Abstract
Introduction
Vidutolimod, a CpG-A TLR9 agonist, was investigated in a phase 1b study (CMP-001-003; ClinicalTrials.gov, NCT03438318) in combination with atezolizumab with and without radiation therapy (RT) in patients with advanced NSCLC.
Methods
Patients with progressive disease after anti–programmed cell death protein 1 or programmed death-ligand 1 therapy received either vidutolimod and atezolizumab (part A) or vidutolimod, atezolizumab, and RT (part B). The primary objective was to evaluate the safety of vidutolimod and atezolizumab with and without RT. Key secondary end point was best objective response rate per Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
Between March 28, 2018, and July 25, 2019, a total of 29 patients were enrolled and received at least one dose of vidutolimod (part A, n = 13; part B, n = 16). Intratumoral injections of vidutolimod were administered successfully, including injection of visceral lesions. The most common treatment-related adverse events (≥30%) were flu-like symptoms and hypotension. No objective responses were observed; 23.1% and 50.0% of the patients in parts A and B, respectively, had stable disease as best response. In parts A and B, 15.4% and 25.0% of the patients, respectively, had tumor shrinkage (<30% decrease in tumor size, nonirradiated). Enrollment was stopped owing to lack of objective responses. In the two patients with initial tumor shrinkage in part A, a strong serum induction of C-X-C motif chemokine ligand 10 was observed.
Conclusions
Vidutolimod and atezolizumab with and without RT had a manageable safety profile, with minimal clinical activity in heavily pretreated patients with programmed cell death protein 1 or programmed death-ligand 1 blockade–resistant NSCLC.
Keywords: Vidutolimod, Atezolizumab, Radiation, TLR9 agonist, NSCLC
Introduction
Immune checkpoint inhibition has revolutionized the treatment paradigm for patients with metastatic NSCLC and is yielding unprecedented benefit.1,2 Nevertheless, a substantial portion of patients do not respond, and many who do respond eventually experience disease progression owing to acquired resistance to programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) blockade.2 The absence of preexisting interferon (IFN) gamma–secreting CD8+ T cells at the tumor margin or within the tumor is one of several postulated mechanisms of resistance to anti–PD-1 or PD-L1 therapies,3, 4, 5, 6 and thus, combination therapies targeting multiple cancer immune evasion pathways may be necessary for an effective antitumor immune response.7
Vidutolimod (previously known as CMP-001) is a CpG-A TLR9 agonist packaged within an immunogenic virus-like particle that induces the production of anti–virus-like particle antibodies, thereby stimulating plasmacytoid dendritic cells (pDCs), resulting in IFN alfa induction and increased tumor regression in preclinical models compared with treatment with naked CpG-A oligonucleotides.8 The activation of pDCs is enhanced by co-stimulation of TLR9 and Fcγ receptor IIA (CD32).9,10 In preclinical studies, pDC activation by CpG oligonucleotides led to cross-priming of antitumor CD8+ T cells, mediated by the transfer of tumor antigens from pDCs to conventional DCs.11 In a phase 1b study in advanced melanoma, intratumoral injection of vidutolimod plus pembrolizumab resulted in reversal of PD-1 blockade resistance, with durable responses and an acceptable safety profile in patients who previously progressed on anti–PD-1 therapy.12
Atezolizumab, a PD-L1 blocking antibody, is approved by the U.S. Food and Drug Administration in multiple indications, including the first-line treatment of adult patients with metastatic NSCLC whose tumors have high PD-L1 expression (PD-L1 ≥50% of tumor cells) and no EGFR or ALK genomic tumor aberrations; atezolizumab is also used in combination with chemotherapy for the first-line treatment of patients with metastatic NSCLC without EGFR or ALK genomic tumor aberrations.13, 14, 15 Radiation therapy (RT) has been found to act as an immunostimulant and immunosuppressive.16, 17, 18 Its immunomodulatory effects are driven by multiple mechanisms, including DNA fragmentation-mediated induction of IFN-stimulated genes, induction of inflammatory cytokines, and induction of immunogenic cell death, resulting in increased antigen presentation.17,18 TLR9 agonists have previously been reported to synergize with RT and improve responses in mouse tumor models19,20 and in humans.21 In lung and colon cancer mouse models, RT resulted in recruitment of pDCs into tumors, whereas intratumoral injection of vidutolimod induced CD4+ and CD8+ T-cell responses in tumors, with local and abscopal antitumor effects.22
On the basis of the complementary immune-activating effects of a TLR9 agonist, PD-1 or PD-L1 inhibitor, and RT, we hypothesized that a combination approach may enhance antitumor immune responses and bring clinical benefit to patients with resistance to PD-1 or PD-L1 blockade.23 Here, we report the results of the phase 1b study, CMP-001-003, evaluating vidutolimod and atezolizumab with and without RT in patients with NSCLC who progressed on previous PD-1 or PD-L1 blockade therapy.
Methods
Study Design and Oversight
CMP-001-003 (NCT03438318) was a multicenter, open-label, two-part, phase 1b study of vidutolimod and atezolizumab with and without RT in patients with advanced NSCLC. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All relevant institutional review boards approved this study, and all patients provided written informed consent.
Patients received vidutolimod and atezolizumab in part A and vidutolimod, atezolizumab, and RT in part B. Each part included a five-patient safety run-in. After a 30-day dose-limiting toxicity (DLT) monitoring period that included the first five doses of vidutolimod, a safety review committee determined whether accrual should continue. Accrual to part B was sequential to part A and contingent on an acceptable safety profile in part A.
Patients
Patients aged 18 years or older with histologically confirmed NSCLC with recurrent or metastatic disease, measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1), and Eastern Cooperative Oncology Group performance status of 0 or 1 were eligible. Progressive disease (PD) on prior PD-1 or PD-L1 blockade was required. Patients with EGFR-activating mutations or ALK gene rearrangements must have received prior standard-of-care treatment and have evidence of PD. At least one extra–central nervous system (CNS), non–bone tumor lesion of at least 1.5 cm amenable to intratumoral injection that was not near or encasing critical structures, such as the major blood vessels, trachea, or nerve bundles, was required. Patients with CNS metastases were eligible for the trial if the metastases had been treated by use of surgery or RT, the patient did not require corticosteroids of greater than 10 mg/d prednisone or the equivalent, the patient was neurologically stable for at least 2 weeks, and the brain magnetic resonance imaging performed within 6 weeks of screening did not reveal progression of CNS disease.
