Abstract
Salinity is one of the causes that limit crop production. Plant Growth Promoting Rhizobacteria (PGPR) are beneficial soil bacteria that play a significant role in promoting plant growth. These microorganisms can produce their effect through the emission of Volatile Organic Compounds (VOCs). Most of the research to study the effects of microbial VOCs on plant growth has been carried out under controlled conditions using partitioned Petri dishes. In this article, we describe an alternative method that has the advantage of allowing long-term trials, being able to let the plant have a greater development in growth and height, without space limitation. In the proposed method, M. piperita were planted in glass jars containing Murashige and Skoog solid media, with a small glass vial containing Hoagland media inserted into the jar. This small vial was inoculated with the specified bacterium and served as the source of bacterial volatiles. This way plants were exposed to mVOCs without having any physical contact with the rhizobacteria.
-
•
The procedure allows studying the effect of microbial VOCs on plant growth.
-
•
It also allows longer trials, being able to let the plant develop more without space limitation.
Keywords: Salt stress, Microbial volatile organic compound, mVOCs, Plant-growth-promoting rhizobacteria, PGPR, Growth Parameters
Method name: Modeling the effect of microbial VOCs on aromatic and medicinal plants grown under saline stress
Graphical abstract
Specifications table
| Subject area: | Agricultural and Biological Sciences |
| More specific subject area: | Modeling the effect of mVOCs on plant growth under salt stress |
| Name of new method: | Modeling the effect of microbial VOCs on aromatic and medicinal plants grown under saline stress |
| Name and reference of original method: | Cappellari, L.R., Chiappero, J., Palermo, T.B., Giordano, W. and Banchio, E. (2020). “Volatile organic compounds from rhizobacteria increase the biosynthesis of secondary metabolites and improve the antioxidant status in Mentha piperita L. grown under salt stress” Agronomy 10 (8), 1094. DOI: https://doi.org/10.3390/agronomy10081094. |
| Resource availability: | N/A |
Method details
Bacterial cultures
Bacillus amyloliquefaciens GB03 (originally described as Bacillus subtilis GB03) [1] strain was grown on LB (Luria-Bertani) medium [2] (Table 1) for routine use and maintained in nutrient broth with 15% glycerol at −80 °C for storage. The bacterial culture was grown overnight at 30 °C and centrifuged at 120 rpm, washed twice in 0.9% NaCl by Eppendorf centrifugation (4300 g, 10 min, 4 °C), re-suspended in sterile water, and adjusted to a final concentration of ∼109 CFU/mL for use as an inoculum.
Table 1.
Medium Luria Bertani (LB) [2].
| NaCl | 5 g |
|---|---|
| Yeast extract | 5 g |
| Triptein | 10 g |
| Distilled water | 1000mL |
Plant micropropagation
Young shoots from Mentha piperita were surface-disinfected by being soaked for 1 min in 17% sodium hypochlorite solution and rinsed 3 x in sterile distilled water. Disinfected shoots were cultured in 100 mL Murashige and Skoog culture medium (MS) [3] (Table 2) containing 0.7%(w/v) agar and 3%(w/v) sucrose. pH adjusted to 5.6–5.8 using concentrated HCl (1 M).
Table 2.
Murashige and Skoog medium [3].
| Macronutrients |
Micronutrients |
||
|---|---|---|---|
| NH4NO3 | 1650 mg | H3BO3 | 6,2 mg |
| KNO3 | 1900 mg | MnSO4 x 4H2O | 15,17 mg |
| MgSO4 x 7H2O | 370 mg | ZnSO4 x 4H2O | 6,98 mg |
| KH2PO4 | 170 mg | Na2MoO4 x 2H2O | 0,25 mg |
| CaCl2 x 2H2O | 440 mg | CuSO4 x H2O | 0,016 mg |
| Na2EDTA | 37,3 mg | CoCl2 x 6H2O | 0,025 mg |
| FeSO4 x 7H2O | 27,8 mg | IK | 0,83 mg |
| Myo-inositol | 100 mg | Hormones | |
| Thiamine HCl | 0,1 mg | Naphthalene Acetic Acid | 0,025 mg |
| Pyridoxine HCl | 0,5 mg | Indolebutyric acid | 0.66 mg |
| Nicotinic Acid | 0,5 mg | ||
| Biotin | 0,01 mg | ||
| Other Compounds | |||
| Glycine | 2 mg | ||
| Sucrose | 30 g | ||
| Agar | 7 g | ||
| Distilled water | 1000mL | ||
After 30 days, apical meristems with foliar primordia, not showing contamination, were aseptically removed from terminal buds of shoots obtained in the previous step. Explants were cultured in test tubes, in 40 mL MS medium with 0.66 mg/L indolebutyric acid (IBA) and 0.025 mg/L naphthalene acetic acid (NAA) [4].
