Abstract
Early diagnosis and appropriate staging workup are crucial for cancer patients. Whole-body magnetic resonance imaging (WB-MRI) has been proposed as another practical whole-body approach for assessing local invasiveness and distant metastases in patients newly diagnosed with cancer. The current study aimed to evaluate the efficacy of WB-MRI in assessing metastasis in patients newly diagnosed with cancer using histopathologic data as the reference method. A prospective observational study was performed from April 2018 to July 2020. MRI sequences were utilized to acquire anatomical and functional images in three orthogonal planes. The discovery was classified as nodal, skeletal and visceral metastases. Patient-based analysis was used for visceral metastasis and region-based for skeletal, systemic and lymph node metastases. A total of 43 consecutive patients (mean age, 56±15.2 years) were assessed successively. In 41 patients, there was a concordance between the WB-MRI and histological confirmation. The most prevalent site of metastasis was the skeletal system (18 patients). There were 12 individuals with liver metastasis, 10 with lung metastasis and 4 with peritoneal metastasis, with just one brain metastatic lesion found. On WB-MRI, 38 lymph node groups were deemed positive. Out of the total, 66 skeletal locations contained metastases. The accuracy of WB-MRI for nodal, skeletal and visceral metastases was (98.45, 100 and 100%, respectively). In conclusion, WB-MRI in three orthogonal planes, including the diffusion-weighted MRI with background body signal suppression sequence, may be utilized efficiently and accurately for assessing metastasis staging and may thus be utilized in patients with newly diagnosed cancer.
Keywords: WB-MRI, WB-DWI, DWIBS, cancer, metastasis, bone scan
Introduction
Metastasis is the leading cause of morbidity and mortality in patients with cancer, accounting for ~90% of cancer-associated deaths. However, the survival rate has recently increased, owing to earlier detection of cancers and developments in treatments (1). Particular cancers tend to metastasize to specific organs, e.g., breast and prostate cancers prominently develop skeletal metastases (2). Regarding visceral metastasis, the liver is the most common site for metastasis, followed by the lung (3).
Early diagnosis, proper staging and an accurate metastatic workup are critical in oncology. The type and stage of cancer are both crucial variables in the prognosis. As tumors may spread to different anatomical locations, a reliable method to detect distant metastases of malignancies is part of a project for guiding future staging and appropriate treatment (4). Cancer care is highly dependent on accurate information on individual tumor spread. For this reason, early diagnosis and assessment of metastatic workup in individual patients require several imaging modalities. However, this method is time-consuming, costy and unpleasant for the patient (5). In high-risk patients, occult metastases are evaluated during staging using chest X-rays, abdominal ultrasounds and bone scintigraphy, while computed tomography (CT), positron emission tomography with CT (PET/CT) and magnetic resonance imaging (MRI) are increasingly being used as they are more advanced and comprehensive in the detection of both primary and metastatic lesions (6). Previous clinical studies have indicated that 18F-fluorodeoxyglucose (18FDG) PET/CT has significantly higher sensitivity and specificity in the diagnosis and staging of certain cancers than CT alone, even though it is more expensive and uses radioactive ions, i.e. 18FDG (6). Whole-body MRI (WB-MRI) has been proposed as another effective whole-body approach for assessing both local invasiveness and distant metastases in patients with newly diagnosed cancers in recent years (7,8). WB-MRI provides several advantages, including the absence of ionizing radiation, high soft-tissue contrast, low cost, the absence of radioactive substances, improved availability and its safe use in patients with renal impairment (6,9). WB-MRI primarily provides structural information (revealing a detailed image of the pathology or lesion) on tumor spread; however, the absence of functional datasets has been resolved by incorporating WB-DWI into medical practice (10). WB-DWI shortens examination interpretation times by directing the radiologist's attention to abnormalities, which may then be investigated on anatomic sequences (11,12). In addition, WB-DWI with background body signal suppression (DWIBS) enables volumetric capture of DWIs of the entire body. This idea differs from traditional DWI, which has been demonstrated to be effective during free breathing and has a crucial role in WB imaging in oncology patients (13). Initial studies on TNM staging of lung cancer using WB-MRI vs. PET/CT reveal that both modalities give adequate accuracy and effectiveness. Whole-body MRI tends to be more effective in identifying brain and liver metastases, whereas PET/CT appears to be more effective in detecting lymph node (LN) and soft tissue metastases (14).