Treatment
The TLR9 agonist vidutolimod was administered subcutaneously once weekly at 5 mg (1 mg/mL) in weeks 1 and 2, intratumorally at 5 mg (1 mg/mL) or 10 mg (2 mg/mL) in weeks 3 to 5, and every 3 weeks (subcutaneously or intratumorally at the investigator’s discretion) thereafter. At week 2 (1 wk after the first vidutolimod injection), PD-L1 blockade with intravenous atezolizumab 1200 mg was administered every 3 weeks. The RT consisted of 20 grays (photons or protons) delivered in five fractions for 5 days beginning more than or equal to 2 days before starting vidutolimod in part B; a vidutolimod injection into the irradiated lesion was required. Treatment continued until PD, unacceptable toxicity, or consent withdrawal. Patients with documented PD in part A had the option of receiving radiation add-on treatment.
The route of administration of vidutolimod (i.e., subcutaneous or intratumoral) beyond week 5 was at the investigator’s discretion. Owing to results from a study evaluating vidutolimod and pembrolizumab in patients with PD-1 blockade–resistant metastatic melanoma which revealed a similar safety profile for the vidutolimod 10-mg intratumoral dose and doses less than 10 mg, previously enrolled patients receiving vidutolimod 5 mg intratumoral injections were dose escalated to 10 mg intratumorally.12 Subsequently enrolled patients also received vidutolimod 10 mg intratumorally. Subcutaneous administration could be performed at any site, but areas of lymphatic drainage of metastatic disease were preferred. Vidutolimod intratumoral injections could be administered to target or to nontarget lesions, although the target lesions were preferred. The target lesions were identified per RECIST v1.1. The irradiated lesions could not be used as the target lesions for response assessment. If the total dose of vidutolimod was to be split across multiple lesions, a minimum of approximately 3 mg should have been injected into each lesion. For the patients in part 2, vidutolimod injection into the irradiated lesion was required.
To reduce symptoms associated with vidutolimod-induced cytokine release, intravenous fluids, nonsteroidal anti-inflammatory drugs, and antiemetics were recommended. For patients who experienced a vidutolimod-related adverse event (AE) of at least grade 3, steroid prophylaxis was recommended for subsequent vidutolimod doses. For the first five vidutolimod dosing visits, patient vital signs were collected before vidutolimod dosing and at 30-minute intervals (±15 min) for 4 hours after dosing; observation periods could be reduced to 1 hour for patients with mild to no AEs at the investigator’s discretion. An internal gross tumor volume plus 5- to 8-mm margin (based on the investigator’s discretion) was used to define the planning target volume for RT. No clinical target volume was specified for RT. The prescribed dose of RT was administered to the planning target volume, with 95% coverage whenever possible. In part A, patients who received RT at the time of PD underwent a 10-day vidutolimod washout period. Methods on DLT assessments are provided in the Supplementary Methods.
Objectives and Assessments
The primary objective was to evaluate the safety of vidutolimod and atezolizumab with and without RT. Treatment-emergent AEs (TEAEs) were coded using the Medical Dictionary for Regulatory Activities, version 20.0, and the severity of TEAEs was classified using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. The investigator assessed the relationship of TEAEs to the combination study treatment (treatment-related AEs [TRAEs]) and not to the individual treatment components.
The secondary objectives were to assess the antitumor activity of the combination treatment and its pharmacodynamic effects on C-X-C motif chemokine ligand 10 (CXCL10). Antitumor activity was measured using best objective response rate, time to response, and duration of response per investigator-assessed RECIST v1.1. Tumor imaging was performed less than or equal to 3 weeks before the first vidutolimod injection, every 9 (±1) weeks from the first vidutolimod injection, and at the end of the treatment.
Exploratory objectives included the systemic pharmacodynamic profile of intratumoral vidutolimod injection and characterization of biomarkers in tumor biopsy specimens and the peripheral blood. Methods on safety parameters assessed are provided in the Supplementary Methods.
Pharmacodynamic and Biomarker Analyses
A magnetic 25-Plex Luminex Assay (catalog #LHC0009M; ThermoFisher Scientific, Waltham, MA) was used to quantitate cytokine and chemokine levels; 4-μm-thick serial sections generated from formalin-fixed paraffin-embedded tumor biopsy tissue were immunostained for CD8 (cytotoxic T cells; catalog #M7103; Dako Agilent, Santa Clara, CA), PD-L1 (catalog #13684; Cell Signaling Technology, Danvers, MA), and corresponding matching isotype controls. Full-scan analysis was performed using Flagship Bioscience’s (Broomfield, CO) proprietary computational tissue analysis imaging software system. The assay was performed by QPS (Newark, DE) according to the manufacturer’s specification, with samples analyzed in triplicate and read on a Bio-Plex 200 (Bio-Rad Laboratories, Hercules, CA).
Concentrations for each biomarker were back-calculated against the corresponding standard curve using five-parameter logistic regression. Staining and analysis were performed by Flagship Biosciences. Slides were stained in a Leica Bond RX Autostainer and scanned on Aperio’s AT Turbo and CS bright-field slide scanning systems (Leica Biosystems, Buffalo Grove, IL). The computational tissue analysis and image analysis platform identified nuclei based on hematoxylin staining and then quantified the intensity of staining for each identified cell. To identify the positive cells, staining-intensity thresholds were set using biomarker-specific algorithms for CD8 and PD-L1. For PD-L1, multiple thresholds of scoring were set (negative, +1, +2, and +3), consistent with manual scoring approaches. All annotations and image analysis markups were assessed by a pathologist to verify performance and accuracy. Stained cell counts and the percentage of positive cells were quantified. PD-L1 expression was quantified from multiple intensity thresholds using the following algorithm that calculated the histology score (H-score). Digital H-scores ranging from 0 to 300 were calculated using the following standard formula: [3 × % cells +3 intensity] + [2 × % cells +2 intensity] + [1 × % cells +1 intensity].
