Abstract
Shilabo District of Somali Regional State in Ethiopia is economically important for livestock production. The indigenous pasturelands are increasingly being invaded by Prosopis juliflora, thereby threatening their livestock production capacity. However, the ecological impact of Prosopis' invasion has yet to be investigated. This study was conducted to examine the effects of Prosopis juliflora on plant species abundance, diversity, and occurrence. Vegetation samples were collected from three 20-m by 20-m sub-plots nested within 100-m by 100-m main plots that were purposefully selected in target study sites and replicated three times in both invaded and uninvaded areas. The study recorded 44 plant species, of which thirteen species occurred in both invaded and uninvaded areas, 13 species in invaded areas and 24 in uninvaded areas. The collected data were analyzed using the Statistical Package for Social Sciences (SPSS) version 26, and an independent t-test was used to determine the statistical significance difference between invaded and uninvaded areas in terms of number, density, important value index, diversity, abundance, evenness, and richness of plant species. These plant parameters were recorded in invaded and uninvaded areas with species numbers of 678.33 and 1763, densities of 226.00 and 366.98/ha, abundances of 254.04 and 409.45, important value indexes of 15.00 and 9.68, Shannon diversity of 1.56 and 3.40, evenness of 0.52, and 0.99, and richness of 1.30 and 1.35, respectively. The uninvaded area had a significantly higher species diversity index (3.40) than the invaded area (1.56; P 0.00). Similarly, the number of plant species, density, abundance, important value index, species evenness, and richness were significantly higher in uninvaded areas than invaded areas (p < 0.000).
Keywords: Native plants, Plant diversity, Prosopis juliflora, Rangeland, Species composition
1. Introduction
Prosopis trees have been widely planted in tropical Africa, Asia, and Australia for the production of fuel wood, fodder, and land rehabilitation [22,23]. Prosopis juliflora (S·W.) D.C. is one of the species that has been introduced, naturalized, and has now become invasive in numerous locations [19,22,25,28].
P. julifora is widely spread due to its prolific planting, seed distribution by livestock, wildlife, and water, persistent accumulation of large viable seeds in soil seed banks, and coppicing [20,25]. P. juliflora can grow into a tree or a shrub, depending on the surrounding environment and genetic makeup [3,22]. However, the shrub form dominates, generating dense thickets that are impenetrable and have a negative ecological impact on the invaded areas by impeding the movement of people and cattle [18].
P. juliflora, which poses a threat to agriculture and rangeland productivity, depletes water supplies, and displaces native plants and animals, is thought to have infected about 4 million hectares in Africa alone [31]. P. Juliflora invasion is estimated to reach 171,655.67 ha in Ethiopia, mainly in the Korahe zone, with trends showing a mean increase of 14,304.64 ha/year between 1989 and 2001 [1]. Prosopis has a variety of detrimental ecological effects, including a reduction in palatable native pasture plants and a threat to wild animals [4,8,13].
Prosopis has the potential to have good ecological consequences by minimizing soil erosion, enhancing soil fertility, and being useful for reclaiming moderately salty soils and degraded fields [27]. Additionally, it's critical to combat desertification in arid areas by storing carbon dioxide and reducing climate change [3,11]. In addition to its positive ecological impacts, P. Juliflora also has positive socioeconomic impacts. Prosopis' most significant socioeconomic impact is related to its replacement of pasture fields and native trees with high browsing values, which are the only sources of feed for the animals in pastoral communities [27].
The purpose of this study is to evaluate the occurrence and abundance of indigenous species in invaded and uninvaded areas to better understand the interactions that are frequently present between P. juliflora and native species in the Korahe zone of the Somali region.
Invaded areas in this zone were estimated to be 171,655.77 ha in 2001 [1]. However, there haven't been any invasion studies done in the area to date. Therefore, the purpose of this study was to investigate how P. juliflora affected the diversity of plants in the rangelands of Shilabo district, Somali Regional State, Ethiopia.
