Dear Editor,
The high diagnostic potential of urinary extracellular vesicles (uEVs) for urogenital disease has been recognized for more than a decade. This is emphasized by the identification of different molecular biomarkers (i.e. protein, mRNA, miRNA, lipids and metabolites) in uEV preparations that may assist the clinical management of prostate, bladder, and renal cancer (Junker et al., 2016). uEV biomarkers for other pathologies like acute and chronic kidney disease of various etiologies, cystic and tubule‐interstitial disease, or for kidney transplantation are also under active investigation (Grange & Bussolati, 2022).
Apart from the growing need for validation studies, the translational potential of uEV biomarkers is hampered by several biological factors. Such factors include the diverse cellular origins of uEVs throughout the renal and urogenital tract, but also the dynamic molecular composition of urine due to hydration status, diet, salt regulation, exercise, and circadian rhythm. In addition to these inherent factors, the reproducibility of uEV analysis is also strongly influenced by logistic variables like the differences in the time of sampling or the preanalytical procedures for handling of urine samples (Erdbrügger et al., 2021).
The general reporting recommendations for EV sample processing and analysis are covered in detail in the Minimal Information for Studies of Extracellular Vesicles (MISEV 2018) position paper (Thery et al., 2018). However, a community consensus on best methodological practices that is tailored to the biofluid‐specific characteristics and requirements is of particular importance for the success of preclinical and clinical studies on biomarker discovery, validation and future use in clinical decision making. To address this need in uEVs research, the Urine Task Force of the Rigor and Standardization Subcommittee of the International Society for Extracellular Vesicles (ISEV) published a position paper summarizing the current state of the art and listing detailed recommendations for improved rigor, reproducibility and inter‐operability in uEV research (Erdbrügger et al., 2021).
