Abstract
Chronic liver disease (CLD) is a continuous process that causes a reduction of liver function lasting more than six months. CLD includes alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), chronic viral infection, and autoimmune hepatitis, which can lead to liver fibrosis, cirrhosis, and cancer. Liver inflammation and oxidative stress are commonly associated with the development and progression of CLD. Molecular signaling pathways such as AMP-activated protein kinase (AMPK), C-Jun N-terminal kinase, and peroxisome proliferator-activated receptors (PPARs) are implicated in the pathogenesis of CLD. Therefore, antioxidant and anti-inflammatory agents from natural products are new potent therapies for ALD, NAFLD, and hepatocellular carcinoma (HCC). In this review, we summarize some powerful products that can be potential applied in all the stages of CLD, from ALD/NAFLD to HCC. The selected agents such as β-sitosterol, curcumin, genistein, and silymarin can regulate the activation of several important molecules, including AMPK, Farnesoid X receptor, nuclear factor erythroid 2-related factor-2, PPARs, phosphatidylinositol-3-kinase, and lysyl oxidase-like proteins. In addition, clinical trials are undergoing to evaluate their efficacy and safety.
Keywords: Chronic liver disease, Alcoholic liver disease, Non-alcoholic fatty liver disease, Hepatocellular carcinoma, Natural products, Inflammation, Oxidative stress, Treatment, Clinical trials
Core Tip: Chronic liver disease (CLD) is a continuous process that causes a reduction of liver function lasting more than six months. CLD can be subclassified into alcoholic liver disease, non-alcoholic fatty liver disease, chronic viral infection, and autoimmune hepatitis, which can lead to liver fibrosis, cirrhosis, and cancer. Liver inflammation and oxidative stress are commonly associated with the development and progression of CLD. Therefore, anti-inflammatory and antioxidant agents are promising drugs for CLD treatment. Clinical trials are undergoing to evaluate their efficacy and safety.
INTRODUCTION
Chronic liver disease (CLD) is a continuous process of inflammation, destruction, and regeneration of liver parenchyma, with a reduction of liver function that lasts more than six months[1]. According to the spectrum of etiologies of CLD, it can be subclassified into alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), chronic viral infection, and autoimmune hepatitis, which can lead to liver fibrosis, cirrhosis, and cancer[2-4].
The spectrum of ALD includes alcoholic fatty liver, alcoholic hepatitis, fibrosis, and cirrhosis[5]. Alcohol drinking history and volume are direct causing factors for ALD, which can progress into hepatocellular carcinoma (HCC, Figure 1), the most common type of primary liver cancer[3]. In addition, factors such as age, gender, genetic variants, chronic virus infection, and smoking contribute to the development and progression of ALD[6,7]. Development of transgenic mouse models of ALD has provided a powerful tool to understand the disease pathogenesis[8]. Cellular and molecular mechanism studies have advanced our knowledge of the pathogenesis of ALD[8,9]. Multiple processes including excessive accumulation of lipids, reactive oxygen species (ROS) production, mitochondrial dysfunction, and cell inflammation and death are involved in ALD pathogenesis[10]. Despite all these efforts, there are no Food and Drug Administration-approved therapies for ALD[11].
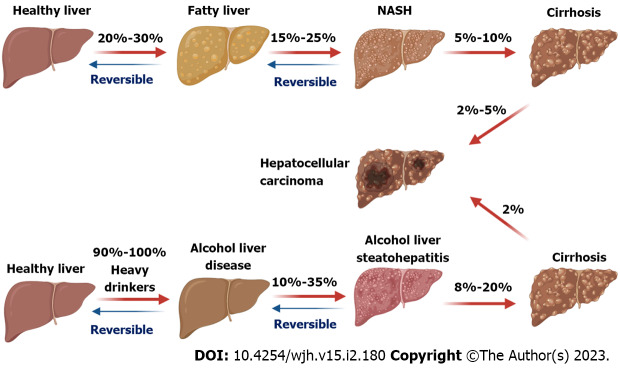
Figure 1.
The development of hepatocellular carcinoma from non-alcoholic fatty liver disease and alcoholic fatty liver disease. The prevalence (20%-30%) of non-alcoholic fatty liver (NAFL) in the world population and the following percentages of NAFL into non-alcoholic steatohepatitis (NASH) (15%-25%), NASH into cirrhosis (5%-10%), and cirrhosis into hepatocellular carcinoma (HCC) (2%-5%) are labeled. Around 90%-100% of heavy drinkers can develop alcoholic liver disease (ALD), then the percentages of progression from simple ALD into alcohol liver steatohepatitis (10%-35%), cirrhosis (8%-20%), and HCC (2%) are shown in the graphic. This cartoon was created using Biorender online tools (https://biorender.com). NASH: Non-alcoholic steatohepatitis.
NAFLD is the most common CLD with a broad spectrum, ranging from non-alcohol fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH) with the progression of liver inflammation and different degrees of fibrosis[12]. NASH also can progression to HCC (Figure 1)[13]. The global prevalence of NAFLD was estimated to be 29.8% [95% confidence interval (CI): 28.6%-31.1%] in 2019[14], and the prevalence is estimated to be 32.4% (95%CI: 29.9-34.9) in 2022[15]. It affects more than 30% of people in the United States[16]. NAFLD is closely associated with other metabolic disorders, including obesity, diabetes, chronic kidney disease, and cardiovascular disease[17,18]. A new nomenclature for NAFLD has been suggested by a group of experts, namely metabolic dysfunction-associated fatty liver disease (MAFLD), which is based on the evidence of hepatic steatosis plus one of the following three criteria, including the presence of overweight or obesity, or presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation[19,20]. However, there are no currently approved medicines for NAFLD or MAFLD treatment[12].
Oxidative stress and inflammation are commonly associated with CLD independent of disease types[21,22]. For example, ethanol consumption can induce alcohol liver steatosis, inflammation, and production of ROS, resulting in the development of ALD with liver inflammation and oxidative stress[23]. In addition to hepatocyte injury, both innate and adaptive immune cells including macrophages, dendritic cells, neutrophils, and lymphocytes are involved in the development of CLD[24,25]. Production of ROS and inflammatory cytokines produced by immune cells under the stimuli of alcohol and diet metabolites, such as cholesterol and acetaldehyde, can further trigger liver oxidative stress, inflammation, and cell apoptosis or death to cause the progression of CLD[26,27].
Treatments, such as lifestyle intervention[28,29], gene editing[30,31], and pharmaceutical therapies[32], can ameliorate or cure CLD at the early stages. However, server condition of CLD requires liver transplantation, which lacks donor availability. Here, the roles of antioxidants and anti-inflammatory agents in CLD treatment, especially for ALD, NAFLD, and HCC, are reviewed. Examples of clinical trials for evaluating the potential efficacies of potential treatment agents are summarized.
DATABASE SEARCHING
The databases of PubMed, Cochrane Library (Wiley), Embase, Web of Science, and Google Scholar from the last five years (from July 2020) were searched for studies by keywords of CLD, ALD, NAFLD, or HCC, and their treatments with anti-oxidative and anti-inflammatory agents. Papers written in English were studied. When reviewing oxidative stress and/or inflammation-related molecules in CLD, the time restriction of the published data was removed.
INFLAMMATION AND OXIDATIVE STRESS IN CLD AND UNDERLYING MOLECULAR MECHANISMS
Inflammation and oxidative stress are commonly associated with each other in the pathogenesis of CLD[33], including ALD, NAFLD, and HCC. Several common signaling pathways are involved in liver inflammation and oxidative stress, such as Toll-like receptor (TLR)/nuclear factor kappa B (NF-κB) and heme oxygenase-1 (HO-1) signaling pathways[34,35]. Dysregulation of lipid metabolism contributes to the pathogenesis of CLD[36,37], which is commonly associated with liver oxidative stress and inflammation. Molecules such as peroxisome proliferator-activated receptors (PPARs) are involved in alcohol or non-alcohol factors-induced lipid metabolism dysregulation and hepatic steatosis[38,39]. In this section, we review some important signaling pathways involved in liver inflammation and oxidative stress during CLD.
AMP-activated protein kinase
AMP-activated protein kinase (AMPK) as a crucial energy sensor plays an important role in energy metabolism in multiple tissues, including the liver[40]. Activation of AMPK by metformin can reduce induced triglyceride accumulation in the livers of mice treated with ethanol compared to control groups[41]. Activation of sirtuin 1 (SIRT1)/Liver kinase B1/AMPK signaling with botulin (a triterpene) treatment reduces serum aminotransferase and triglyceride levels in mice with chronic-binge ethanol[42]. Activation of the AMPK signaling pathway with plant sterol ester of α-linolenic acid can also attenuate endoplasmic reticulum (ER) stress-induced hepatocyte apoptosis in mice with NAFLD[43]. Similarly, stimulating the activation of AMPK by an activator PXL770 reduces de novo lipogenesis in primary mice and human hepatocytes, which can result in the suppression of hepatic steatosis, inflammation, and fibrogenesis in mice with NASH. In addition, PXL770 has a direct inhibitory effect on the production of proinflammatory cytokines and activation of hepatic stellate cells[44].
C-Jun N-terminal kinase
Activation of C-Jun N-terminal kinase (JNK) signaling pathway is involved in lipotoxicity, inflammation, ER stress, and mitochondrial dysfunction. Palmitic acid (PA)-induced activation of JNK/Sab (SH3 domain-binding protein 5) signaling contributes to NASH progression, which is associated with mitochondrial dysfunction, oxidative stress, hepatic steatosis, and inflammation[45].
Deficiency of hypoxia-induced gene domain protein-1α (Higd-1α), a mitochondrial inner membrane protein, promotes free fatty acids (FFAs)-induced apoptosis and oxidative stress in hepatocytes[46]. In this process, the production of cytosolic oxidized mitochondrial DNA (ox-mtDNA) is increased, which induces activation of NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasomes and JNK signaling but decreases fatty acid oxidation (FAO). In contrast, exercise can increase the expression of Higd-1α in the liver to ameliorate hepatic steatosis and inflammation by suppressing ox-mtDNA/NLRP3/JNK pathway[46].
Farnesoid X receptor
Farnesoid X receptor (FXR) is a nuclear receptor that metabolically regulates glucose, bile acid, and lipid metabolism[47,48]. Treatment of Lactobacillus reuteri can ameliorate lipid accumulation in mice with ALD by upregulating FXR expression, which is associated with the upregulation of carbohydrate response element binding protein and downregulation of sterol regulatory element binding transcription factor 1 and cluster of differentiation (CD36)[49]. In addition, the FXR/fibroblast growth factors (FGFs) axis (FGF-15 and FGF-19) also plays a key in the regulation of hepatic inflammation, lipid metabolism, and fibrosis[50,51]. Clinically, treatment of FXR agonist vonafexor also shows anti-fibrotic effects in patients with NASH[52].
Nuclear factor erythroid 2-related factor-2/HO-1
Nuclear factor erythroid 2-related factor-2 (Nrf2) is a key transcription factor that plays a critical role in oxidative stress and inflammatory responses. For example, Nrf2 expression is positively associated with oyster peptide-mediated suppression of inflammation mediated by upregulation of NF-κB signaling and upregulation of antioxidant response in mice with ALD[53]. Activation of Nrf2 is involved in the protective effect of diallyl disulfide against chemical (CCl4)-induced liver injury and oxidative stress[54]. HO-1, an inducible form of antioxidant zyme HO isoforms that regulates heme group degradation, plays an essential role in liver inflammation and oxidative stress[55]. Nrf2 can regulate HO-1 to suppress liver oxidative stress, ER stress, and inflammation[56].
Nrf2 also plays an important role in the pathogenesis of NASH. Activation of Nrf2 can ameliorate liver inflammation, ER stress, iron overload, and lipotoxicity to suppress NASH and oxidative stress, which can be suppressed by transforming growth factor-beta (TGF-β)[57]. Activation of Nrf2 can suppress the expression of ROS and NLRP3 and inhibit Caspase 1/interleukin (IL)-1β and IL-18-mediated inflammation[58]. In addition, pharmacologic activation of Nrf2 by TBE-31, acetylenic tricyclic bis(cyano enone), decreases insulin resistance and liver fat accumulation, inflammation, fibrosis, and oxidative stress in mice with a high-fat plus fructose diet. However, the TBR-31-mediated effect was abolished in Nrf2-null mice[59].
PPARs
PPARs are a group of nuclear receptor proteins that function as ligand-activated receptors to regulate genes in energy metabolism and inflammation. PPARs comprise three subtypes, PPAR-α, PPAR-β/δ, and PPAR-γ, which are pharmaceutical targets for disease treatments[60,61]. These PPARs play important roles in ALD[62], NAFLD[63], hepatitis virus-mediated liver injury[64], and HCC[65].
Activation of PPAR-α by agonist WY-14643 (Pirinixic Acid, Figure 2) ameliorates ethanol-induced liver fat accumulation by increasing FAO[66]. Sustained activation of PPAR-α can decrease obesity and improve insulin resistance to rebuild glucose homeostasis. However, it increases the risk of HCC development due to liver ER stress[67]. Treatment with GW9662, an antagonist of PPAR-γ, significantly decreased lipopolysaccharide (LPS)/TLR4-mediated expression of IL-1β, IL-6, inducible nitric oxide synthase, and nitrite (NO2−) concentration[68].
