Abstract
Several epidemiological studies have clearly identified diabetes mellitus (DM) as a major risk factor for cognitive dysfunction, and it is going to be a major public health issue in the coming years because of the alarming rise in diabetes prevalence across the world. Brain and neural tissues predominantly depend on glucose as energy substrate and hence, any alterations in carbohydrate meta-bolism can directly impact on cerebral functional output including cognition, executive capacity, and memory. DM affects neuronal function and mental capacity in several ways, some of which include hypoperfusion of the brain tissues from cerebrovascular disease, diabetes-related alterations of glucose transporters causing abnormalities in neuronal glucose uptake and metabolism, local hyper- and hypometabolism of brain areas from insulin resistance, and recurrent hypoglycemic episodes inherent to pharmacotherapy of diabetes resulting in neuronal damage. Cognitive decline can further worsen diabetes care as DM is a disease largely self-managed by patients. Therefore, it is crucial to understand the pathobiology of cognitive dysfunction in relation to DM and its management for optimal long-term care plan for patients. A thorough appraisal of normal metabolic characteristics of the brain, how alterations in neural metabolism affects cognition, the diagnostic algorithm for patients with diabetes and dementia, and the management and prognosis of patients when they have this dangerous combination of illnesses is imperative in this context. This evidence-based narrative with the back-up of latest clinical trial reviews elaborates the current understanding on diabetes and cognitive function to empower physicians to manage their patients in day-to-day clinical practice.
Keywords: Diabetes mellitus, Dementia, Cognitive function, Antidiabetic medications, Hyperglycemia, Hypoglycemia
Core Tip: Diabetes mellitus (DM) is a huge risk factor for cognitive dysfunction especially when the glycemic control is inadequate with marked hyperglycemia and recurrent hypoglycemia. Apart from cognitive decline inherent to the disease, presence of other forms of dementia can adversely affect diabetes control and consequently, negatively impact the care of dementia and DM. Appropriate control of DM with a multidisciplinary team approach involving diabetologists, dementia specialists, dieticians and physiotherapists should improve the clinical outcomes of either disease. Judicious and evidence-based adjustments in the antidiabetic medications appropriately tailored for individualised diabetes care with due consideration of patient’s age, severity of dementia and other comorbidities should help to improve care of patients with diabetes and dementia.
INTRODUCTION
Diabetes mellitus (DM) has become a major cause of chronic disease morbidity in the past few decades, and according to the International Diabetes Federation data in the year 2021, approximately 537 million adults across the globe live with the disease[1]. DM can affect any organ system in the body, especially neural tissues and cerebrovascular structures causing various structural and functional disorders of the nervous system. Abnormalities in glucose metabolism including fasting and post-prandial hyp-erglycemia, prediabetic state and frank diabetes can result in neural dysfunction and various acute and chronic nervous system disorders including cognitive decline[2]. Cognitive dysfunction of chronic (and usually irreversible) nature that affects the usual intellectual performance of an individual is considered as dementia.
The World Health Organization (WHO) defines dementia as “a syndrome in which there is deterioration in cognitive function beyond what might be expected from the usual consequences of biological ageing”. According to the latest estimates of WHO, more than 55 million people live with dementia, and about 10 million new cases added to this pool every year[3]. Although dementia often affects the elderly individuals, it is not an unavoidable consequence of biological ageing process. Dementia not only affects the physical, economic, and psychosocial functioning of the individual with the disease but also hugely impacts the carers, families, and the society, and therefore strains the healthcare systems at large.
Based on strong scientific evidence, DM is now identified as one of the major causes, and a potentially modifiable risk factor for the development dementia[2,4,5]. A recent meta-analysis of 122 studies observed that DM poses 1.25- to 1.91-fold higher risk for cognitive impairment and dementia[4]. The study also observed an elevated risk of dementia among subjects with prediabetes, fasting and postprandial hyperglycemia, elevated hemoglobin A1c (HbA1c) and those with abnormal fasting plasma insulin levels. Therefore, it is important to understand the pathobiology of diabetes and cognitive dysfunction to develop appropriate clinical algorithms for management of both the entities in day-to-day clinical practice which is the theme of discussion in this evidence-based review.
REVIEW METHODOLOGY
To compile most up-to-date and the best evidence on the topic of discussion, we performed a PubMed literature search to procure currently available best evidence. For this we used the MeSH terms/key words: “brain metabolism”, “cognition/cognitive function”, “cognitive dysfunction”, “dementia”, “memory loss/memory impairment”, “diabetes mellitus” “type 2 diabetes mellitus/T2DM/T2D”, “type 1 diabetes mellitus/T1DM/T1D”, “pathobiology”, “pathophysiology”, “neuroimaging”, “lifestyle intervention”, “exercise”, “diet”, “antidiabetic medications”, “insulin” “pharmacotherapy”, “bariatric/metabolic surgery”, “prognosis”, “clinical trials” and “diabetes technology”.
The first two authors performed the initial literature search with guidance from the last two authors for initial drafting of the paper with an up-to-date search performed on 10th December 2022 for revising the paper after receiving the reviewer comments from the Journal. We used the Boolean search strategy using terms ‘AND’ or ‘OR’ where necessary to limit the search output to screen relevant abstracts from the web. We limited our literature review to articles published in English language. We used data and points from the most recent systematic reviews, randomised controlled trials (RCTs), clinical practice guidelines, and high-quality review articles to compile the best evidence available to us on DM and cognitive function to write the revision of this narrative review article.
ENERGY METABOLISM IN THE BRAIN
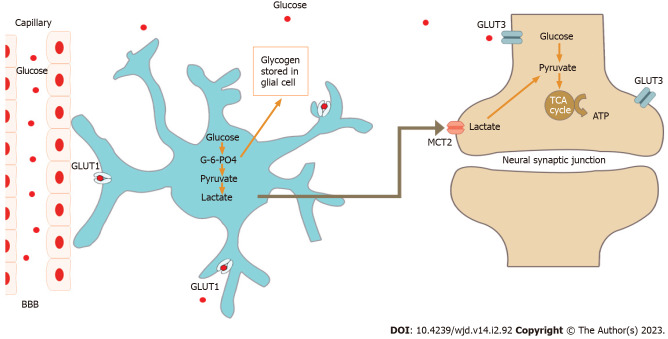
Although brain can use various metabolic substrates for energy production and utilisation, it predominantly uses glucose as the substrate for intermediary metabolism under normal physiological conditions[6,7]. The neuronal functions such as motor commands, sensory perceptions, memory storage, and intellectual output are highly dependent on the basal and on-demand metabolic activity of brain tissue. A graphical representation of normal neuronal glucose utilisation is shown in Figure 1.
Figure 1.
Neuronal utilisation of glucose under normal resting condition. G-6-PO4: Glucose 6-phosphate; BBB: Blood brain barrier; TCA: Tricarboxylic acid; GLUT: Glucose transporter; MCT2: Monocarboxylate transporter.
Astrocytes, the supportive glial cells of the brain, normally take up glucose from circulating blood in the cranial arteries to provide energy substrate to brain for its neural functions and behavioral responses[8]. This astrocyte function as such is under the neuronal control through specific neurotransmitters and their receptors. Experimental animal models revealed that activation of such receptors (for e.g., type-1 cannabinoid receptors associated with mitochondrial membranes in mouse astroglial cells) hampers the brain glucose metabolism with the production of lactate, resulting in alterations in the neuronal functions such as impairment of behavioral responses in social interaction assays[8]. These receptors are potential future targets for genetic and pharmacological manipulation for modulating such responses.
The energy metabolism of brain is highly variable in different areas depending on the neural functions and output of these regions. Most of the neural energy consumption is at the synaptic level for signal production and transmission along with the restoration of membrane potentials after depolarisation[9,10]. A good proportion of brain energy utilization is also for the synthesis of neurotransmitters, axoplasmic transport and the recycling of synaptic vesicles[10-12]. Overall, brain requires about 20% of the total oxygen and 20%-25% of glucose consumption of the body at rest, though the weight of human brain is only about 2% of the body weight[13-15]. However, during situations of stress and higher mental functions involving complex behavioral tasks, the metabolic demand increases further.
To facilitate optimal function of brain areas depending on the degree of neuronal output, supply-according-to-demand mechanisms have evolved through neurovascular and neurometabolic coupling for efficient substrate supply to the brain for fuelling intermediary metabolism[10]. Neurovascular coupling involves increase in blood flow and volume to improve glucose and oxygen supply to the areas of excess neuronal activity following stimulation, while neurometabolic coupling involves the changes in substrate utilization of astrocytes (predominantly by glycolysis) and neurons (predominantly by oxidative metabolism). These mechanisms are developed over centuries of genetic and metabolic adaptations in the evolution of the highly performing intellectual brain of modern man.
Metabolic adaptations of brain
As mentioned above, metabolic activity of the brain varies depending on its neural output for various biological tasks of daily life. Mitochondria are the powerhouses of brain’s energy production as in other body cells, and therefore alterations in mitochondrial function can affect the intellectual performance of human brain in health and disease[15,16]. Mitochondria also functions as mediators of cellular “allostasis”, a process of physiological adaptation of cells in response to various stressors[17]. By its bidirectional communication between stressors and stress mediators, mitochondria confer protective adaptive responses in the cells during period of acute stress[15]. “Neuronal plasticity”, the physiological changes in neural electrical adaptive responses in response to various stimuli, is largely mediated through these metabolic adaptations at the mitochondrial level. However, chronic stressors of any category including alterations in glucose metabolism as observed in chronic hyperglycemia, hypoglycemia, and DM, can cause mitochondrial damage, resulting in neural dysfunction. These metabolic and nonmetabolic chronic stressors result in an “allostatic overload” causing mitochondrial dysfunction and various neurological disorders consequently[15,18].
