Abstract
The high activity of the rrnB P1 promoter in Escherichia coli results from a cis-acting DNA sequence, the UP element, and a trans-acting transcription factor, FIS. In this study, we examine the effects of FIS and the UP element at the other six rrn P1 promoters. We find that UP elements are present at all of the rrn P1 promoters, but they make different relative contributions to promoter activity. Similarly, FIS binds upstream of, and activates, all seven rrn P1 promoters but to different extents. The total number of FIS binding sites, as well as their positions relative to the transcription start site, differ at each rrn P1 promoter. Surprisingly, the FIS sites upstream of site I play a much larger role in transcription from most rrn P1 promoters compared to rrnB P1. Our studies indicate that the overall activities of the seven rrn P1 promoters are similar, and the same contributors are responsible for these high activities, but these inputs make different relative contributions and may act through slightly different mechanisms at each promoter. These studies have implications for the control of gene expression of unlinked multigene families.
The synthesis of ribosomes in bacteria is determined by the rate of synthesis of rRNA and, at high growth rates in Escherichia coli, rRNA promoters account for more than half of the transcription in the cell (10). The large contribution of rRNA transcription to total cellular transcription, the central role played by ribosomes in cell physiology, and the importance of rRNA regulation as a model for the control of global gene expression justify intensive analysis of rRNA promoters.
rRNA is transcribed from two promoters, P1 and P2, at each of the seven rrn operons: rrnA, rrnB, rrnC, rrnD, rrnE, rrnG, and rrnH. However, most previous work on the factors that contribute to the unique strength and regulation of the rrn P1 promoters has been limited to rrnB P1. The rrnB P1 core promoter contains near-consensus −35 and −10 hexamers. Interactions between RNA polymerase (RNAP) and the rrnB P1 core promoter (defined here as −41 to +1 with respect to the transcription start site) are regulated by the concentration of the initiating nucleotide (19) and by the concentration of guanosine tetraphosphate (ppGpp), a nucleotide that inhibits rRNA synthesis in response to amino acid starvation (5, 12). However, the core promoter accounts for <1% of the activity of the full-length promoter (defined here as containing rrnB P1 sequence upstream to −154) (21, 22, 41). The strength of the full-length promoter is attributable to two features: (i) the UP element, an A+T-rich region of DNA located upstream of the −35 hexamer and recognized by the carboxy-terminal domain of the α subunit (αCTD) of RNAP (44); and (ii) FIS, an 11.2-kDa DNA-binding protein that binds as a dimer upstream of the UP element (46, 54), bends each of its binding sites 40 to 90° (18), and interacts with the αCTD of RNAP to activate transcription (8).
UP elements are found at both rRNA (31, 44, 47, 54) and non-rRNA (23) promoters, and the degree of match to the consensus generally correlates with the magnitude of a UP element's effect on transcription (42). Near-consensus UP elements are predicted, based on sequence comparisons, to occur more frequently at stable RNA (rRNA and tRNA) promoters than at other promoters (17). UP elements consist of proximal and/or distal subsites, each of which is capable of interacting with a single αCTD (16, 17). Little is known about the relative effects of UP elements on transcription from the different rRNA promoters.
FIS is the most abundant nucleoid protein during exponential growth (1) and activates transcription not only from rrnB P1 (46) and rrnD P1 (47) but also from many other promoters [e.g., thrU(tufB) (37), proP P2 (53), tyrT (34), and leuV (45)]. DNase I, hydroxyl radical, and dimethyl sulfate footprinting studies identified three FIS binding sites upstream of rrnB P1, centered at −71, −102, and −143 (9, 46). The promoter-proximal FIS site, site I, accounts for most of the activation by FIS at rrnB P1 in vivo; sites II and III increase transcription only marginally (20 to 30%) (9, 46). FIS is assumed to activate all seven rRNA operons (15, 29, 35, 51), but only at rrnB P1 and rrnD P1 has it been shown that the effects of FIS are direct (46, 47). Furthermore, the precise locations of FIS binding sites have not been determined experimentally at rrn P1 promoters other than rrnB P1.