Plantlets obtained from tips were multiplied by single node culture and MS medium prior to autoclaving (20 min, 121 °C). Explants were placed in a growth chamber with controlled conditions of light (16/8-h light/dark cycle), temperature (22 ± 2 °C), and relative humidity (∼70%).
In vitro exposure to mVOCs
Glass jars (250 mL) covered with aluminum foil, containing a clear glass vial with flat bottom (10 mL) inside were sterilized in a stove for 2 cycles of 2 h at 160 °C. Hoagland (Table 3) [5] and Murashige and Skoog (MS) solid media were melted and kept at 25 °C in a thermostatic water bath. Subsequently, in a laminar flow hood, 3 mL of Hoagland medium supplemented with 0.7% (w/v) agar, 3% (w/v) sucrose and NaCl 0, 75 or 100 mM were introduced into the small glass vial with an automatic pipette (1000–5000 µL). Inside the glass jar, 50 mL of Murashige and Skoog medium supplemented with 0.7% (w/v) agar, 3% (w/v) sucrose and NaCl 0, 75 or 100 mM were introduced. The media was allowed to solidify. Subsequently, always working in a laminar flow hood, one node from aseptically cultured M. piperita plantlet were planted on each jar containing solid MS medium. The small vial was inoculated with 50 µL of a bacterial suspension (DO=1) (5 × 107 CFU) [6], [7]. Controls were inoculated with 50 µL of sterile distilled water. Plants were exposed to mVOCs without having any physical contact with the rhizobacteria (Fig. 1).
Table 3.
Hoagland's medium [5].
| CaCl2 1M | 1 mL | Micronutrients | |
|---|---|---|---|
| KCl 1M | 1 mL | H3BO3 | 283 mg |
| MgSO4 1M | 400 µL | MnCl2 | 181 mg |
| KH2PO4 1M | 200 µL | ZnCl2 | 11 mg |
| FeCl3 1M | 200 µL | CuSO4 | 5 mg |
| KNO3 1M | 500 µL | Na2SO4 | 2.5 mg |
| Micronutrients | 200 µL | Distilled water | 100 mL |
| Distilled water | 1000 mL |
Fig. 1.

M. piperita were planted and grown in sterilized glass jars containing MS solid media, with a small glass vial containing Hoagland media inserted into each 250 mL jar. This small vial was inoculated with the specified bacterium.
The glass jars containing plants and bacteria were covered with aluminum foil, sealed with parafilm to avoid contamination, and placed in a growth chamber under controlled conditions of light (16-h/8-h light/dark cycle), temperature (22 ± 2 °C), and relative humidity (∼ 70%). After 30 days, all plants were collected.
Salt stress was generated by the addition of NaCl. MS media (plant growth media) and Hoagland media (bacterial growth media) were supplemented with different salt concentrations: 0, 75, and 100 mM NaCl. For each experimental set, both the plant and bacteria were grown under the same concentration of NaCl but without contact with each other.
Treatments
Salt level concentrations were selected based on previous observations: at lower concentrations (25 and 50 mM), plant growth was not affected, and at higher levels (125 and 150 mM), the rooting capacity decreased significantly.
-
(1)
Control (Not subjected to NaCl, Non-exposed to microbial VOCs).
-
(2)
Subjected to NaCl 75 mM, Non-exposed to microbial VOCs.
-
(3)
Subjected to NaCl 100 mM, Non-exposed to microbial VOCs.
-
(4)
Not subjected to NaCl, Exposure to microbial VOCs.
-
(5)
Subjected to NaCl 75 mM + Exposure to microbial mVOCs.
-
(6)
Subjected to NaCl 100 mM + Exposure to microbial mVOCs.
Plant harvest
The whole plant was carefully removed from its jar, roots were washed to remove the agar, and the following parameters were recorded: shoot length, number of leaves, number of nodes, shoot fresh weight, and root dry weight. Harvested material was frozen in liquid nitrogen and stored at –80 °C until further processing.
