This cohort study attempts to examine the longitudinal association of frailty phenotype with the development of Parkinson disease and to explore the modification role of genetic risk of Parkinson disease in such association.
Key Points
Question
Is physical frailty independently associated with a higher risk of incident Parkinson disease (PD)?
Findings
In this cohort study of 314 998 participants with over 12 years of follow-up, the study team observed that prefrailty and frailty were associated with a 26% and 87% increased risk of PD, respectively. The genetic risk of PD modified the association between frailty and PD.
Meaning
These findings indicate that physical frailty is a potential risk factor for PD and the assessment and management of frailty might have clinical significance in the at-risk population.
Abstract
Importance
Cross-sectional evidence implicates high prevalent frailty in patients with Parkinson disease (PD), whereas the longitudinal association remains unknown.
Objectives
To examine the longitudinal association of the frailty phenotype with the development of PD and to explore the modification role of genetic risk of PD in such an association.
Design, Setting, and Participants
This prospective cohort study launched in 2006 to 2010 with a follow-up of 12 years. Data were analyzed from March 2022 to December 2022. The UK Biobank recruited over 500 000 middle-aged and older adults from 22 assessment centers across the United Kingdom. Participants who were younger than 40 years (n = 101), diagnosed with dementia or PD at baseline, and developed dementia, PD, or died within 2 years from baseline were excluded (n = 4050). Participants who had no genetic data or mismatch between genetic sex and reported gender (n = 15 350), were not of self-reported British White descent (n = 27 850), and had no data for frailty assessment (n = 100 450) or any covariates were also excluded (n = 39 706). The final analysis included 314 998 participants.
Exposures
The physical frailty was assessed by the Fried criteria’s frailty phenotype through 5 domains, ie, weight loss, exhaustion, low physical activity, slow walking speed, and low grip strength. The polygenic risk score (PRS) for PD comprised 44 single-nucleotide variants.
Main Outcomes and Measures
New-onset PD was identified through the hospital admission electronic health records and death register.
Results
Among 314 998 participants (mean age, 56.1 years; 49.1% male), 1916 new-onset PD cases were documented. Compared with nonfrailty, the hazard ratio (HR) of incident PD in prefrailty and frailty was 1.26 (95% CI, 1.15-1.39) and 1.87 (95% CI, 1.53-2.28), respectively, and the absolute rate difference per 100 000 person-years was 1.6 (95% CI, 1.0-2.3) for prefrailty and 5.1 (95% CI, 2.9-7.3) for frailty. Exhaustion (HR, 1.41; 95% CI, 1.22-1.62), slow gait speed (HR, 1.32; 95% CI, 1.13-1.54), low grip strength (HR, 1.27; 95% CI, 1.13-1.43), and low physical activity (HR, 1.12; 95% CI, 1.00-1.25) were associated with incident PD. A significant interaction between frailty and PRS on PD was found and the highest hazard was observed in participants with frailty and high genetic risk.
Conclusions and Relevance
Physical prefrailty and frailty were associated with incident PD independent of sociodemographic factors, lifestyles, multiple morbidities, and genetic background. These findings may have implications for the assessment and management of frailty for PD prevention.
Introduction
Parkinson disease (PD) is the second most prevalent neurodegenerative disease with manifestations of resting tremor, bradykinesia, rigidity, and postural gait disturbance and has the fastest increase in prevalence, death, and disability-adjusted life-years among neurological disorders, according to The Global Burden of Diseases, Injuries, and Risk Factors Study.1 The prevalence of PD in 2016 was 2.4 times higher than the prevalence in 1990.2 The pathophysiology of PD is thought to be neuronal death of dopaminergic neurons and accumulation of Lewy body in neuronal cytoplasm, and the course of PD is considered as irreversible, which emphasizes the importance of prevention. However, few risk factors of PD have been recognized so far.
Frailty is increasingly prevalent and has caught more attention with the aging global population. Frailty is characterized by the decline of physical function in multiple systems and decreased reserve and resistance to stressors.3 Frailty and PD share common features like age-related fatigue, low gait speed, weak grip strength, and similar pathological mechanisms.4,5,6,7,8,9 Previous cross-sectional research investigated that frailty is common in patients with PD10,11,12,13,14,15,16,17 and a study found that the frailty index is associated with both motor and nonmotor features of PD.18 Patients with PD with frailty were more likely associated with cognitive impairment, dementia, orthostatic hypotension, fatigue, hallucinations, and mortality.19,20 However, longitudinal evidence is limited on the association between frailty and incident PD.
