Abstract
Compared with an omnivorous Western diet, plant-based diets containing mostly fruits, vegetables, grains, legumes, nuts and seeds, with restricted amounts of foods of animal origin, are associated with reduced risk and severity of COVID-19. Additionally, inflammatory immune responses and severe acute respiratory symptoms of COVID-19, including pulmonary oedema, shortness of breath, fever and nasopharyngeal infections, are associated with Na toxicity from excessive dietary Na. High dietary Na is also associated with increased risks of diseases and conditions that are co-morbid with COVID-19, including chronic kidney disease, hypertension, stroke, diabetes and obesity. This article presents evidence that low dietary Na potentially mediates the association of plant-based diets with COVID-19 prevention. Processed meats and poultry injected with sodium chloride contribute considerable amounts of dietary Na in the Western diet, and the avoidance or reduction of these and other processed foods in whole-food plant-based (WFPB) diets could help lower overall dietary Na intake. Moreover, high amounts of K in plant-based diets increase urinary Na excretion, and preagricultural diets high in plant-based foods were estimated to contain much lower ratios of dietary Na to K compared with modern diets. Further research should investigate low Na in WFPB diets for protection against COVID-19 and co-morbid conditions.
Keywords: COVID-19 prevention, Low sodium diet, Sodium toxicity, Whole-food plant-based diet, Sodium:potassium ratio, Nutritional epidemiology, Nutritional immunology
A recent systematic review found limited and inconsistent evidence that treating micronutrient deficiencies with high-dose micronutrient supplements, including vitamins A, C, D, and E, and Fe, Zn, and Se, is effective in preventing and hastening recovery from diseases like COVID-19(1). The researchers suggested that maintaining a balanced diet is more important for prevention of COVID-19. Furthermore, obesity and type 2 diabetes prevalence related to consumption of a Western diet high in refined foods increases a population’s risk for COVID-19 severity and mortality(2). Alternatively, plant-based diets that include fruits, vegetables, grains, legumes, nuts and seeds, while restricting foods from animal origins such as meat, dairy products and eggs, are proposed to prevent human disease, as well as reduce environmental damage and eliminate animal suffering associated with an omnivorous Western diet(3). Properly balanced plant-based diets provide adequate sources of protein, Ca, Fe, vitamin D and vitamin B12 (4).
Consumption of a Western diet leads to chronic inflammation by stimulating the innate immune system and impairing adaptive immunity, which reduces defences against viral infections(2). Neuroinflammatory mechanisms associated with peripheral inflammation in COVID-19 are also exacerbated by a poor diet, which can lead to neurodegenerative disease and dementia. Additionally, obese patients with chronic low-grade inflammation related to high adiposity are more susceptible to severe SARS-CoV-2 infection, as are malnourished patients, especially the elderly who lack immune protection provided by a balanced diet(5). During the outbreak of the COVID-19 pandemic in Wuhan, China, 52·7 % of infected patients aged 65 years and older were malnourished and an additional 27·5 % were at risk for malnutrition(6).
‘In both undernutrition and obesity, the ingestion of monotonous diets rich in ultra processed foods may lead to vitamin and mineral deficiencies, impairing the immune system and increasing susceptibility to SARS-CoV-2’(5).
An analysis of COVID-19 patients’ nutritional status in an Iranian population found that low fruit and vegetable intake was significantly associated with disease severity – 63 % of patients consumed between zero to one serving of fruit a day and 77 % reported no daily intake of vegetables(7). On the other hand, the Mediterranean diet and Dietary Approaches to Stop Hypertension (DASH) promote dietary patterns rich in unprocessed fruits and vegetables, and these dietary patterns are associated with anti-inflammatory outcomes(5). A systematic review and meta-analysis of seventeen randomised controlled trials found that adherence to the Mediterranean diet decreased inflammatory markers of endothelial dysfunction and CVD risk, such as C-reactive protein, IL-6 and intracellular adhesion molecule-1(8). The Mediterranean diet is also associated with a protective effect against risk of COVID-19(9). Although research on the DASH diet and COVID-19 is lacking, the DASH diet is noted for reduced inflammation and lower cardiac injury and strain when combined with low Na consumption(10).