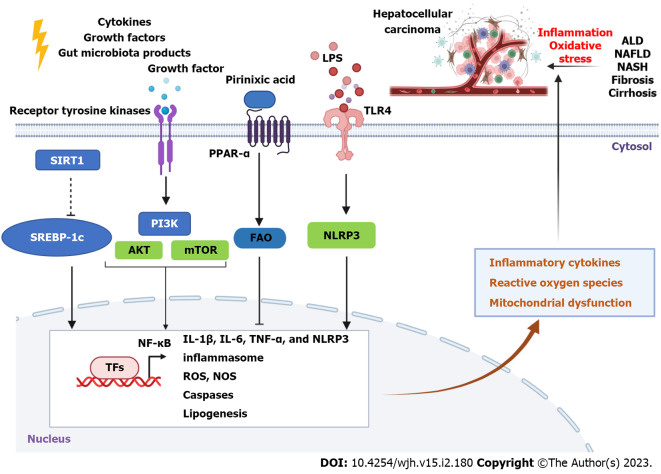
Figure 2.
Molecular signaling pathway in liver inflammation and oxidative stress. Inflammation and oxidative stress are involved in the development of chronic liver diseases such as alcoholic liver disease, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, fibrosis, and cirrhosis into hepatocellular carcinoma. Many factors including cytokines, growth factors, and gut microbiota-derived products such as lipopolysaccharide can activate their receptors such as peroxisome proliferator-activated receptor-α and toll-like receptor 4, resulting in upregulation or inhibition of downstream genes to induce or prevent inflammatory cytokines and production of reactive oxygen species. This cartoon was created using Biorender online tools (https://biorender.com). LPS: Lipopolysaccharide; TLR4: Toll-like receptor 4; ALD: Alcoholic liver disease; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; PPAR-α: Peroxisome proliferator-activated receptor-α; SIRT1: Sirtuin 1; SREBP-1c: Sterol regulatory element binding protein 1c; PI3K: Phosphatidylinositol-3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of rapamycin; FAO: Fatty acid oxidation; NLRP3: NOD-like receptor family pyrin domain containing 3; NF-κB: Nuclear factor kappa B; IL: Interleukin; TNF-α: Tumor necrosis factor-α; NLRP3: NOD-like receptor family pyrin domain containing 3; ROS: Reactive oxygen species; NOS: Nitric oxide synthase.
Treatment with a dual PPAR-α/γ agonist Saroglitazar is able to reduce serum transaminases and 63% of overweight patients with NALFD reduced bodyweight (> 5%)[69]. In addition, many clinical trials have been performed to evaluate the effects of PPARs in ALD. For example, pemafibrate can improve liver function and glucose metabolism in patients with hypertriglyceridemia[70] and decrease liver stiffness in patients with NAFLD measured by magnetic resonance elastography (ClinicalTrials.gov, number: NCT03350165)[71]. Treatments that target PPAR-α such as pemafibrate[71], PPAR-β/δ such as seladelpar[72], and PPAR-γ such as pioglitazone[73,74] show promising efficacy in the clinic for CLD treatment (Figure 3). Meanwhile, a dual PPAR-α/δ agonist elafibranor and a pan-PPAR regulator lanifibranor show promising efficacy for CLD treatment in the clinic[75,76]. For example, a phase 2b clinical trial reveals that treatment of lanifibranor (1200 mg) compared with the placebo can decrease at least 2 points of steatosis, activity, and fibrosis score that incorporates scores for ballooning and inflammation[76].
Figure 3.
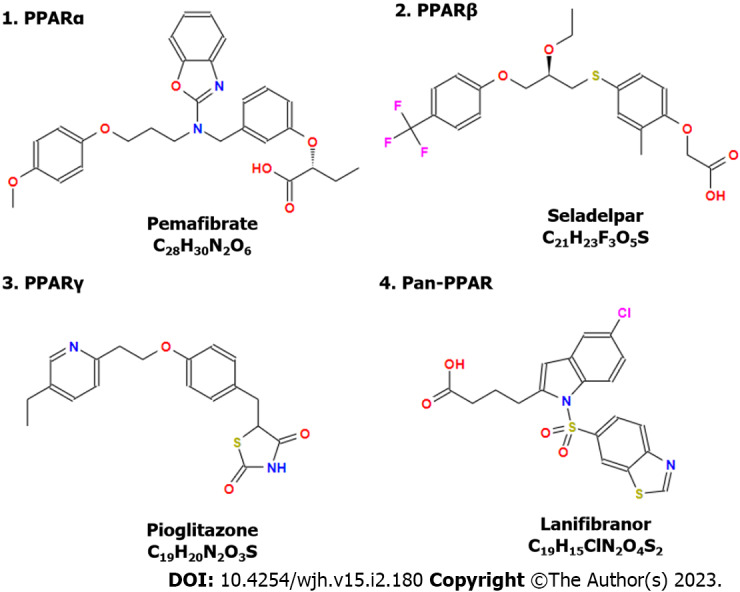
Structures of peroxisome proliferator-activated receptor agonists or modulators applied for the treatment of chronic liver disease. Many peroxisome proliferator-activated receptor regulators have been evaluated in the clinic, showing promising effects in patients with chronic liver disease. All the chemical structures were collected online from the Chemical Book (https://www.chemicalbook.com, accessed on August 10, 2022). PPAR: Peroxisome proliferator-activated receptor.
Phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin
The phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB or AKT)/mammalian target of rapamycin (mTOR) signaling pathway is implicated in the pathogenesis of liver disease and therapy[77,78]. For example, this signaling pathway is involved in the anti-steatosis effect of D-mannose in ALD[79]. Activation of PI3K/AKT/mTOR signaling pathway by arecoline (2.5 μM), an alkaloid ester found in the betel nut palm seeds, promotes the proliferation and migration of HepG2 cells[80]. Acid-sensitive ion channel 1α can upregulate the activation of PI3K/AKT/mTOR signaling pathway to enhance the expression of matrix metalloproteinase (MMP)2 and MMP9 to promote liver cancer cell (HepG2 and SK-Hep1 cells) migration and invasion[81]. One human study also indicates that PI3K is more strongly expressed in tumors than that in cirrhotic livers but not AKT and mTOR, and the expression of PI3K in tumor tissues is independent of etiology[82]. In addition, activation of growth factor receptor protein tyrosine kinases (Figure 2) can result in autophosphorylation on tyrosine residues and subsequent binding and activation of PI3K[83], playing an important role in cancer development. Inhibition or blockade of this signaling pathway can suppress liver fibrosis[84,85] and cancer progression[86,87].
Furthermore, lysyl oxidase family members (LOX) and LOX-like proteins (LOXL1-4) play important roles in liver fibrosis and cancer[88]. Insulin resistance can promote extracellular matrix stabilization by upregulating hepatic production of LOXL2 through upregulation of the expression of Forkhead box protein O1 in NAFLD[89]. In addition, galectins such as galectin-3 also play an essential role in CLD[90-92], including liver fibrosis and cancer. Overall, these molecular signaling pathways are involved in liver inflammation and oxidative stress to promote the development of CLD to HCC (Figure 2).
ANTIOXIDANT AND ANTI-INFLAMMATORY AGENTS IN ALD
Many ingredients from natural products or plants have both antioxidant and anti-inflammatory functions, which are good candidates for CLD treatment. Some of these products may have preventive effects on hepatic steatosis in ALD and NAFLD. For example, diallyl trisulfide (DATS) is a bioactive compound isolated from garlic and can reduce serum levels of aspartate transaminase (AST) and alanine aminotransferase (ALT) and decrease alcohol-induced liver injury[93]. DATS can upregulate PPAR-α expression and down-regulate sterol regulatory element binding protein 1c (SREBP-1c) expression to inhibit hepatic steatosis. Meanwhile, it can reduce liver oxidative stress by increasing antioxidant products and reducing ROS and malondialdehyde (MDA) production in the fatty liver[93]. In this section, we review some promising agents in ALD treatments either in animal models or clinical trials.
β-sitosterol
β-sitosterol is isolated from the roots of Panax ginseng[94]. As a plant sterol, β-sitosterol can reduce alcohol-induced liver injury and oxidative stress via restoration of erythrocyte membrane fluidity, upregulation of glutathione (GSH) activity, and reduction of MDA production. In addition, β-sitosterol can suppress apoptosis-related gene expression by increasing the phosphorylation of PI3K and AKT[95].
Curcumin
Curcumin is an orange-yellow component of turmeric or curry powder isolated from the rhizome of Curcuma longa[96,97]. Supplementation of curcumin can significantly increase the activities of superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) to reduce swimming-induced oxidative stress in mice, by activating Nrf2 signaling pathway[98]. Treatment of curcumin significantly decreases serum levels of ALT, AST, alkaline phosphatase (ALP), gamma-glutamyl transferase, Arginase I, and blood urea nitrogen, while it increases serum levels of Albumin and total protein in ethanol-treated rats compared to the control group[99]. Development of self-assembled micelles of curcumin can be administered by oral delivery to enhance its anti-oxidative stress ability to prevent ALD and gastric mucosa damage[100]. Encapsulation enables to improve the adsorption of curcumin in intestinal epithelial cells and enhance its hepatoprotective effects in rats, via increasing the activity of GPx and decreasing high levels of MDA in the liver[101]. Furthermore, a combined treatment of curcumin and bacicalin shows more protective effects on ALD in rats by reducing liver oxidative damage through activation of the Nrf2/HO-1 signaling pathway[102].
Empagliflozin
Empagliflozin (EMPA) has benefits in cardiovascular, renal, and cerebral diseases, which is potentially mediated through its antioxidant and anti-inflammatory activities. Treatment with EMPA can decrease serum levels of ALT, AST, and ALP. It also increases the activities of GSH and SOD in the liver homogenates and decreases the liver content of MDA and nitric oxide (NO)[103]. Moreover, EMPA can downregulate NF-κB signaling to suppress the expression of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6, which is associated with the upregulation of PPAR-γ, Nrf2, and their target gene HO-1[103].
Gastrodin
Gastrodin is the main bioactive component of Gastrodia elata Blume and displays anti-inflammatory and antioxidant properties. For example, administration of gastrodin (50 or 100 mg/kg) in mice significantly inhibits concanavalin A (ConA)-induced acute hepatitis, partly by suppressing IL-6/Janus Kinase 2/signal transducer and activator of transcription 3 signaling pathway[104]. In addition, treatment with gastrodin ameliorated acetaminophen-induced liver injury in mice. The anti-inflammatory and anti-oxidative stress functions of gastrodin are mediated through the inhibition of signal-regulated kinase/JNK/mitogen-activated protein kinase signaling pathways and hepatic MDA activity, as well as activation of Nrf2 expression and SOD activity[105].
Genistein
Genistein is an isoflavone first isolated from the brooming plant Dyer's Genista tinctoria, which is widely distributed in the Fabaceae family[106-109]. Treatment of genistein at a dose of 0.3 mmol/kg of bodyweight can ameliorate liver fibrosis and apoptosis in mice by suppressing the expression of proinflammatory cytokines such as TNF-α, IL-6, profibrotic cytokines such as TGF-β1, and cell caspase 3[110]. In contrast, another study shows that supplementation of soy proteins significantly decreases serum ALT concentrations and hepatic TNF-α and CD-14 expression and decreases NF-κB protein in casein-based 35% high-fat ethanol liquid diet (EtOH)-treated mice by inhibiting β-catenin signaling[111]. More functional studies of genistein have been performed in NAFLD models, which are discussed in the following section.
Lactoferrin
Lactoferrin (LF) is an iron-binding protein found at relatively high concentrations in mammalian milk[112]. LF displays multiple functions, including antioxidant, anti-cancer, and anti-inflammatory activities. For example, LF treatment can decrease the levels of liver superoxide and suppress liver inflammation in male mice with alcoholic-induced liver injury (ALI) by upregulating the expression of aldehyde dehydrogenase-2 and suppressing overexpression of cytochrome P450 2E1 (CYP2E1)[113]. LF treatment also displays a protective effect in female mice with acute ALI by regulating redox-stress response capacity[114]. The protective effect of LF on ALI is associated with the manipulation of gut microbiota and the modulation of hepatic alcohol metabolism[113].
Selenium
Selenium plays an essential role against oxidation, which is part of the catalytic center of different antioxidant selenoproteins including GPxs and selenoprotein P[115]. The serum levels of selenium are decreased in adult patients with acute and chronic alcoholic-related diseases, accompanied by liver damage and the severity of oxidation[115,116].
Silymarin
Silymarin is an active compound from the extracts of milk thistle (Silybum marianum)[117]. Silymarin displays antioxidant, antifibrotic, anti-inflammatory, and hepatoprotective properties in different types of CLD[118,119], such as ALD. Simultaneous supplementation of silymarin with alcohol treatment can reduce the ethanol-induced increase of serum ALT levels and hepatic microvesicular steatosis and TNF-α expression[120]. Another study on non-human primates also shows that silymarin can prevent the development of alcohol-induced liver fibrosis by decreasing the production of type I collagens[121].
Taraxasterol
Taraxasterol (TAS) is an active ingredient of Taraxacum officinale, which has protective effects on the liver and kidneys by reducing serum levels of ALT and AST, increasing serum and liver SOD and GPx, and maintaining the balance of ion homeostasis[122]. TAS also displays anti-inflammatory function in cultured mouse primary lymphocytes stimulated with Con A and in mice with Con A-induced acute hepatitis[123]. Mechanism studies reveal that TAS inhibits T cell activation and proliferation by suppressing IL-2/IL-2 receptor-mediated downstream signaling pathways[123].
