Highlights
-
•
Small scale longitudinal series performed in a community practice setting.
-
•
Single surgeon performed all the procedures (WS).
-
•
Significant improvements, above MCIDs, observed at 6 months using ODI, VAS and SF-36 scales.
-
•
First independently funded US study on basivertebral nerve ablation.
-
•
First independently funded study on a BVNA FDA-cleared device (INTRACEPT®).
Keywords: Chronic low back pain, Basivertebral nerve ablation, Radiofrequency, Endplate degeneration, Modic changes, Community practice setting, Independently-funded study
Abbreviations: CLBP, Chronic Low Back Pain; BVNA, Basivertebral Nerve Ablation; RF, Radiofrequency; ODI, Oswetry Disability Index; VAS, Visual Analog Scale; RFA, Radio Frequency Ablation; TFESI, Transforaminal Epidural Steroid Injection; MCID, Minimal Clinically Important Differences; SI, Sacroiliac
Abstract
Background
Strong innervation of the vertebral endplates by the basivertebral nerve makes it an ideal target for ablation in the treatment of vertebrogenic low back pain with Modic changes. This data represents the clinical outcomes for 16 consecutively treated patients in a community practice setting.
Methods
Basivertebral nerve ablations were performed on 16 consecutive patients by a single surgeon (WS) utilizing the INTRACEPT® device (Relievant Medsystems, Inc.). Evaluations were performed at baseline, 1 month, 3 months, and 6 months. The Oswestry Disability Index (ODI), Visual Analog Scale (VAS), and SF-36 were recorded in Medrio electronic data capture software. All patients (n = 16) completed the baseline, 1 month, 3 months, and 6 months follow-up.
Results
The ODI, VAS, and SF-36 Pain Component Summary showed statistically significant improvements above minimal clinically important differences at 1 month, 3 months, and 6 months (all p values <0.05). Change in ODI pain impact declined 13.1 points [95% CI: 0.01,27.2] at one month from baseline, 16.5 points [95% CI: 2.5,30.6] at three months from baseline, and 21.1 points [95% CI: 7.0,35.2] six-months from baseline. SF-36 Mental Component Summary also showed some improvements, but with significance only at 3 months (p = 0.0091).
Conclusions
Basivertebral nerve ablation appears to be a durable, minimally invasive treatment for the relief of chronic low back pain that can be successfully implemented in a community practice setting. To our knowledge, this is the first independently funded US study on basivertebral nerve ablation.
Background
Chronic low back pain (CLBP) affects millions of patients worldwide. The standard treatments for CLBP range from conservative interventions to invasive modalities that often result in either temporary relief or only modest pain reduction [1].
Research into the anatomic and pathobiologic understanding of vertebral endplate degeneration has led to the concept of a vertebrogenic pain model [2], as opposed to the typically accepted discogenic pain model [3,4]. This conceptual change has recently gained popularity with mounting evidence that the adjacent vertebral endplates play a significant role in CLBP [5,6]. Multiple independent studies have concluded that Modic type 1 and 2 changes are associated with some types of CLBP [7], [8], [9], [10], [11], [12], [13], [14], [15]. The vertebral body is innervated by the basivertebral nerve which branches from the sinuvertebral nerve and enters posteriorly by way of the basivertebral foramen [16]. It was hypothesized that pain levels could be reduced by interrupting this neural pathway percutaneously [17], [18], [19]. Given that basivertebral nerve ablation (BVNA) is a relatively new spinal procedure for the treatment of CLBP, and in view of the growing amount of research and clinical trials on the topic, we previously published a scoping review to identify the existing clinical evidence for BVNA [20]. In that scoping review, we identified a lack of independent studies [21], [22], [23], which was also confirmed as a limitation in a more recent meta-analysis by Conger et al. [24].
Our scoping review was therefore followed by our own case series of 16 patients treated in our medical practice setting. This work reports on the outcomes gathered at 1, 3, and 6 months post-procedure. Even though there have been a few independent studies on BVNA outside of the US [21], [22], [23], to the best of our knowledge, this is the first independent clinical study on BVNA using the only FDA-cleared device.
Methods
Surgical procedure
This study was HIPAA compliant and conducted with institutional review board approval and participant informed consent (ClinicalTrials.gov Ref #NCT05692440). BVNA was performed on 16 consecutive patients by a single surgeon (WS) utilizing the INTRACEPT® device (Relievant Medsystems, Inc.) as previously described by Fischgrund et al. [25]. BVNA procedures were completed between June 2021 and April 2022.
