinin-1VLa | FLGAIAGVAAKFLPKVFCFITKKC | 24 | 0.166 | 25 | Lithobates vaillanti | (Conlon et al., 2009) |
| Brevinin-2LTb | SILDKIKNVALGVARGAGTGILKALLCKLDKSC | 33 | 0.121 | 30 | Hylarana latouchii | (Wang et al., 2012) |
| Brevinin-2PRb | GLMSLFRGVLKTAGKHIFKNVGGSLLDQAKCKITGEC | 37 | 0.108 | 3 | Rana pirica | (Conlon et al., 2004) |
| Japonicin-2 | FGLPMLSILPKALCILLKRKC | 21 | 0.19 | 12 | Rana japonica | (Isaacson et al., 2002) |
| Palustrin-2CG1 | GLWNTIKEAGKKFAINVLDKIRCGIAGGCKT | 31 | 0.129 | 150 | Amolops chunganensis | (Yang et al., 2012) |
| Palustrin-3a | GIFPKIIGKGIKTGIVNGIKSLVKGVGMKVFKAGLNNIGNTGCNEDEC | 48 | 0.083 | 1 | Rana palustris | (Basir et al., 2000) |
| Ranatuerin-1 | SMLSVLKNLGKVGLGFVACKINKQC | 25 | 0.16 | 20 | R. catesbeiana | (Goraya et al., 1998) |
| XT-1 | GFLGPLLKLAAKGVAKVIPHLIPSRQQ | 27 | 0.148 | 6 | Xenopus tropicalis | (Ali et al., 2001) |
| Charge +5 | ||||||
| Brevinin-2PRd | GLMSVLKGVLKTAGKHIFKNVGGSLLDQAKCKITGQC | 37 | 0.135 | 3 | Rana pirica | (Conlon et al., 2004) |
| Brevinin-2PRe | GLLSVLKGVLKTTGKHIFKNVGGSLLDQAKCKISGQC | 37 | 0.135 | 3 | Rana pirica | (Conlon et al., 2004) |
| Esculentin-2B | GLFSILRGAAKFASKGLGKDLTKLGVDLVACKISKQC | 37 | 0.135 | 1 | Rana berlandieri | (Goraya et al., 2000) |
| Palustrin-1c | ALSILRGLEKLAKMGIALTNCKATKKC | 27 | 0.185 | 28 | Rana palustris | (Basir et al., 2000) |
| Palustrin-1d | ALSILKGLEKLAKMGIALTNCKATKKC | 27 | 0.185 | 28 | Rana palustris | (Basir et al., 2000) |
| Palustrin-3AR | GIFPKIIGKGIVNGIKSLAKGVGMKVFKAGLNNIGNTGCNNRDEC | 45 | 0.111 | 6 | Rana areolata | (Ali et al., 2002) |
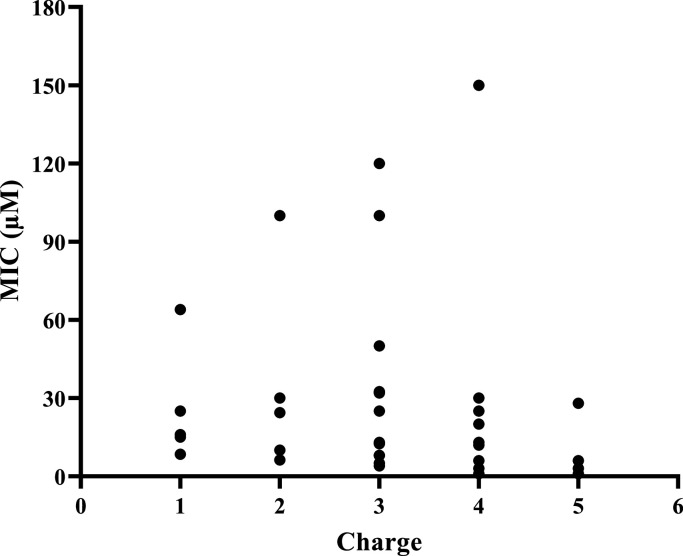
The MIC values of each peptide category were plotted against their corresponding net charges to compare their antibacterial activity (Fig. 2).
