Abstract
Smoking increases lipid levels, including triglycerides, leading to increased cardiovascular disease risk. We performed a meta-analysis to quantify the effects of smoking and smoking cessation on triglyceride levels. The PubMed and Scopus databases were searched to identify studies reporting either triglyceride levels in smokers and non-smokers or the effects of smoking cessation on triglyceride levels. Fixed- and random-effects models were used to perform the analyses when three or more studies/comparisons were available. We identified 169 and 21 studies evaluating the effects of smoking and smoking cessation, respectively, on triglyceride levels. Triglyceride levels were 0.50 mmol/L (95% confidence interval: 0.49–0.50 mmol/L) higher in smokers than non-smokers, but the effect differed widely across studies. No statistically significant effect was observed on triglyceride levels between baseline and 6 weeks (mean difference [MD] = 0.02 [−0.09, 0.12] mmol/L), 2 months (MD = 0.03 [−0.21, 0.27] mmol/L), 3 months (MD = 0.08 [−0.03, 0.21] mmol/L), or 1 year (MD = 0.04 [−0.06, 0.14] mmol/L) after quitting. However, a slightly significant decrease in triglyceride levels was observed at 1 month after cessation (MD = −0.15 [−0.15, −0.01] mmol/L). The results of this meta-analysis provide a basis for understanding the effects of smoking and smoking cessation on triglyceride levels, which could have important implications for public health.
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides
Keywords: Cigarette smoking, Smoking cessation, Triglycerides, Tobacco, Cardiovascular disease
Highlights
-
•
Smoking increases lipid levels, leading to higher cardiovascular disease risk.
-
•
Smokers have higher triglyceride (TG) levels than non-smokers.
-
•
Longer and larger studies are needed to assess how cessation affects TG levels.
1. Introduction
Cigarette smoke contains large amounts of free radicals and pro-oxidants [1], and cigarette smoking is a well-known risk factor for cardiovascular disease (CVD) such as coronary artery disease [2], atherosclerosis, and heart failure [3]. One mechanism through which smoking increases the risk of CVD includes the alteration of lipid levels [3]. Cigarette smoking is associated with increased levels of triglycerides (TGs) and lower levels of high-density lipoprotein cholesterol (HDL-C). However, no significant effects of cigarette smoking on low-density lipoprotein-cholesterol (LDL-C) levels have been observed [4], [5]. Whether the nutritional behavior of smokers also influences their cholesterol levels remains unclear [4]. On average, current smokers have 3% higher total cholesterol levels, approximately 10–15% higher TG levels, and approximately 6.5% lower HDL-C levels than non-smokers [4].
Most international guidelines recommend lowering LDL-C levels to lower the risk of developing CVD, but other lipid molecules have also been associated with CVD [6]. For instance, HDL-C plasma levels were shown to be inversely related to CVD risk [7], including coronary heart disease [6], and this association persists even at very low levels of LDL-C. Additionally, a 1996 meta-analysis performed showed that elevated TG levels were associated with a 14% increased risk of CVD in males and 37% increased risk in females per 1 mmol/L increase in TG levels, after adjustment for HDL-C levels [8].
There is abundant evidence supporting a link between smoking and smoking cessation with HDL-C levels. Two meta-analyses found increased HDL-C levels following smoking cessation [9], [10]; however, no meta-analyses have examined the effects of smoking and smoking cessation on TG levels. Therefore, the aim of the present study was to summarize the available literature through a meta-analysis of the effects of smoking on TG levels.
2. Methods
2.1. Study selection
MEDLINE searches were performed by querying the PubMed and Scopus databases for studies that evaluated the relationship between smoking or smoking cessation and TG levels. The PubMed search was performed on July 9, 2021, using the following query: ("triglycerides"[MeSH Terms] OR "triglycerides"[All Fields]) AND (("smoking"[MeSH Terms] OR "smoking"[All Fields]) OR ("tobacco"[MeSH Terms] OR "tobacco"[All Fields] OR "tobacco products"[MeSH Terms] OR ("tobacco"[All Fields] AND "products"[All Fields]) OR "tobacco products"[All Fields]) OR quitting[All Fields] OR cessation[All Fields]). The search using the Scopus database was performed on the same date, using the following query: “Triglycerides AND (smoking OR tobacco OR cessation OR quitting).”
The abstracts obtained through these searches were screened to confirm whether they contained the relevant information regarding smoking status and TG levels. To verify that all available studies were retrieved, the reference lists of the publications obtained through the original search were checked for any additional articles. Finally, all retrieved articles were assessed according to the following inclusion and exclusion criteria.
The inclusion criteria were as follows: (1) case control, cohorts, or interventional studies such as randomized controlled trials, which included healthy adult subjects; (2) studies reporting measurements of TG levels by smoking exposure (smokers vs. non-smokers and baseline vs. a defined time after smoking cessation) with the following measures available: mean values by group, standard deviation (SD) or standard error (SE), sample size per group, or enough information to allow for the calculation of mean and SD; and (3) studies that were published in English, Spanish, Italian, Portuguese, or German.
The exclusion criteria were as follows: (1) review articles, case reports, or editorials; or (2) studies reporting insufficient data (such as missing values for triglyceride levels by exposure group, SD or SE and sample size; or enough information to calculate means and SD) or those reporting data used in a more recent, included study.
2.2. Data extraction
Two investigators (M.A. and A.vdP.) independently identified relevant articles, extracted the data (mean values by group, SD or SE, sample size per group, or other information to sufficiently allow for the calculation of mean and SD) and discussed disagreements to reach a consensus. The study-specific information that was extracted included the name of the first author, the year of publication, country, study design and population characteristics, number of participants per group, and effect adjustment variables (e.g., age and sex). Estimate-specific information for each exposure group (smokers, non-smokers) and time point (including time after cessation) consisted of TG levels (including mean as well as SD or SE) and group size.
Values reported in mg/dL were converted into mmol/L using the multiplier 0.01129 [11]. Median and range values were converted to mean ± SD using the formulas reported by Hozo et al. [12].
2.3. Statistical analysis
All analyses were performed using the ‘meta’ [13] and ‘dmetar’ [14] packages in R 4.0.5 [15].
To quantify the effects of smoking on TG levels, pooled mean differences (Δ) between smokers and non-smokers (when assessing effects of smoking on TGs) or differences between baseline and follow-up measures after smoking cessation (when assessing the effects of quitting smoking on TG levels) and 95% confidence intervals (CIs) were calculated using the fixed-effects model in the ‘metamean’ function [13]. The ‘metamean’ function uses the inverse variance method for pooling, giving studies with small variance relatively higher weight and studies with larger variance relatively smaller weights [13].
Adjusted estimates were preferred to unadjusted ones. When estimates were provided by strata (e.g., sex or age groups), they were included separately. For the smoking cessation data, estimates were grouped by the duration of quitting and compared using subgroup analyses.
The degree of heterogeneity across estimates was assessed by the I2 value, which describes the percentage of variation across studies that is not caused by sampling error. In general, when interpreting the I2 value, a value less than 25% represents low heterogeneity, a value between 25% and 75% represents moderate heterogeneity, and a value greater than 75% represents substantial heterogeneity [16]. Funnel plot symmetry and Egger’s regression test were used to evaluate publication bias [17]. Statistical significance was assessed at α = 0.05.
To explore possible sources of heterogeneity, the meta-analysis was performed using the random-effects model, and sensitivity analyses were conducted using the ‘InfluenceAnalysis’ function to eliminate studies that contributed the most to heterogeneity. The Baujat plot and forest plot sorted by I2 produced by the ‘InfluenceAnalysis’ function were used to identify the studies with high heterogeneity contribution and low influence on the overall results. These studies represent outliers and were removed to reduce the amount of between-study heterogeneity. Additionally, subgroup analyses were performed based on the definition of smoking, geographical region, study design, period of publication, and sex.
3. Results
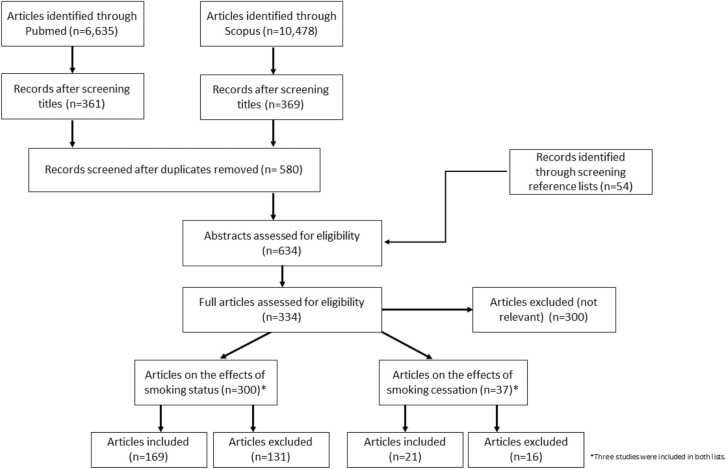
A flow diagram depicting the retrieval process of articles used in the analysis is presented in Fig. 1. We identified 300 publications that presented the effect estimates of smoking status and its association with TG levels. A total of 169 publications reporting 239 effect estimates were included in the analyses, and their characteristics are listed in Supplementary Table 1. The reasons for exclusion were as follows: 1 study had been retracted [18], 1 was published in Japanese [19], 2 compared ever-smokers to non-smokers [20], [21], 2 were duplicates of included studies [22], [23], 2 were conducted on mice [24], [25], 3 lacked non-smoker data [26], [27], [28], 5 used post-prandial data [29], [30], [31], [32], [33], 5 examined effects of other tobacco products (i.e., not cigarettes) [34], [35], [36], [37], [38], 6 presented data regarding the acute effects of smoking on TG levels [39], [40], [41], [42], [43], [44], 8 provided geometric means or log-transformed data [45], [46], [47], [48], [49], [50], [51], [52], 8 had underage populations [53], [54], [55], [56], [57], [58], [59], [60], 16 included diseased populations [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], and 70 publications presented incomplete data [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147]. We identified 21 studies that included data on the effects of smoking cessation and TG levels; their characteristics are listed in Supplementary Table 2. Ten publications were excluded for the following reasons: 1 study compared reducers (those who reduced the amount of cigarettes they smoked) and non-quitters [148], 2 compared immediate changes after smoking [43], [149], 9 did not specify the cessation period [91], [150], [151], [152], [153], [154], [155], [156], [157], 1 was conducted on a diseased population [67], and 3 had incomplete data [158], [159], [160].
