Abstract
Purpose of Review
This paper reviews how sleep is impacted in patients with Prader-Willi syndrome (PWS), focusing on sleep-related breathing disturbances and excessive daytime sleepiness (EDS).
Recent Findings
Hypothalamic dysfunction may underlie several aspects of the PWS phenotype. Central sleep apnea (CSA) can persist beyond infancy. Nocturnal hypoventilation is common and may occur without central or obstructive sleep apnea (OSA). Adenotonsillectomy, a mainstay of OSA treatment, may cause velopharyngeal insufficiency. Growth hormone (GH) is considered safe, but close surveillance for OSA remains important. Cardiac autonomic dysfunction occurs during slow wave sleep and may increase the risk of cardiovascular events. EDS and narcolepsy are also common. Modafinil and pitolisant are treatment options currently being studied.
Summary
Sleep disorders are prevalent in individuals with PWS. Sleep-related breathing disorders present as CSA in infancy and later in life as OSA and hypoventilation. GH therapy has improved the clinical outcomes of patients with PWS, but close surveillance and treatment for OSA is recommended. EDS can persist even after sleep-related breathing disorders are treated, and some individuals may even develop narcolepsy. Early recognition and treatment of sleep-related disorders may prevent morbidity and result in improved survival of patients with PWS.
Keywords: Prader-Willi syndrome, Hypoventilation, Sleep-disordered breathing, Growth hormone, Excessive daytime sleepiness, Narcolepsy
Introduction
Prader-Willi syndrome (PWS) is a genetic disorder that affects about 1/10,000 to 1/30,000 individuals [1, 2••]. It is caused by a deficient expression of imprinted paternal genes on the long arm of chromosome 15. This problem can result from interstitial microdeletion of the paternally inherited 15q11.2-q13, maternal uniparental disomy 15, or, more rarely, due to imprinting defects [3]. Common to all cases of PWS is lack of expression of the small nucleolar ribonucleic acid-116 (SNORD116), which may account for the PWS phenotypes [4]. SNORD116 is also considered a candidate gene for the sleep disturbances experienced by patients with PWS [5].
Individuals with PWS present with infantile hypotonia that results in feeding difficulties and failure to thrive. This is followed in early childhood by excessive, difficult to control eating that often leads to morbid obesity. Characteristic craniofacial features include narrow bifrontal diameter, almond-shaped palpebral fissures, narrow nasal bridge, and thin upper vermillion with down-turned corners of the mouth [1, 2••]. Other features include growth hormone deficiency with subsequent short stature, small hands and feet, hypogonadism, learning difficulties, and challenging behavior patterns [2••].
Recent evidence suggests that PWS pathophysiology is related to hypothalamic dysfunction [6••]. The hypothalamus is essential for respiratory control and sleep/wake regulation. In this paper, we review how PWS impacts sleep in pediatric and adult patients, focusing on the etiology, pathophysiology, management options, and outcomes of sleep-related breathing disturbances. In addition, we address sleep–wake problems, focusing on excessive daytime sleepiness (EDS) and narcolepsy.
Ventilatory Control and the Spectrum of Sleep-Related Breathing Disorders in PWS
For patients with PWS, respiratory disturbances, particularly during sleep, begin in childhood [7]. They are a significant cause of morbidity and mortality. Sleep-related breathing disorders in these patients include central sleep apnea, obstructive sleep apnea, nocturnal hypoxemia, and sleep-related hypoventilation. Patients with PWS have abnormal ventilatory responses to hypoxia and hypercapnia that contribute to the development and severity of their sleep-related breathing disorders [8].
Abnormalities of Ventilatory Control
Hypoxic Ventilatory Response
The hypoxic ventilatory response (HPVR) is controlled by peripheral chemoreceptors located in the carotid bodies that modulate respiratory rate and tidal volume via the central respiratory control centers. The HPVR increases minute ventilation to maintain blood oxygen levels in the normal range under hypoxic conditions and following desaturations related to apneic pauses [8].
Patients with PWS have absent or blunted responses to breathing subatmospheric oxygen (14% FiO2) when compared to healthy controls [9]. Those who mount an HPVR do so by increasing tidal volume rather than respiratory rate, the opposite of the responses in healthy control subjects [9]. Additionally, patients with PWS paradoxically increase, rather than decrease, their respiratory rate in response to breathing a higher concentration of oxygen [10].
Hypercapnic Ventilatory Response
The hypercapneic ventilatory response (HCVR) describes the reaction of the respiratory system to elevated levels of CO2. During extended periods of hypoventilation, elevated levels of CO2 are sensed by peripheral chemoreceptors in the carotid bodies while central chemoreceptors respond to the associated decrease in pH of CSF and body fluids.