The occurrence and development of PD are influenced by genetic and environmental factors, whereas the modification role of genetic predisposition in the association between frailty and PD risk remains largely unknown.8,21 To fill the knowledge gaps mentioned above, we aimed to examine the longitudinal association of frailty with the development of PD within a large prospective UK cohort. We also explored whether the genetic risk of PD modified the association between frailty and PD.
Methods
Study Population
UK Biobank is a large, prospective cohort study including over 500 000 participants aged 40 to 69 years from 22 assessment centers across the UK who were recruited between 2006 and 2010 with a response rate of 5.47% in recruitment.22,23 The study collected rich information, including physical measurement data, economic status, and social activities regarding participants at baseline and during the follow-up, and detailed information can be found online.24 The UK Biobank study was approved by the North West Multi-center research ethics committee (REC reference 11/NW/0382) and written informed consent was provided before participation. We excluded participants who withdrew from UK Biobank or were younger than 40 years old (n = 101) and those diagnosed with PD or dementia at baseline, as well as those who developed PD, developed dementia, or died within 2 years from the baseline (n = 4050). We further excluded participants who had no genetic data or mismatch between genetic sex and self-reported gender (n = 15 350) and were not of White British descent (reported by the participants; n = 27 850) because the genetic instrument we applied was constructed in White participants.25 Those who had no data for frailty assessment (n = 100 450) and missed any data on covariates (n = 39 706) were also excluded. We included 314 998 participants for the final analyses (eFigure 1 in Supplement 1). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Outcomes
We used the algorithm recommended by UK Biobank to identify participants with PD26 and the detailed definition is described in eTable 1 in Supplement 1. The disease information was obtained by the hospital admission electronic health records and death register through linkage with the Hospital Episode Statistics for England, Scottish Morbidity Records for Scotland, or Patient Episode Database for Wales. At the time of our analyses, the censoring dates for the Hospital Episode Statistics for England, Scottish Morbidity Records for Scotland, and Patient Episode Database for Wales were September 30 2021, July 31, 2021, and February 28, 2018, respectively. We calculated the follow-up time from the baseline to the time of PD diagnosis, death, loss to follow-up, or censorship, whichever occurred first.
Physical Frailty Assessment
We adopted the frailty phenotype to assess physical frailty instead of the frailty index or frailty scale, which is more clinically practical and the most frequently used epidemiological measure.27 The frailty phenotype contains 5 criteria, including weight loss, exhaustion, low physical activities, slow walking speed, and low grip strength, which was first described by Fried et al in the Cardiovascular Health Study.28 We modified definitions of several criteria to adapt the data for use in UK Biobank, as previously reported,29,30 and detailed definitions are provided in eTable 2 in Supplement 1. For consistency with previous literature,29,30 participants who met 3 or more criteria were defined as having frailty, those who met 1 or 2 criteria were classified as having prefrailty, and those who did not encounter any criteria were considered to have nonfrailty.
Polygenic Risk Score
The polygenic risk score (PRS) showed the association between genotype and risk of PD by score points and was composed of 44 single-nucleotide variants (SNVs), which were associated with the incidence of PD in White participants found by Chang et al.25 Information regarding these SNVs is provided in eTable 3 in Supplement 1. Detailed information on genotyping and quality control can be found online.31 We used the weighted calculation method to get the Parkinson disease PRS (PD-PRS) as follows:
PRS = (β1 × SNV1 + β2 × SNV2 + ∑ β44 × SNV44) × (44 / sum of the β coefficients), SNVx
This represents the effect allele of the number × SNV. The higher levels of PRS represent the higher genetic susceptibility to PD (eFigure 2 and eTable 4 in Supplement 1).
Covariates
Information on age, sex, smoking status, and alcohol consumption was collected through a self-completed touch-screen questionnaire.22 The Townsend deprivation index was calculated based on the preceding national census output areas in which the participant’s postcode is located and a higher score reflects more socioeconomic deprivation.32 Smoking status was categorized as never, previous, and current. For alcohol consumption, participants were asked, “about how often do you drink alcohol?” and the answers were “daily or almost daily,” “3 or 4 times a week,” “once or twice a week,” “1 to 3 times a month,” “special occasions only,” and “never.” BMI was constructed from height and weight, which were measured by trained nurses. We further considered the number of long-term morbidities (0, 1, 2, 3, 4, and 5 or more), which comprised 42 types of major chronic diseases through the verbal interview in the assessment centers by a physician and a trained nurse. The long-term morbidities were initially developed for a large cross-sectional study in Scotland and subsequently adapted for the UK Biobank.33 We considered the number of long-term morbidities because the index is associated with frailty and life span34 and the detailed definitions are provided in eTable 5 in Supplement 1. We also included assessment centers (22 categories), PD-PRS, genotyping array, and the first 10 principal components of ancestry as covariates. Physical activity was not treated as a confounder because it is one of the components of frailty.
