Key Points
Question
What is the diagnostic accuracy of handheld point-of-care (POC) devices vs laboratory-based quantification for bilirubin in neonates?
Findings
In this systematic review and meta-analysis of data from 10 studies representing 3122 neonates, POC devices tended to underestimate bilirubin levels in neonates, with a pooled mean difference of −14 μmol/L. Precision was limited compared with laboratory-based quantification, with pooled outer confidence bounds of −106 to 78 μmol/L.
Meaning
Although handheld POC bilirubin devices allow for fast bilirubin measurements, this study’s findings suggest that their imprecision limits widespread use for neonatal jaundice management, especially when accurate laboratory-based bilirubin quantification is available.
This systematic review and meta-analysis evaluates the reported diagnostic accuracy of quantification of bilirubin by handheld point-of-care devices vs laboratory-based measures.
Abstract
Importance
Quantification of bilirubin in blood is essential for early diagnosis and timely treatment of neonatal hyperbilirubinemia. Handheld point-of-care (POC) devices may overcome the current issues with conventional laboratory-based bilirubin (LBB) quantification.
Objective
To systematically evaluate the reported diagnostic accuracy of POC devices compared with LBB quantification.
Data Sources
A systematic literature search was conducted in 6 electronic databases (Ovid MEDLINE, Embase, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, CINAHL, and Google Scholar) up to December 5, 2022.
Study Selection
Studies were included in this systematic review and meta-analysis if they had a prospective cohort, retrospective cohort, or cross-sectional design and reported on the comparison between POC device(s) and LBB quantification in neonates aged 0 to 28 days. Point-of-care devices needed the following characteristics: portable, handheld, and able to provide a result within 30 minutes. This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline.
Data Extraction and Synthesis
Data extraction was performed by 2 independent reviewers into a prespecified, customized form. Risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 tool. Meta-analysis was performed of multiple Bland-Altman studies using the Tipton and Shuster method for the main outcome.
Main Outcomes and Measures
The main outcome was mean difference and limits of agreement in bilirubin levels between POC device and LBB quantification. Secondary outcomes were (1) turnaround time (TAT), (2) blood volumes, and (3) percentage of failed quantifications.
Results
Ten studies met the inclusion criteria (9 cross-sectional studies and 1 prospective cohort study), representing 3122 neonates. Three studies were considered to have a high risk of bias. The Bilistick was evaluated as the index test in 8 studies and the BiliSpec in 2. A total of 3122 paired measurements showed a pooled mean difference in total bilirubin levels of −14 μmol/L, with pooled 95% CBs of −106 to 78 μmol/L. For the Bilistick, the pooled mean difference was −17 μmol/L (95% CBs, −114 to 80 μmol/L). Point-of-care devices were faster in returning results compared with LBB quantification, whereas blood volume needed was less. The Bilistick was more likely to have a failed quantification compared with LBB.
Conclusions and Relevance
Despite the advantages that handheld POC devices offer, these findings suggest that the imprecision for measurement of neonatal bilirubin needs improvement to tailor neonatal jaundice management.
Introduction
Neonatal jaundice is a condition caused by elevated unconjugated bilirubin levels. It occurs in up to 80% of neonates and is usually considered benign.1 However, the transient imbalance between bilirubin production and clearance may unpredictably lead to a fast rise of unconjugated bilirubin to levels that are dangerous for the neonate’s developing brain. High unconjugated bilirubin levels carry a risk for both acute and chronic permanent brain damage.2,3 Although largely preventable, severe hyperbilirubinemia remains a major burden for neonatal health, especially in low-resource settings.1,4,5
Early and rapid diagnosis of hyperbilirubinemia is essential to prevent its deleterious effects. Neonatal hyperbilirubinemia can easily be treated by (intensive) phototherapy and, in severe cases, by exchange transfusion.6 In many settings, identification of neonatal hyperbilirubinemia depends on screening via visual inspection of the neonate’s skin color followed by selective laboratory-based bilirubin (LBB) quantification in serum, plasma, or whole blood.7,8
Laboratory-based bilirubin quantification can be performed using a variety of in vitro diagnostic instruments9,10 and is the routine standard for diagnosing hyperbilirubinemia and indicating and monitoring treatment.11 Yet, this approach can require up to 1500 μL of blood and a fully equipped laboratory.12 The turnaround time (TAT), ie, the time between deciding to quantify bilirubin and obtaining the result, can be rather long for LBB quantification. A TAT of hours is common in practice, especially in infants with jaundice cared for outside the hospital setting, in whom bilirubin levels may rise unnoticed.13
In low-resource settings, laboratories may be remote, poorly equipped, and not always able to provide an accurate LBB level.14,15,16 As such, the diagnosis of jaundice in many neonates relies mainly on visual inspection, which is known to be unreliable.17,18 Transcutaneous bilirubin is a fast and reliable method to estimate bilirubin levels in neonates and is useful as a screening instrument.19,20 However, to establish the diagnosis of hyperbilirubinemia and determine the need for treatment, the standard remains a bilirubin quantification in blood. Therefore, the introduction of a reliable, quick, and affordable point-of-care (POC) bilirubin device that is easily transported is much needed, especially in low-resource settings or remote rural areas.16,21
Point-of-care testing is performed near or at the site of a patient and is increasingly being used in hospital and primary care settings. Point-of-care devices rapidly provide results and should facilitate earlier treatment initiation.22,23,24 Moreover, POC devices require a smaller volume of whole blood (eg, 25-50 μL). These characteristics make POC devices an appealing alternative to LBB provided that their reliability sufficiently approaches that of LBB quantification.4
We conducted a systematic review of studies that assessed the diagnostic accuracy of portable, handheld POC bilirubin devices. We performed a meta-analysis to provide an overall assessment of the diagnostic properties of POC bilirubin tests vs LBB quantification. Findings from this review could be instrumental in informing health care professionals about the usefulness and validation of POC devices for bilirubin quantification in everyday neonatal care.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline.25,26 Our review was registered in the International Prospective Register of Systematic Reviews (CRD42021289420).27
Eligibility Criteria
Studies were eligible for inclusion if they were of a prospective cohort, retrospective cohort, or cross-sectional design and compared 1 or more POC devices and LBB measurement for quantifying neonatal bilirubin levels. Point-of-care bilirubin devices were eligible if they were portable, handheld, provided a test result within 30 minutes, and required whole blood or serum to quantify the total bilirubin level. Laboratory-based bilirubin quantification was considered the reference test. The index test needed to be conducted in the same time frame as the reference test. Studies evaluating newborns aged 0 to 28 days were considered eligible.