INTRACEPT® procedure [25]
The procedure is performed unilaterally with the patient in a prone position; either general or conscious sedation is administered. Using standard anatomic landmarks, the location of the entry pedicle at each level to be treated is determined and marked. Under fluoroscopic guidance, an introducer cannula is advanced through the pedicle until the trocar just breaches the posterior vertebral wall. The introducer trocar is exchanged with a smaller plastic cannula/curved nitinol stylet assembly, which facilitates the creation of a curved path from the posterior wall to the pre-determined target located at the terminus of the BVN, located near the center of the vertebral body. Finally, the curved nitinol stylet is removed and an RF probe is introduced positioned at the terminus of the BVN. The bipolar RF probe is activated and the temperature at the tip is maintained at a constant 85 °C for 15 min.
Inclusion/exclusion criteria
The inclusion/exclusion criteria are listed in Table 1. The participant's demographics, pain history, and levels treated are listed in Table 2.
Table 1.
Inclusion/exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adult patients ≥18 years of age. Patients who have experienced chronic low back pain for ≥6 months. Patients who have not responded to at least 6 months of conservative care. Patients with Modic type 1 or 2 changes. |
Patients with severe cardiac or pulmonary disease. Patients with active systemic infection or localized infection in the treatment area. |
Table 2.
Participant's demographics and procedures performed. RFA: medial branch radiofrequency ablation, SI: sacroiliac, TFESI: transforaminal epidural steroid injection. A "Y" on the Spinal Stenosis column indicates a severe or critical, central or foraminal, lumbar spinal stenosis, as noted in the radiologist report.
| Patient # | Age | Sex | Race | Therapeutic Lumbar Interventions Performed Before BVNA | Levels Treated | Spinal Stenosis (Y/N) |
Therapeutic Lumbar Interventions During Follow-Up | ODI Improvement at 6 months |
|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | W | Lumbar discectomy | L4 L5 | N | None | Y |
| 2 | 81 | M | W | Left L4–5 L5-S1 TFESI Bilateral L3–5 RFA L4-L5 discectomy |
L4 | Y | None | Y |
| 3 | 75 | M | W | Bilateral L3–5 RFA Right L4–5 L5-S1 TFESI |
L4 L5 | Y | None | Y |
| 4 | 67 | M | W | Bilateral L3–5 RFA | L4 L5 | N | None | Y |
| 5 | 72 | F | W | Bilateral L3–5 RFA | L4 L5 | N | Bilateral SI joint injection | Y |
| 6 | 83 | M | W | Lumbar fusion & revision Kyphoplasty |
L5 | N | None | Y |
| 7 | 68 | M | W | L4–5 fusion Spinal cord stimulation |
L3 L4 L5 | N | None | Y |
| 8 | 64 | W | H | Right L4–5 L5-S1 TFESI Caudal epidural steroid injection |
L4 L5 | N | None | Y |
| 9 | 71 | M | W | Fusion L3-S1 | L3 L4 | Y | None | Y |
| 10 | 74 | M | W | Bilateral L3–5 RFA | L3 L4 L5 | N | None | N |
| 11 | 84 | M | W | Right L4–5 L5-S1 TFESI | L4 L5 | Y | None | Y |
| 12 | 78 | F | W | Left L4-S1 TFESI | L3 L4 L5 | N | Epidural steroid injection for radicular pain | Y |
| 13 | 74 | M | W | Bilateral L3–5 RFA Epidural steroid injection for radicular pain |
L3 L4 L5 | Y | Epidural steroid injection for radicular pain | N |
| 14 | 77 | M | W | Bilateral L3–5 RFA | L3 L4 L5 | Y | None | Y |
| 15 | 67 | M | W | Left L3-L5 TFESI Left L3-S1 facet injection Left L2-L5 RFA |
L3 L4 L5 | N | Bilateral L3–5 RFA Left SI joint steroid injection |
N |
| 16 | 83 | M | W | Left L4-S1 TFESI Bilateral SI joint injection |
L3 L4 L5 | N | None | N |
Evaluations
A clinical research associate (KM) collected all patient data. Evaluations were performed at baseline, 1 month, 3 months, and 6 months. Clinical data was recorded using the Oswestry Disability Index (ODI), Visual Analog Scale (VAS), and SF-36.
Data capture
Data was recorded in Medrio electronic data capture software (Medrio, San Francisco, CA).
