Abstract
The cultural supernatant of Mycoplasma fermentans induced interleukin-6 production by human gingival fibroblasts. The active entities were divided into hydrophilic and hydrophobic substances. In this study, we purified a 4.1-kilodalton polypeptide from the hydrophilic substances. It reacted with polyclonal antibodies to M. fermentans and activated human macrophages.
Most bacterial modulins, which are involved in inflammatory responses, are cell wall components. However, mycoplasmas, wall-less microbes, stimulate lymphocytes, natural killer cells, and monocytes/macrophages to produce cytokines and chemokines (17). The cytokine production-inducing activity exists mostly in fractions containing hydrophobic compounds such as lipoproteins or lipoglycans (17). One macrophage-stimulating lipopeptide with an approximate molecular mass of 2 kDa has been extracted and isolated from Mycoplasma fermentans cells and is named macrophage-activating lipopeptide 2 (MALP-2) (16). It has been reported that MALP-2 induces production of interleukin-6 (IL-6), IL-8, IL-10, tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein 1, and macrophage inflammatory protein-1 by human monocytes (11). M. fermentans and M. salivarium induce production of IL-6 and IL-8 by human gingival fibroblasts (HGF) (6, 23) and cell surface expression of intercellular adhesion molecule 1 in HGF (3). The activity of M. salivarium exists in cell membrane and intracellular fractions, and a water-soluble active entity (20.6-kDa protein) has been partially purified from intracellular fractions (6). Except for this, water-soluble substances in mycoplasmas capable of activating HGF have not been identified.
In this study, we found that cultural supernatants of M. fermentans induced cytokine production by HGF and we tried to purify and characterize the active entities.
Organisms and culture conditions. M. fermentans
ATCC 19989 was grown in PPLO broth (Difco Laboratories, Detroit, Mich.) supplemented with 2% (vol/vol) horse serum (GIBCO Life Technologies, Inc., Grand Island, N.Y.), 1% (wt/vol) yeast extract (Difco), 1% (wt/vol) d-glucose, 0.002% (wt/vol) phenol red, 0.05% (wt/vol) thallium acetate, and penicillin G (1,000 U/ml). Cultures were incubated at 37°C for 48 h, at which point the growth was in the mid-logarithmic phase, and centrifuged at 100,000 × g for 1 h to separate the cultural supernatant, which was used for purification of the active entities.
Cell cultures.
HGF prepared and used in a previous study (3) were cultured in Dulbecco's modified Eagle's (DME) medium (GIBCO) containing 10% (vol/vol) fetal bovine serum (FBS), penicillin G (100 U/ml), and streptomycin (100 μg/ml) in plastic culture dishes with a medium change every 3 days for 7 to 10 days until the cells reached confluency. The single-cell suspension prepared by trypsin treatment was seeded in wells of a flat-bottom microplate. After incubation for 2 days at 37°C in an atmosphere of 5% CO2, the monolayers were washed three times with DME medium without FBS [DME(−) medium], followed by the addition of a test stimulant in 220 μl of DME(−) medium. Further incubation was done at 37°C for 6 h. The culture plate was then centrifuged at 400 × g for 10 min.
THP-1 (a myelomonocytic cell line, JCRB 0112.1) cells obtained from the Health Science Research Resources Bank (Osaka, Japan) were cultured in RPMI 1640 medium (GIBCO) containing 10% (vol/vol) FBS, penicillin G (100 U/ml), and streptomycin (100 μg/ml) in a plastic culture bottle. The cells were collected by centrifugation at 400 × g for 10 min, washed three times with RPMI 1640 medium without FBS [RPMI(−) medium], and suspended in RPMI(−) medium at a cell concentration of 5 × 106/ml. A 200-μl volume of the cell suspension was transferred to round-bottom wells of a microculture plate, and a test stimulant in 20 μl of RPMI(−) medium was then added to the wells. Further incubation was done at 37°C for 6 h.
Assay for cytokines.
The concentrations of IL-6, TNF-α, and IL-1β in the cultural supernatant were determined by using TiterZyme enzyme-linked immunosorbent assay kits (PerSeptive Diagnostics Inc., Cambridge, Mass.) in accordance with the manufacturer's instructions. The detection limits of the enzyme-linked immunosorbent assay kit are 10.9 pg/ml for IL-6, 28.1 pg/ml for TNF-α, and 2.69 pg/ml for IL-1β.