Although glucose is the predominant energy substrate for human brain in physiological states, brain can use alternate fuels such as ketone bodies, lactate, and medium chain triglycerides when the body’s glucose supply to brain is depleted as in periods of fasting and starvation[19,20]. This imply that the adaptive metabolic neural responses in relation to fasting and nutrient deprivation may have major biological impacts on brain function including cognition and intellectual performance. Recent evidence reveals that improvements in cognitive function, neuronal plasticity, and the resistance of brain to injury and disease occur in response to fasting and calorie restriction[20-22]. On other hand, over-fuelling as in metabolic disorders can result in disease states including neurodegeneration and dementia. Various changes in the central neural circuitry in response to dietary alterations may also modify the gut-brain axis which in turn alter the feeding behavior impacting the metabolic adaptation of brain as evidenced by recent scientific data[23].
BRAIN METABOLISM AND COGNITION
Intellectual capacities of human brain such as memory, mathematical performance, cognition, language, and executive functions are highly dependent on the degree of cerebral metabolic activity[10]. Therefore, any gross alterations in the metabolic milieu of brain are associated with marked changes in the neurocognitive balance in health and disease. Recent evidence suggests that there is a significant reduction in the glucose metabolism and functional connectivity between the intrinsic connectivity networks of brain with ageing, which would explain the age-related cognitive decline and decline in executive functions[24].
Lactate, another energy substrate of the brain, was also recently found to alter neurocognitive functions[25]. This by-product of intermediary metabolism was shown to increase transcription of brain-derived neurotrophic factor in neural cells and neuroglia. Lactate derived from “aerobic glycolysis” by astrocytes was found to enhance memory acquisition and learning-dependent synaptic plasticity in experimental mouse models[26] as shown in Figure 1. The energy demand of brain is often not adequately met by glucose supply from cranial circulation alone during exercise as glucose utilization by skeletal muscles increases substantially. In such situations, brain utilization of locally produced and muscle-derived lactate increases markedly to maintain metabolic demand for the enhanced neural synaptic activity[25].
Marked alterations in the metabolic activity of different areas of human brain is observed in various neurodegenerative disorders. For e.g., in diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and Lewis body dementia (LBD), the inferior parietal lobe was shown to have reduced glucose metabolism and perfusion defects[27-29]. These disorders are associated with significant reduction in cognitive function implying that the metabolic dysfunction has a contributory role in such cognitive decline.
DIABETES AND BRAIN DISORDERS
Glucose being the predominant metabolic substrate for the brain in normal physiological states, abnormalities in glucose homeostasis in diabetes is associated with marked changes in the structural and functional alterations in the brain. Moreover, several brain areas such as hippocampus are very sensitive to local alternations in glucose metabolism inherent to diabetes which may result in neuronal synaptic reorganization[30], and augmented astrocyte proliferation[31]. These in turn can result in cognitive decline of diabetes especially because glucose and insulin are instrumental regulators of cognitive function[32].
There is a well-recognized association between higher glucose levels and the risk of dementia among individuals with or without diabetes as shown by Crane et al[33] in 2013. They observed an 18% higher risk of dementia at 5 years among subjects with a glucose level of 6.4 mmol/L compared to those with a glucose level of 5.5 mmol/L in nondiabetics while the risk of dementia was 40% higher among diabetics with glucose level 10.5 mmol/L compared to those with a level of 8.9 mmol/L. This study clearly demonstrates the linear relationship between higher ambient glucose levels in the central nervous system (CNS) and its potential long-term toxic effects on neurodegeneration resulting in dementia. Another study from the United States also showed a similar risk of cognitive decline (19% excess risk) among patients with diabetes at 20 years compared to nondiabetic individuals[34]. This large cohort study also revealed excess dementia risk among prediabetics, and the duration of abnormal glycaemia had an impact on the degree of cognitive decline in patients with diabetes.
Although the presumed genetic association between type 2 DM (T2DM) and AD (also known as type 3 diabetes) was recently refuted by a well-designed linkage analysis study[35], the two diseases appear to have a strong epidemiological link probably from a causal role of worsening AD in patients with diabetes[36-38]. The metabolic dysregulation within the CNS may accelerate the progression of AD and would explain this association. Even though LBD is found to have no direct association with diabetes[39,40], cognitive decline can be rapid in diabetics with LBD as these patients may not be on appropriate treatment[40]. Diabetes significantly increases the risk of vascular dementia (VaD) owing to the very strong association with cardiovascular disease (CVD), and stroke[41-44]. Regardless of the aetiology of dementia, care of diabetes and that of dementia can be challenging when these diseases co-exist especially in elderly individuals.
Pathophysiology central nervous system disease in diabetes
One of the putative mechanisms for cognitive dysfunction in T2DM is insulin resistance (IR) in the brain[45,46]. Neuronal cells express insulin receptors for its normal functions such as synaptic density and plasticity of dendrites[46,47]. Through various complex mechanisms, insulin receptor signaling improves synaptic and dendritic functions in the CNS to improve cognitive performance[46]. Therefore, central IR in T2DM is often associated with impaired cognitive function. The balance between central insulin sensitivity and IR have also been implicated in the feeding behaviour, satiety and development of obesity in experimental models[46,48,49]. Overnutrition and obesity, which usually lead on to T2DM, were found to be associated with disruption of the blood brain barrier leading to a state of neuroinflammation which in turn results in cognitive dysfunction[46,50,51]. Overnutrition also results in morphological alterations in the hypothalamic neural circuitry that may augment overeating behaviour as a vicious circle aggravating obesity-related pathobiological states[46]. Alterations in gut microbiome commonly observed as part of the adverse eating habits are also associated with CNS neural changes causing cognitive decline[46,52].
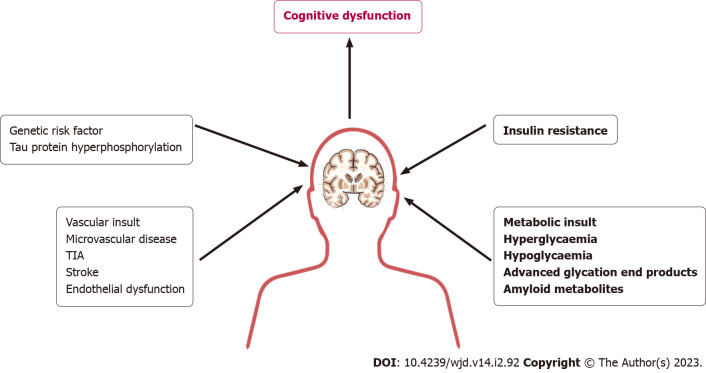
Recurrent hypoglycemia is a common consequence of advanced diabetes especially those on insulin and sulphonylurea. Brain being an organ predominantly using glucose as its metabolic fuel, can have gross impact of hypoglycemic episodes especially when recurrent. The hypoglycemia awareness, partly evoked by neuroglycopenia, gradually diminishes as an adaptive response of recurrent hypoglycemia which will aggravate future risk of more severe hypoglycemic episodes and the consequent complications[53]. Hypoglycemia-induced oxidative stress and neural inflammation can result in structural and functional alterations in vulnerable brain areas causing cognitive impairment[53-55]. Diabetes-induced vasculopathy affects the CNS circulation altering the cerebral blood flow remarkably. Both micro- and macrovascular damage involving the cranial vascular bed from accelerated atherosclerotic process inherent to diabetes are associated with neurocognitive decline and VaD[56-58]. Occurrence of microinfarcts and full-blown strokes are characteristics of longstanding diabetes[56]. Diabetes is identified as one of the most important causes of VaD mandating early diagnosis and proper management to reduce this potential consequence of the disease. A graphical representation of cognitive dysfunction in diabetes is shown in Figure 2.
Figure 2.
A graphical representation of the pathobiology of cognitive dysfunction in patients with diabetes. TIA: Transient ischemic attack.
DIABETES AND COGNITIVE DYSFUNCTION - DIAGNOSTIC EVALUATION
Appropriate diagnostic work up of cognitive dysfunction is especially important in patients with diabetes when compared to other medical problems as management of this metabolic disorder is largely patient-centred with regular glucose self-monitoring, and self-administration of medications including insulin injections. No other human disease needs such intense self-engagement as in diabetic patients for self-monitoring the metabolic parameters, medication compliance, dietary adjustments, and physical activities. Therefore, alterations in the mental functions can have a huge impact on diabetes control which may further affect the cognitive balance in a vicious circle[59,60].
Biochemical evaluation
Periodic measurement of glycated HbA1c is the usual biochemical parameter that enable us to monitor long-term diabetes control in a patient with stable glucose levels without marked fluctuations in daily glycemia. HbA1c level reflects the average glycemic state over a period of 120 d, and therefore wouldn’t always reflect good diabetes control in those with marked variability of glycemia as in patients having recurrent hypoglycaemia alternating with hyperglycaemia. Moreover, HbA1c levels can vary markedly in several conditions such as hemoglobinopathies, chronic kidney disease (CKD), anaemias and use of various medications[61]. Understanding these caveats of monitoring, appropriate use of HbA1c help us to get a reasonable measure of optimal diabetes management in patients with cognitive dysfunction.