Statistical analysis
For the ODI, VAS, and SF-36, we followed published guidelines: The ODI is scored by summing responses to all questions to calculate a total raw score. Raw scores are then converted to percentages. Higher scores indicate more pain. For descriptive purposes, we also categorized the percentages into five categories: (1) 0% to 20%: minimal disability; (2) 21%−40%: moderate disability; (3) 41%−60%: severe disability; (4) 61%−80%: crippled; and (5) 81%−100%: bed bound or exaggerating. The VAS score is determined on a 10-point scale, between the “no pain” anchor (zero) and the highest score (10) that indicates greater pain intensity. Scoring the SF-36 is a three-step process. First, all eight subscores (ranging from 0 to 100) are standardized using a linear z-score transformation. Z-scores are calculated from the means of the general US population sample. Second, z-scores are multiplied by factor coefficients for the physical component summary (PCS) and mental component summary (MCS). Third, t-scores are obtained by multiplying the PCS and MCS sums by 10 and adding 50 to the product to yield a mean of 50 and a standard deviation of 10. Higher scores indicate better health status. To analyze changes over time, we employed mixed, random effects, generalized linear models. The fixed effects were visits: (1) baseline, (2) one month follow-up, (3) three months follow-up, and (4) six months follow-up. The random effect was the subject. For all post-modeling pairwise comparisons, we used a false discovery adjustment (FDR). The FDR method has higher power than the Bonferroni and Tukey HSD method and controls the type I error as well. This is important considering the project's exploratory nature. Statistical significance is found at p<0.05 and R 4.2.2 software was used for all data analysis. The table values are presented as algebraic means. The reported differences, the 95% confidence intervals of the differences, and the plots are presented as marginal or least square means (LS). The LS means are adjusted for the covariates (age and sex).
Results
Demographics and baselines
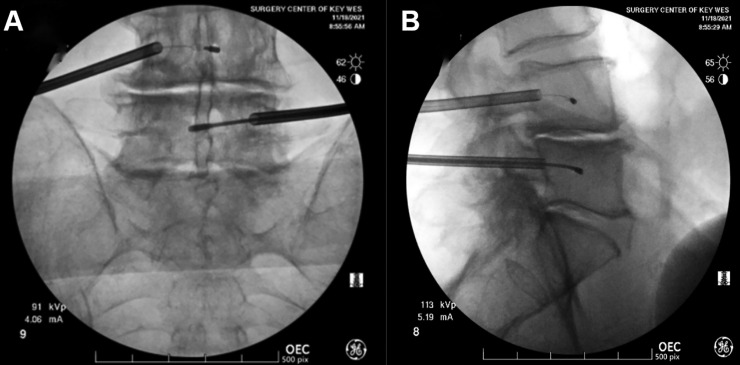
Eighty-one percent (n = 13) of the patients were male, with an average age of 73.3 (SD = 6.32). CLBP duration was greater than 12 months for all patients. No adverse effects were observed in any of the 16 patients studied. Fluoroscopic images representative of the surgical procedure are presented in Fig. 1. Pain impact, as measured by the ODI at baseline, was 44.0% (SD=0.18), with all subjects falling into the moderate disability (50%), severe disability (31.2%), crippled (12.5%), or bedbound (6.25%) category (see method section). The average VAS score at baseline was 7.88 (SD=0.62) on a scale of 0–10. At baseline, SF-36 PCS was very low at 26.2 (SD=5.86). Summary statistics for each scale (ODI, VAS, and SF-36) are presented in Table 3.
Fig. 1.
Representative fluoroscopic image of a BVNA procedure at the L4-L5 level. Front view (A), lateral view (B). BVNA: basivertebral nerve ablation.
Table 3.
Statistics summary. The table values are algebraic means.
