Key Points
Question
What is the association between maternal preconception hepatitis B virus (HBV) infection and congenital heart diseases (CHDs) in offspring?
Findings
In this matched cohort study including 3 690 427 participants, increased CHD risks in offspring were observed among women with preconception previous HBV infection.
Meaning
In this study, maternal preconception previous HBV infection, which might affect the formation of the ovum, was associated with risk of CHDs in offspring.
This cohort study explores the association of maternal preconception hepatitis B virus infection with congenital heart diseases in offspring.
Abstract
Importance
Maternal hepatitis B virus (HBV) infection during early pregnancy has been related to congenital heart diseases (CHDs) in offspring. However, no study to date has evaluated the association of maternal preconception HBV infection with CHDs in offspring.
Objective
To explore the association of maternal preconception HBV infection with CHDs in offspring.
Design, Setting, and Participants
This retrospective cohort study used nearest-neighbor (1:4) propensity score matching of 2013 to 2019 data from the National Free Preconception Checkup Project (NFPCP), a national free health service for childbearing-aged women who plan to conceive throughout mainland China. Women aged 20 to 49 years who got pregnant within 1 year after preconception examination were included, and those with multiple births were excluded. Data were analyzed from September to December 2022.
Exposures
Maternal preconception HBV infection statuses, including uninfected, previous, and new infection.
Main Outcomes and Measures
The main outcome was CHDs, which were prospectively collected from the birth defect registration card of the NFPCP. Logistic regression with robust error variances was used to estimate the association between maternal preconception HBV infection status and CHD risk in offspring, after adjusting for confounding variables.
Results
After matching with a 1:4 ratio, there were 3 690 427 participants included in the final analysis, where 738 945 women were infected with HBV, including 393 332 women with previous infection and 345 613 women with new infection. Approximately 0.03% (800 of 2 951 482) of women uninfected with HBV preconception and women newly infected with HBV carried an infant with CHDs, whereas 0.04% (141 of 393 332) of women with HBV infection prior to pregnancy carried an infant with CHDs. After multivariable adjustment, women with HBV infection prior to pregnancy had a higher risk of CHDs in offspring compared with women who were uninfected (adjusted relative risk ratio [aRR], 1.23; 95% CI, 1.02-1.49). Moreover, compared with couples who were uninfected with HBV prior to pregnancy (680 of 2 610 968 [0.026%]), previously infected women with uninfected men (93 of 252 919 [0.037%]) or previously infected men with uninfected women (43 of 95 735 [0.045%]) had a higher incidence of CHDs in offspring and were significantly associated with a higher risk of CHDs in offspring (previously infected women with uninfected men: aRR, 1.36; 95% CI, 1.09-1.69; previously infected men with uninfected women: aRR, 1.51; 95% CI, 1.09-2.09) with multivariable adjustment, while no significant association was observed between maternal new HBV infection and CHDs in offspring.
Conclusions and Relevance
In this matched retrospective cohort study, maternal preconception previous HBV infection was significantly associated with CHDs in offspring. Moreover, among women with HBV-uninfected husbands, significantly increased risk of CHDs was also observed in previously infected women prior to pregnancy. Consequently, HBV screening and getting HBV vaccination-induced immunity for couples prior to pregnancy are indispensable, and those with previous HBV infection prior to pregnancy should also be taken seriously to decrease the CHDs risk in offspring.
Introduction
Congenital heart disease (CHD) is the most common congenital disease identified in newborns, accounting for roughly one-third of birth defects.1,2,3 Because of significant advances in pediatric cardiac care and therapy, newborns with CHDs are able to live longer and maintain healthier lives.4,5 However, CHDs still have a substantial impact on the health of children and adults; meanwhile, it also brings a heavy burden to society and families.6,7
For years, many CHD-related risk factors have been identified; however, with the complex etiology, most CHDs still cannot be explained, which should be attributed to the interaction of genetic and environmental factors.8,9,10 Since 1941, the first publication of the fatal effects of the rubella virus on the neonatal cardiovascular system, virus infection has been demonstrated to play an important role in CHD development in numerous studies during early pregnancy, including cytomegalovirus, coxsackievirus B3, and some others.11,12,13,14,15,16,17,18 Despite the bulk of these studies showing consistent associations, available information on the association between maternal hepatitis B virus (HBV) infection and CHDs in offspring is limited and contentious with the previous studies during early pregnancy.14,16,17,18 As a significant global public health issue, HBV affects 3.1% to 10.0% of reproductive-aged women in China, a highly endemic area of HBV infection.19,20,21,22,23,24 Although the most recent study has reported that maternal HBV positivity is related to an increased risk of CHDs in offspring in early pregnancy,16 there is little evidence about the association of maternal preconception HBV infection with CHD risk. Given the possible mechanisms for HBV prenatal transmission, such as placental infection and transplacental transmission of HBV25,26 or HBV-DNA existing in infected maternal oocytes or paternal sperms,27,28,29,30,31,32,33 there might be a potential correlation between maternal preconception HBV infection and CHDs in offspring.
