Hepatitis C (HCV) and hepatitis B (HBV) are two major blood-borne viral infections that affect patients with intravenous drug use (IDU). Blood and blood component transfusion and unsafe injection practices are major, whereas surgical procedure and dialysis are minor risk factors for the spread of HBV and HCV.1, 2
With the advent of universal immunization for HBV and regulated blood banking with the screening of blood products, IDU has become the most common risk factor for HCV transmission in Western countries. 3 IDU also emerged as the most crucial risk factor for transmission of HBV and HIV infections over the last few decades.4, 5 As per a 2017 estimation, approximately of 15.6 million people who inject drugs (PWID): 52.3% (95% CI = 42.4–62.1) were HCV antibody positive, 9% (5.1–13.2) were hepatitis B surface antigen (HBsAg) positive, and 17.8% (10.8–24.8) were living with HIV. 6 As per a national survey of 2019, India has an estimated number of around 850,000 PWID, of whom approximately one-third report sharing needles, syringes, or paraphernalia. 7 The pooled prevalence of HCV infection among patients with IDU was around 44.7% in India. 8 Based on a statistical model adopted by the World Health Organization (WHO), to eliminate the public burden of HCV, around 90% of the total HCV infections are to be diagnosed and about 80% of the diagnosed population needs to be treated. However, worldwide, only 20% of HCV patients are diagnosed and only 7% are treated. 9
Reasons for the Large Treatment Gap in the Co-Occurring Substance use Disorders and Hepatitis C
Various reasons exist for such a large treatment gap in the patients with HCV and comorbid substance use disorder (SUD). Those who can access treatment too face several challenges.
Poor Awareness
Poor awareness regarding HCV and its relationship with IDU, stigma (for SUD, IDU, and infection), and accessibility issues are some of the crucial barriers to the treatment of SUD and blood-borne viral infections.9, 10
Barrier from Primary Care Providers (PCPs)
PCPs may not be trained to assess and manage the SUD. Under India’s National Viral Hepatitis Control Program (NVHCP), PCPs are trained to provide a simplified algorithm- based treatment regimen for people living with HCV (PLHCV). 11 However, they have deficits in knowledge about the SUD, and PWID are unlikely to seek care at medical clinics because of perceived stigma, thus delaying referral and aggravating complications in both scenarios. In the community, patients with SUD, especially PWID, are seen as unreliable and manipulative. This can impair their treatment-seeking behavior as they often feel misled or not listened to by medical personnel. 12
Criminalization of SUD
Patients with SUD, especially PWID, face difficulty accessing medical care, especially when they are asymptomatic. Substance use is criminalized in many parts of the world, including India. Patients using substances are often dealt with in the criminal justice system and incarcerated for various reasons. 13 This usually affects the ability to expand access to care for SUD and physical ailments like viral infections. 12
Stringent Treatment-Inclusion Criteria
Historically, the eligibility criteria were extremely stringent for interferon-based treatment of HCV infection. Guidelines required abstinence from the use of illicit opioids and even opioid agonist treatment (OAT). This led to treatment denial, worsening of the clinical condition, and even death. 14 However, there has been a significant improvement in HCV treatment, and we have witnessed a paradigm shift from interferon-based treatment to direct-acting antivirals (DAA). Recent trials included patients on agonist treatment and treatment- naive PWID; the results showed sustained viral response with second and third generation DAAs among 98% of PWID. 15
The Consequences of the Treatment Gap
In SUD patients, untreated HCV infection or delay in its treatment leads to various adverse consequences. Firstly, there is a rapid progression of disease in this group, leading to the rapid development of fibrosis, cirrhosis, and hepatocellular carcinoma. As the patients get infected at an earlier age, there is a huge loss of productivity because of disability-adjusted life years.16 The patients with SUD often engage in high-risk behaviors such as sharing injection equipment, unprotected sexual intercourse with multiple partners, and men having sex with men during “chemsex” parties. These increase the chance of infection with other blood-borne viruses and put other people at risk. 17
What can be Done: Integrated Treatment Approach?