Fig. 1.
Flow diagram for the article retrieval process.
3.1. Effects of smoking status on TG levels
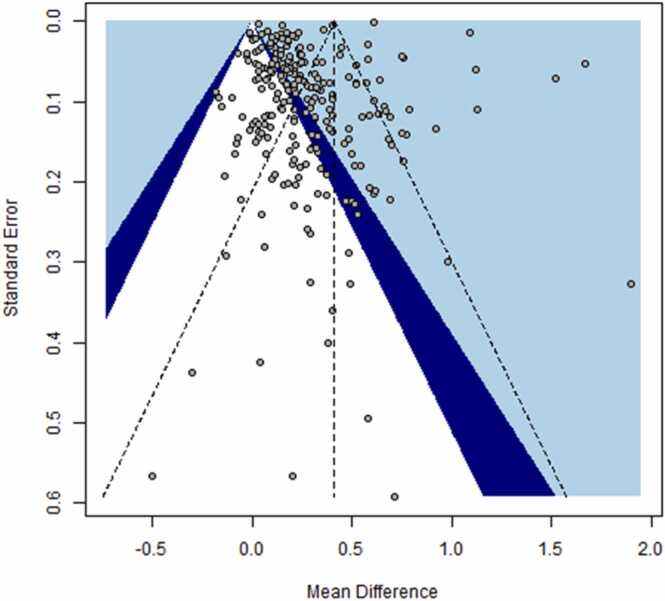
An overview of the 169 included studies can be found in Supplementary Table 1, along with the effect estimates retrieved from these studies. The meta-analysis revealed that the mean TG level of smokers (1.6181 [1.6158, 1.6203] mmol/L) was approximately 38% higher than that of non-smokers (1.1734 [1.1726, 1.1741] mmol/L). This value is higher than previously reported TG levels; a 2010 review stated they were approximately 10% higher in smokers than in non-smokers [4]. The overall meta-analysis (Table 1) revealed a statistically significant increase in mean TG levels in smokers compared to non-smokers, using both fixed-effects (Δ = 0.41 [0.41, 0.42] mmol/L) and random-effects models (Δ = 0.27 [0.23, 0.32] mmol/L). Visual inspection of the funnel plot (Fig. 2) revealed evidence of asymmetry, with several large studies showing significant positive effects. Funnel plot asymmetry was confirmed using Egger’s regression test (intercept = −3.43; 95% CI: −5.20, −1.66, t = −3.08 p = 0.0002), and it may be an indication of publication bias. The heterogeneity of the individual effect estimates was also very high (I2 = 99%).
Table 1.
Results of the meta-analysis conducted on smoking status and triglyceride (TG) level (mmol/L) data from previous studies. All estimates.
| Studies |
Effect Estimates | Mean Differences in TG levels (mmol/L) (smokers – non-smokers) |
|||
|---|---|---|---|---|---|
| Region | Fixed Effects [95% CI] | I2 (%) | Random Effects [95% CI] | ||
| US & Canada | 23 | 34 | 0.08 [0.07, 0.10] | 82 | 0.18 [0.12, 0.23] |
| Europe | 51 | 73 | 0.24 [0.24, 0.25] | 96 | 0.21 [0.17, 0.26] |
| Asia | 43 | 72 | 0.46 [0.46, 0.47] | 100 | 0.29 [0.20, 0.38] |
| Latin America | 5 | 5 | 0.15 [0.00, 0.30] | 36 | 0.17 [− 0.03, 0.36] |
| Middle East | 18 | 21 | 0.11 [0.09, 0.13] | 97 | 0.35 [0.23, 0.46] |
| Indian Subcontinent | 23 | 26 | 0.76 [0.74, 0.79] | 98 | 0.49 [0.30, 0.68] |
| Australia | 2 | 4 | 0.16 [0.03, 0.30] | 11 | 0.18 [0.02, 0.34] |
| Africa | 4 | 4 | 0.13 [0.08, 0.17] | 87 | 0.13 [0.00, 0.26] |
| Study Design | |||||
| Cross Sectional | 143 | 202 | 0.13 [0.12, 0.13] | 98 | 0.26 [0.23, 0.30] |
| Cohort | 19 | 29 | 0.58 [0.58, 0.58] | 99 | 0.27 [0.19, 0.35] |
| Case Control | 5 | 6 | 0.29 [0.25, 0.32] | 97 | 0.40 [0.17, 0.64] |
| RCT | 2 | 2 | NA | NA | NA |
| Period of Publication | |||||
| 1970 s | 4 | 5 | 0.43 [0.38, 0.47] | 95 | 0.30 [0.03, 0.56] |
| 1980 s | 24 | 40 | 0.25 [0.22, 0.28] | 53 | 0.24 [0.19, 0.30] |
| 1990 s | 37 | 54 | 0.17 [0.16, 0.18] | 82 | 0.22 [0.18, 0.26] |
| 2000 s | 51 | 72 | 0.25 [0.24, 0.26] | 99 | 0.23 [0.15, 0.32] |
| 2010 s | 43 | 58 | 0.08 [0.08, 0.09] | 98 | 0.33 [0.28, 0.38] |
| 2020 s | 10 | 10 | 0.59 [0.58, 0.59] | 100 | 0.35 [0.24, 0.47] |
| Smoking Definitiona | |||||
| None/Other | 128 | 163 | 0.16 [0.16, 0.17] | 98 | 0.25 [0.21, 0.28] |
| > 10 CPD | 11 | 12 | 0.37 [0.31, 0.43] | 65 | 0.34 [0.22, 0.46] |
| > 15 CPD | 9 | 11 | 0.41 [0.35, 0.48] | 97 | 0.24 [− 0.19, 0.66] |
| > 20 CPD | 40 | 53 | 0.60 [0.60, 0.61] | 93 | 0.33 [0.26, 0.40] |
| Sexa | |||||
| Males | 82 | 105 | 0.30 [0.29, 0.30] | 98 | 0.31 [0.24, 0.37] |
| Females | 35 | 48 | 0.05 [0.05, 0.06] | 86 | 0.16 [0.12, 0.19] |
| Combined | 70 | 86 | 0.52 [0.52, 0.52] | 99 | 0.29 [0.22, 0.35] |
| Total | 169 | 239 | 0.41 [0.41, 0.42] | 99 | 0.27 [0.23, 0.32] |
The sum of studies does not add to 169, as some studies provided multiple stratum-specific estimates.
Fig. 2.
Funnel plot for studies reporting triglyceride levels in smokers and non-smokers.
Due to the high heterogeneity, we conducted sensitivity analyses, which identified four studies (Park et al., 2021 [161], Chimura et al. [162], Cuschieri et al. [163], and Pasupathi et al. [164]) that had large impacts on between-study heterogeneity. Exclusion of these studies, however, did not substantially reduce the I2 value (I2 = 96%). Thus, we performed subgroup analyses by geographic region, study design, publication period, smoking definition, and sex to further explore possible sources contributing to the high heterogeneity (Table 1). Studies conducted in Australia and Latin America had the lowest heterogeneity (I2 = 11% and I2 = 36%, respectively). Stratification by study design did not substantially decrease heterogeneity (I2 = 89–99%). Studies conducted in the 1980 s had lower heterogeneity (I2 = 53%) than studies published in other periods (I2 = 82–99%). Estimates obtained for females had lower heterogeneity (I2 = 78%) than those for males (98%) or those where both sexes were combined (99%).
We identified 64 comparisons from 48 publications available on individuals smoking more than 15 cigarettes per day (Table 2). The results showed higher mean TG levels in smokers than in non-smokers, and while heterogeneity was slightly lower, it was still considerable (Δ = 0.60 [0.60, 0.61] mmol/L; I2 = 95%). The random-effects model of this meta-analysis yielded similar results for the overall group (Δ = 0.32 [0.25, 0.40] mmol/L). Subgroup analyses of these estimates revealed that USA- and Canada-based studies had lower heterogeneity (I2 = 42%) than studies conducted in other geographic regions (I2 = 86–99%). Significant heterogeneity was also found in studies conducted in the 1970 s (I2 = 92%), 2000 s (I2 = 84%), and 2010 s (I2 = 96%), but not in those conducted in the 1980 s (I2 = 35%) or 1990 s (I2 = 72). While heterogeneity was high in both sex-specific analyses (I2 = 88–93%), it was slightly lower when estimates that combined sexes were included (I2 = 68%).
Table 2.