Fig. 2.
MIC values of different CαAMPs based on charges.
Fig. 2 shows that for most of the CαAMPs (discussed in Table 2), the MIC values are less than 50 µM (E. coli ATCC 25922). Moreover, the antibacterial activity is not proportional to the net charge of CαAMPs obtained from different sources. In this study, we used only one bacterial strain's MIC values. Further investigations may be performed using other strains, although it's difficult to find similar data from published works.
In the same charge group, MIC values may also vary significantly. For example, brevinin-1SPb and ranalexin-1Ca have the same net charge (+3) and peptide length but different MIC values (Bevier et al., 2004; Halverson et al., 2000). This variation may be due to differences in other structural parameters such as hydrophobicity. XT-1 shows 25 times more antibacterial activity than palustrin-2CG1, although they have the same net charge (+4) (Ali et al., 2001; Yang et al., 2012). This may be due to the difference in peptide length, interfacial hydrophobicity, amphiphilicity, and angle subtended by the hydrophobic residues (Hasan et al., 2022). In the case of temporin-PF, the highest helicity-forming peptide showed the strongest antibacterial and hemolytic activities than the other analogs though their charge was the same (+2). Therefore, peptides' helix content and aggregation pattern are directly related to the antimicrobial and hemolytic activities (Zai et al., 2021).
In the next step, we plot charge density and the MIC values to observe the relationship between charge density and the antibacterial activity of CαAMPs for charges from +1 to +5.
According to Fig. 3, most of the MIC values of CαAMPs lie between the charge density of 0.10 to 0.17. Using this bacterial strain, no specific relationship has been found between the charge density and the antimicrobial activity of different categories of CαAMPs. A similar plot should be made using other bacterial species to get a comprehensive relationship between the charge density and the antimicrobial activity.
Fig. 3.
Relationship among charge densities of CαAMPs of different categories and minimal inhibitory concentration (MIC) values against E. coli ATCC 25922.
3. Effect of charge modification on single CαAMPs
To improve the antibacterial activities of CαAMPs, several analogs have been synthesized by altering their net charges. Positively charged amino acids such as Arg, Lys, and His-have been introduced or deducted to modify the net charge of the alpha-helices. This modification can be done in both the helix or the N- and C-terminal. For example, several analogs of amphibian antimicrobial peptides medusin-PT have been synthesized by adding lysine to its nonpolar face to increase the peptide's net charge (Gao et al., 2017). In the case of phylloseptin-PT, net charge was modified by replacing Asn-and His, with Lys-and d-lysin (Gao et al., 2016). On the other hand, positively charged residue Lys-has been added to the N- and C-terminal of magainin 2 to increase the net charge (Bessalle et al., 1992).
Modification of net charge affects both the antibacterial and hemolytic activities of CαAMPs. Increased net charge of CαAMPs will increase their antibacterial activity against Gram-negative and Gram-positive bacteria. An excessively high net charge will lead to increased hemolytic propensity and decreased antimicrobial activity. For example, magainin 2 analogs showed the maximum antimicrobial activity at +5 charge with low hemolytic activity. When the charge is greater than +5, the hemolytic activity rapidly increases while the antibacterial activity decreases (Dathe et al., 2001). In the case of brevinin-1OS, the antibacterial activity decreases with the decrease of net charge. There was a complete loss in antibacterial activity after reducing the net charge from +3 to +1 (Zhou et al., 2020).
The net charge also influences the antibacterial and hemolytic activities of Brevinin-2-related peptide (B2RP: GIWDTIKSMGKVFAGKILQNL) analogs (Conlon et al., 2009). The relationship is shown in Fig. 4.
Fig. 4.
Relationship among the charge of different analogs of Brevinin-2 related peptide (B2RP) with the Minimum Inhibitory Concentration (MIC) against E. coli (ATCC 25726) and hemolysis (LC50) of the human erythrocyte. The LC50 is the mean concentration of peptides producing 50% hemolysis. ★ Marked points indicate greater than the value of that point (> 200).