In this study, a retrospective cohort study using propensity score matching was conducted to evaluate the association of maternal preconception HBV infection statuses with a wide spectrum of CHDs in offspring.
Methods
Data Source
This retrospective cohort was conducted by the National Free Preconception Checkups Project (NFPCP), a national free health service for childbearing-aged women who plan to conceive throughout mainland China, providing preconception examinations and professional counseling. Details about the project-related design, organization, and implementation can be found elsewhere.34,35,36 This study was approved by the Institutional Review Board at the National Research Institute for Family Planning in Beijing, China, and conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from all participants at the beginning of the preconception examination stage. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
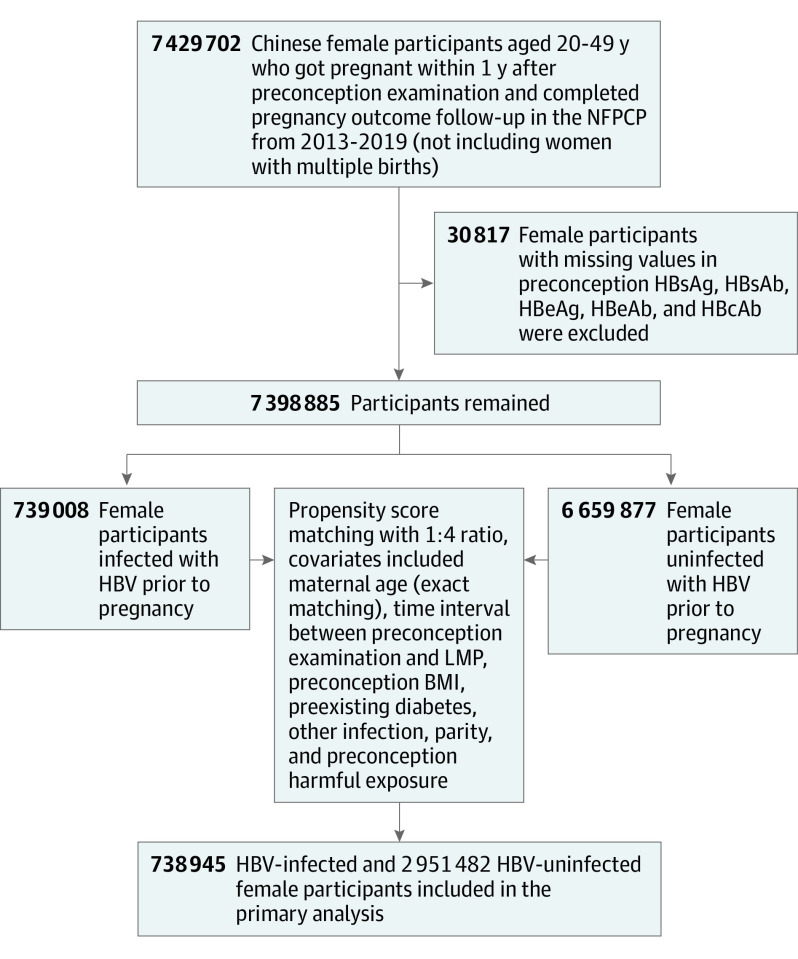
Initially, 7 429 702 women aged 20 to 49 years, who participated from 2013 to 2019 and conceived within 1 year with a singleton before April 2020, were included with the completed pregnancy outcome information. After excluding women who had missing data regarding hepatitis B serological markers, a total of 7 398 885 women were recruited for propensity score matching, with 739 008 women infected with HBV and 6 659 877 women uninfected with HBV.
To minimize the potential bias of maternal preconception HBV infection status on comorbid conditions and confounding, propensity score matching was used, with covariates determined a priori (eTable 2 in Supplement 1). As included covariates with missing values of no more than 5% could be assumed to be missing at random, a new label, unknown, was generated for those variables with missing values. Finally, a 1:4 matched cohort of preconception HBV-infected women, including women with a previous and new infection, and uninfected women was generated with a nearest-neighbor matching algorithm with calipers of 0.02 without replacement; maternal age was matched exactly. Characteristics among the participants before and after matching were also compared (eTable 3 in Supplement 1). The flowchart of the study population selection is shown in the Figure.
Figure. Flowchart of the Study Population Selection.
BMI indicates body mass index; HBcAb, hepatitis B core antibody; HBeAb, hepatitis B envelop antibody; HBeAg, hepatitis B envelop antigen; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; LMP, last menstrual period; NFPCP, National Free Preconception Checkup Project.
Study Exposure and Outcome
Both maternal and paternal blood samples, collected in the preconception examination, were separated and stored at −30 °C and examined using enzyme-linked immunosorbent assay kits for the presence of hepatitis B serological markers: hepatitis B surface antibody (HBsAb), hepatitis B surface antigen (HBsAg), hepatitis B core antibody, hepatitis B envelop antibody, and hepatitis B envelop antigen (HBeAg), which is consistent with the previous study.37 The National Center of Clinical Laboratories for Quality Inspection and Detection was responsible for the external quality assessment and controlled biannually.