Results from the meta-analysis of smoking and triglyceride (TG) level (mmol/L) data from previous studies. Estimates for smokers smoking ≥ 15 cigarettes per day.
| Studies |
Effect Estimates |
Mean Differences in TG levels (mmol/L) (smokers – non-smokers) |
|||
|---|---|---|---|---|---|
| Region | Fixed Effects [95% CI] | I2 (%) | Random Effects [95% CI] | ||
| USA & Canada | 11 | 14 | 0.16 [0.09, 0.22] | 42 | 0.17 [0.07, 0.27] |
| Europe | 14 | 21 | 0.33 [0.31, 0.36] | 89 | 0.22 [0.11, 0.33] |
| Asia | 16 | 21 | 0.61 [0.61, 0.61] | 86 | 0.41 [0.30, 0.51] |
| Middle East | 2 | 3 | 0.82 [0.73, 0.91] | 99 | 0.64 [− 0.28, 1.56] |
| Indian Subcontinent | 4 | 4 | 0.68 [0.56, 0.79] | 95 | 0.66 [0.16, 1.16] |
| Africa | 1 | 1 | NA | NA | NA |
| Study Design | |||||
| Cross Sectional | 42 | 55 | 0.38 [0.35, 0.40] | 92 | 0.31 [0.21, 0.40] |
| Cohort | 6 | 9 | 0.61 [0.61, 0.61] | 95 | 0.42 [0.26, 0.58] |
| Period of Publication | |||||
| 1970 s | 3 | 3 | 0.53 [0.48, 0.58] | 92 | 0.35 [0.05, 0.64] |
| 1980 s | 15 | 23 | 0.19 [0.13, 0.25] | 35 | 0.19 [0.10, 0.27] |
| 1990 s | 14 | 19 | 0.28 [0.24, 0.31] | 72 | 0.28 [0.21, 0.36] |
| 2000 s | 9 | 11 | 0.19 [0.13, 0.25] | 84 | 0.20 [0.04, 0.36] |
| 2010 s | 6 | 7 | 0.82 [ 0.76, 0.88] | 96 | 0.85 [0.52, 118] |
| 2020 s | 1 | 1 | NA | NA | NA |
| Sexa | |||||
| Males | 27 | 32 | 0.38 [0.34, 0.41] | 93 | 0.34 [0.19, 0.48] |
| Females | 10 | 12 | 0.18 [0.14, 0.23] | 88 | 0.15 [0.06, 0.24] |
| Combined | 17 | 20 | 0.61 [0.61, 0.61] | 68 | 0.43 [0.34, 0.51] |
| Total | 48 | 64 | 0.60 [0.60, 0.61] | 95 | 0.32 [0.25, 0.40] |
The sum of studies does not add to 48, as some studies provided multiple stratum-specific estimates.
3.2. Effect of smoking cessation on TG levels
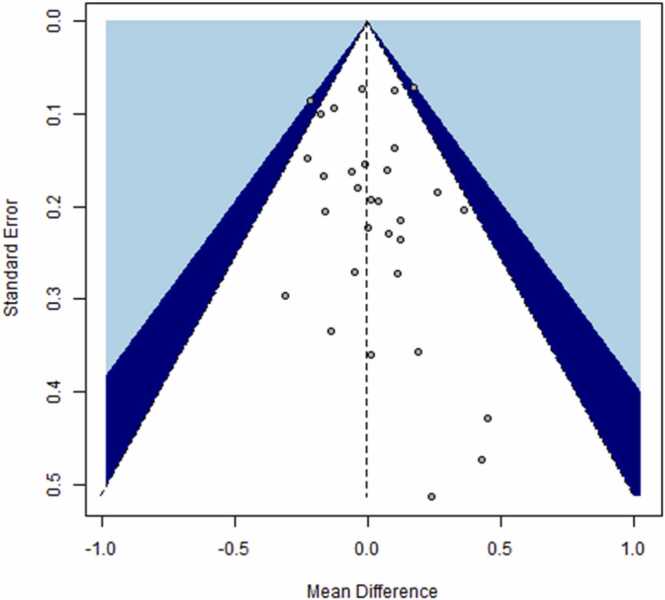
We used 21 studies with 31 estimates to evaluate the effect of smoking cessation on TG levels. An overview of the studies is provided in Supplementary Table 2. The overall meta-analysis showed no statistically significant difference in TG levels from baseline to the end of the observation period after smoking cessation, using either a fixed-effects model (Δ = 0.00 [−0.05, 0.05] mmol/L) or a random-effects model (Δ = −0.01 [−0.06, 0.05] mmol/L) (Table 3). No substantial heterogeneity was identified (I2 = 12%), and visual inspection of the funnel plot (Fig. 3) revealed no evidence of publication bias, which was confirmed using Egger’s regression test (intercept = 0.28, 95% CI: −0.49, 1.05, t = 0.71, p = 0.49).
Table 3.
Smoking cessation and triglyceride (TG) levels (mmol/L).
| Studies |
Effect Estimates | Mean Difference (after quitting – baseline) |
|||
|---|---|---|---|---|---|
| Follow-upa | Fixed Effects [95% CI] | I2 (%) | Random Effects [95% CI] | ||
| 2 weeks | 1 | 1 | NA | NA | NA |
| 3 weeks | 1 | 1 | NA | NA | NA |
| 6 weeks | 3 | 4 | 0.02 [− 0.09, 0.12] | 70 | -0.05 [− 0.27, 0.17] |
| 1 month | 4 | 4 | -0.15 [− 0.29, − 0.01] | 0 | -0.15 [− 0.29, − 0.01] |
| 2 months | 4 | 4 | 0.03 [− 0.21, 0.27] | 0 | 0.03 [− 0.21, 0.27] |
| 3 months | 7 | 11 | 0.08 [− 0.04, 0.19] | 0 | 0.08 [− 0.04, 0.19] |
| 1 year | 3 | 3 | 0.04 [− 0.06, 0.14] | 0 | 0.04 [− 0.06, 0.14] |
| 3 years | 1 | 1 | NA | NA | NA |
| Total | 21 | 31 | 0.00 [− 0.05, 0.05] | 12 | -0.01 [− 0.06, 0.05] |
The sum of studies does not add to 21, as some studies provided estimates for multiple follow-up times.
Fig. 3.
Funnel plot for studies reporting triglyceride levels before and after smoking cessation.
We also conducted subgroup analyses to determine if there were different effects depending on the follow-up duration (Table 3). Three studies provided four comparisons on TG level changes after 6 weeks of cessation [165], [166], [167]. However, pooled analysis revealed no significant difference between TG levels after 6 weeks of smoking cessation and those at baseline (Δ = 0.02 [−0.09, 0.12] mmol/L, I2 = 70%). Heterogeneity was mainly driven by the study by Allen et al. [165], which was the only one to report increased TG levels 6 weeks after smoking cessation. Four studies had a follow-up duration of 1 month after smoking cessation, which revealed a slightly significant decrease in TG levels (Δ = −0.15 [−0.29, –0.01] mmol/L, I2 = 0%). For the 2-month cessation comparison, a non-significant increase in TG levels was found based on four studies [168], [169], [170], [171] (Δ = 0.03 [−0.21, 0.27] mmol/L, I2 = 0%). A total of 7 studies reported 11 comparisons of TG levels after 3 months of cessation [172], [173], [174], [175], [176], [177], [178]. The pooled analysis showed a non-significant increase after this cessation period (Δ = 0.08 [−0.04, 0.19] mmol/L, I2 = 0%). Three studies assessed mean TG levels 1 year after cessation [5], [167], [177], and a non-significant increase in TG levels was found (Δ = 0.04 [−0.06, 0.14] mmol/L, I2 = 0%).
4. Discussion
The aim of this analyses was to quantify the effects of smoking and smoking cessation on serum triglycerides levels. The meta-analysis revealed higher TG levels in smokers than in non-smokers (1.6181 [1.6158, 1.6203] mmol/L vs. 1.1734 [1.1726, 1.1741] mmol/L, respectively). We found that most of the heterogeneity came from European and Asian studies (I2 = 96–100%), studies performed in the 1970 s (I2 = 95) or 2000 s or later (I2 = 99–100%), and studies with male subjects (I2 = 98%) or both sexes combined (I2 = 99). Studies assessing smokers who consumed 15 or more cigarettes per day exhibited lower heterogeneity (I2 = 95%), which was non-negligible. Our analysis of the effect of smoking cessation on TG levels revealed a slightly significant decrease at 1 month after smoking cessation. However, no significant difference in TG levels was observed at 6 weeks, 2 months, 3 months, or 1 year after smoking cessation.
The high heterogeneity in the meta-analysis of smoking status and TG levels can partially be explained by differences in smoking intensity, study design, geographical region, and sex. The notorious intra-individual variability in serum TG levels [179], even after standardizing sampling procedures and laboratory techniques, may also contribute to the heterogeneity seen across studies [180], [181], [182]. TG levels can also vary by up to 25% in healthy fasted subjects when measured 2.5 months apart, and hyper-triglyceridemic individuals can exhibit even greater fluctuations [183]. Besides smoking status, many factors can influence TG levels, including age, body mass index (BMI), oral contraceptive use, stress, alcohol use, and lack of physical exercise [184], [185].
A possible reason for no observable effect after smoking cessation for most follow-up durations might be that weight gain often occurs after quitting smoking [186], [187]. The association between weight gain and TG levels has long been established [188]. Albrink et al. [188] reported results from a prospective cohort of 215 males, where they evaluated the effect of weight gain on various cardiovascular risk parameters. The authors found that in males who gained 4.5 kg or more, TG levels were higher than in those who gained fewer than 4.5 kg during the follow-up period (7.1 mmol/L vs. 4.6 mmol/L). A meta-analysis of the impact of weight loss on lipid parameters [189], which included 64 studies, revealed that for each 1 kg of weight loss, TG levels decreased by 1.93% or 0.017 mmol/L.