Search Strategy and Selection Process
We searched 6 online data sources (Ovid MEDLINE, Embase, Web of Science Core Collection, Google Scholar, Cochrane Central Register of Controlled Trials, and CINAHL) up to December 5, 2022. The search strategy was designed in close collaboration with an experienced biomedical information specialist (W.M.B.) and specifically tailored for each database. The search strategy is available in the eMethods in Supplement 1.
The reference lists of relevant studies and their citations (through Google Scholar) were screened for additional potentially eligible studies that may have been previously missed. There were no restrictions imposed on language, publication date, or time frame of the study.
Records identified were imported into an EndNote (Clarivate) library through electronic extraction. After deduplication using the Bramer method,28 2 reviewers (L.E.H.W. and C.V.H.) manually identified any remaining duplicates. Subsequently, the 2 reviewers independently screened titles and abstracts. Full-text reports of potentially eligible studies were retrieved and assessed for eligibility. Any disagreement was addressed and resolved through discussion or via consulting a third reviewer (J.V.B.).
Data Extraction and Quality Assessment
Data extraction was performed by 2 independent reviewers (L.E.H.W. and C.V.H.) using a prespecified, customized form (eTable 1 in Supplement 1). Disagreements were resolved through discussion or via consulting a third reviewer (J.V.B.). In case of relevant missing information, the corresponding author of the respective study was contacted.
We used the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool29 to evaluate risk of bias and applicability (eTable 2 in Supplement 1). The QUADAS-2 assessment was conducted by the 2 reviewers (L.E.H.W. and C.V.H.) independently. Consensus was reached for all studies on all domains.
Outcomes
Our primary outcome of interest was the mean difference (ie, bias) and limits of agreement (LOAs) in total bilirubin level between the POC device (index test) and LBB quantification (reference test). Secondary outcomes were (1) TAT, (2) blood volumes, and (3) percentage of failed quantifications.
Data Analysis
Our primary analyses involved pooling the overall Bland-Altman statistics to determine the diagnostic accuracy of POC device vs LBB quantification for assessing neonatal bilirubin. Our approach to performing the meta-analysis is based on the method of Tipton and Shuster30 for meta-analysis of Bland-Altman studies, albeit incorporated in a bayesian framework. The advantage of a bayesian framework is making the modeling of several correlated studies easier. Furthermore, it relaxes the reliance on large-sample asymptotic theory, which is important in meta-analysis where the number of studies is often small. All priors where chosen to be weakly informative in order not to have too big an impact on the results.
Tipton and Shuster30 provided a way to estimate population bias (expected difference between the 2 methods), construct pooled LOAs, and calculate the associated uncertainty. To summarize, the pooled LOAs are a function of 3 parameters: (1) population bias (the average difference between 2 tests in the population), (2) the average within-study variation, and (3) the variation in bias across studies. The study-specific estimates of the bias are pooled to give estimates of the population bias and the between-study variance of this bias. The average between-study variation is estimated by pooling the study-specific estimates. The population bias within-study variance and between-study variance can now be used to calculate the LOAs. By the usual definition, the LOAs are defined as the expected bias plus or minus 1.96 times the expected SD of the bias across individuals and study settings. Asymptotically, when the LOAs are constructed this way, the difference in outcomes between the methods when applied in a randomly selected new case has a 95% probability of being between the bounds. However, as the usual LOA definition uses estimates in place of the true parameters of the population, in smaller samples (both in terms of studies and patients within studies), this is not the case. Therefore, we also calculated the outer confidence bounds (CBs) that fully take into account all uncertainty.
Besides pooling all studies, we also anticipated conducting subgroup analyses for each type of POC device separately. Furthermore, some included studies provided multiple Bland-Altman plots for different populations. To account for dependency of these observations, we added an extra hierarchical level to the model (so that there are both study-specific and population-within-study–specific effects of bias and variance).
The data were analyzed using R, version 4.1 (R Foundation for Statistical Computing) and STAN, version 2.26 (STAN Development Team) software. If needed, we converted units of bilirubin levels from mg/dL to μmol/L using a conversion factor of 17.1.
Results
We identified 1439 records through database searching. After removing duplicates, titles and abstracts were screened for 879 records. Twenty-four records were selected for full-text assessment, of which 10 were included in qualitative and quantitative analyses12,31,32,33,34,35,36,37,38,39 (Figure 1).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 Flow Diagram, Including Searches of Databases, Registers, and Other Sources.
POC indicates point-of-care.
Study Characteristics
Table 1 lists the study characteristics of the 10 included studies (representing 3122 neonates), of which 9 used a cross-sectional design12,31,32,33,34,35,36,37,38 and 1 a prospective cohort design39; guidelines for phototherapy40,41,42 used by some studies are also presented. Five studies were performed in Southeast Asia,12,31,32,33,34 3 in Africa,35,36,37 1 in Europe and Africa,38 and 1 in Africa and Southeast Asia.39 Studies were published between 2012 and 2022. Characteristics of the POC devices and LBB quantification are available in eTable 3 in Supplement 1.
