This cohort study investigates if concentrations of serum glial fibrillary acidic protein and neurofilament light chain are associated with and prognostic for disease progression in patients with multiple sclerosis.
Key Points
Question
Are serum glial fibrillary acidic protein (sGFAP) and/or neurofilament light chain (sNfL) concentrations associated with and prognostic for disease progression in patients with multiple sclerosis?
Findings
In this cohort study of 355 patients and 259 healthy controls (contributing 737 and 485 serum samples, respectively), elevated sGFAP z scores (corrected for confounding factors age, sex, and body mass index) identified current disease progression and were associated with future disease progression but not with acute inflammation. In addition, the association of sNfL levels with progression was less pronounced, whereas sNfL levels were strongly increased during relapse activity.
Meaning
Results suggest that sGFAP is more strongly associated than sNfL with disease progression in MS, a finding that has clinical implications for patient management and development of novel drugs.
Abstract
Importance
There is a lack of validated biomarkers for disability progression independent of relapse activity (PIRA) in multiple sclerosis (MS).
Objective
To determine how serum glial fibrillary acidic protein (sGFAP) and serum neurofilament light chain (sNfL) correlate with features of disease progression vs acute focal inflammation in MS and how they can prognosticate disease progression.
Design, Setting, and Participants
Data were acquired in the longitudinal Swiss MS cohort (SMSC; a consortium of tertiary referral hospitals) from January 1, 2012, to October 20, 2022. The SMSC is a prospective, multicenter study performed in 8 centers in Switzerland. For this nested study, participants had to meet the following inclusion criteria: cohort 1, patients with MS and either stable or worsening disability and similar baseline Expanded Disability Status Scale scores with no relapses during the entire follow-up; and cohort 2, all SMSC study patients who had initiated and continued B-cell–depleting treatment (ie, ocrelizumab or rituximab).
Exposures
Patients received standard immunotherapies or were untreated.
Main Outcomes and Measures
In cohort 1, sGFAP and sNfL levels were measured longitudinally using Simoa assays. Healthy control samples served as the reference. In cohort 2, sGFAP and sNfL levels were determined cross-sectionally.
Results
This study included a total of 355 patients (103 [29.0%] in cohort 1: median [IQR] age, 42.1 [33.2-47.6] years; 73 female patients [70.9%]; and 252 [71.0%] in cohort 2: median [IQR] age, 44.3 [33.3-54.7] years; 156 female patients [61.9%]) and 259 healthy controls with a median [IQR] age of 44.3 [36.3-52.3] years and 177 female individuals (68.3%). sGFAP levels in controls increased as a function of age (1.5% per year; P < .001), were inversely correlated with BMI (−1.1% per BMI unit; P = .01), and were 14.9% higher in women than in men (P = .004). In cohort 1, patients with worsening progressive MS showed 50.9% higher sGFAP levels compared with those with stable MS after additional sNfL adjustment, whereas the 25% increase of sNfL disappeared after additional sGFAP adjustment. Higher sGFAP at baseline was associated with accelerated gray matter brain volume loss (per doubling: 0.24% per year; P < .001) but not white matter loss. sGFAP levels remained unchanged during disease exacerbations vs remission phases. In cohort 2, median (IQR) sGFAP z scores were higher in patients developing future confirmed disability worsening compared with those with stable disability (1.94 [0.36-2.23] vs 0.71 [−0.13 to 1.73]; P = .002); this was not significant for sNfL. However, the combined elevation of z scores of both biomarkers resulted in a 4- to 5-fold increased risk of confirmed disability worsening (hazard ratio [HR], 4.09; 95% CI, 2.04-8.18; P < .001) and PIRA (HR, 4.71; 95% CI, 2.05-9.77; P < .001).
Conclusions and Relevance
Results of this cohort study suggest that sGFAP is a prognostic biomarker for future PIRA and revealed its complementary potential next to sNfL. sGFAP may serve as a useful biomarker for disease progression in MS in individual patient management and drug development.
Introduction
The pathogenesis of multiple sclerosis (MS) involves both adaptive and innate immune disease mechanisms. The former is associated with recurring episodes of acute neurologic symptoms, relapses, and formation of localized lesions in the brain and spinal cord caused by invasion of blood-derived immune cells. In contrast, the latter has been suggested to drive more diffuse inflammation and neurodegeneration, also called smoldering MS,1 that clinically presents as disease progression. Although high-efficacy therapies, such as B-cell–depleting treatment (BCDT), result in almost complete suppression of focal lesion formation, their effectiveness for preventing development of long-term disability is modest.2,3 This therapeutic gap is mirrored by a diagnostic unmet need to assess progression. Serum neurofilament light chain (sNfL) is now well established as therapy response marker in active disease4,5,6; however, its capacity to reflect concurrent, or to predict progression, especially when acute inflammatory disease activity is suppressed by high efficacy therapies, is still under debate.4,7,8,9,10,11,12
Glial fibrillary acidic protein (GFAP) is an intermediate filament of astrocytes, equivalent to NfL in neurons, and has been proposed as a biomarker to identify present disease progression and to prognosticate future progression in MS.13,14,15,16,17,18 Early studies measuring GFAP levels in the cerebrospinal fluid (CSF) of patients with MS found a correlation with neurologic disability in subsequent years; however, this was not the case for NfL levels.14 Furthermore, high CSF GFAP levels were associated with faster progression to an Expanded Disability Status Scale (EDSS) score of 3 and 6,19 and levels were higher in primary progressive MS than in relapsing-remitting MS (RRMS).14,20,21 Moreover, there is also evidence of increased GFAP levels in the CSF of patients with progressive MS who had no recent relapses, showing the potential of GFAP levels for measuring pure progression.13 In contrast, although NfL was a sensitive indicator of neuroaxonal injury during acute disease activity, ie, lesion formation and relapses, CSF levels of GFAP remained unaffected in this state.20,22
Based on different methodological approaches in 2 independent patient cohorts followed in the Swiss MS Cohort (SMSC), this study attempted a direct comparison of sGFAP and sNfL levels: how they reflect acute disease activity vs the identification and prognostication of future disease progression and whether their combination provides added value. In cohort 1, we (1) measured their levels in patients who either remained clinically stable or continued to accumulate more disability over time and (2) compared how they are impacted by acute inflammation in a cohort of patients with relapsing forms of MS (RMS). Cohort 2 comprised patients with MS receiving BCDT as a model of optimal suppression of acute disease activity to evaluate how sNfL and sGFAP levels, alone and in combination, are prognostic for future disability worsening and progression independent of relapse activity (PIRA).
Methods
Study Design and Patients With MS
This cohort study, conducted from January 1, 2012, to October 22, 2022, was approved by the ethics committees of all participating centers. Patients in both cohorts provided written informed consent. A description of the SMSC and standard definitions are available in the eMethods in Supplement 1. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Cohort 1
Three groups of patients with MS with extreme phenotypes were compared: patients with either stable MS (stMS) or worsening disability23 had similar baseline EDSS scores and no relapses during the entire follow-up; the focal inflammation group consisted of patients with relapsing MS from whom serum samples were acquired both during active disease phase (relapse and/or contrast-enhancing brain lesions) and remission. Patients with worsening progressive MS or stMS were matched for age, disease duration, EDSS scores, and T2-weighted lesion volume at baseline. Patients with worsening progressive MS presented with at least 1 PIRA event during follow-up. Further details are available in the eMethods in Supplement 1. Cohort 1 included patients of only White race and ethnicity. Other race and ethnic subgroups were too small for meaningful analysis.