When HCVR was tested in subjects with and without PWS, all individuals showed some increase in ventilation in response to hypercapnia, which is the normal response. However, obese subjects with PWS had a blunted HCVR compared to BMI-matched obese controls and non-obese subjects with PWS [9].
Central Sleep Apnea
Central sleep apnea (CSA) is commonly seen in infants and children with PWS up to 2 years of age [11, 12]. The American Academy of Sleep Medicine (AASM) defines central apneas in children as follows: a ≥ 90% decrease in airflow signal from baseline for two breaths’ duration associated with a subsequent arousal or oxygen desaturation of ≥ 3%. In infants under 1 year old, central apneas may also be scored if they are associated with episodes of bradycardia [13]. Central apneas lasting ≥ 20 s are scored regardless of if they are followed by an arousal or desaturation event. Periodic breathing (defined as 3 central apneas of 3-s duration separated by breaths of no more than 20 s) also count towards central apnea scoring [13].
In children with PWS, central apneas occur predominantly during REM sleep and can cause severe intermittent oxyhemoglobin desaturations in some patients [12]. A genotype phenotype investigation showed that PWS patients with chromosomal deletions had a higher incidence of central apneas associated with significant oxygen desaturations (≥ 10%) than patients whose PWS phenotype was caused by another molecular mechanism [14].
CSA may be due to delays in maturation of the central ventilatory control centers or to an abnormal apneic threshold [9]. Congenital adrenal insufficiency is present in up to 60% of patients with PWS and may be related to the increased incidence of CSA [15]. In most cases, CSA improves with time [16]. However, central apneas can persist beyond infancy and may occur concurrently with obstructive apneas or hypoventilation [17•].
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) is common in patients with PWS, particularly after 2 years of age [11], and is highly prevalent in adolescents and adults [18]. Sleep-related symptoms include consistent snoring, pauses in breathing for longer than 5 s, or daytime sleepiness, particularly during incurrent respiratory illness. Some patients are reported to sleep with their necks extended to relieve upper airway obstruction [19]. Patients with PWS have increased risk of OSA for many reasons, including obesity, hypotonia, micrognathia, small oronasopharynx, and viscous secretions [20]. Hypothyroidism, a known cause of OSA, is also seen in some patients with PWS [2••].
The prevalence of OSA in patients with PWS is approximately 80% and many patients are classified as having severe OSA [21, 22•]. Abnormal arousal threshold and arousal responses to hypercapnia and hypoxemia [23, 24] likely contribute to prolonged obstructive apneas and predispose patients to developing sleep related hypoventilation. In a study of 60 adults with PWS, 30% of patients had a sleep-related breathing disorder, most of which were OSA; of these, 8% of patients had previously undiagnosed hypoventilation and only half the cases of OSA were previously identified and treated [25]. Thus, many adult patients with PWS and OSA may not exhibit obvious symptoms, indicating the need for ongoing testing and surveillance in this high risk population.
Sleep-Related Hypoventilation and Hypoxemia
Sleep-related hypoventilation is common in patients with PWS and can occur in absence of either CSA or OSA [17•]. This problem can be related to hypothalamic dysfunction and abnormal respiratory drive, obesity, hypotonia, scoliosis, or some combination of these factors. Hypoventilation out proportion to the degree of OSA has also been reported in children with PWS up to 14 years old [26•]. Hypoventilation is most prominent in REM sleep and may be associated with central hypopneas [17•]. Due to sleep-related hypoventilation, surveillance polysomnography with capnography is required in these patients.
Patients with PWS also have sleep-related hypoxemia, low oxygen desaturation nadirs, and high oxygen-desaturations indices. A genotype–phenotype correlation study showed those patients with PWS due to a chromosomal deletion had on average lower oxygen levels during sleep than patients with PWS due to other genetic mechanisms [22•].
Evaluation of Sleep-Related Breathing Disorders
Overnight polysomnography (PSG) is indicated for all patients diagnosed with PWS to detect sleep-related breathing disorders. The PSG must be performed and interpreted per AASM scoring for age [13]. For all age groups, CO2 monitoring is essential due to the high prevalence of nocturnal hypoventilation. PSG is important prior to initiating growth hormone (GH) treatment, and periodically during GH therapy, as GH can exacerbate adenotonsillar hypertrophy [27, 28]. Patients may not report obvious symptoms of OSA [25, 29], making routine screening more important.