Determination of TPC
Total water-soluble phenolic content was determined using the Folin-Ciocalteu colorimetric reagent [8]. The following steps were performed: the tissue (200 mg) was homogenized in a mortar with 5 ml distilled water (DW), and the homogenate was transferred to a test tube and incubated for 24 h in the dark (test tube were covered with aluminum foil). Next day, carefully 0.5 ml of the supernatant plant extract were transferred to a test tube and incorporated 8 ml DW and 0.5 ml of Folin–Ciocalteu reagent. After 5 min, were added 1 ml of Na2CO3 (20%p/v) solution, and after 1 h, the TPC was determined by colorimetry at a wavelength of 760 nm. Finally, the calibration function to estimate the TPC values was used, expressed in terms of mg gallic acid equivalent per g plant fresh weight (FW).
To determine the TPC, a calibration curve was produced as follows: different volumes from a standard solution of gallic acid (1 mg/ml) was taken, and completed with a corresponding amount of DW, after which, the optical density (OD) at a wavelength of 760 nm was determined. the data was ploted and analyzed by regression statistics, using supplied software packages such as Excel.
Determination of the lipid peroxidation index by quantifying MDA
Lipid peroxidation refers to the oxidative degradation of lipids, which is the process by which free radicals capture electrons from the lipids in cell membranes, resulting in cell damage, with this process proceeding by a free radical chain reaction mechanism. The MDA content was measured following the method of Heath and Packer [9] with some modifications: 50 mg of plant tissue was homogenized in a mortar with 500 µl of 20% (v/v) trichloroacetic acid (TCA). Then, an equal volume of 0.5% thiobarbituric acid (TBA) (0.5 g TBA in 100 mL TCA) was added. The homogenate was incubated at 95 °C in a thermostatic bath for 20 min under a solvent extraction hood, and then was place the samples on ice to stop the reaction. Subsequently, was centrifuged at 15,000 rpm for 15 min at 4 °C, and was record the absorbance of the supernatant at 532 and at 600 nm. The amount of MDA was determined by its molar extinction coefficient (155 mM−1 cm−1) after subtracting the non-specific absorbance at 600 nm from that at 532 nm, with the result expressed as µmol MDA/g FW (grams of fresh weight).
Antioxidant activity against DPPH•
The capacity of radical scavenging in extracts against stable DPPH• (2,2-diphenyl-1-picrylhydrazyl) was determined by the Brand-Williams et al. method [10] with minor modifications. The solution was obtained using 200 mg of frozen aerial parts homogenized in 1 mL of methanol 50%(V/V) which was then incubated in ice for 90 min (vortexed every 30 min) before being centrifuged for 30 min at 15.000 rpm at 4 °C. The supernatant was reserved and diluted in methanol (1:10; V/V), after which, DPPH• 60 µM was added. The extracts were retained in the dark for 30 min at 4 °C, and the absorbance at 515 nm was measured using an UV–visible spectrophotometer. A calibration curve was performed using ascorbic acid. The scavenging capacity of the plant extracts was expressed as mM ascorbic acid equivalents (AAE) per g fresh weight (mM AEE/ g FW).
Method validation
Salinity is an abiotic stress that restricts crop growth due to osmotic and ionic stress [11]. As salinity affects many aspects of the physiology and metabolism of the plants, the presence of soluble salts has a negative consequence for the plant's growth by decreasing the water potential and thus restricting the absorption of water by the roots (osmotic effect). In addition, the absorption of specific saline ions leads to their accumulation in tissues in concentrations at which they can become toxic and induce physiological disorders (ionic toxicity) in the plant, with high concentrations of saline ions being able to modify the absorption of essential nutrients and leading to nutritional imbalances (nutritional effect) [12]. These effects are reflected by a decrease in germination, vegetative growth, and reproductive development [13].
Salinity produces an accumulation of reactive oxygen species (ROS) [12], which can lead to a deterioration of photosynthetic pigments, lipid peroxidation, alterations in selective permeability of cell membranes, protein denaturation, and DNA mutations [14]. Damage to the cell membrane produces small hydrocarbons such as malondialdehyde (MDA), which is a sign of cell membrane damage. Plants have protection and repair systems that mitigate ROS damage. In addition, certain species have evolved protective mechanisms that include enzymatic and non-enzymatic components [15].