P. juliflora is one of the most threatening woody invasive trees and shrubs in the world [3,10]. P. juliflora has just come to light as the worst weed plaguing the pastoral and agro-pastoral populations in Kenya and the rest of eastern Africa [17,19]. P. juliflora reportedly occupies over one million hectares of Ethiopia at the moment [24] and aggressively displacing native tree species and reducing the amount of grazing space [8,26]. However, insufficient attention is paid to the abundance and diversity of plant species in this study area.
2. Materials and methods
2.1. Description of the study area
The Ethiopian Somali regional state has a land area of 340,000 km2, making it the second-largest region in Ethiopia [30]. The total population of this district is 107,590, of whom 67,376 are males and 34,214 are females.
The largest ethnic group identified in the Korahe zone was the Somali people (99.22%), and their economy was mostly focused on the rearing of livestock [29]. The topography of the Shilabo district is predominantly lowland plain with an average altitude of 403 m above sea level and a few foothills of higher altitude. It has a latitude and longitude of 6° 5′ 0″ north and 44° 46′ 0″ east, respectively. The Shilabo district is found at a distance of 1118 km from Addis Ababa and 487.4 km from Jigjiga. Shilabo has a tropical semiarid climate with temperatures ranging from 32 to 35 °C. The area has a bimodal rainfall pattern with two main rainy seasons. The first rainy season occurs from mid-April to the end of June, and the second season occurs from early October to late December.
2.2. Sampling techniques and data collection
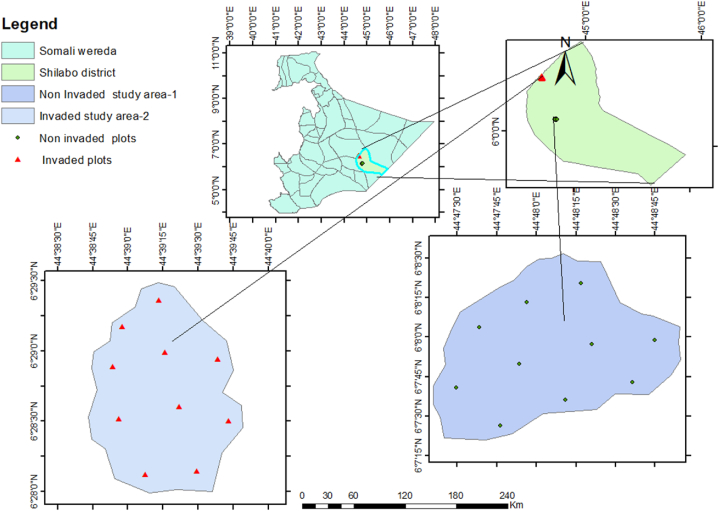
The invaded and uninvaded areas were purposefully selected as study sites based on the predominance of Prosopis juliflora. 100-m x 100-m (1 ha) main plots and three 20-x-20-m sub plots per plot were laid out in three replicates across both study sites (invaded and uninvaded). Thus, in total, 20 plots (10 plots from invaded areas and 10 plots from uninvaded areas) were laid out for the study area (Fig. 1). The distance between the main plots is 100 m.
Fig. 1.
A map of Somali Region showing the study area.
Note: Invaded and uninvaded areas are represented by red and black color dots, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Data collection and processing
In each sub-plot, data on all types of species (herbaceous, tree, shrub, and grass species) was collected, and the geographical location of sub-plots and their altitude were determined with a hand held Global Positioning System (GPS) and recorded. Collected plant data was processed to determine abundance, density [12], and frequency, which were calculated by the formula of [15] as follows:
Frequency, relative frequency, relative density, relative dominance, basal area, and important value index were calculated using the methods described by Ref. [2] as follows:
Where D = DBH
Shannon diversity index and species evenness were calculated using the methods described by Ref. [21] where:
Where n is number of kind of different plant species in study site
, N is total number of species
2.4. Statistical data analysis
The collected data were analyzed using the Statistical Package for Social Sciences (SPSS) version 26. The differences in number, density, important value indexes, diversity, abundance, evenness, and richness of indigenous species between invaded and uninvaded areas were determined with a t-test at a 95% confidence interval.