Treatment of Sleep-Related Breathing Disorders
Once OSA is diagnosed, prompt referral to otolaryngology is recommended. Adenotonsillectomy (AT) is often the first-line treatment for OSA in children. A lateral neck X-ray may be helpful to assess adenotonsillar hypertrophy. OSA rarely resolves spontaneously in patients with PWS, so “watchful waiting” is not recommended. In a 4-year follow-up study of 20 children with PWS and OSA, only 2 children showed spontaneous resolution of their OSA [30]. After AT, children with PWS may have significant reduction in their obstructive apnea hypopnea index (oAHI); however, in many cases, the post-surgical oAHI remains elevated [31] and adjunctive therapy for OSA is required. It is therefore important to complete another PSG after AT to determine if a patient has persistent OSA [32••, 33].
Patients with PWS (especially young children and those with severe OSA, or morbid obesity) are at risk for acute post-operative complications and require close monitoring after AT [32••]. Velopharyngeal insufficiency is a rare complication of AT and may be prudent to counsel patients regarding this and consider speech evaluations before and after AT [32••].
Second-line therapy for OSA is most often continuous positive airway pressure (CPAP) or noninvasive positive pressure ventilation (NPPV) which improves residual OSA when used consistently during sleep [34, 35]. Significant behavioral problems are common in patients with PWS that can make adherence to PAP therapy quite challenging; therefore, we recommend incorporating behavioral therapy to assist with PAP desensitization and improve utilization.
Other components of the workup for OSA include TSH and free T4 levels to screen for hypothyroidism, and echocardiogram to look for pulmonary hypertension (particularly when severe OSA or sleep-related hypoventilation is present) [36]. Patients should also be screened for symptoms of gastroesophageal reflux disease (GERD) as it can worsen OSA [37]. GERD is common in patients with PWS, with a reported incidence of 53% [38].
While NPPV has a back-up rate and is an option for older patients with persistent CSA, NPPV is not usually practical for treating infants with central apneas. Instead, supplemental oxygen is often used as a therapy for central apneas in infants. In one study, oxygen administered to infants with PWS improved their Central Apnea Index (CAI) from median of 4.7 events/h to 2.5 events/h [12] and comparable results have been seen in subsequent studies [11].
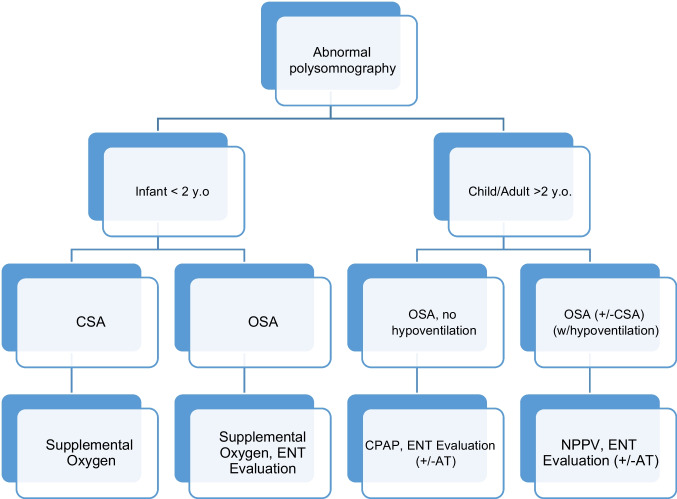
An approach to respiratory management is offered in Fig. 1.
Fig. 1.
Respiratory management of sleep disordered breathing
Growth Hormone Therapy
GH is an FDA-approved treatment for individuals with PWS. It has been postulated to improve resting ventilation and ventilatory response to CO2 by modulating either the peripheral chemoreceptors sensitivity to CO2 or how input is relayed from peripheral chemoreceptors to central respiratory control centers [39].
It was previously suggested that GH therapy may exacerbate OSA due to accelerated growth of lymphoid tissues [27, 28]. More recent reports found no correlation between GH initiation and symptom onset [40] or any clinically significant negative impact on respiration [41•]. In a 2-year, randomized, double-blind, placebo-controlled cross-over study, it was found that there was no significant difference in AHI, CAI, or obstructive apnea index (OAI) in individuals who received GH compared with placebo, affirming the safety of GH therapy [42•]. A cohort of 19 adults who were overweight and with mild or no OSA at baseline who received GH treatment for 1 year showed improvement in sleep efficiency [41•]. In this group, longer apneas and increased oxygen desaturations were reported, but these findings were inconsistent. Most importantly, no deaths were reported [41•].