The present study aimed to determine the efficacy of WB-MRI in assessing metastasis in patients with newly diagnosed cancers using histopathologic data as the reference method.
Patients and methods
Study design
This prospective study was conducted at a single center (Shahid Hemn Teaching Hospital; Sulaimani, Iraq) from April 2018 to July 2020. It was performed on patients newly diagnosed with cancers who have had a whole-body MRI scan.
Inclusion and exclusion criteria
Only patients with malignancies proven by histology and patients with metastatic lesions proven by histopathology or cytology were included. Patients whose MRI sequences were incomplete, low-quality and/or had no histopathological evidence of metastatic lesions were eliminated from the study. Low-quality or incomplete MRI included MRI exams with incomplete sequence(s), which is particularly common in elderly patients who are unable to tolerate the scan, or uncooperative patients who do not obey breathing instructions and motion artifacts that may impair images and lead to lower accuracy.
Measurements
Sensitivity is the proportion of true-positive tests out of all patients with a condition. It was calculated as follows: Sensitivity=(True Positives)/(True Positives + False Negatives).
Specificity is the percentage of true negatives out of all subjects who do not have a disease or condition. Calculation: Specificity=(True Negatives)/(True Negatives + False Positives).
Accuracy measures how correct a diagnostic test identifies and excludes a given condition. Calculation: Accuracy=(True Negatives + True Positives)/(True Negatives + True Positives + False Negatives + False Positives).
Radiological evaluation
In the current study, five sequences were used, including anatomical and functional data between three orthogonal planes (coronal, axial and sagittal). A surface body coil (Philips ACHIEVA; 1.5 Tesla; Philips Medical Systems); was used to acquire the WB-MRI images. The same MR platform was used to scan all patients without giving contrast material. The evaluation lasted for ~1 h. Table I lists the MRI parameters in detail. For improved lesion detection, the DWIBS images were inverted black-and-white grayscale. Conventional sequences were employed to confirm positive DWIBS results or artifacts. On a workstation, two board-certified radiologists with 10 years of expertise in WB-MRI evaluated the findings. Imaging data were categorized based on nodal and distant metastasis and stored in an Excel sheet. The histopathology sample was ordered by a multidisciplinary team or the treating physician for clinical purposes. If histological examination of the samples provided an outcome, no further follow-up was performed. In the case of involvement of just one organ, a sample was taken from the suspicious lesion. In multi-organ metastases, only one lesion was confirmed and all other lesions were considered positive.
Table I.
Whole-body MRI sequences and parameters for oncologic patient evaluation.
| Sequence | T1WI | T1WI | T2WI | T2 STIR | WB-DWIBS |
|---|---|---|---|---|---|
| Plane | Coronal | Sagittal | Axial | Coronal | Coronal |
| Involved regions | Vertex to toes | Whole spine | Vertex to mid-thigh | Vertex to toes | Vertex to mid-thigh |
| Slices, n | 34 | 20 | 152 | 34 | 230 |
| Gap, mm | 1 | 0.4 | 0.6 | 1 | 0 |
| Thickness, mm | 6 | 4 | 6 | 6 | 2 |
| TR, msec | 412 | 500 | 1,000 | 570 | 1,400 |
| TE, msec | 4 | 16 | 80 | 80 | 70 |
| b-value, sec/mm2 | - | - | - | - | 0-800 |
| Phase encoding | Right/left | Feet-head | Anterior-posterior | Right/left | Anterior-posterior |
| Respiratory motion | Breath-hold | Free-breathing | Breath-hold | Breath-hold | Free-breathing |
| Total scan time (min) | 15-20 | 3-6 | 9-10 | 5-10 | 20-25 |
T1WI, T1-weighted imaging; WB-DWIBS, whole-body diffusion-weighted MRI with background body signal suppression sequence; STIR, short tau inversion recovery; TR, repetition time; TE, echo time.
To simplify the process, the WB-MRI was divided into three categories: Nodal, skeletal and visceral metastases. Patient-based analysis was used for visceral metastases, whereas region-based analysis was used for skeletal systems and LN groups.