Telmisartan
Telmisartan (TEL) exhibits similar effects with EMPA on ALD. Treatment of TEL (10 mg/kg/day) decreased serum levels of ALT, AST, and ALP in mice with ALD[124]. In addition, TEL displays anti-inflammatory and antioxidant properties in mice with ALD by increasing the activity of SOD and GPx to reduce liver contents of NO and MDA, upregulating the expression of Nrf-2, PPAR-γ, and Hmox-1, and downregulating NF-κB expression[124].
ANTIOXIDANT AND ANTI-INFLAMMATORY AGENTS IN NAFLD
Hepatic inflammation and oxidative stress are also associated with NAFLD pathogenesis[125]. Therefore, many above-discussed products also display similar bioactive functions against NAFLD.
β-sitosterol
Treatment with β-sitosterol can prevent high-fructose diet-induced macrovesicular hepatic steatosis and inhibit the progression of NAFL to NASH in male rats[126]. Meanwhile, it is also able to inhibit high-fructose diet-induced visceral obesity, hypertriglyceridemia, plasma insulin concentration, and homeostatic model assessment of insulin resistance (HOMA-IR) but increase plasma levels of adiponectin in female rats[127]. Another study shows that in combination with stigmasterol, a dietary phytosterol, β-sitosterol can alleviate a high-fat western-style diet-induced NAFLD in mice post-17-wk treatment, by decreasing hepatic di- and tri-acylglycerols and circulating ceramide levels[128].
Curcumin
Curcumin is a natural polyphenol, which shows anti-inflammatory and antioxidant activities. It can improve insulin resistance and reduce hepatic fat accumulation in dietary obese rat models[129]. Accumulating evidence identifies that curcumin can attenuate hepatic steatosis by suppressing hepatic expression of CD36, PPAR-γ, SREBP-1c, and fatty acid synthase (FAS) in NAFLD mice, through upregulation of Nrf2 and FXR expression and downregulation of liver X receptor α expression[130,131]. In addition, curcumin can induce activation of AMPK and upregulation of PPAR-α, and suppress the high-fat diet (HFD)-induced increase in the expression of SREBP-1, acetyl-CoA carboxylase 1, FAS, and CD36[132]. Meanwhile, curcumin is able to prevent intestinal permeability and suppress LPS/TLR4/NF-κB-mediated inflammatory response to protect against diet-induced hepatic steatosis and inflammation[133]. In addition, curcumin can also suppress NLRP3 inflammasome (Figure 2) and pro–IL-1β synthesis by suppressing LPS-mediated activation of NF-κB signaling pathway[134].
Ex vivo studies also show that treatment of curcumin decreases linoleic acid-induced ROS production and leptin-induced TNF-α expression in human peripheral blood mononuclear cells[135]. A randomized controlled trial in Iran demonstrates that supplementation with curcumin in a phytosomal form (1000 mg/day) significantly reduces body mass index (BMI), waist circumference, and serum levels of AST and ALT[136]. This dose was safe and well tolerated in NAFLD patients[136]. Another double-blind, randomized, placebo-controlled trial displays that daily supplementation of low-dose phospholipid curcumin (250 mg) for 2 mo can significantly decrease hepatic steatosis and serum AST levels in NAFLD patients compared to placebo[137]. In addition, a combined therapy of curcumin (500 mg/day) with piperine, an alkaloid in black pepper with many pharmacological effects on chronic diseases[138], also decreases the severity of NAFLD and serum ALP levels[139]. Large clinical trials are needed for further evaluation of the efficacy of curcumin and its synergistic treatments.
EMPA
EMPA is an inhibitor of sodium-glucose co-transporter 2 (SGLT2), which plays an important role in NAFLD. EMPA treatment can inhibit PA-induced lipid deposition in hepatocytes (HepG2 cells) and HFD-induced hepatic lipid accumulation and inflammation in mice by upregulating the expression of a stress-inducible protein Sestrin2 and activating AMPK-mTOR signaling pathway[140]. Another study demonstrates that EMPA can upregulate the expression of medium-chain acyl-CoA dehydrogenase in NASH liver and PA and glucose-treated hepatocytes by activating AMPK/forkhead box A2 signaling pathway, resulting in a reduction of hepatic lipid deposition in vivo and in vitro[141]. A meta-analysis shows that EMPA can significantly reduce BMI, HOMA-IR, AST, and liver fibrosis in patients with NAFLD[142].
In addition, other SGLT2 inhibitors or gliflozins, such as licogliflozin[143,144] and dapagliflozin[145,146], also can control glycemic production and bodyweight, normalize serum ALT levels, and reduce Fibrosis-4 NAFLD patients with T2DM.
Gastrodin
Gastrodin has been shown to significantly decrease lipid accumulation and inflammatory response in primary mice and human hepatocytes treated with 0.5 mmol/L PA along with 1.0 mmol/L oleic acid. In addition, it ameliorates diet-induced hepatic steatosis and inflammation in mice by activating the AMPK signaling pathway[147]. Gastrodin can also regulate lipid metabolism and display antioxidant effects in larval zebrafish with high-cholesterol diet-induced NAFLD[148].
Genistein
Genistein has been shown to play an important role in NAFLD and NASH treatment. Treatment of genistein reduces the levels of TNF-α and reduces TLR4 mRNA and protein expression and inflammation in the livers of rats with NASH[149]. A combination of genistein with metformin (0.2% + 0.23%) for 3 mo shows a synergistic effect on the reduction of AST, ALT, and TG, liver TG and number of macrophages, and NAFLD activity score (NAS) in HFD-fed mice[150]. The reduction of hepatic steatosis is associated with decreased mRNA levels of lipogenic-related genes SREBP-1c and FAS and upregulated mRNA expression of FAO-related gene carnitine palmitoyl transferase 1[150]. Genistein treatment (16 mg/kg BW/day) for 5 wk can significantly decrease hepatic steatosis, inflammation, and hepatocyte ballooning in ovariectomized rats with high-fat and high-fructose diet-induced NASH[151].
Consumption of dietary isoflavones including genistein is reversely associated with NAFLD, hypertension, and hyperlipidemia in a study on Chinese adults[152]. Molecular mechanism studies show that genistein can suppress the activation of SREBP-1c in FFA-induced fat accumulation in primary human hepatocytes, whereas genistein-mediated upregulation of PPAR-α proteins in normal hepatocytes is abolished in steatotic hepatocytes[153].
LF
LF is an iron-binding protein in mammalian milk and displays multiple functions, including antioxidant, anti-cancer, and anti-inflammatory activities. During NASH progression, LF treatment can inhibit NF-κB activation to downregulate a high-fat diet and chemical dimethylnitrosamine-induced liver injury, inflammation, and fibrosis[154]. Treatment with LF improves insulin sensitivity and reduces hepatic steatosis in ob/ob mice by downregulating SREBP-2. It also regulates hepatocellular iron transport by controlling the hepcidin-ferroportin axis to maintain liver oxidative balance and suppress hepatocyte death[155].
Mastiha
Mastiha is a natural and aromatic resin isolated from the trunk and brunches of mastic trees with antioxidant and anti-inflammatory properties[156]. Mice with diet-induced NASH fed with 0.2% (w/w) Mastiha supplementation for 8 wk can reduce the circulating ALT levels, NAS, hepatic steatosis, and liver collagen production[157]. This study also identifies that Mastiha supplementation changes NASH-induced gut microbiota profile to the diversity and composition of healthy mice. A randomized clinical trial (NCT03135873, www.clinicaltrials.gov) shows that supplementation of Mastiha improves the total antioxidant status (TAS) levels in NAFLD patients with severe obesity compared to that in the corresponding placebo group[158]. The anti-inflammatory function of Mastiha is associated with the expression of microRNA-155 in the plasma of NAFLD patients, which may regulate the differentiation and function of T helper-17 cells[159].
Selenium
Treatment with selenium-enriched green tea extract (200 mg/kg body weight) for 15 wk can significantly reduce body weight gain and visceral fat accumulation in mice with obesity and NAFLD[160]. Reduced serum levels of selenium are independently associated with hepatic fibrosis in NAFLD patients[161]. Another study reveals that selenium deficiency induces hepatic inflammation in pigs by activating the NF-κB signaling pathway, decreasing antioxidant capacity, and increasing ROS levels[162]. Selenium-enriched Lactobacillus acidophilus SNZ 86 (probiotic) can decrease western-style diet-induced hepatic steatosis in mice with NAFLD, by activating autophagy through the upregulation of AMPK/SIRT1 signaling pathway[163]. Co-supplementation of selenium with vitamin B6 can reduce liver lipid synthesis and deposition by increasing the expression of SIRT1 to downregulate SREBP-1c expression (Figure 2) and upregulate PPAR-α expression in HFD-fed rats[164].
Silymarin
The major active compound of silymarin is silybin. Treatment with silybin can significantly decrease lipid accumulation in mice with NAFLD by activating PPAR-α[165]. Since it can partially inhibit the effect of PPAR-α agonist fenofibrate, it is not suggested to be simultaneously applied with PPAR-α agonists. Silymarin also displays a synergistic effect with quercetin on the reduction of lipid accumulation in rat hepatocytes[166]. Silymarin treatment significantly ameliorates high fructose-induced oxidative stress and hepatic steatosis in rats[167]. Silymarin supplementation (560 mg daily) for 8 wk significantly improves serum AST/ALT ratio, ultrasound fatty liver grading, and BMI in patients with morbid obesity and NAFLD[168].
TEL
Treatment with TEL significantly improves fibrosis scores and reduces the levels of serum leptin and its expression in liver tissue[169]. As an angiotensin receptor blocker, it significantly decreases fasting serum-FFA levels and triglyceride-glucose index in patients with NAFLD[170]. TEL displays a similar effect as vitamin E on the reduction of NAS, and improvement of hepatic steatosis, but it has a better effect on the reduction of liver lobular inflammation and hepatocyte ballooning[171]. It can function as a PPAR-γ/α dual agonist to simultaneously improve insulin-sensitivity via activating PPAR-γ and improve lipid metabolism by activating PPAR-α[172].
Delta-tocotrienol
Tocotrienols are natural compounds that belong to one part of two vitamin E components (Tocopherols as another part), including α, β, γ, and δ tocotrienols[173]. Among them, δ-tocotrienol shows strongly anti-inflammatory activity, which can decrease insulin resistance, hepatic steatosis, and serum triglyceride concentrations in rats with diet-induced obesity[174]. Recent studies also show that δ-tocotrienol has anti-cancer properties by regulating angiogenesis and cell proliferation and apoptosis[175].
A human study indicates that oral supplementation of δ-tocotrienol (300 mg, twice daily) for 12 wk significantly decreases serum aminotransferases, high sensitivity C-reactive protein (hs-CRP), and MDA, and fatty liver index (FLI) score compared to placebo[176]. Clinical trials reveal that δ-tocotrienol supplementation results in a significant reduction in plasma glucose, insulin, glycosylated hemoglobin, MDA, high sensitive C-reactive protein, and proinflammatory cytokines (TNF-α and IL-6), and HOMA-IR in pre-diabetic and diabetic patients[177,178]. Another trial also demonstrates that treatment of δ-tocotrienol (300 mg, twice daily) for 24 wk further significantly reduces FLI score, HOMA-IR, and hepatic steatosis than placebo, except decreased serum levels of hs-CRP, MDA, ALT, and AST, without causing adverse events[179].
ANTIOXIDANT AND ANTI-INFLAMMATORY AGENTS IN LIVER CANCER
Both ALD and NAFLD are major contributors to HCC initiation and progression. Therefore, the above-discussed biomolecules may also exhibit anti-HCC effects. For example, treatment of β-sitosterol niosomes, a form of β-sitosterol with polyethylene glycol modification, shows cytotoxicity to HepG2 cells due to increased cellular uptake and displays in vivo anti-HCC ability in Wistar albino rats[180]. Treatment of β-sitosterol-assisted silver nanoparticles (BSS-SNPs) significantly inhibits the proliferation of HepG2 cells and their production of ROS and Nrf2, resulting in the regulation of pro-apoptotic genes such as Bcl-2 Associated X-protein and caspases 3 and 9[181]. Similarly, compounds including curcumin[182], EMPA[183], gastrodin[184], genistein[185], LF[186], selenium[187], silymarin[188], TAS[189], TEL[190], and delta-tocotrienol[191] display anti-HCC effects either in vitro or in vivo, or both (Table 1).
Table 1.