Table 1. Study Characteristics of Included Studies.
| Source | Country | Paired measurements | Study design | Study population | Clinical setting | |
|---|---|---|---|---|---|---|
| Inclusion criteria | Exclusion criteria | |||||
| Boo et al,12 2019 | Malaysia | 561 | Cross-sectional | Gestational age >36 wk; clinical jaundice | With illness (not specified) | Neonatal nurseries of 2 government hospitals |
| Coda Zabetta et al,38 2013 | Egypt | 87 | Cross-sectional | Term and near term; clinically requiring bilirubin determination | NR | Neonatal nurseries of 2 hospitals |
| Italy | 31 | |||||
| Greco et al,36 2017 | Egypt | 126 | Cross-sectional | Gestational age >36 wk; clinical jaundice | NR | Neonatal intensive care unit of a tertiary care referral center |
| Greco et al,39 2018 | Egypt | 130 | Prospective cohort | Gestational age ≥35 wk; postnatal age <28 d; visually jaundiced or signs of acute bilirubin encephalopathy routine screening of bilirubin | NR | 17 Medical centers |
| Indonesia | 530 | |||||
| Nigeria | 168 | |||||
| Vietnam | 630 | |||||
| Kamineni et al,31 2020 | India | 198 | Cross-sectional | Preterm and term neonates; clinically requiring bilirubin measurement; at risk for jaundice | Prior exchange or blood transfusion | Tertiary hospital for women and newborns |
| Keahey et al,35 2017 | Malawi | 63 | Cross-sectional | Age <28 d; at risk for jaundice | NR | Government hospital |
| Rohsiswatmo et al,34 2018 | Indonesia | 94 | Cross-sectional | Gestational age <35 wk; postnatal age ≤14 wk; indication for phototherapy based on IDAI guidelines40 | After phototherapy or exchange transfusion | Neonatal nursery of government hospital |
| Sampurna et al,32 2021 | Indonesia | 126 | Cross-sectional | Gestational age ≥32 wk; postnatal age ≤14 d; birth weight ≥1500 g; clinical jaundice as per Kramer scale (any Kramer score >0)41 | Phototherapy in the preceding 24 h; respiratory or circulatory insufficiency; severe congenital abnormalities | Government hospital |
| Shapiro et al,37 2022 | Malawi | 326 | Cross-sectional | Hospitalized; at risk for jaundice | NR | Neonatal nurseries of 2 government hospitals |
| Thielemans et al,33 2018 | Thailand | 52 | Cross-sectional | Gestational age ≥35 wk; clinical jaundice as per Kramer scale (score >3)41; previous borderline serum bilirubin measurement (ie, ≤50 μmol/L below the treatment threshold of NICE guidelines42) | NR | Field clinic |
Abbreviations: IDAI, Ikatan Dokter Anak Indonesia (Indonesian Pediatric Society); NICE, National Institute for Health and Care Excellence; NR, not reported.
The studies reported a total of 3122 paired measurements. Of the 10 included studies, 8 studied the Bilistick (Bilimetrix srl), a POC test for total bilirubin quantification in 25 μL of whole blood.12,31,32,33,34,36,38,39 The Bilistick consists of a handheld reflectance reader and test strips, which are composed of a blood-plasma filter and nitrocellulose membrane. The blood is loaded on the test strip and filtered. Next, the plasma diffuses to the membrane where bilirubin is measured by the reader through reflectance spectroscopy. The Bilistick requires calibration every 6 months. Two studies evaluated the performance of BiliSpec (3rd Stone Design), a POC test for total bilirubin quantification in 50 μL of whole blood.35,37 BiliSpec, now called BiliDx, consists of a handheld reader and lateral flow card. The lateral flow card separates the blood and plasma (using a fiber plasma separation membrane) and stabilizes the sample so that the bilirubin concentration remains constant over time. Subsequently, the reader measures the light transmitted through the plasma on the card, and the bilirubin level is estimated. BiliSpec calibration cards are housed inside the reader. The calibration process was performed daily during the trials.35,37 There was substantial variation in the reference methods used, and these included blood gas and chemistry analyzers.
Quality Assessment
Four studies31,32,34,36 were considered low risk of poor quality in all domains (Figure 2). Flow and timing were considered at high risk of bias in 3 studies where different laboratory reference methods were used within the study population, potentially signaling verification bias.12,38,39
Figure 2. Quality Assessment of Diagnostic Accuracy Studies 2 Diagram.