To support the implementation of the published recommendations, and enhance their application in daily research practices, here we provide a Quick Reference Card on STORAGE of urinary EVs (Figures 1 and 2, Supplementary File 1). The Quick Reference Card does not substitute a uEVs protocol for storage, isolation or processing but it summarizes the expert community consensus recommendations on the most critical factors affecting storage of fresh or biobank urine and uEVs samples as discussed in the uEV position paper (Erdbrügger et al., 2021). The Card is organized according to six critical stages: Biobanking, Storage of urine prior to processing, Preprocessing, Storage of urinary supernatant and uEVs, Defrosting, and Transportation. Evidence level and reporting priority for each stage are color‐coded in accordance to the findings as described in the ISEV uEVs position paper (Erdbrügger et al., 2021) and according to the MISEV 2018 guidelines (Thery et al., 2018). The Card is intended as an easily accessible guideline tool that can be used during study planning and manuscript preparation, but also as a “bench top” reference during everyday laboratory work.
FIGURE 1.
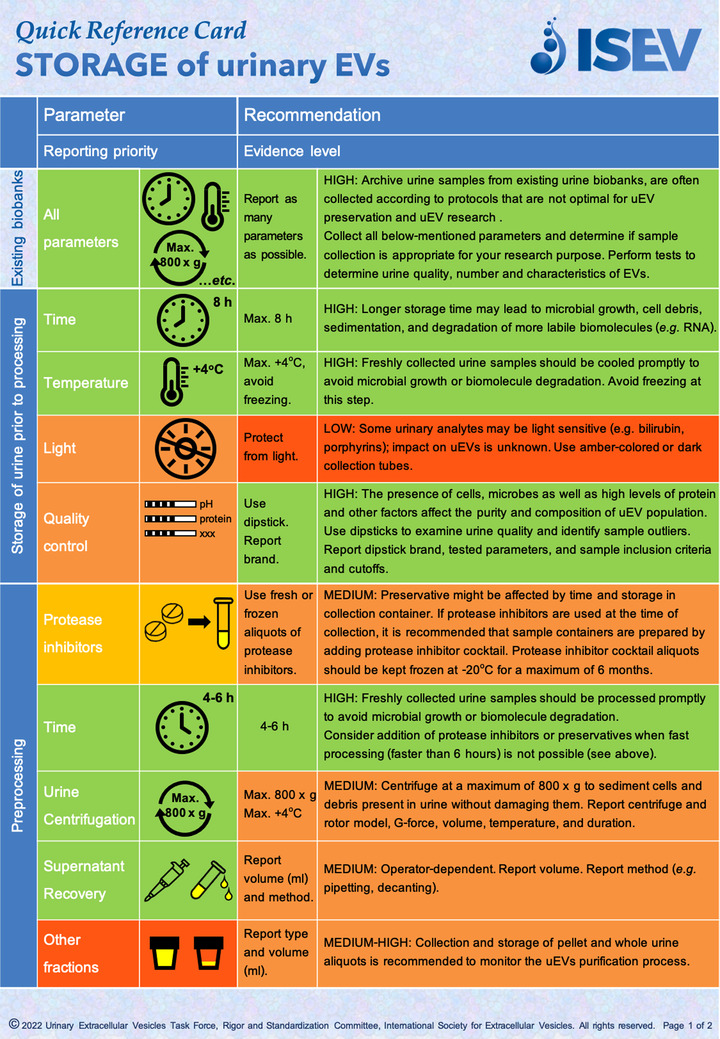
Quick Reference Card “Storage of urinary EVs”, page 1 Storage of urine prior to processing and Pre‐processing steps. Priority and Evidence levels are as reported in (Erdbrügger et al., 2021) and represent expert consensus opinion of the current level of confidence that the parameter is a variable to consider during sample biobanking and data analysis and interpretation.
FIGURE 2.
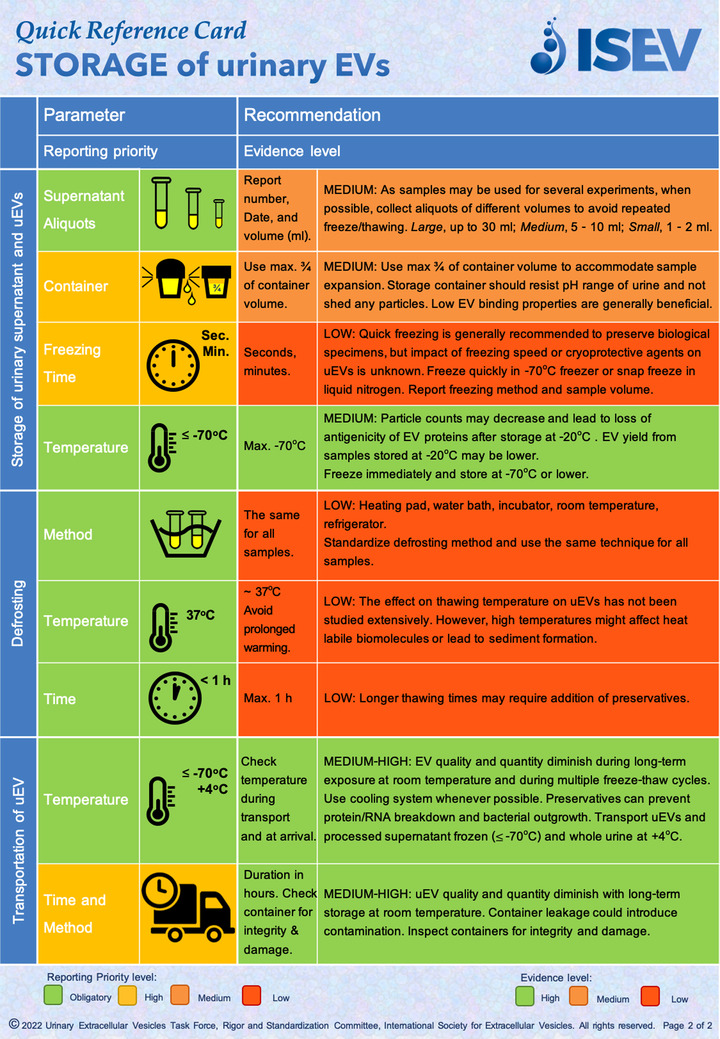
Quick Reference Card “Storage of urinary EVs”, page 2 Storage of urinary supernatant and uEVs, Defrosting, and Transportation of uEVs. Priority and Evidence levels are as reported in (Erdbrügger et al., 2021) and represent expert consensus opinion of the current level of confidence that the parameter is a variable to consider during sample biobanking and data analysis and interpretation.