Botella-Carretero et al. analyzed the association between smoking cessation and weight gain [190] and reported increases in weight, BMI, waist–hip ratio, and diastolic blood pressure after quitting smoking, which were independent of obesity at baseline or the use of nicotine patches or bupropion. Moreover, a systematic review and meta-analysis designed to quantify weight gain after smoking cessation analyzed data from 63,403 quitters and 388,432 continuing smokers and found that the mean weight gain was 4.10 kg [2.69–5.51] among quitters [191]. The authors also reported that, compared to continuing smoking, cessation was associated with absolute weight gain (∆ = 2.61 kg [95% CI: 1.61–3.60]).
Another feasible explanation for the lack of a decrease in TG levels after smoking cessation could be the relatively short follow-up period of only up to 1 year. A recent study by Noh et al. [192] showed that TG levels decreased 3 years after smoking cessation (1.64 ± 0.83 mmol/L vs. 1.47 ± 0.82 mmol/L). A limitation of our baseline vs. time after smoking cessation meta-analysis is that the numbers of evaluated participants in studies typically decrease with longer follow-up duration. Indeed, when examining the sample sizes of the studies included in this meta-analysis, the studies using a follow-up time of 6 weeks after cessation included 467 quitters, while the analyses after 3 months included 446 quitters, and the analyses that were conducted after 1 year included only 356 participants. This was also seen in the study by Noh et al. [192], where only 49 out of the 779 participants quit and were successfully followed for 3 years. To address this issue, larger and longer follow-up studies after smoking cessation are needed to evaluate the impact of quitting on TG levels.
Our analysis has some limitations, for instance it is difficult to adjust for confounding variables that could affect triglyceride levels. While we tried to use adjusted values as much as possible, these were not always available. Another potential limitation is that we only used Pubmed and Scopus as our sources for literature identification, we did also check the reference lists of all identified publications to make sure we had all literature available possible.
Based on the findings of the present meta-analysis, we conclude that smoking is clearly associated with higher TG levels in current smokers than in non-smokers. These data need to be interpreted with caution due to the high heterogeneity encountered in the analyses. A longer follow-up of larger groups of quitters is needed for more comprehensive assessment of the possible effects of smoking cessation on TG levels. Furthermore, the impact of lifestyle and physical changes (e.g., weight gain) on TG levels following smoking cessation should also be investigated.
This study represents the first meta-analysis published on the effects of smoking and smoking cessation on TG levels. The results could be relevant to public health policy and clinicians hoping to improve outcomes for patients after smoking cessation.
Funding
Philip Morris International is the sole source of funding and sponsor of this research. All authors are employees of Philip Morris International, thus, the funder was involved in study design, the collection, analysis and interpretation of data, the writing of the report, and in the decision to submit the article for publication.
CRediT authorship contribution statement
Meagan Antunes: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. Angela van der Plas: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Guillaume de La Bourdonnaye: Validation, Writing – review & editing. Sandrine Pouly: Conceptualization, Validation, Writing – review & editing. Matthew Hankins: Conceptualization, Writing – review & editing, Supervision. Annie Heremans: Resources, Writing – review & editing, Supervision.
Author contributions
Meagan Antunes: was responsible for the data curation; statistical analysis and review and editing of the original draft, Guillaume de La Bourdonnaye was responsible for the data validation and review of the original draft, Sandrine Pouly was responsible for the conceptualization and data validation, Matthew Hankins was responsible for resources and review of the original draft, Annie Heremans was responsible for resources and review of the original draft, Angela van der Plas for the conceptualization, and writing of the original draft.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All authors are employed by Philip Morris International.
Handling Editor: Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.03.001.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data can be found in cited publications and is summarized in supplementary tables.
References
- 1.Pryor W.A., Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y Acad. Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27-8. [DOI] [PubMed] [Google Scholar]
- 2.Celermajer D.S., et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 3.Rempher K.J. Cardiovascular sequelae of tobacco smoking. Crit. Care Nurs. Clin. North Am. 2006;18(1):13–20. doi: 10.1016/j.ccell.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Winkelmann B.R., von Holt K., Unverdorben M. Smoking and atherosclerotic cardiovascular disease: part IV: genetic markers associated with smoking. Biomark. Med. 2010;4(2):321–333. doi: 10.2217/bmm.10.10. [DOI] [PubMed] [Google Scholar]
- 5.Gepner A.D., et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am. Heart J. 2011;161(1):145–151. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alwaili K., et al. High-density lipoproteins and cardiovascular disease: 2010 update. Expert Rev. Cardiovasc. Ther. 2010;8(3):413–423. doi: 10.1586/erc.10.4. [DOI] [PubMed] [Google Scholar]
- 7.Gordon D.J., et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Hokanson J.E., Austin M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 9.Maeda K., Noguchi Y., Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev. Med. 2003;37(4):283–290. doi: 10.1016/s0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 10.Morris C.D., et al. Smoking cessation behaviors among persons with psychiatric diagnoses: results from a population-level state survey. Drug Alcohol Depend. 2014;136:63–68. doi: 10.1016/j.drugalcdep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 11.H.L. Haney EM C. Bougatsos et al. Screening for Lipid Disorders in Children and Adolescents [Internet]. Agency Healthc. Res. Qual. (US) 2007.http://www.ncbi.nlm.nih.gov/books/NBK33478/. [PubMed]
- 12.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with {R}: a practical tutorial. Evid. -Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.M. Harrer et al. dmetar: Companion R. Package Guide 'Doing Meta-Anal. R. ' 2019.
- 15.R Core Team R: a language and environment for statistical computing R. Found. Stat. Comput.: Vienna, Austria 2021.
- 16.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Macaskill P., Walter S.D., Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 2001;20(4):641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 18.Calo W.A., et al. Association of cigarette smoking and metabolic syndrome in a Puerto Rican adult population. J. Immigr. Minor Health. 2012 doi: 10.1007/s10903-012-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misawa K., et al. An epidemiological study on the relationships among HDL-cholesterol, smoking and obesity. Nihon Eiseigaku Zasshi. 1989;44(3):725–732. doi: 10.1265/jjh.44.725. [DOI] [PubMed] [Google Scholar]
- 20.Danesio de Souza J., et al. Lipid profile and associated factors among elderly people, attended at the family health strategy, vicosa/Mg. Nutr. Hosp. 2015;32(2):771–778. doi: 10.3305/nh.2015.32.2.8875. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H., et al. Smoking, obstructive sleep apnea syndrome and their combined effects on metabolic parameters: Evidence from a large cross-sectional study. Sci. Rep. 2017;7(1):8851. doi: 10.1038/s41598-017-08930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senti M.A., Bosch C. Preliminary report: The relationship between smoking and triglyceride- rich lipoproteins is modulated by genetic variation in the glycoprotein IIIa gene. Metabolism. 1998;47(9):1040–1041. doi: 10.1016/s0026-0495(98)90274-8. [DOI] [PubMed] [Google Scholar]
- 23.Nesje L.A., Mjos O.D. Plasma HDL cholesterol and the subclasses HDL2 and HDL3 in smokers and non-smokers. Artery. 1985;13(1):7–18. [PubMed] [Google Scholar]
- 24.Khan A., et al. Cigarette smoking blocks the benefit from reduced weight gain for insulin action by shifting lipids deposition to muscle. Clin. Sci. 2020;134(13):1659–1673. doi: 10.1042/CS20200173. [DOI] [PubMed] [Google Scholar]
- 25.Ma B., et al. Cigarette smoke exposure impairs lipid metabolism by decreasing low-density lipoprotein receptor expression in hepatocytes. Lipids Health Dis. 2020;19(1):88. doi: 10.1186/s12944-020-01276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aldaham S., et al. Smoking status effect on inflammatory markers in a randomized trial of current and former heavy smokers. Int. J. Inflamm. 2015;2015 doi: 10.1155/2015/439396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringsdorf W.M., Jr., Cheraskin E., Medford F.H. Smoking and serum triglycerides. J. Med. Assoc. State Alabama. 1975;45(4):26. [PubMed] [Google Scholar]
