Abstract
Multiple sclerosis is a chronic, demyelinating disease of the CNS. Cognitive impairment is a sometimes neglected, yet common, sign and symptom with a profound effect on instrumental activities of daily living. The prevalence of cognitive impairment in multiple sclerosis varies across the lifespan and might be difficult to distinguish from other causes in older age. MRI studies show that widespread changes to brain networks contribute to cognitive dysfunction, and grey matter atrophy is an early sign of potential future cognitive decline. Neuropsychological research suggests that cognitive processing speed and episodic memory are the most frequently affected cognitive domains. Narrowing evaluation to these core areas permits brief, routine assessment in the clinical setting. Owing to its brevity, reliability, and sensitivity, the Symbol Digit Modalities Test, or its computer-based analogues, can be used to monitor episodes of acute disease activity. The Symbol Digit Modalities Test can also be used in clinical trials, and data increasingly show that cognitive processing speed and memory are amenable to cognitive training interventions.
Introduction
Multiple sclerosis is an inflammatory, demyelinating disease of the CNS, with neurodegeneration being most prominent in progressive phenotypes. The course of multiple sclerosis varies widely. Some patients have a single episode or attack, called a clinically isolated syndrome or a radiologically isolated syndrome, depending on whether the disease manifests clinically or on MRI. Patients with multiple CNS lesions or neurological signs that are separated in time are diagnosed with relapsing-remitting multiple sclerosis. A progressive course refers to accumulating or worsening of neurological disability, independent of relapses. Cognitive impairment can develop insidiously and progress gradually, or decline abruptly during relapses, although this rapid decline has been documented only in the past few years.
In this Review, we endeavour to provide a concise and up-to-date perspective of multiple sclerosis-associated cognitive impairment. We point out that cognitive deficits can arise in isolation from other neurological signs and, when present, are associated with increased risk of future neurological disability. We aim to garner appreciation of how cognitive impairment presents throughout the lifespan, posing unique challenges to the clinical management of paediatric (ie, <18 years) and older patients (ie, >50 years). Easily administered and sensitive tests of cognitive processing speed (CPS) and memory are now available to neurologists. The results of these tests are associated with multiple MRI indices of cerebral disease, including chronic white matter demyelination, acute inflammatory changes, and grey matter atrophy. These tests can be applied routinely to screen for cognitive impairment, monitor disease activity, and assess the effects of treatment. Some restorative and compensatory interventions for cognitive rehabilitation seem to be beneficial. Additional randomised controlled trials with cognition as the primary endpoint are needed to investigate the effects of disease-modifying therapies on cognition.
Prevalence, cognitive profile, and phenotypes
Cognitive deficits can occur in the early stages of multiple sclerosis, even in the absence of other neurological deficits.1, 2 The convention in neuropsychology is to ascribe cognitive impairment to a score where performance falls less than 1·5 SD below normative expectation, after accounting for demographics such as age and education. In diagnosing cognitive impairment, clinicians should account for psychiatric comorbidities, medication side-effects, and multiple sclerosis symptoms that might adversely affect cognitive performance. In two large seminal studies, patients were categorised as having cognitive impairment if their performance was impaired on four of 31 tests3 or two of 11 tests4 in a multidomain neuropsychological test battery. By these, or similar, standards for designating impairment, the prevalence of cognitive impairment in adults with multiple sclerosis ranges from 34% to 65%, varying by research setting and disease course.5, 6, 7, 8
Like all symptoms of multiple sclerosis, cognitive impairment is characterised by high variability between patients. When results are taken together for a group of people with multiple sclerosis,3, 4 CPS, learning, and memory are most frequently involved. Deficits in executive function and visuospatial processing are also reported, but less frequently.3, 4 In particular, in a representative sample of 291 adult patients with any type of multiple sclerosis,4 the frequencies of impairments (varying by test) were as follows: 27–51% in CPS, 54–56% in visual memory, 29–34% in verbal memory, 15–28% in executive function, and 22% in visuospatial processing. Basic language, semantic memory, and attention span are rarely impaired (in about 10% of patients with multiple sclerosis).3, 4 However, some studies suggest that semantic fluency is more often compromised than was previously thought, especially in patients older than 50 years.9, 10
Cognitive impairment occurs in all multiple sclerosis phenotypes:5, 11 estimates are 20–25% of patients with clinically isolated syndrome and radiologically isolated syndrome, 30–45% of patients with relapsing-remitting multiple sclerosis, and 50–75% of patients with secondary progressive multiple sclerosis. Prevalence in primary progressive disease varies greatly, as this phenotype comprises less than 10% of the overall disease population and study samples are small. In patients with radiologically isolated syndrome, in which MRI findings suggestive of multiple sclerosis are incidentally found in an asymptomatic individual,12 cognitive defects can pre-date the appearance of other neurological symptoms and signs and are associated with CNS lesions seen on MRI.6 In a prospective study based on the Norwegian Conscript Service database, male participants who later developed multiple sclerosis showed significantly lower intelligence quotients than did healthy controls at ages 18–19 years, several years before their first symptoms.13 Patients with clinically isolated syndrome or relapsing-remitting multiple sclerosis show similar neuropsychological profiles with prominent involvement of CPS, whereas in progressive forms of the disease, impaired memory and executive function are more common.5, 14
The so-called benign form of multiple sclerosis merits discussion. Patients with benign multiple sclerosis, which is defined by an Expanded Disability Status Scale (EDSS) score of less than 3·0 after at least 15 years since disease onset, can present with cognitive impairment despite preservation of motor and other neurological functions. In a longitudinal study, patients with benign multiple sclerosis and cognitive impairment were more likely to have clinical progression on the basis of EDSS scores (ie, no longer benign status) at follow-up after 5 years15 and 12 years16 than were patients with multiple sclerosis who had preserved cognition. These results reinforce the notion that cognitive impairment can be an early manifestation of the disease, leading to the recommendation that preserved cognitive functioning is an additional requisite for disease course type.17
Paediatric-onset (ie, younger than 18 years at onset) multiple sclerosis represents 2–10% of the disease population, and not only CPS and memory but also verbal intelligence can be affected.18 Decreased intelligence quotient and academic skills in comparison with healthy controls have been noted in several studies,19 suggesting the need for neuropsychological evaluation, special education, or other remedial interventions (panel 1 ). The few longitudinal studies of cognition in people with paediatric-onset multiple sclerosis have yielded inconsistent results because of varying study lengths, time between assessments, and outcome measures. Some studies have shown little change in cognition,20 whereas other studies have shown that up to 56% of tested patients declined on a general cognitive composite score.21 In a 1-year Canadian study of 28 patients,22 relative to 26 age-matched healthy controls, patients with paediatric-onset multiple sclerosis showed reduced age-expected gains. In a population-based longitudinal cohort of 5704 adults with multiple sclerosis in Sweden,7 CPS declined faster in patients with paediatric-onset multiple sclerosis compared with patients with adult onset. Notably, patients with paediatric-onset multiple sclerosis tend to accrue physical disability more slowly than their counterparts with adult onset, despite physical disability commencing at a younger age.23 Therefore, patients with paediatric-onset multiple sclerosis might have cognitive problems that are out of proportion to physical disability.
Panel 1. Paediatric-onset multiple sclerosis: dissociation between physical and cognitive impairment.
A 15-year-old white girl, with 9 years of education, was diagnosed with multiple sclerosis at age 13 years. Symptoms at onset were dysaesthesias in the territory of the right trigeminal nerve. The patient was treated with intramuscular interferon beta 1a but clinical symptoms and activity on MRI persisted, so she was switched to natalizumab at age 18 years, which resulted in complete clinical and MRI stability (Expanded Disability Status Scale score of 1·5).
6 months after diagnosis of multiple sclerosis, and during treatment with interferon beta 1a, psychiatric interview showed that the patient had low self-esteem and difficulties with social integration. School attendance varied because of the fluctuating multiple sclerosis symptoms, and school performance was poor. The patient had no physical disability at this time.
At first presentation with symptoms of multiple sclerosis at age 13 years, neuropsychological testing showed impaired processing speed on the Symbol Digit Modalities Test, impaired memory, and impaired linguistic skills in terms of verbal fluency. After 1 year on natalizumab, repeat neuropsychological testing was stable with some improvement on the Symbol Digit Modalities Test. Neuropsychological test results were used to obtain accommodations and psychological support at school.
This case shows the dissociation between physical and cognitive impairments in paediatric patients with multiple sclerosis, as cognitive impairment can occur in the absence of physical disability. This patient's decreased linguistic abilities, which are seldom seen in adult patients, were a major impediment to school progress. The association of cognitive changes with scholastic difficulties and behavioural problems is well known, but gains can be made with appropriate management strategies.
Taken as a whole, these results emphasise the challenging diagnostic dilemmas and deleterious long-term consequences of early onset of multiple sclerosis. Cerebral damage during the formative years not only might deprive patients of educational and vocational opportunities, but also can disrupt normal neuronal maturation and accrual of cognitive reserve that might buffer the effects of demyelination and atrophy later in life.