Whole Exome Sequencing
DNA was extracted by GeneWiz according to the company’s standard protocols. Sample FASTQ files were first assessed by FASTQC version 0.11.7 and in aggregate by MultiQC as previously described.24,25 Sample FASTQs were aligned by Burrows-Wheeler Alignment tool (version 0.7.17) using Hg38 reference assembly as previously described.26 Reads were sorted and duplicates marked using the bundled Picard tools with GATK version 4.1.8.1.27 Base quality score recalibration was applied27 to the sorted, duplicate-marked alignments. Recalibrated alignments were processed by MuTect28 for single-sample calling; owing to the lack of adjacent normal samples, the germline resource from gnomAD29 was applied, along with the Panel of Normals provided by the Broad Institute (Cambridge, MA), to identify germline variants and sequencing artifacts, respectively. Orientation bias data were emitted and trained by the Learn Orientation Bias module of MuTect to help identify putative artifacts. The emitted MuTect variants and processed orientation bias were integrated into a final callset and were annotated by SnpEff version 5.0.30
Statistical Analysis
The sample size for each part of the study was based on a Simon’s two-stage optimal design by enrolling 12 patients in stage 1 and continuing with enrollment of 23 additional patients in stage 2 only if there were at least two of 12 responders based on RECIST v1.1. The assumptions for the study were if the null hypothesis H0: p ≤ 10% was true, there would be a 10% chance (i.e., α = 0.10) of concluding that the study regimens were promising and should be studied further; if the alternative hypothesis H1: p ≥ 30% was true, there would be a 10% chance (i.e., β = 0.10) of rejecting the study regimens for further study. This was assessed separately for part A and part B.
If the DLT rate exceeded 33% in the first five patients enrolled in each part, further accrual in that part would be halted. If safety run-in established an acceptable safety profile of the treatment regimen in the first five patients, each part would enroll an additional seven patients in stage 1; if an acceptable safety profile was established and more than or equal to two of 12 assessable patients had a RECIST response, each part would enroll an additional 23 patients in stage 2.
All patients who received at least one dose of vidutolimod were included in the analysis. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Patients
Between March 28, 2018, and July 25, 2019, 29 patients with recurrent or metastatic NSCLC were enrolled in the study (part A, 13; part B, 16) (Supplementary Fig. 1). Patient characteristics and prior treatments are summarized in Table 1 and Supplementary Table 1. In parts A and B, the median number of prior lines of therapy was three (range, 1–6), and all patients received prior anti–PD-1 or PD-L1 therapy. Across both parts, the best response to prior anti–PD-1 or PD-L1 treatment was PD in 44.8% of the patients, stable disease in 48.3% of the patients, and PR in 6.9% of the patients. Furthermore, 31% of the patients had PD-L1–negative tumors, 37.9% of the patients had PD-L1–positive tumors, and the status was unknown in 31.0% of the patients.
Table 1.
Patient Demographics and Baseline Characteristics
| Demographic or Characteristics | Part A Vidutolimod + Atezolizumab n = 13 |
Part B Vidutolimod + Atezolizumab + RT n = 16 |
|---|---|---|
| Median age, y (range) | 65 (48–75) | 57 (44–76) |
| Male | female, n (%) | 6 (46.2) | 7 (53.8) | 10 (62.5) | 6 (37.5) |
| ECOG PS 0 | 1, n (%) | 4 (30.8) | 9 (69.2) | 3 (18.8) | 13 (81.3) |
| Baseline disease location(s),a n (%) | ||
| Any lung | 11 (84.6) | 16 (100) |
| Lung only | 3 (23.1) | 1 (6.3) |
| Lung and lymph nodes only | 3 (23.1) | 4 (25.0) |
| Any visceral disease | 5 (38.5) | 4 (25.0) |
| Any CNS disease | 0 | 1 (6.3) |
| Any bone disease | 5 (38.5) | 8 (50.0) |
| Any liver disease | 2 (15.4) | 1 (6.3) |
| PD-L1 status, n (%) | ||
| Negative | 5 (38.5) | 4 (25.0) |
| Positive | 5 (38.5) | 6 (37.5) |
| Unknown | 3 (23.1) | 6 (37.5) |
| Median number of prior systemic cancer treatment regimens, n (range)b | 3 (1–5) | 3 (1–6) |
| 1 Prior therapy, n (%) | 1 (7.7) | 3 (18.8) |
| 2–3 Prior therapies, n (%) | 7 (53.8) | 9 (56.3) |
| ≥4 Prior therapies, n (%) | 5 (38.5) | 4 (25.0) |
| Prior anti–PD-1 or PD-L1 treatment, n (%) | 13 (100) | 16 (100) |
| Monotherapy, n (%) | 11 (84.6) | 6 (37.5) |
| Combination therapy, n (%) | 2 (15.4) | 12 (75.0) |
| Prior anti–PD-1/PD-L1 best response, n (%) | ||
| PR | 1 (7.7) | 1 (6.3) |
| Stable disease | 3 (23.1) | 11 (68.8) |
| PD | 9 (69.2) | 4 (25.0) |
CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PR, partial response; RECIST v1.1, Response Evaluation in Solid Tumors, version 1.1; RT, radiation therapy.
Based on screening RECIST v1.1 target and nontarget lesions. Patients with more than one baseline disease location are included in multiple categories. “Any” indicates that patients may have had lesions in other areas.
Combination treatments, including those administered on the same day, are counted as a single prior treatment regimen.