- 28.Gouveia T.D.S., et al. Smoking history: relationships with inflammatory markers, metabolic markers, body composition, muscle strength, and cardiopulmonary capacity in current smokers. J. Bras. Pneumol. 2020;46(5) doi: 10.36416/1806-3756/e20180353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkeles R.S., et al. Effects of smoking on oral fat tolerance and high density lipoprotein cholesterol. Clin. Sci. 1983;65(6):669–672. doi: 10.1042/cs0650669. [DOI] [PubMed] [Google Scholar]
- 30.Konttinen A., Rajasalmi M. Effect of heavy cigarette smoking on postprandial triglycerides, free fatty acids, and cholesterol. Br. Med. J. 1963;1(5334):850–852. doi: 10.1136/bmj.1.5334.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mero N., et al. Decreased postprandial high density lipoprotein cholesterol and apolipoproteins A-I and E in normolipidemic smoking men: relations with lipid transfer proteins and LCAT activities. J. Lipid Res. 1998;39(7):1493–1502. [PubMed] [Google Scholar]
- 32.Rashidi H.S., Fatahi M. Effects of cigarette smoking on postprandial triglyceride in healthy smokers. J. Diabetes Metab. Disord. 2010;9:21. [Google Scholar]
- 33.Leon-Acuña A., et al. Lifestyle factors modulate postprandial hypertriglyceridemia: from the CORDIOPREV study. Atherosclerosis. 2019;290:118–124. doi: 10.1016/j.atherosclerosis.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Alomari M.A., Khabour O.F., Alzoubi K.H. Metabolic differences between men and women who are long-term users of the water pipe: the Irbid WiHi project. J. Vasc. Nurs. 2020;38(1):18–24. doi: 10.1016/j.jvn.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Hasni Y., et al. Biochemical data and metabolic profiles of male exclusive narghile smokers (ENSs) compared with apparently healthy nonsmokers (AHNSs) Am. J. Men's Health. 2019;13(1) doi: 10.1177/1557988319825754. 1557988319825754-1557988319825754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim T., et al. Association between electronic cigarette use and metabolic syndrome in the Korean general population: a nationwide population-based study. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaik F.B., et al. Quantification of nicotine and cotinine in plasma, saliva, and urine by HPLC method in chewing tobacco users. Asian Pac. J. Cancer Prev. 2019;20(12):3617–3623. doi: 10.31557/APJCP.2019.20.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrestha S., et al. Correlation of urinary cotinine with cardiovascular risk factors in pan masala tobacco users. Indian Heart J. 2019;71(6):459–463. doi: 10.1016/j.ihj.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown A. Paradoxical effects of acute cigarette smoking on plasma antioxidant status in humans. Nutr. Res. 1998;18(9):1499–1519. [Google Scholar]
- 40.Frati A.C., Iniestra F., Ariza C.R. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes Care. 1996;19(2):112–118. doi: 10.2337/diacare.19.2.112. [DOI] [PubMed] [Google Scholar]
- 41.Murchison L.E., Fyfe T. Effects of cigarette smoking on serum-lipids, blood-glucose, and platelet adhesiveness. Lancet. 1966;2(7456):182–184. doi: 10.1016/s0140-6736(66)92472-x. [DOI] [PubMed] [Google Scholar]
- 42.Davis M.C., Matthews K.A. Cigarette smoking and oral contraceptive use influence women's lipid, lipoprotein, and cardiovascular responses during stress. Health Psychol. 1990;9(6):717–736. doi: 10.1037//0278-6133.9.6.717. [DOI] [PubMed] [Google Scholar]
- 43.Gnasso A., et al. Acute influence of smoking on plasma lipoproteins. Klin. Woche. 1984;62(2):36–42. [PubMed] [Google Scholar]
- 44.Gross W., et al. Changes in serum lipids during smoking. Dtsch Med. J. 1970;21(6):368. [PubMed] [Google Scholar]
- 45.Jain R.B., Ducatman A. Associations between smoking and lipid/lipoprotein concentrations among US adults aged >/=20 years. J. Circ. Biomark. 2018;7 doi: 10.1177/1849454418779310. 1849454418779310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slagter S.N., et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013;11:195. doi: 10.1186/1741-7015-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterworth D.M., et al. Contribution of apolipoprotein C-III gene variants to determination of triglyceride levels and interaction with smoking in middle-aged men. Arterioscler. Thromb. Vasc. Biol. 2000;20(12):2663–2669. doi: 10.1161/01.atv.20.12.2663. [DOI] [PubMed] [Google Scholar]
- 48.Teshima K., et al. Cigarette smoking, blood pressure and serum lipids in Japanese men aged 20-39 years. J. Physiol. Anthr. Appl. Hum. Sci. 2001;20(1):43–45. doi: 10.2114/jpa.20.43. [DOI] [PubMed] [Google Scholar]
- 49.Wakabayashi I. Associations of alcohol drinking and cigarette smoking with serum lipid levels in healthy middle-aged men. Alcohol Alcohol. 2008;43(3):274–280. doi: 10.1093/alcalc/agn005. [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi I., Araki Y. Associations of alcohol consumption with blood pressure and serum lipids in Japanese female smokers and nonsmokers. Gend. Med. 2009;6(1):290–299. doi: 10.1016/j.genm.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Sakaguchi C., et al. Differences in levels of biomarkers of potential harm among users of a heat-not-burn tobacco product, cigarette smokers, and never-smokers in Japan: a post-marketing observational study. Nicotine Tob. Res. 2021;23(7):1143–1152. doi: 10.1093/ntr/ntab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato A., et al. Smoking results in accumulation of ectopic fat in the liver. Diabetes Metab. Syndr. Obes. 2019;12:1075–1080. doi: 10.2147/DMSO.S212495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alidzhanova Kh, G., et al., [Clinical and biochemical features of atherosclerosis main risk factors in children of probands with primary hyperlipidemia]. Ter Arkh, 1998. 70(1): p. 19–23. [PubMed]
- 54.Merianos A.L., et al. Tobacco smoke exposure association with lipid profiles and adiposity among U.S. adolescents. J. Adolesc. Health. 2018;62(4):463–470. doi: 10.1016/j.jadohealth.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison J.A., et al. Cigarette smoking, alcohol intake, and oral contraceptives: relationships to lipids and lipoproteins in adolescent school-children. Metabolism. 1979;28(11):1166–1170. doi: 10.1016/0026-0495(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 56.Voors A.W., et al. Smoking, oral contraceptives, and serum lipid and lipoprotein levels in youths. Prev. Med. 1982;11(1):1–12. doi: 10.1016/0091-7435(82)90001-9. [DOI] [PubMed] [Google Scholar]
- 57.Webber L.S., et al. The interaction of cigarette smoking, oral contraceptive use, and cardiovascular risk factor variables in children: the Bogalusa heart study. Am. J. Public Health. 1982;72(3):266–274. doi: 10.2105/ajph.72.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freedman D.S., et al. Cigarette smoking initiation and longitudinal changes in serum lipids and lipoproteins in early adulthood: the Bogalusa heart study. Am. J. Epidemiol. 1986;124(2):207–219. doi: 10.1093/oxfordjournals.aje.a114379. [DOI] [PubMed] [Google Scholar]
- 59.Freedman D.S., et al. Correlates of high density lipoprotein cholesterol and apolipoprotein A-I levels in children. The Bogalusa heart study. Arteriosclerosis. 1987;7(4):354–360. doi: 10.1161/01.atv.7.4.354. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.Y., Lee J.S., Kim Y.H. Handgrip strength and current smoking are associated with cardiometabolic risk in Korean adolescents: a population-based study. Int. J. Environ. Res. Public Health. 2020;17:14. doi: 10.3390/ijerph17145021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruckert E., et al. Relationship between smoking status and serum lipids in a hyperlipidemic population and analysis of possible confounding factors. Clin. Chem. 1992;38(9):1698–1705. [PubMed] [Google Scholar]
- 62.Giudice R., et al. Lifestyle-related risk factors, smoking status and cardiovascular disease. High Blood Press. Cardiovasc. Prev. 2012;19(2):85–92. doi: 10.1007/BF03262458. [DOI] [PubMed] [Google Scholar]
- 63.Hautanen A., et al. Cigarette smoking is associated with elevated adrenal androgen response to adrenocorticotropin. J. Steroid Biochem. Mol. Biol. 1993;46(2):245–251. doi: 10.1016/0960-0760(93)90300-l. [DOI] [PubMed] [Google Scholar]
- 64.Hwang G.Y., et al. The relationship between smoking level and metabolic syndrome in male health check-up examinees over 40 years of age. Korean J. Fam. Med. 2014;35(5):219–226. doi: 10.4082/kjfm.2014.35.5.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwashima Y., et al. Association of hypoadiponectinemia with smoking habit in men. Hypertension. 2005;45(6):1094–1100. doi: 10.1161/01.HYP.0000169444.05588.4c. [DOI] [PubMed] [Google Scholar]
- 66.Lam T.H., et al. Smoking, quitting, and mortality in a Chinese cohort of retired men. Ann. Epidemiol. 2002;12(5):316–320. doi: 10.1016/s1047-2797(01)00258-7. [DOI] [PubMed] [Google Scholar]
- 67.Otsuka F., et al. Smoking cessation is associated with increased plasma adiponectin levels in men. J. Cardiol. 2009;53(2):219–225. doi: 10.1016/j.jjcc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Reddy A.V., et al. Analysis of lipid profile in cancer patients, smokers, and nonsmokers. Dent. Res J. 2016;13(6):494–499. doi: 10.4103/1735-3327.197036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yasue H., et al. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ. J. 2006;70(1):8–13. doi: 10.1253/circj.70.8. [DOI] [PubMed] [Google Scholar]
- 70.Molla G.J., et al. Smoking and diabetes control in adults with type 1 and type 2 diabetes: a nationwide study from the 2018 national program for prevention and control of diabetes of Iran. Can. J. Diabetes. 2020;44(3):246–252. doi: 10.1016/j.jcjd.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Muhsin S.N., S.h. majeed Alnassiri, Muhsin S.N. Studying the levels of some Biochemical variables in blood serum for smoking of Tikrit University students. J. Phys.: Conf. Ser. 2021;1879(2) [Google Scholar]