We have successfully applied this method to study whether mVOCs emitted by Plant Growth Promoting Rhizobacteria (PGPR) have the ability to ameliorate the effects of salt stress in Mentha piperita plants. We subjected M. piperita plants to 3 levels of salt stress (0, 75, and 100 mM NaCl) and exposed them to VOCs emitted by Bacillus amyloliquefaciens GB03, producing the following treatments: (1) Control: 0 NaCl, no exposure to mVOCs; (2) subjected to 75 mM NaCl, without exposure to mVOCs; (3) subjected to 100 mM NaCl, without exposure to mVOCs; (4) plants exposed to Bacillus amyloliquefaciens GB03 VOCs; (5), subjected to 75 mM NaCl + exposure to Bacillus amyloliquefaciens GB03 VOCs or (6) subjected to 100 mM NaCl + exposure to Bacillus amyloliquefaciens GB03 VOCs. These experiments were carried out under sterile conditions as previously described and replicated three times (10 pots per treatment).
The ability of the VOCs emitted by GB03 to mitigate the effects produced by saline stress on plant growth could be evidenced in growth parameters such as stem fresh weight, stem length and root dry weight [6]. (Table 4, Fig. 2,3).
Table 4.
The effect of mVOCs emitted by plant-growth promoting bacteria Bacillus amyloliquefaciens GB03 on shoot fresh weight, shoot length, and root dry weight in M. piperita plants. Means followed by the same letter within a column are not significantly different according to Fisher's LSD test (p < 0.05).
| Treatment | Shoot fresh weight (mg) | Shoot length (cm) | Root dry weight (mg) |
|---|---|---|---|
| 0 mM NaCl | |||
| Control | 224.94±10.94 b | 11.42±0.43 d | 2.55±0.38 a |
| mVOCs GB03 | 272.9 ± 11.14 c | 10.49±0.46 d | 7.33±1.44 c |
| 75 mM NaCl | |||
| Control | 171.23±9.09 a | 5.63±0.38 b | 3.88±0.46 b |
| mVOCs GB03 | 277.78±18.55 c | 6.99±0.32 c | 5.95±0.76 c |
| 100 mM NaCl | |||
| Control | 176.57±8.52 a | 4.00±0.31 a | 4.11±0.46 b |
| mVOCs GB03 | 245.39±23.2 bc | 5.97±0.50 bc | 5.89±0.69 c |
Fig. 2.
Effect of VOCs of B. amyloliquefaciens GB03 on M. piperita plants grown under saline stress conditions.
Fig. 3.
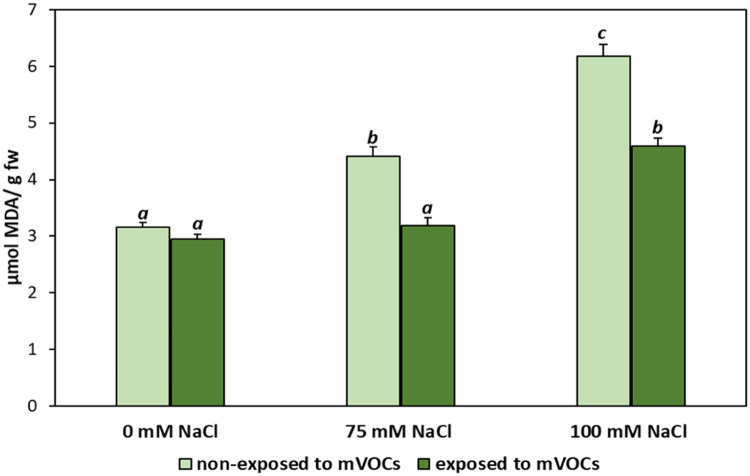
Malondialdehyde (MDA) content in Mentha piperita grown under salt stress media (0, 75, and 100 mM NaCl) and exposed to B. amyloliquefaciens GB03 mVOCs emission (mean ± SE). Values followed by the same letter in a column are not significantly different according to Fisher's LSD test (p < 0.05).
The efficiency of the salt stress treatment was observed in the decrease in shoot fresh weight and shoot length being significantly decreased at both concentrations evaluated (p < 0.05) (Table 4; Fig. 2). The root dry weight was 50 and 60% higher in plants grown at 75 and 100 mM NaCl, respectively. Positive effects of GB03 mVOCs on M. piperita growth were observed. The different growth parameters in plants exposed to mVOCs showed an increase, with shoot fresh weight registering a rise of 20%, and root dry weight of 280% compared to the corresponding control plants (those not exposed to mVOCs; Fig. 3).