If HbA1c is unreliable as in the situations mentioned above, an alternative biochemical test such as fructosamine test may be useful[61,62]. If there is an option for daily monitoring of capillary blood glucose (CBG) on multiple occasions, it provides the best chance of control of glycemia in patients with memory impairment[60]. Moreover, such monitoring would also enable us to optimise glycemic control. Newer glucose monitoring devices also enable calculation of predicted HbA1 levels which can be compared with the measured HbA1c to have idea about the reliability of the test.
Exclusion of other causes cognitive dysfunction such as thyroid disease, vitamin deficiencies and liver disorders by appropriate biochemical and hormonal evaluation is mandatory as part of initial evaluation and follow up care as and when necessary. As these diseases can often co-exist in some patients with diabetes, prompt testing would help timely diagnosis and appropriate management.
Neuroimaging
Neuroimaging is an integral part of routine initial evaluation of cognitive dysfunction in any individual to exclude structural abnormalities of the brain. Again, when there is a rapid unexplained decline in cognition without a clear identifiable reason in patients with known dementia, neuroimaging is warranted to exclude such abnormalities. Even minor unnoticed trauma can be associated with intracranial bleeds in elderly individuals which can be associated with rapid decline in memory function indicating urgent neuroimaging. Amyloid angiopathy is another disorder associated with spontaneous intracerebral bleed which may present similarly with an indication for urgent imaging studies[63].
Computed tomography scan is the usual first line imaging modality in most centres as it is cost effective, easily available, and provides reasonable sensitivity for initial evaluation of most major structural lesions such as stroke, tumors and hematomas[63-65]. Magnetic resonance imaging and positron emission tomography may be necessary for further evaluation of patient’s with cognitive dysfunction for accurate diagnosis of the pathological entity and for follow up management[66,67]. In the evaluation and follow up of patient’s with diabetes and cognitive dysfunction, imaging studies are indicated to exclude the possibility of development of such structural abnormalities described above or the co-existence of other disease entities such as AD, LBD or VaD.
MANAGEMENT OF DIABETES IN DEMENTIA
Owing to the risks associated with hypoglycemia in dementia patients, tight glycemic control is not usually recommended as in a patient without memory problems. Acceptable glucose and HbA1c targets are usually set by healthcare providers depending on the degree of cognitive impairment and other associated co-morbidities in the patient. Patient’s ability for CBG monitoring, and antidiabetic medication self-administration should be assessed promptly on periodic basis to optimise glycemic management. Individualised glycemic targets should be set with due consideration of patient’s situation and comorbid illnesses such as CKD, heart disease and hypoglycemia awareness. If self-care of diabetes is an issue, ensuring of regular assisted care by family members or by care providers becomes essential. In situations where these are not feasible, institutionalised care is recommended.
How diabetes management impacts dementia
Optimal diabetes care was found to be associated with better cognitive outcomes in patients with established dementia based on the data from multiple studies[68-70]. As discussed earlier, the glycemic load and IR in areas of brain associated with processing, storage and retention of memory has impact on cognition and therefore, optimising glycemic management may have significant influence on prognosis of patients with dementia. Prevention of marked hyperglycemia with appropriate adjustments of glycemic management should be tailored to suit the individual requirements of the patient on periodic basis to achieve this goal. While attempting to prevent marked hyperglycemia, all necessary precautions should be taken to prevent hypoglycemic episodes which can negatively impact on neurocognitive function[70,71]. Therefore, immediate and long term targets on clinical and biochemical parameters should be set periodically for glycemic and diabetes control in every patient with established cognitive dysfunction.
Dietary management
Dietary adjustment to optimise adequate nutritional supply while avoiding marked glycemic fluctuations is the corner stone of management of any form of diabetes in patients with the disease. This principle is equally important in patients with diabetes and dementia as nutritional deficits may have a negative impact on neurocognitive outcomes whereas appropriate nutritional interventions may have beneficial effects[72]. Low carbohydrate, high fibre diets with proteins and fat in moderation may be entirely appropriate to dementia patients as in normal subjects though palatability and refusal of timely intake of food can pose problems especially in advanced stages of the illness. A diet plan with due consideration of the sociocultural factors should help to improve adherence to such dietary interventions in cognitively impaired individuals with DM as in normal diabetic patients[73].
Physical activities
Regular moderate intensity physical activity is an integral part of daily management of any individual with DM. Physical activity improves skeletal muscle metabolism which in turn reduces the IR and insulin sensitivity and therefore improves diabetes and cardiometabolic parameters. As most patients with dementia are older individuals, exercise interventions may also improve sarcopenia associated with old age[74,75]. Such interventions improve the cognitive function and also reduce risk of imbalance of ageing and consequent falls. As the metabolism improves with exercise interventions, the diabetes management regime needs to be periodically revisited to avoid the risk of hypoglycemia. Multiple studies clearly demonstrated the remarkable benefits of exercise interventions on long term diabetes control, cognitive functions, and even risk of hypoglycemia in patients with dementia[76-78]. Therefore, an appropriate physical activity program should be considered for all patients with dementia to optimise the management with due consideration of the exercise capacity, co-morbidities and patient cooperation.
Optimising drug therapy
All patients with type 1 DM (T1DM) at onset of the disease, and most patients with T2DM at some stage of the disease would require pharmacotherapy for management of hyperglycemia. Insulin treatment is an absolute requirement for patients with T1DM from the diagnosis whereas many patients with T2DM are largely managed by noninsulin pharmacotherapy. Compliance with medical management can be a major issue in dementia patients with marked memory impairment complicating diabetes care. Therefore, healthcare providers’ responsibility is greater in managing such patients while ensuring adequate glycemic care with the avoidance of over-/undertreatment associated with significant morbidity and even mortality risks.
Insulin and insulin secretagogues (e.g., sulphonylurea and meglitinides) can be associated with significant risk of hypoglycemia while combination treatment of these with other molecules such as metformin, dipeptidyl peptidase-4 inhibitors (DPP-4i) and glucagon like peptide-1 receptor agonists (GLP-1RA) may potentiate the risk of hypoglycemia. The use of latter two molecules may have a beneficial effect in slowing down the cognitive decline in patients with dementia as revealed by a recent meta-analysis[79]. A detailed appraisal of treatment with individual antidiabetic agent in managing patients with dementia is beyond the scope of this review and therefore, readers are recommended to follow standard guidelines with consideration of individual patient characteristics based on the broad principle of avoiding hypoglycemia while optimising glycemic control.
Bariatric surgery
Bariatric procedures are associated with massive improvements in obesity and are the best available treatment modality for patients with obesity especially when associated with comorbidities such as T2DM. A significant proportion of patients achieve remission or reversal of T2DM. Metabolic surgery has been found to be associated with remarkable improvements cognitive function in patients with memory deficits in various observational studies[80-82]. However, with the currently available data, it is difficult to make firm recommendations in the absence of large scale long term follow up data based on RCTs.
Impact of dementia on diabetes care: Gradual decline in the memory deficits over time is the usual long-term consequence of all forms of irreversible dementia. Worsening memory is expected to have a huge impact on diabetes care especially when patients self-manage their diabetes. Medication compliance issues with inappropriate meal timings and improper administration of antidiabetic medications can adversely affect glycemic care with further decline in memory function. The resultant fluctuations in glycemia with uncontrolled hyperglycemia and recurrent hypoglycemic episodes will worsen diabetes-related complications and cause rapid decline in the neurocognitive functions[83].
Medication compliance
Memory impairment is usually associated with a decline in the executive functions of day-to-day living such as self-care, nutritional intake, and monitoring of medical problems such as diabetes early in the course of dementia. Forgetfulness associated with inadequate drug intake is common in patients with cognitive dysfunction and computer assisted cognitive training is shown to improve diabetes self-care in such patients[84]. When compliance issues become marked with poor diabetes self-management and recurrent acute hyperglycemic complications, home-based or institutionalised care support should be considered for supervised glycemic management.
Hypoglycemia management
Improper administration of antidiabetic agents such as insulin and insulin secretagogues without timely food intake can result in marked hypoglycemic episodes which can result in falls, rapid decline in cognitive functions and even death. Prompt review of diabetes drug regime with appropriate changes in the pharmacotherapy should be enforced urgently in such situations. Multidose insulin regime may need to be switched over to once daily long-acting insulin or twice daily mixed insulin regimes may be considered with due consideration of patient’s diet and physical activities[85].
Discontinuation of insulin secretagogues with hypoglycemic potential also need to be considered in presence of erratic meal pattern of patients with moderate to severe dementia[85,86]. Antidiabetic agents with less propensity for hypoglycemia and drugs which need less frequent administration such as DPP-4i and GLP-1RA are preferable in such situations. Although sodium-glucose cotransporter-2 inhibitors are hypo-neutral agents, the use of these agents in patient with advanced dementia needs caution as these patients can get dehydrated due to diuretic effect of these agents. Supervised drug administration by carers and institutionalised care should be considered to improve medication adherence and glycemic care in patients with advanced dementia.