Statistical Analyses
Complete case analysis was applied in this study. Cox proportional hazard regression models were used to evaluate the hazard ratios (HRs) with 95% CIs for the association between physical frailty and incident PD, using follow-up time as the underlying timescale. We adopted the Schoenfeld residual method to check the proportional hazard assumption and no violation was found. Participants with nonfrailty were treated as the reference group and the HRs (95% CIs) for prefrailty and frailty were calculated. We further estimated the absolute rate difference using multivariable log-linear regression models with robust variance estimates to represent the excess number of PD cases per 100 000 person-years35 and 95% CIs were estimated by using the δ method. We also examined the association between 5 components of frailty and the risk of PD individually and mutually. To examine whether the risk of PD correlated linearly with the increasing number of frailty phenotype components, ie, the accumulative effect of frailty components, the restricted cubic spline model was used to fit the association between the number of frailty components and incident PD.
To examine the joint association between frailty and PD-PRS on the hazard of PD, we treated participants with nonfrailty and low PRS as the reference group to conduct a joint analysis on the association between frailty and genetic risk of PD with 3 × 3 groups. The interaction between frailty and PRS tertiles was tested by the fully adjusted model, including a multiplicative interaction term. We also estimated the association between frailty and PD stratified by the tertiles of PD-PRS.
We performed stratified analyses to examine the association between frailty and incident PD by age, sex, Townsend deprivation index, and the number of long-term morbidities. We further examined the robustness of our results by Fine and Gray models for the competing risk. All statistical analyses were performed by SAS version 9.4 (SAS Institute) and R version 4.2.1 (The R Project). A 2-sided P-value <.05 was considered statistically significant.
Results
The study included 314 998 participants (mean age, 56.1 years; 154 739 male [49.1%]) and 13 544 of them were from Wales (4.3%), 23 959 from Scotland (7.6%), and all other participants from England. Baseline demographics and characteristics are shown in Table 1 by frailty phenotype. Among all participants, 10 877 met the criteria for frailty (3.5%), 135 885 for prefrailty (43.1%), and 168 236 for nonfrailty (53.4%). People with prefrailty and frailty were more likely to be older and female, more deprived, and current smokers, and had higher BMI and more long-term morbidities than those with nonfrailty.
Table 1. Baseline Characteristics by Frailty Category, UK Biobank 2006 to 2010.
| Baseline characteristics | Frailty phenotype, No. (%) | ||
|---|---|---|---|
| Nonfrailty | Prefrailty | Frailty | |
| No. of participants | 168 236 | 135 885 | 10 877 |
| Age, mean (SD), y | 55.9 (8.1) | 56.3 (8.1) | 57.9 (7.6) |
| Sex | |||
| Male | 85 164 (50.6) | 65 136 (47.9) | 4439 (40.8) |
| Female | 83 072 (49.4) | 70 749 (52.1) | 6438 (59.2) |
| Townsend deprivation index, median (IQR) | −2.4 (−3.8 to −0.2) | −2.2 (−3.7 to 0.3) | −1.0 (−3.0 to 2.1) |
| Smoking status | |||
| Never | 93 692 (55.7) | 71 783 (52.8) | 4874 (44.8) |
| Former | 59 528 (35.4) | 49 277 (36.3) | 4163 (38.3) |
| Current | 15 016 (8.9) | 14 825 (10.9) | 1840 (16.9) |
| Alcohol consumption | |||
| Daily or most daily | 41 299 (24.6) | 28 525 (21.0) | 1559 (14.3) |
| 3 or 4 Times a week | 46 391 (27.6) | 31 536 (23.2) | 1531 (14.1) |
| Once or twice a week | 43 497 (25.9) | 35 772 (26.3) | 2494 (22.9) |
| 1-3 Times a month | 16 517 (9.8) | 16 139 (11.9) | 1475 (13.6) |
| Never or special occasions only | 20 532 (12.2) | 23 913 (17.6) | 3818 (35.1) |
| BMI,a mean (SD) | 26.4 (4.0) | 28.0 (4.9) | 31.2 (6.6) |
| No. of long-term morbidities | |||
| None | 69 141 (41.1) | 41 464 (30.5) | 1085 (10.0) |
| 1 | 57 581 (34.2) | 45 216 (33.3) | 2234 (20.5) |
| 2 | 27 824 (16.5) | 28 209 (20.8) | 2694 (24.8) |
| 3 | 9878 (5.9) | 13 372 (9.8) | 2278 (20.9) |
| 4 | 2826 (1.7) | 5061 (3.7) | 1373 (12.6) |
| ≥ 5 | 986 (0.6) | 2563 (1.9) | 1213 (11.2) |
Calculated as weight in kilograms divided by height in meters squared.