| Variable | Statistic | Baseline | 1 month | 3 months | 6 months |
|---|---|---|---|---|---|
| ODI (%/100) | Mean (SD) | 0.440 (0.178) | 0.308 (0.276) | 0.274 (0.169) | 0.228 (0.167) |
| Median [Min, Max] | 0.410 [0.240, 0.840] | 0.230 [0, 0.860] | 0.220 [0.0200, 0.680] | 0.220 [0.0200, 0.600] | |
| ODI (%) | Minimal Disability | 0 (0%) | 8 (50.0%) | 6 (37.5%) | 8 (50.0%) |
| Moderate Disability | 8 (50.0%) | 4 (25.0%) | 7 (43.8%) | 6 (37.5%) | |
| Severe Disability | 5 (31.2%) | 0 (0%) | 2 (12.5%) | 2 (12.5%) | |
| Crippled | 2 (12.5%) | 3 (18.8%) | 1 (6.2%) | 0 (0%) | |
| Bed Bound | 1 (6.2%) | 1 (6.2%) | 0 (0%) | 0 (0%) | |
| VAS | Mean (SD) | 7.88 (0.619) | 5.25 (2.62) | 5.69 (2.30) | 4.44 (1.71) |
| Median [Min, Max] | 8.00 [6.00, 9.00] | 6.00 [0, 8.00] | 6.00 [2.00, 10.0] | 4.00 [2.00, 7.00] | |
| SF-36 PCS | Mean (SD) | 26.2 (5.86) | 36.3 (13.6) | 39.4 (9.92) | 42.8 (12.2) |
| Median [Min, Max] | 23.9 [20.6, 41.8] | 32.0 [21.9, 58.9] | 42.3 [23.8, 55.0] | 44.5 [23.0, 58.1] | |
| SF-36 MCS | Mean (SD) | 48.2 (11.9) | 52.0 (12.0) | 57.2 (9.91) | 54.8 (11.3) |
| Median [Min, Max] | 52.6 [29.7, 65.1] | 55.6 [29.2, 70.4] | 59.7 [34.1, 67.1] | 58.9 [23.3, 68.2] | |
ODI
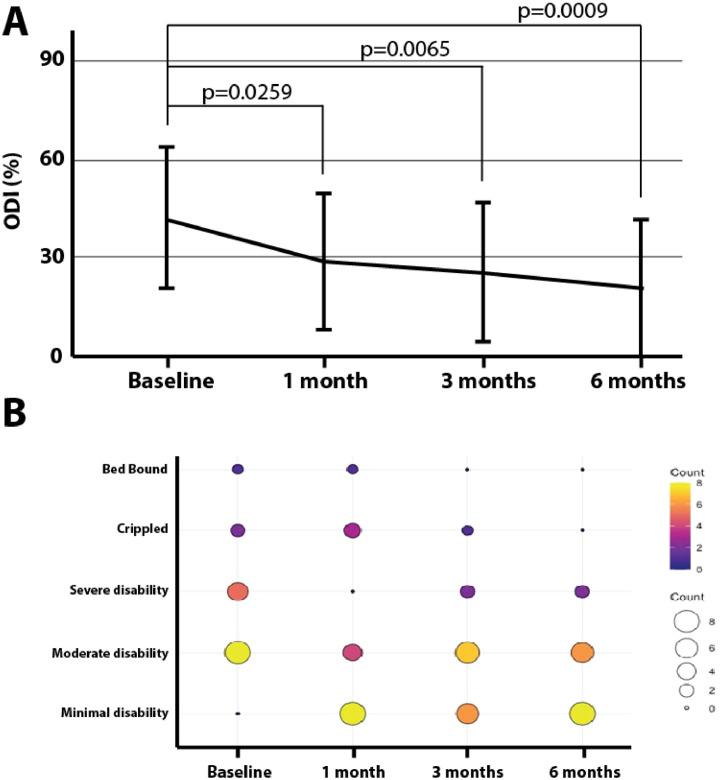
ODI results revealed a significant reduction from baseline not only in the subject's pain impact but also a reduction in the number of crippled/bed-bound individuals (p <0.05). Change in ODI pain impact declined 13.1 points [95% CI: 0.01,27.2] at one month from baseline, 16.5 points [95% CI: 2.5,30.6] at three months from baseline, and 21.1 points [95% CI: 7.0,35.2] at six months from baseline, as shown in Fig. 2A. Additionally, using a Fisher's Exact test, significant improvements in ODI pain categories were also measured (p = 0.005). At baseline, 50% reported being severely disabled, crippled, or bed bound vs. only 12.5% at 6 months (Fig. 2B).
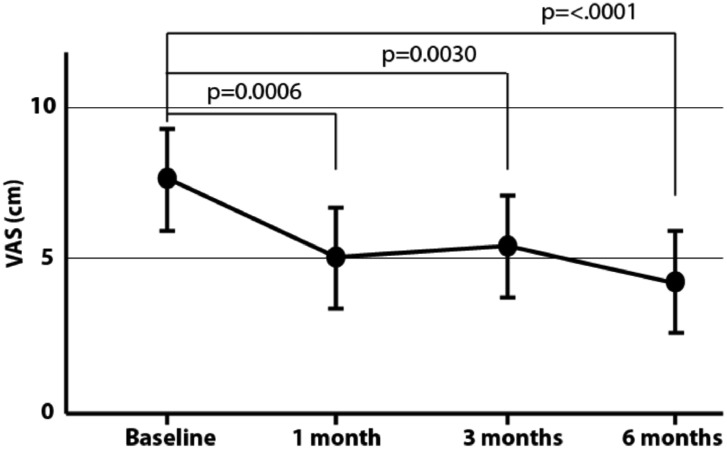
Fig. 3.