PROGNOSIS OF DEMENTIA IN DIABETES
Prognosis of patients with dementia largely depends on the type and the pathobiology of the individual disorders causing cognitive dysfunction. However, prompt diabetes care may alter the course of the disease to some extend because of the impact of altered glucose metabolism on brain structures as mentioned in the previous sections. There is some emerging evidence showing beneficial effects of treatment with antidiabetic medications of the GLP-1RA class for neuroprotection in patients with PD, AD, stroke, and amyotrophic lateral sclerosis[87]. Moreover, optimal diabetes management may help to prevent deterioration of cognitive function in various dementing illnesses in relation to hypo- and hyperglycemic complications of improper diabetes care.
Diabetes types and dementia
Dementia may occur in patients with any of form of diabetes regardless of the type. The degree of cognitive decline in such patients largely depends on the appropriateness of diabetes management as mentioned earlier. As care of both the disorders can impact the management and prognosis of the other, healthcare providers are expected to have good understanding of either disease pathobiology. A multidisciplinary team (MDT) approach involving dementia specialists, physiotherapists, diabetologists and dieticians may help to optimise management of patients with moderate to severe forms of either disease. Moreover, individualised care plans for patients with consideration of their age, sociocultural factors, and comorbidities are important to obtain optimal outcomes.
T1DM and dementia
Patients with longstanding T1DM are at risk of some form of cognitive impairment, and diabetologists caring for such patients should be vigilant in identifying incident cognitive dysfunction in such patients. As a significant proportion of T1DM cases are on basal bolus insulin regimes (once/twice daily long-/ intermediate- acting insulin and mealtime short acting insulin), compliance issues with timing of meals and insulin administration may emerge as serious problems early in the course of dementia. Rapid decline of diabetes control with adverse consequence on cognitive functions are the results of such a situation[88].
Regular CBG monitoring is important in the management T1DM patients to aid variable dose mealtime insulin administration adjusted for their carbohydrate intake. Recently, intermittent scanning of a continuous glucose monitoring device (measuring interstitial tissue glucose) has revolutionised the self-monitoring of glycemic parameters and diabetes care in such patients[89]. Appropriate use of such technology in a supervised setting can potentially mitigate the cognitive decline in relation to poor glycemic care in patients with dementia. Appropriate changes in the insulin regime as mentioned earlier also may be necessary in patients with poor meal compliance and insulin administration issues.
T2DM and dementia
There is some evidence to support the notion that AD may have an association with T2DM based on multiple epidemiological correlation studies[90-92]. Although the pathobiological interlink is not very strong, we have to consider this association while planning management of patients with T2DM, especially because of the constraints imposed on glycemic care by the development of dementia. Appropriate early administration of medications of incretin mimetic class such as DPP-4i and GLP-1RA to optimise diabetes control and prevention of AD will help to some extent[87,93]. Although there has been a signal towards some vague association of metformin use to the development of AD in Asians in a recent meta-analysis[94], the study results have to be interpreted with caution as the data analysed was of low quality and of observational type. Insulin administration issues can be addressed as mentioned earlier.
Other types of diabetes and cognitive function
There is not much data on the incidence and prevalence of cognitive dysfunction in patients with other forms of diabetes such as diabetes in patients with chronic pancreatitis, monogenic diabetes, and syndromic type of diabetes. However, glycemic care can pose similar problems when cognitive decline becomes moderate to severe as in T1DM and T2DM. Nutritional imbalance from pancreatic diabetes and neurological problems in some patients with syndromic diabetes can pose problems in glycemic care. Supportive care with an appropriate MDT approach might help to improve care in such patients.
USE OF NEWER DIABETES TECHNOLOGIES FOR THE CARE OF PATIENTS WITH COGNITIVE DYSFUNCTION
Regular monitoring of CBG can be hectic and add additional burden to patients with dementia. Although use of flash glucose monitoring device can avert the finger pricking, patients with dementia can forget flashing their device resulting in loss of data if not scanned for more than eight hours. The real-time continuous glucose monitoring system (rtCGM) offers benefit in automatically sensing and transmitting the data to the application on the phone. Wireless Innovation for Seniors with Diabetes Mellitus trial compared rtCGM with standard finger prick capillary glucose monitoring in older adults (age > 60 years) with T1DM for prevention of hypoglycaemia and glycaemic control[95]. This trial also included patients with mild cognitive impairment although individuals with advanced dementia were excluded. The rtCGM arm spent less time below the range (blood glucose < 70 mg/dL) and there was also significant reduction in the HbA1c in the rtCGM arm when compared with the control (mean difference of -0.3%; 95% confidence interval: -0.4% to -0.1%; P < 0.001). With the lesson learned during coronavirus disease 2019 pandemic with rtCGM and third party data sharing, these features can help the family members, carers or care givers monitor the glucose level remotely and help the patient with the decision regarding insulin dose calculations[96,97].
Appropriate use of technology can help to manage T1DM and insulin treated T2DM individuals when they develop dementia. Although there is no robust evidence through RCTs, sensibly matching the technology to the individual needs and support system will ease the management[98]. Disposable insulin pens reduce the workload for the patient than the reusable pen that needs periodic change of the cartridge. The use of smart insulin pen automatically uploads the delivered dose in the linked server[99]. The alarm features in these pens can be an additional advantage to remind the timely administration of insulin. Remote review of the doses by the carer can help with titrating the dose, deliver the missed dose and prevent overdosing with insulin. Individuals with mild dementia do manage the insulin pump with ease if they are used to it for a long duration before the dementia settles[100]. The insulin pump with predictive low glucose suspend features can help preventing hypoglycaemia provided that patient does not pull out the pump connections, hence not to be used in patient with moderate to advanced dementia as the risk of diabetic ketoacidosis will be high if there is disconnection. The behaviour of patient with the insulin pump can be studied using saline filled cartridges in the practice for a period of a week or two, and the information derived can help to decide about the individual’s ability to manage the insulin pump.
If the individual manages the insulin pump, then use of hybrid closed loop insulin delivery system can be tried as it can vary insulin basal delivery depending on the blood glucose level rather than the set basal targets and the trials have clearly shown beneficial effects in elderly patients with T1DM[100]. The remote blousing feature with smart phone in some of the hybrid closed insulin technology (e.g., CamAPS FX hybrid closed loop app) will help carers in delivering the correct insulin dose. This third-party insulin delivery via the remote blousing feature should only be used in the countries where such regulation is allowed. Lastly, the use of insulin only bionic pancreas where only qualitative announcement of the meal is required can be an additional tool for management of individual with mild early dementia where complicated carbohydrate counting can be ignored[101]. More trials using technology in patients with early dementia are needed, as there is an increase in elderly patients with T1DM and it is predicted that prevalence of T1DM itself will be doubled by the year 2040[102].
OTHER COMORBIDITIES/MODIFIABLE RISK FACTORS POTENTIALLY IMPACTING COGNITIVE DYSFUNCTION IN DIABETES
Several other coexistent illnesses can exaggerate the risk of cognitive decline in patients with diabetes. Therefore, management of these comorbidities are also very crucial for optimal long-term outcomes.
Hypertension
One of the most common chronic diseases affecting middle-aged and the elderly population is hypertension. It is one of the most common comorbidities in the diabetic populations, especially in those with early onset T2DM with a prevalence of about 67.5%[103]. A recent study showed that the odds ratio for dementia in patients with hypertension is 5.82, and more than 90% dementia patients with diabetes had hypertension[104]. From this observation, it is imperative to obtain prompt blood pressure (BP) control in patients with dementia while considering the risks associated with intense BP reduction such as postural hypotension and falls. Antihypertensive medications modifying the renin-angiotensin-aldosterone system have been recently found to improve executive function, processing speed, verbal memory and composite score compared to other antihypertensive medications in a recent clinical trial[105].
Dyslipidemia
Several studies have shown association between dyslipidemia and dementia especially when present in patients with diabetes[106]. Diabetes (especially T2DM) as such is a strong risk factor for atherosclerotic CVD even in patients with normal lipid levels for nondiabetic individuals. This may be related to presence of more atherogenic low-density lipoprotein cholesterol particles in diabetics making them prone to develop CVD. Accelerated atherosclerosis of the cranial arteries may be an important factor reducing cerebral blood flow and cognitive decline in such patients. However, intense lipid lowering therapy was not associated with better cognitive outcomes in the ACCORD clinical trial[107].
Associated CVD
CVD is an important risk factor for dementia owing to its close association with cerebrovascular disease, stroke, and impaired brain perfusion. Even in those without established cerebrovascular disease or stroke, CVD was found to be associated with higher rates of cognitive decline in a systematic review[108]. The authors observed that severe atherosclerosis posed 59% and atrial fibrillation posed 26% higher risk for development of dementia.
Proteinuria
Both micro- and macroalbuminuria are associated with high risk of generalised vasculopathy and atherosclerotic disease. Diabetes-related microvascular disease affects kidneys early in the course of the disease, especially in patients with poor diabetes control, and tremendously exaggerate the atherosclerotic CVD. A recent meta-analysis showed significant association between albuminuria and cognitive dysfunction[109]. This systematic review involving 16 studies among 127296 participants revealed a 20% excess risk of dementia among patients with albuminuria.
Apolipoprotein ε4 allele
Apolipoprotein (ApoE) is protein that carries the lipid molecules for their transport in human body in the form of apolipoproteins. Historically, career of ApoE ε4 allele has been found to possess strong association with the development of dementia[110]. A recent study involving 206960 participants from the United Kingdom biobank cohort showed that the presence of ApoE ε4 allele was associated an increased risk [hazard ratio (HR) = 1.63] of developing dementia[111]. However, when potentially modifiable risk factors such as hypertension, diabetes and coronary artery disease were clustered in to this risk, the HR increased to 2.20. Table 1 summarises the risk factors for dementia or cognitive decline among patients with diabetes.