Cohort 2
We included all SMSC patients who had initiated and continued BCDT (ocrelizumab or rituximab). sNfL and sGFAP levels were measured in the first sample available 8 months or more after treatment start (median [IQR], 12.2 [10.7-16.8] months). We included patients with RRMS and progressive MS. PIRA was defined by the occurrence of confirmed disability worsening (CDW) events in the absence of relapses between the visit defining baseline of the EDSS worsening event until its confirmation visit at least 6 months later. All other CDW events were defined to be relapse-associated worsening (RAW) events. Cohort 2 included patients of only White race and Hispanic ethnicity. Other race and ethnic subgroups were too small for meaningful analysis.
Healthy Controls
Blood samples from healthy controls (HCs) in the Genome-Wide Association Study of Multiple Sclerosis (GeneMSA24,25) were collected at the University Hospital Basel between July 7, 2004, and May 29, 2007. A family history or current diagnosis of MS, as well as other reported ongoing relevant illnesses (eg, diabetes, arterial hypertension), were considered exclusionary for this group.
sGFAP and sNfL Measurements
Blood samples were collected within 8 days from the clinical visit and stored at −80 °C following standardized procedures.26 sGFAP and sNfL concentrations were measured in duplicate with the ultrasensitive single molecule array (Simoa) technology (Quanterix). In cohort 1, samples were measured using the singleplex Simoa GFAP Discovery Kit on the HD-X analyzer according to the manufacturer’s instructions. sNfL levels had been measured in a previous study4 using the Simoa Nf-Light kit. In cohort 2, samples were measured using the Neurology 2-plex B assay according to manufacturer’s instructions (eFigure 1 in Supplement 1). Further details, including information on magnetic resonance imaging (MRI) assessment methods, are in the eMethods in Supplement 1.
Statistical Analysis
In HCs, the association between log-transformed biomarker concentrations as a dependent variable and age, sex, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) as independent variables were analyzed using mixed models with a random intercept for person. In analogy with age- and BMI-adjusted sNfL reference values,4 we calculated sGFAP z scores additionally adjusted for sex. A more detailed description of the statistical analysis is available in the eMethods in Supplement 1.
Cohort 1
Comparison of sGFAP and sNfL levels in stMS/worsening progressive MS and RMS cohorts vs HCs was performed using a linear mixed model with log-transformed sGFAP or sNfL levels as the dependent variable and age, BMI, sex, and phenotype group (stMS, worsening progressive MS; RMS in either remission or active disease state) as independent variables as well as a random intercept for the person to account for the repeated nature of the data. To assess the association of disease progression with sGFAP or sNfL levels (individual biomarkers as dependent variables), univariable and multivariable models with stMS vs worsening progressive MS status as well as age, sex, BMI, follow-up time, disease duration, disease-modifying treatment, and EDSS scores as independent variables were used. To evaluate the independent association between disease progression or active disease status and sGFAP or sNfL levels that is not explained by the other biomarker, the respective log2-transformed marker was additionally added to these models. The within-person variation of sGFAP or sNfL levels was assessed by the intraclass correlation coefficient (ICC) with 95% CI obtained by bootstrapping. Atrophy rates per year in the combined stMS and worsening progressive MS cohort were assessed with a linear mixed model. The associations between biomarker levels and gray matter volume and white matter volume loss were modeled using interaction terms between log2-transformed baseline sGFAP and sNfL levels, and follow-up time and estimates express the change in annualized atrophy rates per doubling in biomarker concentration. To compare the prognostic power of baseline sGFAP and sNfL levels for PIRA, univariable and multivariable Cox regression models were performed in the combined stMS and worsening progressive MS cohort.
Cohort 2
Biomarker levels in patients with and without later CDW were visualized using box plots and were considered increased compared with HC when being significantly above z = 0 in the univariate Wilcoxon signed rank tests (a z score of 0, corresponding to the 50th percentile, indicates the physiologic mean level of HC4). A cross-sectional analysis was performed using linear models with individual biomarker z score as the dependent variable and demographic and clinical variables as predictors. The association between biomarker levels and time to CDW was investigated using Kaplan-Meier curves and Cox regression models. Receiver operating characteristics (ROC) analyses were performed to identify optimal cut points for sGFAP and sNfL z score values to dichotomize the respective biomarker levels in high and low groups to prognosticate CDW. The performance of a composite of both biomarkers in prognosticating CDW was investigated by categorizing patients into 4 groups according to high and low levels for each biomarker, using the constellation of low sGFAP/low sNfL as a reference.
Sensitivity analyses were performed using only CDW due to PIRA (ie, excluding CDW due to RAW). A 2-sided P value ≤ .05 was considered statistically significant. Analyses were performed in R, version 4.2.0 (R Project for Statistical Computing).
Results
Serum GFAP and sNfL Concentrations in HCs
This study included a total of 355 patients (103 [29.0%] in cohort 1: median [IQR] age, 42.1 [33.2-47.6] years; 30 male individuals [29.1%]; 73 female individuals [70.9%] and 252 [71.0%] in cohort 2: median [IQR] age, 44.3 [33.3-54.7] years; 96 male individuals [38.1%]; 156 female individuals [61.9%]). The cohort of 259 HCs (485 samples) included 177 female individuals (68.3%) and had a median (IQR) age at baseline of 44.3 (36.3-52.3) years. sGFAP levels increased with age (1.5% per year; P < .001) (eFigures 2 and 3 in Supplement 1) and were inversely correlated with BMI (1.1% decrease per BMI unit, estimate 0.989; 95% CI, 0.979-0.998; P = .01). Across all ages, levels were 14.9% higher in women than in men (P = .004). sNfL levels increased by 2.5% per year of age and decreased by 2.2% per unit BMI (estimate 0.978; 95% CI, 0.969-0.986; P < .001) in both sexes. sGFAP and sNfL levels were moderately correlated at baseline (Spearman ρ = 0.47; P < .001).
Cohort 1
At baseline, patients with stMS and worsening progressive MS showed little difference in demographic, clinical, or MRI data, except that treatment with monoclonal antibodies at last follow-up was more frequent in worsening progressive MS; the EDSS score remained stable in stMS (decreased from 3.0 to 2.5 at 7.1 years median follow-up), whereas in worsening progressive MS, it increased from a score of 4.0 to 6.0 with a median follow-up of 6.5 years (Table 1; eFigure 4 in Supplement 1). Worsening progressive MS showed more total brain volume loss (0.28% per year) vs stMS (estimate 0.997; 95% CI, 0.996-0.998; P < .001) (eFigure 5 in Supplement 1). Patients with RMS were more frequently untreated in active vs remission state (Table 1).