During a mean follow-up of 12.3 years (3 860 420 person-years), 1916 PD cases were documented. Compared with nonfrail participants, those with prefrailty and frailty showed a significantly increased risk for the incidence of PD, which was 26% higher in prefrailty (HR, 1.26; 95% CI, 1.15-1.39) and 87% higher in frailty (HR, 1.87; 95% CI, 1.53-2.28) after adjustment for covariates, and the absolute rate differences per 100 000 person-years were 1.6 (95% CI, 1.0-2.3) for prefrailty and 5.1 (95% CI, 2.9-7.3) for frailty (Table 2).
Table 2. Association Between Physical Frailty and Incident Parkinson Disease, UK Biobank 2006 to 2010.
| Characteristic | Nonfrailty | Prefrailty | Frailty |
|---|---|---|---|
| Cases/person-years | 869 of 2 074 604 | 920 of 1 658 307 | 127 of 127 509 |
| Incident cases per 100 000 person-years | 41.9 | 55.5 | 99.6 |
| Model 1,a HR (95% CI) | 1 [Reference] | 1.32 (1.20-1.44) | 2.25 (1.86-2.71) |
| Model 2,b HR (95% CI) | 1 [Reference] | 1.26 (1.15-1.39) | 1.87 (1.53-2.28) |
| Absolute rate difference per 100 000 person-years (95% CI) | 0 [Reference] | 1.6 (1.0-2.3) | 5.1 (2.9-7.3) |
Model 1, adjusted for age, sex, Townsend deprivation index, and assessment centers.
Model 2, included model 1 plus alcohol consumption, smoking status, body mass index, the number of long-term morbidities, Parkinson disease polygenic risk score, genotyping array, and the first 10 principal components of ancestry.
The study team investigated 5 components of frailty and their association with incident PD independently and mutually (Table 3). In the fully adjusted model, the study team observed that exhaustion (HR, 1.41; 95% CI, 1.22-1.62), slow gait speed (HR, 1.32; 95% CI, 1.13-1.54), low grip strength (HR, 1.27; 95% CI, 1.13-1.43), and low physical activity (HR, 1.12; 95% CI, 1.00-1.25) were significantly associated with incident PD, while the null association was observed for weight loss. In addition, the study team found a positive linear association between the accumulative number of frailty components and the incidence of PD (P for nonlinearity = 0.79) (eFigure 3 in Supplement 1), with an HR increasing by 21% (95% CI, 1.15-1.27) each additional component of the frailty phenotype.
Table 3. Individual Components of Frailty and Their Association With Incident Parkinson Disease, UK Biobank 2006 to 2010.
| Characteristic | HR (95% CI) | ||
|---|---|---|---|
| Frailty component | Model 1a | Model 2b | Model 3c |
| Weight loss | 1.04 (0.92-1.18) | 0.99 (0.87-1.12) | 1.00 (0.88-1.13) |
| Exhaustion | 1.70 (1.49-1.94) | 1.50 (1.30-1.72) | 1.41 (1.22-1.62) |
| Low physical activity | 1.23 (1.11-1.37) | 1.19 (1.06-1.32) | 1.12 (1.00-1.25) |
| Slow gait speed | 1.71 (1.48-1.96) | 1.48 (1.28-1.73) | 1.32 (1.13-1.54) |
| Low grip strength | 1.43 (1.27-1.60) | 1.32 (1.18-1.49) | 1.27 (1.13-1.43) |
Model 1, adjusted for age, sex, Townsend deprivation index, and assessment centers.
Model 2, included model 1 plus alcohol consumption, smoking status, body mass index, the number of long-term morbidities, Parkinson disease polygenic risk score, genotyping array, and the first 10 principal components of ancestry.
Model 3, plus model 2 and 5 frailty components (mutual adjustment).
