ABSTRACT
COVID-19 and sepsis pose great challenges to clinicians and growing evidence is demonstrating links between the two conditions. Both can be complicated by acute heart failure. The use of levosimendan in patients with ventricular dysfunction during COVID-19 infection and sepsis has very little evidence. A 46-year-old, hypertensive and obese patient was admitted for severe left ventricular failure and shock during sepsis following a COVID-19 infection. The patient was treated first with norepinephrine, which was partially effective, then with the addition of levosimendan as a continuous 24 hours infusion. Vital signs and echocardiographic systolic performance indices, such as FE, SVi, CI, dP/dT, TAPSE, and tricuspid S-wave velocity, as well as diastolic function, were recorded at access, 12 and 24 hours. After initiation of levosimendan, a rapid improvement in vital signs and systolic and diastolic performance indices was observed, not depending on changes in preload, afterload, and inflammatory status. Blood cultures were negative for the presence of bacteria, thus defining the picture of likely viral sepsis. Cardiac magnetic resonance was determinant, showing a picture of myocarditis sustained by immune processes rather than direct viral injury, which was confirmed by endomyocardial biopsy. In conclusion, this case highlights the efficacy of levosimendan in acute heart failure complicated by shock due to COVID-19-related myocarditis and concomitant sepsis and confirms cardiac magnetic resonance as the gold standard for the diagnosis of myocardial inflammatory disease. To the best of our knowledge, this is the first documented case of effective use of levosimendan in this context.
Keywords: acute heart failure, cardiovascular magnetic resonance imaging, COVID-19 disease, levosimendan, myocarditis, viral sepsis
INTRODUCTION
The COVID-19 pandemic has been and continues to be a difficult trial for health systems and physicians around the world. Similarly, sepsis is a frequent clinical condition in the hospital setting and still presents a challenge to the clinician. Indeed, both conditions are associated with serious and potentially life-threatening consequences. The link between COVID-19 disease and sepsis has been a subject of study from the earliest moments. Growing evidence shows that, in the COVID-19 era, a certain percentage of cases labeled as bacterial sepsis are COVID-19 disease courses that mimic sepsis in phenotype. On the other hand, COVID-19 infection is associated with an increased risk of developing bacterial sepsis. Among the most impactful complications of both COVID-19 disease and sepsis is heart failure, almost always secondary to inflammatory-type damage. Levosimendan represents one of the new milestones in the treatment of acute heart failure, as it is characterized by a positive inotropic effect not associated with increased oxygen consumption. However, there is still little evidence on the efficacy of levosimendan in acute heart failure in course of sepsis, COVID-19-related myocarditis, or both. We present a case of effective levosimendan therapy in a patient with acute heart failure and cardiogenic shock due to acute left ventricular systolic dysfunction during COVID-19-related lymphocytic myocarditis and concomitant sepsis.