Ability of M. fermentans cultural supernatants to induce IL-6 production by HGF.
M. fermentans was grown in medium identical to that described above. A 2-ml aliquot of the culture was taken periodically and divided into two portions. One portion was used to determine the pH and the number of viable cells by using liquid and agar media described by Hayflick (7). The other portion was centrifuged at 100,000 × g for 1 h to separate the cultural supernatant, which was used to determine IL-6 production-inducing activity.
Determination of amounts of proteins, amino groups, and carbohydrates.
The amount of proteins was determined by the method of Dully and Grieve (4), the amount of compounds with amino groups was determined by the colorimetric ninhydrin method using l-arginine as a standard (25), and the amount of carbohydrates was determined by the phenol-sulfuric acid method (5).
SDS-PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 16% polyacrylamide gels by the method of Laemmli (13). Proteins were stained by using a silver stain plus kit (Bio-Rad Laboratories, Hercules, Calif.). Kaleidoscope polypeptide standards (Bio-Rad) were used to estimate molecular weights.
Enzyme treatment.
Proteinase K was purchased from Takara Biomedicals (Otsu, Japan), and lipoprotein lipase (EC 3.1.1.34.) was purchased from Sigma-Aldrich Co. (St. Louis, Mo.). A 0.02:1 (wt/wt) reaction mixture of enzyme and the sample separated from the cultural supernatant of M. fermentans was treated at 37°C for 2 h and then tested for IL-6 production-inducing activity.
Antiserum.
Japanese White rabbits were injected subcutaneously with 2 ml of a 1:1 (vol/vol) mixture of Freund's incomplete adjuvant (Difco) and a cell suspension (500 μg of protein/ml) of M. fermentans grown in PPLO broth (Difco) supplemented with 2% (vol/vol) rabbit serum (GIBCO) on day 0, subcutaneously with the same volume of the same mixture on day 14, and intraperitoneally with 1 ml of the cell suspension on day 21. Sera were drawn before immunization (preimmune serum) and 1 week after the final immunization (anti-M. fermentans serum).
IL-6 production-inducing activity in cultural supernatants of M. fermentans.
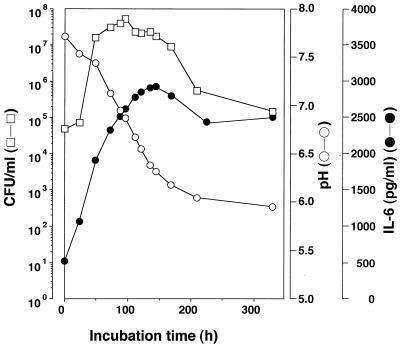
Almost in parallel with viable counts, the IL-6 production-inducing activity increased with prolongation of the incubation time up to 150 h, at which point the activity had reached a maximum (Fig. 1). Then, the activity decreased gradually, to approximately 80% of the maximum at 220 h, and remained at that level for up to 380 h. Activity was not detected in medium not inoculated with the organism.
FIG. 1.
Growth of M. fermentans in liquid medium and IL-6 production-inducing activity in the cultural supernatant. M. fermentans was grown in liquid medium, and a 2-ml aliquot of the culture was taken periodically and divided into two portions. One portion was used to determine the pH (○) and the number of viable cells (CFU/ml, □). The other was centrifuged at 100,000 × g for 1 h to separate the cultural supernatant, which was used to determine the ability to induce IL-6 production by HGF (●).
Purification of active entities.
Proteins in the cultural supernatant were precipitated with ammonium sulfate between 0 and 30%, 30 and 60%, and 60 and 100% saturation and were examined for activity. Most of the activity (97%) was recovered in the proteins precipitating with ammonium sulfate between 30 and 60% saturation (P60, Table 1). In order to characterize the active entities in P60, the effect of enzymes on the activity was investigated. The activity was reduced to 30% by treatment with proteinase K and lipoprotein lipase (Table 2), suggesting that proteins and lipoproteins are involved in expression of the activity.