The WHO has fixed the target to eliminate viral hepatitis as a public health threat by 2030. We should wholeheartedly try to diagnose and treat viral hepatitis to reach the target by the next eight years. To reduce the treatment gap, we should increase the public awareness and availability of screening and improve the availability, accessibility, and acceptability of evidence-based treatments for both HCV and SUD. Integration of treatment for SUD and HCV can help increase treatment access for the substance-using population. 9
Various approaches are used to integrate HCV treatment with SUD care services. In most setups, HCV treatment is integrated at the SUD treatment center. The primary requirement is the coexistence of SUD treatment and facilities for screening, testing, counseling, and treatment of HCV, preferably. The existing medical personnel at the de-addiction clinics and medical units should be trained on both conditions to integrate the services effectively. The focus of an integrated care model includes logistic support for caregivers, information regarding the availability of testing services, counseling for viral hepatitis and HIV infection, and coordination of the pharmacies to dispense the correct regimen of DAAs and SUD treatment. Subsequently, the service should be initiated, and an enhanced service should be formulated to address the stigma, gain the leadership buy-in, and link to care for co-occurring conditions. The aim should be to build a recovery-oriented and patient-centered care system. 18
Integration at SUD Clinic
Here, the SUD treatment clinic provides screening facilities for various blood-borne infections. The specialists offer those with HCV infection further evaluation in-person or through teleconsultation and provide treatment if necessary. HCV seropositive patients on OAT are supplied with DAA.
A Norway-based trial assessed the feasibility of integrating HCV treatment with OAT or community care center (CCC) with the help of a multidisciplinary team. It was found that, although the treatment set up was resource-intensive (because of multidisciplinary team and infrastructure for assessment and treatment), the integrated treatment group had significantly earlier treatment initiation, better treatment coverage, and higher sustained virological response (SVR) compared to those referred to the standard care for HCV. 19 A USA-based exploratory study showed that telemedicine-based integration helps in treatment adherence to DAA in patients on methadone-based OAT. The reported SVR was around 94%. Although the setting appears feasible and the result encouraging, the small sample size may limit the generalizability, and there may be some potential limitations regarding allowance to prescribe DAA through teleconsultation or insurance reimbursement for teleprescription. 20
Integration with Harm Reduction Services
Some experimental setups tried to integrate the service at harm reduction centers. So, the treatment users are not essentially substance-free at the baseline and while initiating DAA. In one such USA-based study, a drop-in center was used to initiate DAA. Most of the patients achieved SVR, and the SVR was associated with treatment adherence but not with substance use or SUD treatment status. 21 This approach appears to be least restrictive and ideal for the difficult-to-reach SUD population. Still, human resources and infrastructure requirements make it challenging to implement.
Integration with Family Physician Clinic
A more decentralized approach has been tried in some setups. Here, a family physician provides screening and treatment for SUD and HCV in the clinic. This decentralization might help in easy access to treatment for both disorders. The family physician’s clinic might provide a less stigmatized environment and better treatment access. In a Swiss study, the integration of treatments for HCV and opioid use disorder (OUD) at the family physician clinic was compared with the one-stop HCV and OUD treatment at opioid substitution clinic. It was found that the necessary workup was incomplete in a considerable proportion of the treatment users at the family physician clinic, decentralized treatment program. 22 On the other hand, various programs have tried to integrate the medication dispensing part with a decentralized aspect of OUD treatment and found encouraging results in treatment adherence and viral suppression. 23
A recent systematic review discussed the effects of full decentralization and integration (testing and treatment at the same site), partial decentralization (testing at the same site and linkage to treatment services), integrated screening and treatment within harm-reduction services, and task shifting for HCV care to nonspecialists. Integrated treatment delivery for PWID was categorized as recruitment from OAT, harm reduction services, and primary care. The results revealed that among PWID, full and partial decentralization were better than no decentralization and integration in terms of detection and treatment uptake for HCV. However, full decentralization showed a better outcome than partial decentralization. The authors observed that SVR achieved by treatment from a nonspecialist did not differ from care by specialists. Although low-middle income countries (LMIC) were underrepresented in this review, the authors could find nine studies in PWID from LMIC. The results from LMIC and high-income countries did not differ. 24 Given that decentralized, integrated care for SUD and HCV is feasible, acceptable, and possibly effective in the LMIC settings, and the existing burden of PWID and HCV in India, we call for immediate action to present and test models of service delivery from an Indian setting. The clinical outcome (such as cure rates, de-addiction targets) and cost-effectiveness should be assessed to evaluate the success of this model. 25
Real-world data from the state of Punjab (India) of an integrated, decentralized, telemedicine supervised care for HCV in PWID showed that more than 90% of patients achieved SVR at 12 weeks, and there were no major adverse events. 26 In this program (“Punjab model”), treatment was delivered at the district hospital level, and additional linkage services were provided to treat OUD. This article discusses another model of integrated care at the addiction treatment services.