CASE
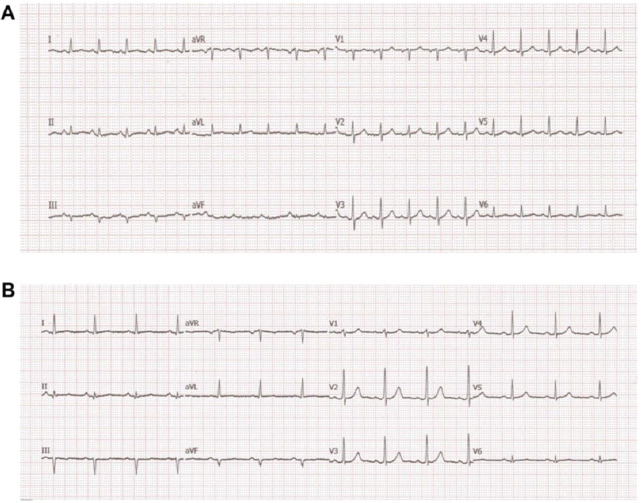
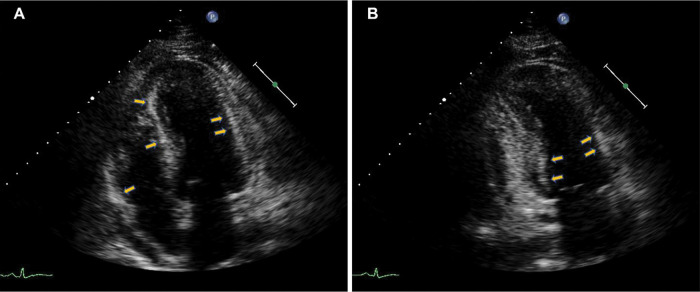
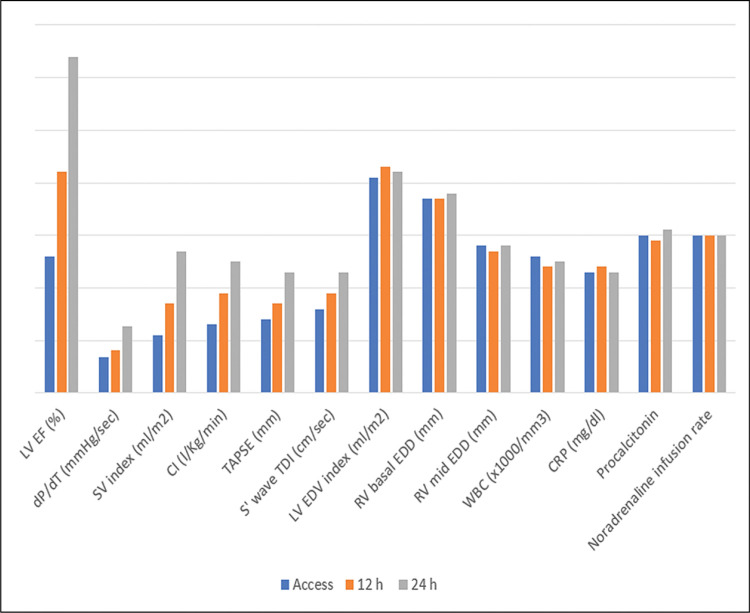
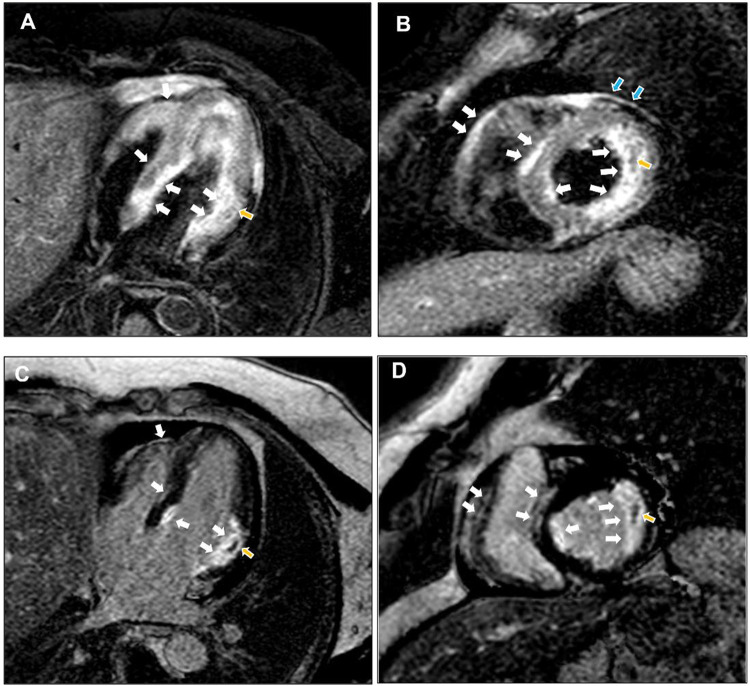
A 46-year-old man with a history of arterial hypertension and obesity as the only known diseases was admitted for fever, hypotension, and marked asthenia. A few weeks earlier, the patient underwent a cardiological examination with an electrocardiogram (ECG) and transthoracic echocardiography (TTE) for hypertension follow-up, showing normal findings. Two weeks prior to hospital admission, a COVID-19 infection characterized only by rhinitis and mild cough had occurred. On admission to our intensive care unit (ICU), the patient was alert, oriented, cooperative, and asthenic. He presented with blood pressure (BP) of 85/55 mmHg and a heart rate (HR) of approximately 120 bpm, arterial oxygen saturation of 85% in spontaneous respiration, and fever with a body temperature of 38.3 C° (Table 1). A femoral central venous catheter (CVC) was positioned. The ECG showed sinus tachycardia with a rate of 122 beats per minute (bpm), diffuse low voltages, and the absence of significant repolarization abnormalities (Fig. 1). The blood test showed neutrophilic leukocytosis with a white blood cell count of 26800 x 1000/mm3, C-reactive protein (CRP) elevation with a value of 17.4 mg/dl, Procalcitonin elevation with a value of 11.7 ng/ml, elevated high sensitivity Troponin (hs-Tn) with a value of 23000 ng/L, elevated brain natriuretic peptide (BNP) with a value of 6890 pg/ml, elevated creatinine with a value of 1.8 mg/dl and transaminases and total bilirubin; reverse transcription-polymerase chain reaction (RT-PCR) nasopharyngeal swab for COVID-19 was negative. COVID-19 IgM antibody test was performed resulting in high IgM levels. The TTE showed normal left ventricular (LV) cavitary dimensions, as expressed by an indexed end-diastolic volume (EDVi) of 41 ml/m2, diffuse LV parietal thickening with thicknesses of 15-16 mm, not present in a TTE performed a few weeks earlier, with several areas of increased myocardial echogenicity (Fig. 2), severely reduced LV global systolic function with an ejection fraction (EF) quantified by Simpson biplane method of 26%, a dP/dT ratio of 672 mmHg/sec, indexed stroke volume (SVi) calculated by velocity-time integral (VTI) method of 11 ml/m2 with a cardiac index (CI) of 1.3 l/min/m2, elements defining the picture of low output. Grade II LV diastolic dysfunction was also present. The TTE also showed normal cavitary dimensions and reduced global right ventricular (RV) systolic function defined by a basal end-diastolic diameter (EDD) of 37 mm and mean diameter of 28 mm, and TAPSE 14 mm tricuspid S-wave velocity at tissue