Table 1. Patient Characteristics of Stable, Worsening Progressive Multiple Sclerosis (MS) and Relapsing MS Sampled During Remission and Active Disease.
| Variable | MS, No. (%) | P value | No. (%) | P value | ||
|---|---|---|---|---|---|---|
| Stable | Worsening progressive | Remission | Active | |||
| No. of patients | 19 | 18 | NA | 66 | NA | |
| Samples, No. | 169 | 184 | 66 | 66 | ||
| No. of samples per patient | 9 (8-10) | 10 (9-12.5) | .10 | NA | NA | NA |
| Follow-up time, median (IQR) [range], y | 7.1 (5.7-8.0) [4.1-9.0] | 6.5 (5.2-7.7) [2.7-8.5] | .40 | NA | NA | NA |
| Sex | ||||||
| Female | 12 (63.2) | 11 (61.1) | <.99 | 50 (75.8) | NA | |
| Male | 7 (36.8) | 7 (38.9) | 16 (24.2) | |||
| Age, median (IQR), y | 44.2 (39.5-49.2) | 43.8 (40.9-53.8) | .78 | 40.6 (30.2-46.4) | 39.9 (29.2-45.4) | .62 |
| Disease category at study entry | ||||||
| RRMS | 18 (94.7) | 10 (55.6) | .02 | 62 (93.9) | 62 (93.9) | .80 |
| Progressive MS | 1 (5.3) | 8 (44.4) | 4 (6.1) | 4 (6.1) | ||
| EDSS score, median (IQR) | 3.0 (2.5-3.8) | 4.0 (3.1-4.4) | .07 | 2.0 (1.5-3.0) | 2.0 (2.0-3.0) | .25 |
| Disease duration, median (IQR), y | 9.4 (6.3-20.1) | 13.70 (7.8-18.7) | .43 | 7.8 (3.8-14.7) | 7.5 (3.4-14.1) | .50 |
| DMT | .09 | .001 | ||||
| Untreated | 3 (15.8) | 7 (38.9) | 8 (12.1) | 23 (34.8) | ||
| Platform | 5 (26.3) | 0 (0) | 4 (7.6) | 9 (13.6) | ||
| Oral | 6 (31.6) | 6 (33.3) | 40 (60.6) | 31 (47.0) | ||
| Monoclonal antibody therapies | 5 (26.3) | 5 (27.8) | 13 (19.7) | 3 (4.5) | ||
| Relapsea | NA | NA | NA | 0 (0) | 36 (54.5) | NA |
| Time since last relapse, median (IQR), d | NA | NA | NA | NA | 16.0 (4.8-22.5) | NA |
| T2w lesion volume, median (IQR), mL | 10.9 (2.7-19.7) | 16.3 (12.8-44.7) | .21 | 5.2 (2.0-14.6) | 5.9 (2.6-17.9) | 0.48 |
| EDSS score at last sampling, median (IQR) | 2.5 (2.0-3.8) | 6.0 (5.6-6.9) | <.001 | NA | NA | NA |
| No. of PIRA events | ||||||
| 0 | 19 (100) | 0 (0) | <.001 | NA | NA | NA |
| 1 | 0 (0) | 6 (33.3) | ||||
| 2 | 0 (0) | 8 (44.4) | ||||
| 3 | 0 (0) | 4 (22.2) | ||||
| DMT at last visit | ||||||
| Untreated | 1 (5.3) | 4 (22.2) | <.001 | NA | NA | NA |
| Platform | 4 (21.1) | 0 (0) | ||||
| Orals | 11 (57.9) | 0 (0) | ||||
| mAB | 3 (15.8) | 14 (77.8) | ||||
| CEL at sample | 1 (0.8) | 2 (1.9) | .83 | NA | NA | NA |
| New/enlarging T2w lesion at sample | 13 (7.7) | 20 (10.9) | .41 | |||
| Presence of CEL | NA | NA | NA | 0 (0) | 30 (45.5) | NA |
| Relapse and CEL | 0 (0) | 9 (13.6) | NA | |||
| T2w lesion volume, median (IQR), mL | 5.2 (2.0-14.6) | 5.9 (2.6-17.9) | .48 | |||
Abbreviations: CEL, contrast-enhancing lesion; DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; mAB, monoclonal antibody therapies; MS, multiple sclerosis; NA, not applicable; PIRA, progression independent of relapse activity; RRMS, relapsing-remitting MS; w, weighted.
Within 30 days.
Comparison of sGFAP and sNfL Concentrations Between Patients and HCs
sGFAP levels were highest in worsening progressive MS (103.0 pg/mL with a 77% increase vs 51.8 pg/mL in HCs; P < .001), followed by RMS in active disease (59.1 pg/mL; P < .001), RMS during remission (52.9 pg/mL; P = .01), and patients with stMS (63.2 pg/mL; P = .12) (eTable 1, eFigure 6 in Supplement 1). Conversely, sNfL levels were highest in active RMS (10.2 pg/mL, namely 98.6% as per adjusted estimate higher than in HCs, 6.3 pg/mL; P < .001), followed by worsening progressive MS (10.9 pg/mL; P < .001), stMS (7.2 pg/mL; P = .03), and RMS in remission (6.7 pg/mL; P < .001).
Serum GFAP and sNfL Levels in Worsening Progressive MS vs stMS
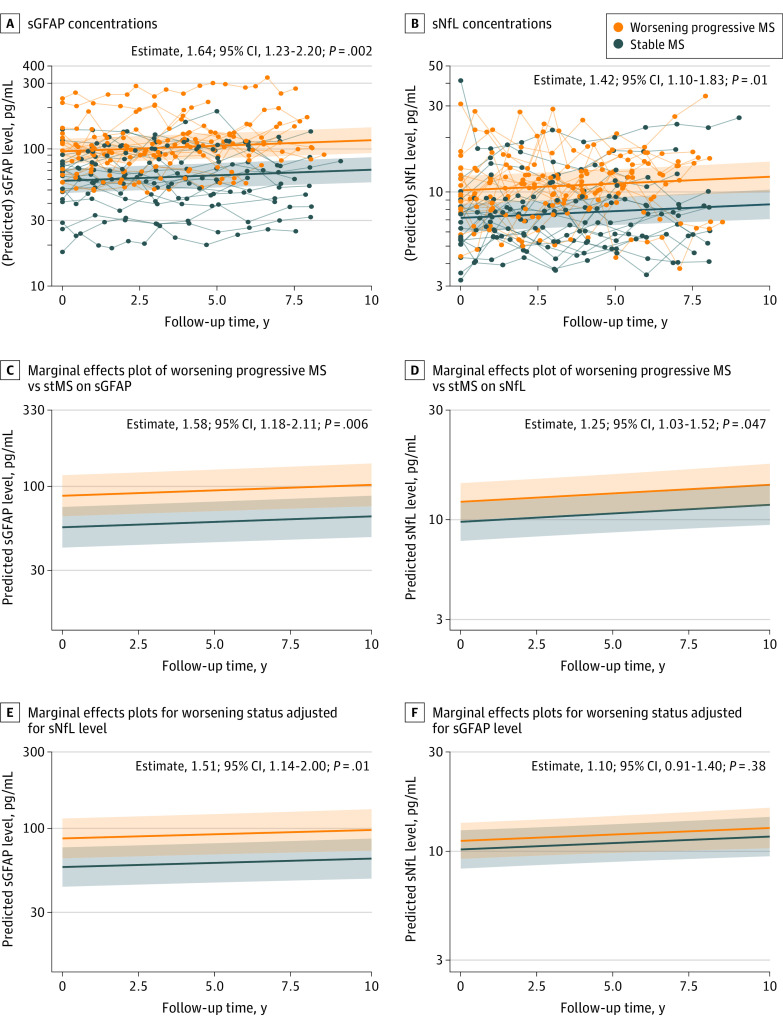
sGFAP and sNfL concentrations were increased by 64.2% and 42.2%, respectively, in worsening progressive MS vs stMS (Table 2, model 1, Figure 1A). After multivariable adjustment, these differences were 57.5% and 24.8%, respectively (Table 2, model 2, Figure 1B), also after additional correction for sNfL (50.9% increase in worsening progressive MS vs stMS), whereas the 25% increase of sNfL levels disappeared after additional sGFAP-level adjustment (Table 2, model 3, Figure 1C). Additional sensitivity analyses adjusting for T2-weighted lesion volume, and number of new and enlarged and contrast-enhancing brain lesions confirmed these results and showed comparably increased sGFAP levels in worsening progressive MS vs stMS (eTable 2 in Supplement 1). sGFAP levels in the worsening progressive MS cohort showed less within-person variability over time (ICC: estimate, 0.91; 95% CI, 0.83-0.94, ie, 91% of the variation in sGFAP levels is explained by variation between patients), whereas for sNfL ICC was 0.80 (95% CI, 0.72-0.85; difference, 11%; 95% CI, 2%-19%; P = .02).