In Fig. 4, the maximum antibacterial activity (less MIC) is observed at charges +4 and +5, further increase in the charge will decrease the activity. Peptides with a net charge of +5 and +6 exhibited almost no hemolytic activity (LC50 = >200 μM). Therefore, the net charge +5 can be considered the optimum charge of B2RP, which provides higher antibacterial activity (low MIC) and lower hemolytic activity (high LC50). However, the MIC values can be changed due to the variation in the bacterial strain. Changing the net charge of the peptides significantly influences the antibacterial and hemolytic activity of the peptide. During the modification of the net charge, one should find out the optimum charge for that particular AMPs by plotting MIC and LC50 values against the corresponding charge.
4. Conclusion
The CαAMPs can be used as a functional antibacterial agent for the current antibiotic resistance situation. The net charge of the peptides greatly regulates the antibacterial activity of these peptides. The CαAMPs from different amphibian species have been classified into five categories from net charge +1 to +5, and the antibacterial activity of each category has been observed using MIC values of E. coli ATCC 25922. Most of the CαAMPs of different charge groups have MIC values less than 50 µM, which lie between the charge density of 0.10 to 0.17. A similar plot should be made using other bacterial species to get a comprehensive relationship between the charge density and the antimicrobial activity. Changing the charge of the peptides influences the antibacterial and hemolytic activity. During the modification of net charge, one should find the optimum charge for that particular AMPs by plotting antibacterial and hemolysis values against the corresponding charge. The antibacterial activity of CαAMPs can also be increased by modulating other structural parameters such as peptide length, charge, amino acid composition, helicity, amphiphilicity, etc. For each modification, however, the optimum changes must be determined using a similar approach. As there is no universal standard for observing the antimicrobial activity of CαAMPs, researchers use varied ways for their study. There is a significant variation in the process of peptide preparation, the selection of microorganisms, the measurement of the MIC values, etc. That's why it's not easy to compare all the available data. There should be a validated standard procedure to determine the antibacterial and hemolytic activities of CαAMPs. The information in this review will be useful in understanding the antibacterial activity of available CαAMPs across species and charge categories. Furthermore, our research implies that creating novel effective antibacterial agents necessitates an appropriate charge adjustment.
Funding
This work didn't get any funding.
CRediT authorship contribution statement
Md. Monirul Islam: Conceptualization, Methodology, Software. Fahim Asif: Data curation, Writing – original draft. Sabbir Uz Zaman: Writing – original draft, Investigation. Md. Kamrul Hasan Arnab: Visualization, Software. Md. Mostafizur Rahman: Validation. Moynul Hasan: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Ali M.F., Lips K.R., Knoop F.C., Fritzsch B., Miller C., Conlon J.M. Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata. Biochim. Biophys. Acta Proteins Proteom. 2002;1601:55–63. doi: 10.1016/s1570-9639(02)00432-6. [DOI] [PubMed] [Google Scholar]
- Ali M.F., Soto A., Knoop F.C., Conlon J.M. Antimicrobial peptides isolated from skin secretions of the diploid frog, Xenopus tropicalis (Pipidae) Biochim. Biophys. acta. 2001;1550:81–89. doi: 10.1016/s0167-4838(01)00272-2. [DOI] [PubMed] [Google Scholar]
- Andreu D., Aschauer H., Kreil G., Merrifield R.B. Solid-phase synthesis of PGLa and isolation of its natural counterpart, PGLa [PGLa-(4-24)] from skin secretion of Xenopus laevis. Eur. J. Biochem. 1985;149:531–535. doi: 10.1111/j.1432-1033.1985.tb08957.x. [DOI] [PubMed] [Google Scholar]