doppler imaging (TDI) of 7.3 cm/sec. The inferior vena cava (IVC) was dilated with a maximum diameter of 26 mm with inspiratory collapse <50%. The right ventricular systolic pressure (RVSP) was 41 mmHg (26+15). The examination also showed the absence of hemodynamically significant valvulopathy and a slight amount of pericardial effusion, not associated with signs of hemodynamic involvement.). The patient was then evaluated by a thoracic computed tomography scan that showed a picture of perivascular aortic and pulmonary adipose tissue imbibition and by abdominal ultrasonography that showed no significant findings. Blood cultures were started, and the patient was then treated with broad-spectrum antibiotic therapy, including INN-daptomycin and piperacillin/tazobactam, crystalloid hydration, and nasal cannula ventilatory therapy with four l/min oxygen flow. Therapy with norepinephrine in continuous intravenous infusion at a dosage of 0.6 micrograms (mcg)/Kg/min was also initiated, obtaining a poor hemodynamic response, despite increasing the dosage to 0.9 mcg/Kg/min, defined by BP of 90-95/60 mmHg and persistence of oliguria. For these reasons, therapy with levosimendan was initiated. Bolus administration was avoided in light of the general clinical condition characterized by renal and hepatic organ damage. Continuous maintenance intravenous infusion was directly initiated at a 0.1 mcg/kg/min dosage. At 12 hours after the start of the infusion, BP had increased to 100/60 mmHg, and HR had decreased to 110 bpm. Further hemodynamic improvement was observed 24 hours after the start of infusion with evidence of BP 125/70 mmHg, HR 95 bpm, diuresis since the start of infusion of 1800 ml. A control with TTE was performed at the 12th and 24th hours after the start of infusion with evidence of clear and progressive improvement of systolic performance indices. In fact, at the 24th hour after the start of the infusion, LV EF was 66%, dP/dT ratio was 1275 mmHg/sec, TAPSE 23 mm, the tricuspid S-wave velocity at TDI was 11.2 cm/sec, SVi was 27 ml/m2 with a CI of 2.5 l/min/m2. LV diastolic function showed improvement from grade II to normal. IVC showed a maximum diameter of 18 mm with complete inspiratory collapse, RVSP was 28 mmHg (23+5) (Table 2), diffuse parietal thickening persisted with increased myocardial reflectivity with unchanged features. Twenty-four hours after the start of levosimendan infusion, no significant changes were observed in LV and RV volumes and inflammation indices showed no substantial differences compared with access (Table 3). Figure 3 depicts the trend of the indices of LV and RV systolic performance as well as the indices of preload, afterload and inflammation over time. Meanwhile, blood