Visual Analog Scale (VAS). We found a significant difference between VAS scores at one-month, three-month, and six-month follow-up visits (p<0.05). From baseline, changes in VAS scores declined 2.62 points [95% CI: 0.83,4.40] at one month, 2.18 points [95% CI: 0.39,3.97] at three months, and 3.44 points [95% CI: 1.64,5.21] at six months.
Fig. 2.
Oswestry Disability Index (ODI). ODI results revealed a significant reduction from baseline in the subject's pain impact but also a reduction in the number of individuals reporting being crippled or bed-bound (p <0.05). Change in ODI pain impact declined 13.1 points [95% CI: 0.01,27.2] at one month from baseline, 16.5 points [95% CI: 2.5,30.6] at three months from baseline, and 21.1 points [95% CI: 7.0,35.2] six-months from baseline (A). Additionally, statistically significant improvement in ODI pain categories was also measured (p = 0.005) (B).
VAS
We found a significant difference between VAS scores at one-month, three months, and six months follow-up visits (p<0.05). From baseline, changes in VAS scores declined 2.62 cm [95% CI: 0.83,4.40] at one month, 2.18 cm [95% CI: 0.39,3.97] at three months, and 3.44 cm [95% CI: 1.64,5.21] at six months.
SF-36
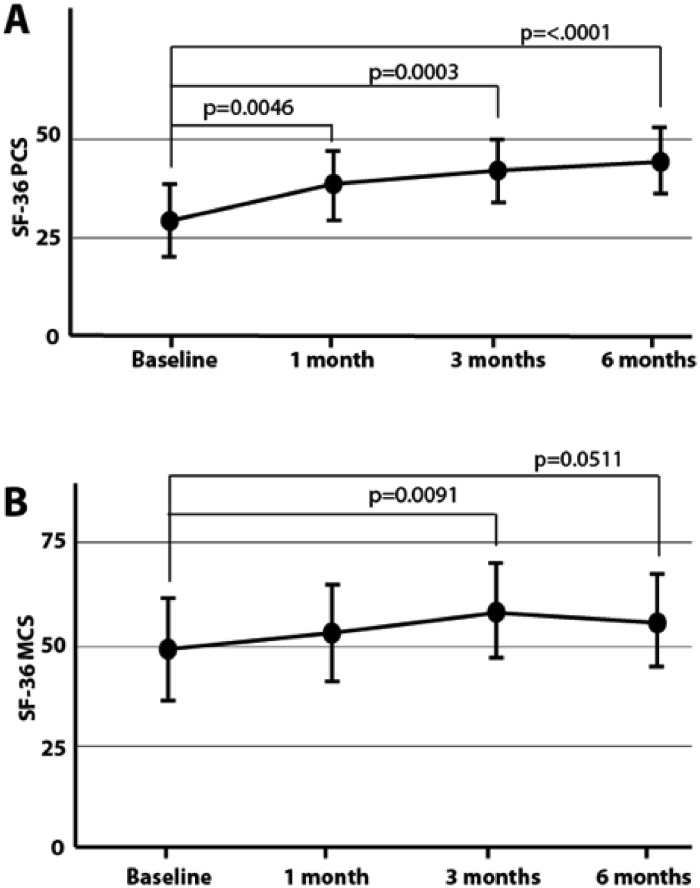
Change in physical functioning, as measured by the SF-36 PCS, improved by 9.9 [95% CI: 1.46,18.5] at one month from baseline, 13.1 [95% CI: 4.6,21.6] three months from baseline, and 16.4 [95% CI: 7.9,25.0] six-months from baseline. A similar trend is found for the SF-36 MCS but is not as pronounced, with significance only at 3 months (p = 0.091). From baseline, SF-36 MCS improved by 8.9 [95% CI: 1.6,16.3] at three months and 6.6 [95% CI: 0.7,13.9] at six months (Fig. 4).
Fig. 4.
SF-36. SF-36 PCS improved by 10.1 points [95% CI: 1.46,18.5] at one month from baseline, 13.1 points [95% CI: 4.6,21.6] three months from baseline, and 16.4 points [95% CI: 7.9,25.0] six-months from baseline (A). A similar trend is found for SF-36 MCS but is not as pronounced. From baseline, SF-36 MCS improved by 8.9 [95% CI: 1.6,16.3] at three months and 6.6 [95% CI: 0.7,13.9] at six months (B).
Discussion
Our results indicate that BVNA is a durable, minimally invasive treatment for the relief of CLBP that can be successfully implemented in a community practice setting.