- Basir Y.J., Knoop F.C., Dulka J., Conlon J.M. Multiple antimicrobial peptides and peptides related to bradykinin and neuromedin N isolated from skin secretions of the pickerel frog, Rana palustris. Biochim. Biophys. Acta. 2000;1543:95–105. doi: 10.1016/s0167-4838(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Batista C.V., Scaloni A., Rigden D.J., Silva L.R., Rodrigues Romero A., Dukor R., Bloch C. A novel heterodimeric antimicrobial peptide from the tree-frog Phyllomedusa distincta. FEBS Lett. 2001;494:85–89. doi: 10.1016/s0014-5793(01)02324-9. [DOI] [PubMed] [Google Scholar]
- Bessalle R., Haas H., Goria A., Shalit I., Fridkin M. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob. Agents Chemother. 1992;36:313–317. doi: 10.1128/aac.36.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevier C.R., Sonnevend A., Kolodziejek J., Nowotny N., Nielsen P.F., Conlon J.M. Purification and characterization of antimicrobial peptides from the skin secretions of the mink frog (Rana septentrionalis) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;139:31–38. doi: 10.1016/j.cca.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Castro M.S., Ferreira T.C.G., Cilli E.M., Crusca E., Jr Mendes-Giannini, M J.S., Sebben A., Fontes W. Hylin a1, the first cytolytic peptide isolated from the arboreal South American frog Hypsiboas albopunctatus (“spotted treefrog”) Peptides. 2009;30:291–296. doi: 10.1016/j.peptides.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Chen Z., Yang X., Liu Z., Zeng L., Lee W., Zhang Y. Two novel families of antimicrobial peptides from skin secretions of the Chinese torrent frog, Amolops jingdongensis. Biochimie. 2012;94:328–334. doi: 10.1016/j.biochi.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Conlon J.Michael, Abraham B., Galadari S., Knoop F.C., Sonnevend A., Pál T. Antimicrobial and cytolytic properties of the frog skin peptide, kassinatuerin-1 and its L- and D-lysine-substituted derivatives. Peptides. 2005;26:2104–2110. doi: 10.1016/j.peptides.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Ahmed E., Condamine E. Antimicrobial properties of brevinin-2-related peptide and its analogs: efficacy against multidrug-resistant Acinetobacter baumannii. Chem. Biol. Drug Des. 2009;74:488–493. doi: 10.1111/j.1747-0285.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Raza H., Coquet L., Jouenne T., Leprince J., Vaudry H., King J.D. Purification of peptides with differential cytolytic activities from the skin secretions of the Central American frog, Lithobates vaillanti (Ranidae) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009;150:150–154. doi: 10.1016/j.cbpc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Sonnevend A., Davidson C., Demandt A., Jouenne T. Host-defense peptides isolated from the skin secretions of the Northern red-legged frog Rana aurora aurora. Dev. Comp. Immunol. 2005;29:83–90. doi: 10.1016/j.dci.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Sonnevend A., Davidson C., Smith D.D., Nielsen P.F. The ascaphins: a family of antimicrobial peptides from the skin secretions of the most primitive extant frog, Ascaphus truei. Biochem. Biophys. Res. Commun. 2004;320:170–175. doi: 10.1016/j.bbrc.2004.05.141. [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Sonnevend A., Patel M., Al-Dhaheri K., Nielsen P.F., Kolodziejek J., Pál T. A family of brevinin-2 peptides with potent activity against Pseudomonas aeruginosa from the skin of the Hokkaido frog, Rana pirica. Regul. Pept. 2004;118:135–141. doi: 10.1016/j.regpep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Conlon J.Michael, Al-Ghaferi N., Abraham B., Jiansheng H., Cosette P., Leprince J., Vaudry H. Antimicrobial peptides from diverse families isolated from the skin of the Asian frog, Rana grahami. Peptides. 2006;27:2111–2117. doi: 10.1016/j.peptides.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Conlon J.Michael, Demandt A., Nielsen P.F., Leprince J., Vaudry H., Woodhams D.C. The alyteserins: two families of antimicrobial peptides from the skin secretions of the midwife toad Alytes obstetricans (Alytidae) Peptides. 2009;30:1069–1073. doi: 10.1016/j.peptides.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Dathe M., Nikolenko H., Meyer J., Beyermann M., Bienert M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 2001;501:146–150. doi: 10.1016/s0014-5793(01)02648-5. [DOI] [PubMed] [Google Scholar]