Table 1.
Risk factors for cognitive decline in diabetes mellitus
|
Risk factors for cognitive decline in diabetes mellitus
| |
| Age > 60 yr | Atherosclerotic cardiovascular disease |
| Presence of ApoE ε4 allele | Uncontrolled hypertension |
| Long duration of diabetes | Proteinuria |
| Poor glycaemic control/high HbA1c | Dyslipidaemia |
| Higher fasting glucose levels | Physical inactivity |
| Recurrent hypoglycaemic episodes | Unhealthy diet |
| Severe insulin resistance | Depression |
ApoE: Apolipoprotein; HbA1c: Hemoglobin A1c.
CLINICAL TRIALS ON MODIFIABLE RISK FACTORS OF COGNITIVE DYSFUNCTION AMONG PATIENTS WITH DIABETES
Several RCTs examined the potential benefits of management of various modifiable risk factors for cognitive decline in patients with diabetes. However, only a small proportion of these studies showed even marginal benefits. Some of the trials even showed the probability of harm in the participants. Therefore, we need much more research input in this area to ensure we have more promising modalities of treatment for diabetic patients with cognitive dysfunction. A list of landmark clinical trials looking at the benefits of potentially modifiable risk factors for managing patients with diabetes and cognitive dysfunction is shown in Table 2.
Table 2.
Landmark randomized controlled trials looking at the benefits of clinical management of modifiable risk factors in diabetes and cognitive dysfunction
|
Intervention/ treatment
|
Study characteristics & benefit(s) of treatment/intervention group
|
Ref.
|
| Treatment with antihypertensives acting on renin angiotensin axis | Better executive function, processing speed, verbal memory and composite score compared to those treated with other antihypertensives | Wharton et al[105], 2022 |
| Intensive BP and lipid control compared to standard treatment (ACCORD trial) | Intense BP control and lipid reduction had no effects on cognitive decline. Moreover, total brain volume was found to be less with intense BP control (systolic BP < 120 mm Hg) than standard treatment after 40 mo | Williamson et al[107], 2014 |
| Liraglutide therapy for T2DM | Activation of different cerebral areas with improved memory, attention, and better scores in all cognitive function tests | Li et al[112], 2021 |
| Intense vs standard BP control (SPRINT trial) | Intense BP control was not associated with improvements in memory or processing speed compared to standard BP reduction | Rapp et al[113], 2020 |
| 10 yr of ILI vs standard care (Look AHEAD trial) | ILI resulted in better odds for emergence of: Decision-making inability (OR = 0.851) and problem solving inability (OR = 0.694) in those without these baseline complaints | Espeland et al[114], 2018 |
| Finnish diabetes prevention study | Middle-aged overweight participants with impaired glucose tolerance showed better cognitive performance with low total fat & saturated fat intake, and frequent physical activities compared to standard lifestyle | Lehtisalo et al[115], 2016 |
BP: Blood pressure; T2DM: Type 2 diabetes mellitus; ILI: Intense lifestyle intervention; OR: Odds ratio.
AREAS OF UNCERTAINTY/EMERGING CONCEPTS
Although optimal glycemic care is expected to ameliorate the cognitive decline associated with hyper- and hypoglycemic care of patients with diabetes and dementia, it is not clear if prompt diabetes control might alter the pathobiology of individual dementing illnesses. The proposed association between T2DM and AD is currently vague, and more studies may shed light on this grey area.
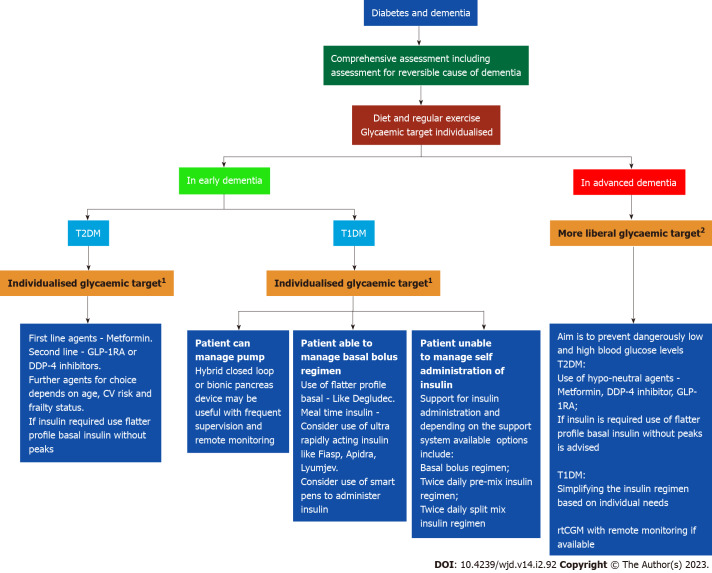
The benefits of observed improvement of cognitive function among patients with massive weight loss following bariatric surgery need additional evidence through largescale RCTs for use in day-to-day clinical practice. The beneficial effects of incretin manipulation by GLP-1RA and DPP-4i on different forms of neurodegenerative disorders such as AD need to be clarified in long term RCTs. The potential risk of metformin use and AD development revealed in some ethnic groups needs further studies as metformin is the first-line drug with other remarkable health benefits when used in patients with T2DM. Figure 3 shows a pragmatic approach to the management of diabetes and dementia in day-to-day clinical practice.
Figure 3.
Practical approach to the management of patient with diabetes and dementia. 1Glycaemic target according to comorbidities to avoid marked glycaemic variability, hypo- and hyperglycaemia. 2Target glucose 7-12 mmol/L ideally (but can range between 5-16 mmol/L especially while on insulin). T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; GLP-1RA: Glucagon like insulinotropic peptide-receptor agonist; DPP-4: Dipeptidyl peptidase-4; CV: Cardiovascular; rtCGM: Real-time continuous glucose monitoring.
CONCLUSION
Development of cognitive dysfunction is a big risk of inadequate diabetes management in patients with any form of diabetes. Onset of dementia can impact diabetes care with the risk of worsening of either disease from inadequate glycemic care. Currently available evidence suggest that optimal diabetes management can have better clinical outcomes among patients with neurocognitive dysfunction. A multidisciplinary approach to management of patients involving diabetologists, dieticians, dementia specialists and physical therapists with appropriate antidiabetic treatment and nonpharmacological interventions may improve diabetes care in patients with diabetes and dementia. If appropriately used, technological advancements can further improve the care of diabetes patients with dementia. More research is needed in these areas as the incidence of both the diseases are increasing globally owing to increasing prevalence of obesity and aged individuals in the global population.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 5, 2022
First decision: December 26, 2022
Article in press: January 16, 2023
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bagheri-Mohammadi S, Iran; Glumac S, Croatia S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Meghna Julian Sebastian, School of Life Sciences, Keele University, Stoke-on-Trent ST5 5BG, United Kingdom.
Shahanas KA Khan, Department of Endocrinology and Metabolism, Lancashire Teaching Hospitals NHS Trust, Preston PR2 9HT, United Kingdom.
Joseph M Pappachan, Department of Endocrinology and Metabolism, Lancashire Teaching Hospitals NHS Trust, Preston PR2 9HT, United Kingdom; Faculty of Science, Manchester Metropolitan University, Manchester M15 6BH, United Kingdom; Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PL, United Kingdom. drpappachan@yahoo.co.in.
Mohammad Sadiq Jeeyavudeen, Department of Endocrinology and Metabolism, University Hospitals of Edinburgh, Edinburgh EH16 4SA, United Kingdom.
References
- 1.IDF Diabetes Atlas. IDF Diabetes Atlas 2021. IDF Atlas 10th Edition. [cited 17 September 2022]. Available from: https://diabetesatlas.org/atlas/tenth-edition/
- 2.Barbiellini Amidei C, Fayosse A, Dumurgier J, Machado-Fragua MD, Tabak AG, van Sloten T, Kivimäki M, Dugravot A, Sabia S, Singh-Manoux A. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA. 2021;325:1640–1649. doi: 10.1001/jama.2021.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Fact sheet: Dementia. [cited 17 September 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia .
- 4.Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. doi: 10.1016/j.arr.2019.100944. [DOI] [PubMed] [Google Scholar]
- 5.Selman A, Burns S, Reddy AP, Culberson J, Reddy PH. The Role of Obesity and Diabetes in Dementia. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23169267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharples PM, Bartlett K, Eyre JA. Cerebral consumption of glucose. Lancet. 1989;1:1142. doi: 10.1016/s0140-6736(89)92421-5. [DOI] [PubMed] [Google Scholar]
- 7.Nordström CH, Forsse A, Jakobsen RP, Mölström S, Nielsen TH, Toft P, Ungerstedt U. Bedside interpretation of cerebral energy metabolism utilizing microdialysis in neurosurgical and general intensive care. Front Neurol. 2022;13:968288. doi: 10.3389/fneur.2022.968288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez-Blasco D, Busquets-Garcia A, Hebert-Chatelain E, Serrat R, Vicente-Gutierrez C, Ioannidou C, Gómez-Sotres P, Lopez-Fabuel I, Resch-Beusher M, Resel E, Arnouil D, Saraswat D, Varilh M, Cannich A, Julio-Kalajzic F, Bonilla-Del Río I, Almeida A, Puente N, Achicallende S, Lopez-Rodriguez ML, Jollé C, Déglon N, Pellerin L, Josephine C, Bonvento G, Panatier A, Lutz B, Piazza PV, Guzmán M, Bellocchio L, Bouzier-Sore AK, Grandes P, Bolaños JP, Marsicano G. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature. 2020;583:603–608. doi: 10.1038/s41586-020-2470-y. [DOI] [PubMed] [Google Scholar]
- 9.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Watts ME, Pocock R, Claudianos C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front Mol Neurosci. 2018;11:216. doi: 10.3389/fnmol.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, Gerencser AA, Kim H, Brand MD, Edwards RH, Nakamura K. The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem. 2015;290:22325–22336. doi: 10.1074/jbc.M115.656405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 14.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morella IM, Brambilla R, Morè L. Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders. Neurosci Biobehav Rev. 2022;142:104892. doi: 10.1016/j.neubiorev.2022.104892. [DOI] [PubMed] [Google Scholar]
- 16.Pei L, Wallace DC. Mitochondrial Etiology of Neuropsychiatric Disorders. Biol Psychiatry. 2018;83:722–730. doi: 10.1016/j.biopsych.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 18.Picard M, Juster RP, Sloan RP, McEwen BS. Mitochondrial Nexus to Allostatic Load Biomarkers. Psychosom Med. 2017;79:114–117. doi: 10.1097/PSY.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB. Brain metabolism during short-term starvation in humans. J Cereb Blood Flow Metab. 1994;14:125–131. doi: 10.1038/jcbfm.1994.17. [DOI] [PubMed] [Google Scholar]
- 20.Brocchi A, Rebelos E, Dardano A, Mantuano M, Daniele G. Effects of Intermittent Fasting on Brain Metabolism. Nutrients. 2022;14 doi: 10.3390/nu14061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Tan HE, Lu Z, Tsang KS, Chung AJ, Zuker CS. Gut-brain circuits for fat preference. Nature. 2022;610:722–730. doi: 10.1038/s41586-022-05266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu K, Niu N, Li X, Chen Y, Wang D, Zhang J, Li H, Wei D, Chen K, Cui R, Zhang Z, Yao L. The characteristics of glucose metabolism and functional connectivity in posterior default network during nondemented aging: relationship with executive function performance. Cereb Cortex. 2022 doi: 10.1093/cercor/bhac248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue X, Liu B, Hu J, Bian X, Lou S. The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: a dual role as an energy supply substrate and a signaling molecule. Nutr Metab (Lond) 2022;19:52. doi: 10.1186/s12986-022-00687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RA, Lone A, Lim H, Martinez F, Frame AK, Scholl TJ, Cumming RC. Aerobic Glycolysis Is Required for Spatial Memory Acquisition But Not Memory Retrieval in Mice. eNeuro. 2019;6 doi: 10.1523/ENEURO.0389-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firbank MJ, Yarnall AJ, Lawson RA, Duncan GW, Khoo TK, Petrides GS, O'Brien JT, Barker RA, Maxwell RJ, Brooks DJ, Burn DJ. Cerebral glucose metabolism and cognition in newly diagnosed Parkinson's disease: ICICLE-PD study. J Neurol Neurosurg Psychiatry. 2017;88:310–316. doi: 10.1136/jnnp-2016-313918. [DOI] [PubMed] [Google Scholar]
- 28.Duran-Aniotz C, Hetz C. Glucose Metabolism: A Sweet Relief of Alzheimer's Disease. Curr Biol. 2016;26:R806–R809. doi: 10.1016/j.cub.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 29.Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, Iannaccone S, Magnani G, Padovani A, Ferini-Strambi L, Perani D. Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimers Res Ther. 2019;11:20. doi: 10.1186/s13195-019-0473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magariños AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci U S A. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saravia FE, Revsin Y, Gonzalez Deniselle MC, Gonzalez SL, Roig P, Lima A, Homo-Delarche F, De Nicola AF. Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. Brain Res. 2002;957:345–353. doi: 10.1016/s0006-8993(02)03675-2. [DOI] [PubMed] [Google Scholar]
- 32.Wrighten SA, Piroli GG, Grillo CA, Reagan LP. A look inside the diabetic brain: Contributors to diabetes-induced brain aging. Biochim Biophys Acta. 2009;1792:444–453. doi: 10.1016/j.bbadis.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy J, de Strooper B, Escott-Price V. Diabetes and Alzheimer's disease: shared genetic susceptibility? Lancet Neurol. 2022;21:962–964. doi: 10.1016/S1474-4422(22)00395-7. [DOI] [PubMed] [Google Scholar]
- 36.Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, Okonkwo OC, La Rue A, Hermann BP, Koscik RL, Jonaitis EM, Sager MA, Asthana S. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Yi Q, Wang Y, Wang J, Yu H, Zhang J, Hu M, Xu J, Wu Z, Hou L, Zhang Z, Zhang Y, Tu Z, Yang K, Guo K, Zhou Y, Geng T, Pan X, Liu G, Song P, Pan A. Long-term glycemic variability and risk of adverse health outcomes in patients with diabetes: A systematic review and meta-analysis of cohort studies. Diabetes Res Clin Pract. 2022;192:110085. doi: 10.1016/j.diabres.2022.110085. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen HE, Sandvik CH, Subhi Y, Grauslund J, Pedersen FN. Relationship between Diabetic Retinopathy and Systemic Neurodegenerative Diseases: A Systematic Review and Meta-analysis. Ophthalmol Retina. 2022;6:139–152. doi: 10.1016/j.oret.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Javanshiri K, Haglund M, Englund E. Cardiovascular Disease, Diabetes Mellitus, and Hypertension in Lewy Body Disease: A Comparison with Other Dementia Disorders. J Alzheimers Dis. 2019;71:851–859. doi: 10.3233/JAD-190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Secnik J, Cermakova P, Fereshtehnejad SM, Dannberg P, Johnell K, Fastbom J, Winblad B, Eriksdotter M, Religa D. Diabetes in a Large Dementia Cohort: Clinical Characteristics and Treatment From the Swedish Dementia Registry. Diabetes Care. 2017;40:1159–1166. doi: 10.2337/dc16-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A Systematic Review of Meta-Analyses that Evaluate Risk Factors for Dementia to Evaluate the Quantity, Quality, and Global Representativeness of Evidence. J Alzheimers Dis. 2019;70:S165–S186. doi: 10.3233/JAD-190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford E, Greenslade N, Paudyal P, Bremner S, Smith HE, Banerjee S, Sadhwani S, Rooney P, Oliver S, Cassell J. Predicting dementia from primary care records: A systematic review and meta-analysis. PLoS One. 2018;13:e0194735. doi: 10.1371/journal.pone.0194735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tun NN, Arunagirinathan G, Munshi SK, Pappachan JM. Diabetes mellitus and stroke: A clinical update. World J Diabetes. 2017;8:235–248. doi: 10.4239/wjd.v8.i6.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Vorst IE, Koek HL, de Vries R, Bots ML, Reitsma JB, Vaartjes I. Effect of Vascular Risk Factors and Diseases on Mortality in Individuals with Dementia: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2016;64:37–46. doi: 10.1111/jgs.13835. [DOI] [PubMed] [Google Scholar]
- 45.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev. 2016;96:1169–1209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Jin K, Chen B, Liu R, Cheng S, Zhang Y, Lu J. Overnutrition Induced Cognitive Impairment: Insulin Resistance, Gut-Brain Axis, and Neuroinflammation. Front Neurosci. 2022;16:884579. doi: 10.3389/fnins.2022.884579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–879. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillemot-Legris O, Muccioli GG. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Więckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U. Western diet as a trigger of Alzheimer's disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 2021;70:101397. doi: 10.1016/j.arr.2021.101397. [DOI] [PubMed] [Google Scholar]