Antioxidant and anti-inflammatory agents for the treatment of hepatocellular carcinoma
|
Molecules
|
Model
|
Function
|
Ref.
|
| β-sitosterol | HepG2 cells; Rat HCC | Treatment of β-sitosterol niosomes displays direct cytotoxicity to HepG2 cells in vitro and anti-HCC ability in rats | [182] |
| Curcumin | HepG2 and SK-Hep-1 cells. A nude mouse xenograft model bearing HepG2 cells | It can inhibit cell proliferation and increase cell apoptosis and cell cycle arrest at the G0/G1 phase of cancer cells by downregulating the expression of BCLAF1 and inhibiting the activation of the PI3K/AKT/GSK-3β pathway | [183] |
| Empagliflozin | DENA-induced HCC in mice | It shows a synergistic effect on the control of angiogenesis, invasion, and metastasis of tumor cells in mice with DENA-induced HCC by inhibiting the expression of MAPKs and reducing liver injury enzymes | [184] |
| Gastrodin | Subcutaneous H22 cells-induced tumor in mice | It can specifically increase the expression of NF-κB downstream genes such as Bcl-xL, Bcl-2, and IL-2 in CD4 but not CD8 T cells | [185] |
| Genistein | TAA-induced HCC in rats | It displays antioxidant and anti-HCC effects by suppressing the versican/PDGF bidirectional axis and protein expression of PKC and ERK-1 | [186] |
| Lactoferrin | DEN-induced HCC in rats | It shows a chemopreventive effect against DEN-induced HCC in rats in a dose-dependent manner by suppressing the expression and activation of AKT | [187] |
| Selenium | TAA-induced HCC in rats | Selenium nanoparticles improve the tumor suppressive effect of sorafenib and overcome drug resistance in rat HCC by inducing apoptosis and targeting AKT/mTOR and NF-κB signaling pathways, as well as epigenetic regulation | [188] |
| Silymarin | DEN/AAF/CCl4 induced HCC in rats | It suppresses cancer cell growth in rats with DEN/AAF/CCl4-induced tumors by inhibiting the expression of Ki-67 and HGF/c-Met, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways | [189] |
| Taraxasterol | HepG2 and Huh7H22 bearing mice | It can suppress tumor cell growth by suppressing Ki67 expression and inducing cell apoptosis via suppressing IL-6/STAT3 signaling pathway, as well as promoting T cell infiltration in tumor tissue | [190] |
| Telmisartan | NDEA-induced HCC in mice | It exerts an anti-HCC effect and increases tumor cell sensitivity to sorafenib treatment by suppressing phosphorylation-induced activation of TAK1 and the ERK1/2 and NF-кB signaling pathways | [191] |
| Delta-tocotrienol | HCC cell lines SK Hep-1 and Huh7 | It promotes the anti-HCC cell activity of IFN-α by increasing ROS and increasing cell apoptosis together with an increased Bax/Bcl-xL ratio. In addition, it can activate Notch1 signaling pathway | [192] |
AKT: Protein kinase B; Bax: Bcl-2-like protein 4; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma extra-large; BCLAF1: BCL-2-associated transcription factor 1; CD4: Cluster of differentiation 4; c-Met: Tyrosine-protein kinase Met; ERK-1/2: Extracellular signal-regulated kinases 1/2; GSK-3β: Glycogen synthase kinase-3β; HCC: Hepatocellular carcinoma; HGF: Hepatocyte growth factor; IL-2: Interleukin 2; Ki-67: Marker of proliferation Ki-67; MAPK: Mitogen-activated protein kinase; mTOR: Mammalian target of rapamycin; NF-κB: Nuclear factor κB; PI3K: Phosphatidylinositol-3-kinase; PDGF: Platelet-derived growth factor; SIRT1: Sirtuin 1; SREBP-1c: Sterol regulatory element binding protein 1c; STAT3: Signal transducer and activator of transcription 3; DENA Diethylnitrosamine; TAA: Thioacetamide; ROS: Reactive oxygen species; NDEA: N-Nitrosodiethylamine; AAF: 2-acetylaminofluorene; CCl4: Carbon tetrachloride.
CLINICAL TRIALS
Clinical trials have been started to evaluate the efficacy of these molecules in CLD (Table 2), such as EMPA[192] and silymarin[193,194]. For example, treatment with EMPA can improve liver steatosis in patients with NAFLD without T2DM[192]. Another trial shows that oral supplementation of genistein (250 mg) for 8 wk can decrease insulin resistance, oxidative stress, and inflammation and improve lipid metabolism in patients with NAFLD[195].
Table 2.
Clinical trials for evaluating the efficacy of compounds in liver disease
|
Treatment
|
Trial number
|
Phase
|
Aims or results
|
| Curcumin | NCT02908152 | 2-3 | To investigate the effects of curcumin supplements on metabolic factors and hepatic fibrosis in NAFLD patients with T2DM |
| NCT04109742 | 2 | To test the effect of curcumin in pediatric patients with NAFLD | |
| Empagliflozin | NCT03867487 | 2 | To evaluate the preliminary feasibility, initial efficacy, and safety of empagliflozin as a SGLT2 inhibitor for treating NAFLD in adolescents with obesity |
| NCT04642261 | 4 | To test the effects of empagliflozin on reducing hepatic fat content as measured by MRI-PDFF in NAFLD patients without DM | |
| Gastrodin | NCT04035824 | 4 | To treat hypertension together with Uncaria |
| Genistein | IRCT201312132480N5 | 3 | Oral supplementation of genistein (250 mg) for 8 wk can decrease insulin resistance, oxidative stress, and inflammation and improve lipid metabolism in patients with NAFLD |
| Lactoferrin | NCT04335058 | None | To test the effect of lactoferrin with iron versus iron alone in the treatment of anemia in CLD |
| Selenium | NCT00271245 | None | To test the effect of selenium in patients with cirrhosis |
| NCT01650181 | 4 | To test the impacts using siliphos-selenium-methionine-alpha lipoic acid plus metformin versus metformin in patients with fatty liver and NASH | |
| Silymarin | NCT00389376 | 1 | An increase in silymarin is observed in NAFLD patients, compared to that in patients with HCV |
| NCT00680407 | 2 | The effect of silymarin on NASH patients remains inconclusive due to the lack of a substantial number of patients | |
| Telmisartan | NCT02213224 | 4 | To evaluate the therapeutic effects of telmisartan and perindopril for NAFLD patients with hypertension |
T2DM: Type 2 diabetes mellitus; NAFLD: Non-alcoholic fatty liver disease; SGLT2: Sodium-glucose cotransporter-2; MRI-PDFF: Magnetic resonance imaging-derived proton density fat fraction; CLD: Chronic liver disease; NASH: Non-alcoholic steatohepatitis; HCV: Hepatitis C virus.
CONCLUSION
CLD is a continuous process that causes a reduction of liver function that lasts more than six months. CLD has a broad spectrum with complex cellular and molecular mechanisms. It can be subclassified into ALD, NAFLD or MAFLD, chronic viral infection, and autoimmune hepatitis, which can lead to liver fibrosis, cirrhosis, and cancer. However, there are no currently available treatments for ALD, NAFLD, and liver fibrosis, except the preventive strategies, such as changes in exercise, diet, and alcohol use. Early preventive strategies predict good outcomes. Patients with advanced ALD and NAFLD require liver transplantation, but without enough donor organs. Liver inflammation and oxidative stress are ubiquitously associated with the development and progression of CLD. Molecular signaling pathways such as AMPK, JNK, and PPAR-mediated signaling pathways are implicated in liver inflammation, oxidative stress, and lipid metabolism. Accumulating studies have demonstrated that natural products with antioxidant and anti-inflammatory functions display therapeutic effects against inflammation, fibrosis, and metabolic disorders, including ALD and NAFLD. These products such as β-sitosterol, curcumin, EMPA, gastrodin, and genistein have shown potential application at all the stages of CLD, from ALD/NAFLD to HCC. In addition, clinical trials that are undergoing to evaluate their efficacy and safety are reviewed. Overall, pre-clinical studies in cell and animal models reveal the protective effects of these agents in CLD. However, more clinical trials are required to evaluate their efficacy and safety.
Natural products, especially antioxidant and anti-inflammatory products, show potent therapeutic alternatives for CLD treatment with their efficacy and low side effects. Remarkably, these products also display anti-HCC functions. However, many pharmaceutical dynamic assays have not been tested, and the potential adverse effects of long-term use of these products are not available. In the future, the synergistic effects of different drugs should be evaluated to treat CLD, due to its complex pathogenic factors.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 9, 2022
First decision: November 23, 2022
Article in press: February 7, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ban Q, China; Prikhodko V, Russia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Chun-Ye Zhang, Christopher S. Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, United States.
Shuai Liu, The First Affiliated Hospital, Zhejiang University, Hangzhou 310006, Zhejiang Province, China.
Ming Yang, Department of Surgery, University of Missouri, Columbia, MO 65211, United States. yangmin@health.missouri.edu.
References
- 1.Sharma A, Nagalli S. Chronic Liver Disease. StatPearls Publishing LLC., 2022. [cited 10 December 2022]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554597 . [PubMed]
- 2.Embade N, Millet O. Molecular Determinants of Chronic Liver Disease as Studied by NMR-Metabolomics. Curr Top Med Chem. 2017;17:2752–2766. doi: 10.2174/1568026617666170707124539. [DOI] [PubMed] [Google Scholar]
- 3.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: spectrum and diagnosis. Transl Gastroenterol Hepatol. 2020;5:19. doi: 10.21037/tgh.2019.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang WJ, Xiao P, Xu HQ, Niu JQ, Gao YH. Growing burden of alcoholic liver disease in China: A review. World J Gastroenterol. 2019;25:1445–1456. doi: 10.3748/wjg.v25.i12.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axley PD, Richardson CT, Singal AK. Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin Liver Dis. 2019;23:39–50. doi: 10.1016/j.cld.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Ferdouse A, Clugston RD. Pathogenesis of Alcohol-Associated Fatty Liver: Lessons From Transgenic Mice. Front Physiol. 2022;13:940974. doi: 10.3389/fphys.2022.940974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking Pathogenic Mechanisms of Alcoholic Liver Disease With Clinical Phenotypes. Gastroenterology. 2016;150:1756–1768. doi: 10.1053/j.gastro.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Wang C, Dai S, Liu Y, Zhang F, Peng C, Li Y. Quercetin Protects Ethanol-Induced Hepatocyte Pyroptosis via Scavenging Mitochondrial ROS and Promoting PGC-1α-Regulated Mitochondrial Homeostasis in L02 Cells. Oxid Med Cell Longev. 2022;2022:4591134. doi: 10.1155/2022/4591134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel F, Parwani K, Patel D, Mandal P. Metformin and Probiotics Interplay in Amelioration of Ethanol-Induced Oxidative Stress and Inflammatory Response in an In Vitro and In Vivo Model of Hepatic Injury. Mediators Inflamm. 2021;2021:6636152. doi: 10.1155/2021/6636152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Yang M. Current Options and Future Directions for NAFLD and NASH Treatment. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22147571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YH, Wu WK, Wu MS. Microbiota-Associated Therapy for Non-Alcoholic Steatohepatitis-Induced Liver Cancer: A Review. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21175999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 16.Atsawarungruangkit A, Laoveeravat P, Promrat K. Machine learning models for predicting non-alcoholic fatty liver disease in the general United States population: NHANES database. World J Hepatol. 2021;13:1417–1427. doi: 10.4254/wjh.v13.i10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perdomo CM, Garcia-Fernandez N, Escalada J. Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate? J Clin Med. 2021;10 doi: 10.3390/jcm10092040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9:372–381. doi: 10.1038/nrgastro.2012.79. [DOI] [PubMed] [Google Scholar]