At the other end of the lifespan, clinicians can be confronted with a differential diagnosis of multiple sclerosis-associated cognitive impairment versus age-associated mild cognitive impairment. The diagnosis is particularly important because some treatments, such as acetylcholinesterase inhibitors, are indicated for people with Alzheimer's disease,24 but not for those with multiple sclerosis.25 Some evidence suggests that the cognitive profile of patients with multiple sclerosis older than 50 years does not differ from that of the general population with multiple sclerosis, although Jakimovski and colleagues9 reported that patients older than 50 years (mean age 62·1 years [SD 6·3]) had impairments on a semantic fluency test, as frequently observed in patients with Alzheimer's disease. Neuropsychological testing that includes comprehensive language assessment and radiological testing that is more specific to other causes of cognitive impairment other than multiple sclerosis (eg, amyloid PET) might be needed in evaluating older patients (ie, older than 50 years) with multiple sclerosis (panel 2 ). Vascular cognitive impairment is characterised by lacunes and widespread ischaemic white matter lesions, affecting CPS and executive function.26 This characterisation is particularly relevant considering that vascular comorbidities such as diabetes, hypertension, and hyperlipidaemia have been increasingly reported in people with multiple sclerosis.27 In sum, for older patients, the differential diagnosis of cognitive impairment due to multiple sclerosis from that of Alzheimer's disease, vascular dementia, and related disorder requires a comprehensive analysis of cognitive profile and advanced imaging.
Panel 2. An older patient: ruling out comorbidities associated with ageing.
A white woman with 14 years of education was diagnosed with primary progressive multiple sclerosis at age 45 years and presented for cognitive evaluation at age 84 years. Primary neurological signs at the time of this assessment were bilateral lower extremity weakness, poor balance, and recurrent falls. Chart review showed slowly progressive clinical decline including cognitive problems and bladder symptoms. She was not receiving a disease-modifying therapy but was receiving baclofen for spasticity. Expanded Disability Status Scale score was 6·5.
Neuropsychology testing at age 84 years showed decline from 3 years earlier in memory and visuospatial processing. This evaluation coincided with neurobehavioural consultation in the dementia centre and the patient was diagnosed with amnestic mild cognitive impairment because of marked forgetfulness, word finding difficulty, and inability for self-care. At age 85 years, re-examination to assess for Alzheimer's disease showed sustained deficits in memory, processing speed (Symbol Digit Modalities Test), and story memory. Testing also showed deficits in verbal fluency. Amyloid PET imaging with 18F-florbetapir showed increased focal uptake within the right occipital grey matter compared with normal uptake by clinical read of the radiologist. Over the 5-year follow-up period (aged 84–89 years), MRI showed 5% whole brain volume loss, 25% increase in lateral ventricle volume, and 10% decrease in hippocampal volume.
This case shows the increasingly common problem of ruling out Alzheimer's disease and related dementias in older patients with multiple sclerosis (>65 years). Here, the diagnosis is uncertain, but the patient is being followed up closely for more evidence of early Alzheimer's disease. The prognosis for Alzheimer's disease is of course very different to that for multiple sclerosis, necessitating an intense life-care plan and with donepezil indicated as therapy in the early stages.
MRI assessment
Early MRI studies showed that the extent of white matter abnormalities visible on T2-weighted MRI did not fully explain the severity of cognitive impairment in patients with multiple sclerosis.28 Subsequent attempts to visualise more subtle damage, by use of more advanced MRI techniques, such as magnetisation transfer, diffusion tensor imaging, and T1 relaxometry, indicated widespread damage to brain tissue that appeared normal on conventional T1-weighted or T2-weighted MRI.29, 30, 31, 32 Other improvements in MRI technology included double inversion recovery, providing visualisation of cortical lesions,33 which were robustly correlated with cognitive decline.34
Of volumetric measures, grey matter volume correlated with cognitive performance in several studies focusing on the deep grey matter,35 mesial temporal cortex,36 and neocortex.37 The clinical significance of damage to deep grey matter structures was further established by studying atrophy38 and diffusivity changes of the thalamus,39 which were both independently correlated with cognitive impairment. Besides the thalamus and the cortical grey matter, hippocampal volume40 and function41 are altered in patients with multiple sclerosis, and the hippocampus is a predilection site for demyelinated lesions.42
In addition to structural damage, studies have increasingly focused on the functional connectivity of grey matter structures, such as the thalamus, hippocampus, and cerebral cortex, by use of resting state functional MRI. These studies noted altered connectivity patterns in patients with multiple sclerosis who had cognitive impairment.43, 44 Although the dynamics found in patients with multiple sclerosis who have cognitive impairment differ from those of healthy controls without multiple sclerosis, the direction of the relationship is inconsistent: increased functional connectivity is noted in some studies,45 and decreased connectivity in others.46 Early in the disease, increased connectivity can signify that neuronal resources are compensating for demyelination and neuronal loss. Later, once these reserve resources are exhausted, connectivity diminishes, and cognitive impairment is more apparent. Overall, these network functional MRI studies indicate that cognitive decline is explained by an accruing destabilisation of the brain network physiology. Whether or not this destabilisation can be halted is a topic of active research.
The MRI techniques described here have greatly helped to provide clues to what underlies cognitive impairment in patients with multiple sclerosis, and all of these metrics correlate with cognitive impairment. The white matter lesions and diffuse damage, as well as the grey matter lesions and atrophy, together lead to the variable symptoms of cognitive impairment in patients with multiple sclerosis. Combined structural and functional imaging measures provide an explanation for the observed clinical heterogeneity.47 At this point, prediction of cognitive decline in individual patients is difficult on the basis of MRI alone. Of the MRI measures that have been investigated, grey matter atrophy seems to be the most reliable marker and patients with more severe structural damage at baseline are at greatest risk for future cognitive impairment.48 When such atrophy is readily apparent, this could alert clinicians to the need for further investigation of a patient's cognitive status, to identify people at risk for employment loss or in need of cognitive rehabilitation.
Cognitive assessment
Clinical neuropsychologists have abandoned lengthy, comprehensive test batteries for patients with multiple sclerosis, in favour of more targeted, sensitive tests, such as the Symbol Digit Modalities Test (SDMT).49 The SDMT presents a symbol–digit pairing key at the top of a page and a series of symbols below, each with a blank space underneath. For 90 s, the examinee orally indicates the matching number associated with a random array of symbols, as rapidly as possible. Originally administered in a written response format, followed by an oral response, Rao50 abandoned the written format to avoid the potential confounders of upper extremity weakness and ataxia. The oral response SDMT has since become the gold standard for assessing cognition in patients with multiple sclerosis.51, 52 Benedict and colleagues53 further developed the test by establishing equivalent, alternate forms to mitigate the effects of associative memory. As noted previously,51, 54 the SDMT is sensitive but non-specific. The test emphasises processing speed, but a patient's performance also depends to some extent on other functions, such as working memory, paired-associate learning, and visual scanning. The more complex a task, the more likely it is to be sensitive to change and cerebral pathology, and this sensitivity might partly explain the importance of the SDMT in clinical and research applications (for a historical perspective see Benedict and colleagues).51
Episodic memory tests emphasising learning over successive trials, followed by a measure of retention or delayed recall after 20–30 min, are a mainstay of clinical neuropsychology. On the Rey Auditory Verbal Learning Test (RAVLT),55 the examiner reads a list of 15 words and asks the patient to recall as many words as possible. There are five learning trials followed by a delayed recall task, in which the patient recalls the same information without another exposure to the word list. Another frequently used verbal memory test, the California Verbal Learning Test (CVLT),56 also discriminates cognitive impairment in patients with multiple sclerosis from otherwise healthy controls. A visual memory test called the Brief Visuospatial Memory Test Revised (BVMTR)57 presents a 2 × 3 matrix of six figures for 10 s. Visual learning is assessed by the rendering of the figures after exposure and, as in the RAVLT or CVLT, patients are asked to retain the same information over 20–30 min. These memory tests (ie, RAVLT, CVLT, and BVMTR) are nearly as effective as the SDMT at distinguishing cognitive impairment in patients with multiple sclerosis from otherwise healthy individuals. In a systematic review, effect size for distinguishing cognitive impairment in people with multiple sclerosis from otherwise healthy controls (Cohen's d) was reported as 1·1 for SDMT, 1·0 for BVMTR, and 0·9 for CVLT.51 Patients with multiple sclerosis rarely show evidence of rapid forgetting on these memory tests, unlike patients with Alzheimer's disease, in whom rapid forgetting of learned information is a hallmark. Rather, in multiple sclerosis, the initial learning most clearly discriminates patients from healthy controls.58 Consequently, only the learning trials of the CVLT and BVMTR were recommended for routine use in multiple sclerosis clinics.52 Importantly, neuropsychological test batteries emphasising CPS and memory correlate with employability59 and other activities of daily living.60
Two consensus conference initiatives recommended optimal brief assessment batteries for multiple sclerosis: the Multiple Sclerosis Outcomes Assessment Consortium (MSOAC)61 and the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS).52 The MSOAC included the SDMT51 as the sole cognitive measure in an attempt to replace the Paced Auditory Serial Addition Test (PASAT)50 as the gold standard for clinical trials, and to facilitate inclusion of cognitive measures in clinical trial design by promoting their acceptance by regulatory agencies. The other MSOAC tests are the Timed 25 Foot Walk,62 the Nine-Hole Peg Test,63 and the Low Contrast Letter Acuity,64 to encompass a range of functions related to neurological disability. By contrast, the BICAMS was an attempt to translate and validate cognitive tests that are simple to administer for the clinical care of patients with multiple sclerosis. Devoted only to cognition, BICAMS includes the SDMT, the BVMTR, and either the RAVLT or CVLT. Progress continues in the international validation of these test batteries.65, 66
All of these tests necessitate extra time in a clinical setting as they are administered by clinical personnel. Naturally, there is interest in the use of a computer or digital interface that could allow a more automated assessment of these key cognitive functions (appendix pp 1–5), and the validity of some computer-assisted tests has been established in patients with multiple sclerosis.67 Computer-mediated tests can offer metrics that are missing from traditional psychometric tests. The automation could ease administration and minimise the need for trained professionals. Some of these tests yield data that are similar whether or not a technician is present.68
Monitoring cognitive function in the clinical setting
Many patients with multiple sclerosis have progressive cognitive decline due to neurodegeneration.69 Gradual slowing on CPS tests such as the SDMT70 is related to a loss of grey matter volume (eg, thalamic atrophy)71 and is partly mitigated by the effects of cognitive reserve.72, 73 Monitoring for progressing cognitive dysfunction is, therefore, one goal for screening in clinical settings.