3. Result and discussion
3.1. Species composition of invaded and uninvaded areas
A total of 2441 plants, representing 44 species from 19 families, were recorded in the study area (Table 1a; Table 1b). Mimosaceae, Burseraceae, Tiliaceae, Malvaceae, Gramineae, Fabaceae, Salvadoraceae, Solanaceae, and Rhammceae families had 498, 388, 273, 206, 158, 151, 102, 90, and 24 species, respectively (Fig. 2). Mimosaceae had the largest number (498) of species, while Rhammceae had the lowest (24) (Fig. 2).
Table 1a.
Number and % occurrence of Species occurring in both in invaded and un-invaded areas.
| S.No | Species Name | Family Name | No of plants found | No of Species in % occurrence |
|---|---|---|---|---|
| 1 | Phionopsis rotundifolia | Asteraceae | 70 | 10.62 |
| 2 | Paspalidium deserotum | Gramineae | 68 | 10.32 |
| 3 | Abutilon fruticosum | Malvaceae | 172 | 26.1 |
| 4 | Acacia bussei | Mimosaceae | 77 | 11.68 |
| 5 | Acacia senegal | Mimosaceae | 96 | 14.57 |
| 6 | Solanumm elastomoides | Solanaceae | 90 | 13.66 |
| 7 | Grewia penicillata | Tiliaceae | 86 | 13.05 |
| Total | 659 | 100 |
Table 1b.
Number and % occurrence of Species occurring in invaded areas.
| S.No | Species Name | Family name | No of plants found | No of Species in % occurrence |
|---|---|---|---|---|
| 1 | Cadaba glandulosa | Burseraceae | 34 | 7.80 |
| 2 | Balanites galabra | Cucurbitaceae | 30 | 6.88 |
| 3 | Cucumis ficifolius | Cucurbitaceae | 12 | 2.75 |
| 4 | Prosopis Juliflora | Fabaceae | 89 | 20.41 |
| 5 | Ziziphus lotus | Gramineae | 30 | 6.88 |
| 6 | Grewia bicolour | Malvaceae | 34 | 7.80 |
| 7 | Acacia horrid | Mimosaceae | 23 | 5.28 |
| 8 | Acacia Nilotica | Mimosaceae | 28 | 6.42 |
| 9 | Grewia villosa | Rhammceae | 24 | 5.50 |
| 10 | Commiphora boiviniana | Tiliaceae | 35 | 8.03 |
| 11 | Crotalaria jijigensis thulin | Tiliaceae | 33 | 7.57 |
| 12 | Grewia ferruginea | Tiliaceae | 29 | 6.65 |
| 13 | Momordica sessifolia | Tiliaceae | 35 | 8.03 |
Fig. 2.
Distribution of the species encountered in the study area among families.
3.2. Plant species in invaded and non invaded areas
There are 44 individual species in the study area (Table 1a, Table 1b; Table 1c). Out of 44 plant species recorded in the study, 7 species occurred in both invaded and uninvaded areas (Table 1a), 13 species were found in invaded areas (Table 1b), and 24 species were from uninvaded areas (Table 1c).
Table 1c.
Number and % occurrence of Species occurring uninvaded areas.