- 19.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 21.Pohl R, Feder S, Haberl EM, Rein-Fischboeck L, Weiss TS, Spirk M, Bruckmann A, McMullen N, Sinal CJ, Buechler C. Chemerin Overexpression in the Liver Protects against Inflammation in Experimental Non-Alcoholic Steatohepatitis. Biomedicines. 2022;10 doi: 10.3390/biomedicines10010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabbia D, Cannella L, De Martin S. The Role of Oxidative Stress in NAFLD-NASH-HCC Transition-Focus on NADPH Oxidases. Biomedicines. 2021;9 doi: 10.3390/biomedicines9060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathur M, Yeh YT, Arya RK, Jiang L, Pornour M, Chen W, Ma Y, Gao B, He L, Ying Z, Xue B, Shi H, Choi Y, Yu L. Adipose lipolysis is important for ethanol to induce fatty liver in the National Institute on Alcohol Abuse and Alcoholism murine model of chronic and binge ethanol feeding. Hepatology. 2022 doi: 10.1002/hep.32675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallio M, Sangineto M, Romeo M, Villani R, Romano AD, Loguercio C, Serviddio G, Federico A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Y, Lian Y, Zheng Q, Huang Z, Gu L, Bi Y, Li J, Huang Y, Wu Y, Chen L. Association among cytokine profiles of innate and adaptive immune responses and clinical-virological features in untreated patients with chronic hepatitis B. BMC Infect Dis. 2020;20:509. doi: 10.1186/s12879-020-05233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petagine L, Zariwala MG, Patel VB. Alcoholic liver disease: Current insights into cellular mechanisms. World J Biol Chem. 2021;12:87–103. doi: 10.4331/wjbc.v12.i5.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai Y, Tan Q, Xv S, Huang S, Wang Y, Li Y, Zeng T, Mo C, Chen Y, Zhou C, Gao L, Lv Z. Ginsenoside Rb1 Alleviates Alcohol-Induced Liver Injury by Inhibiting Steatosis, Oxidative Stress, and Inflammation. Front Pharmacol. 2021;12:616409. doi: 10.3389/fphar.2021.616409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monserrat-Mesquida M, Quetglas-Llabrés M, Bouzas C, Montemayor S, Mascaró CM, Casares M, Llompart I, Gámez JM, Tejada S, Martínez JA, Tur JA, Sureda A. A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease. Antioxidants (Basel) 2022;11 doi: 10.3390/antiox11071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco I, Bianco A, Mirizzi A, Campanella A, Bonfiglio C, Sorino P, Notarnicola M, Tutino V, Cozzolongo R, Giannuzzi V, Aballay LR, Buongiorno C, Bruno I, Osella AR. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients. 2020;13 doi: 10.3390/nu13010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabaleta N, Torella L, Weber ND, Gonzalez-Aseguinolaza G. mRNA and gene editing: Late breaking therapies in liver diseases. Hepatology. 2022;76:869–887. doi: 10.1002/hep.32441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aravalli RN, Steer CJ. CRISPR/Cas9 therapeutics for liver diseases. J Cell Biochem. 2018;119:4265–4278. doi: 10.1002/jcb.26627. [DOI] [PubMed] [Google Scholar]
- 32.Harrison SA, Neff G, Guy CD, Bashir MR, Paredes AH, Frias JP, Younes Z, Trotter JF, Gunn NT, Moussa SE, Kohli A, Nelson K, Gottwald M, Chang WCG, Yan AZ, DePaoli AM, Ling L, Lieu HD. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2021;160:219–231.e1. doi: 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Xu JJ, Li HD, Wu MF, Zhu L, Du XS, Li JJ, Li Z, Meng XM, Huang C, Li J. 3-B-RUT, a derivative of RUT, protected against alcohol-induced liver injury by attenuating inflammation and oxidative stress. Int Immunopharmacol. 2021;95:107471. doi: 10.1016/j.intimp.2021.107471. [DOI] [PubMed] [Google Scholar]
- 34.Yue SR, Tan YY, Zhang L, Zhang BJ, Jiang FY, Ji G, Liu BC, Wang RR. Gynostemma pentaphyllum polysaccharides ameliorate non-alcoholic steatohepatitis in mice associated with gut microbiota and the TLR2/NLRP3 pathway. Front Endocrinol (Lausanne) 2022;13:885039. doi: 10.3389/fendo.2022.885039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ai G, Wu X, Dou Y, Huang R, Zhong L, Liu Y, Xian Y, Lin Z, Li Y, Su Z, Chen J, Qu C. Oxyberberine, a novel HO-1 agonist, effectively ameliorates oxidative stress and inflammatory response in LPS/D-GalN induced acute liver injury mice via coactivating erythrocyte metabolism and Nrf2 signaling pathway. Food Chem Toxicol. 2022;166:113215. doi: 10.1016/j.fct.2022.113215. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Matos MC, Sandhu B, Bonder A, Jiang ZG. Lipoprotein metabolism in liver diseases. Curr Opin Lipidol. 2019;30:30–36. doi: 10.1097/MOL.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 37.Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238. doi: 10.1016/j.molmet.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Z, Deng ZT, Huang S, Ning M, Feng Y, Shen Y, Zhao QS, Leng Y. Alisol B Alleviates Hepatocyte Lipid Accumulation and Lipotoxicity via Regulating RARα-PPARγ-CD36 Cascade and Attenuates Non-Alcoholic Steatohepatitis in Mice. Nutrients. 2022;14 doi: 10.3390/nu14122411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Denning KL, Lu Y. PPARα agonist WY-14,643 induces adipose atrophy and fails to blunt chronic ethanol-induced hepatic fat accumulation in mice lacking adipose FGFR1. Biochem Pharmacol. 2021;192:114678. doi: 10.1016/j.bcp.2021.114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham TH, Lee GH, Jin SW, Lee SY, Han EH, Kim ND, Jeong HG. Puerarin attenuates hepatic steatosis via G-protein-coupled estrogen receptor-mediated calcium and SIRT1 signaling pathways. Phytother Res. 2022;36:3601–3618. doi: 10.1002/ptr.7526. [DOI] [PubMed] [Google Scholar]
- 41.Xie F, Zhong Y, Wang D, So KF, Xiao J, Lv Y. Metformin protects against ethanol-induced liver triglyceride accumulation by the LKB1/AMPK/ACC pathway. Mol Biol Rep. 2022;49:7837–7848. doi: 10.1007/s11033-022-07610-y. [DOI] [PubMed] [Google Scholar]
- 42.Bai T, Yang Y, Yao YL, Sun P, Lian LH, Wu YL, Nan JX. Betulin alleviated ethanol-induced alcoholic liver injury via SIRT1/AMPK signaling pathway. Pharmacol Res. 2016;105:1–12. doi: 10.1016/j.phrs.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Han H, Xue T, Li J, Guo Y, Li X, Wang L, Pei L, Zheng M. Plant sterol ester of α-linolenic acid improved non-alcoholic fatty liver disease by attenuating endoplasmic reticulum stress-triggered apoptosis via activation of the AMPK. J Nutr Biochem. 2022;107:109072. doi: 10.1016/j.jnutbio.2022.109072. [DOI] [PubMed] [Google Scholar]
- 44.Gluais-Dagorn P, Foretz M, Steinberg GR, Batchuluun B, Zawistowska-Deniziak A, Lambooij JM, Guigas B, Carling D, Monternier PA, Moller DE, Bolze S, Hallakou-Bozec S. Direct AMPK Activation Corrects NASH in Rodents Through Metabolic Effects and Direct Action on Inflammation and Fibrogenesis. Hepatol Commun. 2022;6:101–119. doi: 10.1002/hep4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Y, Xu J, Huang P, Yang L, Liu Y, Li Y, Wang J, Song H, Zheng P. Scoparone Improves Nonalcoholic Steatohepatitis Through Alleviating JNK/Sab Signaling Pathway-Mediated Mitochondrial Dysfunction. Front Pharmacol. 2022;13:863756. doi: 10.3389/fphar.2022.863756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu JY, Chen M, Mu WJ, Luo HY, Guo L. Higd1a facilitates exercise-mediated alleviation of fatty liver in diet-induced obese mice. Metabolism. 2022;134:155241. doi: 10.1016/j.metabol.2022.155241. [DOI] [PubMed] [Google Scholar]
- 47.Panzitt K, Wagner M. FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166133. doi: 10.1016/j.bbadis.2021.166133. [DOI] [PubMed] [Google Scholar]
- 48.Jiao Y, Lu Y, Li XY. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol Sin. 2015;36:44–50. doi: 10.1038/aps.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Y, Xiang X, Liu C, Cai T, Li T, Chen Y, Bai J, Shi H, Zheng T, Huang M, Fu W. Transcriptomic Analysis Reveals Lactobacillus reuteri Alleviating Alcohol-Induced Liver Injury in Mice by Enhancing the Farnesoid X Receptor Signaling Pathway. J Agric Food Chem. 2022;70:12550–12564. doi: 10.1021/acs.jafc.2c05591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Kang W, Liu S, Li J, Liu J, Chen X, Gan F, Huang K. Gut microbiota-bile acid-intestinal Farnesoid X receptor signaling axis orchestrates cadmium-induced liver injury. Sci Total Environ. 2022;849:157861. doi: 10.1016/j.scitotenv.2022.157861. [DOI] [PubMed] [Google Scholar]
- 51.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, Alnouti Y, Fouts DE, Stärkel P, Loomba R, Coulter S, Liddle C, Yu RT, Ling L, Rossi SJ, DePaoli AM, Downes M, Evans RM, Brenner DA, Schnabl B. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67:2150–2166. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratziu V, Harrison SA, Loustaud-Ratti V, Bureau C, Lawitz E, Abdelmalek M, Alkhouri N, Francque S, Girma H, Darteil R, Couchoux H, Wolf M, Sanyal A, Vonderscher J, Scalfaro P. Hepatic and renal improvements with FXR agonist vonafexor in individuals with suspected fibrotic NASH. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.10.023. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Yu H, Xing R, Li P. Hepatoprotective Effect of Oyster Peptide on Alcohol-Induced Liver Disease in Mice. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23158081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174–185. doi: 10.1016/j.fct.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Origassa CS, Câmara NO. Cytoprotective role of heme oxygenase-1 and heme degradation derived end products in liver injury. World J Hepatol. 2013;5:541–549. doi: 10.4254/wjh.v5.i10.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Guan W, Zhang N, Wang Y, Tian Y, Sun H, Li X, Liu J. Lactobacillus plantarum Lp2 improved LPS-induced liver injury through the TLR-4/MAPK/NFκB and Nrf2-HO-1/CYP2E1 pathways in mice. Food Nutr Res. 2022;66 doi: 10.29219/fnr.v66.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bathish B, Robertson H, Dillon JF, Dinkova-Kostova AT, Hayes JD. Nonalcoholic steatohepatitis and mechanisms by which it is ameliorated by activation of the CNC-bZIP transcription factor Nrf2. Free Radic Biol Med. 2022;188:221–261. doi: 10.1016/j.freeradbiomed.2022.06.226. [DOI] [PubMed] [Google Scholar]
- 58.Biao Y, Chen J, Liu C, Wang R, Han X, Li L, Zhang Y. Protective Effect of Danshen Zexie Decoction Against Non-Alcoholic Fatty Liver Disease Through Inhibition of ROS/NLRP3/IL-1β Pathway by Nrf2 Signaling Activation. Front Pharmacol. 2022;13:877924. doi: 10.3389/fphar.2022.877924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma RS, Harrison DJ, Kisielewski D, Cassidy DM, McNeilly AD, Gallagher JR, Walsh SV, Honda T, McCrimmon RJ, Dinkova-Kostova AT, Ashford MLJ, Dillon JF, Hayes JD. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2) Cell Mol Gastroenterol Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner N, Wagner KD. The Role of PPARs in Disease. Cells. 2020;9 doi: 10.3390/cells9112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Decara J, Rivera P, López-Gambero AJ, Serrano A, Pavón FJ, Baixeras E, Rodríguez de Fonseca F, Suárez J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front Pharmacol. 2020;11:730. doi: 10.3389/fphar.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y, Lu Y. Alcoholic fatty liver is blunted by rFGF21 administration in mice lacking adipose FGFR1: The role of FGF21 in PPARα-mediated regulation of adipose tissue mass. Biochem Biophys Res Commun. 2022;619:84–89. doi: 10.1016/j.bbrc.2022.05.099. [DOI] [PubMed] [Google Scholar]
- 63.Pan J, Zhou W, Xu R, Xing L, Ji G, Dang Y. Natural PPARs agonists for the treatment of nonalcoholic fatty liver disease. Biomed Pharmacother. 2022;151:113127. doi: 10.1016/j.biopha.2022.113127. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Che Y, Wang S, Wang J, Liu X, Kou B, Guan Y, Chen D, Shi Y. ASPP2 reduction attenuates HBV induced chronic liver damage: A hybrid mouse model study. Biochem Biophys Res Commun. 2022;610:61–69. doi: 10.1016/j.bbrc.2022.03.109. [DOI] [PubMed] [Google Scholar]
- 65.Ning Z, Guo X, Liu X, Lu C, Wang A, Wang X, Wang W, Chen H, Qin W, Zhou L, Ma C, Du J, Lin Z, Luo H, Otkur W, Qi H, Chen D, Xia T, Liu J, Tan G, Xu G, Piao HL. USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma. Nat Commun. 2022;13:2187. doi: 10.1038/s41467-022-29846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, Denning KL, Lu Y. PPARα agonist WY-14,643 induces the PLA2/COX-2/ACOX1 pathway to enhance peroxisomal lipid metabolism and ameliorate alcoholic fatty liver in mice. Biochem Biophys Res Commun. 2022;613:47–52. doi: 10.1016/j.bbrc.2022.04.132. [DOI] [PubMed] [Google Scholar]