- Ebenhan T., Gheysens O., Kruger H.G., Zeevaart J.R., Sathekge M.M. Antimicrobial peptides: their role as infection-selective tracers for molecular imaging. BioMed Res. Int. 2014 doi: 10.1155/2014/867381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Wu D., Wang L., Lin C., Ma C., Xi X., Shaw C. Targeted modification of a novel amphibian antimicrobial peptide from Phyllomedusa tarsius to enhance its activity against MRSA and microbial biofilm . Front. Microbiol. 2017;8:628. doi: 10.3389/fmicb.2017.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Wu D., Xi X., Wu Y., Ma C., Zhou M., Shaw C. Identification and characterisation of the antimicrobial peptide, phylloseptin-PT, from the skin secretion of Phyllomedusa tarsius, and comparison of activity with designed, cationicity-enhanced analogues and diastereomers. Molecules. 2016;21:1667. doi: 10.3390/molecules21121667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya J., Wang Y., Li Z., O'flaherty M., Knoop F.C., Platz J.E., Conlon J.M. Peptides with antimicrobial activity from four different families isolated from the skins of the North American frogs Rana luteiventris. Eur. J. Biochem. 2000;267:894–900. doi: 10.1046/j.1432-1327.2000.01074.x. [DOI] [PubMed] [Google Scholar]
- Goraya Jadvinder, Knoop F.C., Conlon J.M. Ranatuerins: antimicrobial peptides isolated from the skin of the American Bullfrog, Rana catesbeiana. Biochem. Biophys. Res. Commun. 1998;250:589–592. doi: 10.1006/bbrc.1998.9362. [DOI] [PubMed] [Google Scholar]
- Halverson T., Basir Y.J., Knoop F.C., Conlon J.M. Purification and characterization of antimicrobial peptides from the skin of the North American green frog Rana clamitans. Peptides. 2000;21:469–476. doi: 10.1016/s0196-9781(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Hancock R.E., Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- Hasan M., Hossain F., Dohra H., Yamazaki M. Role of interfacial hydrophobicity in antimicrobial peptide magainin 2-induced nanopore formation. Biochem. Biophys. Res. Commun. 2022;630:50–56. doi: 10.1016/j.bbrc.2022.08.094. [DOI] [PubMed] [Google Scholar]
- Hasan M., Islam M.M., Rahman M.M. A Review on structure - activity relationship of antimicrobial peptide magainin 2. Dhaka Univ. J. Pharm. Sci. 2022;20:427–434. [Google Scholar]
- Isaacson T., Soto A., Iwamuro S., Knoop F.C., Conlon J.M. Antimicrobial peptides with atypical structural features from the skin of the Japanese brown frog Rana japonica. Peptides. 2002;23:419–425. doi: 10.1016/s0196-9781(01)00634-9. [DOI] [PubMed] [Google Scholar]
- Lee T.H., N. Hall K., Aguilar M.I. Antimicrobial peptide structure and mechanism of action: a focus on the role of membrane structure. Curr. Top. Med. Chem. 2016;16:25–39. doi: 10.2174/1568026615666150703121700. [DOI] [PubMed] [Google Scholar]
- Leite J.R.S.A., Silva L.P., Rodrigues M.I.S., Prates M.V., Brand G.D., Lacava B.M., Bloch C., Jr. Phylloseptins: a novel class of anti-bacterial and anti-protozoan peptides from the Phyllomedusa genus. Peptides. 2005;26:565–573. doi: 10.1016/j.peptides.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Magalhães B.S., Melo J.A.T., Leite J.R.S.A., Silva L.P., Prates M.V., Vinecky F., Bloch C., Jr. Post-secretory events alter the peptide content of the skin secretion of Hypsiboas raniceps. Biochem. Biophys. Res. Commun. 2008;377:1057–1061. doi: 10.1016/j.bbrc.2008.10.102. [DOI] [PubMed] [Google Scholar]