Cognitive deficits are also a sign of acute disease activity. Relapses are defined as new or worsening neurological signs or symptoms lasting longer than 24 h, in the absence of fever or infection.74, 75 Relapses are commonly treated by use of a short course of corticosteroid therapy at a high dose, oral or intravenous methylprednisolone,76 prednisone, or adrenocorticotropic hormone.77, 78 Relapses are typically diagnosed by sensory signs and physical manifestations via neurological examination. Early case reports and uncontrolled observational studies79, 80, 81, 82 suggested that acute changes in cognition could signify a relapse. However, these studies either did not control for cognitive level before the relapse or did not include a comparison group to control for practice effects.
Morrow and colleagues83 evaluated monthly SDMT values used to screen for progressive multifocal leukoencephalopathy in the STRATA study of patients receiving natalizumab for multiple sclerosis. The analysis showed a transient decline during relapses, followed by partial recovery. However, the specific neurological deficits shown by the patients who relapsed were not described, and it is certainly possible that impairments that could affect cognitive performance, such as optic neuritis, severe ataxia, upper extremity paresis, or other signs, might have affected the results. Benedict and colleagues84 studied patients who were having a relapse and who had symptoms and signs of cognitive impairment by self-report, informant-report, or clinician impression. Patients with optic neuritis, upper extremity paresis, severe ataxia, or spinal cord signs that might compromise cognitive testing were excluded. Compared with non-relapsing patients, SDMT scores declined from baseline, and subsequently normalised, or nearly so, after corticosteroid treatment. These results were replicated in patients with multiple sclerosis who were treated with a 5-day course of adrenocorticotropic hormone gel (5 mL),85 which is a less common treatment of acute disease activity in multiple sclerosis.78
Acute disease activity can be identified by gadolinium enhancement on MRI and can be deemed clinically silent, especially if the patient's cognitive status is not assessed. Pardini and colleagues86 retrospectively studied patients undergoing the SDMT and MRI at 6-month intervals. Isolated cognitive relapse was defined as SDMT decline and stable EDSS. Patients had gadolinium enhancement on MRI and partly recovered on the SDMT at 6-month follow-up, suggesting that the SDMT decline is a meaningful marker of active disease. These results were replicated by the same research group and the SDMT decline was associated with observer-reported difficulty in day-to-day living activities,87 reported by use of the informant-report Multiple Sclerosis Neuropsychological Screening Questionnaire.88
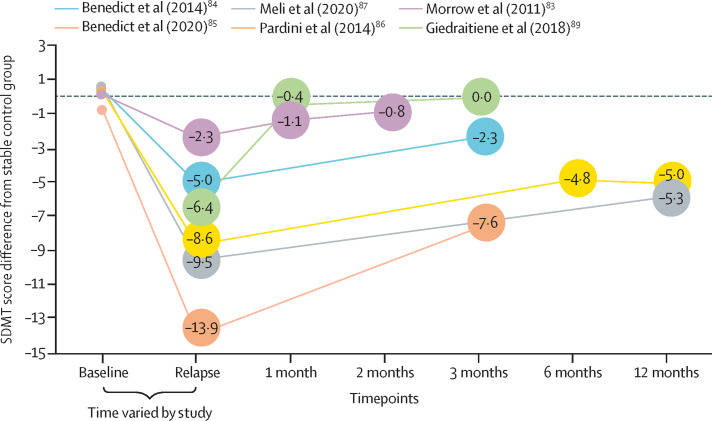
Figure 1 shows decline and recovery curves in patients with some form of cognitive relapse; only SDMT data are displayed, as it is the only test that provides results that are consistently associated with relapses. The growing literature on cognitive relapses covers both conventionally defined relapses during which the patient also has cognitive impairment, and isolated cognitive relapses, in which the only other indicator of active disease is gadolinium enhancement on MRI. Multiple sclerosis relapses with cognitive manifestations or isolated cognitive relapses can markedly affect daily function, especially where employment is concerned (panel 3 ; figure 2 ). Recovery is often incomplete, as improvement lags behind the performance of stable (no relapse activity) control patients at months 1,83 3,84 and 6 of follow-up.86, 87 The extent to which incomplete recovery rather than neurodegeneration accounts for progressive cognitive decline requires further scrutiny. Also, the scope of impairment is unknown. Some research suggests that memory declines during relapse,89, 90 although only SDMT, which measures CPS, reliably shows impairment associated with relapse in all studies.
Figure 1.
SDMT decline and recovery curves in patients with cognitive relapse
Change from baseline in the difference between relapsing (defined by clinical diagnosis of gadolinium enhancement on MRI) patients and stable patients in raw SDMT score. For each study, the mean of the stable control group is subtracted from the mean SDMT score of the relapsing group. The relapsing and stable groups are generally well matched at baseline with the difference in scores ranging from −0·7 to 0·6. In each study, the relapsing group recovers after the relapse timepoint, to varying degrees, seldom returning to a difference score of 0. SDMT=Symbol Digit Modalities Test.
Panel 3. A middle-aged patient: cognitive relapse.
A 53-year-old white man, with 12 years of education, had been diagnosed with relapsing-remitting multiple sclerosis 27 years previously. The patient had been stable for several years in his 50s without a disease-modifying therapy, with an Expanded Disability Status Scale score of 2·5.
When evaluated by neuropsychologists, he had been diagnosed with a new clinical relapse, his Expanded Disability Status Scale score increasing to 4·0. Psychiatric interview showed marked dysphoria and apathy. Brain MRI showed 13 gadolinium enhancing lesions (figure 2; not all of which are visible here). Work status monitoring showed criticism from his employers for errors and formal discipline for poor performance.
Earlier baseline assessment at age 49 years showed normal Timed 25 Foot Walk, mild impairment on the Symbol Digit Modalities Test, and slowed Nine-Hole Peg Test in the non-dominant hand. At relapse, aged 53 years, Symbol Digit Modalities Test score dropped 6 points to 43 points, but all other performance metrics (ie, Timed 25 Foot Walk and Nine-Hole Peg Test) were stable. Symbol Digit Modalities Test was still impaired at recovery, despite resolution of enhancing lesions on MRI at 6 months, when the patient reported improvement in fatigue and ambulation, and his Expanded Disability Status Scale score had partly recovered to 3·5. Following this cognitive relapse, the patient was placed on a high-efficacy medication, fingolimod.
This case shows the role of cognitive testing in identifying and managing acute multiple sclerosis disease activity. The relapse was primarily diagnosed via cognitive decline, evidenced by patient and caregiver report and employer complaints. Symptoms and work performance stabilised, with resolution of gadolinium enhancement on MRI, but the Symbol Digit Modalities Test score remained impaired, probably reflecting incomplete recovery of cognitive relapse.
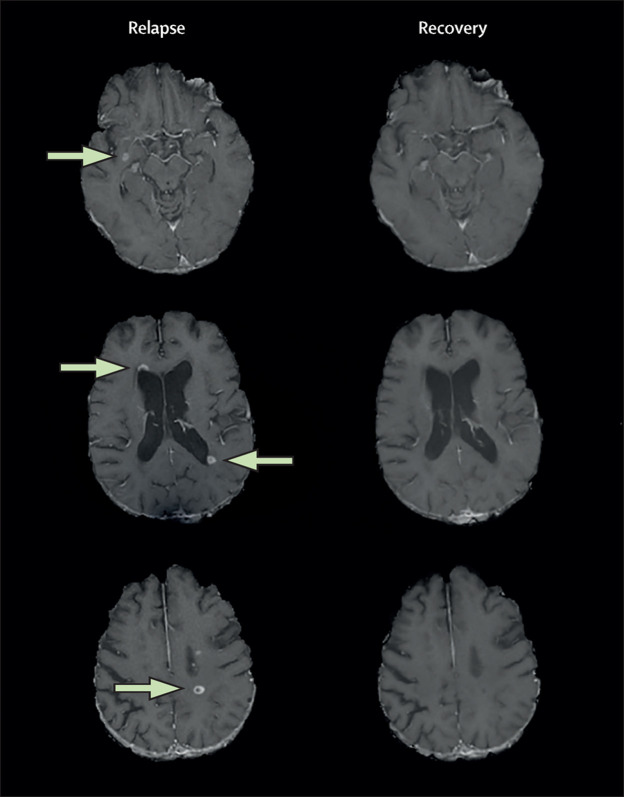
Figure 2.