- 67.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, Zhu Y, Borensztajn J, Reddy JK. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012;26:628–638. doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baumann A, Burger K, Brandt A, Staltner R, Jung F, Rajcic D, Lorenzo Pisarello MJ, Bergheim I. GW9662, a peroxisome proliferator-activated receptor gamma antagonist, attenuates the development of non-alcoholic fatty liver disease. Metabolism. 2022;133:155233. doi: 10.1016/j.metabol.2022.155233. [DOI] [PubMed] [Google Scholar]
- 69.Padole P, Arora A, Sharma P, Chand P, Verma N, Kumar A. Saroglitazar for Nonalcoholic Fatty Liver Disease: A Single Centre Experience in 91 Patients. J Clin Exp Hepatol. 2022;12:435–439. doi: 10.1016/j.jceh.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yokote K, Yamashita S, Arai H, Araki E, Matsushita M, Nojima T, Suganami H, Ishibashi S. Effects of pemafibrate on glucose metabolism markers and liver function tests in patients with hypertriglyceridemia: a pooled analysis of six phase 2 and phase 3 randomized double-blind placebo-controlled clinical trials. Cardiovasc Diabetol. 2021;20:96. doi: 10.1186/s12933-021-01291-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, Tanigawa R, Iizuka M, Iida Y, Loomba R. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1263–1277. doi: 10.1111/apt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, Doerffel Y, Gitlin N, Gordon SC, Odin JA, Sheridan D, Wörns MA, Clark V, Corless L, Hartmann H, Jonas ME, Kremer AE, Mells GF, Buggisch P, Freilich BL, Levy C, Vierling JM, Bernstein DE, Hartleb M, Janczewska E, Rochling F, Shah H, Shiffman ML, Smith JH, Choi YJ, Steinberg A, Varga M, Chera H, Martin R, McWherter CA, Hirschfield GM. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 73.Gastaldelli A, Sabatini S, Carli F, Gaggini M, Bril F, Belfort-DeAguiar R, Positano V, Barb D, Kadiyala S, Harrison S, Cusi K. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int. 2021;41:2659–2670. doi: 10.1111/liv.15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Della Pepa G, Russo M, Vitale M, Carli F, Vetrani C, Masulli M, Riccardi G, Vaccaro O, Gastaldelli A, Rivellese AA, Bozzetto L. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Diabetes Res Clin Pract. 2021;178:108984. doi: 10.1016/j.diabres.2021.108984. [DOI] [PubMed] [Google Scholar]
- 75.Sven M F, Pierre B, Manal F A, Quentin M A, Elisabetta B, Vlad R, Philippe HM, Bruno S, Jean-Louis J, Jean-Louis A. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp Clin Trials. 2020;98:106170. doi: 10.1016/j.cct.2020.106170. [DOI] [PubMed] [Google Scholar]
- 76.Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, Loomba R, Harrison SA, Balabanska R, Mateva L, Lanthier N, Alkhouri N, Moreno C, Schattenberg JM, Stefanova-Petrova D, Vonghia L, Rouzier R, Guillaume M, Hodge A, Romero-Gómez M, Huot-Marchand P, Baudin M, Richard MP, Abitbol JL, Broqua P, Junien JL, Abdelmalek MF NATIVE Study Group. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 77.Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C, Liu S, Yang M. Hepatocellular Carcinoma and Obesity, Type 2 Diabetes Mellitus, Cardiovascular Disease: Causing Factors, Molecular Links, and Treatment Options. Front Endocrinol (Lausanne) 2021;12:808526. doi: 10.3389/fendo.2021.808526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu M, Chen Y, Deng F, Chang B, Luo J, Dong L, Lu X, Zhang Y, Chen Z, Zhou J. D-Mannose Regulates Hepatocyte Lipid Metabolism via PI3K/Akt/mTOR Signaling Pathway and Ameliorates Hepatic Steatosis in Alcoholic Liver Disease. Front Immunol. 2022;13:877650. doi: 10.3389/fimmu.2022.877650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie H, Jing R, Liao X, Chen H, Xie X, Dai H, Pan L. Arecoline promotes proliferation and migration of human HepG2 cells through activation of the PI3K/AKT/mTOR pathway. Hereditas. 2022;159:29. doi: 10.1186/s41065-022-00241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Liang J, Cao N, Gao J, Xie Y, Zhou S, Tang X. ASIC1α up-regulates MMP-2/9 expression to enhance mobility and proliferation of liver cancer cells via the PI3K/AKT/mTOR pathway. BMC Cancer. 2022;22:778. doi: 10.1186/s12885-022-09874-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diniz PHC, Silva SDC, Vidigal PVT, Xavier MAP, Lima CX, Faria LC, Ferrari TCA. Expression of MAPK and PI3K/AKT/mTOR Proteins according to the Chronic Liver Disease Etiology in Hepatocellular Carcinoma. J Oncol. 2020;2020:4609360. doi: 10.1155/2020/4609360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Yuan Z, He J, Xie T, Zhou M, Chen TT, Shi LP, He Y, Wang J, Shao M, Che JY. Effects and mechanisms of ziqi ruangan decoction on hepatic fibrosis. Pak J Pharm Sci. 2021;34:2101–2107. [PubMed] [Google Scholar]
- 85.Li HG, You PT, Xia Y, Cai Y, Tu YJ, Wang MH, Song WC, Quan TM, Ren HY, Liu YW, Dan HX, Xu SQ. Yu Gan Long Ameliorates Hepatic Fibrosis by Inhibiting PI3K/AKT, Ras/ERK and JAK1/STAT3 Signaling Pathways in CCl(4)-induced Liver Fibrosis Rats. Curr Med Sci. 2020;40:539–547. doi: 10.1007/s11596-020-2211-3. [DOI] [PubMed] [Google Scholar]
- 86.Wang S, Wu Y, Liu M, Zhao Q, Jian L. DHW-208, A Novel Phosphatidylinositol 3-Kinase (PI3K) Inhibitor, Has Anti-Hepatocellular Carcinoma Activity Through Promoting Apoptosis and Inhibiting Angiogenesis. Front Oncol. 2022;12:955729. doi: 10.3389/fonc.2022.955729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung YY, Um JY, Sethi G, Ahn KS. Fangchinoline abrogates growth and survival of hepatocellular carcinoma by negative regulation of c-met/HGF and its associated downstream signaling pathways. Phytother Res. 2022;36:4542–4557. doi: 10.1002/ptr.7573. [DOI] [PubMed] [Google Scholar]
- 88.Chen W, Yang A, Jia J, Popov YV, Schuppan D, You H. Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology. 2020;72:729–741. doi: 10.1002/hep.31236. [DOI] [PubMed] [Google Scholar]
- 89.Dongiovanni P, Meroni M, Baselli GA, Bassani GA, Rametta R, Pietrelli A, Maggioni M, Facciotti F, Trunzo V, Badiali S, Fargion S, Gatti S, Valenti L. Insulin resistance promotes Lysyl Oxidase Like 2 induction and fibrosis accumulation in non-alcoholic fatty liver disease. Clin Sci (Lond) 2017;131:1301–1315. doi: 10.1042/CS20170175. [DOI] [PubMed] [Google Scholar]
- 90.Zetterberg FR, MacKinnon A, Brimert T, Gravelle L, Johnsson RE, Kahl-Knutson B, Leffler H, Nilsson UJ, Pedersen A, Peterson K, Roper JA, Schambye H, Slack RJ, Tantawi S. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J Med Chem. 2022;65:12626–12638. doi: 10.1021/acs.jmedchem.2c00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herrera-Marcos LV, Martínez-Beamonte R, Macías-Herranz M, Arnal C, Barranquero C, Puente-Lanzarote JJ, Gascón S, Herrero-Continente T, Gonzalo-Romeo G, Alastrué-Vera V, Gutiérrez-Blázquez D, Lou-Bonafonte JM, Surra JC, Rodríguez-Yoldi MJ, García-Gil A, Güemes A, Osada J. Hepatic galectin-3 is associated with lipid droplet area in non-alcoholic steatohepatitis in a new swine model. Sci Rep. 2022;12:1024. doi: 10.1038/s41598-022-04971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sideras K, de Man RA, Harrington SM, Polak WG, Zhou G, Schutz HM, Pedroza-Gonzalez A, Biermann K, Mancham S, Hansen BE, Bart Takkenberg R, van Vuuren AJ, Pan Q, Ijzermans JNM, Sleijfer S, Sprengers D, Dong H, Kwekkeboom J, Bruno MJ. Circulating levels of PD-L1 and Galectin-9 are associated with patient survival in surgically treated Hepatocellular Carcinoma independent of their intra-tumoral expression levels. Sci Rep. 2019;9:10677. doi: 10.1038/s41598-019-47235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen LY, Chen Q, Cheng YF, Jin HH, Kong DS, Zhang F, Wu L, Shao JJ, Zheng SZ. Diallyl trisulfide attenuates ethanol-induced hepatic steatosis by inhibiting oxidative stress and apoptosis. Biomed Pharmacother. 2016;79:35–43. doi: 10.1016/j.biopha.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Lee DG, Lee J, Kim KT, Lee SW, Kim YO, Cho IH, Kim HJ, Park CG, Lee S. High-performance liquid chromatography analysis of phytosterols in Panax ginseng root grown under different conditions. J Ginseng Res. 2018;42:16–20. doi: 10.1016/j.jgr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Z, Wu A, Jin H, Liu F. β-Sitosterol attenuates liver injury in a rat model of chronic alcohol intake. Arch Pharm Res. 2020;43:1197–1206. doi: 10.1007/s12272-020-01271-w. [DOI] [PubMed] [Google Scholar]
- 96.Lu W, Khatibi Shahidi F, Khorsandi K, Hosseinzadeh R, Gul A, Balick V. An update on molecular mechanisms of curcumin effect on diabetes. J Food Biochem. 2022;46:e14358. doi: 10.1111/jfbc.14358. [DOI] [PubMed] [Google Scholar]
- 97.Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer (Review) Mol Med Rep. 2019;19:23–29. doi: 10.3892/mmr.2018.9665. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Wang J, Jing Z, Ordovas JM, Shen L. Anti-fatigue and anti-oxidant effects of curcumin supplementation in exhaustive swimming mice via Nrf2/Keap1 signal pathway. Curr Res Food Sci. 2022;5:1148–1157. doi: 10.1016/j.crfs.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farashbandi AL, Shariati M, Mokhtari M. Comparing the Protective Effects of Curcumin and Ursodeoxycholic Acid after Ethanol-Induced Hepatotoxicity in Rat Liver. Ethiop J Health Sci. 2021;31:673–682. doi: 10.4314/ejhs.v31i3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bao S, Zhang Y, Ye J, Zhu Y, Li R, Xu X, Zhang Q. Self-assembled micelles enhance the oral delivery of curcumin for the management of alcohol-induced tissue injury. Pharm Dev Technol. 2021;26:880–889. doi: 10.1080/10837450.2021.1950185. [DOI] [PubMed] [Google Scholar]
- 101.Kim SG, Suh HJ, Han SH, Lee HS, Kim HW, Kim H. Encapsulated Curcumin Enhances Intestinal Absorption and Improves Hepatic Damage in Alcoholic Liver Disease-Induced Rats. Prev Nutr Food Sci. 2019;24:410–417. doi: 10.3746/pnf.2019.24.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X, Chang X, Zhan H, Zhang Q, Li C, Gao Q, Yang M, Luo Z, Li S, Sun Y. Curcumin and Baicalin ameliorate ethanol-induced liver oxidative damage via the Nrf2/HO-1 pathway. J Food Biochem. 2020:e13425. doi: 10.1111/jfbc.13425. [DOI] [PubMed] [Google Scholar]
- 103.Abdelhamid AM, Elsheakh AR, Abdelaziz RR, Suddek GM. Empagliflozin ameliorates ethanol-induced liver injury by modulating NF-κB/Nrf-2/PPAR-γ interplay in mice. Life Sci. 2020;256:117908. doi: 10.1016/j.lfs.2020.117908. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Y, Chen J, Yao Z, Gu X. Gastrodin ameliorates Concanavalin A-induced acute hepatitis via the IL6/JAK2/STAT3 pathway. Immunopharmacol Immunotoxicol. 2022;44:925–934. doi: 10.1080/08923973.2022.2093741. [DOI] [PubMed] [Google Scholar]
- 105.Liao CC, Yu HP, Chou AH, Lee HC, Hu LM, Liu FC. Gastrodin Alleviates Acetaminophen-Induced Liver Injury in a Mouse Model Through Inhibiting MAPK and Enhancing Nrf2 Pathways. Inflammation. 2022;45:1450–1462. doi: 10.1007/s10753-021-01557-1. [DOI] [PubMed] [Google Scholar]
- 106.Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, Zhilina OM, Garsiya ER, Smeriglio A, Trombetta D, Pons DG, Martorell M, Cardoso SM, Razis AFA, Sunusi U, Kamal RM, Rotariu LS, Butnariu M, Docea AO, Calina D. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid Med Cell Longev. 2021;2021:3268136. doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suksri K, Semprasert N, Limjindaporn T, Yenchitsomanus PT, Kooptiwoot S, Kooptiwut S. Cytoprotective effect of genistein against dexamethasone-induced pancreatic β-cell apoptosis. Sci Rep. 2022;12:12950. doi: 10.1038/s41598-022-17372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang W, Zhang L, Zhang X. Anti-atherosclerotic effects of genistein in preventing ox-low-density lipoprotein-induced smooth muscle-derived foam cell formation via inhibiting SRC expression and L-Ca channel currents. Ann Transl Med. 2022;10:700. doi: 10.21037/atm-22-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jafari A, Esmaeilzadeh Z, Khezri MR, Ghasemnejad-Berenji H, Pashapour S, Sadeghpour S, Ghasemnejad-Berenji M. An overview of possible pivotal mechanisms of Genistein as a potential phytochemical against SARS-CoV-2 infection: A hypothesis. J Food Biochem. 2022;46:e14345. doi: 10.1111/jfbc.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao L, Zhang N, Yang D, Yang M, Guo X, He J, Wu W, Ji B, Cheng Q, Zhou F. Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model. Nutrients. 2018;10 doi: 10.3390/nu10111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mercer KE, Pulliam CF, Hennings L, Cleves MA, Jones EE, Drake RR, Ronis MJJ. Diet Supplementation with Soy Protein Isolate, but Not the Isoflavone Genistein, Protects Against Alcohol-Induced Tumor Progression in DEN-Treated Male Mice. Adv Exp Med Biol. 2018;1032:115–126. doi: 10.1007/978-3-319-98788-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soliman SA, Emeish WFA, Abdel-Hafeez HH. Lactoferrin improves the immune response and resistance of silver carp, a hematological, light (histochemical and immunohistochemical), fluorescent, and scanning electron microscopic study. Microsc Res Tech. 2022;85:3565–3581. doi: 10.1002/jemt.24208. [DOI] [PubMed] [Google Scholar]