- 52.Cope EC, LaMarca EA, Monari PK, Olson LB, Martinez S, Zych AD, Katchur NJ, Gould E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J Neurosci. 2018;38:8889–8904. doi: 10.1523/JNEUROSCI.0789-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCrimmon RJ. Consequences of recurrent hypoglycaemia on brain function in diabetes. Diabetologia. 2021;64:971–977. doi: 10.1007/s00125-020-05369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ. Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab. 2009;297:E194–E201. doi: 10.1152/ajpendo.91041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehtewish H, Arredouani A, El-Agnaf O. Diagnostic, Prognostic, and Mechanistic Biomarkers of Diabetes Mellitus-Associated Cognitive Decline. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biessels GJ, Nobili F, Teunissen CE, Simó R, Scheltens P. Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol. 2020;19:699–710. doi: 10.1016/S1474-4422(20)30139-3. [DOI] [PubMed] [Google Scholar]
- 58.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luchsinger JA, Palmas W, Teresi JA, Silver S, Kong J, Eimicke JP, Weinstock RS, Shea S. Improved diabetes control in the elderly delays global cognitive decline. J Nutr Health Aging. 2011;15:445–449. doi: 10.1007/s12603-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobson AM, Ryan CM, Braffett BH, Gubitosi-Klug RA, Lorenzi GM, Luchsinger JA, Trapani VR, Bebu I, Chaytor N, Hitt SM, Farrell K, Lachin JM DCCT/EDIC Research Group. Cognitive performance declines in older adults with type 1 diabetes: results from 32 years of follow-up in the DCCT and EDIC Study. Lancet Diabetes Endocrinol. 2021;9:436–445. doi: 10.1016/S2213-8587(21)00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sodi R, McKay K, Dampetla S, Pappachan JM. Monitoring glycaemic control in patients with diabetes mellitus. BMJ. 2018;363:k4723. doi: 10.1136/bmj.k4723. [DOI] [PubMed] [Google Scholar]
- 62.Conlin PR, Colburn J, Aron D, Pries RM, Tschanz MP, Pogach L. Synopsis of the 2017 U.S. Department of Veterans Affairs/U.S. Department of Defense Clinical Practice Guideline: Management of Type 2 Diabetes Mellitus. Ann Intern Med. 2017;167:655–663. doi: 10.7326/M17-1362. [DOI] [PubMed] [Google Scholar]
- 63.Rodrigues MA, Samarasekera N, Lerpiniere C, Humphreys C, McCarron MO, White PM, Nicoll JAR, Sudlow CLM, Cordonnier C, Wardlaw JM, Smith C, Al-Shahi Salman R. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018;17:232–240. doi: 10.1016/S1474-4422(18)30006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ball EL, Sutherland R, Squires C, Mead GE, Religa D, Lundström E, Cheyne J, Wardlaw JM, Quinn TJ, Shenkin SD. Predicting post-stroke cognitive impairment using acute CT neuroimaging: A systematic review and meta-analysis. Int J Stroke. 2022;17:618–627. doi: 10.1177/17474930211045836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodworth DC, Scambray KA, Corrada MM, Kawas CH, Sajjadi SA. Neuroimaging in the Oldest-Old: A Review of the Literature. J Alzheimers Dis. 2021;82:129–147. doi: 10.3233/JAD-201578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker AF, Ohlhauser L, Scarapicchia V, Smart CM, Szoeke C, Gawryluk JR. A Systematic Review of Neuroimaging Studies Comparing Individuals with Subjective Cognitive Decline to Healthy Controls. J Alzheimers Dis. 2022;86:1545–1567. doi: 10.3233/JAD-215249. [DOI] [PubMed] [Google Scholar]
- 67.Grueso S, Viejo-Sobera R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer's disease dementia: a systematic review. Alzheimers Res Ther. 2021;13:162. doi: 10.1186/s13195-021-00900-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabatabaei Malazy O, Bandarian F, Qorbani M, Mohseni S, Mirsadeghi S, Peimani M, Larijani B. The effect of metformin on cognitive function: A systematic review and meta-analysis. J Psychopharmacol. 2022;36:666–679. doi: 10.1177/02698811211057304. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Sun L, He G, Gang X, Zhao X, Wang G, Ning G. Cerebral perfusion alterations in type 2 diabetes mellitus - a systematic review. Front Neuroendocrinol. 2021;62:100916. doi: 10.1016/j.yfrne.2021.100916. [DOI] [PubMed] [Google Scholar]
- 70.McMillan JM, Mele BS, Hogan DB, Leung AA. Impact of pharmacological treatment of diabetes mellitus on dementia risk: systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2018;6:e000563. doi: 10.1136/bmjdrc-2018-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang L, Zhu M, Ji J. Association between hypoglycemia and dementia in patients with diabetes: a systematic review and meta-analysis of 1.4 million patients. Diabetol Metab Syndr. 2022;14:31. doi: 10.1186/s13098-022-00799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu J, Tan LJ, Lee JE, Shin S. Association between the mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front Nutr. 2022;9:946361. doi: 10.3389/fnut.2022.946361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lenart-Bugla M, Łuc M, Pawłowski M, Szcześniak D, Seifert I, Wiegelmann H, Gerhardus A, Wolf-Ostermann K, Rouwette EAJA, Ikram MA, Brodaty H, Jeon YH, Maddock J, Marseglia A, Melis RJF, Samtani S, Wang HX, Welmer AK, Vernooij-Dassen M, Rymaszewska J. What Do We Know about Social and Non-Social Factors Influencing the Pathway from Cognitive Health to Dementia? Brain Sci. 2022;12 doi: 10.3390/brainsci12091214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Act. 2019;16:10. doi: 10.1186/s11556-019-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, Aubertin-Leheudre M, Bernabei R, Cadore EL, Cesari M, Chen LK, de Souto Barreto P, Duque G, Ferrucci L, Fielding RA, García-Hermoso A, Gutiérrez-Robledo LM, Harridge SDR, Kirk B, Kritchevsky S, Landi F, Lazarus N, Martin FC, Marzetti E, Pahor M, Ramírez-Vélez R, Rodriguez-Mañas L, Rolland Y, Ruiz JG, Theou O, Villareal DT, Waters DL, Won Won C, Woo J, Vellas B, Fiatarone Singh M. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25:824–853. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
- 76.Cai YH, Wang Z, Feng LY, Ni GX. Effect of Exercise on the Cognitive Function of Older Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front Hum Neurosci. 2022;16:876935. doi: 10.3389/fnhum.2022.876935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang JH, Lu L, Li JY, Qu XY, Li J, Qian S, Wang YQ, Jia RX, Wang CS, Xu Y. Contributions of Modifiable Risk Factors to Dementia Incidence: A Bayesian Network Analysis. J Am Med Dir Assoc. 2020;21:1592–1599.e13. doi: 10.1016/j.jamda.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Dyer AH, Briggs R, Mockler D, Gibney J, Kennelly SP. Non-pharmacological interventions for cognition in patients with Type 2 diabetes mellitus: a systematic review. QJM. 2020;113:155–161. doi: 10.1093/qjmed/hcz053. [DOI] [PubMed] [Google Scholar]
- 79.Jin Y, Zhao H, Hou Y, Song G. The effects of dipeptidyl peptidase-4 inhibitors and glucagon-like peptide 1 receptor agonists on cognitive functions in adults with type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2020;57:1129–1144. doi: 10.1007/s00592-020-01529-1. [DOI] [PubMed] [Google Scholar]
- 80.Morledge MD, Pories WJ. Bariatric surgery and cognitive impairment. Obesity (Silver Spring) 2021;29:1239–1241. doi: 10.1002/oby.23187. [DOI] [PubMed] [Google Scholar]
- 81.Handley JD, Williams DM, Caplin S, Stephens JW, Barry J. Changes in Cognitive Function Following Bariatric Surgery: a Systematic Review. Obes Surg. 2016;26:2530–2537. doi: 10.1007/s11695-016-2312-z. [DOI] [PubMed] [Google Scholar]
- 82.Ashrafian H, Harling L, Darzi A, Athanasiou T. Neurodegenerative disease and obesity: what is the role of weight loss and bariatric interventions? Metab Brain Dis. 2013;28:341–353. doi: 10.1007/s11011-013-9412-4. [DOI] [PubMed] [Google Scholar]
- 83.Chen NC, Chen CL, Shen FC. The Risk Factors of Severe Hypoglycemia in Older Patients with Dementia and Type 2 Diabetes Mellitus. J Pers Med. 2022;12 doi: 10.3390/jpm12010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silverman JM, Zhu CW, Schmeidler J, Lee PG, Alexander NB, Guerrero-Berroa E, Beeri MS, West RK, Sano M, Nabozny M, Karran M. Does computerized cognitive training improve diabetes self-management and cognition? Diabetes Res Clin Pract. 2022;195:110149. doi: 10.1016/j.diabres.2022.110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeCarlo K, Wallia A, Kang RH, Cooper A, Cherupally M, Harris SA, Aikman C, Liss DT, Ackermann RT, O'Brien MJ. Initiating second-line antidiabetic medication among older adults with type 2 diabetes on Metformin. BMC Geriatr. 2022;22:97. doi: 10.1186/s12877-022-02792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wojszel ZB, Kasiukiewicz A. A retrospective cross-sectional study of type 2 diabetes overtreatment in patients admitted to the geriatric ward. BMC Geriatr. 2019;19:242. doi: 10.1186/s12877-019-1256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X, Qiang Q, Li N, Feng P, Wei W, Hölscher C. Neuroprotective Mechanisms of Glucagon-Like Peptide-1-Based Therapies in Ischemic Stroke: An Update Based on Preclinical Research. Front Neurol. 2022;13:844697. doi: 10.3389/fneur.2022.844697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Creo AL, Cortes TM, Jo HJ, Huebner AR, Dasari S, Tillema JM, Lteif AN, Klaus KA, Ruegsegger GN, Kudva YC, Petersen RC, Port JD, Nair KS. Brain functions and cognition on transient insulin deprivation in type 1 diabetes. JCI Insight. 2021;6 doi: 10.1172/jci.insight.144014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Zou C, Na H, Zeng W, Li X. Effect of Different Glucose Monitoring Methods on Bold Glucose Control: A Systematic Review and Meta-Analysis. Comput Math Methods Med. 2022;2022:2851572. doi: 10.1155/2022/2851572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, Huang J, Tao J. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 2021;58:671–685. doi: 10.1007/s00592-020-01648-9. [DOI] [PubMed] [Google Scholar]