Brain MRI of a 53-year-old man with relapsing-remitting multiple sclerosis
Contrast axial brain MRI scan images obtained from the patient (described in panel 3) during and after relapse with documented cognitive impairment. MRI was obtained on a 3·0T GE Signa Excite HD 12 Twin-Speed scanner (GE, Milwaukee, WI, USA). At both timepoints, T1-weighted images were acquired with a single-dose intravenous bolus of 0·1 mmol/kg gadolinium-pentetic acid 5 min after injection. The lesions that appear by use of this method represent a breakdown of the blood–brain barrier, allowing myelin-reactive T cells to enter the CNS and cause a cascade of inflammatory changes that lead to oedema, demyelination, and axonal loss. During the relapse timepoint, several gadolinium-enhancing MRI brain lesions of 0·8 cm3 or greater volume were present (arrows). In total, 13 lesions were identified but not all are visible in these sections. The gadolinium-enhancing lesions were no longer observable at the recovery timepoint, indicating a recovery of the blood–brain barrier, although some tissue damage (not shown) might persist.
Treatment of cognitive impairment
The mission of the MSOAC was to improve outcome measures in phase 3 clinical trials, which are typically annualised relapse rate or sustained progression of neurological disability, as defined by the EDSS. Generally speaking, first-line disease-modifying therapies (eg, interferon beta or glatiramer acetate) show benefit over placebo for the traditional outcomes of annualised relapse rate and disability progression, and newer escalation disease-modifying therapies (eg, fingolimod, ocrelizumab, or ozanimod) are more efficacious than the older therapies.91 In a meta-analysis that included any study examining change in cognitive performance, which encompassed 55 cohorts from 44 studies, disease-modifying therapies showed gains in either SDMT or PASAT with a small to medium effect size.92 Only in the past 5 years has SDMT become a frequent tertiary or exploratory outcome in phase 3 trials.92 Post-hoc analysis of SDMT results from the DECIDE study93 showed significantly greater mean improvement in patients given daclizumab compared with patients given interferon beta 1a, although daclizumab is no longer being pursued as a possible therapy for multiple sclerosis owing to side-effects. Although other SDMT analyses are yet to be published, research in this area is ongoing, as is evident from conference abstracts over the past 2 years.94, 95, 96 It would seem, at least so far, that although the differences in SDMT between first-line and escalation therapies are significant, they represent small effects and are not recommended as a factor for clinical decision making.92
There are no approved medications for the treatment of cognitive symptoms in patients with multiple sclerosis, although the literature is beset by methodological limitations. A systematic review97 found insufficient evidence for drugs focusing on the relief of cognitive impairment in patients with multiple sclerosis. The best evidence was noted for fampridine (with SDMT being the primary outcome), with a single class I study showing a transient effect,98 although lower class trials of fampridine yielded inconsistent results.99 Adequately powered and controlled studies, with cognition as a primary outcome, are needed to properly examine the effects of pharmacological agents on cognitive function.
Stronger evidence exists on cognitive training in patients with multiple sclerosis (table ). Restorative approaches rely on repetitive training for targeted cognitive functions (eg, processing speed and working memory), often via computerised tasks in clinic settings or at home via remotely guided training (telerehabilition).103 A meta-analysis covering 20 randomised controlled trials and 982 participants found a moderate effect size among treated patients.111 Small to moderate effects were reported for CPS, executive function, and memory. However, effects waned after training, suggesting the need for booster sessions. Although several computerised programmes are available, RehaCom has emerged as the programme most studied for people with multiple sclerosis. In several randomised controlled studies in patients with multiple sclerosis, benefits were observed in the areas of attention, CPS, memory, and executive function.101 Effects were observed immediately following treatment and up to 2 years post-treatment.112 Restorative approaches have shown that improved cognition (eg, attention and executive function) is associated with changes in brain activation and connectivity.113 This association raises the question of whether a patient's response to restorative cognitive rehabilitation is related to their baseline reserve capabilities, as indicated by behavioural proxy measures or functional MRI indices of connectivity. Preliminary work has suggested that this relationship might well exist. Brain HQ showed greater benefit on SDMT in patients with multiple sclerosis who had a high cognitive reserve, low disruption of white matter tracts, and functional MRI profiles of connectivity that appeared closest to those of people without multiple sclerois.114
Table.
Suggested approaches to cognitive retraining in patients with multiple sclerosis
| Description | RCT characteristics | Treatment duration | Test used | Effect size* | |
|---|---|---|---|---|---|
| Restorative approaches | |||||
| RehaCom100 | Computer programme with training modules for various cognitive functions; available in 27 languages; easy to administer; requires a therapist | 23 patients with RRMS; RehaCom (n=12) vs active placebo (n=11); clinic-based training in attention and information processing; therapist supervised | 6 weeks, two sessions per week, 1 h per session | Paced Visual Serial Addition Test | Large |
| RehaCom101 | Computer programme with training modules for various cognitive functions; available in 27 languages; easy to administer; requires a therapist | 58 patients with RRMS; RehaCom (n=32) vs usual clinical care (n=26); training in attention and information processing in clinic-based or community samples; therapist supervised | 10 weeks, two sessions per week, 1 h per session | CNS Vital Signs | Medium |
| RehaCom102 | Computer programme with training modules for various cognitive functions; available in 27 languages; easy to administer; requires a therapist | 36 patients with SPMS; RehaCom (n=19) vs active sham placebo (n=17); training was multimodal and home based but supervised by a therapist | 10 weeks, two sessions per week, 1 h per session | Brief International Cognitive Assessment for Multiple Sclerosis | Large |
| BrainHQ103 | Adaptive computer programme providing exercises targeting speed, attention, working memory, and executive functions | 89 patients with RRMS, 35 with SPMS, and seven with PPMS; adaptive training (n=74) vs active placebo (n=61); home based, remotely supervised; results unavailable for four patients | 12 weeks, five sessions per week, 1 h per session | Neuropsychological composite score | Small to medium |
| Attention Process Training104 | Adaptive programme of tasks and exercises targeting focused, sustained, selective, alternating, and divided attention | 88 patients with RRMS; Attention Process Training (n=55) vs active placebo (n=33); home based but with supervision | 12 weeks, two sessions per week, 1 h per session | Paced Auditory Serial Addition Test | Medium |
| Speed of Processing Training105 | Computerised drill and practice designed specifically to improve processing speed | 21 patients with RRMS; speed of processing training (n=12) vs no treatment control (n=9); community based | 5 weeks, 10 sessions, 30–45 min per session | Digit Symbol Coding | Large |
| Compensatory approaches | |||||
| Modified Story Memory Technique106 | Participants trained to use context and imagery to improve learning and memory; computer-assisted administration | 86 patients with impaired learning, of which 55 patients had RRMS; modified Story Memory Technique (n=41) vs active placebo (n=45); community based and therapist delivered | 5 weeks, two sessions per week, 45–60 min per session | California Verbal Learning Test-II | Medium to large |
| Mental Visual Imagery107 | Six 2 h individual sessions of visual imagery training | 20 patients with RRMS; visual imagery training group (n=10) vs active placebo (n=10); clinic based with supervision | 3–6 weeks, one or two sessions per week, six 2 h sessions | Adapted autobiographical interview | Large |
| General compensatory strategies108 | Training in compensatory strategies, explanations on different kinds of internal and external aids, mnemonics, mental reviews, and error-free learning | 60 patients with multiple sclerosis; memory treatment (n=20) vs placebo (n=20; relaxation) vs control (n=20; information only); group intervention with four people per group; clinic based with supervision | 8 weeks, one session per week, 1 h per session | Brief Repeatable Battery of Neuropsychological Tests | Large |
| Self-generated learning109 | Behavioural sessions training in the use of self-generated learning techniques | 24 patients with RRMS, four with SPMS, seven with PPMS; treatment group (n=19) vs active placebo (n=16); community based with supervision | Six 1 h sessions | Contextual Memory Test | Large |
Approaches to cognitive rehabilitation for patients with multiple sclerosis based on current knowledge.6, 13, 14 Articles were selected to show the breadth of available intervention techniques. RCT=randomised controlled trial. RRMS=relapsing-remitting multiple sclerosis. SPMS=secondary progressive multiple sclerosis. PPMS=primary progressive multiple sclerosis.
The effects vary by intervention type and cognitive tests administered. Effect size was calculated by use of a web calculator,110 with Cohen's d as the measure of standardised effect size. We defined Cohen's d 0·2–0·4 as small, 0·5–0·7 as medium, and 0·8 or greater as large.