TABLE 1.
IL-6 production-inducing activity in proteins separated from supernatants of M. fermentans cultures by ammonium sulfate
| Concn of ammonium sulfate used to separate proteins (%) | Total amt of protein (mg) | Sp acta (U/mg of protein) | Total U of activity (%) |
|---|---|---|---|
| 0–30 | 15.7 | 2.1 | 33.0 (0.8) |
| 30–60 (P60) | 72.1 | 58.7 | 4,232.3 (97.0) |
| 60–100 | 24.9 | 3.8 | 94.7 (2.2) |
One unit of activity is defined as the amount of protein required to induce production of 1 ng of IL-6. Each value is the mean of two separate experiments.
TABLE 2.
Effects of proteinase K and lipoprotein lipase on IL-6 production-inducing activity of P60
| Treatment | Sp acta in U/mg of protein (%) |
|---|---|
| None | 58.7 ± 6.4 (100) |
| Proteinase K | 17.4 ± 2.3 (30) |
| Lipoprotein lipase | 18.0 ± 7.5 (31) |
One unit of activity is defined as the amount of protein required to induce production of 1 ng of IL-6. One-hundred-microliter volumes of fractions obtained from the initial reversed-phase chromatography (60 μg of protein) were pretreated at 37°C for 2 h with 1.2 μg each of proteinase K and lipoprotein lipase, and then 20 μl of the reaction mixture was used for stimulation of HGF. Each value is the mean and standard deviation of three separate experiments.
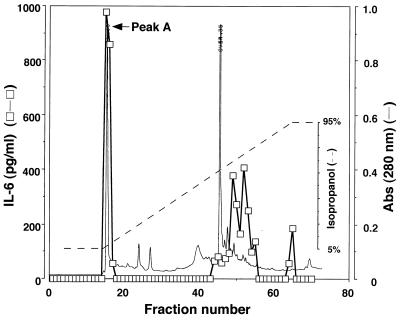
P60 was fractionated by reversed-phase high-performance liquid chromatography (HPLC) with a preparative Nucleosil 120-7 C18 column (10 by 300 mm; Chemco Scientific Co. Ltd., Osaka, Japan). Fractionation was carried out by using the following program: at time zero, 5% N,N′-dimethylformamide (DMF)–95% milli-Q water (MQW); at 15 min, 5% DMF–95% MQW; at 60 min, 5% DMF–95% 2-propanol; and at the end, 5% DMF–95% 2-propanol. Each fraction was dried in vacuo at 45°C, dissolved in MQW, and examined for IL-6 production-inducing activity. The activity was recovered in fractions with two peaks eluted at hydrophilic and hydrophobic regions. The hydrophobic fractions seemed to contain lipoproteins or lipopeptides that had already been characterized (16). However, hydrophilic substances in M. fermentans capable of inducing production of cytokines have not been identified. Therefore, we were very much interested in the active entities in the hydrophilic fractions, peak A (Fig. 2).
FIG. 2.
Protein profile (solid line) obtained by reversed-phase chromatography of P60 on an HPLC column. P60 was applied to a preparative Nucleosil 120-7 C18 column (10 by 300 mm; Chemco Scientific Co. Ltd.). Fractionation was carried out by using the following program: at time zero, 5% DMF–95% MQW; at 15 min, 5% DMF–95% MQW; at 60 min, 5% DMF–95% 2-propanol; and at the end, 5% DMF–95% 2-propanol. The flow rate was 1.0 ml/min. Each fraction was dried in vacuo at 45°C, dissolved in MQW, and examined for IL-6 production-inducing activity (□). Abs, absorbance.
The effect of polymyxin B on the IL-6 production-inducing activity of peak A was investigated to eliminate the possibility that the activity was attributable to lipopolysaccharides that might contaminate peak A. HGF were preincubated with polymyxin B (0, 5,000, and 10,000 U/ml) because polymyxin B is an antibiotic that destroys the biological activities of lipopolysaccharides and then stimulated with peak A at 37°C for 6 h. The activity was not affected by polymyxin B (data not shown).