Statistical analysis
To strengthen the identification of the collected datasets, statistical analysis using two softwares, SPSS (version 25; IBM Corporation) and Excel 2016 (Microsoft Corporation), was performed. The presence or absence of metastasis was used to classify the findings. Using 2x2 cross-tabulation data, the sensitivity, specificity and accuracy of WB-MRI were estimated.
Results
Patient characteristic
A WB-MRI scan was performed on 153 patients diagnosed with primary cancers. Only 43 individuals with distant metastatic lesions, confirmed by histology, were included. WB-MRI was used to evaluate a total of 43 consecutive patients (24 males and 19 females) who had just been diagnosed with cancer, with a mean age of 56±15.2 (range, 18-83) years. Histopathological confirmation was used as the gold standard. The present study included a variety of primary tumors. Breast cancer was the most prevalent primary tumor, accounting for 16.2%. Breast cancer was the most common primary tumor in seven cases followed by prostate cancer in six cases. Table II provides an account of the locations/types of primary tumors within the cohort.
Table II.
Types/locations of primary tumors in the patients (n=43).
| Primary malignancy | N |
|---|---|
| Melanoma | 4 |
| Sarcoma | 4 |
| Bronchogenic cancer | 2 |
| Prostate cancer | 6 |
| Breast cancer | 7 |
| Endometrial cancer | 3 |
| Ovarian cancer | 2 |
| Colorectal cancer | 2 |
| Thyroid carcinoma | 3 |
| Carcinoma of unknown primary origin | 6 |
| Neuroendocrine tumor | 1 |
| Mesothelioma | 1 |
| Testicular tumor | 2 |
| Total | 43 |
Efficacy
According to the patient-based method, there was a concordance between the WB-MRI and histological confirmation in 41 of 43 cases (95.3%). The skeletal system was the most prevalent location of metastasis. There was a concordance between WB-MRI and histological confirmation of visceral metastases. There were no false-positive or false-negative outcomes recorded. On WB-MRI, 12 patients had hepatic metastasis, 10 had pulmonary metastasis, 4 had peritoneal metastasis and only one had brain metastasis.
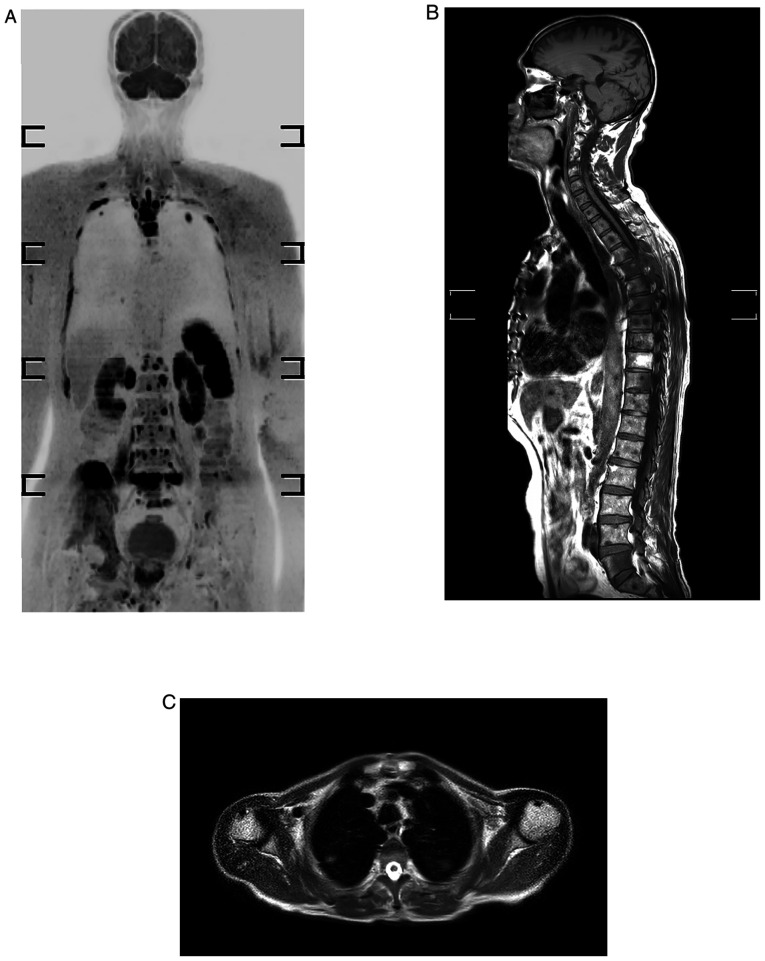
For the LNs, a region-based analysis was performed. A total of 258 areas were evaluated on 43 individuals. Lymphadenopathy was defined as any LN measuring >10 mm in the short axis diameter or any abnormal LN of any size that had newly appeared. WB-MRI verified 38 of the 258 LNs as positive, whereas histology confirmed 36. Two LN areas were first considered to be negative on imaging but turned out to be positive on histology. The presence or absence of metastases was determined in all skeletal areas. WB-MRI was used to evaluate 301 regions of the 43 