- 91.Diniz Pereira J, Gomes Fraga V, Morais Santos AL, Carvalho MDG, Caramelli P, Braga Gomes K. Alzheimer's disease and type 2 diabetes mellitus: A systematic review of proteomic studies. J Neurochem. 2021;156:753–776. doi: 10.1111/jnc.15166. [DOI] [PubMed] [Google Scholar]
- 92.Kanthi A, Singh D, Manjunath NK, Nagarathna R. Changes in Electrical Activities of the Brain Associated with Cognitive Functions in Type 2 Diabetes Mellitus: A Systematic Review. Clin EEG Neurosci. 2022:15500594221089106. doi: 10.1177/15500594221089106. [DOI] [PubMed] [Google Scholar]
- 93.Erbil D, Eren CY, Demirel C, Küçüker MU, Solaroğlu I, Eser HY. GLP-1's role in neuroprotection: a systematic review. Brain Inj. 2019;33:734–819. doi: 10.1080/02699052.2019.1587000. [DOI] [PubMed] [Google Scholar]
- 94.Zhang JH, Zhang XY, Sun YQ, Lv RH, Chen M, Li M. Metformin use is associated with a reduced risk of cognitive impairment in adults with diabetes mellitus: A systematic review and meta-analysis. Front Neurosci. 2022;16:984559. doi: 10.3389/fnins.2022.984559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, Bhargava A, Bode BW, Carlson A, Chaytor NS, Fox DS, Goland R, Hirsch IB, Kruger D, Kudva YC, Levy C, McGill JB, Peters A, Philipson L, Philis-Tsimikas A, Pop-Busui R, Shah VN, Thompson M, Vendrame F, Verdejo A, Weinstock RS, Young L, Miller KM Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group. Effect of Continuous Glucose Monitoring on Hypoglycemia in Older Adults With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2020;323:2397–2406. doi: 10.1001/jama.2020.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gothong C, Singh LG, Satyarengga M, Spanakis EK. Continuous glucose monitoring in the hospital: an update in the era of COVID-19. Curr Opin Endocrinol Diabetes Obes. 2022;29:1–9. doi: 10.1097/MED.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klarskov CK, Lindegaard B, Pedersen-Bjergaard U, Kristensen PL. Remote continuous glucose monitoring during the COVID-19 pandemic in quarantined hospitalized patients in Denmark: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:968. doi: 10.1186/s13063-020-04872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allen NA, Litchman ML, May AL. Using advanced diabetes technologies in patients with dementia in assisted living facilities: Case studies. Cogent Med. 2017;4:1411632. [Google Scholar]
- 99.Masierek M, Nabrdalik K, Janota O, Kwiendacz H, Macherski M, Gumprecht J. The Review of Insulin Pens-Past, Present, and Look to the Future. Front Endocrinol (Lausanne) 2022;13:827484. doi: 10.3389/fendo.2022.827484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boughton CK, Hartnell S, Thabit H, Mubita WM, Draxlbauer K, Poettler T, Wilinska ME, Hood KK, Mader JK, Narendran P, Leelarathna L, Evans ML, Hovorka R. Hybrid closed-loop glucose control compared with sensor augmented pump therapy in older adults with type 1 diabetes: an open-label multicentre, multinational, randomised, crossover study. Lancet Healthy Longev. 2022;3:e135–e142. doi: 10.1016/S2666-7568(22)00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bionic Pancreas Research Group. Russell SJ, Beck RW, Damiano ER, El-Khatib FH, Ruedy KJ, Balliro CA, Li Z, Calhoun P, Wadwa RP, Buckingham B, Zhou K, Daniels M, Raskin P, White PC, Lynch J, Pettus J, Hirsch IB, Goland R, Buse JB, Kruger D, Mauras N, Muir A, McGill JB, Cogen F, Weissberg-Benchell J, Sherwood JS, Castellanos LE, Hillard MA, Tuffaha M, Putman MS, Sands MY, Forlenza G, Slover R, Messer LH, Cobry E, Shah VN, Polsky S, Lal R, Ekhlaspour L, Hughes MS, Basina M, Hatipoglu B, Olansky L, Bhangoo A, Forghani N, Kashmiri H, Sutton F, Choudhary A, Penn J, Jafri R, Rayas M, Escaname E, Kerr C, Favela-Prezas R, Boeder S, Trikudanathan S, Williams KM, Leibel N, Kirkman MS, Bergamo K, Klein KR, Dostou JM, Machineni S, Young LA, Diner JC, Bhan A, Jones JK, Benson M, Bird K, Englert K, Permuy J, Cossen K, Felner E, Salam M, Silverstein JM, Adamson S, Cedeno A, Meighan S, Dauber A. Multicenter, Randomized Trial of a Bionic Pancreas in Type 1 Diabetes. N Engl J Med. 2022;387:1161–1172. doi: 10.1056/NEJMoa2205225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, Donaghue KC International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group, Magliano DJ, Maniam J, Orchard TJ, Rai P, Ogle GD. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741–760. doi: 10.1016/S2213-8587(22)00218-2. [DOI] [PubMed] [Google Scholar]
- 103.TODAY Study Group. Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385:416–426. doi: 10.1056/NEJMoa2100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagar SD, Pemu P, Qian J, Boerwinkle E, Cicek M, Clark CR, Cohn E, Gebo K, Loperena R, Mayo K, Mockrin S, Ohno-Machado L, Ramirez AH, Schully S, Able A, Green A, Zuchner S SEEC Consortium, Jordan IK, Meller R. Investigation of hypertension and type 2 diabetes as risk factors for dementia in the All of Us cohort. Sci Rep. 2022;12:19797. doi: 10.1038/s41598-022-23353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wharton W, Anderson A, Hayden KM, Carmichael OT, Clark JM, Luchsinger JA, Espeland M, Yasar S. Effect of renin-angiotensin system antihypertensive medication use on cognitive function in diabetes mellitus with obesity or overweight: An ancillary study to the Action for Health in Diabetes (Look AHEAD) trial. Diabetes Obes Metab. 2022;24:2443–2453. doi: 10.1111/dom.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li XY, Zhang M, Xu W, Li JQ, Cao XP, Yu JT, Tan L. Midlife Modifiable Risk Factors for Dementia: A Systematic Review and Meta-analysis of 34 Prospective Cohort Studies. Curr Alzheimer Res. 2019;16:1254–1268. doi: 10.2174/1567205017666200103111253. [DOI] [PubMed] [Google Scholar]
- 107.Williamson JD, Launer LJ, Bryan RN, Coker LH, Lazar RM, Gerstein HC, Murray AM, Sullivan MD, Horowitz KR, Ding J, Marcovina S, Lovato L, Lovato J, Margolis KL, Davatzikos C, Barzilay J, Ginsberg HN, Linz PE, Miller ME Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Investigators. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324–333. doi: 10.1001/jamainternmed.2013.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stefanidis KB, Askew CD, Greaves K, Summers MJ. The Effect of Non-Stroke Cardiovascular Disease States on Risk for Cognitive Decline and Dementia: A Systematic and Meta-Analytic Review. Neuropsychol Rev. 2018;28:1–15. doi: 10.1007/s11065-017-9359-z. [DOI] [PubMed] [Google Scholar]
- 109.Li H, Zhao S, Wang R, Gao B. The association between cognitive impairment/dementia and albuminuria: a systematic review and meta-analysis. Clin Exp Nephrol. 2022;26:45–53. doi: 10.1007/s10157-021-02127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 111.Calvin CM, Conroy MC, Moore SF, Kuzma E, Littlejohns TJ. Association of Multimorbidity, Disease Clusters, and Modification by Genetic Factors With Risk of Dementia. JAMA Netw Open. 2022;5:e2232124. doi: 10.1001/jamanetworkopen.2022.32124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Q, Jia M, Yan Z, Li Q, Sun F, He C, Li Y, Zhou X, Zhang H, Liu X, Bu X, Gao P, He H, Zhao Z, Zhu Z. Activation of Glucagon-Like Peptide-1 Receptor Ameliorates Cognitive Decline in Type 2 Diabetes Mellitus Through a Metabolism-Independent Pathway. J Am Heart Assoc. 2021;10:e020734. doi: 10.1161/JAHA.120.020734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rapp SR, Gaussoin SA, Sachs BC, Chelune G, Supiano MA, Lerner AJ, Wadley VG, Wilson VM, Fine LJ, Whittle JC, Auchus AP, Beddhu S, Berlowitz DR, Bress AP, Johnson KC, Krousel-Wood M, Martindale-Adams J, Miller EC, Rifkin DE, Snyder JK, Tamariz L, Wolfgram DF, Cleveland ML, Yang M, Nichols LO, Bryan RN, Reboussin DM, Williamson JD, Pajewski NM SPRINT Research Group. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol. 2020;19:899–907. doi: 10.1016/S1474-4422(20)30319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Espeland MA, Dutton GR, Neiberg RH, Carmichael O, Hayden KM, Johnson KC, Jeffery RW, Baker LD, Cook DR, Kitzman DW, Rapp SR Action for Health in Diabetes (Look AHEAD) Research Group. Impact of a Multidomain Intensive Lifestyle Intervention on Complaints About Memory, Problem-Solving, and Decision-Making Abilities: The Action for Health in Diabetes Randomized Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2018;73:1560–1567. doi: 10.1093/gerona/gly124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lehtisalo J, Lindström J, Ngandu T, Kivipelto M, Ahtiluoto S, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Eriksson JG, Uusitupa M, Tuomilehto J, Luchsinger J Finnish Diabetes Prevention Study. Association of Long-Term Dietary Fat Intake, Exercise, and Weight with Later Cognitive Function in the Finnish Diabetes Prevention Study. J Nutr Health Aging. 2016;20:146–154. doi: 10.1007/s12603-015-0565-1. [DOI] [PubMed] [Google Scholar]