Compared with plant-based diets, the Western diet contributes additional sources of Na from meat and poultry. Na is added to processed meats, and sodium chloride is injected into poultry during processing to improve texture and water-holding capacity(11), which can increase the Na content of a four-ounce serving of chicken from 50–75 mg to 400 mg(12). A Canadian study found that Na was an added ingredient in 72 % of poultry products(13). Next to pizza consumed by young people between 6 and 19 years of age, and yeast breads consumed by people 51 years and older, chicken and chicken mixed dishes provided the most Na of all food categories among all age groups in an analysis of data from the US National Health and Nutrition Examination Survey (NHANES) 2003–2008(14).
A recent systematic review of global nations found that ultraprocessed food was most commonly consumed in the USA and Great Britain, with the least consumption in Italy, which the researchers attributed to the Italian population’s greater adherence to a traditional Mediterranean diet(15). In spite of this, Italy has experienced a high rate of COVID-19 infections(16). However, Italy is also known to have high dietary salt intake(17). Consumers may add salt to whole-food recipes prepared with few or no foods processed with Na, and people often employ salt as a condiment at meals, emphasising an important point that not all whole-food diets are low in Na. Additionally, an Action on Salt survey of plant-based and vegan foods served in UK restaurants and fast-food outlets found a high level of salt in most items(18). Dietary Na intake in various cultures and nations needs to be considered in research investigating the association of COVID-19 prevention with whole-food plant-based diets (WFPB).
The present article proposes that COVID-19 prevention associated with a WFPB diet is potentially mediated by high intake of unprocessed fruits, vegetables, whole grains, legumes, nuts and seeds, with low intake of ultraprocessed foods, Na and foods of animal origin. A grounded theory method was used when writing this paper to search and select relevant research findings from the peer-reviewed literature on dietary Na, WFPB diets and COVID-19(19). A comparative analysis of the selected findings was used to conceptualise themes and synthesise the present grounded theory narrative.
Sodium toxicity and COVID-19
Sodium toxicity and COVID-19 symptoms
Na toxicity is a condition within the body caused by poisonous effects of excessive Na intake, as in overdose death from acute salt poisoning(20). Na toxicity can also occur from large amounts of Na ingested over long periods(21). A daily Na dietary intake greater than 2300 mg is associated with increased risks of CVD and other chronic diseases(22). Furthermore, Na toxicity has been associated with the nutritional epidemiology and nutritional immunology of COVID-19(23). The effects of Na toxicity on mechanisms of COVID-19 symptoms are summarised in this section.
Acute respiratory distress syndrome is a fatal condition in COVID-19 that blocks the air sacs of the lungs with a gummy yellow fluid causing severe shortness of breath and reduced arterial oxygen saturation(24). Of relevance, lung fluid in acute respiratory distress syndrome appears identical to lung fluid in pulmonary oedema(25). Recent research confirms that pulmonary oedema is a pathological feature in severe cases of COVID-19(26). Furthermore, infusions of excess sodium chloride into the lungs have been found to cause pulmonary oedema(27) – potentially relating acute respiratory distress syndrome and pulmonary oedema in COVID-19 to effects of Na toxicity from excessive sodium chloride and retained fluid that may accumulate in the lungs.
Other symptoms of COVID-19 include nasal sinus congestion, headache and fever which are also associated with Na toxicity. According to the WHO, sinuses in acute sinusitis are blocked with fluid which can cause headaches(28). Fluid blockage in sinuses may be due to hypervolemia and nasal mucosal oedema from Na toxicity. Migraine headache pain is also associated with COVID-19(29), and Na permeability through the blood–cerebral spinal fluid barrier as well as through the blood−brain barrier are increased in migraine(30). Fever is listed among adverse effects of pharmaceutical sodium chloride tablets(31). Furthermore, fever occurs from the pyrogen action of sodium chloride injected into laboratory animals, which may affect control of hyperthermia due to an imbalance between Ca and Na ions in the anterior hypothalamus(32).