The shoot fresh weight in plants grown under 75 mM salt stress and exposed to mVOCs was 60% greater than in plants subjected to 75 mM NaCl but not exposed to mVOCs, with the same tendency observed in plants subjected to NaCl 100 mM (p<0.05). The same positive effect was observed for the shoot length in plants grown under salt stress and exposed to mVOCs (Table 4; Fig. 2). Root dry weight was also significantly increased by exposure to mVOCs when plants were subjected to both concentrations of salt stress conditions.
An increase in the ROS accumulation in plant leaves caused a rise in MDA, a product of membrane lipid peroxidation [16]. MDA content indicates the level of cell membrane damage, which is often applied to estimate plant tolerance to osmotic stress [17] (Fig. 3). The lipid peroxidation increased 1.4 and 2-fold in 75 and 100 mM NaCl treated plants, respectively, in relation to control plants. For plants treated with mVOCs and subjected to salt stress, the MDA content was approximately 25% lower than for plant stress and not treated with mVOCs.
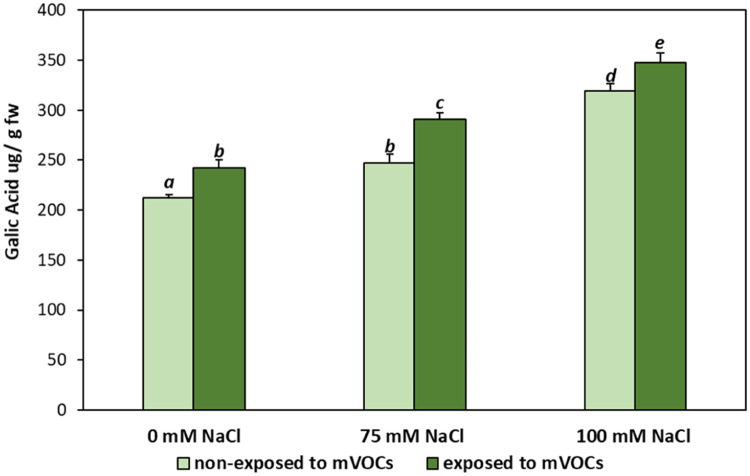
Phenolic compounds are antioxidants that may be required for scavenging ROS and protecting the lipid membrane from oxidative stress [12]. The level of Total Phenolic Content (TPC) in plants subjected to salt stress conditions increased with the severity of the NaCl concentration, both in plants exposed and not exposed to mVOCs. In plants grown under salt conditions (75 or 100 mM), the TPC levels rose by 15 and 50%, respectively, in relation to control plants (Fig. 4). In addition, the plants subjected to both concentrations of NaCl and treated with GB03 VOCs registered an increase in TPC compared to non-exposed plants (p < 0.05), but no statistically significant interaction effect was found (p > 0.05). The highest TPC concentrations were detected in plants treated with salt 100 mM and mVOCs.
Fig. 4.
Total phenolic content of Mentha piperita plants grown under salt stress media (0, 75, and 100 mM NaCl) and exposed to B. amyloliquefaciens GB03 mVOCs emission (mean ± SE). Values followed by the same letter in a column are not significantly different according to Fisher's LSD test (p < 0.05).
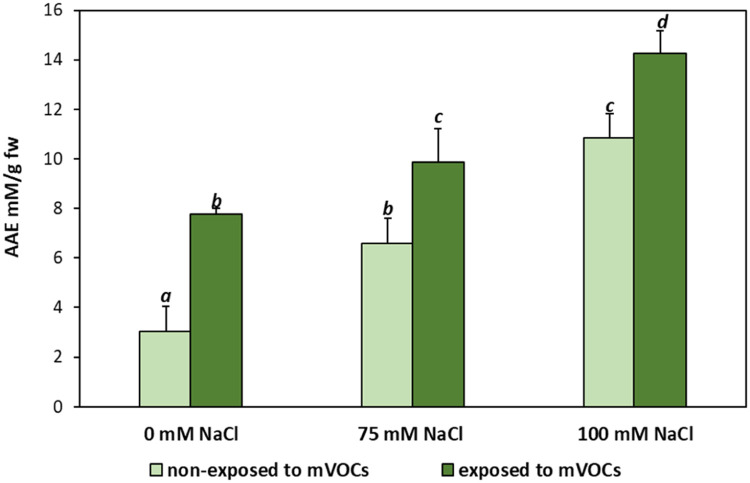
The accumulation of non-enzymatic antioxidants was stimulated by the salt stress (Fig. 5). Total antioxidant content is expressed as mM of ascorbic acid equivalents (AEE). The technique was carried out through the use of the free radical DPPH• (2,2-diphenyl-1-picrylhydrazyl) (Fig. 6). The highest levels of antioxidant activity were observed when plants were exposed to VOCs and grown under 100 mM NaCl conditions.