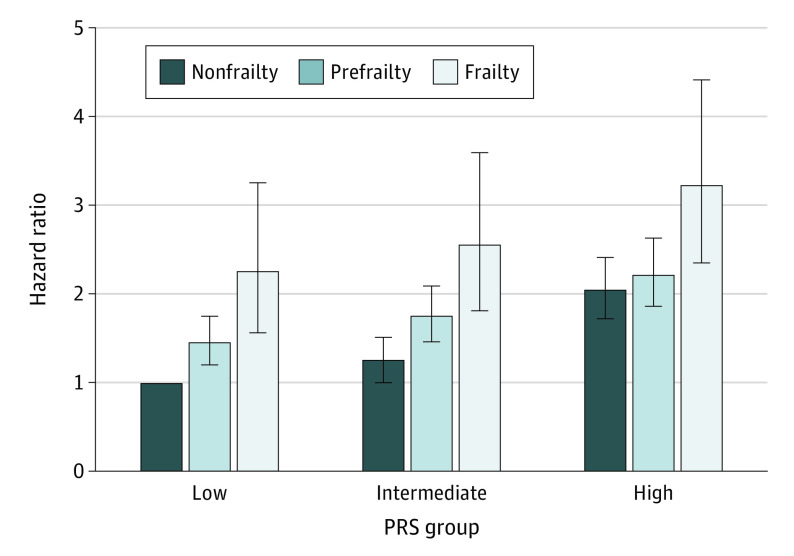
The study team observed a positive association of PD-PRS tertiles with the risk of PD (eTable 4 in Supplement 1) and further assessed the joint association of frailty phenotype and PRS on incident PD (Figure; eTable 6 in Supplement 1). Participants with frailty and the highest tertile of PRS had the highest risk of PD (HR, 3.22; 95% CI, 2.35-4.41) in contrast with those with nonfrailty and the lowest tertile of PRS. Using frailty as a reference, participants with nonfrailty had decreased risk of incident PD by 43% (HR, 0.57; 95% CI, 0.39-0.84), 55% (HR, 0.45; 95% CI, 0.32-0.65), and 42% (HR, 0.58; 95% CI, 0.42-0.79) in low-, intermediate-, and high-PRS groups, respectively (Table 4).
Figure. Risk of Incident Parkinson Disease According to Genetic Risk and Frailty Phenotype.
Model adjusted for age, sex, Townsend deprivation index, assessment centers, alcohol consumption, smoking status, body mass index, the number of long-term morbidities, genotyping array, and the first 10 principal components of ancestry. PRS indicates polygenic risk score.
Table 4. Risk of Incident Parkinson Disease According to Frailty Status Within Polygenic Risk Category, UK Biobank 2006 to 2010a.
| Frailty status | PRS (HR [95% CI]) | P value for interactiona | ||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Frailty | 1 [Reference] | 1 [Reference] | 1 [Reference] | .008 |
| Prefrailty | 0.78 (0.54-1.12) | 0.65 (0.46-0.91) | 0.64 (0.47-0.87) | |
| Nonfrailty | 0.57 (0.39-0.84) | 0.45 (0.32-0.65) | 0.58 (0.42-0.79) | |
Model adjusted for age, sex, Townsend deprivation index, assessment centers, alcohol consumption, smoking status, body mass index, the number of long-term morbidities, genotyping array, and the first 10 principal components of ancestry.
P value for the interaction between frailty status and polygenic risk score categories.
In stratified analyses, the significant association between frailty and PD was more pronounced in participants with more long-term conditions (P for interaction = .02) (eTable 7 in Supplement 1). The study results remained robust after the adjustment by the Fine and Gray model for the competing risk (eTable 8 in Supplement 1).
Discussion
In this large prospective cohort study, we found that physical frailty was associated with subsequent PD risk independent of sociodemographic factors, lifestyles, multiple morbidities, and genetic background. Compared with participants without frailty, those with prefrailty and frailty had a 26% and 87% higher risk of PD, respectively. In addition, we observed an interaction between frailty and genetic risk on incident PD and the highest risk of incident PD was observed in those with frailty and high PRS.
To our knowledge, this is the first large prospective study investigating the longitudinal association between frailty and the incidence of PD and exploring the genetic interaction in such association. Previous studies found that frailty was common in adults with PD. In a claims-based cross-sectional study of 62 786 beneficiaries with PD, 55.3% were diagnosed with frailty.17 The findings support other studies with a small sample size of patients with PD showing frailty was highly prevalent in PD.11,13,15,16 A case-control study found significant increments in the mean number of frailty phenotypes and cumulative frailty index in patients with PD compared with controls.14 Another cross-sectional research study in the UK investigated that the prevalence of frailty in a PD cohort (HR, 35.6%; 95% CI,: 27.0%-45.2%) was significantly higher than the controls cohort (HR, 5.2%; 95% CI, 3.2%-8.1%; P < .001).12 A cross-sectional study of Hellenic Longitudinal Investigation of Aging and Diet conducted by Ntanasi et al,10 including 1765 participants, found that the prevalence of PD in those who were frail defined by the Fried frailty phenotype or Fried frailty index was 4 times (odds ratio, 4.09; 95% CI, 1.54-10.89) and 12 times (odds ratio, 12.16; 95% CI, 5.46-27.09) higher than nonfrail participants. However, little longitudinal evidence has been reported on physical frailty and incident PD. In our research, prefrailty and frailty were significantly associated with an increased risk of incident PD. It was robust after adjustment by age and sex, sociodemographic factors, behavior factors, long-term morbidities, and genetic background.