Virion aggregates are normally shed through the upper nasal passages of the mucosal immune system, but aggregates of SARS-CoV-2 may accumulate in the upper nasal passages due to impaired mucociliary clearance from the ciliostasis effect of sodium chloride(33). Prolonged mucociliary clearance was found in COVID-19 patients compared with other patients with non-nasal symptoms(34). Moreover, viral sepsis occurs as the accumulation of viruses and other particles in the upper nasal passages enter the bloodstream(35), and sepsis was increased in elderly patients with increased concentrations of plasma Na in hypernatremia(36). A link between sepsis and excess Na could explain the occurrence of sepsis in COVID-19 patients potentially related to Na toxicity(35).
Sodium toxicity and COVID-19 immnue response
Na toxicity also affects immune response mechanisms in COVID-19, summarised as follows. Changed immune responses that promote inflammation and organ damage are associated with sodium chloride intake, which increase the release of inflammatory cytokines: TNF-α, IL-6 and macrophage inflammatory protein-2(37). Of relevance, responses to RNA viruses like SARS-CoV-2 are similarly associated with increased secretion of cytokines, IL-6 and TNF-α, with fewer antiviral responses and more pro-inflammatory responses(38).
Increased levels of sodium chloride stimulate T-cell proliferation and lower anti-inflammatory responses – pro-inflammatory M1 macrophages are increased and anti-inflammatory M2 macrophages are suppressed by increased levels of sodium chloride. Moreover, sodium chloride enhances IL-4 and IL-13 production and suppresses interferon-γ (IFN-γ) produced in memory T cells(39). Furthermore, clearance of extracellular pathogens is assisted by IL-17-producing helper T cells (Th17), and increased levels of sodium chloride activate a kinase signalling pathway that induces development of Th17 cells(40).
Of significance, human receptor angiotensin-converting enzyme 2, a binding site for SARS-CoV-2, is found in alveolar macrophage cell membranes in the respiratory tract and in other immune system cells(41), implying that angiotensin-converting enzyme 2 provides a protective mechanism in the endocytosis and lysis of pathogens. But this protection could be reduced as angiotensin-converting enzyme 2 expression is lowered by high sodium chloride dietary intake, as demonstrated in laboratory animal experiments(42).
Plant-based diets and COVID-19
Plant-based diets that are high in unprocessed fruits, vegetables, grains, legumes, nuts and seeds and low in animal-based food products were associated with reduced severity of COVID-19 in a case–control study of frontline healthcare workers from six countries(43). Compared with workers who reported following a plant-based diet, higher amounts of meat and poultry consumed by other workers was associated with several fold greater odds of moderate to severe COVID-19. Healthcare workers who did not follow a plant-based diet consumed 24 % more fish and seafood, 91·6 % more poultry, and 192·3 % more red and processed meats than other workers. Researchers of the study concluded that future studies are needed with more detailed data of macro- and micronutrient dietary intake associated with COVID-19 severity(43), which includes dietary intake of Na and K.
A randomised controlled trial found that participants assigned to dietary patterns categorised as vegan (no animal-based foods), vegetarian (permits eggs and dairy products) and pescovegetarian (permits seafood) had lower Dietary Inflammatory Index (DII) scores compared with participants assigned to semivegetarian and omnivorous dietary patterns(44). Importantly, although all groups followed diets high in Na, intake of dietary Na reported at the end of the 6-month trial was lowest in the vegan group that consumed no meat, poultry or seafood.
More recently, a healthy diet was assessed using a Plant-Based Diet Score, and the diet was associated with lower risk and severity of COVID-19 in participants from the COVID-19 Symptom Study(45). The healthful Plant-Based Diet Index (hPDI) used in the study places emphasis on fruits, vegetables and whole grains. The association of a poor diet with COVID-19 risk in the study was stronger among people of lower socio-economic status (SES). Relatedly, a systematic review and meta-analysis of socio-economic determinants of dietary Na intake in high-income countries found that ‘people of low SES consume more Na than do people of high SES’(46). This implies that the increased risk and severity of COVID-19 in people with lower SES is associated with a poor diet that may also be high in Na.
However, in addition to diet, many social determinants are also associated with risk of COVID-19, such as reduced access to healthcare services, crowded environments with greater exposure to the coronavirus, and stress and co-morbid conditions associated with poverty(47). Additional social determinants include food insecurity, unavailability of healthy and affordable food, unemployment, discrimination, crime and violence, lack of quality education, and poor health literacy(48). More research is needed to investigate how social determinants interact with high-Na dietary patterns associated with COVID-19 risk in people with lower SES.