All patients received at least one dose of vidutolimod subcutaneously, and the median number of subcutaneous vidutolimod injection visits was two (range, part A: 2–12; part B: 1–5). The median number of vidutolimod intratumoral injection visits was three (range, part A: 1–12; part B: 1–6). The most common sites of vidutolimod-injected lesions were the lung or pleura (part A, 38.5%; part B, 31.3%) or the lymph nodes (part A, 30.8%; part B, 31.3%). Only one patient in part B did not receive intratumoral vidutolimod injection owing to early PD. All patients in part B received RT, and RT was administered to one patient in part A after progression as allowed per the protocol. Details on the administered study treatments are summarized in Supplementary Table 2.
Safety
No DLTs were reported during the safety run-in period in stage 1 for either part of the study. In part A, all 13 patients (100.0%) had one or more TRAE and six patients (46.2%) had grade 3 or 4 TRAEs. The most common any-grade TRAEs were pyrexia (46.2%), hypotension (38.5%), chills (30.8%), headache (30.8%), and fatigue (23.1%). Dyspnea was the most frequent grade 3 or 4 TRAE (two of 13 patients; 15.4%); the two incidences occurred after the second intratumoral dose (n = 1) and third intratumoral dose (n = 1) of vidutolimod, respectively. One patient (7.7%) experienced treatment-related pneumonitis of at least grade 3, which was the only TRAE that resulted in study discontinuation. Pneumonitis developed after the first intratumoral injection to the anterior mediastinal prevascular lymph node.
In part B, 14 patients (87.5%) had at least one any-grade TRAE and eight patients (50.0%) had grade 3 or 4 TRAEs. The most common TRAEs were hypotension (56.3%), pyrexia (56.3%), chills (37.5%), and anemia (31.3%), and the most common grade 3 or 4 TRAEs were hypotension (25.0%), anemia (12.5%), and hypokalemia (12.5%). No patient discontinued the study treatment owing to TRAEs in part B.
Across both parts, TRAEs of grade 3 hypotension occurred in five patients after the second intratumoral dose (n = 4) or after the third intratumoral dose (n = 1) of vidutolimod. Four of these patients were hospitalized for 24 to 48 hours, depending on comorbidities and other clinical events. Hypotension occurred once in two patients, twice in one patient, and three times in one patient; each patient was hospitalized for hypotension once. Of the four patients hospitalized, one was in part A and the other three were in part B; tumor sites injected were the right pleural mass (part A), the left lung, the upper lobe of the right lung, and the left inguinal lymph node (part B). One patient with refractory grade 3 hypotension was treated with tocilizumab and high-dose steroids per institutional protocol. All grade 3 hypotension events resolved, and the patients recovered. All patients had received the recommended prophylaxis regimen (e.g., intravenous fluids, nonsteroidal anti-inflammatory drugs, antiemetics) before the vidutolimod injection, and two patients had received steroids. No treatment-related deaths were observed in either part. The TRAEs are summarized in Table 2, and the overall safety summary is presented in Supplementary Table 3.
Table 2.
Treatment-Related Adverse Events
| Incidence, n (%) | Part A Vidutolimod + Atezolizumab n = 13 |
Part B Vidutolimod + Atezolizumab + RT n = 16 |
||||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | Any Grade | Grade 3 | Grade 4 | |
| Patients with ≥1 TRAE | 13 (100.0) | 4 (30.8) | 2 (15.4) | 14 (87.5) | 7 (43.8) | 1 (6.3) |
| TRAEs with ≥15% incidence in any parta | ||||||
| Pyrexia | 6 (46.2) | 0 | 0 | 9 (56.3) | 1 (6.3) | 0 |
| Hypotension | 5 (38.5) | 1 (7.7) | 0 | 9 (56.3) | 4 (25.0) | 0 |
| Chills | 4 (30.8) | 0 | 0 | 6 (37.5) | 1 (6.3) | 0 |
| Fatigue | 3 (23.1) | 1 (7.7) | 0 | 3 (18.8) | 0 | 0 |
| Anemia | 1 (7.7) | 0 | 0 | 5 (31.3) | 2 (12.5) | 0 |
| Headache | 4 (30.8) | 0 | 0 | 1 (6.3) | 0 | 0 |
| Hypophosphatemia | 2 (15.4) | 1 (7.7) | 0 | 3 (18.8) | 0 | 0 |
| Injection site pain | 2 (15.4) | 0 | 0 | 2 (12.5) | 0 | 0 |
| Injection site reaction | 2 (15.4) | 0 | 0 | 2 (12.5) | 0 | 0 |
| Platelet count decreased | 1 (7.7) | 0 | 0 | 3 (18.8) | 0 | 0 |
| Tachycardia | 2 (15.4) | 0 | 0 | 2 (12.5) | 1 (6.3) | 0 |
| Hypokalemia | 1 (7.7) | 0 | 0 | 2 (12.5) | 1 (6.3) | 1 (6.3) |
| Back pain | 2 (15.4) | 1 (7.7) | 0 | 0 | 0 | 0 |
| Dyspnea | 2 (15.4) | 2 (15.4) | 0 | 0 | 0 | 0 |
| Injection site rash | 2 (15.4) | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 2 (15.4) | 1 (7.7) | 0 | 0 | 0 | 0 |
Note: No treatment-related deaths were reported.
RT, radiation therapy; TRAE, treatment-related adverse event.
Or ≥2 patients of grade ≥3 in any part.
The most common cause of treatment discontinuation was PD, determined by either RECIST v1.1 (part A, nine of 13 [69.2%]; part B, seven of 16 [43.8%]) or the treating physician clinically (part A, two of 13 [15.4%]; part B, seven of 16 [43.8%]). Three patients withdrew consent; one patient had radiologically confirmed PD on a prior scan (part A), one patient withdrew consent and died 11 days after withdrawing, and one patient withdrew for unknown reasons before undergoing postbaseline imaging.
