Abstract
Penile urethral swabs collected from PCR-confirmed Chlamydia trachomatis-infected, C. trachomatis-uninfected, and non-C. trachomatis-infected, nongonococcal urethritis-infected males were analyzed for cytokine, total immunoglobulin (Ig), and specific antibody levels by enzyme-linked immunosorbent assay. Differential cellular components of the swab transport medium were also enumerated for the same groups. Although low, the levels of C. trachomatis-specific IgA and IgG antibodies and interleukin 8 cytokine were significantly higher in C. trachomatis-infected individuals. There were no significant differences in the levels of seven additional cytokines evaluated.
Chlamydial infections affect more than 89 million people per year (8) globally. In much of the developing world, Chlamydia trachomatis causes blinding trachoma and sexually transmitted diseases (STDs) worldwide. Chlamydial infections are the most common bacterial STD (4) in the United States, with approximately 4 million new cases per year.
Recent studies using the C. trachomatis-infected mouse model have characterized the immunoregulatory response as Th1 type, reflecting production of gamma interferon (IFN-γ) and cell-mediated immunity, which are critical components of an effective immune response (13, 21). Despite the plethora of information on immune responses to C. trachomatis in animal models, there exists a paucity of information about the C. trachomatis-induced response(s) in humans. In the present study, we have evaluated human male urethral specimens for characteristic evidence of a C. trachomatis immune response in a well-defined patient population.
(A portion of these results was presented at the Ninth International Symposium on Human Chlamydial Infection, Napa Valley, Calif., 1998.)
The University of Alabama at Birmingham's Institutional Review Board and the Quality Improvement Office of the Jefferson County Department of Health approved the present study. Included in the study were 142 men, aged 13 to 46 years (median age, 25 years), who attended the Jefferson County Department of Health STD Clinic in Birmingham, Ala. The population consisted of 100 African Americans, 39 Caucasians, and 3 Hispanics. Patients were classified as symptomatic (dysuria with or without urethral discharge) or asymptomatic based on patient complaints and clinical findings. The total enrollment was divided into three groups. The C. trachomatis-infected group (n = 71) was defined as PCR positive for C. trachomatis only. A second group (n = 15) contained patients with nongonococcal urethritis (NGU) who were C. trachomatis PCR negative and was identified as non-C. trachomatis–NGU. The third group was considered uninfected controls (n = 56) who were not infected with C. trachomatis, Neisseria gonorrhoeae, or Treponema pallidum, based on laboratory findings, and were negative for human immunodeficiency virus.
A Dacron-tipped stainless-steel shaft swab was used to sample the penile urethra and was placed into 1.5 ml of 2-SP transport medium (23). The transport medium was used to evaluate C. trachomatis cell culture; C. trachomatis PCR (23); enzyme-linked immunosorbent assay for cytokines (27) interleukin 1β (IL-1β), IL-2, IL-6, IL-8, IL-10, IL-12 (p70), IL-18, transforming growth factor-β (TGF-β), tumor necrosis factor alpha (TNF-α) (R&D Systems, Minneapolis, Minn.), IL-4, and IFN-γ (PharMingen, San Diego, Calif.); immunoglobulin (Ig) (11, 19); and antigen-specific antibody (Labsystems, Helsinki, Finland) (22).
In some previously published studies, semen had been used to determine the presence of C. trachomatis and C. trachomatis-specific antibody levels (16, 17, 26). However, this fluid is predominantly derived from sources other than the urethra and thus may not reflect the primary site of infection (24). The results from the present study probably portray the local responses to C. trachomatis more accurately than do evaluations of other body fluids such as urine and semen, which only pass transiently through the site of infection, the male urethra.