culture results were available, all negative. After obtaining clinical stability, the CVC was removed and a culture of its tip was performed, also with negative results. The patient was evaluated with cardiac magnetic resonance imaging (CMR). Steady-state free precession-CINE (SSFp-CINE), double inversion recovery/T1 weighted (DIR/T1w), triple inversion recovery/T2 weighted (TIR/T2w), EGE (early gadolinium enhancement), and LGE (late gadolinium enhancement) sequences were performed. Normal LV and RV volumes and systolic function were confirmed in the SSFp-CINE sequences. There was evidence of mild hypokinesia of the mid and basal segments of the lateral and inferolateral walls in the same sequences. In the TIR/T2w sequences, there was increased signal intensity defined as the ratio of skeletal muscle intensity > 1.9, suggesting edema, spread to almost all LV myocardial segments, with a higher ratio seen with subendocardial localization at the basal and mid segments of the interventricular septum, both on the right and the left side of it, the inferior basal wall, and the basal and mid segments of the lateral and inferolateral walls, in the latter case with nearly total transmurality. In these last segments, in the area characterized by enhancement, a stria with an isointense signal compared with healthy myocardium was found, suggesting a spared area. Furthermore, edema and EGE/LGE, involving basal segments of the free wall of the RV and the pericardium at the lateral level, were detected. (Fig. 4). CMR findings defined a picture compatible with immune-mediated myocarditis. An endomyocardial biopsy performed two days later confirmed a painting of lymphocytic myocarditis. Given the subendocardial distribution of edema and LGE, a result that did not allow us to exclude an ischemic origin, we proceeded to perform coronary arteriography, which showed normal coronary circulation. After 21 days of hospitalization, the patient was discharged with excellent hemodynamic compensation and normal laboratory, electrocardiographic, and echocardiographic findings. TTE performed at 1 and 3 months after discharge showed normal findings, particularly normal LV and RV systolic and diastolic function.
Table 1.
Vital signs on access and at 12 and 24 hours.
| access | 12th hour | 24th hour | |
|---|---|---|---|
| BP (mmHg) | 85/50 | 100/60 | 125/70 |
| HR (bpm) | 122 | 110 | 95 |
| BT (C°) | 38.3 | 37.6 | 37.9 |
Fig. 1.
ECG. Diffuse low voltage in the acute phase with gradual normalization over a period of about 20 days was observed.
Fig. 2.
TTE. Several areas of increased myocardial echogenicity (arrows) involving the ventricular septum, the anterior, lateral, inferior walls and the RV free wall were detected.
Table 2.