To evaluate the association of different maternal preconception HBV infection statuses with CHDs risk in offspring, women were split into 3 groups based on the clinical guideline38 (eTable 1 in Supplement 1): uninfected (none of serum hepatitis B serological markers positive or only serum HBsAb positive), previous infection (both serum HBsAg and HBeAg negative), or new infection (serum HBsAg positive).
The outcome was any CHDs (including atrial septal defect, ventricular septal defect, atrioventricular septal defect, patent ductus arteriosus, Tetralogy of Fallot, pulmonary stenosis, and transposition of the great arteries), based on the birth defect registration card from the NFPCP, which is the same as the national health birth defect registration card.
Covariates
The propensity score matching and multivariable adjustment analyses included a set of covariates, some of which were previously identified as risk factors for CHDs. Demographic covariates include parental age, the time interval between examination and last menstrual period (LMP) (within 6 months or 6 months to 1 year), maternal nationality (Han or other), drinking (yes or no), smoking (yes or no), preconception harmful exposure (including exposure history of radial, high temperature, pesticide, new decoration, paint, other organic solvents, fever, common cold, or passive smoking; yes or no), history of adverse pregnancy outcomes (including spontaneous abortion, induced abortion, or preterm birth; yes or no), parental family history of CHDs (yes or no), parity (nulliparous or multiparous), maternal folic acid intake (yes or no), early pregnancy common cold (yes or no), passive smoking (yes or no), and medication (treatment use or no use).
Preconception clinical covariates included maternal body mass index (underweight [less than 18.5; calculated as weight in kilograms divided by height in meters squared], normal [18.5-23.9], overweight [24.0-27.9], or obese [28.0 or more]), diabetes (yes or no), hypertension (yes or no), other infection (including Neisseria gonorrhoeae, Chlamydia trachomatis, Toxoplasma gondii, cytomegalovirus, Treponema pallidum, or Rubella virus by detecting their specific-serum antibodies; yes or no), paternal serum HBsAg status (positive or negative), and HBV infection prior to pregnancy (yes or no); Men were also classified into 3 groups, which were similar to the classification of maternal preconception HBV infection status: uninfected, previous infection, and new infection). Details about the covariates’ definition are shown in eTable 2 in Supplement 1. All these tests were measured consists with National Guide to Clinical Laboratory Procedures.
Statistical Analysis
Categorical variables were demonstrated as counts and percentages, and continuous variables were expressed as means and SDs or medians and IQRs. The difference between each categorical and continuous variable was assessed by using the χ2 test, Fisher exact test, or Kruskal-Wallis test.
In this matched cohort, the association between maternal preconception HBV infection status and CHDs in offspring was estimated using logistic regression with robust error variances. Separate models, fitted with or without adjustment for the listed covariates (eTable 2 in Supplement 1), were used to estimate the relative risk ratios (RRs) and 95% CIs of maternal preconception HBV infection with CHDs risk in offspring.
Considering the infectivity of HBV within couples and the potential impact of HBV infection on the formation of the gamete, paternal HBV infection might influence the association of maternal HBV infection prior to pregnancy with the risk of CHD in offspring. Therefore, participants were further divided into 9 different groups, and couples uninfected with HBV prior to pregnancy were defined as the reference group. All the covariates adjusted in the model are presented in eTable 2 in Supplement 1. All statistical analyses were performed using R software version 4.1.0 (R Foundation for Statistical Computing) with the packages tidyverse version 1.3.1, MatchIt version 4.3.2, lmtest version 0.9-40, and sandwich version 3.0-2.
Results
Initially, 7 398 885 participants were identified, including 1822 infants with CHDs in the cohort. Of 739 008 women infected with HBV prior to pregnancy, 604 408 (81.8%) got pregnant within 6 months, and more than half were nulliparous (437 463 of 739 008 [59.2%]), which was similar to the uninfected women before matching; however, 37 726 (5.1%) had preexisting diabetes, and 102 054 (13.8%) had harmful preconception exposure, which was higher than uninfected women. After propensity score matching (2 951 482 women uninfected with HBV and 738 945 women infected with HBV), characteristics were all well balanced between groups (eTable 3 in Supplement 1).
Among 2 951 482 women uninfected with HBV (80.0%), the median (IQR) length of time from baseline examination to LMP was 2.24 (0.79-4.89) months, with approximately 0.03% (800 of 2 951 482) carrying an infant with a CHD. In 393 332 women with prepregnant previous HBV infection (10.7%), the median (IQR) length of time from baseline examination to LMP was 2.38 (0.89-4.99) months, with approximately 0.04% (141 of 393 332) carrying an infant with a CHD. In 345 613 women with new HBV infection prior to pregnancy (9.3%), the median (IQR) length of time from baseline examination to LMP was 2.21 (0.83-4.79) months, with approximately 0.03% (90 of 345 613) carrying an infant with a CHD. Details of the baseline characteristics are shown in Table 1.