Our study also found that components of frailty, including exhaustion, low physical activity, slow gait speed, and low grip strength may correlate with PD. A study investigated that in the frailty population, dopamine-related genotype has a stronger association with slow gait speed, which is not driven by the extreme gait slowing among frailty.36 A probable explanation is that frailty may trigger dopamine-related genotypes to develop PD. One hypothesis associated with fatigue with dopamine imbalance has been used as evidence to explain how fatigue can occur.37 Dopaminergic neuron death is a characteristic pathology of PD and these studies may support our results about slow gait speed and exhaustion. Indeed, slow gait speed and low grip strength are associated with the motor symptom of PD on bradykinesia and stiffness, which may indicate that motor symptoms develop before PD manifests.38 Interestingly, the International Parkinson and Movement Disorder Society defined prodromal PD to describe the phenomenon that symptoms present before clinical diagnosis of PD.39 Frailty phenotype, such as slow gait speed, may also associate with the function of the brain and muscle through the vascular system,40 which often influences the cerebra and muscles when aging and cognitive impairment is associated with cerebral vasculature as well.41 As for exhaustion, the nigrostriatal pathway is a possible way to link frailty and PD, as it is the basic pathological mechanism of PD8 and related to the regulation of exercise fatigue.
We applied PRS to examine the association between the PRS of PD and frailty phenotype in the incidence of PD in the UK Biobank. We observed that a higher risk of incident PD was associated with worse frail status and higher PD-PRS and a significant interaction between frailty and PRS categories in both joint analyses and stratified analyses, prompting the gene-environment interaction in PD occurrence. In addition, among all PRS tertiles, a significantly decreased risk of incident PD was found in participants without frailty. Our results suggest that improvement of physical frailty would benefit individuals even with a high genetic risk of PD.
Existing studies have found that the brain renin-angiotensin system (RAS) influences the development of PD by regulating aging and neuroinflammation and brain RAS plays an important role in the progression of dopaminergic neuron degeneration.42 In addition, RAS regulates various factors, including inflammation, oxidant state, vascular regulations, mitochondria dysfunctions, and apoptosis, which are relevant to frailty. It could be seen from this that RAS might be an essential factor linking frailty and PD. Besides, it could be through the gut-brain axis and intestinal function,6,43 inflammation,4,8 dysfunction of mitochondria,7,8 and deficiency in dopaminergic system function5,8 to combine frailty and PD. Assessment and screening for physical frailty may have clinical implications for PD prevention. Our findings support that frailty might be a valuable tool in PD screening. In addition, when patients present with prefrailty or frailty, strategies to change patients’ lifestyles should be tailored to prevent or delay the development of PD.
Strengths and Limitations
Our study has several strengths. Given the few longitudinal studies on the association between physical frailty and incident PD, we provide firsthand prospective evidence in this regard. The large sample size and long-term follow-up increase the credibility of our results. Furthermore, the overwhelming information provided by the UK Biobank favors us considering a large array of confounders, especially the genetic predisposition of PD. Our study is subjected to some limitations. First, some risk factors of PD, like exposure to the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine, could not be considered in the statistical model as there are no relevant data in UK Biobank. Second, the UK Biobank was subject to a low response rate (5.47%) and selection bias. However, recent evidence from a prospective cohort study and individual participant meta-analysis indicated, that despite a very low response rate, risk factor associations in the UK Biobank seem to be generalizable.44 Third, we excluded many participants because of the missing data on frailty assessments and covariates which may introduce potential bias. We applied a complete case analysis that can give biased estimates when the data are not missing completely randomly. However, we did not observe any chance of data being missing related to any of the variables involved in our analysis. Fourth, except for grip strength, frailty components and some covariates were self-reported, and reporting bias might exist. However, a study about self-reported frailty components found that characteristics of frailty are similar regardless of whether self-reported or test-based measures.45 Fifth, our study only included White participants and the associations we observed between frailty and PD are warranted replicated in other ethnicities. Additionally, the direct causation inference should be cautious because of the study’s observational nature.