Echocardiographic parameters on access and at 12 and 24 hours.
| T0 | 12th hour | 24th hour | |
|---|---|---|---|
| LV EDV index (ml/m2) | 41 | 43 | 42 |
| LV EF (%) | 26 | 42 | 64 |
| dP/dT (mmHg/sec) | 672 | 808 | 1275 |
| SV index (ml/m2) | 11 | 17 | 27 |
| CI (l/Kg/min) | 1.3 | 1.87 | 2.5 |
| LV DF | Grade II | Grade II | Grade I |
| RV basal EDD (mm) | 37 | 37 | 39 |
| RV mid EDD (mm) | 28 | 27 | 29 |
| TAPSE (mm) | 14 | 17 | 23 |
| S' wave TDI (cm/sec) | 7.3 | 9 | 11.2 |
| IVC diameter (mm) | 26 | 20 | 18 |
| IVC collapse (%) | <50 | <50 | 100 |
| RSVP (mmHg) | 46 | 38 | 28 |
Table 3.
Blood test parameters on access and at 12 and 24 hours.
| access | 12th hour | 24th hour | |
|---|---|---|---|
| WBC (x1000/mm3) | 26800 | 24300 | 25000 |
| CRP (mg/dl) | 23 | 24 | 21 |
| Procalcitonin (ng/ml) | 11.7 | 12.1 | 11.6 |
| Hs Tn (ng/L) | 23000 | 17000 | 9000 |
| BNP (pg/ml) | 6890 | 3740 | 1080 |
Fig. 3.
This graph depicts the improvement in left ventricular systolic performance indices over time. Of note, concomitant with this improvement was no substantial change in afterload, as expressed by LV and RV size, afterload in relation to Norepinephrine rate infusion, and indices of inflammation.
Fig. 4.
CMR. In the TIR/t2w sequences, there was an increase in signal intensity compatible with edema, spread to almost all LV myocardial segments, with a higher ratio seen with subendocardial localization to the basal and middle segments of the interventricular septum, both on the right and left sides of it, the inferior basal wall, and the basal and middle segments of the lateral and inferolateral walls, in the latter case with almost total transmurality. In addition, edema was seen involving the basal segments of the RV free wall and the pericardium at the lateral level (A and B, white arrows). To the areas of edema, corresponded enhancement in the EGE (not shown) and LGE sequences (C and D, white arrows). At the level of the lateral wall, in the area characterized by edema and enhancement, there was a stria with the same signal intensity as in healthy myocardium suggesting a spared area (yellow arrows).
DISCUSSION
Sepsis is a clinical condition still representing a challenge for the clinician as well as a high economic impact condition for national systems worldwide. This condition has an estimated incidence of more than 49 million cases per year, of which about one-fifth die [1] and is frequently underdiagnosed, misdiagnosed or diagnosed with delay and is often associated with serious and potentially fatal consequences [2]. The link between COVID-19 disease and sepsis represents one of the most widely considered issues being studied since the beginning of the pandemic era. While it has been shown that COVID-19 infection may be a risk factor for the development of bacterial sepsis, likely due to an immune system imbalance during the COVD-19 infection [3], on the other hand, growing evidence is showing how, in a certain percentage of cases COVID-19 presents acute manifestations, including those regarding blood tests, very similar to those of bacterial sepsis, most likely due to cytokines and other factors that are typically released during sepsis [4,5] leading to the definition of viral sepsis in the course of COVID-19 infection [5]. This phenomenon often leads to misdiagnosis of bacterial sepsis when the patient is actually in the course of viral sepsis [6]. In this case, the negative result of blood and CVC cultures performed could suggest the hypothesis that the inflammatory manifestations were due to viral sepsis with immune mechanisms sequelae of COVID-19 infection rather than bacterial sepsis. Among the most feared complications of sepsis, multi-organ failure is described, including acute cardiac failure, which plays a central role in the pathogenesis of other organ failures such as renal and hepatic failure [7,8]. On the other hand, COVID-19 infection is also associated with acute cardiovascular manifestations, even major ones such as myocarditis [9]. Several modalities by which COVID-19 disease determines myocardial inflammation or injury have been hypothesized. Among the most accredited hypotheses are direct viral damage, systemic inflammation, myocarditis mediated by immune mechanisms and exaggerated cytokine dismission [10]. A certain percentage of cases of myocarditis related to COVID-19 present heart failure at onset, in turn