Table 1. Baseline Characteristics Among Different Hepatitis B Virus (HBV) Infection Status Groups.
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| Uninfected women (n = 2 951 482) | Women with previous infection (n = 393 332) | Women with new infection (n = 345 613) | ||
| Time interval between examination and LMP, median (IQR), mo | 2.24 (0.79-4.89) | 2.38 (0.89-4.99) | 2.21 (0.83-4.79) | NA |
| Paternal age at LMP, y | ||||
| Mean (SD) | 28.60 (4.72) | 29.20 (4.83) | 28.90 (4.82) | <.001b |
| Median (IQR) | 28 (25-31) | 28 (26-32) | 28 (25-31) | |
| Maternal nationality | ||||
| Han | 2 731 028 (92.5) | 360 986 (91.8) | 313 431 (90.7) | <.001 |
| Other | 134 008 (4.6) | 24 690 (6.3) | 24 499 (7.1) | |
| Missing data | 86 446 (2.9) | 7656 (1.9) | 7683 (2.2) | |
| Maternal smoking | ||||
| No | 2 924 510 (99.1) | 388 431 (98.8) | 342 936 (99.2) | <.001 |
| Yes | 8538 (0.3) | 1367 (0.3) | 792 (0.2) | |
| Missing data | 18 434 (0.6) | 3534 (0.9) | 1885 (0.6) | |
| Maternal early pregnancy common cold | ||||
| No | 2 937 873 (99.5) | 391 225 (99.5) | 344 197 (99.6) | <.001 |
| Yes | 13 609 (0.5) | 2107 (0.5) | 1416 (0.4) | |
| Maternal early pregnancy medication | ||||
| No | 2 922 933 (99.0) | 388 408 (98.8) | 342 298 (99.0) | <.001 |
| Yes | 28 549 (1.0) | 4924 (1.2) | 3315 (1.0) | |
| Paternal serum HBsAg status | ||||
| Negative | 2 745 540 (93.0) | 334 768 (85.1) | 295 572 (85.5) | <.001 |
| Positive | 155 620 (5.3) | 48 420 (12.3) | 42 654 (12.4) | |
| Missing data | 50 322 (1.7) | 10 144 (2.6) | 7387 (2.1) | |
| Paternal HBV infection status | ||||
| Uninfected | 2 611 648 (88.5) | 253 012 (64.3) | 260 010 (75.2) | <.001 |
| Previous infection | 95 778 (3.3) | 76 573 (19.5) | 28 576 (8.3) | |
| New infection | 153 975 (5.2) | 47 944 (12.2) | 42 242 (12.2) | |
| Missing data | 90 081 (3.0) | 15 803 (4.0) | 14 785 (4.3) | |
| Maternal family history of CHD | ||||
| No | 2 949 871 (99.9) | 392 998 (99.9) | 345 428 (99.9) | <.001 |
| Yes | 1611 (0.1) | 334 (0.1) | 185 (0.1) | |
| Paternal family history of CHD | ||||
| No | 2 950 245 (99.9) | 393 091 (99.9) | 345 446 (99.9) | <.001 |
| Yes | 1237 (0.04) | 241 (0.1) | 167 (0.1) | |
| Maternal early pregnancy folacin use | ||||
| No | 400 877 (13.6) | 66 942 (17.0) | 62 408 (18.1) | <.001 |
| Yes | 2 491 885 (84.4) | 320 613 (81.5) | 277 583 (80.3) | |
| Missing data | 58 720 (2.0) | 5777 (1.5) | 5622 (1.6) | |
| Newborn with CHDs | ||||
| No | 2 950 682 (99.9) | 393 191 (99.9) | 345 523 (99.9) | .007 |
| Yes | 800 (0.03) | 141 (0.04) | 90 (0.03) | |
Abbreviations: CHDs, congenital heart diseases; HBsAg, hepatitis B surface antigen; LMP, last menstrual period; NA, not available.
The χ2 test was used unless otherwise indicated.
The Kruskal-Wallis test was used.
Compared with uninfected women, women with HBV infection prior to pregnancy had a higher risk of carrying an infant with a CHD, with an adjusted RR (aRR) of 1.23 (95% CI, 1.02-1.49); however, no significant association was found between maternal preconception new HBV infection with CHDs in offspring (aRR, 0.99; 95% CI, 0.79-1.24) (Table 2).
Table 2. Association Between Maternal Preconception Hepatitis B Virus (HBV) Infection and Congenital Heart Diseases in Offspring.
| Maternal preconception HBV infection status | Women, No. (%) | RR (95% CI) | ||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | ||
| Uninfected | 800 (0.027) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Previous HBV infection | 141 (0.036) | 1.32 (1.11-1.58) | 1.31 (1.09-1.57) | 1.23 (1.02-1.49) |
| New HBV infection | 90 (0.026) | 0.96 (0.77-1.19) | 0.99 (0.80-1.24) | 0.99 (0.79-1.24) |
Abbreviation: RR, relative risk ratio.