Sodium:potassium ratio
High intake of dietary K, which is abundant in fresh fruits and vegetables, was found to counter effects of high salt intake associated with hypertension and inflammation(49). K induces natriuresis, or Na excretion by the kidneys, and a low Na:K urinary excretion ratio is more strongly associated with low blood pressure than Na or K levels alone(50). Compared with modern diets, preagricultural diets that emphasised whole plant-based foods were estimated to have much lower dietary Na:K ratios, providing preagricultural humans with approximately 600 mg of Na and 7000 mg of K a day(51). WFPB diets are not the only source of adequate K – health authorities encourage the general public to consume five to six servings of fruits and vegetables a day to meet K needs(52). Nevertheless, considering modern global food consumption patterns that favour high salt intake and low K, implementing dietary guidelines for Na and K from the WHO (under 2000 mg Na and at least 3510 mg K for adults per d) ‘will be an enormous challenge for global public health’(53).
Modern Na dietary intake is higher than K intake in China(54), and metabolic syndrome in Chinese adults is associated with a higher dietary Na:K ratio(55). Moreover, hypokalemia (serum K < 3·5 mEq/l) was highly prevalent in patients hospitalised with COVID-19 in Italy(56), China(57) and Spain(58). Likewise, hyponatremia is also common in COVID-19 patients (serum Na < 135 mEq/l), but more research is needed to determine whether the type of hyponatremia associated with COVID-19 is hypervolemic hyponatremia caused by water retention related to excess Na(23,59). Interactions between Na and K in WFPB diets should be investigated in the prevention of COVID-19.
Plant-based diets, sodium and COVID-19 co-morbidities
Severe cases of COVID-19 are often co-morbid with chronic kidney disease(60), hypertension(61), and stroke(62), and plant-based diets and dietary Na restriction have been found to be effective in the prevention of these diseases and conditions. Plant-based diets are recommended for prevention and management of chronic kidney disease(63–68), and dietary Na restriction has also been found beneficial in chronic kidney disease(69–72).
Hypertension is effectively managed by low Na diets(73–75) as well as by plant-based diets(76–78). Na restricted diets have also been associated with reduced risk of stroke(79,80), as have plant-based diets(81). However, an exception to decreased stroke risk occurs in association with plant-based diets of low quality(82), possibly related to salt additives in processed foods like refined grain products and snack foods.
Other co-morbidities of severe COVID-19 include diabetes(83) and obesity(84), and plant-based diets are associated with reduced risk of diabetes(85–87) and obesity(88,89). High dietary Na is also associated with diabetes(90,91) and obesity(92,93). Collectively, the above-cited studies suggest an association between Na, plant-based diets, and diseases and conditions co-morbid with severe COVID-19.
Conclusions
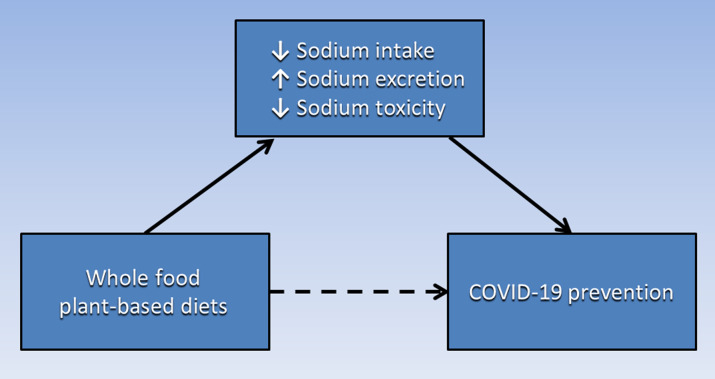
Based on the evidence presented in this paper, Fig. 1 proposes a causative pathway in which the association of WFPB diets with COVID-19 prevention (dashed arrow) is potentially mediated by the combination of low Na intake, increased Na excretion and reduced Na toxicity. Future studies should examine the Na content of WFPB diets and the impact of reduced dietary Na on prevention of COVID-19 and co-morbid diseases. Additionally, the interaction of Na and K and the dietary Na:K ratio in WFPB diets should be further investigated for the prevention of these diseases. Finally, other components of WFPB diets may also contribute causative pathways to COVID-19 prevention, such as high fibre and low fat content, as well as vitamins, minerals and phytonutrients. These additional WFPB diet components should be investigated separately and in combination with Na and K.