Table 2. Multivariable Mixed Linear Models Investigating the Association of Worsening Status (Stable Multiple Sclerosis [MS] vs Worsening Progressive MS) With Log-Transformed Serum Glial Fibrillary Acidic Protein (sGFAP) and Serum Neurofilament Light Chain (sNfL) Levels.
| Model | Sample, No. | sGFAP, median (IQR), pg/mL | Estimate (95% CI)a | P value | sNfL, median (IQR), pg/mL | Estimate (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Model 1: simple | |||||||
| Follow-up time | NA | NA | 1.019 (1.011-1.026) | <.001 | NA | 1.017 (1.008-1.027) | <.001 |
| Progression | |||||||
| Stable MS | 169 | 63.2 (43.4-90.7) | NA | NA | 7.1 (5.4-9.4) | NA | NA |
| Worsening progressive MS | 184 | 103.0 (81.3-132.5) | 1.642 (1.226-2.199) | .002 | 10.9 (8.2-13.9) | 1.422 (1.104-1.831) | .01 |
| Model 2: multivariable | |||||||
| Age at baseline | NA | NA | 1.008 (0.993-1.023) | .35 | NA | 1.019 (1.009-1.029) | .002 |
| Follow-up time | NA | NA | 1.016 (1.007-1.025) | <.001 | NA | 1.019 (1.008-1.030) | .001 |
| Sex | |||||||
| Female | 224 | 87.7 (57.3-109.7) | 1.026 (0.764-1.378) | .87 | 8.4 (6.3-10.9) | 0.875 (0.725-1.058) | .21 |
| Male | 129 | 84.3 (57.7-121.1) | NA | 11.8 (5.8-16.7) | NA | ||
| BMIb | NA | NA | 0.991 (0.973-1.008) | .32 | NA | 0.969 (0.953-0.985) | <.001 |
| Disease duration at baseline | NA | NA | 1.002 (0.985-1.018) | .86 | NA | 1.005 (0.994-1.016) | .40 |
| DMT | |||||||
| Untreated | 48 | 97.4 (63.8-112.9) | NA | NA | 11.7 (8.7-16.4) | NA | NA |
| Platform | 40 | 68.6 (57.7-90.4) | 1.191 (1.048-1.356) | .009 | 9.7 (6.3-17.4) | 0.956 (0.821-1.142) | .59 |
| Orals | 118 | 74.7 (39.4-97.4) | 1.032 (0.933-1.139) | .54 | 7.7 (5.3-9.5) | 0.921 (0.811-1.039) | .20 |
| mAB | 147 | 103.6 (68.4-136.5) | 1.080 (0.997-1.171) | .06 | 9.4 (6.8-12.8) | 0.938 (0.842-1.035) | .22 |
| EDSS score | NA | NA | 1.011 (0.982-1.041) | .46 | NA | 1.002 (0.969-1.039) | .92 |
| Progression | |||||||
| Stable MS | 169 | 63.2 (43.4-90.7) | NA | NA | 7.1 (5.4-9.4) | NA | NA |
| Worsening progressive MS | 184 | 103.0 (81.3-132.5) | 1.575 (1.178-2.106 | .006 | 10.9 (8.2-13.9) | 1.248 (1.024-1.521) | .05 |
| Model 3: plus sNfL/sGFAP | |||||||
| Age at baseline | NA | NA | 1.004 (0.990-1.0.19) | .59 | NA | 1.016 (1.007-1.026) | .004 |
| Follow-up time | NA | NA | 1.012 (1.004-1.021) | .005 | NA | 1.014 (1.003-1.025) | .01 |
| Sex | |||||||
| Female | 224 | 87.7 (57.3-109.7) | 1.053 (0.792-1.400) | .74 | 8.4 (6.3-10.9) | 0.868 (0.725-1.040) | .17 |
| Male | 129 | 84.3 (57.7-121.1) | NA | NA | 11.8 (5.8-16.7) | NA | NA |
| BMIb | NA | NA | 0.996 (0.979-1.013) | .66 | NA | 0.973 (0.958-0.989) | .002 |
| Disease duration at baseline | NA | NA | 1.001 (0.985-1.017) | .94 | NA | 1.005 (0.994-1.1015) | .42 |
| DMT | |||||||
| Untreated | 48 | 97.4 (63.8-112.9) | NA | NA | 11.7 (8.7-16.4) | NA | NA |
| Platform | 40 | 68.6 (57.7-90.4) | 1.214 (1.072-1.377) | .003 | 9.7 (6.3-17.4) | 0.907 (0.782-1.082) | .24 |
| Oral | 118 | 74.7 (39.4-97.4) | 1.045 (0.948-1.151) | .37 | 7.7 (5.3-9.5) | 0.917 (0.812-1.032) | .17 |
| mAB | 147 | 103.6 (68.4-136.5) | 1.090 (1.008-1.179) | .03 | 9.4 (6.8-12.8) | 0.918 (0.827-1.010) | .10 |
| EDSS score | NA | NA | 1.012 (0.984-1.041) | .41 | NA | 0.999 (0.968-1.036) | .98 |
| sNfL per doubling, pg/mL | NA | NA | 1.141 (1.079-1.207) | <.001 | NA | NA | NA |
| sGFAP per doubling, pg/mL | NA | NA | NA | NA | NA | 1.217 (1.120-1.315) | <.001 |
| Progression | |||||||
| Stable MS | 169 | 63.2 (43.4-90.7) | NA | NA | 7.1 (5.4-9.4) | NA | NA |
| Worsening progressive MS | 184 | 103.0 (81.3-132.5) | 1.509 (1.139-1.998) | .01 | 10.9 (8.2-13.9) | 1.099 (0.905-1.339) | .38 |
Abbreviations: BMI, body mass index; DMT, disease modifying treatment; EDSS, Expanded Disability Status Scale; mAB, monoclonal antibody therapies; NA, not applicable; sGFAP, serum glial fibrillary acidic protein; sNfL, serum neurofilament light chain.