Diagnostic Accuracy of POC Devices Compared With LBB Quantification
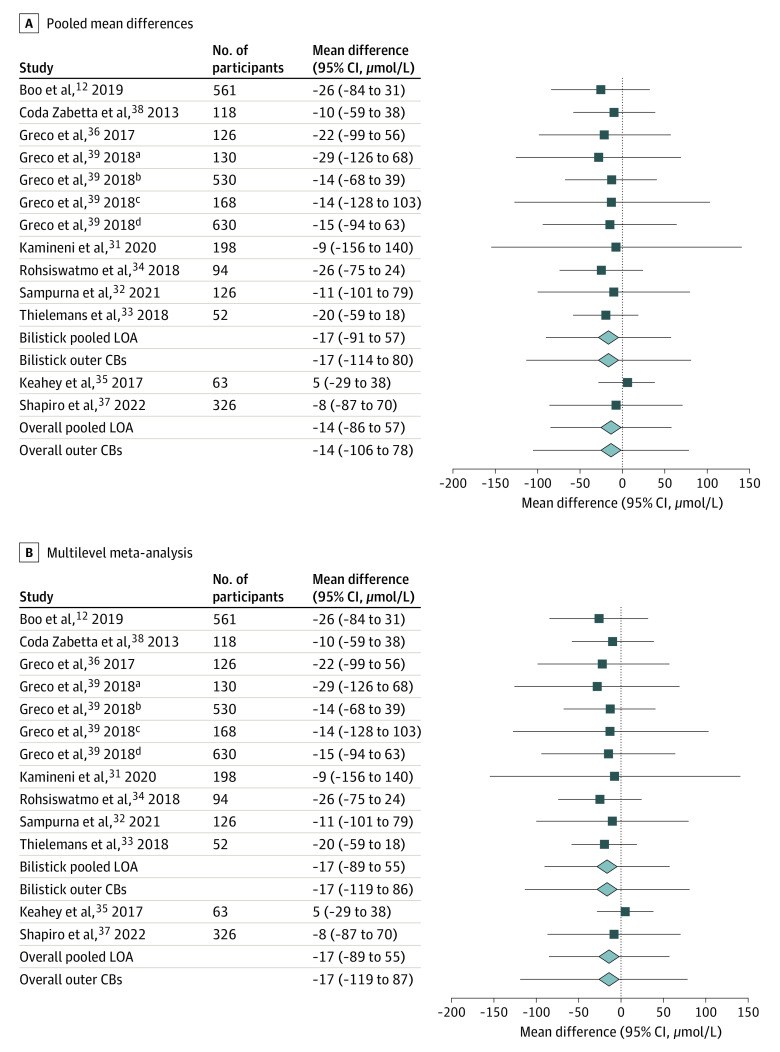
All 8 studies involving the Bilistick reported an underestimation of the POC device compared with LBB quantification for assessing neonatal bilirubin.12,31,32,33,34,36,38,39 The 2 studies evaluating the BiliSpec had contrasting outcomes with regard to bias35,37 (Table 2). Results from 2 studies were presented in a single Bland-Altman plot, even though the data were retrieved from patients in different hospitals that used a different reference method to measure total serum bilirubin.12,38 One study was performed in 17 medical centers in 4 countries, and results were presented in 4 Bland-Altman plots for these 4 countries.39 Thirteen effect estimates derived from the 10 studies evaluating POC bilirubin devices were meta-analyzed. The pooled mean difference between the POC bilirubin device and LBB quantification was −14 μmol/L, with the pooled 95% LOA ranging from −86 to 57 μmol/L and 95% CBs of −106 to 78 μmol/L (Figure 3A). Meta-analysis was performed for the Bilistick separately. Eleven effect estimates from the 8 studies were evaluated for the Bilistick. The pooled estimate of the mean difference between the Bilistick and LBB quantification was −17 μmol/L, with the pooled 95% LOA ranging from −91 to 57 μmol/L and 95% CBs of −114 to 80 μmol/L. Due to the small number of eligible studies evaluating the BiliSpec (n = 2), we could not conduct our preplanned subgroup analysis. eTable 4 in Supplement 1 shows the summary of findings.
Table 2. Primary and Secondary Outcomes Reported in Included Studies.
| Source | MD (bias) (SD)a | Corresponding LOAs, μmol/La | Variability of POC device across different bilirubin concentrations | TAT, POC vs LBB | Percentage of failed quantifications, POC vs LBB | Missed diagnosis by POC | |
|---|---|---|---|---|---|---|---|
| UL | LL | ||||||
| Boo et al,12 2019 | −26.5 (−29.4) | 31.2 | −84.1 | Underestimation was also observed for values >300 μmol/L | LBB quantification: 98 (range, 24-424) min and 114 (range, 34-1039); POC: 2 min; hospital LBB quantification was significantly longer (P < .001) | Bilistick: 15.9 due to high hematocrit and blood clotting; Jendrassik-Grof method: 1.3; sample rejected by laboratory method | Above treatment threshold, the sensitivity was 0.74, ie, approximately 26% missed diagnoses; sensitivity lower at higher Bilistick cutoff values |
| Coda Zabetta et al,38 2013 | −10.3 (24.1) | 38.0 | −58.7 | Underestimation was observed more frequently at values >342 μmol/L | NR | Bilistick: NR; Jendrassick-Grof method and diazo method: NR | NR |
| Greco et al,36 2017 | −22.2 (39.3) | 56.4 | −99.2 | No specific comment | NR | Bilistick: 6.8 due to technical problems; diazo method: 2.5 due to technical problems | NR |
| Greco et al,39 2018 | Egypt: −29.1 (49.6) | 68.4 | −126.5 | Underestimation was stable over a wide range of bilirubin values | NR | Bilistick: 1.5 due to technical problems; diazo method or direct spectrophotometry: NR | Above treatment threshold, the sensitivity was 0.70, ie, approximately 30% missed diagnoses |
| Indonesia: −13.7 (27.4) | 39.3 | −68.4 | |||||
| Nigeria: −13.7 (58.1) | 102.6 | −128.2 | |||||
| Vietnam: −15.4 (39.3) | 63.3 | −94.0 | |||||
| Kamineni et al,31 2020 | −8.6 (75.2) | 140.2 | −155.6 | Underestimation was observed across all ranges of bilirubin levels | NR | Bilistick: 6.2 due to hemolysis; direct spectrophotometry: NR | NR |
| Keahey et al,35 2017 | 5.1 (17.1) | 37.6 | −29.1 | No specific comment | NR | BiliSpec: NR; direct spectrophotometry: NR | NR |
| Rohsiswatmo et al,34 2018 | −25.6 (25.1)b | 23.6 | −74.9 | Underestimation was observed more frequently at higher bilirubin levels | NR | Bilistick: NR; chemical oxidation method: NR | If treatment decisions would have been based on the POC device, then 11 of 94 infants who needed treatment would have been missed (approximately 12%) |
| Sampurna et al,32 2021 | −11.0 (46.0) | 79.0 | −101.0 | No specific comment | NR | Bilistick: 10.7 due to different device error messages; diazo method: NR | If treatment decisions would have been based on the POC device, then 10 of 39 infants who needed treatment would have been missed (approximately 26%) |
| Shapiro et al,37 2022 | −8.2 (NR) | 70.4 | −87.0 | No specific comment | NR | BiliSpec: 9 due to strip not adequately filling; direct spectrophotometry: 9 due to different device error messages or missing measurements | If treatment decisions would have been based on the POC device, 90.7% would have resulted in the same decision as the LBB quantification, ie, approximately 9% missed diagnoses |
| Thielemans et al,33 2018 | −20.0 (NR) | 18.0 | −59.0 | No specific comment | NR | Bilistick: 48.6% due to different device error messages; direct spectrophotometry: NR | NR |
Abbreviations: LBB, laboratory-based bilirubin; LL, lower limit; LOA, limit of agreement; MD (bias), mean difference; NR, not reported; POC, point-of-care; TAT, turnaround time; UL, upper limit.