Defining the appropriate minimal clinically important difference (MCID) for the various outcomes we collected in this study (ODI, VAS and SF-36) is critical since a statistically significant difference might not always be clinically relevant. There are a range of MCIDs that have been used depending on the type of procedure performed. For example, a VAS difference of 3 cm has been considered MCID in the context of pain management in an emergency setting [26], while in the context of BVNA, the recent ASPN guidelines refer to an MCID of 2 cm for the VAS [27]. Identifying a single agreed-upon MCID score for the ODI is a known difficult task [28]. Some studies have used MCID values with a very large range from 7 to 51 points for patients with spinal conditions [29]. In the context of BVNA, a 15 point MCID for the ODI has been used in previous studies using the INTRACEPT® device [30]. This MCID was based on a study from Copay et al. 2008 [31] in which the MCID for ODI was set at 12.8 in the context of lumbar surgeries. The improvements we observed in our study at 6 months compared to baseline were well-above the aforementioned MCIDs of 15 points for the ODI and 2 cm for the VAS. In the Copay et al. article, a few referenced studies support reporting data as mean changes and considering a change of one-half standard deviation as clinically meaningful [31]. In our study, all statistically significant mean changes from baseline are larger than one-half standard deviation (which is 5.0). If we use Copay's suggested MCID of 4.9, which is about one-half standard deviation improvement, our SF-36-PCS data also exceeds this threshold at all timepoints.
As mentioned in the results section, the average age of the patient population in this study was 73.3. This is significantly older than the average patient age of previous studies on the INTRACEPT® procedure. The average age in the SMART and INTRACEPT® trials was indeed 47 and 50, respectively [25,32]. Despite their older age, possible additional comorbidities, and likely multifactorial sources of pain, our patients demonstrated a21.1 ODI points decrease compared to 20.8 points decrease in the SMART trial at 6 months. The VAS improvement in our study was strong (3.44 cm reduction vs. 2.99 cm in the SMART trial), see Schnapp et al. 2022 for a detailed scoping review of the previously published BVNA studies [20]. Commercial insurance products are not yet widely covering this procedure. For this reason, our real-world study cohort was skewed to a much older Medicare-aged population.
About 25% of the patients did not show ODI improvements at 6 months (Table 4). Interestingly, these are all patients that would have been excluded from past studies, such as the INTRACEPT® trial, due to their low baseline ODI, the presence of severe stenosis, or the large number of affected levels. Among these 4 non-responders, patient #13 had severe spinal stenosis at 3 levels and was not a candidate for open decompression. It is, however, important to note that 5 other patients with severe foraminal or central stenosis did show improvements at 6 months (Table 2). Patient #15 had degenerative endplates at 5 levels, but only the L3-L5 levels were treated. These results emphasize that more research to define the right inclusion and exclusion criteria is critical to patient outcomes. For example, the mere presence of stenosis appears to be insufficient as an exclusion criteria. Several recent studies have also looked further into the INTRACEPT® trial data in an attempt to anticipate outcomes based on patient demographics, clinical characteristics, pain location, and exacerbating activities [33]. Boody, et al. suggested in their study that a low baseline ODI could be a predictor for non-favorable outcomes [33]. Notably, 3 of the 4 patients that showed no ODI improvements at 6 months had among the lowest baselines of our study (28%, see Table 4). Among the 16 patients included in our study, 5 had a baseline ODI <30 and only 2 of these showed improvements at 6 months.
Table 4.
Summary of the 4 patients that did not have ODI improvements after the BVNA procedure at 6 months.
| Patient # | Baseline ODI | 1 month ODI | 3 months ODI | 6 months ODI | Possible explanations for poor ODI outcomes |
|---|---|---|---|---|---|
| 10 | 28% | 38% | 18% | 32% | Low baseline ODI. |
| 13 | 28% | 40% | 42% | 40% | Low baseline ODI. Severe central and foraminal stenosis at 3 levels. |
| 15 | 46% | 68% | 48% | 60% | Degenerative endplates from L1-L5, but only L3-L5 levels were treated with BVNA. |
| 16 | 28% | 6% | 18% | 32% | Low baseline ODI. |
Our study has several limitations in that it is a small-scale study following only 16 patients, with no controls as has been done in the past in much larger studies [32]. In keeping with our real-world setting, therapeutic procedures were not specifically withheld post-BVNA. There were 4 patients who received such therapeutic procedures as outlined in Table 2. Patient #5 received a medial branch radiofrequency ablation and steroid injections during the 6 months follow-up, but only after ODI had already dropped to a minimal level (22% at 3 months). One of the patients who did not benefit from BVNA (patient #15) also received medial branch radiofrequency ablation (RFA) and steroid injections during the 6 months follow-up, which did not result in any further ODI improvements. These 2 procedures are therefore unlikely to have had any significant impact on the study. Two other patients had epidural injections to treat leg pain (patients #12 and #13, Table 2). Patient #12 had pre-existing radicular symptoms prior to BVNA. Although patient #12 had significant improvement of axial lumbar pain, the pre-existing left radicular symptoms remained unchanged. It is for this reason that the patient elected to resume lumbar epidural injections 5 months after BVNA. Patient #13 was a non-responder to BVNA (Table 4). This patient had been previously treated with lumbar epidural injections with modest improvement and elected to resume lumbar epidural injections when no response to BVNA was noted. Unfortunately, the patient did not respond to subsequent epidural injections either.