Fig. 5.
Antioxidant activity expressed as ascorbic acid equivalents (AAE) in Mentha piperita grown under salt stress media (0, 75, and 100 mM NaCl) and exposed to B. amyloliquefaciens GB03 mVOCs emission (mean ± SE). Values followed by the same letter in a column are not significantly different according to Fisher's LSD test (p < 0.05).
Funding
This work was supported by grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT).
CRediT authorship contribution statement
Lorena del Rosario Cappellari: Investigation, Validation, Data curation, Writing – original draft. Samanta Soledad Gil: Investigation, Visualization, Data curation. Tamara Belen Palermo: Data curation. Jimena Sofia Palermo: Visualization. Romina Meneguzzi: Data curation. Walter Giordano: Funding acquisition. Erika Banchio: Supervision, Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Maria Julia Banchio for editorial assistance.
Footnotes
Related research article
For a published article:
Cappellari, L.R., Chiappero, J., Palermo, T.B., Giordano, W. and Banchio, E. (2020). “Volatile organic compounds from rhizobacteria increase the biosynthesis of secondary metabolites and improve the antioxidant status in Mentha piperita L. grown under salt stress” Agronomy 10 (8), 1094. https://doi.org/10.3390/agronomy10081094.
Data availability
Data will be made available on request.
References
- 1.Choi S.K., Jeong H., Kloepper J.W., Ryu C.M. Genome sequence of Bacillus amyloliquefaciens GB03, an active ingredient of the first commercial biological control product. Genome Announc. 2014;2:01092–01098. doi: 10.1128/genomeA.01092-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luria S.E., Burrous J.W. Hybridization between Escherichia coli and Shigella. J. Bacteriol. 1955;74:461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murashige T., Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 4.Santoro M., Zygadlo J., Giordano W., Banchio E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita) Plant Physiol. Biochem. 2011;49:1077–1082. doi: 10.1016/j.plaphy.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. Univ. Calif. Coll. Agric. Exp. Sta. Circ. Berkeley. 1938:347–353. [Google Scholar]
- 6.Cappellari L.R., Banchio E. Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J. Plant Growth Regul. 2019;39:764–775. doi: 10.1007/s00344-019-10020-3. [DOI] [Google Scholar]
- 7.Cappellari L.R., Chiappero J., Palermo T.B., Giordano W., Banchio E. Volatile organic compounds from rhizobacteria increase the biosynthesis of secondary metabolites and improve the antioxidant status in Mentha piperita L. grown under salt stress. Agronomy. 2020;10(8):1094. doi: 10.3390/agronomy10081094. [DOI] [Google Scholar]
- 8.Singleton V.L., Rossi A.J. Colorimetry of total phenolics with phosphomolybdic-Phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 9.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;25:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 10.Brand-Williams W., Cuvelier M.E., Berset C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- 11.Negrão S., Schmöckel S.M., Tester M. Evaluating physiological responses of plants to salinity stress. Ann Bot. 2017;119:1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J.K. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu X.M., Zhang H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015;6:774. doi: 10.3389/fpls.2015.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 15.Khan N., Bano A., Ali S., Babar M.A. Crosstalk amongst phytohormones from plant and PGPR under biotic and abiotic stresses. Plant Growth Reg. 2020;90:189–203. doi: 10.1007/s10725-020-00571-x. [DOI] [Google Scholar]
- 16.Yazici I., Tuerkan I., Sekmen A.H., Demiral T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007;61:49–57. doi: 10.1016/j.envexpbot.2007.02.010. [DOI] [Google Scholar]
- 17.Miao B.H., Han X.G., Zhang W.H. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 2010;105:967–973. doi: 10.1093/aob/mcq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.