- 72.Onalan E., Gozel N. The association between the prevalence of cigarette smoking and complications in patients with type 2 diabetes. Prog. Nutr. 2020;22:415–419. [Google Scholar]
- 73.Pascoe M., et al. Serum cholesterol, body mass index and smoking status do not predict long-term cognitive impairment in elderly stroke patients. J. Neurol. Sci. 2019;406 doi: 10.1016/j.jns.2019.116476. [DOI] [PubMed] [Google Scholar]
- 74.Szwarcbard N., et al. The association of smoking status with glycemic control, metabolic profile and diabetic complications- Results of the Australian National Diabetes Audit (ANDA) J. Diabetes Complicat. 2020;34(9) doi: 10.1016/j.jdiacomp.2020.107626. [DOI] [PubMed] [Google Scholar]
- 75.Trofor L., et al. Evaluation of oxidative stress in smoking and nonsmoking patients diagnosed with anxious-depressive disorder. Farmacia. 2020;68(1):82–89. [Google Scholar]
- 76.Zhang B., et al. The interaction effects of smoking and polycyclic aromatic hydrocarbons exposure on the prevalence of metabolic syndrome in coke oven workers. Chemosphere. 2020;247 doi: 10.1016/j.chemosphere.2020.125880. [DOI] [PubMed] [Google Scholar]
- 77.Asvold B.O., et al. Causal associations of tobacco smoking with cardiovascular risk factors: a Mendelian randomization analysis of the HUNT Study in Norway. Int. J. Epidemiol. 2014;43(5):1458–1470. doi: 10.1093/ije/dyu113. [DOI] [PubMed] [Google Scholar]
- 78.Berns M.A., de Vries J.H., Katan M.B. Dietary and other determinants of lipoprotein levels within a population of 315 Dutch males aged 28 and 29. Eur. J. Clin. Nutr. 1990;44(7):535–544. [PubMed] [Google Scholar]
- 79.Connelly P.W., et al. The prevalence of hyperlipidemia in women and its association with use of oral contraceptives, sex hormone replacement therapy and nonlipid coronary artery disease risk factors. Canadian Heart Health Surveys Research Group. Can. J. Cardiol. 1999;15(4):419–427. [PubMed] [Google Scholar]
- 80.Davila E.P., et al. Prevalence and risk factors for metabolic syndrome in Medellin and surrounding municipalities, Colombia, 2008-2010. Prev. Med. 2013;56(1):30–34. doi: 10.1016/j.ypmed.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 81.Gordon T., et al. Changes associated with quitting cigarette smoking: the Framingham Study. Am. Heart J. 1975;90(3):322–328. doi: 10.1016/0002-8703(75)90320-8. [DOI] [PubMed] [Google Scholar]
- 82.Najder A. Sense of coherence, smoking status, biochemical cardiovascular risk factors and body mass in blue collar workers-short report. Am. J. Mens. Health. 2018;12(4):894–899. doi: 10.1177/1557988317748393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chularojanamontri V., et al. Effects of cigarette smoking and alcohol consumption on blood lipid variables. J. Med. Assoc. Thai. 1985;68(10):503–507. [PubMed] [Google Scholar]
- 84.Gamit K.N., Gohel M.G., Gonsa P.M. RN, Effects of smoking on lipids profile. Int. J. Curr. Res. Rev. 2013;5(17):36–42. [Google Scholar]
- 85.S.P.d.C Odebrecht Vargas Nunes, Odebrecht Vargas M.R., Mendoca Vargas H., Bueno Rezende Machado M., Batista Fonseca R.C., Dodd I.C., Berk, M S. Clinical characteristics and smoking cessation: an analysis of sex and depressive disorders differences. Addict. Disord. Treat. 2013;12(3):158–165. [Google Scholar]
- 86.Peacock R.E., et al. Variation at the lipoprotein lipase and apolipoprotein AI-CIII gene loci are associated with fasting lipid and lipoprotein traits in a population sample from Iceland: interaction between genotype, gender, and smoking status. Genet. Epidemiol. 1997;14(3):265–282. doi: 10.1002/(SICI)1098-2272(1997)14:3<265::AID-GEPI5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 87.Senti M., Aubo C., Bosch M. The relationship between smoking and triglyceride-rich lipoproteins is modulated by genetic variation in the glycoprotein IIIa gene. Metabolism. 1998;47(9):1040–1041. doi: 10.1016/s0026-0495(98)90274-8. [DOI] [PubMed] [Google Scholar]
- 88.Thelle D.S., et al. Blood lipids in middle-aged British men. Br. Heart J. 1983;49(3):205–213. doi: 10.1136/hrt.49.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burnette M.M., et al. Smoking cessation, weight gain, and changes in cardiovascular risk factors during menopause: the Healthy Women Study. Am. J. Public Health. 1998;88(1):93–96. doi: 10.2105/ajph.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen C.C., et al. Association among cigarette smoking, metabolic syndrome, and its individual components: the metabolic syndrome study in Taiwan. Metabolism. 2008;57(4):544–548. doi: 10.1016/j.metabol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 91.Green M.S., Harari G. A prospective study of the effects of changes in smoking habits on blood count, serum lipids and lipoproteins, body weight and blood pressure in occupationally active men. The Israeli CORDIS Study. J. Clin. Epidemiol. 1995;48(9):1159–1166. doi: 10.1016/0895-4356(95)00005-o. [DOI] [PubMed] [Google Scholar]
- 92.Halfon S.T., Green M.S., Heiss G. Smoking status and lipid levels in adults of different ethnic origins: the Jerusalem lipid research clinic program. Int. J. Epidemiol. 1984;13(2):177–183. doi: 10.1093/ije/13.2.177. [DOI] [PubMed] [Google Scholar]
- 93.Haustein K.O., et al. Comparison of the effects of combined nicotine replacement therapy vs. cigarette smoking in males. Nicotine Tob. Res. 2003;5(2):195–203. doi: 10.1080/146222003100073676. [DOI] [PubMed] [Google Scholar]
- 94.Jenkins C.D., Zyzanski S.J., Rosenman R.H. Biological, psychological, and social characteristics of men with different smoking habits. Health Serv. Rep. 1973;88(9):834–843. [PMC free article] [PubMed] [Google Scholar]
- 95.Jossa F., et al. Correlates of high-density lipoprotein cholesterol in a sample of healthy workers. Prev. Med. 1991;20(6):700–712. doi: 10.1016/0091-7435(91)90065-c. [DOI] [PubMed] [Google Scholar]
- 96.Koyama H., Ogawa M., Suzuki S. Relationship between total cholesterol and high-density lipoprotein cholesterol and the effects of physical exercise, alcohol consumption, cigarette smoking and body mass index. J. Nutr. Sci. Vitam. 1990;36(4):377–385. doi: 10.3177/jnsv.36.4-supplementi_377. [DOI] [PubMed] [Google Scholar]
- 97.Loprinzi P.D., et al. Healthy lifestyle characteristics and their joint association with cardiovascular disease biomarkers in US adults. Mayo Clin. Proc. 2016;91(4):432–442. doi: 10.1016/j.mayocp.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 98.Lubin J.H., et al. Synergistic and Non-synergistic Associations for Cigarette Smoking and Non-tobacco Risk Factors for Cardiovascular Disease Incidence in the Atherosclerosis Risk In Communities (ARIC) Study. Nicotine Tob. Res. 2017;19(7):826–835. doi: 10.1093/ntr/ntw235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakanishi N., et al. Association of lifestyle with serum lipid levels: a study of middle-aged Japanese men. J. Epidemiol. 2000;10(4):216–225. doi: 10.2188/jea.10.216. [DOI] [PubMed] [Google Scholar]
- 100.Shennan N.M., Seed M., Wynn V. Variation in serum lipid and lipoprotein levels associated with changes in smoking behaviour in non-obese Caucasian males. Atherosclerosis. 1985;58(1–3):17–25. doi: 10.1016/0021-9150(85)90052-8. [DOI] [PubMed] [Google Scholar]
- 101.Simpson A.J., et al. The effects of chronic smoking on the fibrinolytic potential of plasma and platelets. Br. J. Haematol. 1997;97(1):208–213. doi: 10.1046/j.1365-2141.1997.d01-2137.x. [DOI] [PubMed] [Google Scholar]
- 102.Stamford B.A., et al. Cigarette smoking, exercise and high density lipoprotein cholesterol. Atherosclerosis. 1984;52(1):73–83. doi: 10.1016/0021-9150(84)90157-6. [DOI] [PubMed] [Google Scholar]
- 103.Takeuchi T., et al. Association of metabolic syndrome with smoking and alcohol intake in Japanese men. Nicotine Tob. Res. 2009;11(9):1093–1098. doi: 10.1093/ntr/ntp106. [DOI] [PubMed] [Google Scholar]
- 104.Wannamethee G., Shaper A.G. Blood lipids: the relationship with alcohol intake, smoking, and body weight. J. Epidemiol. Community Health. 1992;46(3):197–202. doi: 10.1136/jech.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilsgaard T., Arnesen E. Change in serum lipids and body mass index by age, sex, and smoking status: the Tromso study 1986-1995. Ann. Epidemiol. 2004;14(4):265–273. doi: 10.1016/j.annepidem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 106.Wang W., et al. Cigarette smoking has a positive and independent effect on testosterone levels. Hormones. 2013;12(4):567–577. doi: 10.14310/horm.2002.1445. [DOI] [PubMed] [Google Scholar]
- 107.Wakabayashi I. Relationship between smoking and cardiometabolic index in middle-aged men. Clin. Lab. 2016;62(6):1045–1051. doi: 10.7754/clin.lab.2015.150939. [DOI] [PubMed] [Google Scholar]
- 108.Cetin I.Y., Sahin B., Sahin S., Etikan I. Serum lipid and lipoprotein levels, dyslipidemia prevalence, and the factors that influence these parameters in a Turkish population living in the province of Tokat. Turk. J. Med. Sci. 2010;40(5):771–782. [Google Scholar]
- 109.Cowan L.D., et al. Demographic, behavioral, biochemical, and dietary correlates of plasma triglycerides. Lipid research clinics program prevalence study. Arteriosclerosis. 1985;5(5):466–480. doi: 10.1161/01.atv.5.5.466. [DOI] [PubMed] [Google Scholar]