Model 1 was a crude model.
Model 2 was adjusted for maternal family history of congenital heart diseases and paternal HBV infection status.
Model 3 was adjusted for maternal family history of congenital heart diseases, nationality, early pregnancy passive smoking, early pregnancy common cold, early pregnancy medication, early pregnancy folacin use, paternal HBV infection status, age, and family history of congenital heart diseases.
Considering the probable confounding effect of paternal HBV infection status prior to pregnancy on the association of maternal HBV infection with risk of CHDs in offspring, a further factorial design analysis was performed. As Table 3 demonstrates, compared with couples who were uninfected with HBV prior to pregnancy (680 of 2 610 968 [0.026%]), previously infected women with uninfected men (93 of 252 919 [0.037%]) or previously infected men with uninfected women (43 of 95 735 [0.045%]) had a higher incidence of CHDs in offspring and were significantly associated with a higher risk of CHDs in offspring (previously infected women with uninfected men: aRR, 1.36; 95% CI, 1.09-1.69; previously infected men with uninfected women: aRR, 1.51; 95% CI, 1.09-2.09) with multivariable adjustment. Although the aRRs of previously or newly infected couples, couples consisting of previously infected women and newly infected men, or couples consisting of newly infected women with previously infected men did not reach statistical significance, possibly due to the small sample size, relatively higher incidences of CHDs in offspring were still found compared with couples who were uninfected with HBV prior to pregnancy.
Table 3. Association Between the Combination of Parental Hepatitis B Virus (HBV) Infection Status on Congenital Heart Diseases in Offspringa.
| Measure | Men | ||
|---|---|---|---|
| Uninfected | Previous HBV infection | New HBV infection | |
| Uninfected women | |||
| Participants, No. | 2 610 968 | 95 735 | 153 938 |
| CHD cases, No. (%) | 680 (0.026) | 43 (0.045)b | 37 (0.024) |
| aRR (95% CI) | 1 [Reference] | 1.51 (1.09-2.09) | 0.93 (0.67-1.30) |
| Previous HBV infection | |||
| Participants, No. | 252 919 | 76 548 | 47 930 |
| CHDs cases, No. (%) | 93 (0.037)b | 25 (0.033) | 14 (0.029) |
| aRR (95% CI) | 1.36 (1.09-1.69) | 1.10 (0.72-1.66) | 1.08 (0.64-1.84) |
| New HBV infection | |||
| Participants, No. | 259 942 | 28 568 | 42 228 |
| CHDs cases, No. (%) | 68 (0.026) | 8 (0.028) | 14 (0.033) |
| aRR (95% CI) | 1.00 (0.78-1.29) | 1.04 (0.52-2.09) | 1.28 (0.75-2.18) |
Abbreviations: aRR, adjusted relative risk ratio; CHDs, congenital heart diseases.
Model was adjusted for maternal family history of CHDs, nationality, early pregnancy passive smoking, early pregnancy common cold, early pregnancy medication, early pregnancy folacin use, paternal age, and family history of CHDs.
The χ2 test was used for CHD incidence comparison.
Discussion
This matched retrospective cohort study, conducted in a population-based cohort with more than 7.3 million Chinese reproductive-aged women, demonstrates the association of maternal preconception HBV infection with risk of CHDs in offspring, allowing us to evaluate the associations of different infection statuses with risk of CHDs. In the current study, a potential link between maternal preconception previous HBV infection and a significantly increased risk of CHDs in offspring was observed. Although paternal HBV infection prior to pregnancy would also increase the risk of CHDs in offspring, an interaction effect between maternal and paternal HBV infection was not observed.
The main finding in this study is that increased risks of CHDs in offspring were observed among women with HBV infection, and women with the previous infection had a relatively higher risk. To date, only a few studies have reported an association between maternal HBV infection during early pregnancy and risk of CHDs in offspring, with inconsistent results (Liang et al14: n = 5381; odds ratio [OR], 1.38; 95% CI, 0.33-5.72; Wang et al16: n = 44 048; OR, 2.21; 95% CI, 1.66-2.95; Lai et al17: n = 1522; OR, 1.10; 95% CI, 0.46-26 088; and Guo et al18: n = 367; OR, 1448.83; 95% CI, 6.37-329 730.35), which might due to the different sample sizes and definitions of HBV infection. Detailed information on these studies is shown in eTable 4 in Supplement 1. Most noteworthy, to our knowledge, no study has been conducted to assess the association between maternal preconception HBV infection and risk of CHDs in offspring with a detailed definition of HBV infection status until now. This study focused on maternal preconception HBV infection and demonstrated that maternal preconception HBV infection could significantly increase the risk of CHDs in offspring, which is similar to findings from a 2022 Chinese cohort study16 conducted during early pregnancy including 44 048 pregnant women, which found that maternal HBV infection in early pregnancy was implicated in CHDs in offspring (RR , 2.21; 95% CI, 1.66-2.95). Moreover, our study indicated that women with a preconception previous infection had a relatively higher risk of CHDs in offspring than those with a new HBV infection, which might be because of increased attention and treatment in newly infected women. This study provided important evidence about the association between maternal preconception HBV infection and risk of CHDs in offspring, indicating that women who had a previous HBV infection prior to pregnancy should be paid more attention during the pregnancy period in case of the occurrence of CHDs in offspring.