Estimates are back transformed and represent multiplicative effects.
Calculated as weight in kilograms divided by height in meters squared.
Figure 1. Serum Glial Fibrillary Acidic Protein (GFAP) and Serum Neurofilament Light Chain (sNfL) in Worsening Progressive Multiple Sclerosis (MS) and Stable MS.
Concentrations of sGFAP (A) and sNfL (B) in worsening progressive MS vs stable MS (stMS) over follow-up time. sGFAP and sNfL concentrations were increased by 64.2% and 42.2%, respectively, in worsening progressive MS vs stMS. Thin lines connect longitudinal data points of individual patients; thick lines show the group regression lines from Table 2, model 1. Only the regression lines are predicted. Estimates including 95% CI and P value of differences between worsening progressive MS vs stMS are added to the plots (B). Marginal effects plots of multivariable mixed models showing the association of worsening progressive MS vs stMS with sGFAP (C) and sNfL levels (D) over follow-up time. After adjustment for potential confounders, sGFAP and sNfL concentrations were increased by 57.5% and 24.8%, respectively, in worsening progressive MS vs stMS (Table 2, model 2). Marginal effects plots for worsening status with additional adjustment for sNfL (E) and sGFAP (F). Additional correction for sNfL levels had a minor association with the difference of sGFAP levels between stMS vs worsening progressive MS status (50.9% increase); however, additional correction for sGFAP eliminated the association of progression status with sNfL levels (Table 2, model 3).
sGFAP and sNfL Levels in RMS During Active Disease and Remission
sNfL concentrations were 58.4% increased in active disease vs remission, whereas this difference was 7.3% for sGFAP levels (eTable 3 in Supplement 1, model 1). After adjustment for potential confounders, these differences were 53.2% and 4.8%, respectively (eTable 3 in Supplement 1, model 2). Additional correction for sGFAP levels did not influence the association of focal inflammation status with sNfL levels (50.6% increase in active vs remission state), whereas association with sGFAP levels remained insignificant (eTable 3 in Supplement 1, model 3).
Association of Baseline sGFAP and sNfL Levels With Brain Volume Loss and PIRA
Each doubling of baseline sGFAP levels was associated with an additional loss of gray matter volume (−0.24% per year; 95% CI, −0.35% to −0.12%; P < .001) but not white matter volume (0.05%; 95% CI, −0.09% to 0.18%; P = .48), whereas doubling of baseline sNfL levels was associated with an additional loss of white matter volume (−0.26%; 95% CI, −0.38% to −0.15%; P < .001) but not gray matter volume (−0.01%; 95% CI, −0.11 to 0.09; P = .78) (eTable 4, eFigure 7 in Supplement 1). Baseline values of sGFAP levels had a better prognostic capacity for future PIRA (HR per doubling, 3.88; 95% CI, 1.69-8.86; P = .001; ie, an almost 4-fold risk of PIRA by doubling of baseline sGFAP concentration) than sNfL levels (HR, 1.77; 95% CI, 1.11-2.83; P = .02). In a combined model, with additional adjustment for age, sex, BMI, and disease duration, these findings were confirmed: sGFAP levels (HR, 3.63; 95% CI, 1.46-9.04; P = .006) and sNfL levels (HR, 1.90; 95% CI, 0.86-4.19; P = .11).
Cohort 2
Cohort Characteristics
We included 252 patients receiving BCDT who were relapse-free in the 6 months prior to sampling (ie, baseline). The majority of patients presented with RRMS (181 of 252 [71.8%]), whereas the remaining had progressive MS (34 [13.5%] secondary progressive MS; 37 [14.7%] primary progressive MS). A total of 43 of 252 (17.1%) experienced CDW during follow-up, of which 39 (90.7%) were due to PIRA and 4 (9.3%) due to RAW (eTable 5 in Supplement 1).
sGFAP and sNfL Levels and Development of Future CDW
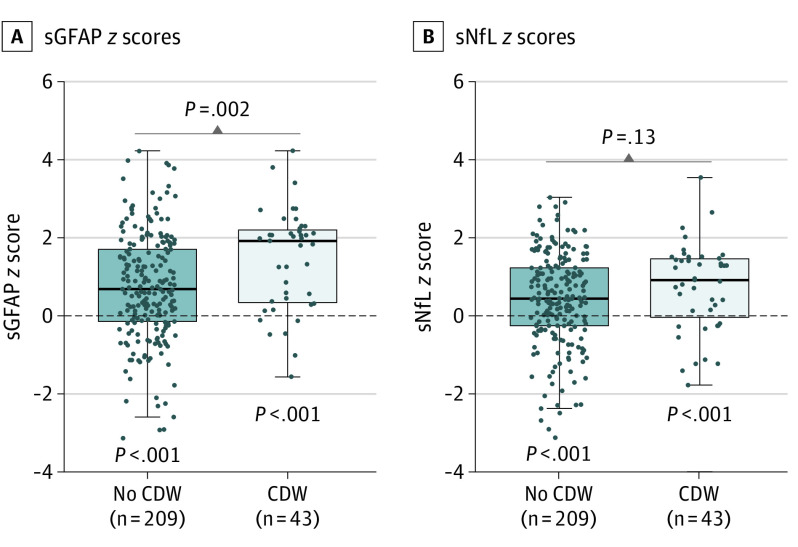
In patients with MS overall, sGFAP levels were strongly increased compared with those of HCs (z score = 0) with a median (IQR) of 0.82 (−0.05 to 1.95) z score units above normal (P < .001), whereas the increase of sNfL levels was less pronounced (0.50; IQR, −0.25 to 1.32; P < .001). Development of CDW was associated with a higher sGFAP z score 12.2 (IQR, 10.7-16.8) months after BCDT start than in patients without future CDW (1.94; IQR, 0.36-2.23 vs 0.71; IQR, −0.13 to 1.73) (Figure 2A). Although sNfL z score were less but still significantly increased vs that in HCs, the difference between patients with vs those without CDW development was not significant (Figure 2B). This pattern was similar when RAW events were excluded (sGFAP levels: PIRA, 1.98; IQR, 0.33-2.27 vs no PIRA, 0.71; IQR, −0.11 to 1.73; P = .003; sNfL levels: PIRA, 1.09; IQR, 0.14-1.49 vs no PIRA, 0.44; IQR, −0.25 to 1.23; P = .04).