- 110.Ge J., et al. Smoking dose modifies the association between C242T polymorphism and prevalence of metabolic syndrome in a Chinese population. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0031926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kauma H., et al. Sex difference in the regulation of plasma high density lipoprotein cholesterol by genetic and environmental factors. Hum. Genet. 1996;97(2):156–162. doi: 10.1007/BF02265258. [DOI] [PubMed] [Google Scholar]
- 112.Kawamoto R., et al. Smoking status is associated with serum high molecular adiponectin levels in community-dwelling Japanese men. J. Atheroscler. Thromb. 2010;17(4):423–430. doi: 10.5551/jat.3681. [DOI] [PubMed] [Google Scholar]
- 113.Kondo T., et al. Multilevel analyses of effects of variation in body mass index on serum lipid concentrations in middle-aged Japanese men. Nagoya J. Med. Sci. 2009;71(1–2):19–28. [PMC free article] [PubMed] [Google Scholar]
- 114.Lemos-Santos M.G., et al. Waist circumference and waist-to-hip ratio as predictors of serum concentration of lipids in Brazilian men. Nutrition. 2004;20(10):857–862. doi: 10.1016/j.nut.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 115.McKarns S.C., et al. Blood parameters associated with atherogenic and thrombogenic risk in smokers and nonsmokers with similar life-styles. Mod. Pathol. 1995;8(4):434–440. [PubMed] [Google Scholar]
- 116.Scragg R., et al. Serum lipid levels in a New Zealand multicultural workforce. New Z. Med. J. 1993;106(952):96–99. [PubMed] [Google Scholar]
- 117.Szkup M., et al. Influence of cigarette smoking on hormone and lipid metabolism in women in late reproductive stage. Clin. Interv. Aging. 2018;13:109–115. doi: 10.2147/CIA.S140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Missoni S., et al. Smoking habits according to metabolic traits in an island population of the eastern Adriatic Coast. Coll. Antropol. 2013;37(3):745–753. [PubMed] [Google Scholar]
- 119.Nakamura S., et al. Blood pressure, levels of serum lipids, liver enzymes and blood glucose by aldehyde dehydrogenase 2 and drinking habit in Japanese men. Environ. Health Prev. Med. 2006;11(2):82–88. doi: 10.1007/BF02898147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni W.Q., et al. Serum lipids and associated factors of dyslipidemia in the adult population in Shenzhen. Lipids Health Dis. 2015;14:71. doi: 10.1186/s12944-015-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okada R., et al. Modification of the effect of smoking on cholesterol in Japanese carriers of a PTPN11 polymorphism. Mol. Med. Rep. 2008;1(4):595–598. [PubMed] [Google Scholar]
- 122.Rodriguez C., et al. Comparison of modifiable determinants of lipids and lipoprotein levels among African-Americans, Hispanics, and Non-Hispanic Caucasians > or =65 years of age living in New York City. Am. J. Cardiol. 2002;89(2):178–183. doi: 10.1016/s0002-9149(01)02197-x. [DOI] [PubMed] [Google Scholar]
- 123.Schwartz D., et al. Serum lipids, smoking and body build. Study of 7,972 actively employed males. Rev. Eur. Etud. Clin. Biol. 1971;16(6):529–536. [PubMed] [Google Scholar]
- 124.Shelley J.M., et al. Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann. Epidemiol. 1998;8(1):39–45. doi: 10.1016/s1047-2797(97)00123-3. [DOI] [PubMed] [Google Scholar]
- 125.Baek W., et al. Concurrent smoking and alcohol consumers had higher triglyceride glucose indices than either only smokers or alcohol consumers: a cross-sectional study in Korea. Lipids Health Dis. 2021;20(1):49. doi: 10.1186/s12944-021-01472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bermudez V., et al. Cigarette smoking and metabolic syndrome components: a cross-sectional study from Maracaibo City. Venez. F1000Res. 2018;7:565. doi: 10.12688/f1000research.14571.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Graff-Iversen S., Tverdal A., Stensvold I. Cardiovascular risk factors in Norwegian women using oral contraceptives: results from a cardiovascular health screening 1985-88. Contraception. 1996;53(6):337–344. doi: 10.1016/0010-7824(96)00082-0. [DOI] [PubMed] [Google Scholar]
- 128.Gudnason V., Thormar K., Humphries S.E. Interaction of the cholesteryl ester transfer protein I405V polymorphism with alcohol consumption in smoking and non-smoking healthy men, and the effect on plasma HDL cholesterol and apoAI concentration. Clin. Genet. 1997;51(1):15–21. doi: 10.1111/j.1399-0004.1997.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 129.Gueli N., et al. The influence of lifestyle on cardiovascular risk factors. Analysis using a neural network. Arch. Gerontol. Geriatr. 2005;40(2):157–172. doi: 10.1016/j.archger.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Halmenschlager G., et al. Evaluation of the effects of cigarette smoking on testosterone levels in adult men. J. Sex. Med. 2009;6(6):1763–1772. doi: 10.1111/j.1743-6109.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 131.He J., et al. Effect of migration and related environmental changes on serum lipid levels in southwestern Chinese men. Am. J. Epidemiol. 1996;144(9):839–848. doi: 10.1093/oxfordjournals.aje.a009018. [DOI] [PubMed] [Google Scholar]
- 132.Huang J.H., et al. Lifestyle factors and metabolic syndrome among workers: the role of interactions between smoking and alcohol to nutrition and exercise. Int. J. Environ. Res. Public Health. 2015;12(12):15967–15978. doi: 10.3390/ijerph121215035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jossa F., et al. Coffee and serum lipids: findings from the Olivetti Heart Study. Ann. Epidemiol. 1993;3(3):250–255. doi: 10.1016/1047-2797(93)90027-2. [DOI] [PubMed] [Google Scholar]
- 134.Karabudak E., Türközü D., Köksal E. Association between coffee consumption and serum lipid profile. Exp. Ther. Med. 2015;9(5):1841–1846. doi: 10.3892/etm.2015.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mansour M., et al. Prevalence and associations of behavioural risk factors with blood lipids profile in Lebanese adults: findings from WHO STEPwise NCD cross-sectional survey. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-026148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moradinazar M., et al. Association between dyslipidemia and blood lipids concentration with smoking habits in the Kurdish population of Iran. BMC Public Health. 2020;20(1):673. doi: 10.1186/s12889-020-08809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muga M.A., et al. Association of lifestyle factors with blood lipids and inflammation in adults aged 40 years and above: a population-based cross-sectional study in Taiwan. BMC Public Health. 2019;19(1):1346. doi: 10.1186/s12889-019-7686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh S., et al. Cigarette type or smoking history: which has a greater impact on the metabolic syndrome and its components? Sci. Rep. 2020:10. doi: 10.1038/s41598-020-67524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rao Ch, S., Subash E. The effect of chronic tobacco smoking and chewing on the lipid profile. J. Clin. Diagn. Res.: JCDR. 2013;7(1):31–34. doi: 10.7860/JCDR/2012/5086.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rarau P., et al. Socio-economic status and behavioural and cardiovascular risk factors in Papua New Guinea: a cross-sectional survey. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song W., et al. Mediating effects of lipids on the association between smoking and coronary artery disease risk among Chinese. Lipids Health Dis. 2020;19(1):149. doi: 10.1186/s12944-020-01325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao Y., et al. Relationship between multiple healthy lifestyles and serum lipids among adults in rural China: A population-based cross-sectional study. Prev. Med. 2020;138 doi: 10.1016/j.ypmed.2020.106158. [DOI] [PubMed] [Google Scholar]
- 143.Maas S.C.E., et al. Smoking-related changes in DNA methylation and gene expression are associated with cardio-metabolic traits. Clin. Epigenet. 2020;12(1):157. doi: 10.1186/s13148-020-00951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park S., Kang S. Alcohol, carbohydrate, and calcium intakes and smoking interactions with APOA5 rs662799 and rs2266788 were associated with elevated plasma triglyceride concentrations in a cross-sectional study of Korean Adults. J. Acad. Nutr. Diet. 2020;120(8):1318–1329. doi: 10.1016/j.jand.2020.01.009. e1. [DOI] [PubMed] [Google Scholar]
- 145.Salah Abd- Al kader O. Nicotine adds risk factors to the cardiovascular system and increases mortality and sudden cardiac arrest. Med. Leg. Update. 2020;20(3):563–568. [Google Scholar]
- 146.Wu T., Sonoda S., Liu H. Unprocessed red meat intakes are associated with increased inflammation, triglycerides and HDL cholesterol in past smokers. Nutr. Diet. 2020;77(2):182–188. doi: 10.1111/1747-0080.12555. [DOI] [PubMed] [Google Scholar]
- 147.Rosoff D.B., et al. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: A multivariable Mendelian randomization study. PLOS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Balkau B., et al. The impact of 3-year changes in lifestyle habits on metabolic syndrome parameters: the D.E.S.I.R study. Eur. J. Cardiovasc Prev. Rehabil. 2006;13(3):334–340. doi: 10.1097/01.hjr.0000214614.37232.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yeung D.L. Relationships between cigarette smoking, oral contraceptives, and plasma vitamins A, E, C, and plasma triglycerides and cholesterol. Am. J. Clin. Nutr. 1976;29(11):1216–1221. doi: 10.1093/ajcn/29.11.1216. [DOI] [PubMed] [Google Scholar]
- 150.Rabkin S. Relationship between weight change and the reduction or cessation of cigarette smoking. Int J. Obes. 1984;8(6):665–673. [PubMed] [Google Scholar]