In order to determine whether our information about rrn P1 promoters extends beyond rrnB P1, we have defined the inputs to transcription from the other six P1 promoters. We measured the effects of the UP element and the transcription factor FIS at all seven rrn P1 promoters in vivo and in vitro. Our major conclusions are that all seven rrn P1 promoters have UP elements and are activated by FIS, but the relative contributions of these cis- and trans-acting factors to transcription differ significantly at the individual rrn P1 promoters.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. All DNA sequences in the text and figures are written 5′ to 3′ and refer to the nontemplate strand. EcoRI-HindIII fragments containing promoter sequences were constructed by PCR as described previously (42). Promoters with an UP element from one operon and a core promoter from another (“hybrid UP element promoters”) were constructed by PCR with an upstream primer containing an EcoRI site and an UP element sequence from one operon, and ca. 20 bp of core promoter sequence from a different operon used as the PCR template. “Hybrid FIS site promoters” with FIS sites from one operon (operon 1) and a UP element and core promoter from another (operon 2) were constructed in a two-step PCR process. In the first round of PCR, the upstream primer contained ca. 20 bp of sequence from upstream of −61 from operon 1 and ca. 20 bp of sequence from downstream of −61 from operon 2, which was used as the PCR template. The downstream primer contained a HindIII site adjacent to, and containing, the transcription start site (position +1). In a second round of PCR, operon 1 was used as a PCR template, the upstream primer contained an EcoRI site and a 20-bp sequence containing the final upstream endpoint, and the product of the first PCR was used as the downstream primer.
TABLE 1.
Strains and plasmids in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Strains | ||
| VH1000 | RLG3499 = MG1655 lacZ lacI pyrE+ | 19 |
| RJ1617 | MC1000 fis::kan-767 | 27 |
| RLG3669 | BL21 (DE3) pLysS | Novagen |
| λ system I lysogens | ||
| RLG4297 | VH1000 λ rrnB P1 (−88 to +1)-lacZ | This work |
| RLG4787 | VH1000 λ rrnE P1 (−89 to +1)-lacZ | This work |
| RLG4788 | VH1000 λ rrnE P1 (−209 to +1)-lacZ | This work |
| RLG4789 | VH1000 λ rrnE P1 (−61 to +1)-lacZ | This work |
| RLG4999 | VH1000 λ M13 polylinker-lacZ | This work |
| RLG5940 | RLG4999 fis::kan-767 | This work |
| RLG5949 | VH1000 λ rrnB P1 (−154 to +1)-lacZ | This work |
| RLG5950 | VH1000 λ rrnABC P1 (−61 to +1)-lacZ | This work |
| RLG6150 | VH1000 λ rrnG P1 (−199 to +1)-lacZ | This work |
| RLG6152 | VH1000 λ rrnH P1 (−205 to +1)-lacZ | This work |
| RLG6153 | VH1000 λ rrnH P1 (−62 to +1)-lacZ | This work |
| RLG6360 | VH1000 λ rrnG P1 (−61 to +1)-lacZ | This work |
| RLG6361 | RLG4297 fis::kan-767 | This work |
| RLG6362 | RLG5949 fis::kan-767 | This work |
| RLG6363 | RLG5950 fis::kan-767 | This work |
| RLG6369 | RLG6150 fis::kan-767 | This work |
| RLG6370 | RLG6152 fis::kan-767 | This work |
| RLG6371 | RLG6153 fis::kan-767 | This work |
| RLG6372 | RLG6360 fis::kan-767 | This work |
| RLG6373 | RLG6390 fis::kan-767 | This work |
| RLG6374 | RLG6392 fis::kan-767 | This work |
| RLG6375 | RLG6504 fis::kan-767 | This work |
| RLG6376 | RLG6394 fis::kan-767 | This work |
| RLG6377 | RLG6505 fis::kan-767 | This work |
| RLG6378 | RLG6396 fis::kan-767 | This work |
| RLG6379 | RLG6397 fis::kan-767 | This work |
| RLG6380 | RLG6398 fis::kan-767 | This work |
| RLG6381 | RLG6368 fis::kan-767 | This work |
| RLG6390 | VH1000 λ rrnG P1 (−86 to +1)-lacZ | This work |
| RLG6392 | VH1000 λ rrnH P1 (−82 to +1)-lacZ | This work |
| RLG6394 | VH1000 λ rrnA P1 (−199 to +1)-lacZ | This work |
| RLG6396 | VH1000 λ rrnC P1 (−201 to +1)-lacZ | This work |
| RLG6397 | VH1000 λ rrnD P1 (−81 to +1)-lacZ | This work |
| RLG6398 | VH1000 λ rrnD P1 (−198 to +1)-lacZ | This work |
| RLG6503 | VH1000 λ rrnD P1 (−60 to −42)-rrnB P1 (−41 to +1)-lacZ | This work |