Fig. 1.
The association of WFPB diets with COVID-19 prevention (dashed arrow) is potentially mediated by low sodium intake, increased sodium excretion and reduced sodium toxicity. WFPB, whole-food plant-based.
Acknowledgements
This research received no external funding.
The author declares no conflict of interest.
References
- 1. James PT, Ali Z, Armitage AE, et al. (2021) The role of nutrition in COVID-19 susceptibility and severity of disease: a systematic review. J Nutr 151, 1854–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butler MJ & Barrientos RM (2020) The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun 87, 53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alcorta A, Porta A, Tárrega A, et al. (2021) Foods for plant-based diets: challenges and innovations. Foods 10, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuso PJ, Ismail MH, Ha BP, et al. (2013) Nutritional update for physicians: plant-based diets. Permanente J 17, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morais AHA, Aquino JS, da Silva-Maia JK, et al. (2021) Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr 125, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li T, Zhang Y, Gong C, et al. (2020) Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr 74, 871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karimi S, Tondro A, Hematpour B, et al. (2021) Evaluation of nutritional status and health behaviors of patients infected with COVID-19. J Family Med Prim Care 10, 3459–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwingshackl L & Hoffmann G (2014) Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 24, 929–939. [DOI] [PubMed] [Google Scholar]
- 9. Perez-Araluce R, Martínez-González MÁ, Gea A, et al. (2022) Components of the Mediterranean diet and risk of COVID-19. Front Nutr 8, 805533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juraschek SP, Kovell LC, Appel LJ, et al. (2021) Effects of diet and sodium reduction on cardiac injury, strain, and inflammation: the DASH-Sodium trial. J Am Coll Cardiol 77, 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petracci M, Bianchi M, Mudalal S, et al. (2013) Functional ingredients for poultry meat products. Trends Food Sci Technol 33, 27–39. [Google Scholar]
- 12. Conis E (2009) The Hidden Salt in Chicken. Los Angeles Times. https://www.latimes.com/archives/la-xpm-2009-jun-22-he-nutrition22-story.html (accessed January, 2022).
- 13. Parpia AS, L’Abbé M, Goldstein M, et al. (2018) The impact of additives on the phosphorus, potassium, and sodium content of commonly consumed meat, poultry, and fish products among patients with chronic kidney disease. J Ren Nutr 28, 83–90. [DOI] [PubMed] [Google Scholar]
- 14. Drewnowski A & Rehm CD (2013) Sodium intakes of US children and adults from foods and beverages by location of origin and by specific food source. Nutrients 5, 1840–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marino M, Puppo F, Del Bo’ C, et al. (2021) A systematic review of worldwide consumption of ultra-processed foods: findings and criticisms. Nutrients 13, 2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cook DJ, Marshall JC & Fowler RA (2020) Critical illness in patients with COVID-19: mounting an effective clinical and research response. JAMA 323, 1559–1560. [DOI] [PubMed] [Google Scholar]
- 17. Donfrancesco C, Lo Noce C, Russo O, et al. (2020) Abstract P352: trend of salt consumption in Italy from 2008 to 2018: preliminary results of the Cuore project. Circulation 141, AP352–AP352. [Google Scholar]
- 18. actiononsalt.org.uk (2020) Salt Content of Vegan and Plant-Based Meals Served in the Out of Home Sector. https://www.actiononsalt.org.uk/media/action-on-salt/news/surveys/2020/Action-on-Salt-Report---Salt-Content-of-Vegan-&-Plant-Based-Food.pdf (accessed January, 2022).
- 19. Wolfswinkel JF, Furtmueller E & Wilderom CPM (2013) Using grounded theory as a method for rigorously reviewing literature. Eur J Inf Syst 22, 45–55. [Google Scholar]
- 20. Mehlenbacher G, Garbach D, Eggleston W, et al. (2020) Death from salt and baking soda ingestion. Toxicol Commun 4, 15–17. [Google Scholar]
- 21. Soloway RAG (2022) Sodium: Too Much of a Good Thing. The Dangers of Sodium Poisoning. poison.org. https://www.poison.org/articles/sodium-too-much-of-a-good-thing (accessed January, 2022).