Conclusions
We demonstrated a significant association of prefrailty and frailty with the incidence of PD over 12 years of follow-up. Such association was modified by the genetic risk of PD, where the highest risk of PD was observed in those with frailty and high-genetic risk. Integrating frailty assessment into the primary prevention of PD may favor the identification of high-risk individuals.
eTable 1. Disease Definitions in UK Biobank Study
eTable 2. Frailty Definition and Cut-off Points in UK Biobank Study
eTable 3. Information of 44 SNPs for PD in UK Biobank
eTable 4. Association between PD-PRS and Incident PD
eTable 5. Definition and List of Long-Term Morbidities
eTable 6. Risk of incident PD according to genetic risk and frailty phenotype
eTable 7. Stratified Analysis for the Association between Physical Frailty and Incidence of PD
eTable 8. Association of Frailty and PD Using Fine & Gray Models for Competing Risk
eFigure 1. Flow Chart
eFigure 2. The Curve of Density Distribution and Weighted Polygenic Risk Score
eFigure 3. The Accumulation of Frailty Phenotype Components and Risk of PD
Data sharing statement
References
- 1.Feigin VL, Abajobir AA, Abate KH, et al. ; GBD 2015 Neurological Disorders Collaborator Group . Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877-897. doi: 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Parkinson’s Disease Collaborators . Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939-953. doi: 10.1016/S1474-4422(18)30295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365-1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 4.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi: 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Seiffert P, Derejczyk J, Kawa J, et al. Frailty phenotype and the role of levodopa challenge test in geriatric inpatients with mild parkinsonian signs. Biogerontology. 2017;18(4):641-650. doi: 10.1007/s10522-017-9716-6 [DOI] [PubMed] [Google Scholar]
- 6.Di Sabatino A, Lenti MV, Cammalleri L, Corazza GR, Pilotto A. Frailty and the gut. Dig Liver Dis. 2018;50(6):533-541. doi: 10.1016/j.dld.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Andreux PA, van Diemen MPJ, Heezen MR, et al. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci Rep. 2018;8(1):8548. doi: 10.1038/s41598-018-26944-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284-2303. doi: 10.1016/S0140-6736(21)00218-X [DOI] [PubMed] [Google Scholar]
- 9.Ebina J, Ebihara S, Kano O. Similarities, differences and overlaps between frailty and Parkinson’s disease. Geriatr Gerontol Int. 2022;22(4):259-270. doi: 10.1111/ggi.14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntanasi E, Maraki M, Yannakoulia M, et al. Frailty and prodromal Parkinson’s disease: results from the HELIAD study. J Gerontol A Biol Sci Med Sci. 2021;76(4):622-629. doi: 10.1093/gerona/glaa191 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed NN, Sherman SJ, Vanwyck D. Frailty in Parkinson’s disease and its clinical implications. Parkinsonism Relat Disord. 2008;14(4):334-337. doi: 10.1016/j.parkreldis.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Peball M, Mahlknecht P, Werkmann M, et al. Prevalence and associated factors of sarcopenia and frailty in Parkinson’s disease: a cross-sectional study. Gerontology. 2019;65(3):216-228. doi: 10.1159/000492572 [DOI] [PubMed] [Google Scholar]
- 13.Özer FF, Akin S, Gültekin M, Zararsiz GE, Soylu AE. Frailty in patients with Parkinson’s disease: associations with disability and timed up and go. Noro Psikiyatr Ars. 2019;58(3):206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan AH, Hew YC, Lim SY, et al. Altered body composition, sarcopenia, frailty, and their clinico-biological correlates, in Parkinson’s disease. Parkinsonism Relat Disord. 2018;56:58-64. doi: 10.1016/j.parkreldis.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 15.Roland KP, Jakobi JM, Jones GR, Powell C. Quality of life as a determinant of frailty phenotype in community-dwelling persons with Parkinson’s disease. J Am Geriatr Soc. 2012;60(3):590-592. doi: 10.1111/j.1532-5415.2011.03862.x [DOI] [PubMed] [Google Scholar]
- 16.Lin WC, Huang YC, Leong CP, et al. Associations between cognitive functions and physical frailty in patients with Parkinson’s disease. Front Aging Neurosci. 2019;11:283. doi: 10.3389/fnagi.2019.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham DS, Pham Nguyen TP, Willis AW. Claims-based frailty and outcomes: applying an aging measure to older adults with Parkinson’s disease. Mov Disord. 2021;36(8):1871-1878. doi: 10.1002/mds.28561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belvisi D, Canevelli M, Costanzo M, et al. The role of frailty in Parkinson’s disease: a cross-sectional study. J Neurol. 2022;269(6):3006-3014. doi: 10.1007/s00415-021-10873-3 [DOI] [PubMed] [Google Scholar]