GH therapy can result in improvement in ventilatory control and inspiratory drive [39]. In an Australian multicenter retrospective study, there was no significant increase in median oAHI following initiation of GH in children with PWS [43]. One study showed worsening of sleep disturbance after initiation of GH therapy; however, on average, affected patients had higher IGF-1 levels and respiratory issues, so they may have had other causes of morbidity [28]. Nonetheless, because a high rate of sleep disordered breathing is present in patients with PWS, providers should screen patients for OSA with PSG prior to initiation of GH [44]. Given the benefits of GH in patients with PWS, every attempt should be made to treat OSA with the goal of continuing GH therapy. Current guidelines advise that clinicians should repeat a PSG 6 to 10 weeks after GH initiation, particularly in children (who may not demonstrate obvious symptoms of sleep apnea) and in patients whose breathing-related symptoms worsen [28, 45]. Other groups recommend repeating PSG within the first 3–6 months of GH initiation [44] as well as annually during continued GH treatment depending on evolving symptoms and clinical presentation [46]. One study found the development of moderate to severe OSA in 13% of children after the initiation of GH, supporting the need for surveillance PSG after starting treatment [43].
Consequences of Sleep-Related Breathing Disorders
The estimated mortality rate of individuals with PWS is 1 to 4% per year and respiratory failure is the leading cause of death for all age groups [47]. Some deaths are reportedly related to sleep apnea or hypoventilation [19, 48]. There is also an increased risk of death from cardiopulmonary causes in those with the maternal disomy 15 subtype, although the mechanism for this is unknown [47]. Pulmonary hypertension, a known consequence of untreated OSA, is also reported in patients with PWS [49]. Other consequences of untreated OSA include increased risk of cardiovascular events and metabolic disease [50]. Adrenal insufficiency may be a contributing factor to sudden death, especially during periods of acute stress and infection [15]. Stress may also increase the frequency of central apneas in patients with PWS [51].
The behavioral problems exhibited by patients with PWS may be secondary to their sleep disorders, but causation has not been established. One study showed that increased OSA severity was associated with autistic-like behavior and impulsiveness in children with PWS [52]. While these features may be due to PWS itself, they may be worsened by OSA, which is known to increase daytime agitation and impulsiveness in children.
Cardiac Autonomic Control During Sleep
Children with PWS have impaired parasympathetic modulation during slow wave sleep, resulting in high heart rates in absence of cardiovascular disease and independent of obesity [53••]. This is important as cardiac death is the second most common cause of mortality in patients with PWS [47]. Patients with PWS should have regular cardiovascular assessments, starting in childhood [53••, 54].
Excessive Daytime Sleepiness and Narcolepsy
EDS unexplained by sleep disordered breathing is reported in some patients with PWS. Among respondents in the Global PWS Registry, 55% reported having EDS or narcolepsy. 6.1% of participants in the registry reported carrying a diagnosis of narcolepsy and 8.8% carried a diagnosis of cataplexy [55••]. While OSA is known to be associated with EDS, there is increasing evidence that it is not the only contributor in patients with PWS. Notably, some patients with PWS have persistent EDS even after weight loss and improvement in sleep disordered breathing, suggesting that primary hypersomnia is a characteristic feature of PWS [56]. Hypothalamic dysfunction may be a cause in some patients [57].
Some individuals with PWS have a narcolepsy-like presentation with sleep-onset REM periods, and sometimes cataplexy [58]. Low levels of orexin (also called hypocretin), a neurotransmitter produced in the hypothalamus that regulates appetite and sleep, is implicated in narcolepsy type I (formerly called narcolepsy with cataplexy). This disease is caused by the degeneration of hypothalamic neurons that produce orexin [59], resulting in very low or undetectable levels of orexin in the CSF. Interestingly, patients with PWS who exhibit EDS also have lower levels of orexin compared to normal individuals, but not as low as those with narcolepsy type I [60]. As opposed to the pathophysiology of narcolepsy type I, the number of hypothalamic orexin neurons is not reduced in individuals with PWS [61], suggesting that it is disruptions in the pathways connecting these hypothalamic neurons to the brainstem and cerebral cortex that are responsible for the lower orexin levels seen in these patients [62]. One study, showed that hypoxemia and hypercarbia inhibited hypothalamic orexin neurons in rats, suggesting that sleep disordered breathing may increase the risk of developing EDS [63]. Recognition and treatment of sleep disordered breathing is therefore of utmost importance to prevent further degradation of what may be an already impaired neuronal signaling system.
Diagnosing narcolepsy in patients with PWS can be challenging, especially as the diagnosis requires exclusion (or treatment) of other confounding sleep disorders, such as OSA. Patients with PWS sometimes have difficulty with adherence to PAP therapy and their OSA may never truly resolve, which leads to challenges making the diagnosis of narcolepsy based on current clinical guidelines. A Multiple Sleep Latency Test (MSLT) should be performed in all patients who have significant hypersomnolence out of proportion to their OSA. Ideally, the MSLT should be completed after patients have been established on appropriate PAP therapy, and PAP should be used during the overnight PSG preceding MSLT testing.