- 113.Li D, He Q, Yang H, Du Y, Yu K, Yang J, Tong X, Guo Y, Xu J, Qin L. Daily Dose of Bovine Lactoferrin Prevents Ethanol-Induced Liver Injury and Death in Male Mice by Regulating Hepatic Alcohol Metabolism and Modulating Gut Microbiota. Mol Nutr Food Res. 2021;65:e2100253. doi: 10.1002/mnfr.202100253. [DOI] [PubMed] [Google Scholar]
- 114.Li D, Hu Z, He Q, Guo Y, Chong Y, Xu J, Qin L. Lactoferrin Alleviates Acute Alcoholic Liver Injury by Improving Redox-Stress Response Capacity in Female C57BL/6J Mice. J Agric Food Chem. 2021;69:14856–14867. doi: 10.1021/acs.jafc.1c06813. [DOI] [PubMed] [Google Scholar]
- 115.Ojeda ML, Nogales F, Del Carmen Gallego-López M, Carreras O. Binge drinking during the adolescence period causes oxidative damage-induced cardiometabolic disorders: A possible ameliorative approach with selenium supplementation. Life Sci. 2022;301:120618. doi: 10.1016/j.lfs.2022.120618. [DOI] [PubMed] [Google Scholar]
- 116.Ojeda ML, Rua RM, Murillo ML, Carreras O, Nogales F. Binge drinking during adolescence disrupts Se homeostasis and its main hepatic selenoprotein expression. Alcohol Clin Exp Res. 2015;39:818–826. doi: 10.1111/acer.12707. [DOI] [PubMed] [Google Scholar]
- 117.Federico A, Dallio M, Loguercio C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules. 2017;22 doi: 10.3390/molecules22020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Said ES, Mohammed AH, Ali HM, Babiker AY, Alnughaymishi R, Althaqeel NZ, Ahmed AS. Evaluation of hepatoprotective effect of Nebivolol and sodium copper Chlorophyllin on CCL4-induced hepatotoxicity in mice. Eur Rev Med Pharmacol Sci. 2022;26:1717–1728. doi: 10.26355/eurrev_202203_28241. [DOI] [PubMed] [Google Scholar]
- 119.Aghemo A, Alekseeva OP, Angelico F, Bakulin IG, Bakulina NV, Bordin D, Bueverov AO, Drapkina OM, Gillessen A, Kagarmanova EM, Korochanskaya NV, Kucheryavii UA, Lazebnik LB, Livzan MA, Maev IV, Martynov AI, Osipenko MF, Sas EI, Starodubova A, Uspensky YP, Vinnitskaya EV, Yakovenko EP, Yakovlev AA. Role of silymarin as antioxidant in clinical management of chronic liver diseases: a narrative review. Ann Med. 2022;54:1548–1560. doi: 10.1080/07853890.2022.2069854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30:407–413. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336–339. doi: 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 122.Yousefi Ghale-Salimi M, Eidi M, Ghaemi N, Khavari-Nejad RA. Antiurolithiatic effect of the taraxasterol on ethylene glycol induced kidney calculi in male rats. Urolithiasis. 2018;46:419–428. doi: 10.1007/s00240-017-1023-9. [DOI] [PubMed] [Google Scholar]
- 123.Ye XJ, Xu R, Liu SY, Hu B, Shi ZJ, Shi FL, Zeng B, Xu LH, Huang YT, Chen MY, Zha QB, He XH, Ouyang DY. Taraxasterol mitigates Con A-induced hepatitis in mice by suppressing interleukin-2 expression and its signaling in T lymphocytes. Int Immunopharmacol. 2022;102:108380. doi: 10.1016/j.intimp.2021.108380. [DOI] [PubMed] [Google Scholar]
- 124.Abdelhamid AM, Elsheakh AR, Suddek GM, Abdelaziz RR. Telmisartan alleviates alcohol-induced liver injury by activation of PPAR-γ/ Nrf-2 crosstalk in mice. Int Immunopharmacol. 2021;99:107963. doi: 10.1016/j.intimp.2021.107963. [DOI] [PubMed] [Google Scholar]
- 125.Yang M, Kimchi ET, Staveley-O'Carroll KF, Li G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gumede NM, Lembede BW, Nkomozepi P, Brooksbank RL, Erlwanger KH, Chivandi E. β-Sitosterol mitigates the development of high-fructose diet-induced nonalcoholic fatty liver disease in growing male Sprague-Dawley rats. Can J Physiol Pharmacol. 2020;98:44–50. doi: 10.1139/cjpp-2019-0295. [DOI] [PubMed] [Google Scholar]
- 127.Gumede NM, Lembede BW, Brooksbank RL, Erlwanger KH, Chivandi E. β-Sitosterol Shows Potential to Protect Against the Development of High-Fructose Diet-Induced Metabolic Dysfunction in Female Rats. J Med Food. 2020;23:367–374. doi: 10.1089/jmf.2019.0120. [DOI] [PubMed] [Google Scholar]
- 128.Feng S, Dai Z, Liu AB, Huang J, Narsipur N, Guo G, Kong B, Reuhl K, Lu W, Luo Z, Yang CS. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1274–1284. doi: 10.1016/j.bbalip.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R. Preventive effect of curcumin on inflammation, oxidative stress and insulin resistance in high-fat fed obese rats. J Complement Integr Med. 2016;13:137–143. doi: 10.1515/jcim-2015-0070. [DOI] [PubMed] [Google Scholar]
- 130.Yan C, Zhang Y, Zhang X, Aa J, Wang G, Xie Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed Pharmacother. 2018;105:274–281. doi: 10.1016/j.biopha.2018.05.135. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, Cheng F, Luo Y, Zhan Z, Hu P, Ren H, Tang H, Peng M. PEGylated Curcumin Derivative Attenuates Hepatic Steatosis via CREB/PPAR-γ/CD36 Pathway. Biomed Res Int. 2017;2017:8234507. doi: 10.1155/2017/8234507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Um MY, Hwang KH, Ahn J, Ha TY. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 2013;113:152–157. doi: 10.1111/bcpt.12076. [DOI] [PubMed] [Google Scholar]
- 133.Feng D, Zou J, Su D, Mai H, Zhang S, Li P, Zheng X. Curcumin prevents high-fat diet-induced hepatic steatosis in ApoE(-/-) mice by improving intestinal barrier function and reducing endotoxin and liver TLR4/NF-κB inflammation. Nutr Metab (Lond) 2019;16:79. doi: 10.1186/s12986-019-0410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hasanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar A. Curcumin: an inflammasome silencer. Pharmacol Res. 2020;159:104921. doi: 10.1016/j.phrs.2020.104921. [DOI] [PubMed] [Google Scholar]
- 135.Inzaugarat ME, De Matteo E, Baz P, Lucero D, García CC, Gonzalez Ballerga E, Daruich J, Sorda JA, Wald MR, Cherñavsky AC. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS One. 2017;12:e0172900. doi: 10.1371/journal.pone.0172900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res (Stuttg) 2017;67:244–251. doi: 10.1055/s-0043-100019. [DOI] [PubMed] [Google Scholar]
- 137.Mirhafez SR, Azimi-Nezhad M, Dehabeh M, Hariri M, Naderan RD, Movahedi A, Abdalla M, Sathyapalan T, Sahebkar A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv Exp Med Biol. 2021;1308:25–35. doi: 10.1007/978-3-030-64872-5_3. [DOI] [PubMed] [Google Scholar]
- 138.Derosa G, Maffioli P, Sahebkar A. Piperine and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;928:173–184. doi: 10.1007/978-3-319-41334-1_8. [DOI] [PubMed] [Google Scholar]
- 139.Mirhafez SR, Dehabeh M, Hariri M, Farimani AR, Movahedi A, Naderan RD, Jamialahmadi T, Simental-Mendía LE, Sahebkar A. Curcumin and Piperine Combination for the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind Randomized Placebo-Controlled Trial. Adv Exp Med Biol. 2021;1328:11–19. doi: 10.1007/978-3-030-73234-9_2. [DOI] [PubMed] [Google Scholar]
- 140.Ma Y, Zhang G, Kuang Z, Xu Q, Ye T, Li X, Qu N, Han F, Kan C, Sun X. Empagliflozin activates Sestrin2-mediated AMPK/mTOR pathway and ameliorates lipid accumulation in obesity-related nonalcoholic fatty liver disease. Front Pharmacol. 2022;13:944886. doi: 10.3389/fphar.2022.944886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Shen QL, Xin Q, Sun B, Zhang S, Fang QH, Shi YX, Niu WY, Lin JN, Li CJ. MCAD activation by empagliflozin promotes fatty acid oxidation and reduces lipid deposition in NASH. J Mol Endocrinol. 2022;69:415–430. doi: 10.1530/JME-22-0022. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Liu X, Zhang H, Wang X. Efficacy and Safety of Empagliflozin on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2022;13:836455. doi: 10.3389/fendo.2022.836455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line Treatment for Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Who Do Not Respond to Incretin-Based Therapies Including Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors. Clin Drug Investig. 2016;36:313–319. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 144.Harrison SA, Manghi FP, Smith WB, Alpenidze D, Aizenberg D, Klarenbeek N, Chen CY, Zuckerman E, Ravussin E, Charatcharoenwitthaya P, Cheng PN, Katchman H, Klein S, Ben-Ari Z, Mendonza AE, Zhang Y, Martic M, Ma S, Kao S, Tanner S, Pachori A, Badman MK, He Y, Ukomadu C, Sicard E. Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study. Nat Med. 2022;28:1432–1438. doi: 10.1038/s41591-022-01861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qiao P, Jia Y, Ma A, He J, Shao C, Li X, Wang S, Yang B, Zhou H. Dapagliflozin protects against nonalcoholic steatohepatitis in db/db mice. Front Pharmacol. 2022;13:934136. doi: 10.3389/fphar.2022.934136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He K, Li J, Xi W, Ge J, Sun J, Jing Z. Dapagliflozin for nonalcoholic fatty liver disease: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2022;185:109791. doi: 10.1016/j.diabres.2022.109791. [DOI] [PubMed] [Google Scholar]
- 147.Wan J, Zhang Y, Yang D, Liang Y, Yang L, Hu S, Liu Z, Fang Q, Tian S, Ding Y. Gastrodin Improves Nonalcoholic Fatty Liver Disease Through Activation of the Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway. Hepatology. 2021;74:3074–3090. doi: 10.1002/hep.32068. [DOI] [PubMed] [Google Scholar]
- 148.Ahmad O, Wang B, Ma K, Deng Y, Li M, Yang L, Yang Y, Zhao J, Cheng L, Zhou Q, Shang J. Lipid Modulating Anti-oxidant Stress Activity of Gastrodin on Nonalcoholic Fatty Liver Disease Larval Zebrafish Model. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yin Y, Liu H, Zheng Z, Lu R, Jiang Z. Genistein can ameliorate hepatic inflammatory reaction in nonalcoholic steatohepatitis rats. Biomed Pharmacother. 2019;111:1290–1296. doi: 10.1016/j.biopha.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 150.Zamani-Garmsiri F, Hashemnia SMR, Shabani M, Bagherieh M, Emamgholipour S, Meshkani R. Combination of metformin and genistein alleviates non-alcoholic fatty liver disease in high-fat diet-fed mice. J Nutr Biochem. 2021;87:108505. doi: 10.1016/j.jnutbio.2020.108505. [DOI] [PubMed] [Google Scholar]
- 151.Witayavanitkul N, Werawatganon D, Chayanupatkul M, Klaikeaw N, Sanguanrungsirikul S, Siriviriyakul P. Genistein and exercise modulated lipid peroxidation and improved steatohepatitis in ovariectomized rats. BMC Complement Med Ther. 2020;20:162. doi: 10.1186/s12906-020-02962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang X, Wang Y, Xu W, Lan L, Li Y, Wang L, Sun X, Yang C, Jiang Y, Feng R. Dietary isoflavones intake is inversely associated with non-alcoholic fatty liver disease, hyperlipidaemia and hypertension. Int J Food Sci Nutr. 2022;73:60–70. doi: 10.1080/09637486.2021.1910630. [DOI] [PubMed] [Google Scholar]
- 153.Seidemann L, Krüger A, Kegel-Hübner V, Seehofer D, Damm G. Influence of Genistein on Hepatic Lipid Metabolism in an In Vitro Model of Hepatic Steatosis. Molecules. 2021;26 doi: 10.3390/molecules26041156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aoyama Y, Naiki-Ito A, Xiaochen K, Komura M, Kato H, Nagayasu Y, Inaguma S, Tsuda H, Tomita M, Matsuo Y, Takiguchi S, Takahashi S. Lactoferrin Prevents Hepatic Injury and Fibrosis via the Inhibition of NF-κB Signaling in a Rat Non-Alcoholic Steatohepatitis Model. Nutrients. 2021;14 doi: 10.3390/nu14010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo C, Xue H, Guo T, Zhang W, Xuan WQ, Ren YT, Wang D, Chen YH, Meng YH, Gao HL, Zhao P. Recombinant human lactoferrin attenuates the progression of hepatosteatosis and hepatocellular death by regulating iron and lipid homeostasis in ob/ob mice. Food Funct. 2020;11:7183–7196. doi: 10.1039/d0fo00910e. [DOI] [PubMed] [Google Scholar]