complicated by cardiogenic shock in about 50% of cases [11]. Levosimendan represents one of the new cornerstones in treating acute heart failure and its efficacy has also been studied and confirmed in cardiogenic shock (12 Buerkem). One of the main advantages of levosimendan over other inotropes is represented by a positive inotropic effect not associated with increased oxygen consumption due to its mechanism of action of sensitizing myocyte Troponin C to calcium, regardless of intracellular calcium concentration. However, levosimendan also has a pulmonary and systemic vasodilator effect due to its action on adenosine triphosphate-dependent mitochondrial potassium channels [13]; thus, its use should be very cautious and, in any case, used in conjunction with the administration of a vasoconstrictor when in the presence of conditions characterized by a vasodilatory component such as septic shock. Less evidence is available regarding the use of levosimendan in septic shock and its benefits are uncertain in these cases [14]. However, the studies conducted on levosimendan have considered patients with anamnestic chronic heart failure in the decompensation phase of ischemic, primary, and valvular origin in the vast majority of cases. In these studies, otherwise healthy patients who presented with an acute reduction in global left ventricular systolic function in the course of a septic state are poorly represented, as well as patients with heart failure secondary to myocarditis. Even lesser evidence is available regarding the use of levosimendan in acute left ventricular systolic dysfunction during COVID-19-related myocarditis. In our patient, since the first evaluation, acute LV dysfunction could be attributed with sufficient certainty to an inflammatory process, considering the presence of echocardiographic elements suggestive of myocardial inflammation and the fact that routine echocardiography a few weeks earlier showed normal findings. Indeed, the genesis of hypotension and subsequent shock was less defined. These, in fact, could have depended both on the acute cardiac dysfunction and the presence of sepsis. In any case, the concomitant administration of norepinephrine allowed the opportunity to use levosimendan with a good margin of safety in light of the above reasons. The improvement in LV and RV systolic performance indices can be legitimately attributed to the levosimendan effect in this case. First, LV and RV volumes were essentially stable throughout the evaluation, making it unlikely that the functional improvement was due to different preload conditions. Carrying the same concept to the afterload state, Noradrenaline posology was not changed over the described time frame. Last but not least, two elements deserve to be considered. One is represented by the fact that the value of the inflammatory indices at the 24th hour from the start of levosimendan infusion remained substantially superimposable on the one detected at access; the other element consists in the fact that the TTE signs suggestive of myocardial edema, such as diffuse parietal thickening with increased myocardial echogenicity [15], persisted at the 24th-hour control. These findings make it unlikely that the improvement in the hemodynamic picture was secondary to a significant improvement in the general condition of a non-cardiovascular nature, specifically the inflammatory state. The improvement in RV systolic function confirms the effectiveness of levosimendan in increasing the inotropic state of both ventricles [16]. In the first instance, the reduction in RVSP may be interpreted as consensual with the improvement in global LV systolic function resulting in improved pulmonary postcapillary pressures. However, the known vasodilator effect of levosimendan on the pulmonary circulation may have contributed to this as well [17]. Regarding the positive trend that LV diastolic function had, some considerations can be made. In the first instance, the simplest and most direct hypothesis is that improving diastolic function could be consensual to that of systolic function [18]. However, levosimendan therapy could also represent at least a concomitant cause of the phenomenon, as demonstrated by some evidence showing that levosimendan could positively affect LV diastolic function even independently of any improvement in systolic function [19]. Finally, a reduction in