It is also essential to measure a wake CO2 level by arterial or capillary blood gas in any patient with PWS, obesity, and hypersomnia. Chronic respiratory failure with hypoventilation can cause EDS, headaches, and other symptoms, as well as contribute to cardiovascular stress and pulmonary hypertension. When daytime CO2 levels are significantly elevated (e.g., pCO2 > 50 mmHg), initiation of NPPV may be indicated. Blood gas testing can expedite the diagnosis of chronic hypoventilation compared to waiting for completion of PSG.
Modafinil, a central stimulant, may be helpful in the treatment of patients with PWS and EDS in the absence of sleep disordered breathing. An open-label pilot study of 10 patients with PWS and EDS showed significant improvement in sleepiness symptoms using the Epworth Sleepiness scale [64]. Pitolisant may also improve the EDS and neurospsychiatric symptoms in PWS patients by acting on the H3 receptor to potentiate the central activity of histaminergic neurons [65, 66•]. At the time of this review, the use of pitolisant for EDS in patients is not yet FDA-approved.
Conclusions
Sleep disorders are prevalent throughout the lifetime of individuals with PWS. Sleep-related breathing disorders present as CSA in infancy and OSA and hypoventilation in older children, adolescents, and adults. GH therapy has improved the clinical outcomes of patients with PWS, but close surveillance for the development or worsening of OSA is of utmost importance. EDS can persist even after sleep-related breathing disorders are treated and some individuals may develop narcolepsy. Early recognition and treatment of sleep disorders may prevent and improve survival of patients with PWS and is therefore a critical component for medical management for these patients.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 2.Butler MG, Miller JL, Forster JL. Prader-Willi syndrome - clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. 2019;15:207–244. doi: 10.2174/1573396315666190716120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38:1249–1263. doi: 10.1007/s40618-015-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieth E, Eddiry S, Gaston V, et al. Highly restricted deletion of the SNORD116 region is implicated in Prader-Willi syndrome. Eur J Hum Genet. 2015;23:252–255. doi: 10.1038/ejhg.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassi G, Priano L, Maggi S, et al. Deletion of the Snor D116/S no RD116 alters sleep in mice and patients with Prader-Willi syndrome. Sleep. 2016 doi: 10.5665/sleep.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021;9:235–246. doi: 10.1016/S2213-8587(21)00002-4. [DOI] [PubMed] [Google Scholar]
- 7.Einfeld SL, Kavanagh SJ, Evans EJ, Tonge BJ, Taffe J (2002) Mortality in Prader-Willi syndrome NIH Public Access. Van [DOI] [PMC free article] [PubMed]
- 8.Gillett E, Perez I. Disorders of sleep and ventilatory control in Prader-Willi syndrome. Diseases. 2016;4:23. doi: 10.3390/diseases4030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arens R, Gozal D, Omlin KJ, Livingston FR, Liu J, Keens TG, Ward SLD. Hypoxic and hypercapnic ventilatory responses in Prader-Willi syndrome. J Appl Physiol. 1994 doi: 10.1152/jappl.1994.77.5.2224. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D, Arens R, Omlin KJ, Ward SLD, Keens TG. Absent peripheral chemosensitivity in Prader-Willi syndrome. J Appl Physiol. 1994 doi: 10.1152/jappl.1994.77.5.2231. [DOI] [PubMed] [Google Scholar]
- 11.Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi syndrome. PLoS ONE. 2014 doi: 10.1371/journal.pone.0101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urquhart DS, Gulliver T, Williams G, Harris MA, Nyunt O, Suresh S. Central sleep-disordered breathing and the effects of oxygen therapy in infants with Prader-Willi syndrome. Arch Dis Child. 2013 doi: 10.1136/archdischild-2012-303441. [DOI] [PubMed] [Google Scholar]
- 13.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.6. American Academy of Sleep Medicine, Darien, Illinois. Darien, Illinois: American Academy of Sleep Medicine. 2011.