Our study demonstrates the feasibility and benefits of the BVNA procedure when performed in a community practice setting, and emphasizes the need to further study the best inclusion and exclusion criteria. To our knowledge, this is the first independently funded US study on BVNA and the first independently funded study on the INTRACEPT® procedure, which is the only FDA-cleared BVNA device.
Declarations of Competing Interests
“The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.” This work was internally funded by Neuroscience Associates, Key West, FL. The institution has no interest or role in the design, writing, collection, or analysis of the data presented. There was no external funding in the preparation of this manuscript.
Acknowledgments
Short summary sentence
This work describes the 6-month results of an independent case series for the efficacy and safety of basivertebral nerve ablation as a treatment modality for chronic low back pain in a community practice setting.
Acknowledgements
We wish to thank Dr. Patrick C. Hardigan, Ph.D. (Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Davie, Florida) for his expert contribution to the statistical analysis performed.
Footnotes
FDA device/drug status: Not applicable.Author disclosures: WS: Nothing to disclose. KM: Nothing to disclose. GJ-RD: Nothing to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2023.100201.
Appendix. Supplementary materials
References
- 1.Chen S., Chen M., Wu X., et al. Global, regional and national burden of low back pain 1990-2019: a systematic analysis of the Global Burden of Disease study 2019. J Orthop Transl. 2022;32:49–58. doi: 10.1016/j.jot.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dieën J.H., Weinans H., Toussaint H.M. Fractures of the lumbar vertebral endplate in the etiology of low back pain: a hypothesis on the causative role of spinal compression in aspecific low back pain. Med Hypotheses. 1999;53(3):246–252. doi: 10.1054/mehy.1998.0754. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y.G., Guo T.M., Guo X., Wu S.X. Clinical diagnosis for discogenic low back pain. Int J Biol Sci. 2009;5(7):647–658. doi: 10.7150/ijbs.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galbusera F., van Rijsbergen M., Ito K., Huyghe J.M., Brayda-Bruno M., Wilke H.J. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur Spine J. 2014;23(Suppl 3):S324–S332. doi: 10.1007/s00586-014-3203-4. [DOI] [PubMed] [Google Scholar]
- 5.Lotz J.C., Fields A.J., Liebenberg E.C. The role of the vertebral end plate in low back pain. Global Spine J. 2013;3(3):153–164. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braithwaite I., White J., Saifuddin A., Renton P., Taylor B.A. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7(5):363–368. doi: 10.1007/s005860050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herlin C., Kjaer P., Espeland A., et al. Modic changes-Their associations with low back pain and activity limitation: a systematic literature review and meta-analysis. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson K.J., Dagher A.P., Eckel T.S., Clark M., Reinig J.W. Modic changes on MR images as studied with provocative diskography: clinical relevance–a retrospective study of 2457 disks. Radiology. 2009;250(3):849–855. doi: 10.1148/radiol.2503080474. [DOI] [PubMed] [Google Scholar]
- 9.Weishaupt D., Zanetti M., Hodler J., et al. Painful lumbar disk derangement: relevance of endplate abnormalities at MR imaging. Radiology. 2001;218(2):420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- 10.Fritzell P., Hägg O., Wessberg P., Nordwall A. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976) 2001;26(23):2521–2532. doi: 10.1097/00007632-200112010-00002. discussion 32–4. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen J., Karppinen J., Niinimäki J., et al. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet Disord. 2015;16:98. doi: 10.1186/s12891-015-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schistad E.I., Espeland A., Rygh L.J., Røe C., Gjerstad J. The association between Modic changes and pain during 1-year follow-up in patients with lumbar radicular pain. Skeletal Radiol. 2014;43(9):1271–1279. doi: 10.1007/s00256-014-1928-0. [DOI] [PubMed] [Google Scholar]