patients, with 66 sites considered positive. The accuracy for detecting bone metastases was 100%. There were no false-positive or false-negative results for skeletal and visceral metastases. The sagittal plane is strongly recommended in oncology because spine curvatures may be responsible for partial volume effects that conceal certain lesion on coronal plane. In the current study, the accuracy for bone metastasis was 100%; this high result is explained by the efficacy of DWBIS and the provision of images in three orthogonal planes (Fig. 1).
Figure 1.
Whole-body MRI of a 69-year-old male who developed prostate cancer presented with skeletal and lung metastases. (A) Inverted black-white coronal diffusion-weighted MRI with background body signal suppression sequence displaying multiple rib, vertebral and right iliac bone lesions, and also pulmonary lesions at upper lobes bilaterally. (B) T1-weighted image displaying hypointense dorsal and lumbar skeletal lesions. (C) Axial T2-weightedimage showing abnormal focal pulmonary lesions.
The study found that WB-MRI was 100% accurate in diagnosing skeletal and visceral metastases but only 98.45% accurate in diagnosing LN metastases. The sensitivity, specificity and accuracy of visceral, skeletal and nodal metastases are all provided in Table III.
Table III.
Sensitivity, specificity and accuracy of visceral, skeletal and nodal metastases based on histological findings.
| MRI finding | Visceral metastasis | Bone metastasis | Nodal metastasis |
|---|---|---|---|
| Sensitivity, % | 100 | 100 | 94.74 |
| Specificity, % | 100 | 100 | 99.09 |
| Accuracy, % | 100 | 100 | 98.45 |
Discussion
A proper staging work-up and monitoring are required for assessing prognosis and management strategies in patients with cancer (15). Bone scans and PET scans are commonly utilized in the initial work-up in patients with cancer to evaluate metastases. These techniques expose the cancer-affected patient to potentially hazardous radiation (16). A previous study has been performed to demonstrate the utility of WB-MRI in resolving this dilemma, as MRI is generally more readily available and is a radiation-free technique. Adding to that, MRI may detect metastatic lesions in bone before the occurrence of changes in bone metabolism, making them visible on bone scans (16). WB-MRI has been used for both primary staging and monitoring of different malignancies, particularly cancers that commonly metastasize to the bone, brain and abdominal organs, such as breast cancer and colorectal cancer (17). WB-MRI has also been used as a diagnostic tool for imaging of various hematologic malignancies and bone marrow pathologies, such as lymphoma and multiple myeloma (17).