MultidisciplinaRy IntEgrated Care fOr CooccUrRing Substance use Disorder and HEpatitis C(RECOURSE) from an Indian Setting
The Ministry of Health and Family Welfare, Government of India, has been running a Drug De-addiction Program (DDAP) since 1988 to 1989. Drug De-addiction and Treatment Centre, Postgraduate Institute of Medical Education and Research, Chandigarh was one of the first six addiction treatment centers created under the DDAP. The center has been identified as a center of excellence by the Ministry and mandated to carry out clinical research, training, and advocacy at the national and regional levels. We have outpatient (OP), inpatient, and community-based treatment services. The OP setting has a multidisciplinary team—with psychiatrists, psychiatry trainees, social workers, nurses, specialized addiction counselors (psychologists), and pharmacists. The clinic has an on-site laboratory facility. A vast majority of the clients (>95%) are treated in the OP setting. The center caters to the population of the union territory of Chandigarh and six neighboring states of North India. According to the national survey, one of the neighboring states (Uttar Pradesh) topped the list of clients needing treatment (per one lakh or 0.1 million population) for alcohol, cannabis, and OUD. The immediate neighbors (Punjab and Haryana) were the next two states with regard to the treatment need for OUD. 7 Moreover, as the clinic is situated within a general hospital setting, clients with medical comorbidities are linked with appropriate medical services. Likewise, clients are also referred from other medico-surgical specialties. The liver clinic at our institution was the first nodal center for delivery of the unique hub and spoke model of care, with 25 peripheral sites providing locally available, telesupervised care to patients with HCV. 13
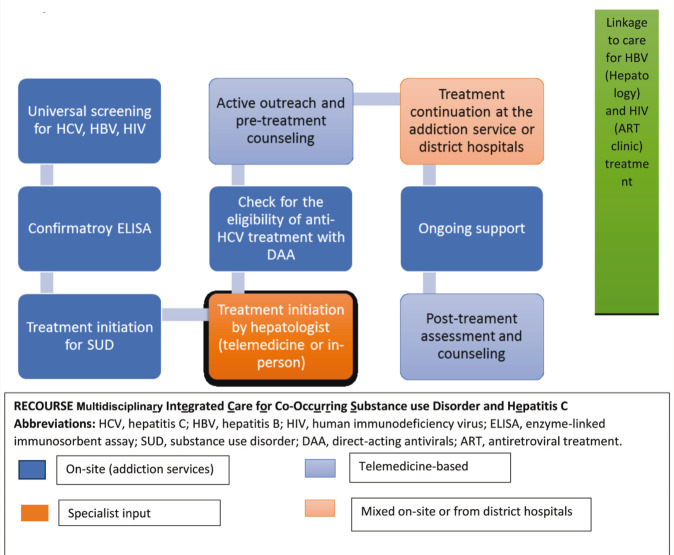
A previous study from our center showed that nearly a third (31%) of the PWID had HCV seropositive status. Among those with noninjection drug use (NIDU), the prevalence of seropositivity was 3.2%. 27 The global estimate of HCV seroprevalence was 2.8%. 28 It was not surprising that HCV seroprevalence was significantly higher in PWID, but higher seropositivity among NIDU is also noteworthy. To address the problem of co-occurring SUD and HCV, since June 2021, we have started a decentralized testing and integrated treatment program, RECOURSE. It has some unique features such as the provision of universal screening for all blood-borne viral infections, active outreach, hybrid care (in-person and telemedicine), coordination with a state-run HCV treatment program, and linkage to additional services for HBV and HIV. The objectives of RECOURSE are: (a) to test all patients with SUD for HCV seropositive status, (b) to maximize initiation of anti-HCV treatment in eligible patients, (c) to minimize anti-HCV treatment discontinuation, (d) initiation and continuation of treatment for SUD, and (e) capacity building of staff working at the addiction treatment services. The Program has the Following Components:
On-Site Universal Screening for Blood-Borne Viral Hepatitis and HIV
We approach all patients registered to the center for an initial immunochromatography-based screening for blood-borne viral hepatitis (HBV, HCV) and HIV at the on-site laboratory.