In 12 patients, 65 doses of vidutolimod were injected into one or more visceral lesions (lung [n = 8], pleura [n = 4], kidney [n = 1], and liver [n = 1]) (Supplementary Table 2); TEAEs on the day of injection were reported in five of these patients. The TEAEs reported in patients after a lung lesion injection (n = 3) were mild injection site pain; mild intermittent fever and chills, injection site pain, and moderate constipation; and mild tachycardia, moderate chills, hypotension, fatigue, and anorexia. In the patient with TEAEs after kidney lesion injection (n = 1), mild chills, nasal congestion, fatigue, and lower extremity edema were reported. In the patient with TEAEs after liver lesion injection (n = 1), mild hot flushes, chills, tachycardia, diaphoresis, fever, injection site pain, back pain, abdominal pain, and moderate hypotension were reported. Except for injection site pain, these AEs were likely treatment related and not procedure related. There were no hospitalizations owing to visceral injections, but three patients experienced serious AEs on the day of the visceral injection that were considered treatment related (any component) but not injection procedure related.
Antitumor Activity
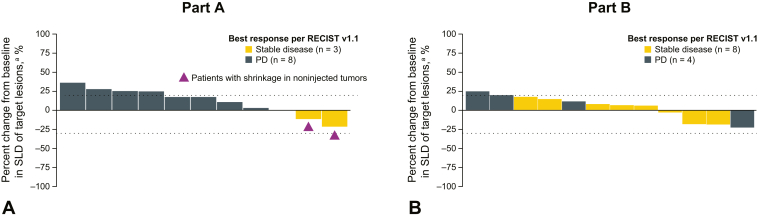
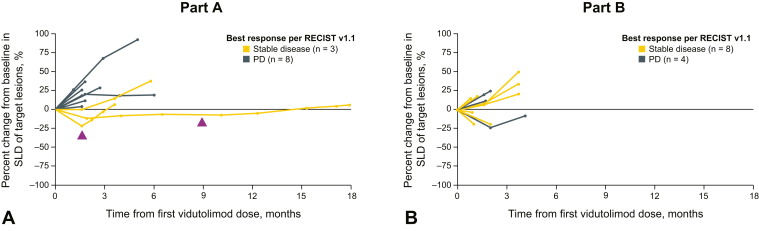
At the time of database lock (April 15, 2020), all patients had discontinued the study treatment. No partial or complete responses were observed in either part (Table 3). In part A (vidutolimod + atezolizumab), three patients (23.1%) had stable disease and eight patients (61.5%) had PD. Two of the patients with stable disease as best response with vidutolimod plus atezolizumab had tumor shrinkage (two of 13 [15.4%]) (Figs. 1A and B, and 2A and B). Two patients were not assessable for response; one had pneumonitis that required treatment discontinuation and one died owing to clinical PD before the follow-up imaging. One patient with PD in part A received RT after progression but had further progression on a subsequent scan (<2 mo after RT) and discontinued the study treatment. In part B (vidutolimod + atezolizumab + RT), 50.0% of the patients (eight of 16) had stable disease as best response and 31.3% (five of 16) had PD. Four patients (three with stable disease and one with PD) had tumor shrinkage in part B (four of 16; 25.0%) (Figs. 1A and B, and 2A and B). Three patients were not assessable for response; one discontinued treatment owing to clinical deterioration before having a postbaseline scan, one withdrew consent, and one had incomplete postbaseline imaging. Median progression-free survival (PFS) per investigator-assessed RECIST v1.1 was 1.8 months (95% confidence interval, 1.1–2.9) and 1.7 months (95% confidence interval, 1.3–2.2) in parts A and B, respectively.
Table 3.
Antitumor Activity of Vidutolimod and Atezolizumab With and Without RT
| Antitumor Activity Measure | Part A Vidutolimod + Atezolizumab n = 13 |
Part B Vidutolimod + Atezolizumab + RT n = 16 |
|---|---|---|
| Best ORR by RECIST v1.1, INV-assessed, % (95% CI) | 0 (0–24.7) | 0 (0–20.6) |
| CR, n (%) | 0 | 0 |
| PR, n (%) | 0 | 0 |
| Stable disease, n (%) | 3 (23.1) | 8 (50.0) |
| PD, n (%) | 8 (61.5) | 5 (31.3) |
| NE,a n (%) | 2 (15.4) | 3 (18.8) |
| Median PFS INV-assessed, mo (95% CI) | 1.8 (1.1–2.9) | 1.7 (1.3–2.2) |
CI, confidence interval; CR, complete response; INV, investigator; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST v1.1, Response Evaluation in Solid Tumors, version 1.1; RT, radiation therapy.
Two patients in part A and two patients in part B discontinued before follow-up imaging was performed; one patient in part B had incomplete postbaseline imaging.
Figure 1.
Investigator-assessed antitumor activity of vidutolimod and atezolizumab with and without RT in NSCLC. Maximum percent change in SLD of target lesions from baseline in patients treated in part A (A) and part B (B). aTwo patients in part A and four in part B had missing scans or incomplete postbaseline imaging and were excluded from this assessment. PD, progressive disease; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; RT, radiation therapy; SLD, sum of longest diameter.
Figure 2.
Antitumor activity of vidutolimod and atezolizumab with and without RT in NSCLC over time. Percent change from baseline in SLD of target lesions over time in part A (A) and part B (B). In parts A and B, two patients and four patients were excluded from the plot owing to missing scans or incomplete postbaseline imaging, respectively. PD, progressive disease; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; RT, radiation therapy; SLD, sum of longest diameter.
Pharmacodynamics
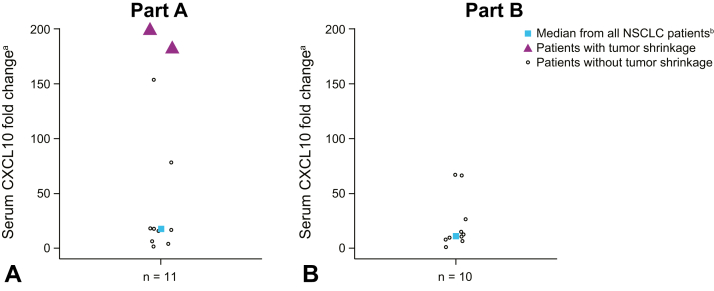
Levels of CXCL10 were used to detect the intended biological effect of TLR9 activation in tumor-associated pDCs leading to their secretion of type I IFN within 24 hours after intratumoral vidutolimod injection (Fig. 3A and B). CXCL10 was induced in most patients; however, the two patients in part A (vidutolimod + atezolizumab) with tumor shrinkage displayed a higher induction of CXCL10 than other patients in both parts. The change in CXCL10 in these two patients was also larger than the median fold change of CXCL10 in responders from a previous study (NCT02680184) evaluating vidutolimod plus pembrolizumab in patients with PD-1 blockade–resistant advanced melanoma.