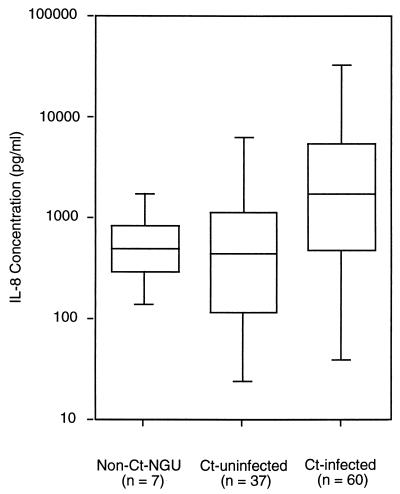
We examined swab specimens in transport medium for the presence of cytokines in the control group, the non-C. trachomatis–NGU group, and the C. trachomatis-positive NGU group (Table 1). Only IL-8 was significantly increased (P < 0.0001) in C. trachomatis-PCR-positive subjects (Fig. 1). Furthermore, levels of the Th1-associated cytokines, IL-2, IFN-γ, and IL-18, as well as Th2 cytokines, IL-6 and IL-10, and the inflammatory cytokine IL-1β in the urethral swab fluid of C. trachomatis-infected males did not differ significantly from those in the control group. IL-4, TGF-β, and TNF-α were below the detection limits of this method in all specimens.
TABLE 1.
Cytokine levels in urethral swab specimens
| Patient group | Cytokine level [mean ± SEM (n)]
|
||||||
|---|---|---|---|---|---|---|---|
| IL-1β (pg/ml) | IL-2 (pg/ml) | IL-6 (pg/ml) | IL-10 (pg/ml) | IL-12 (p70) (pg/ml) | IL-18 (ng/ml) | IFN-γ (pg/ml) | |
| Uninfected | 26.0 ± 4.8 (13) | 57.5 ± 20.6 (15) | 152 ± 37.4 (4) | 6.6 ± 1.3 (12) | 354 ± 139 (11) | 5.8 ± 0.5 (37) | 0.6 ± 0.1 (10) |
| Non-C. trachomatis–NGU | 42.6 ± 9.7 (7) | 69.6 ± 26.0 (7) | Not done | 7.0 ± 0.69 (7) | 392 ± 160 (6) | Not done | 0.7 ± 0.1 (7) |
| C. trachomatis infected | 49.1 ± 13.8 (23) | 50.3 ± 11.6 (19) | 94.3 ± 14.6 (6) | 16.0 ± 7.7 (18) | 140 ± 43.1 (16) | 7.6 ± 0.9 (40) | 1.6 ± 0.6 (24) |
| P valuea | |||||||
| Uninfected vs infected | 0.73 | 0.72 | 0.17 | 0.98 | 0.21 | 0.32 | 0.092 |
| Non-C. trachomatis–NGU vs infected | 0.13 | 0.53 | Not done | 0.34 | 0.47 | Not done | 0.36 |
Differences for all comparisons were considered significant at P < 0.05 (Mann-Whitney U test).
FIG. 1.
Comparison of IL-8 levels in non-C. trachomatis–NGU, C. trachomatis-uninfected, and C. trachomatis-infected males. The minimum, maximum, and quartiles (25%, 50%, and 75%) are shown for each group. Statistically significant differences were found in IL-8 levels in males infected with C. trachomatis compared with those who were not C. trachomatis infected (P < 0.0001).
Serum specimens from 9 C. trachomatis-infected and 17 C. trachomatis-uninfected males were evaluated for the same battery of cytokines tested (except for IL-18) in the urethral swab medium (data not shown). No statistically significant difference was found between the serum cytokine levels of the two groups except for IL-8; IL-8 levels were dramatically lower in sera of C. trachomatis-infected males than in those of C. trachomatis-uninfected males (P = 0.0016).
Cellular components from urethral specimens less than 24 h old in 2-SP were concentrated by cytospin centrifugation onto glass slides. Differential counts of lymphocytes, monocytes, and polymorphonuclear leukocytes (PMN) from swab specimen samples were compared for the three groups (data not shown). The numbers of monocytes in both the C. trachomatis-infected (n = 14) and the non-C. trachomatis–NGU (n = 7) males were lower than those in the uninfected males (n = 11) (P = 0.036 and 0.038, respectively). The total numbers of lymphocytes were equal among the groups. Compared to the uninfected group, the non-C. trachomatis–NGU group had higher numbers of PMN (P = 0.038) but the C. trachomatis-infected group did not. Six asymptomatic males (five non-C. trachomatis–NGU and one C. trachomatis positive) had no leukocytes observed on the cytocentrifuged slide specimens.