- Mattute B., Knoop F.C., Conlon J.M. Kassinatuerin-1: a peptide with broad-spectrum antimicrobial activity isolated from the skin of the hyperoliid frog, Kassina senegalensis. Biochem. Biophys. Res. Commun. 2000;268:433–436. doi: 10.1006/bbrc.2000.2136. [DOI] [PubMed] [Google Scholar]
- Michael C.A., Dominey-Howes D., Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front. Public Health. 2014;2:145. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento A., Chapeaurouge A., Perales J., Sebben A., Sousa M.V., Fontes W., Castro M.S. Purification, characterization and homology analysis of ocellatin 4, a cytolytic peptide from the skin secretion of the frog Leptodactylus ocellatus. Toxicon. 2007;50:1095–1104. doi: 10.1016/j.toxicon.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Nascimento A.C.C., Zanotta L.C., Kyaw C.M., Schwartz E.N.F., Schwartz C.A., Sebben A., Castro M.S. Ocellatins: new antimicrobial peptides from the skin secretion of the South American frog Leptodactylus ocellatus (Anura: Leptodactylidae) Protein J. 2004;23:501–508. doi: 10.1007/s10930-004-7877-z. [DOI] [PubMed] [Google Scholar]
- Otvos L. Antibacterial peptides and proteins with multiple cellular targets. J. Pept. Sci. 2005;11:697–706. doi: 10.1002/psc.698. [DOI] [PubMed] [Google Scholar]
- Park J.M., Jung J.E., Lee B.J. Antimicrobial peptides from the skin of a Korean frog, Rana rugosa. Biochem. Biophys. Res. Commun. 1994;205:948–954. doi: 10.1006/bbrc.1994.2757. [DOI] [PubMed] [Google Scholar]
- Park S., Park S.-.H., Ahn H.-.C., Kim S., Kim S.S., Lee B.J., Lee B.-.J. Structural study of novel antimicrobial peptides, nigrocins, isolated from Rana nigromaculata. FEBS Lett. 2001;507:95–100. doi: 10.1016/s0014-5793(01)02956-8. [DOI] [PubMed] [Google Scholar]
- Park S.C., Kim J.Y., Jeong C., Yoo S., Hahm K.S., Park Y. A plausible mode of action of pseudin-2, an antimicrobial peptide from Pseudis paradoxa. Biochim. Biophys. Acta. 2011;1808:171–182. doi: 10.1016/j.bbamem.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Prates M.V., Sforça M.L., Regis W.C.B., Leite J.R.S.A., Silva L.P., Pertinhez T.A., Bloch C., Jr The NMR-derived solution structure of a new cationic antimicrobial peptide from the skin secretion of the anuran Hyla punctata. J. Biol. Chem. 2004;279:13018–13026. doi: 10.1074/jbc.M310838200. [DOI] [PubMed] [Google Scholar]
- Prates M.V., Sforça M.L., Regis W.C.B., Leite J.R.S.A., Silva L.P., Pertinhez T.A., Bloch C., Jr. The NMR-derived solution structure of a new cationic antimicrobial peptide from the skin secretion of the anuran Hyla punctata. J. Biol. Chem. 2004;279:13018–13026. doi: 10.1074/jbc.M310838200. [DOI] [PubMed] [Google Scholar]
- Read A.F., Woods R.J. Antibiotic resistance management. Evol. Med. Public Health. 2014;2014:147. doi: 10.1093/emph/eou024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith L.A., King J.D., Nielsen P.F., Sonnevend A., Conlon J.M. An antimicrobial peptide from the skin secretions of the mountain chicken frog Leptodactylus fallax (Anura:Leptodactylidae) Regul. Pept. 2005;124:173–178. doi: 10.1016/j.regpep.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Rozek T. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis. Eur. J. Biochem. 2000;267:5330–5341. doi: 10.1046/j.1432-1327.2000.01536.x. [DOI] [PubMed] [Google Scholar]
- Rozek T., Wegener K.L., Bowie J.H., Olver I.N., Carver J.A., Wallace J.C., Tyler M.J. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis the solution structure of aurein 1.2. Eur. J. Biochem. 2000;267:5330–5341. doi: 10.1046/j.1432-1327.2000.01536.x. [DOI] [PubMed] [Google Scholar]
- Rozwandowicz M., Brouwer M.S.M., Fischer J., Wagenaar J.A., Gonzalez-Zorn B., Guerra B., Hordijk J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018;73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- Sani M.A., Separovic F. How membrane-active peptides get into lipid membranes. Acc. Chem. Res. 2016;49:1130–1138. doi: 10.1021/acs.accounts.6b00074. [DOI] [PubMed] [Google Scholar]