On-Site Sample Collection for Confirmatory Tests
We also collect blood samples for confirmatory enzyme-linked immunosorbent assay (ELISA) tests for HCV, HBV, and HIV and arrange to send these samples to the concerned laboratories on the same day.
On-Site Treatment for SUD
The multidisciplinary OP team starts standard SUD treatment. Patients with a history of injection drug use (and those with moderate to severe opioid dependence) are started on buprenorphine-based OAT.
Figure 1. Flow-Diagram for the RECOURSE Program.
Active Outreach and Pretreatment Counseling for HCV
The senior registrar (psychiatrist) communicates the ELISA-positive test results directly to the patients, either during in-person consultations or by telephone, within a week of registration to the center. We check the identity of the patients to minimize breaches in confidentiality while communicating the test results. We also counsel patients regarding the need for treatment and positive treatment outcomes, provide information and practical support for early treatment initiation, and address individual concerns.
Treatment Initiation
During the second in-person visit to the addiction treatment center, blood samples are drawn for HCV viral load, complete blood count, and liver function tests. These investigations are prerequisites for HCV treatment initiation. Patients meet the doctors from the hepatology department of our hospital for treatment initiation either in-person or by teleconsultation. All those who are domiciles of Punjab can directly access treatment from either the nodal center or from the nearest district hospitals under the NVHCP, which offers free-of-cost DAA treatment. Current drug use status does not influence the decision of treatment initiation. At this stage, with patients’ consent, we may engage the caregivers too in treatment.
Ongoing Support
We periodically monitor patients clinically and by laboratory investigations, liver function tests, and complete hemograms. We also check adherence to DAA. In case of nonadherence, the senior registrars explore reasons and manage as per the need (e.g., difficulty in fetching medications from the hepatology department is dealt with by dispensing medication from the addiction clinic).
Posttreatment Counseling and Outreach
After three months of treatment initiation, patients are contacted on the phone to check for treatment adherence and overall side effects and inform the need for SVR at three months after treatment completion.
Ongoing On-Site Training
We have an in-built ongoing capacity building for the senior registrars, pharmacists, and laboratory personnel. The experts from hepatology are accessible for teleconsultation as and when required.
Additional Linkage to Care
Patients with HBsAg-positive status are linked to the hepatology clinic. HIV seropositive patients are referred to the antiretroviral treatment (ART) clinic situated in a different block of the same hospital.
RECOURSE requires a coordinated multidisciplinary team of psychiatrists, hepatologists, nurses, laboratory personnel, and pharmacists. Patients’ data are updated in real-time in a Google Sheet that is shared only with the treating consultants and senior registrars. In the next phase, we would like to completely decentralize treatment initiation and dispensing for HCV. Linkage to hepatology services will be reserved only for the difficult-to-treat patients (e.g., those with an abnormal liver function test, who develop serious side effects on DAA, and with nonresponse to the standard DAA regime).
Conclusion
Integrating SUD and HCV treatment is the hour’s need to help realize the WHO’s objective to eliminate viral hepatitis as a public health problem by 2030. Successful integration would improve the health status of this difficult-to-reach group and help contain the spread of the infection in the community. The availability of relatively low-cost and safer DAA treatment for HCV and evidence-based SUD treatment services has made it possible to decentralize and integrate co-occurring SUD and HCV care even in the LMIC. The RECOURSE program described in this article is perhaps the first in India to lay out a model of integrated care for co-occurring SUD and HCV at an addiction treatment service. The model appears to be viable and acceptable. In the future, we will test and report the real-world effectiveness of our program.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Dhiman RK, Grover GS, and Premkumar M. Hepatitis C elimination: A public health perspective. Curr Treat Options Gastroenterol, 2019; 17: 367–377. [DOI] [PubMed] [Google Scholar]
- 2.Premkumar M and Kumar Chawla Y. Chronic hepatitis B: Challenges and successes in India. Clin Liver Dis (Hoboken), 2021; 18: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shire NJ and Sherman KE. Epidemiology of hepatitis C virus: A battle on new frontiers. Gastroenterol Clin North Am, 2015; 44: 699–716. [DOI] [PubMed] [Google Scholar]
- 4.Alavian SM. New globally faces of hepatitis B and C in the world. Gastroenterol Hepatol Bed Bench, 2011; 4: 171–174. [PMC free article] [PubMed] [Google Scholar]
- 5.Patel P, Borkowf CB, Brooks JT, et al. Estimating per-act HIV transmission risk: A systematic review. AIDS, 2014; 28: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob Health, 2017; 5: e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambekar A, Agrawal A, Rao R, et al. Magnitude of substance use in India. Ministry of Social Justice and Empowerment, Government of India, 2019. [Google Scholar]
- 8.Goel A, Seguy N, and Aggarwal R. Burden of hepatitis C virus infection in India: A systematic review and meta-analysis. J Gastroenterol Hepatol, 2019; 34: 321–329. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva, Switzerland: WHO Press; July 2018. [PubMed] [Google Scholar]
- 10.Tandon T and Collective L. Drug policy in India. IDPC briefing paper, February 2015.