- 156.Soulaidopoulos S, Tsiogka A, Chrysohoou C, Lazarou E, Aznaouridis K, Doundoulakis I, Tyrovola D, Tousoulis D, Tsioufis K, Vlachopoulos C, Lazaros G. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients. 2022;14 doi: 10.3390/nu14030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kannt A, Papada E, Kammermeier C, D'Auria G, Jiménez-Hernández N, Stephan M, Schwahn U, Madsen AN, Østergaard MV, Dedoussis G, Francino MP MAST4HEALTH consortium. Mastiha (Pistacia lentiscus) Improves Gut Microbiota Diversity, Hepatic Steatosis, and Disease Activity in a Biopsy-Confirmed Mouse Model of Advanced Non-Alcoholic Steatohepatitis and Fibrosis. Mol Nutr Food Res. 2019;63:e1900927. doi: 10.1002/mnfr.201900927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kanoni S, Kumar S, Amerikanou C, Kurth MJ, Stathopoulou MG, Bourgeois S, Masson C, Kannt A, Cesarini L, Kontoe MS, Milanović M, Roig FJ, Beribaka M, Campolo J, Jiménez-Hernández N, Milošević N, Llorens C, Smyrnioudis I, Francino MP, Milić N, Kaliora AC, Trivella MG, Ruddock MW, Medić-Stojanoska M, Gastaldelli A, Lamont J, Deloukas P, Dedoussis GV, Visvikis-Siest S. Nutrigenetic Interactions Might Modulate the Antioxidant and Anti-Inflammatory Status in Mastiha-Supplemented Patients With NAFLD. Front Immunol. 2021;12:683028. doi: 10.3389/fimmu.2021.683028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Amerikanou C, Papada E, Gioxari A, Smyrnioudis I, Kleftaki SA, Valsamidou E, Bruns V, Banerjee R, Trivella MG, Milic N, Medić-Stojanoska M, Gastaldelli A, Kannt A MAST4HEALTH, Dedoussis GV, Kaliora AC. Mastiha has efficacy in immune-mediated inflammatory diseases through a microRNA-155 Th17 dependent action. Pharmacol Res. 2021;171:105753. doi: 10.1016/j.phrs.2021.105753. [DOI] [PubMed] [Google Scholar]
- 160.Zhou DD, Mao QQ, Li BY, Saimaiti A, Huang SY, Xiong RG, Shang A, Luo M, Li HY, Gan RY, Li HB, Li S. Effects of Different Green Teas on Obesity and Non-Alcoholic Fatty Liver Disease Induced by a High-Fat Diet in Mice. Front Nutr. 2022;9:929210. doi: 10.3389/fnut.2022.929210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abdallah AAM, Abdelrahman MM, Attia HMAS, Hafez A, Anwar Rashed S, Amin YA, Hemdan SB. Decreased Serum zinc, selenium, and vitamin E as possible risk factors of hepatic fibrosis in non-alcoholic fatty liver disease. Nutr Health. 2022:2601060221103032. doi: 10.1177/02601060221103032. [DOI] [PubMed] [Google Scholar]
- 162.Tang C, Li S, Zhang K, Li J, Han Y, Zhan T, Zhao Q, Guo X, Zhang J. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020;36:101519. doi: 10.1016/j.redox.2020.101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pant R, Sharma N, Kabeer SW, Sharma S, Tikoo K. Selenium-Enriched Probiotic Alleviates Western Diet-Induced Non-alcoholic Fatty Liver Disease in Rats via Modulation of Autophagy Through AMPK/SIRT-1 Pathway. Biol Trace Elem Res. 2022 doi: 10.1007/s12011-022-03247-x. [DOI] [PubMed] [Google Scholar]
- 164.Zhang Q, Zhou X, Zhang J, Li Q, Qian Z. Selenium and vitamin B(6) cosupplementation improves dyslipidemia and fatty liver syndrome by SIRT1/SREBP-1c pathway in hyperlipidemic Sprague-Dawley rats induced by high-fat diet. Nutr Res. 2022;106:101–118. doi: 10.1016/j.nutres.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 165.Cui S, Pan XJ, Ge CL, Guo YT, Zhang PF, Yan TT, Zhou JY, He QX, Cheng LH, Wang GJ, Hao HP, Wang H. Silybin alleviates hepatic lipid accumulation in methionine-choline deficient diet-induced nonalcoholic fatty liver disease in mice via peroxisome proliferator-activated receptor α. Chin J Nat Med. 2021;19:401–411. doi: 10.1016/S1875-5364(21)60039-0. [DOI] [PubMed] [Google Scholar]
- 166.Stephen Robert JM, Peddha MS, Srivastava AK. Effect of Silymarin and Quercetin in a Miniaturized Scaffold in Wistar Rats against Non-alcoholic Fatty Liver Disease. ACS Omega. 2021;6:20735–20745. doi: 10.1021/acsomega.1c00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mengesha T, Gnanasekaran N, Mehare T. Hepatoprotective effect of silymarin on fructose induced nonalcoholic fatty liver disease in male albino wistar rats. BMC Complement Med Ther. 2021;21:104. doi: 10.1186/s12906-021-03275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mirhashemi SH, Hakakzadeh A, Yeganeh FE, Oshidari B, Rezaee SP. Effect of 8 Weeks milk thistle powder (silymarin extract) supplementation on fatty liver disease in patients candidates for bariatric surgery. Metabol Open. 2022;14:100190. doi: 10.1016/j.metop.2022.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang QZ, Liu YL, Wang YR, Fu LN, Zhang J, Wang XR, Wang BM. Effects of telmisartan on improving leptin resistance and inhibiting hepatic fibrosis in rats with non-alcoholic fatty liver disease. Exp Ther Med. 2017;14:2689–2694. doi: 10.3892/etm.2017.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wasta Esmail VA, Al-Nimer MSM, Mohammed MO. Effects of Orlistat or Telmisartan on the Serum Free Fatty Acids in Non-alcoholic Fatty Liver Disease Patients: An Open-Labeled Randomized Controlled Study. Turk J Gastroenterol. 2022;33:421–426. doi: 10.5152/tjg.2020.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alam S, Abrar M, Islam S, Kamal M, Hasan MJ, Khan MAS, Ahmad N. Effect of telmisartan and vitamin E on liver histopathology with non-alcoholic steatohepatitis: A randomized, open-label, noninferiority trial. JGH Open. 2020;4:663–669. doi: 10.1002/jgh3.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Devan AR, Nair B, Kumar AR, Nath LR. An insight into the role of telmisartan as PPAR-γ/α dual activator in the management of nonalcoholic fatty liver disease. Biotechnol Appl Biochem. 2022;69:461–468. doi: 10.1002/bab.2123. [DOI] [PubMed] [Google Scholar]
- 173.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78:2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wong WY, Ward LC, Fong CW, Yap WN, Brown L. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur J Nutr. 2017;56:133–150. doi: 10.1007/s00394-015-1064-1. [DOI] [PubMed] [Google Scholar]
- 175.Wang H, Yan W, Sun Y, Yang CS. δ-Tocotrienol is the Most Potent Vitamin E Form in Inhibiting Prostate Cancer Cell Growth and Inhibits Prostate Carcinogenesis in Ptenp-/- Mice. Cancer Prev Res (Phila) 2022;15:233–245. doi: 10.1158/1940-6207.CAPR-21-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pervez MA, Khan DA, Ijaz A, Khan S. Effects of Delta-tocotrienol Supplementation on Liver Enzymes, Inflammation, Oxidative stress and Hepatic Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Turk J Gastroenterol. 2018;29:170–176. doi: 10.5152/tjg.2018.17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suleman F, Khan DA, Pervez MA, Aamir M. Effects of delta-tocotrienol supplementation on glycaemic control in individuals with prediabetes: A randomized controlled study. J Pak Med Assoc. 2022;72:4–7. doi: 10.47391/JPMA.966. [DOI] [PubMed] [Google Scholar]
- 178.Mahjabeen W, Khan DA, Mirza SA, Pervez MA. Effects of delta-tocotrienol supplementation on Glycemic Control, oxidative stress, inflammatory biomarkers and miRNA expression in type 2 diabetes mellitus: A randomized control trial. Phytother Res. 2021;35:3968–3976. doi: 10.1002/ptr.7113. [DOI] [PubMed] [Google Scholar]
- 179.Pervez MA, Khan DA, Slehria AUR, Ijaz A. Delta-tocotrienol supplementation improves biochemical markers of hepatocellular injury and steatosis in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled trial. Complement Ther Med. 2020;52:102494. doi: 10.1016/j.ctim.2020.102494. [DOI] [PubMed] [Google Scholar]
- 180.Nisha R, Kumar P, Gautam AK, Bera H, Bhattacharya B, Parashar P, Saraf SA, Saha S. Assessments of in vitro and in vivo antineoplastic potentials of β-sitosterol-loaded PEGylated niosomes against hepatocellular carcinoma. J Liposome Res. 2021;31:304–315. doi: 10.1080/08982104.2020.1820520. [DOI] [PubMed] [Google Scholar]
- 181.Kathiswar Raj R, Ezhilarasan D, Rajeshkumar S. β-Sitosterol-assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Mater Res A. 2020;108:1899–1908. doi: 10.1002/jbm.a.36953. [DOI] [PubMed] [Google Scholar]
- 182.Bai C, Zhao J, Su J, Chen J, Cui X, Sun M, Zhang X. Curcumin induces mitochondrial apoptosis in human hepatoma cells through BCLAF1-mediated modulation of PI3K/AKT/GSK-3β signaling. Life Sci. 2022;306:120804. doi: 10.1016/j.lfs.2022.120804. [DOI] [PubMed] [Google Scholar]
- 183.Abdelhamid AM, Saber S, Youssef ME, Gaafar AGA, Eissa H, Abd-Eldayem MA, Alqarni M, Batiha GE, Obaidullah AJ, Shahien MA, El-Ahwany E, Amin NA, Etman MA, Kaddah MMY, Abd El-Fattah EE. Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: Emerging approach for new application. Biomed Pharmacother. 2022;145:112455. doi: 10.1016/j.biopha.2021.112455. [DOI] [PubMed] [Google Scholar]
- 184.Shu G, Yang T, Wang C, Su H, Xiang M. Gastrodin stimulates anticancer immune response and represses transplanted H22 hepatic ascitic tumor cell growth: Involvement of NF-κB signaling activation in CD4+ T cells. Toxicol Appl Pharmacol. 2013;269:270–279. doi: 10.1016/j.taap.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 185.El-Far YM, Khodir AE, Emarah ZA, Ebrahim MA, Al-Gayyar MMH. Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. Redox Rep. 2022;27:9–20. doi: 10.1080/13510002.2022.2031515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hegazy RR, Mansour DF, Salama AA, Abdel-Rahman RF, Hassan AM. Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats. Pharmacol Rep. 2019;71:879–891. doi: 10.1016/j.pharep.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 187.Al-Noshokaty TM, Mesbah NM, Abo-Elmatty DM, Abulsoud AI, Abdel-Hamed AR. Selenium nanoparticles overcomes sorafenib resistance in thioacetamide induced hepatocellular carcinoma in rats by modulation of mTOR, NF-κB pathways and LncRNA-AF085935/GPC3 axis. Life Sci. 2022;303:120675. doi: 10.1016/j.lfs.2022.120675. [DOI] [PubMed] [Google Scholar]
- 188.Yassin NYS, AbouZid SF, El-Kalaawy AM, Ali TM, Almehmadi MM, Ahmed OM. Silybum marianum total extract, silymarin and silibinin abate hepatocarcinogenesis and hepatocellular carcinoma growth via modulation of the HGF/c-Met, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways. Biomed Pharmacother. 2022;145:112409. doi: 10.1016/j.biopha.2021.112409. [DOI] [PubMed] [Google Scholar]
- 189.Ren F, Zhang Y, Qin Y, Shang J, Wang Y, Wei P, Guo J, Jia H, Zhao T. Taraxasterol prompted the anti-tumor effect in mice burden hepatocellular carcinoma by regulating T lymphocytes. Cell Death Discov. 2022;8:264. doi: 10.1038/s41420-022-01059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saber S, Khodir AE, Soliman WE, Salama MM, Abdo WS, Elsaeed B, Nader K, Abdelnasser A, Megahed N, Basuony M, Shawky A, Mahmoud M, Medhat R, Eldin AS. Telmisartan attenuates N-nitrosodiethylamine-induced hepatocellular carcinoma in mice by modulating the NF-κB-TAK1-ERK1/2 axis in the context of PPARγ agonistic activity. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1591–1604. doi: 10.1007/s00210-019-01706-2. [DOI] [PubMed] [Google Scholar]
- 191.Lucci A, Vera MC, Comanzo CG, Lorenzetti F, Ferretti AC, Ceballos MP, Quiroga AD, Alvarez ML, Carrillo MC. Delta-tocotrienol enhances the anti-tumor effects of interferon alpha through reactive oxygen species and Erk/MAPK signaling pathways in hepatocellular carcinoma cells. Can J Physiol Pharmacol. 2022;100:453–463. doi: 10.1139/cjpp-2021-0606. [DOI] [PubMed] [Google Scholar]
- 192.Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, Khamseh ME. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv Ther. 2020;37:4697–4708. doi: 10.1007/s12325-020-01498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schrieber SJ, Hawke RL, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, Meyers CM, Doo E, Fried MW. Differences in the disposition of silymarin between patients with nonalcoholic fatty liver disease and chronic hepatitis C. Drug Metab Dispos. 2011;39:2182–2190. doi: 10.1124/dmd.111.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Navarro VJ, Belle SH, D'Amato M, Adfhal N, Brunt EM, Fried MW, Reddy KR, Wahed AS, Harrison S Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS One. 2019;14:e0221683. doi: 10.1371/journal.pone.0221683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Amanat S, Eftekhari MH, Fararouei M, Bagheri Lankarani K, Massoumi SJ. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin Nutr. 2018;37:1210–1215. doi: 10.1016/j.clnu.2017.05.028. [DOI] [PubMed] [Google Scholar]