- 22. hsph.harvard.edu (2020) Salt and Sodium. Harvard T.H. Chan School of Public Health. https://www.hsph.harvard.edu/nutritionsource/salt-and-sodium/ (accessed January, 2022).
- 23. Brown RB (2021) Sodium toxicity in the nutritional epidemiology and nutritional immunology of COVID-19. Medicine 57, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begley S (2020) With Ventilators Running Out, Doctors Say the Machines are Overused for COVID-19. statnews.com. https://www.statnews.com/2020/04/08/doctors-say-ventilators-overused-for-covid-19/ (accessed January, 2022).
- 25. Carlson RW, Schaeffer RC Jr, Michaels SG, et al. (1979) Pulmonary edema fluid. Spectrum of features in 37 patients. Circulation 60, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 26. Cui X, Chen W, Zhou H, et al. (2021) Pulmonary edema in COVID-19 patients: mechanisms and treatment potential. Front Pharmacol 12, 1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hutchin P, Terzi R, Hollandsworth L, et al. (1969) Pulmonary congestion following infusion of large fluid loads in thoracic surgical patients. Ann Thorac Surg 8, 339–347. [DOI] [PubMed] [Google Scholar]
- 28. WHO (2020) Allergic Rhinitis and Sinusitis. World Health Organization. https://www.who.int/respiratory/other/Rhinitis_sinusitis/en/ (accessed January, 2022).
- 29. Sampaio Rocha-Filho PA & Magalhães JE (2020) Headache associated with COVID-19: frequency, characteristics and association with anosmia and ageusia. Cephalalgia 40, 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghaffari H, Grant SC, Petzold LR, et al. (2020) Regulation of CSF and brain tissue sodium levels by the blood-CSF and blood-brain barriers during migraine. Front Comput Neurosci 14, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. drugs.com (2020) Sodium Chloride Side Effects. Drugs.com. https://www.drugs.com/sfx/sodium-chloride-side-effects.html (accessed January, 2022).
- 32. Feldberg W & Saxena P (1970) Mechanism of action of pyrogen. J Physiol 211, 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu Y, Tong J, Meng F, et al. (2018) Ciliostasis of airway epithelial cells facilitates influenza A virus infection. Vet research 49, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koparal M, Kurt E, Altuntas EE, et al. (2021) Assessment of mucociliary clearance as an indicator of nasal function in patients with COVID-19: a cross-sectional study. Eur Arch Oto-Rhino-Laryngol 278, 1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li H, Liu L, Zhang D, et al. (2020) SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 395, 1517–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Freitas G, Gudur A, Vela-Ortiz M, et al. (2019) Where there is sodium there may be sepsis. J Community Hospital Intern Med Perspective 9, 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Afsar B, Kuwabara M, Ortiz A, et al. (2018) Salt intake and immunity. Hypertension 72, 19–23. [DOI] [PubMed] [Google Scholar]
- 38. Vabret N, Britton GJ, Gruber C, et al. (2020) Immunology of COVID-19: current state of the science. Immunity 52, 910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matthias J, Maul J, Noster R, et al. (2019) Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment. Sci Transl Med 11, eaau0683. [DOI] [PubMed] [Google Scholar]
- 40. Wu C, Yosef N, Thalhamer T, et al. (2013) Induction of pathogenic T H 17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubins JB (2003) Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Respir Crit Care Med 67, 103–104. [DOI] [PubMed] [Google Scholar]
- 42. Berger RCM, Vassallo PF, de Oliveira Crajoinas R, et al. (2015) Renal effects and underlying molecular mechanisms of long-term salt content diets in spontaneously hypertensive rats. PLoS One 10, e0141288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim H, Rebholz CM, Hegde S, et al. (2021) Plant-based diets, pescatarian diets and COVID-19 severity: a population-based case–control study in six countries. BMJ Nutr Prev Health 4, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turner-McGrievy GM, Wirth MD, Shivappa N, et al. (2015) Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutr Res 35, 97–106. [DOI] [PubMed] [Google Scholar]
- 45. Merino J, Joshi AD, Nguyen LH, et al. (2021) Diet quality and risk and severity of COVID-19: a prospective cohort study. Gut 70, 2096–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Mestral C, Mayén AL, Petrovic D, et al. (2017) Socioeconomic determinants of sodium intake in adult populations of high-income countries: a systematic review and meta-analysis. Am J Public Health 107, e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel JA, Nielsen FBH, Badiani AA, et al. (2020) Poverty, inequality and COVID-19: the forgotten vulnerable. Public na 183, 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. health.gov (2022) Social Determinants of Health. Healthy People 2030 U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion. https://health.gov/healthypeople/priority-areas/social-determinants-health (accessed January, 2022).