Figure 3.
CXCL10 fold change with vidutolimod and atezolizumab with and without RT in NSCLC. CXCL10 fold change was assessed in the serum samples collected after week 3 and week 8 vidutolimod injections in parts A (A) and B (B). aValues capped at 200 for plotting purposes. bMedians calculated based on maximal fold change from baseline after either week 3 or week 8 vidutolimod injections. CXCL10, C-X-C motif chemokine ligand 10; RT, radiation therapy.
Exploratory Biomarker Analyses
PD-L1 expression, CD303+ cells, and CD8+ T cells were assessed before and after treatment with vidutolimod and atezolizumab with and without RT (Supplementary Fig. 2A-C). No consistent increase in PD-L1 expression, CD303+ cells, and CD8+ T-cell infiltration was observed.
Whole exome sequencing was performed for patients with available tumor biopsy samples (n = 11) to determine genetic alterations in EGFR, KEAP1, and STK11 (Supplementary Table 4). Pathogenic mutations as identified using SnpEff version 5.0 were detected in posttreatment biopsies from three patients; two patients with PD from part A had an STK11 mutation (p.Glu199∗; n = 1) or frameshift mutation in KEAP1 (p.Ala510fs; n = 1) and one patient with stable disease from part B also had an STK11 mutation (p.Lys84∗), with a PFS of 3.7 months.
Discussion
Immunotherapy alone or in combination with chemotherapy is widely used as a first-line treatment for advanced NSCLC in patients without a targetable oncogene driver alteration,13,31, 32, 33, 34, 35 but efficacious second-line treatment options after progression on PD-1 or PD-L1 blockade remain limited to chemotherapy.1 CMP-001-003 is the first phase 1b study assessing vidutolimod and atezolizumab with and without RT in patients with PD-1 or PD-L1 blockade–resistant NSCLC. Consistent with findings in advanced melanoma,12 vidutolimod and atezolizumab with and without RT had a manageable safety profile. Intratumoral injections of vidutolimod into the visceral lesions were safely performed. In contrast with findings of the clinical activity of vidutolimod plus pembrolizumab in metastatic melanoma,12 vidutolimod and atezolizumab with and without RT had modest pharmacodynamic and clinical activity, with no objective responses observed and a median PFS of less than 2.0 months in this heavily pretreated NSCLC patient population.
The limited activity observed was potentially owing to the patient population studied and the patients' molecular makeup. Most patients were heavily pretreated, receiving a median of three prior systemic therapies; furthermore, most patients had a best response of PD (44.8%) or stable disease (48.3%) to prior anti–PD-1 or PD-L1. These factors are consistent with primary resistance to PD-1 or PD-L1 blockade. The PD-L1 status or expression level was unknown in 31.0% of the patients in this study. The differences in disease biology of NSCLC and the mutational status of the tumor may also affect the response to immunotherapy.36,37 Worse survival outcomes have been reported for patients with STK11 and KEAP1 mutations in NSCLC.38, 39, 40, 41
RT was not able to overcome resistance to immunotherapy in this study population. In a previous phase 2 study in NSCLC, RT plus pembrolizumab had a greater clinical benefit than pembrolizumab alone in a subgroup analysis of 43 patients with PD-L1–negative tumors who were naive to PD-1 or PD-L1 blockade.42 In the current study, RT was unable to restore antitumor immunity and induce responses outside the radiation field in patients (part B, N = 16) with PD-1 or PD-L1 blockade–resistant NSCLC. These findings suggest that the immunosuppressive role of RT may have negatively affected vidutolimod activity. In addition, irradiated lesions were not included in the RECIST antitumor assessment, and the RT regimen under investigation had not been standardized.43 Furthermore, RT was administered using 20-gray doses in five fractions more than 1 week before PD-1 blockade therapy; however, an alternative dose, frequency, or timing of RT administration may improve clinical activity.42, 43, 44 In Welsh et al.,44 stereotactic body RT was more effective than conventional RT in patients with advanced NSCLC.
These clinical activity findings are consistent with those of previously reported clinical trials of TLR9 agonists in combination with other therapies in patients with advanced NSCLC who had PD after chemotherapy. A phase 2 study of PF-3512676 plus erlotinib revealed a median PFS of 1.6 months compared with 1.7 months with erlotinib alone in patients with advanced NSCLC, and study enrollment was halted at the interim analysis owing to lack of efficacy.45 The IMO-2055 was studied in combination with erlotinib and bevacizumab in a phase 1b dose-escalation trial in patients with advanced NSCLC; only 15% of the patients achieved a partial response, likely owing to the presence of an activating EGFR mutation in their tumors46; thus, the IMO-2055 is no longer in development for NSCLC. Key differences between these studies and CMP-001-003 include that vidutolimod was primarily administered intratumorally after the initial subcutaneous dosing whereas the others were administered subcutaneously throughout the study; in addition, patients in these studies were not administered PD-1 or PD-L1 inhibitors.45,46 The rationale for intratumoral delivery was to augment immune cell infiltration and activation in the tumor microenvironment. In CMP-001-003, we observed evidence of systemic immune cell activation by induction of CXCL10, but this did not translate into antitumor efficacy. Therefore, it is likely that the lack of antitumor activity of vidutolimod and atezolizumab was related to biological characteristics of PD-1 blockade-refractory NSCLC.