parietal edema could also have contributed to improving diastolic function. However, the latter hypothesis seems less likely in this context because echocardiographic elements suggestive of myocardial edema/inflammation persisted even at 24th hours. As for the inflammatory process leading to the acute reduction in left ventricular systolic and diastolic function, in this case, the differential diagnosis should include bacterial myocarditis, sepsis-related cardiac dysfunction and myocarditis related to COVID-19 infection, in turn, possibly expressed as myocarditis with direct viral injury and myocarditis mediated by immune pathways, or both. The hypothesis of myocarditis sustained by bacteria is in itself unlikely in this context in light of the serial search for bacteria in the blood and on the CVC, all of which were negative as already mentioned. The search for the presence of COVID-19 by RT-PCR nasopharyngeal swab, which was negative, together with the detection of high values of IgM anti-COVID-19, correlates well with the hypothesis of phlogistic involvement likely secondary to infection by COVID-19 but sustained by an immune process rather than by direct viral damage [20,21]. However, CMR imaging played a key role in this case. First, the absence of subepicardial involvement made myocarditis from direct viral or bacterial damage very unlikely. On the other hand, subendocardial involvement is not typical of a septic heart; in fact, this finding is typically an expression of ischemic damage. Nevertheless, subendocardial involvement may also represent an atypical and rarer pattern related to myocarditis, particularly for the types sustained by immune mechanisms [22]. Endomyocardial biopsy, showing lymphocytic myocarditis, confirmed the latter hypothesis. As mentioned above, subendocardial localization is typically related to ischemic damage. Thus, given the normal angiographic picture under stable conditions, it is not excluded that there may also have been a coronary vasospastic component induced by the inflammation, as already shown by some evidence [23]. Nevertheless, the involvement of basal segments with sparing of middle and apical ones, the mild regional kinetics abnormalities despite the nearly total transmurality in some segments, the mid-wall myocardial sparing zone into the damaged myocardium are elements that make the ischemic hypothesis less likely in this case. The behavior of ECG was characterized by the absence of ischemic repolarization changes and new Q waves, diffuse low voltage in the acute phase with gradual normalization despite the constant quantity of the pericardial effusion, which appears to be in keeping with the presence of diffuse myocardial edema [24] with gradual resolution rather than the development of ischemic necrosis. To the best of our knowledge, this is the first documented case of effective use of levosimendan in this context.
CONCLUSIONS
Levosimendan was found to be effective towards both left ventricular and right ventricular systolic function and left ventricular diastolic function in this case of acute heart failure due to COVID-19 related myocarditis in the course of viral sepsis. Further studies are needed to confirm the efficacy of this drug in this population. The case confirms the role of cardiac magnetic resonance imaging as the gold standard for diagnosing and defining inflammatory myocardial disease before the endomyocardial biopsy stages. The diagnosis of bacterial sepsis in the COVID-19 era, especially in critically ill patients, is more complex and must always consider the possibility of viral sepsis in the differential diagnosis. Further studies are needed to delineate better the aspects that will allow an early differential diagnosis between the two conditions.
Key messages
Both COVID-19 and sepsis can be complicated by acute heart failure and growing evidence is demonstrating links between the two conditions.
The use of Levosimendan still has very little evidence in those contexts.
We present a case that highlights the efficacy of levosimendan in acute heart failure complicated by shock due to COVID-19-related myocarditis and concomitant sepsis and confirms CMR as the gold standard for the diagnosis of myocardial inflammatory disease.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Conflict of interest
All the authors declare no conflict of interest.
Founding
Not founded.