The MD (bias) and corresponding LOAs of Bilistick bilirubin – LBB, meaning that the difference between Bilistick bilirubin and LBB is plotted against the mean of Bilistick bilirubin and LBB. We converted units of bilirubin levels from mg/dL to μmol/L using a conversion factor of 17.1 (ie, 1 mg/dL = 17.1 μmol/L).
Quantification before phototherapy treatment.
Figure 3. Forest Plots of Pooled Mean Difference (Bias), Limits of Agreement (LOAs), and Outer Confidence Bounds (CBs).
aEstimates from Egypt.
bEstimates from Indonesia.
cEstimates from Nigeria.
dEstimates from Vietnam.
For the multilevel meta-analysis of these estimates, we clustered the results from Greco et al.39 Results from the multilevel meta-analysis were similar to those from the primary analysis (Figure 3B): For all POC devices, the mean difference was −17 μmol/L, with the pooled LOA ranging from −89 to 55 μmol/L and 95% CBs of −119 to 87 μmol/L. For the Bilistick, the mean difference was −17 μmol/L, with the pooled 95% LOA ranging from −89 to 55 μmol/L and 95% CBs of −119 to 86 μmol/L.
Turnaround Time
Only 1 study by Boo et al12 reported on the TAT difference (Table 2). In the 2 participating hospitals, the median TATs of the LBB quantification were 98 minutes (range, 24-424 minutes) and 114 minutes (range, 34-1039 minutes). Compared with the mean TAT of the Bilistick of 2 minutes, the hospital LBB quantification was approximately 60 times longer (P < .001). Eight studies reported on the time interval between when the blood was loaded on the test strip and when the bilirubin level was displayed on the POC’s screen: 2 minutes for the BiliSpec35 and between 100 seconds and 3 minutes for the Bilistick.12,31,33,34,36,38,39
Blood Volumes
All studies evaluating the Bilistick reported a blood volume of 25 μL for sampling12,31,32,33,34,36,38,39; blood volume for the BiliSpec was 50 μL.35,37 Four studies reported blood volumes ranging between 50 and 1500 μL for the reference test in the laboratory12,33,34,37 (eTable 3 in Supplement 1). Blood volume needed was 40 to 60 times less for POC devices than for LBB quantification.
Percentage of Failed Quantifications
The reported percentage of failed quantifications of the Bilistick varied between 1.5% and 48.6% across studies, with a median of 8.75% (IQR, 5.02%-24.07%).12,31,32,33,36,39 Common error messages were high hematocrit (>65%) and hemolysis. One study reported 9% failed measurements of the BiliSpec due to the strip not adequately being filled.37 The reported percentage of error rates of the laboratory quantification varied between 1.3% and 2.5% and were due to technical malfunction.12,36
Discussion
In this systematic review and meta-analysis, we assessed the accuracy of portable, handheld POC bilirubin devices, ie, the Bilistick and BiliSpec. Overall, the POC devices tended to underestimate the neonatal bilirubin level compared with conventional LBB quantification methods. Furthermore, the pooled estimates revealed that these POC bilirubin devices are imprecise, where the calculated outer confidence bounds were substantial, ie, −106 to 78 μmol/L for all of the POC devices and −114 to 80 μmol/L for the Bilistick. The multilevel meta-analysis showed similar results to the primary analysis, strengthening our confidence in the results. Bilirubin test results were up to approximately 60 times faster through POC devices compared with LBB quantification, whereas the volumes of the used blood samples were 40 to 60 times less using POC devices. Failed measurements occurred in up to 49% of measurements for the Bilistick,33 which was attributed to weather conditions (high humidity) and seemed not representative for updated versions in similar climates.32 In studies comparing error rates between POC device and LBB quantification, the POC devices were more likely to fail.
The inaccuracy of the POC device quantification should be considered in light of the accuracy of conventional LBB quantification methods that are generally considered as the clinical reference methods for bilirubin quantification. Although viewed as the reference standard, several studies reported rather high interlaboratory variability and inaccuracy of LBB levels measured in value-assigned commutable specimens as well as in patient samples, signaling inaccuracy and imprecision.10,43,44,45,46,47 The substantial inconsistencies encountered when quantifying bilirubin on different commonly used multianalysis instruments underline the timelessness of Mather’s statement that “bilirubin determinations are perhaps the most notoriously unreliable of any in clinical chemistry.”48(p350) The inaccuracy and high variability of POC bilirubin test results may, at least in part, originate from hemolysis or be affected by the relatively high hemoglobin concentration in the newborn.33 To this end, the Bilistick displays a warning message in case of too much hemolysis.