| RLG6504 | VH1000 λ rrnA P1 (−81 to +1)-lacZ | This work |
| RLG6505 | VH1000 λ rrnC P1 (−81 to +1)-lacZ | This work |
| RLG6512 | VH1000 λ rrnABC P1 (−61 to −42)-rrnD P1 (−41 to +1)-lacZ | This work |
| RLG6513 | VH1000 λ rrnD P1 (−61 to +1)-lacZ | This work |
| RLG6516 | VH1000 λ rrnB P1 (−154 to −62)-rrnD P1 (−61 to +1)-lacZ | This work |
| RLG6517 | VH1000 λ rrnD P1 (−198 to −62)-rrnABC P1 (−61 to +1)-lacZ | This work |
| RLG6522 | VH1000 λ rrnE P1 (−209 to −62)-rrnABC P1 (−61 to +1)-lacZ | This work |
| RLG6523 | VH1000 λ rrnE P1 (−88 to −62)-rrnABC P1 (−61 to +1)-lacZ | This work |
| RLG6524 | VH1000 λ rrnB P1 (−154 to −62)-rrnE P1 (−61 to +1)-lacZ | This work |
| RLG6525 | VH1000 λ rrnB P1 (−88 to −62)-rrnE P1 (−61 to +1)-lacZ | This work |
| RLG6527 | RLG6513 fis::kan-767 | This work |
| RLG6528 | VH1000 λ rrnB P1 (−82 to +1)-lacZ | This work |
| λ system II lysogens | ||
| RLG6352 | VH1000 λ rrnE P1 “CA” (−41/+1)-lacZ | This work |
| RLG6353 | VH1000 λ rrnH P1 “CA” (−41/+1)-lacZ | This work |
| RLG6354 | VH1000 λ rrnG P1 “CA” (−41/+1)-lacZ | This work |
| RLG6358 | VH1000 λ rrnABC P1 “CA” (−41/+1)-lacZ | This work |
| RLG6502 | VH1000 λ rrnD P1 “CA” (−41/+1)-lacZ | This work |
| Plasmids | ||
| pRJ1077 | pET11a with fis | 39 |
| pRLG770 | Vector (no insert) | 46 |
| pRLG5943 | rrnB P1 (−154 to +1) in pRLG770 | This work |
| pRLG4782 | rrnE P1 (−209 to +1) in pRLG770 | This work |
| pRLG5945 | rrnG P1 (−199 to +1) in pRLG770 | This work |
| pRLG5947 | rrnH P1 (−205 to +1) in pRLG770 | This work |
| pRLG6385 | rrnA P1 (−199 to +1) in pRLG770 | This work |
| pRLG6387 | rrnC P1 (−201 to +1) in pRLG770 | This work |
| pRLG6398 | rrnD P1 (−198 to +1) in pRLG770 | This work |
EcoRI-HindIII fragments were inserted into pRLG770 (46) for in vitro binding and transcription studies and/or were inserted into one of two phage λ lacZ fusion systems for in vivo studies. lacZ fusion system I is able to tolerate very strong promoters but exhibits relatively high background activity; system II has a much lower background but cannot tolerate very strong promoters (41).
The first A of the HindIII site (AAGCTT) was positioned to serve as the transcription start site for the cloned P1 promoter constructs of rrnA, rrnB, rrnC, rrnE, rrnG, and rrnH. The rrnD P1 promoter constructs contain the HindIII sequence immediately downstream of the +1 transcription start site (G). Therefore, the transcribed sequences were identical for the rrn P1 promoters starting with ATP; transcripts from rrnD P1 started with a GTP and were 1 bp longer than those from the other rrn P1 promoters.
Core promoter constructs (−41 to +1) contained a 2-bp CA insertion between the EcoRI site (GAATTC) and the −41 endpoint. The 2-bp CA insert, originally introduced during cloning using synthetic “linkers” (21, 41), reduced promoter activity relative to a construct with the EcoRI site immediately adjacent to −41 (data not shown). Presumably, the EcoRI site functions as a weak UP element (17), and the CA insertion moves the EcoRI site out of phase for interactions with αCTD.
Promoter activity determinations.
Promoter activities in vivo were determined from lacZ fusions as described previously (see above and references 6 and 32). β-Galactosidase activities were measured from cells grown for at least three generations in exponential growth (to an optical density at 600 nm [OD600]of 0.3 to 0.35), when FIS levels are maximal (2, 4). The activities of promoters in system I are reported directly in Miller units with background activity subtracted (determined from RLG4999). In order to derive a factor for converting system II activities to system I activities, we compared four promoters tolerated by both fusions systems. The activities of rrnB P1 (−41 to +1), rrnB P1 (−41 to +50), rrnD P1 (−41 to +1), and lacUV5 (−46 to +1) differed by 6.1-, 7.7-, 5.3-, and 7.7-fold, respectively, in the two systems, with an average of 6.7-fold, which was used as the conversion factor.