Peak A was then fractionated by size-exclusion HPLC with an Asahipak GS-520 column (7.6 by 500 mm; Asahi Chemical Industry Co. Ltd., Tokyo, Japan). The fractionation was done by elution with MQW. Each fraction was dried in vacuo at 45°C, dissolved in MQW, and examined for IL-6 production-inducing activity. The activity was recovered in fractions with a major peak (peak B).
Peak B was further fractionated by reversed-phase HPLC with an ODP-50 column (4.6 by 250 mm; Shoko Co. Ltd., Tokyo, Japan). Fractionation was carried out by using the following program: at time zero, 0.05% trifluoroacetic acid (TFA)–20% acetonitrile; at 5 min, 0.05% TFA–20% acetonitrile; at 20 min, 0.05% TFA–80% acetonitrile; and at the end, 0.05% TFA–80% acetonitrile. Each fraction was dried in vacuo at 45°C, dissolved in MQW, and examined for IL-6 production-inducing activity. The activity was recovered in fractions with a major peak (peak C).
Properties of the active entities in peak C.
Peak C activated macrophages to produce TNF-α and IL-1β (data not shown). Peak C was found to contain substances with amino groups and carbohydrates. The specific activity of peak C calculated on the basis of the amount of amino group-containing substances was about 75-fold higher than that of P60, while the activity calculated on the basis of the amount of carbohydrate-containing substances was lower than that of P60 (Table 3).
TABLE 3.
Purification of IL-6 production-inducing substances from supernatants of M. fermentans cultures
| Sample | Purificationa
|
|
|---|---|---|
| Amino group | Carbohydrate | |
| P60 | 1.0 | 1.0 |
| Peak A | NTb | 0.9 |
| Peak B | 57.2 | 0.6 |
| Peak C | 75.1 | 0.7 |
Purification is defined as the specific activity calculated on the basis of the amount of amino group-containing substances or carbohydrate-containing substances.
NT, not tested.
The effect of lipoprotein lipase on the IL-6 production-inducing activity of peak C was investigated. A 0.02:1 (wt/wt) reaction mixture of lipoprotein lipase and peak C was treated at 37°C for 2 h and then tested for the activity. It was found that lipoprotein lipase had no effect on the activity of peak C (data not shown).
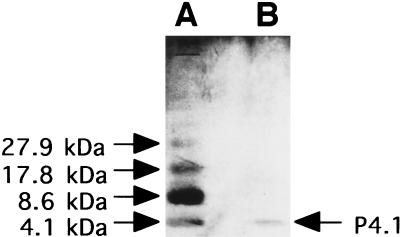
SDS-PAGE of peak C revealed one dense band with a molecular mass of 4.1 kDa (Fig. 3). Thus, the active entity in peak C was found to be a 4.1-kDa polypeptide (P4.1).
FIG. 3.
SDS-PAGE. SDS-PAGE of polypeptide standards (A; 4.0 μg of protein) and peak C (B; 0.3 μg of protein) was performed with a 16% polyacrylamide gel. Proteins were stained by using a silver stain plus kit (Bio-Rad).
M. fermentans cells and peak C were spotted onto two nitrocellulose membranes. To avoid nonspecific binding of antibodies, each of the membranes was blocked with 5% skim milk in phosphate-buffered saline. One membrane was then reacted with anti-M. fermentans serum, and the other was reacted with preimmune serum. Each of antibodies was detected by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G and a DAB substrate (Vector Laboratories, Inc., Burlingame, Calif.). Anti-M. fermentans serum, but not preimmune serum, reacted with M. fermentans cells and peak C (Fig. 4). The fact that only anti-M. fermentans serum reacted with peak C demonstrated that P4.1 was derived from M. fermentans.
FIG. 4.
Detection of substances reacted with anti-M. fermentans serum. M. fermentans cells (A, 4 μg of protein; B, 0.4 μg of protein) and peak C (C, 0.06 μg of protein) were spotted onto two nitrocellulose membranes. Each of the membranes was blocked with 5% skim milk in phosphate-buffered saline. One membrane was then reacted with anti-M. fermentans serum, and the other was reacted with preimmune serum. Each of antibodies was detected by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G and a DAB substrate (Vector Laboratories, Inc.).