- 151.Rabkin S.W., Boyko E., Streja D.A. Relationship of weight loss and cigarette smoking to changes in high-density lipoprotein cholesterol. Am. J. Clin. Nutr. 1981;34(9):1764–1768. doi: 10.1093/ajcn/34.9.1764. [DOI] [PubMed] [Google Scholar]
- 152.Richard F., et al. Effect of smoking cessation on lipoprotein A-I and lipoprotein A-I:A-II levels. Metabolism. 1997;46(6):711–715. doi: 10.1016/s0026-0495(97)90018-4. [DOI] [PubMed] [Google Scholar]
- 153.Sharip A., Firek A., Tonstad S. The Effects of Smoking Cessation on the Risk Factors for the Metabolic Syndrome: A Follow-Up Study of Veterans. J. Smok. Cessat. 2017;12(3):143–152. [Google Scholar]
- 154.Smith I., et al. The influence of smoking cessation and hypertriglyceridaemia on the progression of peripheral arterial disease and the onset of critical ischaemia. Eur. J. Vasc. Endovasc. Surg. 1996;11(4):402–408. doi: 10.1016/s1078-5884(96)80170-5. [DOI] [PubMed] [Google Scholar]
- 155.Tamura U., et al. Changes in Weight, cardiovascular risk factors and estimated risk of coronary heart disease following smoking cessation in Japanese male workers: HIPOP-OHP study. J. Atheroscler. Thromb. 2010;17(1):12–20. doi: 10.5551/jat.1800. [DOI] [PubMed] [Google Scholar]
- 156.Yoon C., et al. Effects of smoking cessation and weight gain on cardiovascular disease risk factors in Asian male population. Atherosclerosis. 2010;208(1):275–279. doi: 10.1016/j.atherosclerosis.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 157.Zhang P., et al. Influence of smoking cessation on carotid artery wall elasticity evaluated by echo-tracking. J. Clin. Ultrasound. 2012;40(6):352–356. doi: 10.1002/jcu.21920. [DOI] [PubMed] [Google Scholar]
- 158.Stubbe I., Eskilsson J., Nilsson-Ehle P. High-density lipoprotein concentrations increase after stopping smoking. Br. Med. J. Clin. Res. Ed. 1982;284(6328):1511–1513. doi: 10.1136/bmj.284.6328.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Evolahti A., Hultcrantz M., Collins A. Psychosocial work environment and lifestyle as related to lipid profiles in perimenopausal women. Climacteric. 2009;12(2):131–145. doi: 10.1080/13697130802521290. [DOI] [PubMed] [Google Scholar]
- 160.Toffolo M.C.F., et al. Alteration of inflammatory adipokines after four months of smoking abstinence in multidisciplinary intervention program. Nutr. Hosp. 2018;35(2):434–441. doi: 10.20960/nh.1548. [DOI] [PubMed] [Google Scholar]
- 161.Park S., et al. Smoking, development of or recovery from metabolic syndrome, and major adverse cardiovascular events: a nationwide population-based cohort study including 6 million people. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0241623. e0241623-e0241623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chimura Y., Daimon T., Wakabayashi I. Proneness to high blood lipid-related indices in female smokers. Lipids Health Dis. 2019;18(1) doi: 10.1186/s12944-019-1050-3. 113-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cuschieri S., et al. Relationship of past, present, and passive smoking with sociodemographic, anthropometric, biochemical, and dysglycemic profiles. J. Diabetes. 2019;11(1):87–89. doi: 10.1111/1753-0407.12844. [DOI] [PubMed] [Google Scholar]
- 164.Pasupathi P., Saravanan G., Farook J. Oxidative stress bio markers and antioxidant status in cigarette smokers compared to nonsmokers. J. Pharm. Sci. Res. 2009;1(2):55–62. [Google Scholar]
- 165.Allen S.S., et al. Effects of treatment on cardiovascular risk among smokeless tobacco users. Prev. Med. 1995;24(4):357–362. doi: 10.1006/pmed.1995.1058. [DOI] [PubMed] [Google Scholar]
- 166.Masarei J.R., et al. Effect of smoking cessation on serum apolipoprotein A-I and A-II concentrations. Pathology. 1991;23(2):98–102. doi: 10.3109/00313029109060805. [DOI] [PubMed] [Google Scholar]
- 167.Stamford B.A., et al. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am. J. Clin. Nutr. 1986;43(4):486–494. doi: 10.1093/ajcn/43.4.486. [DOI] [PubMed] [Google Scholar]
- 168.Eliasson B., et al. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur. J. Clin. Investig. 1997;27(5):450–456. doi: 10.1046/j.1365-2362.1997.1330680.x. [DOI] [PubMed] [Google Scholar]
- 169.Lee S.S., et al. The changes of blood glucose control and lipid profiles after short-term smoking cessation in healthy males. Psychiatry Investig. 2011;8(2):149–154. doi: 10.4306/pi.2011.8.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nilsson P., et al. Effects of smoking cessation on insulin and cardiovascular risk factors--a controlled study of 4 months' duration. J. Intern. Med. 1996;240(4):189–194. doi: 10.1046/j.1365-2796.1996.16844000.x. [DOI] [PubMed] [Google Scholar]
- 171.Ponciano-Rodriguez G., et al. Early changes in the components of the metabolic syndrome in a group of smokers after tobacco cessation. Metab. Syndr. Relat. Disord. 2014;12(4):242–250. doi: 10.1089/met.2014.0007. [DOI] [PubMed] [Google Scholar]
- 172.Komiyama M., et al. Analysis of factors that determine weight gain during smoking cessation therapy. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Korhonen T., et al. Smoking cessation program with exercise improves cardiovascular disease biomarkers in sedentary women. J. Women’s Health. 2011;20(7):1051–1064. doi: 10.1089/jwh.2010.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ludviksdottir D., et al. Effects of nicotine nasal spray on atherogenic and thrombogenic factors during smoking cessation. J. Intern Med. 1999;246(1):61–66. doi: 10.1046/j.1365-2796.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- 175.Niaura R., et al. Exercise, smoking cessation, and short-term changes in serum lipids in women: a preliminary investigation. Med. Sci. Sport. Exerc. 1998;30(9):1414–1418. doi: 10.1097/00005768-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 176.Tonstad S., Urdal P. Does short-term smoking cessation reduce plasma total homocysteine concentrations? Scand. J. Clin. Lab. Investig. 2002;62(4):279–284. doi: 10.1080/003655102760145834. [DOI] [PubMed] [Google Scholar]
- 177.van den Berkmortel F.W., et al. Smoking or its cessation does not alter the susceptibility to in vitro LDL oxidation. Eur. J. Clin. Investig. 2000;30(11):972–979. doi: 10.1046/j.1365-2362.2000.00739.x. [DOI] [PubMed] [Google Scholar]
- 178.Kato T., et al. Varenicline-assisted smoking cessation decreases oxidative stress and restores endothelial function. Hypertens. Res. 2014;37(7):655–658. doi: 10.1038/hr.2014.52. [DOI] [PubMed] [Google Scholar]
- 179.Demacker P.N., et al. Intra-individual variation of serum cholesterol, triglycerides and high density lipoprotein cholesterol in normal humans. Atherosclerosis. 1982;45(3):259–266. doi: 10.1016/0021-9150(82)90227-1. [DOI] [PubMed] [Google Scholar]
- 180.Glover E.D., et al. A randomized, controlled trial to assess the efficacy and safety of a transdermal delivery system of nicotine/mecamylamine in cigarette smokers. Addiction. 2007;102(5):795–802. doi: 10.1111/j.1360-0443.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- 181.Ebbert J.O., et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–163. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bolt D.M., et al. Why two smoking cessation agents work better than one: role of craving suppression. J. Consult Clin. Psychol. 2012;80(1):54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Evins A.E., et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J. Clin. Psychopharmacol. 2007;27(4):380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- 184.Culhane M.A., et al. Predictors of early abstinence in smokers with schizophrenia. J. Clin. Psychiatry. 2008;69(11):1743–1750. doi: 10.4088/jcp.v69n1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang A., et al. A study on the factors influencing triglyceride levels among adults in Northeast China. Sci. Rep. 2018;8(1):6388. doi: 10.1038/s41598-018-24230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.S A.L., et al. Effect of intensity of cigarette smoking on haematological and lipid parameters. J. Clin. Diagn. Res. 2014;8(7):BC11–BC13. doi: 10.7860/JCDR/2014/9545.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park K.H., Shin D.G., Cho K.H. Dysfunctional lipoproteins from young smokers exacerbate cellular senescence and atherogenesis with smaller particle size and severe oxidation and glycation. Toxicol. Sci. 2014;140(1):16–25. doi: 10.1093/toxsci/kfu076. [DOI] [PubMed] [Google Scholar]
- 188.Albrink M.J., Meigs J.W., Granoff M.A. Weight gain and serum triglycerides in normal men. New Engl. J. Med. 1962;266:484–489. doi: 10.1056/NEJM196203082661003. [DOI] [PubMed] [Google Scholar]
- 189.Dattilo A.M., Kris-Etherton P.M. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am. J. Clin. Nutr. 1992;56(2):320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 190.Botella-Carretero J.I., et al. Weight gain and cardiovascular risk factors during smoking cessation with bupropion or nicotine. Horm. Metab. Res. 2004;36(3):178–182. doi: 10.1055/s-2004-814343. [DOI] [PubMed] [Google Scholar]
- 191.Tian J., et al. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes. Rev. 2015;16(10):883–901. doi: 10.1111/obr.12304. [DOI] [PubMed] [Google Scholar]
- 192.Noh J.M., et al. Changes in the serum level of high density lipoprotein-cholesterol after smoking cessation among adult men. Korean J. Fam. Med. 2012;33(5):305–310. doi: 10.4082/kjfm.2012.33.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data can be found in cited publications and is summarized in supplementary tables.