Strategy-based compensatory approaches emphasise manualised behavioural therapy that is administered by a therapist for individuals or groups. The modified Story Memory Technique was the first compensatory approach to be published, providing class I evidence for efficacy.106 The modified Story Memory Technique trains patients to use context and imagery as strategies to improve the retention of information, up to 6 months post-treatment.106 After 5 weeks of training, relative to placebo, patients with memory impairment (n=86) recalled significantly more words over five learning trials on the CVLT (ie, a moderate to large effect), which was the primary endpoint in the study. Self-reported and family-reported everyday functioning, the secondary endpoint, also showed improvements. This treatment effect was associated with increased brain activation and functional connectivity in areas associated with learning and memory.115, 116
Examples of other strategy-based approaches include mental imagery, musical mnemonics, goal attainment training, and general compensatory strategies (table). Despite the increased degree and breadth of cognitive impairment in patients with progressive forms of multiple sclerosis, most rehabilitation studies have focused on relapsing-remitting multiple sclerosis. Studies suggest that both restorative102 and compensatory117 approaches are also useful in treating cognitive impairment in people with a progressive course, although these studies are scarce and there are exceptions.118 As patients with progressive disease have less cognitive reserve and grey matter volume, they might be less able to benefit from a restorative approach than patients with relapsing-remitting multiple sclerosis. The promising results of cognitive retraining in patients with multiple sclerosis provide an opportunity for an improved understanding of the underlying mechanisms associated with treatment response, which should be investigated further in future research.
Some investigators are combining cognitive rehabilitation with other interventions to maximise effects, such as cognitive behavioural therapy,118 transcranial direct current stimulation,119 and aerobic exercise.120 These combined approaches, although promising, require further research before they are ready for clinical practice.
As a single-modality intervention, exercise training would seem a viable approach in patients with multiple sclerosis, considering the cognitive benefits of aerobic fitness, physical activity, and exercise in healthy older adults. Motl and colleagues121 have called for randomised controlled trials in patients with multiple sclerosis that adopt the same interventions that were shown to be successful in the gerontology literature. Although there is some preliminary evidence that aerobic, resistance, balance, and other modes of exercise training might improve cognition, in multiple sclerosis, this conclusion seems premature. A 2011 systematic review by Motl and colleagues121 examined cognitive outcomes and noted conflicting evidence and methodological concerns, such as studies were not designed specifically to improve cognition, inadequate statistical power, and an absence of transferability to quality of life outcomes. Notably, studies that did include cognition as a primary outcome often showed a beneficial effect on cognition, whereas those that did not include cognition as a primary outcome often reported negative or no effects. A 2016 update to this review reported mostly positive findings.120 However, none of the 21 new studies in the update recruited patients with cognitive impairment a priori. Yet 16 of 21 (76%) studies were randomised controlled trials, most of which used neuropsychological tests as the primary outcome. Taken together, these findings suggest that claims that exercise training can be used to treat cognitive impairment in patients with multiple sclerosis are premature.
Future directions for research into exercise and cognition should include examining the effects of interventions that might improve cognition on brain activity via neuroimaging. For example, in a pilot investigation of the effects of 12 weeks of supervised, progressive exercise training for walking on a treadmill, the investigators assessed verbal learning and memory in eight ambulatory patients with multiple sclerosis and investigated the treatment's effect on the hippocampus by use of advanced neuroimaging (eg, magnetic resonance elastography).122 Results showed that exercise training improved learning and memory, and that this improvement was strongly associated with hippocampal activity.
Conclusions and future directions
Cognitive impairment is no longer regarded as a rare or poorly measured sign in patients with multiple sclerosis. Easily applied and sensitive tests, such as the SDMT, make cognitive appraisal accessible in neurology clinics and phase 3 trials. Defective CPS and impaired learning and memory are associated with the core pathological elements of multiple sclerosis on MRI. Most closely tied to cognitive impairment are regional grey matter atrophy, neural network disruption, and poor reserve-based compensatory mechanisms, as shown by functional MRI. Although slowed processing is the hallmark of cognitive impairment in all phenotypes, patients with paediatric-onset multiple sclerosis also struggle to develop linguistic skills and cognitive reserve, affecting academic performance and resilience later in life (panel 1). At the other end of the lifespan, older patients (>50 years) with multiple sclerosis are increasingly brought to the neuropsychology clinic with what might be age-associated memory complaints. In some cases, the patient's cognitive impairment can be difficult to distinguish from that of early Alzheimer's disease or related dementias (panel 2). Therefore, although simple neuropsychological tests are useful for routine care for patients with multiple sclerosis, a need exists for more comprehensive assessment in these multiple sclerosis subpopulations.
The course of cognitive decline needs further study. We know that cognitive impairment occurs in patients with radiologically isolated syndrome, clinically isolated syndrome, or even the so-called benign form of multiple sclerosis. Clinicians should bear in mind that cognitive impairment can present in patients with multiple sclerosis in the absence of other neurological signs or symptoms. Cognitive impairment is a common cause of work problems123 and job loss124 and bodes poorly for the risk of future disability worsening. How much of this impairment reflects neurodegeneration that might occur in otherwise stable patients is unknown, as is the degree to which worsening cognition reflects incomplete recovery from acute, demyelinating lesions. In reviewing the literature regarding relapses, we have distinguished between relapses in which cognitive manifestations are in addition to other relapse signs, and in which cognition is the only deficit—in both cases, patients might not fully recover and would require follow-up. Well designed longitudinal studies that account for the effects of cognitive relapses from early in the course of the disease, with healthy demographically matched controls, are sorely needed.
The accessibility of neuropsychological testing has increased substantially over the past decade, as shown by the MSOAC61 and BICAMS52 initiatives. Additionally, the range of platforms is rapidly expanding for the appraisal of cognition, including telemedicine125 and computerised neuropsychological assessment devices.67 Although more research is needed to establish the validity of many computerised neuropsychological assessment devices, in our opinion, the time has clearly come for CPS tests to be used in clinical routine for varying purposes. As testing becomes more commonplace, documented cognitive deficits will be increasingly appreciated by clinicians as another clinical sign of acute or subacute disease activity. Administration of the SDMT or a similar task is recommended by consensus for baseline testing soon after diagnosis, annually, or as indicated for clinical purposes.126 We concur with this opinion. The SDMT is the neuropsychological test that is most widely recommended because of its sensitivity, reliability, and predictive validity in patients with multiple sclerosis. To avoid overexposure to the same test, different versions of the SDMT (eg, adaptations for computerised neuropsychological assessment devices) should be used to screen for cognitive relapse, assess the effects of treatments, or for other purposes. The SDMT is increasingly applied in phase 3 clinical trials and efforts are underway to adjust the EDSS to account for cognition.127
We suggest that the SDMT is a biomarker of disease activity. Early cognitive impairment is a harbinger of future neurological disability and employment loss. Accounting for cognitive relapse could enhance statistical power in clinical trials in which the annualised relapse rate is the primary outcome. In the clinic, ignoring or missing cognitive changes could delay offering rehabilitative therapies.
If cognitive impairment is missed, patients might be deprived of effective treatment. Debate continues about symptomatic pharmacological therapies, although some studies are encouraging.98 There is a growing literature supporting the effects of cognitive training and these treatments should be made available to patients with multiple sclerosis (table). Considering research findings indicating that atrophy of key grey matter hubs and network disruption occur early in multiple sclerosis, interventions for cognitive impairment should also be applied early in the disease course.
In conclusion, the literature emboldens us to recommend the routine appraisal of cognitive function in patients with multiple sclerosis for both clinical and research purposes. Such evaluation will broaden the understanding of situations in which no other evidence of disease activity is reported, identify patients in need of early intervention, and enhance the appreciation of the clinical relevance of disease-modifying therapies. Tests measuring CPS, such as the SDMT, are reproducible, sensitive, and easily applied, and testing will most certainly become more accessible with technological developments.
Search strategy and selection criteria
We searched PubMed, MEDLINE, PsycInfo, and Embase databases for papers published between Jan 1, 2014, and May 1, 2020, using the search terms “multiple sclerosis” AND ([“cognition” or “neuropsychological test” or “cognitive function” or “cognitive impairment” or “memory” or “processing speed” or “executive function”] or [“MRI” or “rehabilitation”]). There were no language restrictions. We also identified articles through citations and reference lists, review articles, and the authors' own published research. The final reference list was generated on the basis of the relevance of papers to the topics that are discussed in this Review.
Contributors
RHBB was the lead writer and originated the concept, design, table, and figures for the paper. MPA, JD, and JJGG contributed equally to writing, table format, and concepts throughout the paper.
Declaration of interests
RHBB received honoraria, speaking, or consulting fees from Biogen, Celgene, EMD Serono, Genentech, Medday, Novartis, and Roche; research support from Biogen, Genentech, and Novartis; and royalties from Psychological Assessment Resources. MPA received honoraria, speaking, or consulting fees and research grants from Merck, Biogen, Roche, Novartis, and Sanofi Genzyme. JD received honoraria, speaking, or consulting fees from Biogen, Celgene, Novartis, Sanofi, and MedRhythm; and grant funding from Biogen and EMD Serono. JJGG has received research grants from Biogen, Celgene, Merck, Medday, and Novartis.