Mycoplasmal lipoproteins activate lymphocytes, monocytes/macrophages, and fibroblasts, and the activity resides in the N-terminal lipopeptide moieties (16, 22). Lipoproteins are not released in a water-soluble form from intact mycoplasma cells because they are anchored to the cell membranes by the N-terminal lipid moiety. Therefore, we were interested in mycoplasma-derived and hydrophilic substances capable of activating mammalian cells because we thought that hydrophilic substances are capable of interacting with target cells more easily and play more important pathological roles than hydrophobic substances such as lipoproteins. Recently, we have partially purified and characterized hydrophilic substances from M. salivarium that are responsible for induction of IL-6 production by HGF (6). In this sense, we were very much interested in the finding that the cultural supernatant of M. fermentans induced IL-6 production by HGF. The activity existed in hydrophilic and hydrophobic substances in the cultural supernatant. Hydrophobic substances are possibly lipoproteins or lipopeptides liberated in some way from cell membranes of M. fermentans and solubilized in the cultural supernatant, possibly by association with serum albumin in horse serum, an essential ingredient of growth medium. The active entity in hydrophilic substances was found to be a 4.1-kDa peptide, named P4.1, with carbohydrates (Table 3). P4.1 is presumably a product of M. fermentans or, less likely, a fragment separated from proteins or lipoproteins in cell membranes of the organism by some enzymes, because the activity increased almost in parallel with viable counts in cultures and reached a maximum at the end of the log phase (Fig. 1).
P4.1 activates TNF-α production by macrophages. MALP-2 has been purified from the cell membrane of M. fermentans (16) and is thought to be the N-terminal lipopeptide moiety released from lipoproteins degraded by some proteolytic enzyme. Therefore, it is very likely that the cultural supernatant of M. fermentans contains lipopeptides such as MALP-2. Lipopeptides are possibly eluted at the hydrophobic region in reversed-phase HPLC. However, P4.1 was eluted at the hydrophilic region and is resistant to lipoprotein lipase. Therefore, it is speculated that P4.1 is another activator of macrophages.
M. fermentans was first isolated (19, 20) from the human lower genital tract and is thought to be a common inhabitant of the genital tract. M. fermentans has been suggested to be associated with rheumatoid arthritis (RA) and AIDS because M. fermentans was also isolated from RA patients (32), detected in synovial fluid of RA patients by PCR methods (9, 21), and isolated from AIDS patients (10, 14).
Large amounts of cytokines such as TNF-α, IL-1β, IL-6, and IL-8 are produced in the inflamed rheumatoid synovial fluid of RA patients and play crucial roles in the pathophysiology of RA (28). The finding that P4.1 and lipoproteins induce IL-6 production by HGF suggests that they are capable of inducing IL-6 production by synovial fibroblasts.
Many studies on AIDS have suggested that mycoplasmas, including M. fermentans, are involved for some reasons as possible cofactors in AIDS pathogenesis. For example, higher titers of antibodies to these mycoplasmas have been detected in human immunodeficiency virus-infected patients than in noninfected individuals (30), and M. fermentans has been shown to have the ability to invade host cells (15, 29) and induce TNF-α secretion by monocytes, which is known to activate human immunodeficiency virus replication (18). The finding that P4.1 induces TNF-α production by macrophages suggests that it may play some pathological role in AIDS.
Periodontitis is an inflammatory disorder characterized by bone resorption. IL-6 is an inflammatory cytokine that plays an etiological role in this disease. IL-6 alone does not induce bone resorption by osteoclast formation, but soluble IL-6 receptors trigger the formation in the presence of IL-6 (12). Porphyromonas gingivalis, a gram-negative, anaerobic bacterium, is thought to be one of the pathogens in periodontitis. P. gingivalis possesses the ability to induce IL-6 production by HGF (27). Baker et al. reported that IL-6 production induced by infection with P. gingivalis contributes to alveolar bone loss (1). P4.1 also induces IL-6 production by HGF. In addition, M. fermentans has been detected in human saliva (2, 24). This suggests that P4.1 plays some etiological role in periodontitis.