Over the past decades, there have been advances in the understanding of CHD risk factors, but most investigations focused on maternal factors, while paternal factors were often neglected. To our knowledge, no previous studies have evaluated the interaction effect of both spouses’ HBV infection prior to pregnancy on risk of CHDs in offspring. HBV infection has been reported to affect not only oocytes but also sperm, and there may be an interaction effect of spouses’ preconception HBV infection statuses.27,28,29,30,31,32,33 In this study, increased CHD risks were observed only in women previously infected with HBV with uninfected men or previously infected men with uninfected women prior to pregnancy. Moreover, previously infected men with uninfected women had a higher prevalence of CHDs in offspring than previously infected women with uninfected men. Although other groups did not demonstrate significant results, which might be due to the small sample size or those with newly infected seeking treatment, different levels of increased CHD risk in offspring under various combinations of maternal and paternal preconception HBV infection were observed, which hinted that HBV infection might have a longer harmful effect on the formation of the gamete and the growth of the fertilized egg, which is worth further studies in the future. Therefore, paternal HBV infection status prior to pregnancy should not be overlooked, and couples should be encouraged to maintain an HBV-free status.
In China, although HBV vaccination has been incorporated into the Chinese immunization program since the early 1990s, considering the serum HBsAb titers may decrease gradually with age, both women and men are recommended to regularly monitor their HBV infection status, and HBV-related health education should be strengthened, especially for couples who plan to conceive, to lower the risk of CHDs in offspring. In addition, for participants with a previous infection, even though they were not contagious, they should also be taken seriously to lower the risk of CHDs in offspring.
The etiology of CHDs is recognized to be complicated and multifactorial. Although the precise mechanism of CHDs cannot be well established, the association between maternal preconception HBV infection and CHDs in offspring might be explained by the following mechanisms. First, HBV could infect the ovum and replicate in it. HBV DNA sequences are also able to integrate into its’ chromosomes and be active during the embryo development,27,32,33,39,40 which might result in abnormal embryonic heart development consequently. Second, maternal preconception HBV infection may alter the epigenome profile in newborns,41 which could influence embryonic heart development.
Strengths and Limitations
To our knowledge, this matched retrospective cohort study was the first to evaluate the association between maternal preconception HBV infection and risk of CHDs in offspring. The association can be well evaluated with the propensity score matching and multivariate adjustment. Second, compared with previous studies during early pregnancy, our study provided more detailed classifications to better evaluate the association of the different maternal preconception HBV infection statuses with the risk of CHDs in offspring, providing important new evidence, especially for preconception care. Furthermore, our study demonstrated that paternal HBV infection status might also play an important role in increasing the CHDs risk, which needs to be better explored in the future.
However, our study has some limitations. First, missing diagnosis of CHDs within 28 days after delivery should be a concern. Second, with an unmeasured load of HBV DNA, the association of different HBV DNA loads with the risk of CHDs in offspring could not be assessed. Third, detailed CHD subtypes were not recorded, so we cannot evaluate the association of maternal preconception HBV infection with the risks of different CHD subtypes in offspring. Fourth, as with previous studies, not all potential confounders were adjusted, and the possibly unmeasured or unknown covariates could not be ruled out. Furthermore, because detailed maternal early pregnancy medication history was not well documented, the influence of antiviral medicines in infected women on the association between maternal preconception HBV infection and CHD cannot be assessed. In addition, no detailed records were available relating to complications in pregnancy, especially gestational diabetes, which might lead to a potentially biased estimation of the association of maternal preconception HBV infection with CHDs in offspring. Additionally, NFPCP, the basement cohort, provides preconception counseling, and women or men with a new HBV infection status were recommended to go to the hospital for further treatment; however, these medical records were not collected in this study, which may lead to an underestimation of the association of maternal preconception new HBV infection with the risk of CHDs in offspring.