Supplementary Material
References
- 1.Benedict RHB, DeLuca J, Enzinger C, Geurts JJG, Krupp LB, Rao SM. Neuropsychology of multiple sclerosis: looking back and moving forward. J Int Neuropsychol Soc. 2017;23:832–842. doi: 10.1017/S1355617717000959. [DOI] [PubMed] [Google Scholar]
- 2.Glanz BI, Holland CM, Gauthier SA, et al. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult Scler. 2007;13:1004–1010. doi: 10.1177/1352458507077943. [DOI] [PubMed] [Google Scholar]
- 3.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 4.Benedict RHB, Cookfair D, Gavett R, et al. Validity of the Minimal Assessment of Cognitive Function In Multiple Sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006;12:549–558. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 5.Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler. 2017;23:1258–1267. doi: 10.1177/1352458516674367. [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Hakiki B, Goretti B, et al. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology. 2012;78:309–314. doi: 10.1212/WNL.0b013e31824528c9. [DOI] [PubMed] [Google Scholar]
- 7.McKay KA, Manouchehrinia A, Berrigan L, Fisk JD, Olsson T, Hillert J. Long-term cognitive outcomes in patients with pediatric-onset vs adult-onset multiple sclerosis. JAMA Neurol. 2019;76:1028–1034. doi: 10.1001/jamaneurol.2019.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duquin JA, Parmenter BA, Benedict RH. Influence of recruitment and participation bias in neuropsychological research among MS patients. J Int Neuropsychol Soc. 2008;14:494–498. doi: 10.1017/S1355617708080624. [DOI] [PubMed] [Google Scholar]
- 9.Jakimovski D, Weinstock-Guttman B, Roy S, et al. Cognitive profiles of aging in multiple sclerosis. Front Aging Neurosci. 2019;11:105. doi: 10.3389/fnagi.2019.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandstadter R, Fabian M, Leavitt VM, et al. Word-finding difficulty is a prevalent disease-related deficit in early multiple sclerosis. Mult Scler. 2019 doi: 10.1177/1352458519881760. published online Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnen A, Landmeyer NC, Bürkner PC, Wiendl H, Meuth SG, Holling H. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568–578. doi: 10.1016/j.neubiorev.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 12.De Stefano N, Giorgio A, Tintoré M, et al. Radiologically isolated syndrome or subclinical multiple sclerosis: MAGNIMS consensus recommendations. Mult Scler. 2018;24:214–221. doi: 10.1177/1352458517717808. [DOI] [PubMed] [Google Scholar]
- 13.Cortese M, Riise T, Bjørnevik K, et al. Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol. 2016;80:616–624. doi: 10.1002/ana.24769. [DOI] [PubMed] [Google Scholar]
- 14.Branco M, Ruano L, Portaccio E, et al. Aging with multiple sclerosis: prevalence and profile of cognitive impairment. Neurol Sci. 2019;40:1651–1657. doi: 10.1007/s10072-019-03875-7. [DOI] [PubMed] [Google Scholar]
- 15.Portaccio E, Stromillo ML, Goretti B, et al. Neuropsychological and MRI measures predict short-term evolution in benign multiple sclerosis. Neurology. 2009;73:498–503. doi: 10.1212/WNL.0b013e3181b351fd. [DOI] [PubMed] [Google Scholar]
- 16.Razzolini L, Portaccio E, Stromillo ML, et al. The dilemma of benign multiple sclerosis: can we predict the risk of losing the “benign status”? A 12-year follow-up study. Mult Scler Relat Disord. 2018;26:71–73. doi: 10.1016/j.msard.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Rovaris M, Riccitelli G, Judica E, et al. Cognitive impairment and structural brain damage in benign multiple sclerosis. Neurology. 2008;71:1521–1526. doi: 10.1212/01.wnl.0000319694.14251.95. [DOI] [PubMed] [Google Scholar]
- 18.Amato MP, Krupp LB, Charvet LE, Penner I, Till C. Pediatric multiple sclerosis: cognition and mood. Neurology. 2016;87(suppl 2):S82–S87. doi: 10.1212/WNL.0000000000002883. [DOI] [PubMed] [Google Scholar]
- 19.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology. 2008;70:1891–1897. doi: 10.1212/01.wnl.0000312276.23177.fa. [DOI] [PubMed] [Google Scholar]
- 20.Charvet LE, O'Donnell EH, Belman AL, et al. Longitudinal evaluation of cognitive functioning in pediatric multiple sclerosis: report from the US Pediatric Multiple Sclerosis Network. Mult Scler. 2014;20:1502–1510. doi: 10.1177/1352458514527862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amato MP, Goretti B, Ghezzi A, et al. Neuropsychological features in childhood and juvenile multiple sclerosis: five-year follow-up. Neurology. 2014;83:1432–1438. doi: 10.1212/WNL.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 22.Till C, Racine N, Araujo D, et al. Changes in cognitive performance over a 1-year period in children and adolescents with multiple sclerosis. Neuropsychology. 2013;27:210–219. doi: 10.1037/a0031665. [DOI] [PubMed] [Google Scholar]
- 23.Waldman A, Ness J, Pohl D, et al. Pediatric multiple sclerosis: clinical features and outcome. Neurology. 2016;87(suppl 2):S74–S81. doi: 10.1212/WNL.0000000000003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wimo A, Winblad B, Shah SN, Chin W, Zhang R, McRae T. Impact of donepezil treatment for Alzheimer's disease on caregiver time. Curr Med Res Opin. 2004;20:1221–1225. doi: 10.1185/030079902125004349. [DOI] [PubMed] [Google Scholar]
- 25.Krupp LB, Christodoulou C, Melville P, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology. 2011;76:1500–1507. doi: 10.1212/WNL.0b013e318218107a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. 2018;31:36–43. doi: 10.1097/WCO.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 27.Marrie RA, Fisk J, Tremlett H, et al. Differing trends in the incidence of vascular comorbidity in MS and the general population. Neurol Clin Pract. 2016;6:120–128. doi: 10.1212/CPJ.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foong J, Rozewicz L, Chong WK, Thompson AJ, Miller DH, Ron MA. A comparison of neuropsychological deficits in primary and secondary progressive multiple sclerosis. J Neurol. 2000;247:97–101. doi: 10.1007/pl00007804. [DOI] [PubMed] [Google Scholar]
- 29.Comi G, Rovaris M, Leocani L, Martinelli V, Filippi M. Assessment of the damage of the cerebral hemispheres in MS using neuroimaging techniques. J Neurol Sci. 2000;172(suppl 1):S63–S66. doi: 10.1016/s0022-510x(99)00282-8. [DOI] [PubMed] [Google Scholar]
- 30.Rovaris M, Iannucci G, Falautano M, et al. Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. J Neurol Sci. 2002;195:103–109. doi: 10.1016/s0022-510x(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 31.Deloire MS, Salort E, Bonnet M, et al. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:519–526. doi: 10.1136/jnnp.2004.045872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrenken H, Geurts JJ, Knol DL, et al. Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology. 2006;240:811–820. doi: 10.1148/radiol.2403050569. [DOI] [PubMed] [Google Scholar]
- 33.Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 34.Nelson F, Datta S, Garcia N, et al. Intracortical lesions by 3T magnetic resonance imaging and correlation with cognitive impairment in multiple sclerosis. Mult Scler. 2011;17:1122–1129. doi: 10.1177/1352458511405561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houtchens MK, Benedict RHB, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–1223. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- 36.Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80:201–206. doi: 10.1136/jnnp.2008.148403. [DOI] [PubMed] [Google Scholar]
- 37.Amato MP, Bartolozzi ML, Zipoli V, et al. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93. doi: 10.1212/01.wnl.0000129544.79539.d5. [DOI] [PubMed] [Google Scholar]
- 38.Bisecco A, Rocca MA, Pagani E, et al. Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Hum Brain Mapp. 2015;36:2809–2825. doi: 10.1002/hbm.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benedict RH, Hulst HE, Bergsland N, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult Scler. 2013;19:1478–1484. doi: 10.1177/1352458513478675. [DOI] [PubMed] [Google Scholar]
- 40.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 41.Hulst HE, Schoonheim MM, Roosendaal SD, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp. 2012;33:2268–2280. doi: 10.1002/hbm.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geurts JJ, Bö L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 43.Meijer KA, Eijlers AJC, Douw L, et al. Increased connectivity of hub networks and cognitive impairment in multiple sclerosis. Neurology. 2017;88:2107–2114. doi: 10.1212/WNL.0000000000003982. [DOI] [PubMed] [Google Scholar]
- 44.d'Ambrosio A, Valsasina P, Gallo A, et al. Reduced dynamics of functional connectivity and cognitive impairment in multiple sclerosis. Mult Scler. 2020;26:476–488. doi: 10.1177/1352458519837707. [DOI] [PubMed] [Google Scholar]
- 45.Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- 46.Tona F, Petsas N, Sbardella E, et al. Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology. 2014;271:814–821. doi: 10.1148/radiol.14131688. [DOI] [PubMed] [Google Scholar]
- 47.Meijer KA, van Geest Q, Eijlers AJC, Geurts JJG, Schoonheim MM, Hulst HE. Is impaired information processing speed a matter of structural or functional damage in MS? Neuroimage Clin. 2018;20:844–850. doi: 10.1016/j.nicl.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eijlers AJC, van Geest Q, Dekker I, et al. Predicting cognitive decline in multiple sclerosis: a 5-year follow-up study. Brain. 2018;141:2605–2618. doi: 10.1093/brain/awy202. [DOI] [PubMed] [Google Scholar]