Secretory leukocyte protease inhibitor (SLPI) is a product of the innate immune system and is present in many human secretions, including tears, nasal secretions, cervical mucus, and seminal fluid (1, 5, 20), but it has not been described in penile urethral fluid. As a consequence of PMN activity associated with C. trachomatis infections, we expected that neutrophil elastase would be increased, as reported previously in C. trachomatis-proven male urethral infections or urethritis (6). Elastase can damage the epithelium and possibly interfere with host defenses (29). Because SLPI acts as a major inhibitor of neutrophil elastase in secretions (3), has microbicidal activity (12), and is stable in an acidic environment (7), we compared levels of SLPI in the urethral specimens of all three groups. Although SLPI was found in all specimens from all three groups, no increased levels of this protein were detected in the C. trachomatis-infected patients. In light of the total numbers of PMN in both the C. trachomatis-infected and -uninfected males (P = 0.13), the measured levels of SLPI were not unexpected.
Igs have been reported as an important component in the immune response to C. trachomatis in animals and humans (14, 28). Total IgA, IgA1, IgA2, IgG, and IgM levels were higher in the urethral swab fluids from C. trachomatis-infected males than in swab samples from the C. trachomatis-uninfected males (for all Igs, P < 0.050 [Table 2]); a significant increase in the levels of total secretory IgA (S-IgA) in the urethral swab fluids was observed in the C. trachomatis-infected group compared to the uninfected group (P = 0.0071). Levels of total IgA1, IgA2, and S-IgA were not determined for non-C. trachomatis–NGU patients. The source of the increased levels of Igs in the C. trachomatis-infected male urethra may be local production or transudation of plasma proteins into the genital tract secretions.
TABLE 2.
Total Ig concentrations in urethral swab specimens
| Patient group | Concn [ng/ml mean ± SEM (n)] of:
|
|||||
|---|---|---|---|---|---|---|
| IgA | IgA1 | IgA2 | S-IgA | IgG | IgM | |
| Uninfected | 898 ± 245 (15) | 582 ± 178 (15) | 252 ± 64 (15) | 1,046 ± 349 (15) | 1,113 ± 247 (13) | 53 ± 15 (12) |
| Non-C. trachomatis–NGU | 3,329 ± 810 (7) | Not done | Not done | Not done | 4,310 ± 2,061 (7) | 133 ± 64 (7) |
| C. trachomatis infected | 1,503 ± 244 (18) | 846 ± 121 (17) | 994 ± 190 (17) | 2,761 ± 507 (18) | 24,007 ± 12,183 (28) | 394 ± 104 (28) |
| P valuea | ||||||
| Uninfected vs infected | 0.035 | 0.045 | 0.014 | 0.0071 | <0.0001 | 0.0001 |
| Non-C. trachomatis–NGU vs infected | 0.002 | Not done | Not done | Not done | 0.015 | 0.26 |
Mann-Whitney U test.
Total levels of serum IgA, IgG, and IgM were not significantly elevated in the C. trachomatis-infected group compared to the uninfected group (data not shown) (for all Igs, P > 0.20). There was no difference in serum Ig levels for the non-C. trachomatis–NGU subjects compared with the C. trachomatis-uninfected subjects (data not shown) (for all Igs, P > 0.70).
As measured by a commercial serum antibody detection assay, optical density units of C. trachomatis-specific IgA and IgG in urethral swab transport medium were higher in C. trachomatis-infected males than in C. trachomatis-uninfected males (P < 0.0001 [Table 3]). Sera from 9 C. trachomatis-infected individuals and 11 uninfected individuals (non-C. trachomatis–NGU subjects were not included) revealed no differences in specific antibody levels (P > 0.5).
TABLE 3.