The question remains whether the POC bilirubin devices are fit for clinical decision making. Despite the short TAT, ease of use, and relatively low costs, their use should be conditional upon the reliability to produce accurate bilirubin results. Theoretically, use of POC bilirubin devices entails a risk of missing any neonate with jaundice who actually needs phototherapy or, in the case of overestimation, starting phototherapy too early. The risk of misclassification was encountered and acknowledged by several of the studies.32,34,35,37,39 Moreover, POC devices require near-perfect conditions for optimal use and results in terms of humidity, preanalytic conditions (eg, saturation of test strip or membrane), and hematocrit. Despite the risk of misclassification, POC bilirubin devices might be useful when diagnosing neonatal hyperbilirubinemia solely on visual inspection or when laboratory facilities are remote, which might be the case in some low-resource settings.
Precise quantification of POC bilirubin is highly clinically relevant because treatment thresholds in current international guidelines for the management of neonatal hyperbilirubinemia are based on bilirubin concentrations.49 To avoid unnecessary escalation of care, it is important that the measured bilirubin level approaches the true level, irrespective of the applied method. Our recommendations are based on 10 studies, of which 3 were considered as having a high risk of bias based on QUADAS-2 assessment (Figure 2). Future research should focus on assessing the proportional bias of the POC bilirubin devices for multiple concentrations of bilirubin. Our findings suggest that handheld POC bilirubin devices still need to be optimized in order to overcome their limitations. To support their future assessment, the total allowable error and clinically relevant limits of bilirubin quantification should be defined in international guidelines, including specific recommendations for handheld POC bilirubin devices. To harmonize POC bilirubin results with LBB methods, incorporation of POC bilirubin devices into an external quality assessment program seems sensible analogous to existing external quality assessment for LBB methods. While awaiting such data, test results of POC bilirubin readings should be interpreted cautiously.
Strengths and Limitations
To our knowledge, this systematic review and meta-analysis is the first of existing data on 2 POC devices for neonatal bilirubin quantification, which are currently available and CE (Conformité Européene) marked. We used a comprehensive search strategy, including several electronic databases and reference and citation searches. Moreover, we used a unique approach for the data synthesis wherein correlation coefficients were used to measure the relationship between variables without giving any information on the meaningful clinical association.50 In 1983, Bland and Altman51 underlined these shortcomings and provided a new approach to method comparison studies. We focused on Bland-Altman method comparison studies, which are frequently used in laboratory medicine for the validation of a new method.52 This systematic review is one of the first meta-analyses to use the unified framework for Bland-Altman meta-analysis provided by Tipton and Shuster,30 allowing us to calculate the LOAs and their associated outer confidence bounds (measures of uncertainty). Our data provide clinically relevant information for all contemporary and future users of POC bilirubin instruments involved in the care of infants with jaundice.
We acknowledge several limitations of this study. First, we were not able to calculate the variability across different bilirubin concentrations of the POC measurements, as the necessary data were not available from the original reports included in our review. Two studies reported that underestimation by the POC device was observed more frequently at higher bilirubin levels34,38 (eTable 3 in Supplement 1). This underestimation by the device is important because it carries a risk of undertreatment. Especially at high levels of bilirubin, POC bilirubin quantification should be accurate to minimize the risk of undertreatment. Second, overall data on the diagnostic accuracy to indicate the need for treatment could not be retrieved. The largest study on the Bilistick reported a sensitivity of 70.8% (95% CI, 65.0%-75.7%) for diagnosis of hyperbilirubinemia requiring treatment, with a specificity of 98.5% (95% CI, 97.7%-99.1%) and similar positive and negative predictive values of approximately 93%,39 and another multicenter study showed sensitivity, specificity, and positive and negative predictive values of 0.74, 0.84, 0.67, and 0.88, respectively, for any bilirubin value above treatment thresholds.12 Five studies reported on the number of infants with hyperbilirubinemia necessitating treatment that would have been missed by the POC device (9%-30%)12,32,34,37,39 (Table 2). Third, the variety of the applied clinical reference method with reported inaccuracies for bilirubin quantification may be considered as a limitation of this systematic review, although it may also strengthen the external validation of our findings. Fourth, most of the 3122 neonates represented in this review were late preterm and term neonates. As such, generalizability of the findings to preterm and very preterm newborns is limited.
Conclusions
Quantification of bilirubin levels using POC devices requires less blood volume and produces faster results compared with conventional LBB quantification. Despite these advantages, our findings in this systematic review and meta-analysis suggest that the percentage of failed quantifications and their imprecision for measurement of neonatal bilirubin need to be improved in POC devices.