| S.No | Species Name | Family Name | No of plants found | No of Species in % occurrence |
|---|---|---|---|---|
| 1 | Ruellia patula | Acanthaceae | 43 | 3.19 |
| 2 | Aerva javanica | Amaranthaceae | 51 | 3.79 |
| 3 | Balanites scillin | Balaniteceae; | 51 | 3.79 |
| 4 | Cordia gharaf | Boraginaceae | 47 | 3.49 |
| 5 | Commiphora boiviniana | Burseraceae | 52 | 3.86 |
| 6 | Commiphora borensis | Burseraceae | 31 | 2.30 |
| 7 | Commiphora gawlalo | Burseraceae | 57 | 4.23 |
| 8 | Commiphora kua | Burseraceae | 76 | 5.65 |
| 9 | Commiphora playfairi | Burseraceae | 62 | 4.61 |
| 10 | Commiphora rustrata | Burseraceae | 76 | 5.65 |
| 11 | Boscia coriacea | Capparaceae | 71 | 5.27 |
| 12 | Terminalia orbicularis | Combretaceae | 59 | 4.38 |
| 13 | Ipomoea donaldsonii | Convolvulaceae | 61 | 4.53 |
| 14 | Cordeauxia edulis hemsl | Fabaceae | 62 | 4.61 |
| 15 | Enneapogon elegans | Gramineae | 60 | 4.46 |
| 16 | Acacia benaderensis | Mimosaceae | 60 | 4.46 |
| 17 | Acacia edgeworthii | Mimosaceae | 43 | 3.19 |
| 18 | Acacia mellifera | Mimosaceae | 72 | 5.35 |
| 19 | Acacia nubica | Mimosaceae | 52 | 3.86 |
| 20 | Acacia tortilis | Mimosaceae | 47 | 3.49 |
| 21 | Sesamothamus rivea | Pedaliaceae | 56 | 4.16 |
| 22 | Dobera glabra | Salvadoraceae | 58 | 4.31 |
| 23 | Salvadora persica | Salvadoraceae | 44 | 3.27 |
| 24 | Grewia tenax | Tiliaceae | 55 | 4.09 |
3.3. Plant species diversity indices, evenness, and richness
There are 678.33 average species in invaded areas. In uninvaded areas, there are 1763 average species (Table 2). The average density of species in invaded areas was 226/ha, and that of univaded areas was 366.98/ha (Table 2). The average density of the species in the uninvaded area (366.98 ha) was significantly (P < 0.000) greater than the density of species in the invaded area (226.00 ha). This was due to the competition for resources with native plants.
Table 2.
Species diversity indices in invaded and un-invaded areas.
| Sites | Diversity parameters |
||||||
|---|---|---|---|---|---|---|---|
| Nav | Den. | AB | IVI | H | SE | SR | |
| Invaded | 678.33 | 226.00 | 254.04 | 15.00 | 1.56 | 0.52 | 1.30 |
| Un-invaded | 1763.00 | 366.98 | 409.45 | 9.68 | 3.40 | 0.99 | 1.35 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
P: significance level at 5%; Nav: average number of species; Den: species density (number of species per hectare).
AB: species abundance; IVI: important value index; H: Shannon diversity index; SE: species evenness; and SR: species Richness.
[9] Stated that Prosopis juliflora can suppress the growth of grasses under its canopy and increase biodiversity by delaying seed germination and reducing plant growth in terms of roots, shoots, leaf area, stem diameter, and plant height. As a result, density was lower in invaded areas than un-invaded areas. It also suppresses biodiversity by computing both resources and the natural environment [16]. Similarly [14], stated that the density of plant species decreases as the density of Prosopis Juliflora increases.
Table 2 revealed that Shannon's diversity index was significantly lower in invaded (H’ = 1.56) than in uninvaded (H’ = 3.40) areas (P < 0.000). The result was in line with [16], who reported a lower Shannon diversity index in invaded areas than that of uninvaded areas. The findings were comparable to those reported by Ref. [14], who found that high species richness was recorded under non-Prosopis juliflora sites rather than areas with Prosopis juliflora in Gujarat, India.
The species evenness and richness of plant species in invaded and uninvaded areas were 0.52 and 0.99 and 1.30 and 1.35, respectively. This indicated that species richness and evenness were significantly higher in uninvaded areas than invaded areas (Table 2). This is due to the fact that Prosopis juliflora invasion might affect the growth of native plants, inhibit light penetration, and release allelopathic chemicals [5,7].
Un-invaded areas had significantly higher average densities (366.98) and abundances (409.45) than invaded areas (N = 678; Den = 226.00/ha; AB = 254.04) (Table 2). This is because un-invaded areas have a greater number of individual species than invaded areas.
The important value index (15.00) was significantly (p = 0.000) higher in invaded areas than that of un-invaded areas (9.68) (Table 2). The higher important value index showed that there are fewer species in invaded areas than un-invaded areas. This result is in line with the result of [6], who stated that high IVI was attributed to a few species, and these species are those that are well adapted to the high pressure of disturbance, natural and environmental factors, and the effect of local communities.