C. trachomatis-specific antibodies in urethral swab specimens and serum
| Patient group | Amt [absorbance units, mean ± SEM (n)] of:
|
|||
|---|---|---|---|---|
| Urethral IgA | Urethral IgG | Serum IgA | Serum IgG | |
| Uninfected | 0.144 ± 0.03 (30) | 0.076 ± 0.004 (33) | 0.577 ± 0.252 (11) | 0.852 ± 0.298 (11) |
| C. trachomatis infected | 0.548 ± 0.129 (33) | 0.187 ± 0.047 (33) | 0.640 ± 0.232 (9) | 1.031 ± 0.294 (9) |
| Pa | <0.0001 | <0.001 | 0.66 | 0.50 |
P value determined by comparison of uninfected to infected males (Mann-Whitney U test).
To our knowledge, this study provides the first comprehensive survey of the local response associated with human chlamydial infection. Compared to the uninfected control group, the C. trachomatis-infected group had higher levels of IL-8, total Ig, and C. trachomatis-specific antibodies at the locally affected area. However, our findings differ from those of investigators using chlamydia-infected animal models that reflect a typical Th1-type response to initial and repeat infection (21). The present study reports only a mild cellular inflammatory response directly associated with C. trachomatis infection.
Only IL-8 levels were elevated in C. trachomatis-infected men compared with the two uninfected groups (Fig. 1). The IL-8 levels for the non-C. trachomatis–NGU group were comparable to IL-8 levels detected in the C. trachomatis-negative group. IL-8 is a CXC chemokine that is produced by epithelial cells, monocytes, and neutrophils and appears to mediate the recruitment of neutrophils to areas of inflammation or infection (2, 10). Rasmussen et al. (25) have shown that primary endocervical epithelial cells as well as cell lines (HeLa, SiHa, and HT-29) are capable of secreting IL-8 when infected with C. trachomatis in vitro. Hang et al. (9) have detected IL-8 by cytokine staining in urethral epithelial tissue from both disease-free and diseased subjects. These results suggest that epithelial cells lining the urethra not only produce IL-8 normally but also, when infected with C. trachomatis, produce it at increased levels.
The cytokine IL-18 has been implicated as important in the regulation of both innate immunity and acquired immune responses (18). Lu et al. (15) have shown that cell lines infected with C. trachomatis do in fact produce the active form of IL-18 after cleaving its proform with caspase 1. However, in the present study, there was no statistically significant difference in the levels of IL-18 between C. trachomatis-infected and -uninfected males (Table 1).
Hedges et al. (11) reported minor antibody responses in cervical mucus from women infected with N. gonorrhoeae. Similarly, as indicated here, immune responses in the urogenital tract to the sexually acquired pathogen C. trachomatis appear to be limited in magnitude. Although low, the levels of C. trachomatis-specific antibodies were however higher in the urogenital tract than those found in the systemic compartment. The results of this study indicate that C. trachomatis infection of the male urogenital tract induces low immune and minimal cytokine responses against the infecting organism and that the male urogenital tract may be considered a weak inductive site.
Acknowledgments
This work was supported by NIH Public Health Service grants AI-28147 and AI-34970.
We thank Shirley Prince and Rose Kulhavy for their excellent laboratory assistance.