eMethods. Search Terms
eTable 1. Data Extraction Table
eTable 2. Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2)
eTable 3. Characteristics of the Point-of-Care (POC) Devices and Laboratory-Based Bilirubin (LBB) Quantification Reported in Included Studies
eTable 4. Summary of Results and Subgroup Analysis
Data Sharing Statement
References
- 1.Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74(suppl 1):86-100. doi: 10.1038/pr.2013.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Pichon JB, Riordan SM, Watchko J, Shapiro SM. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs). Curr Pediatr Rev. 2017;13(3):199-209. doi: 10.2174/1573396313666170815100214 [DOI] [PubMed] [Google Scholar]
- 3.Johnson L, Bhutani VK. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol. 2011;35(3):101-113. doi: 10.1053/j.semperi.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 4.Greco C, Arnolda G, Boo NY, et al. Neonatal jaundice in low- and middle-income countries: lessons and future directions from the 2015 Don Ostrow Trieste Yellow Retreat. Neonatology. 2016;110(3):172-180. doi: 10.1159/000445708 [DOI] [PubMed] [Google Scholar]
- 5.Olusanya BO, Ogunlesi TA, Slusher TM. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch Dis Child. 2014;99(12):1117-1121. doi: 10.1136/archdischild-2013-305506 [DOI] [PubMed] [Google Scholar]
- 6.Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89(11):873-878. [PubMed] [Google Scholar]
- 7.de Boer J, Zondag L. Multidisciplinaire richtlijn Postnatale Zorg: Verloskundige basiszorg voor moeder en kind. Koninklijke Nederlandse Organisatie van Verloskundigen; 2018. [Google Scholar]
- 8.Dijk PH, de Vries TW, de Beer JJA; Dutch Pediatric Association . Richtlijn ‘Preventie, diagnostiek en behandeling van hyperbilirubinemie bij de pasgeborene, geboren na een zwangerschapsduur van meer dan 35 weken’. Ned Tijdschr Geneeskd. 2009;153:A93. [PubMed] [Google Scholar]
- 9.Hulzebos CV, Vitek L, Coda Zabetta CD, et al. Diagnostic methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments. Pediatr Res. 2021;90(2):277-283. doi: 10.1038/s41390-021-01546-y [DOI] [PubMed] [Google Scholar]
- 10.Greene DN, Liang J, Holmes DT, Resch A, Lorey TS. Neonatal total bilirubin measurements: still room for harmonization. Clin Biochem. 2014;47(12):1112-1115. doi: 10.1016/j.clinbiochem.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 11.Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. 2018;2(8):610-620. doi: 10.1016/S2352-4642(18)30139-1 [DOI] [PubMed] [Google Scholar]
- 12.Boo NY, Chang YF, Leong YX, et al. The point-of-care Bilistick method has very short turn-around-time and high accuracy at lower cutoff levels to predict laboratory-measured TSB. Pediatr Res. 2019;86(2):216-220. doi: 10.1038/s41390-019-0304-0 [DOI] [PubMed] [Google Scholar]
- 13.van der Geest BAM, Rosman AN, Bergman KA, et al. Severe neonatal hyperbilirubinaemia: lessons learnt from a national perinatal audit. Arch Dis Child Fetal Neonatal Ed. 2022;107(5):527-532. doi: 10.1136/archdischild-2021-322891 [DOI] [PubMed] [Google Scholar]
- 14.Olusanya BO, Ogunlesi TA, Kumar P, et al. Management of late-preterm and term infants with hyperbilirubinaemia in resource-constrained settings. BMC Pediatr. 2015;15:39. doi: 10.1186/s12887-015-0358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anticona Huaynate CF, Pajuelo Travezaño MJ, Correa M, et al. Diagnostics barriers and innovations in rural areas: insights from junior medical doctors on the frontlines of rural care in Peru. BMC Health Serv Res. 2015;15:454. doi: 10.1186/s12913-015-1114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florkowski C, Don-Wauchope A, Gimenez N, Rodriguez-Capote K, Wils J, Zemlin A. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM)—does it leverage any advantage in clinical decision making? Crit Rev Clin Lab Sci. 2017;54(7-8):471-494. doi: 10.1080/10408363.2017.1399336 [DOI] [PubMed] [Google Scholar]
- 17.Moyer VA, Ahn C, Sneed S. Accuracy of clinical judgment in neonatal jaundice. Arch Pediatr Adolesc Med. 2000;154(4):391-394. doi: 10.1001/archpedi.154.4.391 [DOI] [PubMed] [Google Scholar]
- 18.Okolie F, South-Paul JE, Watchko JF. Combating the hidden health disparity of kernicterus in Black infants: a review. JAMA Pediatr. 2020;174(12):1199-1205. doi: 10.1001/jamapediatrics.2020.1767 [DOI] [PubMed] [Google Scholar]
- 19.Dani C, Hulzebos CV, Tiribelli C. Transcutaneous bilirubin measurements: useful, but also reproducible? Pediatr Res. 2021;89(4):725-726. doi: 10.1038/s41390-020-01242-3 [DOI] [PubMed] [Google Scholar]
- 20.Okwundu C, Bhutani VK, Smith J, Esterhuizen TM, Wiysonge C. Predischarge transcutaneous bilirubin screening reduces readmission rate for hyperbilirubinaemia in diverse South African newborns: a randomised controlled trial. S Afr Med J. 2020;110(3):249-254. doi: 10.7196/SAMJ.2020.v110i3.14186 [DOI] [PubMed] [Google Scholar]
- 21.Slusher TM, Zipursky A, Bhutani VK. A global need for affordable neonatal jaundice technologies. Semin Perinatol. 2011;35(3):185-191. doi: 10.1053/j.semperi.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 22.Abel G. Current status and future prospects of point-of-care testing around the globe. Expert Rev Mol Diagn. 2015;15(7):853-855. doi: 10.1586/14737159.2015.1060126 [DOI] [PubMed] [Google Scholar]
- 23.McPartlin DA, O’Kennedy RJ. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: potential and limitations. Expert Rev Mol Diagn. 2014;14(8):979-998. doi: 10.1586/14737159.2014.960516 [DOI] [PubMed] [Google Scholar]