- 19.Smith N, Brennan L, Gaunt DM, Ben-Shlomo Y, Henderson E. Frailty in Parkinson’s disease: a systematic review. J Parkinsons Dis. 2019;9(3):517-524. doi: 10.3233/JPD-191604 [DOI] [PubMed] [Google Scholar]
- 20.McMillan JM, Michalchuk Q, Goodarzi Z. Frailty in Parkinson’s disease: a systematic review and meta-analysis. Clin Park Relat Disord. 2021;4:100095. doi: 10.1016/j.prdoa.2021.100095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs BM, Belete D, Bestwick J, et al. Parkinson’s disease determinants, prediction and gene-environment interactions in the UK Biobank. J Neurol Neurosurg Psychiatry. 2020;91(10):1046-1054. doi: 10.1136/jnnp-2020-323646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen N, Sudlow C, Downey P, et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1(3):123-126. doi: 10.1016/j.hlpt.2012.07.003 [DOI] [Google Scholar]
- 24.UK Biobank . Enabling you vision to improve public health. Accessed February 9, 2023. https://www.ukbiobank.ac.uk/
- 25.Chang D, Nalls MA, Hallgrímsdóttir IB, et al. ; International Parkinson’s Disease Genomics Consortium; 23andMe Research Team . A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet. 2017;49(10):1511-1516. doi: 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biobank U . Algorithmically-defined outcomes (ADOs) Version 2.0. Accessed February 3, 2023. https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_main.pdf
- 27.Hanlon P, Fauré I, Corcoran N, et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020;1(3):e106-e116. doi: 10.1016/S2666-7568(20)30014-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 29.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323-e332. doi: 10.1016/S2468-2667(18)30091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petermann-Rocha F, Lyall DM, Gray SR, et al. Associations between physical frailty and dementia incidence: a prospective study from UK Biobank. Lancet Healthy Longev. 2020;1(2):e58-e68. doi: 10.1016/S2666-7568(20)30007-6 [DOI] [PubMed] [Google Scholar]
- 31.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster HME, Celis-Morales CA, Nicholl BI, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3(12):e576-e585. doi: 10.1016/S2468-2667(18)30200-7 [DOI] [PubMed] [Google Scholar]
- 33.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37-43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 34.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323-e332. doi: 10.1016/S2468-2667(18)30091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 36.Mance S, Rosso A, Bis J, Studenski S, Bohnen N, Rosano C. Catechol-O-Methyltransferase genotype, frailty, and gait speed in a biracial cohort of older adults. J Am Geriatr Soc. 2021;69(2):357-364. doi: 10.1111/jgs.16842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52. doi: 10.3389/fneur.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jagadeesan AJ, Murugesan R, Vimala Devi S, et al. Current trends in etiology, prognosis and therapeutic aspects of Parkinson’s disease: a review. Acta Biomed. 2017;88(3):249-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611. doi: 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 40.Dumurgier J, Elbaz A, Ducimetière P, Tavernier B, Alpérovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iadecola C, Duering M, Hachinski V, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. 2019;73(25):3326-3344. doi: 10.1016/j.jacc.2019.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cosarderelioglu C, Nidadavolu LS, George CJ, et al. Brain renin-angiotensin system at the intersect of physical and cognitive frailty. Front Neurosci. 2020;14:586314. doi: 10.3389/fnins.2020.586314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Travagli RA, Browning KN, Camilleri M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2020;17(11):673-685. doi: 10.1038/s41575-020-0339-z [DOI] [PubMed] [Google Scholar]
- 44.Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theou O, O’Connell MD, King-Kallimanis BL, O’Halloran AM, Rockwood K, Kenny RA. Measuring frailty using self-report and test-based health measures. Age Ageing. 2015;44(3):471-477. doi: 10.1093/ageing/afv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Disease Definitions in UK Biobank Study
eTable 2. Frailty Definition and Cut-off Points in UK Biobank Study
eTable 3. Information of 44 SNPs for PD in UK Biobank
eTable 4. Association between PD-PRS and Incident PD
eTable 5. Definition and List of Long-Term Morbidities
eTable 6. Risk of incident PD according to genetic risk and frailty phenotype
eTable 7. Stratified Analysis for the Association between Physical Frailty and Incidence of PD
eTable 8. Association of Frailty and PD Using Fine & Gray Models for Competing Risk
eFigure 1. Flow Chart
eFigure 2. The Curve of Density Distribution and Weighted Polygenic Risk Score
eFigure 3. The Accumulation of Frailty Phenotype Components and Risk of PD
Data sharing statement