4. Conclusion
The findings suggested that if the P. juliflora invasion is not addressed, there is a risk of decimation of important fodder species those that are common in uninvaded areas but completely absent in invaded areas. As a result, immediate actions on invasion and its effects on plant diversity are required.
Author contribution statement
Dagnachew Bezaredie: Conceived and designed the experiments; Performed the experiments; Wrote the paper.Zawde Tadesse: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.Zemenu Tadesse: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to send their appreciation and thanks to the research, publication, and technology transfer directorate of Kebridehar University for their assistance and guidance support.
Contributor Information
Dagnachew Bezaredie, Email: danibezaredie@gmail.com.
Zawde Tadesse, Email: zawdetadesse2014@gmail.com.
Zemenu Tadesse, Email: kelemutadese2008@gmail.com.
References
- 1.Abdulahi A., Wudad A., Babege K. Invasion of Prosopis juliflora and its impact on pastoralists' livelihoods in korahey zone of Somali regional state, Ethiopia. J. Soc. Sci. 2020;48 [Google Scholar]
- 2.Ajayi S., Obi R. Tree species composition, structure and importance value index (IVI) of okwangwo division, cross river national park, Nigeria. Int. J. Sci. Res. 2016;5:85–93. [Google Scholar]
- 3.Berhanu A., Tesfaye G. The Prosopis dilemma, impacts on dryland biodiversity and some controlling methods. Journal of the Drylands. 2006;1:158–164. [Google Scholar]
- 4.Dubale A. 2008. Invasive Plants and Food Security: the Case of Prosopis Juliflora in the Afar Region of Ethiopia. accessed March 10, 2014) [Google Scholar]
- 5.El Keblawy A. Impact of crop residues on seed germination of native desert plants grown as weeds. Afr. J. Biotechnol. 2012;11:7836–7842. [Google Scholar]
- 6.Erenso F., Maryo M., Abebe W. Floristic composition, diversity and vegetation structure of woody plant communities in Boda dry evergreen Montane Forest, West Showa, Ethiopia. Int. J. Biodivers. Conserv. 2014;6:382–391. [Google Scholar]
- 7.Getachew S., Demissew S., Woldemariam T. Allelopathic effects of the invasive Prosopis juliflora (Sw.) DC. on selected native plant species in Middle Awash, Southern Afar Rift of Ethiopia. Management of Biological Invasions. 2012;3:105–114. [Google Scholar]
- 8.Haile Z.M. 2008. Invasion of Prosopis Juliflora (SW.) DC and Rural Livelihoods the Case of Afar Pastoralists at Middle Awash Area of Ethiopia. [Google Scholar]
- 9.Hundessa N., Fufa A. Distribution and socio-economic impacts of Prosopis juliflora in east shewa and west arsi zones, Ethiopia. International Journal of African and Asian Studies. 2016;24:31–41. [Google Scholar]
- 10.Hussain M.I., Shackleton R.T., El-Keblawy A. Del mar trigo pérez, M., gonzález, L., invasive mesquite (Prosopis juliflora), an allergy and health challenge. Plants. 2020;9:141. doi: 10.3390/plants9020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jama B. vol. 1. World Agroforestry Centre; Nairobi: 2005. Agroforestry in the Drylands of Eastern Africa: a Call to Action. ICRAF Working Paper-No; pp. 8–13. [Google Scholar]
- 12.Keating M.J., Feldman J.D. Visual deprivation and intertectal neuronal connexions in Xenopus laevis. Proc. R. Soc. Lond. B Biol. Sci. 1975;191:467–474. doi: 10.1098/rspb.1975.0139. [DOI] [PubMed] [Google Scholar]
- 13.Kebede A., Coppock D.L. 2009. Pastoral Livestock Facilitate Dispersal of Prosopis Juliflora in a Ethiopian Wildlife Reserve. [Google Scholar]
- 14.Kumar S., Mathur M. Impact of invasion by Prosopis juliflora on plant communities in arid grazing lands. Trop. Ecol. 2014;55:33–46. [Google Scholar]