To conclude, we present a novel format of communication for EV study guidelines and recommendations that can also be applied to other topics within, but importantly also outside the field of urinary EVs. Ultimately, by using this format, we endeavor to enhance adherence to pre‐analytical best practice guidelines in order to promote reproducibility and, above all, the translational potential of uEV studies.
CONFLICTS OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: M.v.R., C.S., C.G., J.W., T.T., M.D., A.B., B.G., A.L., C.B., D.B., U.E., E.M.U. Writing, original draft preparation: M.v.R., E.M.U.; Writing, review and editing: M.v.R., C.S., C.G., J.W., T.T., M.D., A.B., B.G., A.L., C.B., D.B., U.E., E.M.U.
All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary File 1. Quick Reference Card: Storage of Urinary EVs
ACKNOWLEDGEMENTS
This work was supported by the Alpe d'HuZes grant “IMMPROVE” of the Dutch Cancer Society (grant #EMCR2015‐8022), by the Norges Forskningsråd, Kreftforeningen and Helse Sør‐Øst RHF (NO), by the NIH, National Heart, Lung, and Blood Institute, Award number K23‐HL‐126101 and by the Dutch Kidney Foundation (Nierstichting), Award number: CP18.05.
van Royen, M. E. , Soekmadji, C. , Grange, C. , Webber, J. P. , Tertel, T. , Droste, M. , Buescher, A. , Giebel, B. , Jenster, G. , Llorente, A. , Blijdorp, C. J. , Burger, D. , Erdbrügger, U. , & Martens‐Uzunova, E. S. (2023). The quick reference card “Storage of urinary EVs” – A practical guideline tool for research and clinical laboratories. Journal of Extracellular Vesicles, 12, e12277. 10.1002/jev2.12286
Martin E. van Royen, Carolina Soekmadji, Cristina Grange, Jasson P. Webber, Tobias Tertel, Marvin Droste, Anja Buescher, Bernd Giebel, Guido Jenster, Alicia Llorente, Charles J. Blijdorp, Dylan Burger, Uta Erdbrügger, and Elena S. Martens‐Uzunova: Equal contributions
REFERENCES
- Erdbrügger, U. , Blijdorp, C. J. , Bijnsdorp, I. V. , Borras, F. E. , Burger, D. , Bussolati, B. , Byrd, J. B. , Clayton, A. , Dear, J. W. , Falcon‐Perez, J. M. , Grange, C. , Hill, A. F. , Holthöfer, H. , Hoorn, E. J. , Jenster, G. , Jimenez, C. R. , Junker, K. , Klein, J. , Knepper, M. A. , … Martens‐Uzunova, E. S. (2021). Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 10, e12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange, C. , & Bussolati, B. (2022). Extracellular vesicles in kidney disease. Nature Reviews Nephrology, 18, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, K. , Heinzelmann, J. , Beckham, C. , Ochiya, T. , & Jenster, G. (2016). Extracellular vesicles and their role in urologic malignancies. European Urology, 70, 323–331. [DOI] [PubMed] [Google Scholar]
- Thery, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , Atkin‐Smith, G. K. , Ayre, D. C. , Bach, J.‐M. , Bachurski, D. , Baharvand, H. , Balaj, L. , Baldacchino, S. , Bauer, N. N. , Baxter, A. A. , Bebawy, M. , … Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1. Quick Reference Card: Storage of Urinary EVs