- 13.Jensen T.S., Karppinen J., Sorensen J.S., Niinimäki J., Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17(11):1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudli S., Fields A.J., Samartzis D., Karppinen J., Lotz J.C. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–3734. doi: 10.1007/s00586-016-4459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudli S., Sing D.C., Hu S.S., et al. ISSLS prize in basic science 2017: intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J. 2017;26(5):1362–1373. doi: 10.1007/s00586-017-4955-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey J.F., Liebenberg E., Degmetich S., Lotz J.C. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat. 2011;218(3):263–270. doi: 10.1111/j.1469-7580.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorio M., Clerk-Lamalice O., Beall D.P., Julien T. International society for the advancement of spine surgery guideline-intraosseous ablation of the basivertebral nerve for the relief of chronic low back pain. Int J Spine Surg. 2020;14(1):18–25. doi: 10.14444/7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tieppo Francio V., Sayed D. StatPearls; Treasure Island (FL): 2022. Basivertebral Nerve Ablation. [PubMed] [Google Scholar]
- 19.Tieppo Francio V., Sherwood D., Twohey E., et al. Developments in minimally invasive surgical options for vertebral pain: basivertebral nerve ablation - a narrative review. J Pain Res. 2021;14:1887–1907. doi: 10.2147/JPR.S287275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnapp W., Martiatu K., Delcroix G.J. Basivertebral nerve ablation for the treatment of chronic low back pain: a scoping review of the literature. Pain Physician. 2022;25(4):E551–EE62. [PubMed] [Google Scholar]
- 21.De Vivo A.E., D'Agostino G., D'Anna G., et al. Intra-osseous basivertebral nerve radiofrequency ablation (BVA) for the treatment of vertebrogenic chronic low back pain. Neuroradiology. 2021;63(5):809–815. doi: 10.1007/s00234-020-02577-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.S., Adsul N., Yudoyono F., et al. Transforaminal epiduroscopic basivertebral nerve laser ablation for chronic low back pain associated with Modic changes: a preliminary open-label study. Pain Res Manag. 2018;2018 doi: 10.1155/2018/6857983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishchenko I.V., Garmish A.R., Kravchuk L.D., Saponenko A.I. Radiofrequency ablation of the basivertebral nerve in the treatment of chronic low back pain: analysis of a small clinical series. Hirurgia Pozvonochnika. 2021;18(3):61–67. [Google Scholar]
- 24.Conger A., Burnham T.R., Clark T., Teramoto M., McCormick Z.L. The effectiveness of intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebrogenic low back pain: an updated systematic review with single-arm meta-analysis. Pain Med. 2022;23(Suppl 2):S50–S62. doi: 10.1093/pm/pnac070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischgrund J.S., Rhyne A., Franke J., et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2018;27(5):1146–1156. doi: 10.1007/s00586-018-5496-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.S., Hobden E., Stiell I.G., Wells G.A. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. 2003;10(10):1128–1130. doi: 10.1111/j.1553-2712.2003.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 27.Sayed D., Naidu R.K., Patel K.V., et al. Best practice guidelines on the diagnosis and treatment of vertebrogenic pain with basivertebral nerve ablation from the american society of pain and neuroscience. J Pain Res. 2022;15:2801–2819. doi: 10.2147/JPR.S378544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwind J., Learman K., O'Halloran B., Showalter C., Cook C. Different minimally important clinical difference (MCID) scores lead to different clinical prediction rules for the Oswestry disability index for the same sample of patients. J Man Manip Ther. 2013;21(2):71–78. doi: 10.1179/2042618613Y.0000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung M, Saltzman CL, Kendall R, et al. What Are the MCIDs for PROMIS, NDI, and ODI Instruments Among Patients With Spinal Conditions? Clin Orthop Relat Res. 2018;476(10):2027–2036. doi: 10.1097/CORR.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koreckij T., Kreiner S., Khalil J.G., et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-Month treatment arm results. N Am Spine Soc J. 2021;8 doi: 10.1016/j.xnsj.2021.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copay A.G., Glassman S.D., Subach B.R., Berven S., Schuler T.C., Carreon L.Y. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Khalil J.G., Smuck M., Koreckij T., et al. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019;19(10):1620–1632. doi: 10.1016/j.spinee.2019.05.598. [DOI] [PubMed] [Google Scholar]
- 33.Boody B.S., Sperry B.P., Harper K., Macadaeg K., McCormick Z.L. The relationship between patient demographic and clinical characteristics and successful treatment outcomes after basivertebral nerve radiofrequency ablation: a pooled cohort study of three prospective clinical trials. Pain Med. 2022;23(Suppl 2):S2–S13. doi: 10.1093/pm/pnac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.