Figure 2. Serum Glial Fibrillary Acidic Protein (sGFAP) and Serum Neurofilament Light Chain (sNfL) z Scores in Patients With and Without Confirmed Disease Worsening During Follow-up While Receiving B-Cell–Depleting Therapy in Comparison to Healthy Controls.
Box plot representation of sGFAP z scores (A) and sNfL z scores (B). Dashed lines indicate mean values in healthy controls (ie, z score = 0) and P values below indicate whether observed values differ from z scores 0 (Wilcoxon signed rank test). In patients with MS (without and with future confirmed disease worsening [CDW] development), sGFAP levels were increased compared with healthy controls (z scores healthy controls = 0; P < .001 for both), whereas the increase of sNfL was less pronounced (P < .001 for both). Development of CDW was associated with higher sGFAP z scores, which was not the case for sNfL.
Next, we explored which demographic and disease-related variables were associated with increased biomarker levels in patients receiving BCDT compared with HCs using multivariable models (ie, using biomarker z scores as dependent variable (eTable 6 in Supplement 1). Models on the absolute sGFAP and sNfL concentrations are included in eTable 7 in Supplement 1. The model for sGFAP z score explained 13.3% of the variance and was driven by female sex, younger age, higher EDSS, and whether the patient developed CDW while receiving BCDT (CDW status in eTable 6 in Supplement 1). The same model with sNfL z score as the outcome explained 1.8% of its variance. Specifically, only sGFAP z scores, but not those of sNfL, were linked to the EDSS score and future CDW. Again, findings were similar in the PIRA only set (not shown).
Prognostic Value of sGFAP and sNfL Levels for Future CDW
Time-to-event analyses showed that 1 sGFAP z-score unit increase led to a 1.36-fold (HR, 1.36; 95% CI, 1.09-1.69; P = .006) increased risk of CDW (after correction for covariates: HR, 1.32; 95% CI, 1.06-1.66; P = .01). For sNfL z score, a numerically higher risk was found (HR, 1.25; 95% CI, 0.95-1.65; P = .11; after correction: HR, 1.27; 95% CI, 0.95-1.71; P = .11). When combining both sGFAP and sNfL z scores in 1 model, sGFAP was associated with disease worsening: HR, 1.34 (95% CI, 1.03-1.73; P = .03), but not sNfL (HR, 1.04; 95% CI, 0.75-1.43; P = .82).
Next, we used different z score cut points to see whether their increase was associated quantitatively to the risk of CDW. sGFAP z score cut points of 1, 1.5, and 2 led to gradually increasing CDW hazard ratios ranging from 2.1 to 3.4 (eFigure 8A in Supplement 1). The associations were all significant for sGFAP, whereas for sNfL (eFigure 8B in Supplement 1), findings were less strong.
sGFAP and Prognostication of Worsening in a Combined Analysis of sNfL and sGFAP Levels
The risk of CDW in patients with high sGFAP levels (ie, z score >1.8, cutoff optimized in ROC analysis) compared with low sGFAP levels was 3-fold increased (HR, 3.25; 95% CI, 1.78-5.93; P < .001) in a time-to-event analysis. Patients with high sNfL levels (ie, z score >1.3) showed a 2-fold increased risk of future CDW (HR, 2.26; 95% CI, 1.24-4.14; P = .008) vs patients with low sNfL levels.
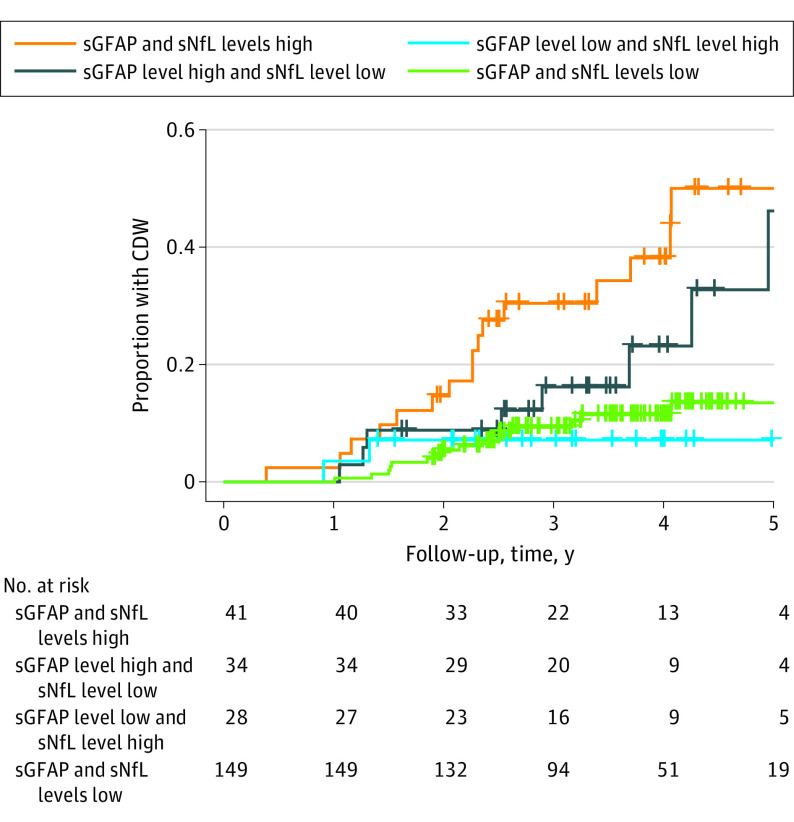
The combination of high sGFAP/high sNfL levels was associated with a 4-fold increased risk of worsening compared with low sGFAP/low sNfL levels (HR, 4.09; 95% CI, 2.04-8.18; P < .001 and PIRA only: HR, 4.71; 95% CI, 2.05-9.77; P < .001), that of high sGFAP/low sNfL levels showed slightly reduced association (Figure 3) (PIRA only: HR, 2.28; 95% CI, 0.92-5.64; P = .08). In contrast, the combination of low sGFAP/high sNfL levels did not show an increased risk for future CDW (PIRA only: HR, 1.17; 95% CI, 0.34-4.10; P = .80). The Kaplan-Meier analysis indicated that 4 years after initiation of treatment, 38% (95% CI, 20%-53%) of patients in the high sGFAP/high sNfL group will have CDW, compared with 23% (95% CI, 3%-39%) in the high sGFAP/low sNfL group, whereas this will be the case only in 11% (95% CI, 6%-16%) if they fall into the low sGFAP/low sNfL group.
Figure 3. Kaplan-Meier Curves Using Combined Biomarker Data to Predict Time to Confirmed Disease Worsening (CDW).
Optimized cutoffs of serum glial fibrillary acidic protein (sGFAP) and serum neurofilament light chain (sNfL) z scores from receiver operating characteristic curve analysis, based on the Youden index, were used to dichotomize patient groups. High sGFAP/high sNfL levels were associated with a 4-fold (hazard ratio [HR], 4.09; 95% CI, 2.04-8.18; P < .001) increased risk of CDW compared with low sGFAP/low sNfL levels. The combination of high sGFAP/low sNfL levels showed a slightly reduced risk (HR, 2.32; 95% CI, 0.99-5.42; P = .05). The combination of low sGFAP/high sNfL levels, however, did not show an increased risk on CDW (HR, 1.03; 95% CI, 0.30-3.53; P = .97).