Temporomandibular disorder is a disease in which pain and impaired mandibular movement appear to arise directly from degenerative or inflammatory changes within the temporomandibular joint, but its precise pathogenesis has not been elucidated. It has been suggested that the development of temporomandibular disorder may be traced to a single traumatic event that occurred before the manifestation of symptoms of the disease (26). However, we detected M. fermentans in the synovial fluid of patients with temporomandibular disorder by PCR (31). Recently, Henry et al. also reported that they detected some microbes, including M. fermentans, in temporomandibular joints (8). Therefore, P4.1 may play an etiological role in temporomandibular disorder. For these reasons, M. fermentans may be involved in the pathogenicity of some oral diseases.
This study has demonstrated that M. fermentans produces a hydrophilic polypeptide capable of inducing the production of inflammatory cytokines other than lipoproteins.
Acknowledgments
This work was partially supported by Grants-in-Aid for Scientific Research (C) (10671762), which were provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Baker P J, Dixon M, Evans R T, Dufour L, Johnson E, Roopenian D C. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chingbingyong M I, Hughes C V. Detection of Mycoplasma fermentans in human saliva with a polymerase chain reaction based assay. Arch Oral Biol. 1996;41:311–314. doi: 10.1016/0003-9969(96)84556-0. [DOI] [PubMed] [Google Scholar]
- 3.Dong L, Shibata K-I, Sawa Y, Hasebe A, Yamaoka Y, Yoshida S, Watanabe T. Transcriptional activation of mRNA of intercellular adhesion molecule-1 and induction of its cell surface expression in normal human gingival fibroblasts by Mycoplasma salivarium and Mycoplasma fermentans. Infect Immun. 1999;67:3061–3065. doi: 10.1128/iai.67.6.3061-3065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dully J R, Grieve P A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975;64:136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- 5.Fox J D, Robyt J F. Miniaturization of three carbohydrate analyses using a microsample plate reader. Anal Biochem. 1991;195:93–96. doi: 10.1016/0003-2697(91)90300-i. [DOI] [PubMed] [Google Scholar]
- 6.Hasebe A, Shibata K-I, Domon H, Dong L, Watanabe T. Partial purification and characterization of the active entity responsible for inducing interleukin-6 production by human gingival fibroblasts from Mycoplasma salivarium cells. Microbiol Immunol. 1999;43:1003–1008. doi: 10.1111/j.1348-0421.1999.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965;23(Suppl. 1):285–303. [PubMed] [Google Scholar]
- 8.Henry C H, Hughes C V, Gerard H C, Hudson A P, Wolford L M. Reactive arthritis: preliminary microbiologic analysis of the human temporomandibular joint. J Oral Maxillofac Surg. 2000;58:1137–1142. doi: 10.1053/joms.2000.9575. [DOI] [PubMed] [Google Scholar]
- 9.Johnson S, Sidebottom D, Bruckner F, Collins D. Identification of Mycoplasma fermentans in synovial fluid samples from arthritis patients with inflammatory disease. J Clin Microbiol. 2000;38:90–93. doi: 10.1128/jcm.38.1.90-93.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katseni V L, Gilroy C B, Ryait B K, Ariyoshi K, Bieniasz P D, Taylor-Robinson D. Mycoplasma fermentans in individuals seropositive and seronegative for HIV-1. Lancet. 1993;341:271–273. doi: 10.1016/0140-6736(93)92617-3. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann A, Mühlradt P F, Gemsa D, Sprenger H. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect Immun. 1999;67:6303–6308. doi: 10.1128/iai.67.12.6303-6308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotake S, Sato K, Kim K J, Takahashi N, Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Suda T, Kashiwazaki S. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lo S C, Shih J W, Newton P B, 3rd, Wong D M, Hayes M M, Benish J R, Wear D J, Wang R Y. Virus-like infectious agent (VLIA) is a novel pathogenic mycoplasma: Mycoplasma incognitus. Am J Trop Med Hyg. 1989;41:586–600. doi: 10.4269/ajtmh.1989.41.586. [DOI] [PubMed] [Google Scholar]