- 49.Smith A. Western Psychological Services; Los Angeles, CA: 1982. Symbol Digit Modalities Test: manual. [Google Scholar]
- 50.Rao SM. National Multiple Sclerosis Society; New York, NY: 1991. A manual for the brief, repeatable battery of neuropsychological tests in multiple sclerosis. [Google Scholar]
- 51.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:721–733. doi: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langdon DW, Amato MP, Boringa J, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) Mult Scler. 2012;18:891–898. doi: 10.1177/1352458511431076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benedict RH, Smerbeck A, Parikh R, Rodgers J, Cadavid D, Erlanger D. Reliability and equivalence of alternate forms for the Symbol Digit Modalities Test: implications for multiple sclerosis clinical trials. Mult Scler. 2012;18:1320–1325. doi: 10.1177/1352458511435717. [DOI] [PubMed] [Google Scholar]
- 54.Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology. 2018;90:278–288. doi: 10.1212/WNL.0000000000004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- 56.Delis DC, Kramer JH, Kaplan E, Ober BA. 2nd edn. The Psychological Corporation; San Antonio, TX: 2000. Califorina Verbal Learning Test. [Google Scholar]
- 57.Benedict RHB. Psychological Assessment Resources; Odessa, FL: 1997. Brief Visuospatial Memory Test revised professional manual. [Google Scholar]
- 58.Stegen S, Stepanov I, Cookfair D, et al. Validity of the California Verbal Learning Test-II in multiple sclerosis. Clin Neuropsychol. 2010;24:189–202. doi: 10.1080/13854040903266910. [DOI] [PubMed] [Google Scholar]
- 59.Benedict RHB, Drake AS, Irwin LN, et al. Benchmarks of meaningful impairment on the MSFC and BICAMS. Mult Scler. 2016;22:1874–1882. doi: 10.1177/1352458516633517. [DOI] [PubMed] [Google Scholar]
- 60.Goverover Y, Chiaravalloti N, DeLuca J. Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) and performance of everyday life tasks: actual reality. Mult Scler. 2016;22:544–550. doi: 10.1177/1352458515593637. [DOI] [PubMed] [Google Scholar]
- 61.LaRocca NG, Hudson LD, Rudick R, et al. The MSOAC approach to developing performance outcomes to measure and monitor multiple sclerosis disability. Mult Scler. 2018;24:1469–1484. doi: 10.1177/1352458517723718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Motl RW, Cohen JA, Benedict R, et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:704–710. doi: 10.1177/1352458517690823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feys P, Lamers I, Francis G, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler. 2017;23:711–720. doi: 10.1177/1352458517690824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balcer LJ, Raynowska J, Nolan R, et al. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:734–747. doi: 10.1177/1352458517690822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldman MD, LaRocca NG, Rudick RA, et al. Evaluation of multiple sclerosis disability outcome measures using pooled clinical trial data. Neurology. 2019;93:e1921–e1931. doi: 10.1212/WNL.0000000000008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corfield F, Langdon D. A systematic review and meta-analysis of the Brief Cognitive Assessment for Multiple Sclerosis (BICAMS) Neurol Ther. 2018;7:287–306. doi: 10.1007/s40120-018-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wojcik CM, Beier M, Costello K, et al. Computerized neuropsychological assessment devices in multiple sclerosis: a systematic review. Mult Scler. 2019;25:1848–1869. doi: 10.1177/1352458519879094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wojcik CM, Rao SM, Schembri AJ, et al. Necessity of technicians for computerized neuropsychological assessment devices in multiple sclerosis. Mult Scler. 2020;26:109–113. doi: 10.1177/1352458518813287. [DOI] [PubMed] [Google Scholar]
- 69.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 70.Healy BC, Barker L, Bakshi R, et al. Trajectories of Symbol Digit Modalities Test performance in individuals with multiple sclerosis. Mult Scler. 2020 doi: 10.1177/1352458520913439. published online March 31. [DOI] [PubMed] [Google Scholar]
- 71.Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler. 2016;22:1327–1336. doi: 10.1177/1352458515616204. [DOI] [PubMed] [Google Scholar]
- 72.Benedict RH, Morrow SA, Weinstock Guttman B, Cookfair D, Schretlen DJ. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc. 2010;16:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- 73.Fuchs TA, Wojcik C, Wilding GE, et al. Trait conscientiousness predicts rate of longitudinal SDMT decline in multiple sclerosis. Mult Scler. 2020;26:245–252. doi: 10.1177/1352458518820272. [DOI] [PubMed] [Google Scholar]
- 74.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berkovich RR. Acute multiple sclerosis relapse. Continuum (Minneap Minn) 2016;22:799–814. doi: 10.1212/CON.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 76.Lattanzi S, Cagnetti C, Danni M, Provinciali L, Silvestrini M. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J Neurol. 2017;264:1697–1704. doi: 10.1007/s00415-017-8505-0. [DOI] [PubMed] [Google Scholar]
- 77.Comi G, Radaelli M. Oral corticosteroids for multiple sclerosis relapse. Lancet. 2015;386:937–939. doi: 10.1016/S0140-6736(15)00072-0. [DOI] [PubMed] [Google Scholar]
- 78.Berkovich R, Bakshi R, Amezcua L, et al. Adrenocorticotropic hormone versus methylprednisolone added to interferon β in patients with multiple sclerosis experiencing breakthrough disease: a randomized, rater-blinded trial. Ther Adv Neurol Disord. 2017;10:3–17. doi: 10.1177/1756285616670060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prado FM, Kosac VA, Dib JG. Cognitive relapse in multiple sclerosis: report of 3 cases. Mult Scler J. 2012;18:1830–1831. [Google Scholar]
- 80.Patzold T, Schwengelbeck M, Ossege LM, Malin JP, Sindern E. Changes of the MS functional composite and EDSS during and after treatment of relapses with methylprednisolone in patients with multiple sclerosis. Acta Neurol Scand. 2002;105:164–168. doi: 10.1034/j.1600-0404.2002.1o135.x. [DOI] [PubMed] [Google Scholar]
- 81.Foong J, Rozewicz L, Quaghebeur G, Thompson AJ, Miller DH, Ron MA. Neuropsychological deficits in multiple sclerosis after acute relapse. J Neurol Neurosurg Psychiatry. 1998;64:529–532. doi: 10.1136/jnnp.64.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozakbas S, Cagiran I, Ormeci B, Idiman E. Correlations between multiple sclerosis functional composite, expanded disability status scale and health-related quality of life during and after treatment of relapses in patients with multiple sclerosis. J Neurol Sci. 2004;218:3–7. doi: 10.1016/j.jns.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Morrow SA, Jurgensen S, Forrestal F, Munchauer FE, Benedict RH. Effects of acute relapses on neuropsychological status in multiple sclerosis patients. J Neurol. 2011;258:1603–1608. doi: 10.1007/s00415-011-5975-3. [DOI] [PubMed] [Google Scholar]
- 84.Benedict RH, Morrow S, Rodgers J, et al. Characterizing cognitive function during relapse in multiple sclerosis. Mult Scler. 2014;20:1745–1752. doi: 10.1177/1352458514533229. [DOI] [PubMed] [Google Scholar]
- 85.Benedict RH, Pol J, Yasin F, et al. Recovery of cognitive function after relapse in multiple sclerosis. Mult Scler. 2020 doi: 10.1177/1352458519898108. published online Jan 23. [DOI] [PubMed] [Google Scholar]
- 86.Pardini M, Uccelli A, Grafman J, Yaldizli Ö, Mancardi G, Roccatagliata L. Isolated cognitive relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:1035–1037. doi: 10.1136/jnnp-2013-307275. [DOI] [PubMed] [Google Scholar]
- 87.Meli R, Roccatagliata L, Capello E, et al. Ecological impact of isolated cognitive relapses in MS. Mult Scler. 2020;26:114–117. doi: 10.1177/1352458518813722. [DOI] [PubMed] [Google Scholar]
- 88.Benedict RHB, Cox D, Thompson LL, Foley F, Weinstock-Guttman B, Munschauer F. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler. 2004;10:675–678. doi: 10.1191/1352458504ms1098oa. [DOI] [PubMed] [Google Scholar]
- 89.Giedraitiene N, Kaubrys G, Kizlaitiene R. Cognition during and after multiple sclerosis relapse as assessed with the Brief International Cognitive Assessment for Multiple Sclerosis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-26449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giedraitiene N, Kaubrys G. Distinctive pattern of cognitive disorders during multiple sclerosis relapse and recovery based on computerized CANTAB tests. Front Neurol. 2019;10:572. doi: 10.3389/fneur.2019.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalincik T, Manouchehrinia A, Sobisek L, et al. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain. 2017;140:2426–2443. doi: 10.1093/brain/awx185. [DOI] [PubMed] [Google Scholar]
- 92.Landmeyer NC, Bürkner PC, Wiendl H, et al. Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis: a meta-analysis. Neurology. 2020;94:e2373–e2383. doi: 10.1212/WNL.0000000000009522. [DOI] [PubMed] [Google Scholar]
- 93.Benedict RH, Cohan S, Lynch SG, et al. Improved cognitive outcomes in patients with relapsing-remitting multiple sclerosis treated with daclizumab beta: results from the DECIDE study. Mult Scler. 2018;24:795–804. doi: 10.1177/1352458517707345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benedict RH, de Seze J, Hauser SL, et al. Impact of ocrelizumab on cognition in patients at increased risk of progressive disease. 2018 Annual Meeting of the Consortium of Multiple Sclerosis Centers; Nashville, TN, USA; May 30–June 2, 2018 (abstr 209).