Additional investigation into vidutolimod-treated patients with NSCLC and patients with melanoma may provide insight into the difference in clinical response rates between these two populations. Given the strong induction of CXCL10 observed in a subset of patients with tumor shrinkage (n = 2) in this study, immune activation may be achievable in PD-1 or PD-L1 blockade–resistant advanced NSCLC but may require a novel combination approach. In a previous study in patients with advanced melanoma receiving vidutolimod and pembrolizumab, a trend toward higher serum levels of CXCL10 was observed in patients with complete response, partial response, or stable disease compared with those patients with PD.47
In conclusion, vidutolimod and atezolizumab with and without RT were found to have modest pharmacodynamic and clinical activities in this heavily pretreated patient population with PD-1 or PD-L1 blockade–resistant NSCLC and a manageable safety profile consistent with studies in melanoma. This study reveals that intratumoral injections of vidutolimod, including injections of the visceral lesions, are safe, supporting further development and evaluation of vidutolimod plus PD-1 or PD-L1 blockade in other tumor types. Studies in melanoma (NCT04698187 and NCT04695977), head and neck squamous cell carcinoma (NCT04633278), cutaneous squamous cell carcinoma, Merkel cell carcinoma, and triple-negative breast cancer (NCT04916002) are ongoing.
CRediT Authorship Contribution Statement
Marcelo Vailati Negrao: Conceptualization, Resources, Data curation, Formal analysis, Supervision, Validation, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Vassiliki A. Papadimitrakopoulou: Conceptualization, Investigation, and Writing—review and editing.
Andrew C. Price: Investigation and Writing—review and editing.
Alda L. Tam: Investigation, Methodology, and Writing—review and editing.
Muhammad Furqan: Conceptualization, Formal analysis, Investigation, Methodology, Writing—review and editing.
Sandeep T. Laroia: Writing—review and editing.
Erminia Massarelli: Conceptualization and Writing—review and editing.
Jose Pacheco: Investigation and Writing—review and editing.
John V. Heymach: Supervision, Investigation, and Writing—review and editing.
Anne S. Tsao: Writing—review and editing.
Gary V. Walker: Supervision, Investigation, Visualization, Writing—original draft, and Writing—review and editing.
Lalit Vora: Writing—review and editing.
David Mauro: Writing—review and editing.
Heather Kelley: Conceptualization, Formal analysis, and Writing—review and editing.
James E. Wooldridge: Resources, Formal analysis, Investigation, Writing—original draft, Writing—review and editing.
Arthur M. Krieg: Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Jiaxin Niu: Conceptualization, Resources, Data curation, Formal analysis, Supervision, Validation, Investigation, Methodology, Writing—review and editing.
Acknowledgments
This study was sponsored by Checkmate Pharmaceuticals, Inc., a wholly owned subsidiary of Regeneron Pharmaceuticals, Inc. Atezolizumab was provided by F. Hoffmann-La Roche Ltd. Medical writing assistance was provided by Cindy M. Rigby, PhD, of ApotheCom (San Francisco, California, USA) and funded by Checkmate Pharmaceuticals, Inc. (Cambridge, Massachusetts, now Tarrytown, New York). The authors thank the patients and their families and all investigators and study site personnel.
Data Availability
Qualified researchers may request access to the study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication have been approved by major health authorities (e.g., Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency), if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.
Footnotes
The affiliations of Dr. Papadimitrakopoulou, Dr. Mauro, and Ms. Kelley are that at the time of the study.
Disclosure: Dr. Negrao has received institutional research funding from Mirati, Novartis, Checkmate, Ziopharm, Alaunos, AstraZeneca, Genentech, and Pfizer; has received paid meals from Ziopharm; and is a consultant for Mirati and Merck or Merck Sharp & Dohme. Dr. Papadimitrakopoulou is an employee of Pfizer Inc.; has received honoraria from F. Hoffman-La Roche; has received research funding and has been a scientific advisor for AstraZeneca, Bristol Myers Squibb, Eli Lilly, Novartis, Merck, F. Hoffman-La Roche, Nektar Therapeutics, Janssen, and Bristol Myers Squibb; has been a scientific advisor for AbbVie, Araxes, Arrys Therapeutics, Bolt Therapeutics, Clovis Oncology, Exelixis, G2 Innovation, Gritstone, Ideaya, Leeds Biolabs, Loxo Oncology, Takeda, Tesaro, and TRM Oncology; and has received research funding from Checkmate and Incyte. Dr. Tam has received research grants from Guerbet, Boston Scientific, and Johnson & Johnson; has been a consultant for Cello Therapeutics, Boston Scientific, Endocare, and Johnson & Johnson; and has received honoraria from AstraZeneca. Dr. Furqan has served on the scientific advisory board of AbbVie, Mirati, Janssen Oncology, Jazz Pharmaceuticals, and AstraZeneca; and has received research funding from AstraZeneca and Celgene (now part of Bristol Myers Squibb). Massarelli has served on the speakers’ bureau for AstraZeneca, Eli Lilly, Takeda, and Merck and as an advisory board ad hoc consultant for Janssen, Eli Lilly, Bristol Myers Squibb, Sanofi, and Merck. Dr. Pacheco has received honoraria from Takeda and Genentech; has been an advisor or consultant for AstraZeneca, Gerson Lehrman Group, Hengrui Pharmaceuticals, Jazz Pharmaceuticals, Novartis, Pfizer, Takeda, Sanofi, and Silverback Therapeutics; and has received research grant or funding from Pfizer. Dr. Heymach has been an advisor or consultant for AstraZeneca, Eli Lilly, Bio-Tree, Boehringer Ingelheim, Catalyst, Genentech, GlaxoSmithKline, Guardant Health, Hengrui Pharmaceuticals, Eli Lilly, Novartis, Spectrum, EMD Serono, Sanofi, and Gritstone; has received research funding from AstraZeneca, GlaxoSmithKline, and Spectrum; and has received royalties from Spectrum. Dr. Tsao has served as an advisor to Genentech, EMD Serono, Merck, Bristol Myers Squibb, Eli Lilly, Roche, Novartis, Ariad, Seagen, AstraZeneca, Boehringer Ingelheim, Sellas Life Sciences, and Takeda; has received research support from Millennium, Bristol Myers Squibb, EMD Serono, Polaris, Pfizer, Epizyme, and Seagen; and has received royalties from UpToDate and Wiley. Walker has served as an advisor to Galera Therapeutics. Dr. Mauro is a former employee of Checkmate Pharmaceuticals. Ms. Kelley is a paid consultant for Checkmate Pharmaceuticals. Dr. Wooldridge and Dr. Krieg are employees of Checkmate Pharmaceuticals. Dr. Niu has been an advisor or consultant for Boehringer Ingelheim, Merck, AstraZeneca, Blueprint Medicines, ImmVira, Johnson & Johnson, Takeda, Exelixis, BeiGene, and Mirati. The remaining authors declare no conflict of interest.