Discussion
The long-term course of disability in MS is driven by 2 partly independent pathomechanisms: focal lesional activity and brain-diffuse neurodegeneration.23 sNfL has been established in recent years as a biomarker of ongoing neuronal damage in the course of the former process, whereas its association with progression as the clinical manifestation of the latter is relatively weaker.12 The need for a biomarker that specifically reflects current and prognosticates future disability due to pure progression/PIRA has become urgent on the background that disability worsening often continues despite almost complete suppression of acute disease activity under high-efficacy therapies.2,3 Increased CSF levels of GFAP have been proposed first by Axelsson et al14 as a specific biomarker for progression. However, this finding was based on repetitive CSF analysis, which has precluded its entry into routine practice to close this diagnostic gap. Second, the relative contribution of lesional activity and RAW vs PIRA to the overall progression could not be determined in the mixed RRMS and progressive MS population studied. In this cohort study, we attempted to resolve the question about the mechanistic source of GFAP increase in MS by 2 orthogonal methodological approaches where relapse activity was absent in worsening progressive MS and stMS (cohort 1) or lesional activity and relapses were suppressed by BCDT in a mixed MS population (cohort 2). Current results suggest that increased levels of sGFAP were associated with pure progression/PIRA, although this biomarker is largely inert to acute disease activity. Higher baseline sNfL levels were prognostic for white matter volume loss, and baseline sGFAP specifically prognosticated gray matter loss, a previously proposed proxy for disease progression.27,28 These findings from serum analysis are fully congruent with those of Axelsson et al in CSF.14
The increase of GFAP levels in the course of MS progression appears to result from astrocyte proliferation/activation and possibly injury.29 This seems to be a brain-diffuse process, affecting mainly the normal-appearing white matter resulting in decreased diffusion tensor imaging derived measures.16,30 In return, the minor increase of sNfL seen, eg, in patients with worsening progressive MS may result from continuous neuronal loss outside of acute lesion formation as part of the pathogenesis of progression due to subclinical neuroinflammation in chronic active lesions and in normal-appearing white matter.31
The association of sGFAP levels with future CDW and imaging features of progression is further supported by studies using serum samples.16,17,32,33 However, these were incompletely controlled for confounding factors such as sex, age, and BMI, which resulted in significant overlap in GFAP levels across different MS groups and also controls, thus limiting clinical usefulness. Moreover, the comparison of raw biomarker concentrations vs z scores as an outcome highlights the advantage of the latter in terms of pathogenetic relevance and ease of interpretability; covariates explained 29% of variation in raw sNfL concentration but only 1.8% of the variation in sNfL z score. For sGFAP levels, covariates similarly explained 25% of the variation in sGFAP concentrations but additionally also 13% in the variation of sGFAP z scores. Using corrected z scores, instead of absolute concentrations, to assess change of these biomarkers compared with normal values, thus substantially increase the sensitivity for detecting pathologic values, a prerequisite for its use in individual patients.
Important from a clinical perspective is that prognostication of future disability can be made based on a single GFAP measurement and from a biofluid (serum) that is easily accessible in clinical practice. A further aspect in our data set for the clinical use of sGFAP levels is the establishment of normative values of sGFAP that allow to define aberrations from physiological values corrected for confounding factors. Although age and BMI were known confounders, also based on the experience from the establishment of normative values for sNfL,4 the 15% increase of sGFAP values in women vs men was an unexpected finding. Third, the combined evaluation of sNfL and sGFAP levels provides the highest predictive power for disability worsening, specifically in years 2 to 4, as a reflection of a comprehensive coverage of biological processes leading to disability worsening.
Limitations
This study has some limitations. One limitation is that we studied almost exclusively the effect of anti-CD20 antibodies as high-efficacy therapy but less so other types of disease-modifying treatments of this efficacy level (eg, natalizumab). Such evaluations will be necessary to expand on the limited data available whether disease-modifying treatment can lead to decrease of sGFAP levels34 as a potential sign of attenuation of astrogliosis or pathological astrocyte activation. Second, the current normative database is derived from a relatively small cohort of HCs, where the impact of subclinical comorbidities could not be explored. A much larger cohort of persons, including those with other neurologic disease, may be needed to establish robust normal values for sGFAP levels.
Conclusions
In summary, the findings of this cohort study suggest that sGFAP levels may serve as a biomarker that reflects specifically chronic disease processes conveyed by astrocytes that manifest as pure progression/PIRA in MS. With this property, sGFAP levels are complementary to sNfL, whose levels are strongly associated with neuronal damage due to lesional disease activity.
eMethods
eTable 1. Multivariable Mixed Models Testing Associations Between sGFAP and sNfL and Age, Sex, BMI, and MS Extreme Phenotypes vs Healthy Controls
eTable 2. Sensitivity Analysis of Multivariable Mixed Linear Models Investigating the Association Between Worsening Status and sGFAP Levels (Left) and sNfL Levels (Right) With Additional Correction for MRI Variables
eTable 3. Multivariable Mixed Linear Models Investigating the Effect of Focal Inflammation (Remission vs Active State) on sGFAP Levels (Left) and sNfL Levels (Right)
eTable 4. Multivariable Mixed Models to Assess the Association Between BL sGFAP and BL sNfL and Longitudinal GMV or WMV
eTable 5. Patient Characteristics at Time of Sample Collection (Baseline)
eTable 6. Multivariable Linear Models Investigating the Effect of Demographic and MS-Related Characteristics on sGFAP Z Scores (Left) and sNfL Z Scores (Right)
eTable 7. Multivariable Linear Models Investigating the Effect of Demographic and MS-Related Characteristics on sGFAP (Left) and sNfL Concentrations (Right)
eFigure 1. Comparison of sNfL Results From the Nf-Light Kit (Singleplex) and Neurology 2-Plex B Assay (Duplex) (n: 480)
eFigure 2. Associations Between Age (A), BMI (B), and Sex (C) and sGFAP Concentrations in Healthy Controls
eFigure 3. Serum GFAP (Left) and sNfL (Right) and Age in Healthy Controls Stratified by Sex
eFigure 4. EDSS Score Over Time in Stable MS and Worsening Progressive MS
eFigure 5. Total Brain Volume Loss in Stable MS and Worsening Progressive MS
eFigure 6. Serum GFAP (A) and sNfL (B) in Different MS Groups vs Healthy Controls
eFigure 7. Associations of sGFAP and sNfL With Gray (A) and White Matter (B) Atrophy
eFigure 8. Hazard Ratios for CDW Using Increasing Z Score Cut Points for sGFAP (A) and sNfL (B)
eReferences
Data Sharing Statement
References