- 15.Lo S C, Hayes M M, Wang R Y, Pierce P F, Kotani H, Shih J W. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415–1418. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- 16.Mühlradt P F, Kieβ M, Meyer H, Süβmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1138–1144. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg Z F, Fauci A S. Induction of expression of HIV in latently or chronically infected cells. AIDS Res Hum Retrovir. 1989;5:1–4. doi: 10.1089/aid.1989.5.1. [DOI] [PubMed] [Google Scholar]
- 19.Ruiter M, Wentholt H M M. Isolation of a pleuropneumonia-like organism in primary fusospirochetal gangrene of the penis. J Investig Dermatol. 1950;15:301–304. doi: 10.1038/jid.1950.104. [DOI] [PubMed] [Google Scholar]
- 20.Ruiter M, Wentholt H M M. Isolation of a pleuropneumonia-like organism in a case of fusospirillary vulvovaginitis. Acta Dermato-Venereol. 1953;33:123–129. [PubMed] [Google Scholar]
- 21.Schaeverbeke T, Gilroy C B, Bebear C, Dehais J, Taylor-Robinson D. Mycoplasma fermentans, but not M. penetrans, detected by PCR assays in synovium from patients with rheumatoid arthritis and other rheumatic disorders. J Clin Pathol. 1996;49:824–828. doi: 10.1136/jcp.49.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata K-I, Hasebe A, Into T, Yamada M, Watanabe T. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J Immunol. 2000;165:6538–6544. doi: 10.4049/jimmunol.165.11.6538. [DOI] [PubMed] [Google Scholar]
- 23.Shibata K-I, Hasebe A, Sasaki T, Watanabe T. Mycoplasma salivarium induces interleukin-6 and interleukin-8 in human gingival fibroblasts. FEMS Immunol Med Microbiol. 1998;19:275–283. doi: 10.1111/j.1574-695X.1997.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 24.Shibata K-I, Kaga M, Kudo M, Dong L, Hasebe A, Domon H, Sato Y, Oguchi H, Watanabe T. Detection of Mycoplasma fermentans in saliva sampled from infants, preschool and school children, adolescents and adults by a polymerase chain reaction-based assay. Microbiol Immunol. 1999;43:521–525. doi: 10.1111/j.1348-0421.1999.tb02437.x. [DOI] [PubMed] [Google Scholar]
- 25.Shibata K-I, Watanabe T. Carboxypeptidase activity in human mycoplasmas. J Bacteriol. 1986;168:1045–1047. doi: 10.1128/jb.168.2.1045-1047.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solberg W K, Hansson T L, Nordströrm B. The temporomandibular joint in young adults at autopsy: a morphologic classification and evaluation. J Oral Rehabil. 1985;12:303–321. doi: 10.1111/j.1365-2842.1985.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 27.Steffen M J, Holt S C, Ebersole J L. Porphyromonas gingivalis induction of mediator and cytokine secretion by human gingival fibroblasts. Oral Microbiol Immunol. 2000;15:172–180. doi: 10.1034/j.1399-302x.2000.150305.x. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Tetsuka T, Yoshida S, Watanabe N, Kobayashi M, Matsui N, Okamoto T. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1 beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000;465:23–27. doi: 10.1016/s0014-5793(99)01717-2. [DOI] [PubMed] [Google Scholar]
- 29.Taylor-Robinson D, Davies H A, Sarathchandra P, Furr P M. Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int J Exp Pathol. 1991;72:705–714. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R Y, Shih J W, Grandinetti T, Pierce P F, Hayes M M, Wear D J, Alter H J, Lo S C. High frequency of antibodies to Mycoplasma penetrans in HIV-infected patients. Lancet. 1992;340:1312–1316. doi: 10.1016/0140-6736(92)92493-y. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe T, Shibata K-I, Yoshikawa T, Dong L, Hasebe A, Domon H, Kobayashi T, Totsuka Y. Detection of Mycoplasma fermentans in synovial fluids of temporomandibular joints of patients with disorders in the joints. FEMS Immunol Med Microbiol. 1998;22:241–246. doi: 10.1111/j.1574-695X.1998.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 32.Williams M H, Brostoff J, Roitt I M. Possible role of Mycoplasma fermentans in the pathogenesis of rheumatoid arthritis. Lancet. 1970;ii:277–280. doi: 10.1016/s0140-6736(70)91328-0. [DOI] [PubMed] [Google Scholar]