Cite this article as: Negrao MV, Papadimitrakopoulou VA, Price AC, et al. Vidutolimod in combination with atezolizumab with and without radiation therapy in patients with programmed cell death protein 1/programmed death-ligand 1 blockade–resistant advanced NSCLC. JTO Clin Res Rep. 2023;3:100423.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100423.
Supplementary Data
References
- 1.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: non-small cell lung cancer, Version 5.2022. September 26, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 2.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullock B.L., Kimball A.K., Poczobutt J.M., et al. Tumor-intrinsic response to IFNγ shapes the tumor microenvironment and anti-PD-1 response in NSCLC. Life Sci Alliance. 2019;2 doi: 10.26508/lsa.201900328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tumeh P.C., Harview C.L., Yearley J.H., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers M., Lunceford J., Nebozhyn M., et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristescu R., Mogg R., Ayers M., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362:eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Lemke-Miltner C.D., Blackwell S.E., Yin C., et al. Antibody opsonization of a TLR9-agonist–containing viruslike particle enhances in situ immunization. J Immunol. 2020;204:1386–1394. doi: 10.4049/jimmunol.1900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Båve U., Magnusson M., Eloranta M.L., Perers A., Alm G.V., Rönnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 10.Means T.K., Latz E., Hayashi F., Murali M.R., Golenbock D.T., Luster A.D. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu C., Peng P., Loschko J., et al. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc Natl Acad Sci U S A. 2020;117:23730–23741. doi: 10.1073/pnas.2002345117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribas A., Medina T., Kirkwood J.M., et al. Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 2021;11:2998–3007. doi: 10.1158/2159-8290.CD-21-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 14.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 15.TECENTRIQ . Genentech, Inc; South San Francisco, CA: 2022. (atelzolizumab) injection for Intravenous Use. [Google Scholar]
- 16.Formenti S.C., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lhuillier C., Rudqvist N.P., Elemento O., Formenti S.C., Demaria S. Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med. 2019;11:40. doi: 10.1186/s13073-019-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theurich S., Rothschild S.I., Hoffmann M., et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol Res. 2016;4:744–754. doi: 10.1158/2326-6066.CIR-15-0156. [DOI] [PubMed] [Google Scholar]
- 19.Milas L., Mason K.A., Ariga H., et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 20.Mason K.A., Ariga H., Neal R., et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2005;11:361–369. [PubMed] [Google Scholar]
- 21.Brody J.D., Ai W.Z., Czerwinski D.K., et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younes A.I., Barsoumian H.B., Sezen D., et al. Addition of TLR9 agonist immunotherapy to radiation improves systemic antitumor activity. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2020.100983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walshaw R.C., Honeychurch J., Choudhury A., Illidge T.M. Toll-like receptor agonists and radiation therapy combinations: an untapped opportunity to induce anticancer immunity and improve tumor control. Int J Radiat Oncol Biol Phys. 2020;108:27–37. doi: 10.1016/j.ijrobp.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Babraham Informatics. Fast QC. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 25.Ewels P., Magnusson M., Lundin S., Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Auwera G.A., Carneiro M.O., Hartl C., et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11. doi: 10.1002/0471250953.bi1110s43. 10.11-11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cibulskis K., Lawrence M.S., Carter S.L., et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karczewski K.J., Francioli L.C., Tiao G., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cingolani P., Platts A., Wang le L., et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadgeel S., Rodríguez-Abreu D., Speranza G., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 32.Hellmann M.D., Paz-Ares L., Bernabe Caro R., et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 33.Paz-Ares L., Vicente D., Tafreshi A., et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 35.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 36.Cooper W.A., Lam D.C., O’Toole S.A., Minna J.D. Molecular biology of lung cancer. J Thorac Dis. 2013;5(suppl 5):S479–S490. doi: 10.3978/j.issn.2072-1439.2013.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Sheikh M.S. Melanoma: molecular pathogenesis and therapeutic management. Mol Cell Pharmacol. 2014;6:228. [PMC free article] [PubMed] [Google Scholar]
- 38.Skoulidis F., Arbour K.C., Hellmann M.D., et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J Clin Oncol. 2019;37(suppl 15) 102–102. [Google Scholar]
- 39.Skoulidis F., Byers L.A., Diao L., et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arbour K.C., Jordan E., Kim H.R., et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skoulidis F., Goldberg M.E., Greenawalt D.M., et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theelen W.S.M.E., Peulen H.M.U., Lalezari F., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT Phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y., Gao M., Huang Z., Yu J., Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol. 2020;13:105. doi: 10.1186/s13045-020-00940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh J., Menon H., Chen D., et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belani C.P., Nemunaitis J.J., Chachoua A., et al. Phase 2 trial of erlotinib with or without PF-3512676 (CPG 7909, a toll-like receptor 9 agonist) in patients with advanced recurrent EGFR-positive non-small cell lung cancer. Cancer Biol Ther. 2013;14:557–563. doi: 10.4161/cbt.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith D.A., Conkling P., Richards D.A., et al. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol Immunother. 2014;63:787–796. doi: 10.1007/s00262-014-1547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luke J.J., Bao R., Kirkwood J.M., et al. CMP-001 demonstrates improved response in noninflamed anti-PD-1 refractory melanoma and response is associated with serum CXCL10. Cancer Res. 2021;81(suppl 13) Abstract nr CT032. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to the study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication have been approved by major health authorities (e.g., Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency), if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.