- 1.Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the real MS. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi: 10.1177/17562864211066751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cree BAC, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team . Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cree BAC, Hollenbach JA, Bove R, et al. ; University of California, San Francisco MS-EPIC Team . Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653-666. doi: 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benkert P, Meier S, Schaedelin S, et al. ; NfL Reference Database in the Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 5.Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner S, Steffen F, Uphaus T, et al. ; KKNMS consortium . Clinical implications of serum neurofilament in newly diagnosed MS patients: a longitudinal multicentre cohort study. EBioMedicine. 2020;56:102807. doi: 10.1016/j.ebiom.2020.102807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gafson AR, Jiang X, Shen C, et al. Serum neurofilament light and multiple sclerosis progression independent of acute inflammation. JAMA Netw Open. 2022;5(2):e2147588. doi: 10.1001/jamanetworkopen.2021.47588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thebault S, Reaume M, Marrie RA, et al. High or increasing serum NfL is predictive of impending multiple sclerosis relapses. Mult Scler Relat Disord. 2022;59:103535. doi: 10.1016/j.msard.2022.103535 [DOI] [PubMed] [Google Scholar]
- 9.Bridel C, Leurs CE, van Lierop ZYGJ, et al. Serum neurofilament light association with progression in natalizumab-treated patients with relapsing-remitting multiple sclerosis. Neurology. 2021;97(19):e1898-e1905. doi: 10.1212/WNL.0000000000012752 [DOI] [PubMed] [Google Scholar]
- 10.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359-1366. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manouchehrinia A, Stridh P, Khademi M, et al. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(23):e2457-e2467. doi: 10.1212/WNL.0000000000009571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leppert D, Kropshofer H, Häring DA, et al. Blood neurofilament light in progressive multiple sclerosis: post hoc analysis of 2 randomized controlled trials. Neurology. 2022;98(21):e2120-e2131. doi: 10.1212/WNL.0000000000200258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norgren N, Sundström P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63(9):1586-1590. doi: 10.1212/01.WNL.0000142988.49341.D1 [DOI] [PubMed] [Google Scholar]
- 14.Axelsson M, Malmeström C, Nilsson S, Haghighi S, Rosengren L, Lycke J. Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. J Neurol. 2011;258(5):882-888. doi: 10.1007/s00415-010-5863-2 [DOI] [PubMed] [Google Scholar]
- 15.Petzold A, Eikelenboom MJ, Gveric D, et al. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain. 2002;125(pt 7):1462-1473. doi: 10.1093/brain/awf165 [DOI] [PubMed] [Google Scholar]
- 16.Högel H, Rissanen E, Barro C, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler. 2020;26(2):210-219. doi: 10.1177/1352458518819380 [DOI] [PubMed] [Google Scholar]
- 17.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):14798. doi: 10.1038/s41598-018-33158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18(3):158-172. doi: 10.1038/s41582-021-00616-3 [DOI] [PubMed] [Google Scholar]
- 19.Martínez MAM, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler. 2015;21(5):550-561. doi: 10.1177/1352458514549397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mañé-Martínez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid in different types of multiple sclerosis. J Neuroimmunol. 2016;299:112-117. doi: 10.1016/j.jneuroim.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Gunnarsson M, Malmeström C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83-89. doi: 10.1002/ana.22247 [DOI] [PubMed] [Google Scholar]
- 22.Burman J, Zetterberg H, Fransson M, Loskog AS, Raininko R, Fagius J. Assessing tissue damage in multiple sclerosis: a biomarker approach. Acta Neurol Scand. 2014;130(2):81-89. doi: 10.1111/ane.12239 [DOI] [PubMed] [Google Scholar]
- 23.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. doi: 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 26.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914-1922. doi: 10.1212/WNL.0b013e3181c47cc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(9):1144-1150. doi: 10.1001/archneurol.2009.174 [DOI] [PubMed] [Google Scholar]
- 28.Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 2022;79(7):682-692. doi: 10.1001/jamaneurol.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prineas JW, Lee S. Multiple sclerosis: destruction and regeneration of astrocytes in acute lesions. J Neuropathol Exp Neurol. 2019;78(2):140-156. doi: 10.1093/jnen/nly121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraste M, Bezukladova S, Matilainen M, et al. Increased serum glial fibrillary acidic protein associates with microstructural white matter damage in multiple sclerosis: GFAP and DTI. Mult Scler Relat Disord. 2021;50(January):102810. doi: 10.1016/j.msard.2021.102810 [DOI] [PubMed] [Google Scholar]
- 31.Maggi P, Kuhle J, Schädelin S, et al. Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology. 2021;97(6):e543-e553. doi: 10.1212/WNL.0000000000012326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelhak A, Hottenrott T, Morenas-Rodríguez E, et al. Glial activation markers in CSF and serum from patients with primary progressive multiple sclerosis: potential of serum GFAP as disease severity marker? Front Neurol. 2019;10(March):280. doi: 10.3389/fneur.2019.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayrignac X, Le Bars E, Duflos C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10(1):10923. doi: 10.1038/s41598-020-67934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhle J, Maceski AM, Meinert R, et al. Plasma neurofilament light chain and glial fibrillary acidic protein levels are prognostic of disability worsening: a biosignature that helps in differentiating active from nonactive SPMS. Poster presented at: the American Academy of Neurology Meeting; April 21, 2021; online. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Multivariable Mixed Models Testing Associations Between sGFAP and sNfL and Age, Sex, BMI, and MS Extreme Phenotypes vs Healthy Controls
eTable 2. Sensitivity Analysis of Multivariable Mixed Linear Models Investigating the Association Between Worsening Status and sGFAP Levels (Left) and sNfL Levels (Right) With Additional Correction for MRI Variables
eTable 3. Multivariable Mixed Linear Models Investigating the Effect of Focal Inflammation (Remission vs Active State) on sGFAP Levels (Left) and sNfL Levels (Right)
eTable 4. Multivariable Mixed Models to Assess the Association Between BL sGFAP and BL sNfL and Longitudinal GMV or WMV
eTable 5. Patient Characteristics at Time of Sample Collection (Baseline)
eTable 6. Multivariable Linear Models Investigating the Effect of Demographic and MS-Related Characteristics on sGFAP Z Scores (Left) and sNfL Z Scores (Right)
eTable 7. Multivariable Linear Models Investigating the Effect of Demographic and MS-Related Characteristics on sGFAP (Left) and sNfL Concentrations (Right)
eFigure 1. Comparison of sNfL Results From the Nf-Light Kit (Singleplex) and Neurology 2-Plex B Assay (Duplex) (n: 480)
eFigure 2. Associations Between Age (A), BMI (B), and Sex (C) and sGFAP Concentrations in Healthy Controls
eFigure 3. Serum GFAP (Left) and sNfL (Right) and Age in Healthy Controls Stratified by Sex
eFigure 4. EDSS Score Over Time in Stable MS and Worsening Progressive MS
eFigure 5. Total Brain Volume Loss in Stable MS and Worsening Progressive MS
eFigure 6. Serum GFAP (A) and sNfL (B) in Different MS Groups vs Healthy Controls
eFigure 7. Associations of sGFAP and sNfL With Gray (A) and White Matter (B) Atrophy
eFigure 8. Hazard Ratios for CDW Using Increasing Z Score Cut Points for sGFAP (A) and sNfL (B)
eReferences
Data Sharing Statement