- San Millan A. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 2018;26:978–985. doi: 10.1016/j.tim.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Simmaco M., Mignogna G., Barra D., Bossa F. Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J. Biol. Chem. 1994;269:11956–11961. [PubMed] [Google Scholar]
- Simmaco M., Mignogna G., Canofeni S., Miele R., Mangoni M.L., Barra D. Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur. J. Biochem. 1996;242:788–792. doi: 10.1111/j.1432-1033.1996.0788r.x. [DOI] [PubMed] [Google Scholar]
- Sun D., Jeannot K., Xiao Y., Knapp C.W. Editorial: horizontal gene transfer mediated bacterial antibiotic resistance. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Ohe Y., Okubo T., Kakegawa T., Tatemoto K. Isolation and characterization of novel antimicrobial peptides, rugosin A, B and C from the skin of the frog, Rana rugosa. Biochem. Biophys. Res. Commun. 1995;212:249. doi: 10.1006/bbrc.1995.1963. -248. [DOI] [PubMed] [Google Scholar]
- Takahashi D., Shukla S.K., Prakash O., Zhang G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie. 2010;92:1236–1241. doi: 10.1016/j.biochi.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Wang X., Song Y., Li J., Liu H., Xu X., Lai R., Zhang K. A new family of antimicrobial peptides from skin secretions of Rana pleuraden. Peptides. 2007;28:2069–2074. doi: 10.1016/j.peptides.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Wang H., Yu Z., Hu Y., Yu H., Ran R., Xia J., Liu J. Molecular cloning and characterization of antimicrobial peptides from skin of the broad-folded frog, Hylarana latouchii. Biochimie. 2012;94:1317–1326. doi: 10.1016/j.biochi.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Wegener K.L., Wabnitz P.A., Carver J.A., Bowie J.H., Chia B.C., Wallace J.C., Tyler M.J. Host defence peptides from the skin glands of the Australian blue mountains tree-frog Litoria citropa. Solution structure of the antibacterial peptide citropin 1.1. Eur. J. Biochem. 1999;265:627–637. doi: 10.1046/j.1432-1327.1999.00750.x. [DOI] [PubMed] [Google Scholar]
- Wong H., Bowie J.H., Carver J.A. The solution structure and activity of caerin 1.1, an antimicrobial peptide from the Australian green tree frog, Litoria splendida. Eur. J. Biochem. 1997;247:545–557. doi: 10.1111/j.1432-1033.1997.00545.x. [DOI] [PubMed] [Google Scholar]
- Yang X., Xia J., Yu Z., Hu Y., Li F., Meng H., Wang H. Characterization of diverse antimicrobial peptides in skin secretions of Chungan torrent frog Amolops chunganensis. Peptides. 2012;38:41–53. doi: 10.1016/j.peptides.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Zai Y., Xi X., Ye Z., Ma C., Zhou M., Chen X., Siu S.W.I., Chen T., Wang L., Kwok H.F. Aggregation and its influence on the bioactivities of a novel antimicrobial peptide, temporin-PF, and its analogues. Int. J. Mol. Sci. 2021;22:4509. doi: 10.3390/ijms22094509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. U. S. A. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang L. RL: antimicrobial peptides. Curr. Biol. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhou M., Wang L., McGrath S., Chen T., Chen X., Shaw C. Phylloseptin-1 (PSN-1) from Phyllomedusa sauvagei skin secretion: a novel broad-spectrum antimicrobial peptide with antibiofilm activity. Mol. Immunol. 2010;47:2030–2037. doi: 10.1016/j.molimm.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Zhou X., Liu Y., Gao Y., Wang Y., Xia Q., Zhong R., Ma C., Zhou M., Xi X., Shaw C., Chen T., Wu D., Kwok H.F., Wang L. Enhanced antimicrobial activity of N-terminal derivatives of a novel brevinin-1 peptide from the skin secretion of Odorrana schmackeri. Toxins (Basel) 2020;12:484. doi: 10.3390/toxins12080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.