REFERENCES
- 1.Abe T, Kobayashi N, Yoshimura K, Trapnell B C, Kim H, Hubbard R C, Brewer M T, Thompson R C, Crystal R G. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Investig. 1991;87:2207–2215. doi: 10.1172/JCI115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Birrer P, McElvaney N G, Gillissen A, Hoyt R F, Bloedow D C, Hubbard R C, Crystal R G. Intravenous recombinant secretory leukoprotease inhibitor augments antineutrophil elastase defense. J Appl Physiol. 1992;73:317–323. doi: 10.1152/jappl.1992.73.1.317. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States 1998. Morb Mortal Wkly Rep. 1999;47:1–16. [PubMed] [Google Scholar]
- 5.Franken C, Meijer C J, Dijkman J H. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989;37:493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- 6.Fraser P A, Teasdale J, Gan K S, Eglin R, Scott S C, Lacey C J. Neutrophil enzymes in urine for the detection of urethral infection in men. Genitourin Med. 1995;71:176–179. doi: 10.1136/sti.71.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritz H. Human mucus proteinase inhibitor (human MPI). Human seminal inhibitor I (HUSI-I), antileukoprotease (ALP), secretory leukocyte protease inhibitor (SLPI) Biol Chem Hoppe-Seyler. 1988;369:79–82. [PubMed] [Google Scholar]
- 8.Gerbase A C, Rowley J T, Heymann D H, Berkley S F, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74:S12–S16. [PubMed] [Google Scholar]
- 9.Hang L, Wullt B, Shen Z, Karpman D, Svanborg C. Cytokine repertoire of epithelial cells lining the human urinary tract. J Urol. 1998;159:2185–2192. doi: 10.1016/S0022-5347(01)63303-2. [DOI] [PubMed] [Google Scholar]
- 10.Hedges S, Bjarndottir M, Agace W, Svanborg C. Immunoregulatory cytokines modify Escherichia coli induced uroepithelial cell–IL-6 and IL-8 responses. Cytokine. 1996;8:686–697. doi: 10.1006/cyto.1996.0091. [DOI] [PubMed] [Google Scholar]
- 11.Hedges S R, Sibley D A, Mayo M S, Hook III E W, Russell M W. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 12.Hiemstra P S, Maassen R J, Stolk J, Heinzel-Wieland R, Steffens G J, Dijkman J H. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igietseme J U, Uriri I M, Kumar S N, Ananaba G A, Ojior O O, Momodu I A, Candal D H, Black C M. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–4035. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunimoto D, Brunham R C. Human immune response and Chlamydia trachomatis infection. Rev Infect Dis. 1985;7:665–673. doi: 10.1093/clinids/7.5.665. [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Shen C, Brunham R C. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J Immunol. 2000;165:1463–1469. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig M, Hausmann G, Hausmann W, Scriba M, Zimmermann O, Fischer D, Thiele D, Weidner W. Chlamydia trachomatis antibodies in serum and ejaculate of male patients without acute urethritis. Ann Urol. 1996;30:139–146. [PubMed] [Google Scholar]
- 17.Mazzoli S, Meacci F, Cosco E, Poggiali D. Clinical consequences of immune responses to chlamydia in men. Infect Dis Obstet Gynecol. 1996;4:136–142. doi: 10.1155/S1064744996000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McInnes I B, Gracie J A, Leung B P, Wei X Q, Liew F Y. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000;21:312–315. doi: 10.1016/s0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- 19.Mestecky J, Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 20.Mooren H W, Kramps J A, Franken C, Meijer C J, Dijkman J A. Localization of a low-molecular-weight bronchial protease inhibitor in the peripheral human lung. Thorax. 1983;38:180–183. doi: 10.1136/thx.38.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison R P, Morrison S G. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narvanen A, Puolakkainen M, Wu H, Kino K, Suni J. Detection of antibodies to Chlamydia trachomatis with peptide-based species-specific enzyme immunoassay. Infect Dis Obstet Gynecol. 1997;5:349–354. doi: 10.1155/S1064744997000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parks K S, Dixon P B, Richey C M, Hook E W., III Spontaneous clearance of Chlamydia trachomatis infection in untreated patients. Sex Transm Dis. 1997;24:229–235. doi: 10.1097/00007435-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Pudney J, Anderson D J. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samra Z, Soffer Y, Pansky M. Prevalence of genital chlamydia and mycoplasma infection in couples attending a male infertility clinic. Eur J Epidemiol. 1994;10:69–73. doi: 10.1007/BF01717455. [DOI] [PubMed] [Google Scholar]
- 27.Steffen M J, Ebersole J L. Sequential ELISA for cytokine levels in limited volumes of biological fluids. BioTechniques. 1996;21:504–509. doi: 10.2144/96213rr04. [DOI] [PubMed] [Google Scholar]
- 28.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogelmeier C, Hubbard R C, Fells G A, Schnebli H P, Thompson R C, Fritz H, Crystal R G. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Investig. 1991;87:482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]