REFERENCES
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet . 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldens M, Schout M, Hammond NE, et al. Sepsis incidence and mortality are underestimated in Australian intensive care unit administrative data. Med J Aust . 2018;209(6):255–260. doi: 10.5694/mja18.00168. [DOI] [PubMed] [Google Scholar]
- 3.Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics . 2020;52(11):549–557. doi: 10.1152/physiolgenomics.00089.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koçak Tufan Z, Kayaaslan B, Mer M. COVID-19 and Sepsis. Turk J Med Sci . 2021;51(SI-1):3301–3311. doi: 10.3906/sag-2108-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet . 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D, Wang Q, Zhang H, et al. Viral sepsis is a complication in patients with Novel Corona Virus Disease (COVID-19) Med Drug Discov . 2020;8:100057. doi: 10.1016/j.medidd.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt A. Sepsis: an overview of the signs, symptoms, diagnosis, treatment and pathophysiology. Emerg Nurse . 2019;27(5):32–41. doi: 10.7748/en.2019.e1926. [DOI] [PubMed] [Google Scholar]
- 8.Karakike E, Giamarellos-Bourboulis EJ, Kyprianou M, et al. Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis. Crit Care Med . 2021;49(12):2042–2057. doi: 10.1097/CCM.0000000000005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal V, Sarfraz Z, Sarfraz A, et al. COVID-19 Infection and Myocarditis: A State-of-the-Art Systematic Review. J Prim Care Community Health . 2021;12:21501327211056800. doi: 10.1177/21501327211056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babapoor-Farrokhran S, Gill D, Walker J, et al. Myocardial injury and COVID-19: Possible mechanisms. Life Sci . 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haussner W, DeRosa AP, Haussner D, et al. COVID-19 associated myocarditis: A systematic review. Am J Emerg Med . 2022;51:150–155. doi: 10.1016/j.ajem.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buerkem B, Lemm H, Krohe K, et al. Levosimendan in the treatment of cardiogenic shock. Minerva Cardioangiol . 2010;58(4):519–530. [PubMed] [Google Scholar]
- 13.Toller WG, Stranz C. Levosimendan, a new inotropic and vasodilator agent. Anesthesiology . 2006;104(3):556–569. doi: 10.1097/00000542-200603000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Chang W, Xie JF, Xu JY, et al. Effect of levosimendan on mortality in severe sepsis and septic shock: a meta-analysis of randomised trials. BMJ Open . 2018;8(3):e019338. doi: 10.1136/bmjopen-2017-019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammirati E, Frigerio M, Adler ED, et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail. 2020;13(11):e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Wei Z, Zhang C, et al. The effect of levosimendan on right ventricular function in patients with heart dysfunction: a systematic review and meta-analysis. Sci Rep . 2021;11(1):24097. doi: 10.1038/s41598-021-03317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieg AD, Suleiman S, Bünting NA, et al. Levosimendan reduces segmental pulmonary vascular resistance in isolated perfused rat lungs and relaxes human pulmonary vessels. PLoS One. 2020;15(5):e0233176. doi: 10.1371/journal.pone.0233176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waggoner AD, Faddis MN, Gleva MJ, et al. Cardiac resynchronization therapy acutely improves diastolic function. J Am Soc Echocardiogr . 2005;18(3):216–220. doi: 10.1016/j.echo.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Long YX, Cui DY, Kuang X, et al. Effect of Levosimendan on Ventricular Systolic and Diastolic Functions in Heart Failure Patients: A Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol . 2021;77(6):805–813. doi: 10.1097/FJC.0000000000001010. [DOI] [PubMed] [Google Scholar]
- 20.Nicol M, Cacoub L, Baudet M, et al. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail . 2020;7(6):4371–4376. doi: 10.1002/ehf2.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JH, Xu XQ, Zhu YJ, et al. Subendocardial Involvement as an Underrecognized Cardiac MRI Phenotype in Myocarditis. Radiology . 2022;302(1):61–69. doi: 10.1148/radiol.2021211276. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging . 2013;6(5):833–9. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 24.Buttà C, Zappia L, Laterra G, et al. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: A comprehensive review. Ann Noninvasive Electrocardiol . 2020;25(3):e12726. doi: 10.1111/anec.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]