- 24.Patel K, Suh-Lailam BB. Implementation of point-of-care testing in a pediatric healthcare setting. Crit Rev Clin Lab Sci. 2019;56(4):239-246. doi: 10.1080/10408363.2019.1590306 [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1:7. doi: 10.1186/2046-4053-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240-243. doi: 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 30.Tipton E, Shuster J. A framework for the meta-analysis of Bland-Altman studies based on a limits of agreement approach. Stat Med. 2017;36(23):3621-3635. doi: 10.1002/sim.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamineni B, Tanniru A, Vardhelli V, et al. Accuracy of Bilistick (a point-of-care device) to detect neonatal hyperbilirubinemia. J Trop Pediatr. 2020;66(6):630-636. doi: 10.1093/tropej/fmaa026 [DOI] [PubMed] [Google Scholar]
- 32.Sampurna MTA, Rani SAD, Sauer PJJ, Bos AF, Dijk PH, Hulzebos CV. Diagnostic properties of a portable point-of-care method to measure bilirubin and a transcutaneous bilirubinometer. Neonatology. 2021;118(6):678-684. doi: 10.1159/000518653 [DOI] [PubMed] [Google Scholar]
- 33.Thielemans L, Hashmi A, Priscilla DD, et al. Laboratory validation and field usability assessment of a point-of-care test for serum bilirubin levels in neonates in a tropical setting. Wellcome Open Res. 2018;3:110. doi: 10.12688/wellcomeopenres.14767.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohsiswatmo R, Oswari H, Amandito R, et al. Agreement test of transcutaneous bilirubin and Bilistick with serum bilirubin in preterm infants receiving phototherapy. BMC Pediatr. 2018;18(1):315. doi: 10.1186/s12887-018-1290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keahey PA, Simeral ML, Schroder KJ, et al. Point-of-care device to diagnose and monitor neonatal jaundice in low-resource settings. Proc Natl Acad Sci U S A. 2017;114(51):E10965-E10971. doi: 10.1073/pnas.1714020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greco C, Iskander IF, Akmal DM, et al. Comparison between Bilistick system and transcutaneous bilirubin in assessing total bilirubin serum concentration in jaundiced newborns. J Perinatol. 2017;37(9):1028-1031. doi: 10.1038/jp.2017.94 [DOI] [PubMed] [Google Scholar]
- 37.Shapiro A, Anderson J, Mtenthaonga P, et al. Evaluation of a point-of-care test for bilirubin in Malawi. Pediatrics. 2022;150(2):e2021053928. doi: 10.1542/peds.2021-053928 [DOI] [PubMed] [Google Scholar]
- 38.Coda Zabetta CD, Iskander IF, Greco C, et al. Bilistick: a low-cost point-of-care system to measure total plasma bilirubin. Neonatology. 2013;103(3):177-181. doi: 10.1159/000345425 [DOI] [PubMed] [Google Scholar]
- 39.Greco C, Iskander IF, El Houchi SZ, et al. ; Study Team . Diagnostic performance analysis of the point-of-care Bilistick system in identifying severe neonatal hyperbilirubinemia by a multi-country approach. EClinicalMedicine. 2018;1:14-20. doi: 10.1016/j.eclinm.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosim MS, Yunanto A, Dewi R, Sarosa GI, Usman A. Buku Ajar Neonatologi. Ikatan Dokter Anak Indonesia; 2008. [Google Scholar]
- 41.Kramer LI. Advancement of dermal icterus in the jaundiced newborn. AJDC. 1969;118(3):454-458. doi: 10.1001/archpedi.1969.02100040456007 [DOI] [PubMed] [Google Scholar]
- 42.National Collaborating Centre for Women’s and Children’s Health. Neonatal Jaundice: Clinical Guideline. National Institute for Health and Care Excellence ; 2010. Accessed January 8, 2022. https://www.nice.org.uk/cg98/evidence/full-guideline-245411821
- 43.Vreman HJ, Verter J, Oh W, et al. Interlaboratory variability of bilirubin measurements. Clin Chem. 1996;42(6, pt 1):869-873. doi: 10.1093/clinchem/42.6.869 [DOI] [PubMed] [Google Scholar]
- 44.Cobbaert C, Weykamp C, Hulzebos CV. Bilirubin standardization in the Netherlands: alignment within and between manufacturers. Clin Chem. 2010;56(5):872-873. doi: 10.1373/clinchem.2009.142059 [DOI] [PubMed] [Google Scholar]
- 45.Lo SF, Jendrzejczak B, Doumas BT; College of American Pathologists . Laboratory performance in neonatal bilirubin testing using commutable specimens: a progress report on a College of American Pathologists study. Arch Pathol Lab Med. 2008;132(11):1781-1785. doi: 10.5858/132.11.1781 [DOI] [PubMed] [Google Scholar]
- 46.Grohmann K, Roser M, Rolinski B, et al. Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics. 2006;117(4):1174-1183. doi: 10.1542/peds.2005-0590 [DOI] [PubMed] [Google Scholar]
- 47.Mather A. Reliability of bilirubin determinations in icterus of the newborn infant. Pediatrics. 1960;26:350-354. doi: 10.1542/peds.26.3.350 [DOI] [PubMed] [Google Scholar]
- 48.Hollis S. Analysis of method comparison studies. Ann Clin Biochem. 1996;33(pt 1):1-4. doi: 10.1177/000456329603300101 [DOI] [PubMed] [Google Scholar]
- 49.Kemper AR, Newman TB, Slaughter JL, et al. Clinical Practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2022;150(3):e2022058859 doi: 10.1542/peds.2022-058859 [DOI] [PubMed] [Google Scholar]
- 50.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. doi: 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 51.Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015;25(2):141-151. doi: 10.11613/BM.2015.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124(4):1193-1198. doi: 10.1542/peds.2009-0329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Terms
eTable 1. Data Extraction Table
eTable 2. Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2)
eTable 3. Characteristics of the Point-of-Care (POC) Devices and Laboratory-Based Bilirubin (LBB) Quantification Reported in Included Studies
eTable 4. Summary of Results and Subgroup Analysis
Data Sharing Statement