- 14.Torrado M, Araoz V, Baialardo E, et al. Clinical-etiologic correlation in children with Prader-Willi syndrome (PWS): an interdisciplinary study. Am J Med Genet A. 2007 doi: 10.1002/ajmg.a.31520. [DOI] [PubMed] [Google Scholar]
- 15.de Lind Van, Wijngaarden RFA, Joosten KFM, van den Berg S, Otten BJ, de Jong FH, Sweep CGJ, de Weerd AW, Hokken-Koelega ACS. The relationship between central adrenal insufficiency and sleep-related breathing disorders in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2009;94:2387–2393. doi: 10.1210/jc.2008-2808. [DOI] [PubMed] [Google Scholar]
- 16.Khayat A, Narang I, Bin-Hasan S, Amin R, Al-Saleh S. Longitudinal evaluation of sleep disordered breathing in infants with Prader-Willi syndrome. Arch Dis Child. 2017 doi: 10.1136/archdischild-2016-311298. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer J, Davey MJ, Nixon GM. Sleep-disordered breathing in school-aged children with Prader-Willi syndrome. J Clin Sleep Med. 2022 doi: 10.5664/jcsm.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yee BJ, Buchanan PR, Mahadev S, Banerjee D, Liu PY, Phillips C, Loughnan G, Steinbeck K, Grunstein RR. Assessment of sleep and breathing in adults with Prader-Willi syndrome: a case control series. J Clin Sleep Med. 2007 doi: 10.5664/jcsm.27028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiholzer U. Deaths in children with Prader-Willi syndrome: a contribution to the debate about the safety of growth hormone treatment in children with PWS. Horm Res. 2005;63:33–39. doi: 10.1159/000082745. [DOI] [PubMed] [Google Scholar]
- 20.Tan HL, Urquhart DS. Respiratory complications in children with Prader Willi syndrome. Paediatr Respir Rev. 2017;22:52–59. doi: 10.1016/j.prrv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Sedky K, Bennett DS, Pumariega A. Prader willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med. 2014;10:403–409. doi: 10.5664/jcsm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozsezen B, Emiralioglu N, Özön A, et al. Sleep disordered breathing in patients with Prader willi syndrome: impact of underlying genetic mechanism. Respir Med. 2021 doi: 10.1016/j.rmed.2021.106567. [DOI] [PubMed] [Google Scholar]
- 23.Livingston FR, Arens R, Bailey SL, Keens TG, Ward SLD. Hypercapnic arousal responses in Prader-Willi syndrome. Chest. 1995 doi: 10.1378/chest.108.6.1627. [DOI] [PubMed] [Google Scholar]
- 24.Arens R, Gozal D, Burrell BC, Bailey SL, Bautista DB, Keens TC, Davidson Ward SL. Arousal and cardiorespiratory responses to hypoxia in Prader-Willi syndrome. Am J Respir Crit Care Med. 1996 doi: 10.1164/ajrccm.153.1.8542130. [DOI] [PubMed] [Google Scholar]
- 25.Ghergan A, Coupaye M, Leu-Semenescu S, Attali V, Oppert JM, Arnulf I, Poitou C, Redolfi S. Prevalence and phenotype of sleep disorders in 60 adults with prader–Willi syndrome. Sleep. 2017 doi: 10.1093/sleep/zsx162. [DOI] [PubMed] [Google Scholar]
- 26.Abel F, Tan HL, Negro V, Bridges N, Carlisle T, Chan E, Laverty A, Miligkos M, Samuels M, Kaditis AG. Hypoventilation disproportionate to OSAS severity in children with Prader-Willi syndrome. Arch Dis Child. 2019 doi: 10.1136/archdischild-2017-314282. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet G, Deal CL, Crock PA, Robitaille Y, Oligny LL. Sudden death in growth hormone-treated children with Prader-Willi syndrome. J Pediatr. 2004 doi: 10.1016/j.jpeds.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 28.Miller J, Silverstein J, Shuster J, Driscoll DJ, Wagner M. Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab. 2006;91:413–417. doi: 10.1210/jc.2005-1279. [DOI] [PubMed] [Google Scholar]
- 29.Vandeleur M, Davey MJ, Nixon GM. Are sleep studies helpful in children with Prader-Willi syndrome prior to commencement of growth hormone therapy? J Paediatr Child Health. 2013;49:238–241. doi: 10.1111/jpc.12109. [DOI] [PubMed] [Google Scholar]
- 30.Wong SB, Yang MC, Tzeng IS, Tsai WH, Lan CC, Tsai LP. Progression of obstructive sleep apnea syndrome in pediatric patients with Prader-Willi syndrome. Children. 2022 doi: 10.3390/children9060912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CH, Hsu WC, Ko JY, Yeh TH, Lin MT, Kang KT. Adenotonsillectomy for the treatment of obstructive sleep apnea in children with Prader-Willi syndrome: a meta-analysis. Otolaryngology - Head and Neck Surgery (United States) 2020;162:168–176. doi: 10.1177/0194599819893115. [DOI] [PubMed] [Google Scholar]