Conclusions
In conclusion, our study provided new evidence of associations between maternal preconception HBV infection and risk of CHDs in offspring among Chinese childbearing-aged women, indicating the necessity of HBV screening in preparation for pregnancy, and the importance of staying free of HBV infection for women. For those women with previous HBV infection, even if they were not contagious, they should also be taken seriously to decrease the risk of CHDs in offspring.
eTable 1. Classification of Hepatitis B Virus Infection Status
eTable 2. Definition and Classification of Covariates
eTable 3. Baseline Characteristics of Female Participants in the Cohort Before and After Propensity Score Matching
eTable 4. Detailed Information on Previous Studies
Data Sharing Statement
References
- 1.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241-2247. doi: 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 2.Bouma BJ, Mulder BJ. Changing landscape of congenital heart disease. Circ Res. 2017;120(6):908-922. doi: 10.1161/CIRCRESAHA.116.309302 [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455-463. doi: 10.1093/ije/dyz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tennant PWG, Pearce MS, Bythell M, Rankin J. 20-Year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649-656. doi: 10.1016/S0140-6736(09)61922-X [DOI] [PubMed] [Google Scholar]
- 5.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163-172. doi: 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- 6.Gurvitz MZ, Inkelas M, Lee M, Stout K, Escarce J, Chang R-K. Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood. J Am Coll Cardiol. 2007;49(8):875-882. doi: 10.1016/j.jacc.2006.09.051 [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Chen L, Yang T, et al. Congenital heart disease and risk of cardiovascular disease: a meta-analysis of cohort studies. J Am Heart Assoc. 2019;8(10):e012030. doi: 10.1161/JAHA.119.012030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins KJ, Correa A, Feinstein JA, et al. ; American Heart Association Council on Cardiovascular Disease in the Young . Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995-3014. doi: 10.1161/CIRCULATIONAHA.106.183216 [DOI] [PubMed] [Google Scholar]
- 9.Overall JC Jr. Intrauterine virus infections and congenital heart disease. Am Heart J. 1972;84(6):823-833. doi: 10.1016/0002-8703(72)90077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierpont ME, Brueckner M, Chung WK, et al. ; American Heart Association Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Genomic and Precision Medicine . Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation. 2018;138(21):e653-e711. doi: 10.1161/CIR.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma V, Goessling LS, Brar AK, Joshi CS, Mysorekar IU, Eghtesady P. Coxsackievirus B3 infection early in pregnancy induces congenital heart defects through suppression of fetal cardiomyocyte proliferation. J Am Heart Assoc. 2021;10(2):e017995. doi: 10.1161/JAHA.120.017995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Z, Wang L, Yang T, et al. Maternal viral infection and risk of fetal congenital heart diseases: a meta-analysis of observational studies. J Am Heart Assoc. 2019;8(9):e011264. doi: 10.1161/JAHA.118.011264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia YQ, Zhao KN, Zhao AD, et al. Associations of maternal upper respiratory tract infection/influenza during early pregnancy with congenital heart disease in offspring: evidence from a case-control study and meta-analysis. BMC Cardiovasc Disord. 2019;19(1):277. doi: 10.1186/s12872-019-1206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Q, Gong W, Zheng D, Zhong R, Wen Y, Wang X. The influence of maternal exposure history to virus and medicine during pregnancy on congenital heart defects of fetus. Environ Sci Pollut Res Int. 2017;24(6):5628-5632. doi: 10.1007/s11356-016-8198-4 [DOI] [PubMed] [Google Scholar]
- 15.Brown GC. Maternal virus infection and congenital anomalies. Arch Environ Health. 1970;21(3):362-365. doi: 10.1080/00039896.1970.10667251 [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Li Q, Chen L, et al. Maternal viral infection in early pregnancy and risk of congenital heart disease in offspring: a prospective cohort study in Central China. Clin Epidemiol. 2022;14:71-82. doi: 10.2147/CLEP.S338870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai J, Han L, Xu S. Discussion on risk factors of congenital heart disease in newborns. J Prevent Med Inf. 2001;17:370-371. doi: 10.3969/j.issn.1006-4028.2001.05.033 [DOI] [Google Scholar]
- 18.Guo J, Hong X, Yao M. Analysis of risk factors for congenital heart disease in newborns. Chin J Neonatol. 2010;25:76-79. doi: 10.3969/j.issn.1673-6710.2010.02.006 [DOI] [Google Scholar]