- 95.Benedict RHB, Cree B, Tomic D, et al. Impact of siponimod on cognition in patients with secondary progressive multiple sclerosis: results from phase 3 EXPAND study. 70th Annual Meeting of the American Academy of Neurology; Los Angeles, CA, USA; April 21–27, 2018 (abstr 004).
- 96.Deluca J, Cohen J, Cree BAC, et al. Sustained improvement in cognitive processing speed in multiple sclerosis patients completing 18 months of ozanimod treatment: results from the phase 3 SUNBEAM trial. Mult Scler J. 2019;25:N22. [Google Scholar]
- 97.Chen MH, Goverover Y, Genova HM, DeLuca J. Cognitive efficacy of pharmacologic treatments in multiple sclerosis: a systematic review. CNS Drugs. 2020;34:599–628. doi: 10.1007/s40263-020-00734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Giglio L, De Luca F, Gurreri F, et al. Effect of dalfampridine on information processing speed impairment in multiple sclerosis. Neurology. 2019;93:e733–e746. doi: 10.1212/WNL.0000000000007970. [DOI] [PubMed] [Google Scholar]
- 99.Satchidanand N, Drake A, Smerbeck A, et al. Dalfampridine benefits ambulation but not cognition in multiple sclerosis. Mult Scler. 2020;26:91–98. doi: 10.1177/1352458518815795. [DOI] [PubMed] [Google Scholar]
- 100.Cerasa A, Gioia MC, Valentino P, et al. Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabil Neural Repair. 2013;27:284–295. doi: 10.1177/1545968312465194. [DOI] [PubMed] [Google Scholar]
- 101.Messinis L, Nasios G, Kosmidis MH, et al. Efficacy of a computer-assisted cognitive rehabilitation intervention in relapsing-remitting multiple sclerosis patients: a multicenter randomized controlled trial. Behav Neurol. 2017;2017 doi: 10.1155/2017/5919841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Messinis L, Kosmidis MH, Nasios G, et al. Do secondary progressive multiple sclerosis patients benefit from computer- based cognitive neurorehabilitation? A randomized sham controlled trial. Mult Scler Relat Disord. 2020;39 doi: 10.1016/j.msard.2020.101932. [DOI] [PubMed] [Google Scholar]
- 103.Charvet LE, Yang J, Shaw MT, et al. Cognitive function in multiple sclerosis improves with telerehabilitation: results from a randomized controlled trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amato MP, Goretti B, Viterbo RG, et al. Computer-assisted rehabilitation of attention in patients with multiple sclerosis: results of a randomized, double-blind trial. Mult Scler. 2014;20:91–98. doi: 10.1177/1352458513501571. [DOI] [PubMed] [Google Scholar]
- 105.Chiaravalloti ND, Goverover Y, Costa SL, et al. A pilot study examining speed of processing training (SPT) to improve processing speed in persons with multiple sclerosis. Front Neurol. 2018;9:685. doi: 10.3389/fneur.2018.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiaravalloti ND, Moore NB, Nikelshpur OM, DeLuca J. An RCT to treat learning impairment in multiple sclerosis: the MEMREHAB trial. Neurology. 2013;81:2066–2072. doi: 10.1212/01.wnl.0000437295.97946.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ernst A, Sourty M, Roquet D, et al. Benefits from an autobiographical memory facilitation programme in relapsing-remitting multiple sclerosis patients: a clinical and neuroimaging study. Neuropsychol Rehabil. 2018;28:1110–1130. doi: 10.1080/09602011.2016.1240697. [DOI] [PubMed] [Google Scholar]
- 108.Mousavi S, Zare H, Etemadifar M, Taher Neshatdoost H. Memory rehabilitation for the working memory of patients with multiple sclerosis (MS) J Clin Exp Neuropsychol. 2018;40:405–410. doi: 10.1080/13803395.2017.1356269. [DOI] [PubMed] [Google Scholar]
- 109.Goverover Y, Chiaravalloti N, Genova H, DeLuca J. A randomized controlled trial to treat impaired learning and memory in multiple sclerosis: the self-GEN trial. Mult Scler. 2018;24:1096–1104. doi: 10.1177/1352458517709955. [DOI] [PubMed] [Google Scholar]
- 110.Psychometrica Computation of effect sizes. https://www.psychometrica.de/effect_size.html
- 111.Lampit A, Heine J, Finke C, et al. Computerized cognitive training in multiple sclerosis: a systematic review and meta-analysis. Neurorehabil Neural Repair. 2019;33:695–706. doi: 10.1177/1545968319860490. [DOI] [PubMed] [Google Scholar]
- 112.Mattioli F, Bellomi F, Stampatori C, Capra R, Miniussi C. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler. 2016;22:222–230. doi: 10.1177/1352458515587597. [DOI] [PubMed] [Google Scholar]
- 113.Filippi M, Riccitelli G, Mattioli F, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures—an explorative study. Radiology. 2012;262:932–940. doi: 10.1148/radiol.11111299. [DOI] [PubMed] [Google Scholar]
- 114.Fuchs TA, Ziccardi S, Benedict RHB, et al. Functional connectivity and structural disruption in the default-mode network predicts cognitive rehabilitation outcomes in multiple sclerosis. J Neuroimaging. 2020;30:523–530. doi: 10.1111/jon.12723. [DOI] [PubMed] [Google Scholar]
- 115.Dobryakova E, Wylie GR, DeLuca J, Chiaravalloti ND. Functional brain activity 6 months after memory retraining in multiple sclerosis: the MEMREHAB trial. Brain Imaging Behav. 2014;8:403–406. doi: 10.1007/s11682-014-9309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leavitt VM, Wylie GR, DeLuca J, Chiaravalloti ND. Increased functional connectivity within memory networks following memory rehabilitation in multiple sclerosis. Brain Imaging Behav. 2014;8:394–402. doi: 10.1007/s11682-012-9183-2. [DOI] [PubMed] [Google Scholar]
- 117.Chiaravalloti ND, Moore NB, DeLuca J. The efficacy of the modified Story Memory Technique in progressive MS. Mult Scler. 2020;26:354–362. doi: 10.1177/1352458519826463. [DOI] [PubMed] [Google Scholar]
- 118.Martínez-González AE, Piqueras JA. Long-term effectiveness of combined cognitive-behavioral and neuropsychological intervention in a case of multiple sclerosis. Neurocase. 2015;21:584–591. doi: 10.1080/13554794.2014.960425. [DOI] [PubMed] [Google Scholar]
- 119.Charvet L, Shaw M, Dobbs B, et al. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation. 2018;21:383–389. doi: 10.1111/ner.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sandroff BM, Motl RW, Scudder MR, DeLuca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26:271–294. doi: 10.1007/s11065-016-9324-2. [DOI] [PubMed] [Google Scholar]
- 121.Motl RW, Sandroff BM, Benedict RH. Cognitive dysfunction and multiple sclerosis: developing a rationale for considering the efficacy of exercise training. Mult Scler. 2011;17:1034–1040. doi: 10.1177/1352458511409612. [DOI] [PubMed] [Google Scholar]
- 122.Sandroff BM, Johnson CL, Motl RW. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology. 2017;59:61–67. doi: 10.1007/s00234-016-1767-x. [DOI] [PubMed] [Google Scholar]
- 123.Frndak SE, Kordovski VM, Cookfair D, et al. Disclosure of disease status among employed multiple sclerosis patients: association with negative work events and accommodations. Mult Scler. 2015;21:225–234. doi: 10.1177/1352458514540971. [DOI] [PubMed] [Google Scholar]
- 124.Strober L, Chiaravalloti N, Moore N, et al. Unemployment in multiple sclerosis (MS): utility of the MS Functional Composite and cognitive testing. Mult Scler. 2014;20:112–115. doi: 10.1177/1352458513488235. [DOI] [PubMed] [Google Scholar]
- 125.Barcellos LF, Bellesis KH, Shen L, et al. Remote assessment of verbal memory in MS patients using the California Verbal Learning Test. Mult Scler. 2018;24:354–357. doi: 10.1177/1352458517694087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. 2018;24:1665–1680. doi: 10.1177/1352458518803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saccà F, Costabile T, Carotenuto A, et al. The EDSS integration with the Brief International Cognitive Assessment for Multiple Sclerosis and orientation tests. Mult Scler. 2017;23:1289–1296. doi: 10.1177/1352458516677592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.