False-positive results, particularly in enlarged LNs due to inflammatory processes or normal-sized LNs carrying micro-metastases, may decrease MRI or CT sensitivity and specificity. Comparison of WB-MRI to other imaging modalities, such as PET-CT, bone scintigraphy and conventional cross-sectional imaging, has been performed in numerous studies (18-20). Barchetti et al (21) compared WB-MRI to PET-CT and discovered that it had a sensitivity, specificity and accuracy of 99, 98 and 98%, respectively. Regarding visceral metastasis, a recent meta-analysis reported that DWI had a sensitivity of 82.8% and specificity of 80.1% for detecting lung nodules (22). MRI sensitivity and specificity are reduced when pulmonary nodules are <10 mm in size. Regier et al (23)observed a sensitivity of 97% for nodules with a diameter of >10 mm, while it dropped to 86% for nodules 6-9 mm in size and 43.8% for lesions 5 mm or less. Goda et al (24) discovered a sensitivity, specificity and accuracy of 64, 88 and 76%, respectively, and an accuracy of 100% for hepatic lesions. DWIBS was used in the present study instead of DWI, which is more sensitive than DWI, as demonstrated in a recent study published by Eissawy et al (25). In the current study, WB-MRI demonstrated 100% accuracy for visceral metastases. The high percentages obtained in the current study, notably in detecting lung lesions, may be attributed to the use of DWIBS, which allows for free-breathing scanning of moving visceral organs and lesions. In metastatic cancer, there is a limited indication that the lesions detected by WB-MRI contain pathologically viable tumor cells (26). However, in the present study, histology confirmed the radiological findings.
The DWIBS sequence increases the detection of pulmonary lesions. Usuda et al (27) performed a study on 55 patients with lung cancer, concluding that the DWIBS sequence may identify multiple metastatic lesions across the body and differentiate malignancy from benignity in only one examination. Although DWIBS alone may be somewhat sensitive, characterization of lesions is not always achievable due to impeded diffusion in both malignant and nonmalignant processes. To avoid false-positive and false-negative outcomes, correlation with morphologic imaging data is required. Another factor that improves diagnostic accuracy in the present study is the use of a surface coil rather than a main magnet coil, which considerably improves the pulmonary spatial resolution, as indicated by Paruthikunnan et al (28).
Primary malignancies frequently metastasize to LNs, and LN involvements have an impact on patient management (29). WB-DWI offers a functional imaging component that may enhance LN characterization by providing information on tissue characteristics over a wide field at appropriate acquisition times, making it a practical staging and screening method (30). WB-DWI was suggested as an alternative to conventional 18FDG PET/CT for lymphoma staging (31). DWI reveals microstructural and cellular changes in malignant vs. normal LNs. Goda et al (24) determined that the sensitivity, specificity and accuracy for LN detection were 77, 85 and 83%, respectively. Changing the size parameters to bigger or smaller cut-offs may affect sensitivity and specificity, as a low cut-off value would increase sensitivity but reduce specificity (24). This may explain why the sensitivity of WB-DWI-MRI varies from 60 to 90% in different trials (32,33). Schmidt et al (17) reported that WB-MRI detected 92% of LNs with diameters >12 mm. However, the detection accuracy decreased to 67% for LNs sized 6-12 mm. The findings of Sigovan et al (34)indicated that DWI had high sensitivity, specificity and accuracy (91, 83 and 85%, respectively) in distinguishing benign from malignant enlarged mediastinal LNs. The present study found that WB-MRI has 98.45% accuracy in diagnosing LN metastases. Porta-hepatis LNs had the maximum precision. The lowest precision was recorded in mediastinal LNs, where image quality may be compromised by pulsation artifacts (24). Compared with WB-MRI, PET/CT appears to have an increased sensitivity for neoplastic axillary and mediastinal LNs (18).
The most prevalent malignant bone lesion is bone metastasis. Skeletal involvement occurs in 30-70% of all patients with cancer (35). Currently, 99mTc-phosphonate-based scintigraphy is a well-established approach for screening for skeletal metastases in the body. However, in the absence of an osteoblastic response, lesions may be undetectable in the early stage of the disease. In addition, misperception of tracer uptake in healing fractures or degenerative illness may result in false-positive results. The diagnostic performance of MRI for skeletal metastases has been compared to bone scintigraphy in several studies with greater specificity and sensitivity in the early detection of skeletal metastases (36). Recent meta-analyses have indicated that WB-MRI has higher diagnostic accuracy than bone scan and CT in identifying primary and metastatic lesions in patients with prostate cancer (37,38). The sensitivity and specificity of WB-MRI in detecting bone metastases were reported to be 95%, whereas the values obtained for bone scan were only 78 and 85%, and for CT, only 77 and 83%, respectively (35,37,38). Goda et al (24) demonstrated that WB-DWI detected bone lesions with a sensitivity, specificity and accuracy of 88, 94 and 92%, respectively. According to a meta-analysis conducted by Yang et al (36), the sensitivity rates for PET/CT, CT, MRI and bone scintigraphy were 89.7, 72.9, 90.6 and 86.0%, respectively, while the specificity rates were 96.8, 94.8, 95.4 and 81.4%, respectively. This increased precision may even help in determining a more suitable prognosis for each patient. The acquisition of the whole spine sequence enhances the image accuracy, since pathological fractures may be obscured on the coronal plane. The sagittal plane is strongly suggested in oncology because spine curvatures may be responsible for partial volume effects that conceal certain coronal plane lesions. In the current study, the accuracy for bone metastasis was 100%; this high result is explained by the efficacy of DWBIS and the provision of images in three orthogonal planes.