- 32.Clements AC, Dai X, Walsh JM, Sterni LM, Prichett L, Boss EF, Seal SM, Ryan MA. Outcomes of adenotonsillectomy for obstructive sleep apnea in Prader-Willi syndrome: systematic review and meta-analysis. Laryngoscope. 2021 doi: 10.1002/lary.28922. [DOI] [PubMed] [Google Scholar]
- 33.Hoban TF, Friedman NR. Polysomnography should be required both before and after adenotonsillectomy for childhood sleep disordered breathing. J Clin Sleep Med. 2007 doi: 10.5664/jcsm.27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sforza E, Krieger J, Geisert J, Kurtz D. Sleep and breathing abnormalities in a case of Prader-Willi syndrome. Acta Paediatr Scand: The effects of acute continuous positive airway pressure treatment; 1991. [DOI] [PubMed] [Google Scholar]
- 35.Smith IE, King MA, Siklos PWL, Shneerson JM. Treatment of ventilatory failure in the Prader-Willi syndrome. Eur Respir J. 1998 doi: 10.1183/09031936.98.11051150. [DOI] [PubMed] [Google Scholar]
- 36.Maloney MA, Ward SLD, Su JA, Durazo-Arvizu RA, Breunig JM, Okpara DU, Gillett ES. Prevalence of pulmonary hypertension on echocardiogram in children with severe obstructive sleep apnea. J Clin Sleep Med. 2022 doi: 10.5664/jcsm.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu ZH, Yang XP, Niu X, Xiao XY, Chen X. The relationship between obstructive sleep apnea hypopnea syndrome and gastroesophageal reflux disease: a meta-analysis. Sleep and Breathing. 2019 doi: 10.1007/s11325-018-1691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeves R, Strøm F, Sandvik L, Nordgarden H. Gastro-oesophageal reflux - an important causative factor of severe tooth wear in Prader-Willi syndrome? Orphanet J Rare Dis. 2018 doi: 10.1186/s13023-018-0809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindgren AC, Hellström LG, Ritzén EM, Milerad J. Growth hormone treatment increases CO2 response, ventilation and central inspiratory drive in children with Prader-Willi syndrome. Eur J Pediatr. 1999 doi: 10.1007/s004310051246. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann M, Laemmer C, Woelfle J, Fimmers R, Gohlke B. Sleep-disordered breathing in children with Prader-Willi syndrome in relation to growth hormone therapy onset. Horm Res Paediatr. 2020;93:85–93. doi: 10.1159/000506943. [DOI] [PubMed] [Google Scholar]
- 41.Shukur HH, Hussain-Alkhateeb L, Farholt S, Nørregaard O, Jørgensen AP, Hoybye C. Effects of growth hormone treatment on sleep-related parameters in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2021;106:E3634–E3643. doi: 10.1210/clinem/dgab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donze SH, de Weerd AW, van den Bossche RAS, Joosten KFM, Hokken-Koelega ACS. Sleep-related breathing disorders in young adults with Prader-Willi syndrome: a placebo-controlled, crossover GH trial. J Clin Endocrinol Metab. 2019;104:3931–3938. doi: 10.1210/jc.2019-00391. [DOI] [PubMed] [Google Scholar]
- 43.Caudri D, Nixon GM, Nielsen A, et al. Sleep-disordered breathing in Australian children with Prader-Willi syndrome following initiation of growth hormone therapy. J Paediatr Child Health. 2022;58:248–255. doi: 10.1111/jpc.15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deal CL, Tony M, Hoÿbye C, et al. Growth hormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2012-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCandless SE, Saal HM, Braddock SR, Enns G, Gruen JR, Perrin JM, Saul RA, Tarini BA. Clinical report - health supervision for children with Prader-Willi syndrome. Pediatrics. 2011 doi: 10.1542/peds.2010-2820. [DOI] [PubMed] [Google Scholar]
- 46.Berini J, Spica Russotto V, Castelnuovo P, et al. Growth hormone therapy and respiratory disorders: long-term follow-up in PWS children. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1831. [DOI] [PubMed] [Google Scholar]
- 47.Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2017;19:635–642. doi: 10.1038/gim.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proffitt J, Osann K, McManus B, Kimonis VE, Heinemann J, Butler MG, Stevenson DA, Gold JA. Contributing factors of mortality in Prader-Willi syndrome. Am J Med Genet A. 2019 doi: 10.1002/ajmg.a.60688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emanuel H, Kaplan K. 846 pulmonary arterial hypertension as a sequela of untreated obstructive sleep apnea in a patient with Prader-Willi syndrome. Sleep. 2021 doi: 10.1093/sleep/zsab072.843. [DOI] [Google Scholar]