- 49. Crouch SH, Botha-Le Roux S, Delles C, et al. (2021) Inflammation and salt in young adults: the African-PREDICT study. Eur J Nutr 60, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wei K-Y, Gritter M, Vogt L, et al. (2020) Dietary potassium and the kidney: lifesaving physiology. Clin Kidney J 13, 952–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eaton SB, Eaton SB 3rd , Konner MJ, et al. (1996) An evolutionary perspective enhances understanding of human nutritional requirements. J Nutr 126, 1732–1740. [DOI] [PubMed] [Google Scholar]
- 52. Steptoe A, Perkins-Porras L, McKay C, et al. (2003) Behavioural counselling to increase consumption of fruit and vegetables in low income adults: randomised trial. BMJ 326, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Drewnowski A, Rehm CD, Maillot M, et al. (2015) The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open 5, e006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li Y, Zhang P, Wu J, et al. (2020) Twenty-four–hour urinary sodium and potassium excretion and their associations with blood pressure among adults in China. Hypertens 76, 1580–1588. [DOI] [PubMed] [Google Scholar]
- 55. Li X, Guo B, Jin D, et al. (2018) Association of dietary sodium:potassium ratio with the metabolic syndrome in Chinese adults. Br J Nutr 120, 612–618. [DOI] [PubMed] [Google Scholar]
- 56. Alfano G, Ferrari A, Fontana F, et al. (2021) Hypokalemia in patients with COVID-19. Clin Exp Nephrol 25, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen D, Li X, Song Q, et al. (2020) Assessment of Hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Network Open 3, e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moreno-Pérez O, Leon-Ramirez J-M, Fuertes-Kenneally L, et al. (2020) Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: a case series of 306 Mediterranean patients. Int J Infect Dis 100, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fortune BE & Garcia-Tsao G (2013) Hypervolemic hyponatremia: clinical significance and management. Clin Liver Dis 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng Y, Luo R, Wang K, et al. (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muhamad S-A, Ugusman A, Kumar J, et al. (2021) COVID-19 and hypertension: the what, the why, and the how. Front Physiol 12, 665064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nannoni S, de Groot R, Bell S, et al. (2021) Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 16, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Campbell TM & Liebman SE (2019) Plant-based dietary approach to stage 3 chronic kidney disease with hyperphosphataemia. BMJ Case Rep 12, e232080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dupuis L, Brown-Tortorici A, Kalantar-Zadeh K, et al. (2021) A mini review of plant-based diets in hemodialysis. Blood Purif 50, 672–677. [DOI] [PubMed] [Google Scholar]
- 65. González-Ortiz A, Xu H, Avesani CM, et al. (2020) Plant-based diets, insulin sensitivity and inflammation in elderly men with chronic kidney disease. J Nephrol 33, 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Joshi S, Hashmi S, Shah S, et al. (2020) Plant-based diets for prevention and management of chronic kidney disease. Curr Opin Nephrol Hypertens 29, 16–21. [DOI] [PubMed] [Google Scholar]
- 67. Kalantar-Zadeh K, Joshi S, Schlueter R, et al. (2020) Plant-dominant low-protein diet for conservative management of chronic kidney disease. Nutrients 12, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim H, Caulfield LE, Garcia-Larsen V, et al. (2019) Plant-based diets and incident CKD and kidney function. Clin J Am Soc Nephrol 14, 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bovée DM, Cuevas CA, Zietse R, et al. (2020) Salt-sensitive hypertension in chronic kidney disease: distal tubular mechanisms. Am J Physiol Renal Physiol 319, F729–F745. [DOI] [PubMed] [Google Scholar]
- 70. Garofalo C, Borrelli S, Provenzano M, et al. (2018) Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients 10, 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nerbass FB, Pecoits-Filho R, McIntyre NJ, et al. (2015) High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur J Clin Nutr 69, 786–790. [DOI] [PubMed] [Google Scholar]
- 72. Valtuille R (2021) Potential novel benefits of sodium restriction in chronic kidney disease. Curr Hypertens Rev 17, 59–66. [DOI] [PubMed] [Google Scholar]