Each imaging modality has limitations that may lead to bias in the efficacy of WB-MRI. The long scanning duration in the present study is a limitation, particularly for uncooperative and elderly patients. The only criterion for LN evaluation is the size; as the LN is a highly cellular tissue, it exhibited high signal intensity on the DWIBS sequence, regardless of the size. Further limitations of the present study are the relatively small sample size and the exclusion of pulmonary lesions >10 mm in size. The current study's main strength is that histopathology data are employed as the gold standard for included patients. To the best of our knowledge, no previous study has compared WB-MRI to histology.
In conclusion, WB-MRI in three orthogonal planes, including the DWIBS sequence, may be utilized efficiently and accurately to examine patients with malignancies for metastasis. Further studies in the area of this issue are required.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contribution
RJR was a major contributor to the conception of the study. FHK and MNH were involved in the literature review and the writing of the manuscript. RQS and AMS contributed to the conception and the design of the study. SFA, SHA and SN analysed and interpreted the data. RQS and SHK were involved in the literature review, the design of the study, the revision of the manuscript and in the processing of the figures. KKM, SMM and SHM approved the final manuscript version to be published. SHT and SSO confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The Arab Board Scientific Committee (Sulaimani branch, Iraq) and the local ethical committee of the University of Sulaimani/College of Medicine (Sulaimani, Iraq) approved this study (no. 2018-02). Written informed consent to participate in the study was obtained from the patients.
Patient consent for publication
All patients provided written informed consent regarding the publication of their data and images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Guan X. Cancer metastases: Challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heindel W, Gübitz R, Vieth V, Weckesser M, Schober O, Schäfers M. The diagnostic imaging of bone metastases. Dtsch Arztebl Int. 2014;111:741–747. doi: 10.3238/arztebl.2014.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imam K, Bluemke DA. MR imaging in the evaluation of hepatic metastases. Magn Reson Imaging Clin N Am. 2000;8:741–756. [PubMed] [Google Scholar]
- 4.Guimarães MD, Noschang J, Teixeira SR, Santos MK, Lederman HM, Tostes V, Kundra V, Oliveira AD, Hochhegger B, Marchiori E. Whole-body MRI in pediatric patients with cancer. Cancer Imaging. 2017;17(6) doi: 10.1186/s40644-017-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlemmer HP, Schäfer J, Pfannenberg C, Radny P, Korchidi S, Müller-Horvat C, Nägele T, Tomaschko K, Fenchel M, Claussen CD. Fast whole-body assessment of metastatic disease using a novel magnetic resonance imaging system: Initial experiences. Invest Radiol. 2005;40:64–71. doi: 10.1097/01.rli.0000149250.37033.7c. [DOI] [PubMed] [Google Scholar]
- 6.Godinho MV, Lopes FPPL, Costa FM. Whole-body magnetic resonance imaging for the assessment of metastatic breast cancer. Cancer Manag Res. 2018;10:6743–6756. doi: 10.2147/CMAR.S167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauenstein TC, Semelka RC. Emerging techniques: Whole-body screening and staging with MRI. J Magn Reson Imaging. 2006;24:489–498. doi: 10.1002/jmri.20666. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt GP, Haug AR, Schoenberg SO, Reiser MF. Whole-body MRI and PET-CT in the management of cancer patients. Eur Radiol. 2006;16:1216–1225. doi: 10.1007/s00330-006-0183-8. [DOI] [PubMed] [Google Scholar]