- 50.Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015 doi: 10.1016/j.wjorl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beauloye V, Dhondt K, Buysse W, et al. Evaluation of the hypothalamic-pituitary-adrenal axis and its relationship with central respiratory dysfunction in children with Prader-Willi syndrome Rare endocrinological diseases. Orphanet J Rare Dis. 2015 doi: 10.1186/s13023-015-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Donoghue FJ, Camfferman D, Kennedy JD, Martin AJ, Couper T, Lack LD, Lushington K, McEvoy RD. Sleep-disordered breathing in Prader-Willi syndrome and its association with neurobehavioral abnormalities. J Pediatr. 2005 doi: 10.1016/j.jpeds.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Brito LC, Queiroga T, Franco RR, Passone CGB, Lopes MC, Shea SA, Bueno C, Soster LMSFA. Cardiac autonomic control during non-REM and REM sleep stages in paediatric patients with Prader-Willi syndrome. J Sleep Res. 2021 doi: 10.1111/jsr.13165. [DOI] [PubMed] [Google Scholar]
- 54.Marcus KA, van Alfen-Van Der Velden JA, Otten BJ, Weijers G, Yntema HG, de Korte CL, Kapusta L. Cardiac evaluation in children with Prader-Willi syndrome. Acta Paediatrica Int J Paediatr. 2012 doi: 10.1111/j.1651-2227.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 55.Shelkowitz E, Gantz MG, Ridenour TA, Scheimann AO, Strong T, Bohonowych J, Duis J. Neuropsychiatric features of Prader-Willi syndrome. Am J Med Genet A. 2022;188:1457–1463. doi: 10.1002/ajmg.a.62662. [DOI] [PubMed] [Google Scholar]
- 56.Harris JC, Allen RP (1996) Is excessive daytime sleepiness characteristic of Prader-Willi syndrome? The effects of weight change. Arch Pediatr Adolesc Med. 10.1001/archpedi.1996.02170370066011 [DOI] [PubMed]
- 57.Camfferman D, Doug McEvoy R, O’Donoghue F, Lushington K. Prader Willi Syndrome and excessive daytime sleepiness. Sleep Med Rev. 2008 doi: 10.1016/j.smrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Vela-Bueno A, Kales A, Soldatos CR, Dobladez-Blanco B, Campos-Castello J, Espino-Hurtado P, Olivan-Palacios J. Sleep in the Prader-Willi syndrome: clinical and polygraphic findings. Arch Neurol. 1984 doi: 10.1001/archneur.1984.04050150072020. [DOI] [PubMed] [Google Scholar]
- 59.Siegel JM, Moore R, Thannickal T, Nienhuis R. A brief history of hypocretin/orexin and narcolepsy. Neuropsychopharmacology. 2001 doi: 10.1016/S0893-133X(01)00317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omokawa M, Ayabe T, Nagai T, Imanishi A, Omokawa A, Nishino S, Sagawa Y, Shimizu T, Kanbayashi T. Decline of CSF orexin (hypocretin) levels in Prader-Willi syndrome. Am J Med Genet A. 2016 doi: 10.1002/ajmg.a.37542. [DOI] [PubMed] [Google Scholar]
- 61.Fronczek R, Lammers GJ, Balesar R, Unmehopa UA, Swaab DF. The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader-Willi syndrome. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2005-0296. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi M, Miyata R, Tanuma N. Decrease in acetylcholinergic neurons in the pedunculopontine tegmental nucleus in a patient with Prader-Willi syndrome. Neuropathology. 2011 doi: 10.1111/j.1440-1789.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 63.Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Hypoxia and hypercapnia inhibit hypothalamic orexin neurons in rats. J Neurophysiol. 2016 doi: 10.1152/jn.00196.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Cock VC, Diene G, Molinas C, le Masson VD, Kieffer I, Mimoun E, Tiberge M, Tauber M. Efficacy of modafinil on excessive daytime sleepiness in Prader-Willi syndrome. Am J Med Genet A. 2011 doi: 10.1002/ajmg.a.34047. [DOI] [PubMed] [Google Scholar]
- 65.Pennington S, Stutzman D, Sannar E. Pitolisant in an adolescent with Prader-Willi syndrome. J Pediatr Pharmacol Ther. 2021 doi: 10.5863/1551-6776-26.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pullen LC, Picone M, Tan L, Johnston C, Stark H. Cognitive improvements in children with Prader-Willi syndrome following pitolisant treatment—patient reports. J Pediatr Pharmacol Ther. 2019 doi: 10.5863/1551-6776-24.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]