- 15.Mahajan M., Fatima S. Frequency, abundance, and density of plant species by list count quadrat method. International Journal of Multidisciplinary Research. 2017;3:1–8. [Google Scholar]
- 16.Maheshnaik B., Baranidharan K. Vijayabhama. Impact of invasive alien species-Prosopis juliflora on floral diversity of Sathyamangalam tiger reserve, Tamil Nadu, India. Biodiversity Int J. 2018;2:579–585. [Google Scholar]
- 17.Maundu P., Kibet S., Morimoto Y., Imbumi M., Adeka R. Mpact of Prosopis juliflora on Kenya's semi-arid and arid ecosystems and local livelihoods. Biodiversity. 2009;10:33–50. [Google Scholar]
- 18.Muturi G.M., Poorter L., Mohren G.M.J., Kigomo B.N. Ecological impact of Prosopis species invasion in Turkwel riverine forest, Kenya. J. Arid Environ. 2013;92:89–97. doi: 10.1016/j.jaridenv.2013.01.010. [DOI] [Google Scholar]
- 19.Mwangi E., Swallow B. Prosopis juliflora invasion and rural livelihoods in the Lake Baringo area of Kenya. Conserv. Soc. 2008;6:130–140. [Google Scholar]
- 20.Mworia J.K., Kinyamario J.I., Omari J.K., Wambua J.K. Patterns of seed dispersal and establishment of the invader Prosopis juliflora in the upper floodplain of Tana River, Kenya. Afr. J. Range Forage Sci. 2011;28:35–41. doi: 10.2989/10220119.2011.571402. [DOI] [Google Scholar]
- 21.Omayio D., Mzungu E., Kakamega K. Modification of shannon-wiener diversity index towards quantitative estimation of environmental wellness and biodiversity levels under a non-comparative Scenario. J. Environ. Earth Sci. 2019;9:46–57. [Google Scholar]
- 22.Pasiecznik N.M. HDRA; Coventry: 2001. The Prosopis Julifora-Prosopis Pallida Complex: a Monograph. [Google Scholar]
- 23.Rettberg S., Müller-Mahn D. In: Changing Deserts: Integrating People and Their Environment. Mol L., Sternberg T., editors. The White Horse Press; 2012. Human–environment interactions: the invasion of Prosopis juliflora in the drylands of northeast Ethiopia; pp. 297–316. [DOI] [Google Scholar]
- 24.Ryan F. US forest service technical assistance trip to Ethiopia: invasive species management. Rep. Submitt. to USAID. 2011 [Google Scholar]
- 25.Shiferaw W., Demissew S., Bekele T., Aynekulu E., Pitroff W. Invasion of Prosopis juliflora and its effects on soil physicochemical properties in Afar region, Northeast Ethiopia. International Soil and Water Conservation Research. 2021;9:631–638. doi: 10.1016/j.iswcr.2021.04.003. [DOI] [Google Scholar]
- 26.Steele P., Breithaupt J., Labrada R. 2009. Increased Food Security: Control and Management of Prosopis. 2009. [Google Scholar]
- 27.Tessema Y.A. Ecological and economic dimensions of the paradoxical invasive species-Prosopis juliflora and policy challenges in Ethiopia. J. Econ. Sustain. Dev. 2012;3 [Google Scholar]
- 28.van Klinken R.D., Hoffmann J.H., Zimmermann H.G., Roberts A.P. In: Biological Control of Tropical Weeds Using Arthropods. Muniappan R., Reddy G.V.P., Raman A., editors. Cambridge University Press; 2009. Prosopis species (leguminosae) pp. 353–377. [DOI] [Google Scholar]
- 29.Wudad A., Abdulahi A. Expansion of Prosopis juliflora and land use land cover change in korahey zone of Somali regional state, eastern Ethiopia. J. Mater. Environ. Sci. 2021;12:728–737. [Google Scholar]
- 30.Zerga B., Bikila Workineh B.W., Teketay D., Woldetsadik M. ijird; 2018. Rangeland Degradation and Rehabilitation Efforts in the Somali National Regional State, Eastern Ethiopia: A Review; p. 7. [DOI] [Google Scholar]
- 31.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (Apache) IV: hospital mortality assessment for today's critically ill patients. Crit. Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]