- 9.Bisschop C, de Heer EC, Brouwers AH, Hospers GAP, Jalving M. Rational use of 18F-FDG PET/CT in patients with advanced cutaneous melanoma: A systematic review. Crit Rev Oncol Hematol. 2020;153(103044) doi: 10.1016/j.critrevonc.2020.103044. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Li Q, Nie W, Liu S. Diagnostic value of whole-body diffusion-weighted magnetic resonance imaging for detection of primary and metastatic malignancies: A meta-analysis. Eur J Radiol. 2014;83:338–344. doi: 10.1016/j.ejrad.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Nievelstein RA, Littooij AS. Whole-Body MRI in Pediatric Oncology. Img Ped Oncol. 2019:107–135. [Google Scholar]
- 12.Pasoglou V, Michoux N, Tombal B, Lecouvet F. Optimising TNM staging of patients with prostate cancer using WB-MRI. J Belg Soc Radiol. 2016;100(101) doi: 10.5334/jbr-btr.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): Features and potential applications in oncology. Eur Radiol. 2008;18:1937–1952. doi: 10.1007/s00330-008-0968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puls R, Kühn JP, Ewert R, Hosten N. Whole-body magnetic resonance imaging for staging of lung cancer. Front Radiat Ther Oncol. 2010;42:46–54. doi: 10.1159/000262459. [DOI] [PubMed] [Google Scholar]
- 15.Akay S, Kocaoglu M, Emer O, Battal B, Arslan N. Diagnostic accuracy of whole-body diffusion-weighted magnetic resonance imaging with 3.0 T in detection of primary and metastatic neoplasms. J Med Imaging Radiat Oncol. 2013;57:274–282. doi: 10.1111/1754-9485.12026. [DOI] [PubMed] [Google Scholar]
- 16.Kachewar SG. Using DWIBS MRI technique as an alternative to bone scan or PET scan for whole-body imaging in oncology patients. Acta Radiol. 2011;52(788) doi: 10.1258/ar.2011.110144. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt GP, Reiser MF, Baur-Melnyk A. Whole-body MRI for the staging and follow-up of patients with metastasis. Eur J Radiol. 2009;70:393–400. doi: 10.1016/j.ejrad.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt GP, Baur-Melnyk A, Haug A, Heinemann V, Bauerfeind I, Reiser MF, Schoenberg SO. Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PET-CT. Eur J Radiol. 2008;65:47–58. doi: 10.1016/j.ejrad.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Sohaib SA, Koh DM, Barbachano Y, Parikh J, Husband JE, Dearnaley DP, Horwich A, Huddart R. Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin Radiol. 2009;64:362–367. doi: 10.1016/j.crad.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Ciliberto M, Maggi F, Treglia G, Padovano F, Calandriello L, Giordano A, Bonomo L. Comparison between whole-body MRI and Fluorine-18-Fluorodeoxyglucose PET or PET/CT in oncology: A systematic review. Radiol Oncol. 2013;47:206–218. doi: 10.2478/raon-2013-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barchetti F, Stagnitti A, Megna V, Al Ansari N, Marini A, Musio D, Monti ML, Barchetti G, Tombolini V, Catalano C, Panebianco V. Unenhanced whole-body MRI versus PET-CT for the detection of prostate cancer metastases after primary treatment. Eur Rev Med Pharmacol Sci. 2016;20:3770–3776. [PubMed] [Google Scholar]
- 22.Li B, Li Q, Chen C, Guan Y, Liu S. A systematic review and meta-analysis of the accuracy of diffusion-weighted MRI in the detection of malignant pulmonary nodules and masses. Acad Radiol. 2014;21:21–29. doi: 10.1016/j.acra.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Regier M, Schwarz D, Henes FO, Groth M, Kooijman H, Begemann PG, Adam G. Diffusion-weighted MR-imaging for the detection of pulmonary nodules at 1.5 Tesla: Intraindividual comparison with multidetector computed tomography. J Med Imaging Radiat Oncol. 2011;55:266–274. doi: 10.1111/j.1754-9485.2011.02263.x. [DOI] [PubMed] [Google Scholar]
- 24.Goda HH, Abd Elkareem HA, Ahmed EA, Megally HI, Khalaf MI, Taha AM, Mohamed HEG. Whole body diffusion-weighted MRI in detection of metastasis and lymphoma: A prospective longitudinal clinical study. Egypt J Radiol Nucl Med. 2020;51:1–2. [Google Scholar]