- 19.Lao TT, Sahota DS, Law LW, Cheng YK, Leung TY. Age-specific prevalence of hepatitis B virus infection in young pregnant women, Hong Kong Special Administrative Region of China. Bull World Health Organ. 2014;92(11):782-789. doi: 10.2471/BLT.13.133413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Fang W, Fan L, et al. Hepatitis B surface antigen prevalence among 12,393 rural women of childbearing age in Hainan Province, China: a cross-sectional study. Virol J. 2013;10:25. doi: 10.1186/1743-422X-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Li RT, Wang Y, Liu Q, Zhou YH, Hu Y. Seroprevalence of hepatitis B surface antigen among pregnant women in Jiangsu, China, 17 years after introduction of hepatitis B vaccine. Int J Gynaecol Obstet. 2010;109(3):194-197. doi: 10.1016/j.ijgo.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Li H, Ji Y, et al. Seroprevalence of hepatitis B surface antigen and hepatitis B e antigen among childbearing-age women in Mianyang, China. J Infect Dev Ctries. 2015;9(7):770-779. doi: 10.3855/jidc.6938 [DOI] [PubMed] [Google Scholar]
- 23.Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Maternal hepatitis B surface antigen status and incidence of pre-eclampsia. J Viral Hepat. 2013;20(5):343-349. doi: 10.1111/jvh.12037 [DOI] [PubMed] [Google Scholar]
- 24.Sheng QJ, Wang SJ, Wu YY, Dou XG, Ding Y. Hepatitis B virus serosurvey and awareness of mother-to-child transmission among pregnant women in Shenyang, China: an observational study. Medicine (Baltimore). 2018;97(22):e10931. doi: 10.1097/MD.0000000000010931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol. 2007;13(26):3625-3630. doi: 10.3748/wjg.v13.i26.3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol. 2004;10(3):437-438. doi: 10.3748/wjg.v10.i3.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang TH, Zhang QJ, Xie QD, Zeng LP, Zeng XF. Presence and integration of HBV DNA in mouse oocytes. World J Gastroenterol. 2005;11(19):2869-2873. doi: 10.3748/wjg.v11.i19.2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Liu W, Zhang M, Wang M, Wu H, Lu M. Effect of hepatitis B virus infection on sperm quality and outcomes of assisted reproductive techniques in infertile males. Front Med (Lausanne). 2021;8:744350. doi: 10.3389/fmed.2021.744350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han TT, Huang JH, Gu J, Xie QD, Zhong Y, Huang TH. Hepatitis B virus surface protein induces sperm dysfunction through the activation of a Bcl2/Bax signaling cascade triggering AIF/Endo G-mediated apoptosis. Andrology. 2021;9(3):944-955. doi: 10.1111/andr.12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng L, Sun P, Xie X, et al. Hepatitis B virus surface protein induces oxidative stress by increasing peroxides and inhibiting antioxidant defences in human spermatozoa. Reprod Fertil Dev. 2020;32(14):1180-1189. doi: 10.1071/RD20130 [DOI] [PubMed] [Google Scholar]
- 31.Zhou XL, Sun PN, Huang TH, Xie QD, Kang XJ, Liu LM. Effects of hepatitis B virus S protein on human sperm function. Hum Reprod. 2009;24(7):1575-1583. doi: 10.1093/humrep/dep050 [DOI] [PubMed] [Google Scholar]
- 32.Hu XL, Zhou XP, Qian YL, Wu GY, Ye YH, Zhu YM. The presence and expression of the hepatitis B virus in human oocytes and embryos. Hum Reprod. 2011;26(7):1860-1867. doi: 10.1093/humrep/der103 [DOI] [PubMed] [Google Scholar]
- 33.Nie R, Jin L, Zhang H, Xu B, Chen W, Zhu G. Presence of hepatitis B virus in oocytes and embryos: a risk of hepatitis B virus transmission during in vitro fertilization. Fertil Steril. 2011;95(5):1667-1671. doi: 10.1016/j.fertnstert.2010.12.043 [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Wang Q, Shen H. Design of the national free proception health examination project in China. Zhonghua Yi Xue Za Zhi. 2015;95(3):162-165. [PubMed] [Google Scholar]
- 35.Wei Y, Xu Q, Yang H, et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: a population-based cohort study in China. PLoS Med. 2019;16(10):e1002926. doi: 10.1371/journal.pmed.1002926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Guo T, Fu J, et al. Preconception thyrotropin levels and risk of adverse pregnancy outcomes in Chinese women aged 20 to 49 years. JAMA Netw Open. 2021;4(4):e215723. doi: 10.1001/jamanetworkopen.2021.5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Zhang S, Liu M, Wang Q, Shen H, Zhang Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. Lancet Glob Health. 2017;5(6):e624-e632. doi: 10.1016/S2214-109X(17)30142-0 [DOI] [PubMed] [Google Scholar]
- 38.Obstetrics Subgroup CSoO, Gynecology CMA, Chinese Society of Perinatal Medicine CMA . 2020 Clinical guidelines on prevention of mother-to-child transmission of hepatitis B virus. J Clin Hepatol. 2020;36(7):1474-1481. doi: 10.3760/cma.j.cn112141-20200213-00101 [DOI] [PubMed] [Google Scholar]
- 39.Ye F, Yue Y, Li S, et al. Presence of HBsAg, HBcAg, and HBVDNA in ovary and ovum of the patients with chronic hepatitis B virus infection. Am J Obstet Gynecol. 2006;194(2):387-392. doi: 10.1016/j.ajog.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 40.Heby O. DNA methylation and polyamines in embryonic development and cancer. Int J Dev Biol. 1995;39(5):737-757. [PubMed] [Google Scholar]
- 41.Cheng Q, Zhao B, Huang Z, et al. Epigenome-wide study for the offspring exposed to maternal HBV infection during pregnancy, a pilot study. Gene. 2018;658:76-85. doi: 10.1016/j.gene.2018.03.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Classification of Hepatitis B Virus Infection Status
eTable 2. Definition and Classification of Covariates
eTable 3. Baseline Characteristics of Female Participants in the Cohort Before and After Propensity Score Matching
eTable 4. Detailed Information on Previous Studies
Data Sharing Statement