- 73. Graudal NA, Hubeck-Graudal T & Jurgens G (2020) Effects of low sodium diet v. high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 12, CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grillo A, Salvi L, Coruzzi P, et al. (2019) Sodium intake and hypertension. Nutrients 11, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Juraschek SP, Miller ER, Weaver CM, et al. (2017) Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol 70, 2841–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aljuraiban G, Chan Q, Gibson R, et al. (2020) Association between plant-based diets and blood pressure in the INTERMAP study. BMJ Nutr Prev Health 3, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gibbs J, Gaskin E, Ji C, et al. (2021) The effect of plant-based dietary patterns on blood pressure: a systematic review and meta-analysis of controlled intervention trials. J Hypertens 39, 23–37. [DOI] [PubMed] [Google Scholar]
- 78. Joshi S, Ettinger L & Liebman SE (2020) Plant-based diets and hypertension. Am J Lifestyle Med 14, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jayedi A, Ghomashi F, Zargar MS, et al. (2019) Dietary sodium, sodium-to-potassium ratio, and risk of stroke: a systematic review and nonlinear dose-response meta-analysis. Clin Nutr 38, 1092–1100. [DOI] [PubMed] [Google Scholar]
- 80. Willey J, Gardener H, Cespedes S, et al. (2017) Dietary sodium to potassium ratio and risk of stroke in a multiethnic urban population: the Northern Manhattan Study. Stroke 48, 2979–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Campbell T (2017) A plant-based diet and stroke. J Geriatr Cardiol: JGC 14, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baden MY, Shan Z, Wang F, et al. (2021) Quality of plant-based diet and risk of total, ischemic, and hemorrhagic stroke. Neurol 96, e1940–e1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Landstra CP & de Koning EJP (2021) COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front Endocrinol 12, 649525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sjögren L, Stenberg E, Thuccani M, et al. (2021) Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden—a cohort study. PLoS One 16, e0257891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen Z, Drouin-Chartier J-P, Li Y, et al. (2021) Changes in plant-based diet indices and subsequent risk of type 2 diabetes in women and men: three U.S. prospective cohorts. Diabetes Care 44, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jardine MA, Kahleova H, Levin SM, et al. (2021) Perspective: plant-based eating pattern for type 2 diabetes prevention and treatment: efficacy, mechanisms, and practical considerations. Adv Nutr 12, 2045–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McMacken M & Shah S (2017) A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol: JGC 14, 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Najjar RS & Feresin RG (2019) Plant-based diets in the reduction of body fat: physiological effects and biochemical insights. Nutrients 11, 2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tran E, Dale HF, Jensen C, et al. (2020) Effects of plant-based diets on weight status: a systematic review. Diabetes Metab Syndrome Obes: Targets Ther 13, 3433–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hao G, Liu K, Halbert JD, et al. (2020) Dietary sodium and potassium and risk of diabetes: a prospective study using data from the China Health and Nutrition Survey. Diabetes Metab 46, 377–383. [DOI] [PubMed] [Google Scholar]
- 91. Lin Y, Chattopadhyay K, Yang X, et al. (2021) Association between dietary salt and plasma glucose, insulin and hemoglobin A1c levels among type 2 diabetes patients in Eastern China. Diabetes Metab Syndr Obes 14, 4811–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fang K, He Y, Fang Y, et al. (2021) Relationship of sodium intake with overweight/obesity among Chinese children and adolescents: data from the CNNHS 2010–2012. Int J Environ Res Public Health 18, 4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Grimes CA, Bolton KA, Booth AB, et al. (2021) The association between dietary sodium intake, adiposity and sugar-sweetened beverages in children and adults: a systematic review and meta-analysis. Br J Nutr 126, 409–427. [DOI] [PubMed] [Google Scholar]