- 25.Eissawy MG, Saadawy AMI, Farag K, Akl T, Kamr WH. Accuracy and diagnostic value of diffusion-weighted whole body imaging with background body signal suppression (DWIBS) in metastatic breast cancer. Egypt J Radiol Nucl Med. 2021;52(74) [Google Scholar]
- 26.Iwamura H, Kaiho Y, Ito J, Anan G, Satani N, Matsuura T, Tamura R, Murakami K, Koyama K, Sato M. Evaluation of tumor viability for primary and bone metastases in metastatic castration-resistant prostate cancer using whole-body magnetic resonance imaging. Case Rep Urol. 2018;2018(4074378) doi: 10.1155/2018/4074378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usuda K, Iwai S, Yamagata A, Iijima Y, Motono N, Matoba M, Doai M, Yamada S, Ueda Y, Hirata K, et al. Diffusion-weighted whole-body imaging with background suppression (DWIBS) is effective and economical for detection of metastasis or recurrence of lung cancer. Thorac Cancer. 2021;12:676–684. doi: 10.1111/1759-7714.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paruthikunnan SM, Kadavigere R, Karegowda LH. Accuracy of whole-body DWI for metastases screening in a diverse group of malignancies: Comparison with conventional cross-sectional imaging and nuclear scintigraphy. AJR Am J Roentgenol. 2017;209:477–490. doi: 10.2214/AJR.17.17829. [DOI] [PubMed] [Google Scholar]
- 29.Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J Nucl Med. 2004;45:1509–1518. [PubMed] [Google Scholar]
- 30.Tunariu N, Blackledge M, Messiou C, Petralia G, Padhani A, Curcean S, Curcean A, Koh DM. What's new for clinical whole-body MRI (WB-MRI) in the 21st century. Brit J Radiol. 2020;93(20200562) doi: 10.1259/bjr.20200562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharuzhyk S, Zhavrid E, Dziuban A, Sukolinskaja E, Kalenik O. Comparison of whole-body MRI with diffusion-weighted imaging and PET/CT in lymphoma staging. Eur Radiol. 2020;30:3915–3923. doi: 10.1007/s00330-020-06732-w. [DOI] [PubMed] [Google Scholar]
- 32.Balbo-Mussetto A, Cirillo S, Bruna R, Gueli A, Saviolo C, Petracchini M, Fornari A, Lario CV, Gottardi D, De Crescenzo A, Tarella C. Whole-body MRI with diffusion-weighted imaging: A valuable alternative to contrast-enhanced CT for initial staging of aggressive lymphoma. Clin Radiol. 2016;71:271–279. doi: 10.1016/j.crad.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Albano D, Patti C, La Grutta L, Agnello F, Grassedonio E, Mulè A, Cannizzaro G, Ficola U, Lagalla R, Midiri M, Galia M. Comparison between whole-body MRI with diffusion-weighted imaging and PET/CT in staging newly diagnosed FDG-avid lymphomas. Eur J Radiol. 2016;85:313–318. doi: 10.1016/j.ejrad.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Sigovan M, Akl P, Mesmann C, Tronc F, Si-Mohamed S, Douek P, Boussel L. Benign and malignant enlarged chest nodes staging by diffusion-weighted MRI: An alternative to mediastinoscopy? Brit J Radiol. 2018;91(20160919) doi: 10.1259/bjr.20160919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: A meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 36.Engelhard K, Hollenbach HP, Wohlfart K, von Imhoff E, Fellner FA. Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol. 2004;14:99–105. doi: 10.1007/s00330-003-1968-7. [DOI] [PubMed] [Google Scholar]
- 37.Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skelet Radiol. 2014;43:1503–1513. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 38.Liu LP, Cui LB, Zhang XX, Cao J, Chang N, Tang X, Qi S, Zhang XL, Yin H, Zhang J. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bone malignancy: Evidence from a meta-analysis. Medicine (Baltimore) 2015;94(e1998) doi: 10.1097/MD.0000000000001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.