- 11.McGowan CE and Fried MW. Barriers to hepatitis C treatment. Liver int, 2012; 32: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva, Switzerland: WHO Press; 2018. [PubMed] [Google Scholar]
- 13.Dhiman RK, Grover GS, Premkumar M, et al. Decentralized care with generic direct-acting antivirals in the management of chronic hepatitis C in a public health care setting. J Hepatol, 2019; 71: 1076–1085. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson J. Former addicts face barriers to treatment for HCV. JAMA, 2001; 285: 1003–1005. [DOI] [PubMed] [Google Scholar]
- 15.Messina V, Onorato L, Di Caprio G, et al. Directly acting antiviral-based treatment for HCV-infected persons who inject drugs: A multicenter real-life study. Life, 2021; 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DJ, Combellick J, Jordan AE, et al. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. Int J Drug Policy, 2015; 26: 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Midgard H, Weir A, Palmateer N, et al. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol, 2016; 65: S33–S45. [DOI] [PubMed] [Google Scholar]
- 18.A Guide to Integrating HCV Services into Opioid Treatment Programs. 1st ed, https://attcnetwork.org/sites/default/files/2020-07/Guide%20to%20Integrating%20HCV%20Services%20into%20Opioid%20Treatment%20-%20July%2024-1.pdf (2020. accessed November 10, 2021).
- 19.Fadnes LT, Aas CF, Vold JH, et al. Integrated treatment of hepatitis C virus infection among people who inject drugs: A multicenter randomized controlled trial (INTRO-HCV). PLoS Med, 2021; 18: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talal AH, Andrews P, Mcleod A, et al. Integrated, co-located, telemedicine-based treatment approaches for hepatitis C virus management in opioid use disorder patients on methadone. Clin Infect Dis, 2019; 69: 323–331. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis, 2020; 71: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bregenzer A, Conen A, Knuchel J, et al. Management of hepatitis C in decentralised versus centralised drug substitution programmes and minimally invasive point-of-care tests to close gaps in the HCV cascade. Swiss Med Wkly, 2017; 147: w14544. [DOI] [PubMed] [Google Scholar]
- 23.Lucas GM, Mullen BA, Weidle PJ, et al. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis, 2006; 42: 1628–1635. [DOI] [PubMed] [Google Scholar]
- 24.Oru E, Trickey A, Shirali R, et al. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: A global systematic review and meta-analysis. Lancet Glob Health, 2021; 9: e431–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chugh Y, Premkumar M, Grover GS, et al. Cost-effectiveness and budget impact analysis of facility-based screening and treatment of hepatitis C in Punjab state of India. BMJ Open, 2021; 11: e042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhiman RK, Grover GS, Premkumar M, et al. Outcomes of real-world integrated HCV microelimination for people who inject drugs: An expansion of the Punjab Model. E Clin Med, 2021; 41: 101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu D, Sharma AK, Gupta S, et al. Hepatitis C virus (HCV) infection & risk factors for HCV positivity in injecting & non-injecting drug users attending a de-addiction centre in northern India. Indian J Med Res, 2015; 142: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology, 2013; 57: 1333–1342. [DOI] [PubMed] [Google Scholar]