The slightly lower rrnB P1 UP element effect in vivo reported here relative to the 30-fold effect reported previously (41, 42) appears to be attributable to strain differences (NK5031 in the previous reports, VH1000 here) and in the background subtraction used for calculating the activity of the promoter lacking a UP element. Furthermore, the magnitude of FIS-dependent activation reported here is slightly higher than the four- to fivefold value reported previously (9). This difference is attributable primarily to a difference in the construct used for calculating the activity of a promoter-lacZ fusion lacking FIS sites; i.e., the activity of an rrnB P1 construct with a −61 upstream endpoint is slightly lower than that of a construct with an endpoint of −88 containing the Δ72 mutation, a 1-bp deletion within the FIS site.
In vitro transcription reactions were carried out in 10-μl volumes containing 1 nM supercoiled plasmid DNA template, 170 mM NaCl, 10 mM Tris-Cl (pH 8.0), 10 mM MgCl2, 1 mM dithiothreitol, and 100 μg of bovine serum albumin/ml, 500 μM ATP and GTP, 50 μM CTP, 10 mM UTP, and 1 μCi of [α-32P]UTP (NEN/DuPont). FIS concentrations were varied from 0 to 400 nM or from 0 to 800 nM. Transcription was initiated by addition of 1 nM RNAP (a generous gift of R. Landick). After 15 min, reactions were terminated with an equal volume of 95% formamide, 10 mM EDTA, 0.05% xylene cyanol, and 0.05% bromophenol blue. Samples were electrophoresed on 7 M urea–5% acrylamide gels. Dried gels were visualized and quantified by phosphorimaging (ImageQuant Software; Molecular Dynamics).
FIS purification.
FIS was purified from RLG3669 containing pRJ1077 using a procedure obtained from R. Johnson (University of California at Los Angeles). One liter of cells was grown in Luria broth (LB) to an OD600 of 0.7, and FIS protein expression was then induced with 1 mM IPTG for 1 h at 37°C. Cells were pelleted, washed in cold 20 mM Tris-Cl (pH 7.5) and 0.15 M NaCl, and resuspended in half their final volume in 50 mM Tris-Cl (pH 8.0) and 10% sucrose. Cells were lysed in a final volume that was five times their mass. Phenylmethylsulfonyl fluoride (0.1 mM), 2 mM dithiothreitol, 15 mM EDTA, and 0.5 M NaCl (final concentrations) were added, and then cells were lysed by sonication. DNA was removed with polyethyleneimine (PEI) and pelleted by centrifugation at 25,000 × g for 30 min. Residual PEI was removed with 0.2 volumes of phosphocellulose slurry. The lysate was dialyzed against 0.2 M NaCl-HSB buffer (20 mM Tris-Cl [pH 7.5], 0.1 mM EDTA, 10% glycerol), passed through an SP Sepharose (1 ml) column, and eluted with a linear gradient of 0.2 to 1.0 M NaCl. Fractions containing FIS were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), dialyzed against 0.2 M NaCl-HSB buffer overnight, subjected to fast-protein liquid chromatography by using a Resource-S (1-ml) column, and eluted with a 30 ml of a gradient of 0.2 to 0.7 M NaCl. Fractions containing FIS were pooled and dialyzed overnight into 0.5 M NaCl–20 mM Tris-Cl (pH 7.5)–0.1 mM EDTA–50% glycerol. FIS concentrations were determined with the Bradford protein assay reagent using bovine serum albumin as a standard and was confirmed by SDS-PAGE.
DNase I footprinting.
pRLG770 plasmid derivatives containing promoter sequences from rrnA P1 (−199 to +1), rrnC P1 (−201 to +1), rrnD P1 (−198 to +1), rrnE P1 (−209 to +1), rrnG P1 (−199 to +1), and rrnH P1 (−205 to +1) were digested with HindIII, 32P end labeled with Sequenase (U.S. Biochemicals) and [α-32P]dATP on the nontemplate strand, and digested with EcoRI. The resulting promoter fragments were isolated from 5% acrylamide gels and purified by using Elutip-D columns (Schleicher & Schuell). DNase I footprinting was carried out and analyzed as described previously (43, 46).
RESULTS
Sequences of rrn P1 promoters.
Studies on rrnB P1 indicated that sequences upstream of −154 contributed little to promoter activity (21). Therefore, we examined sequences no further upstream than about −200 at the other promoters. To limit the potential for variation in reporter gene activity in our promoter-lacZ fusions attributable to differential mRNA stability, we chose a downstream endpoint of position +1 for the constructs from the different operons (see Materials and Methods).
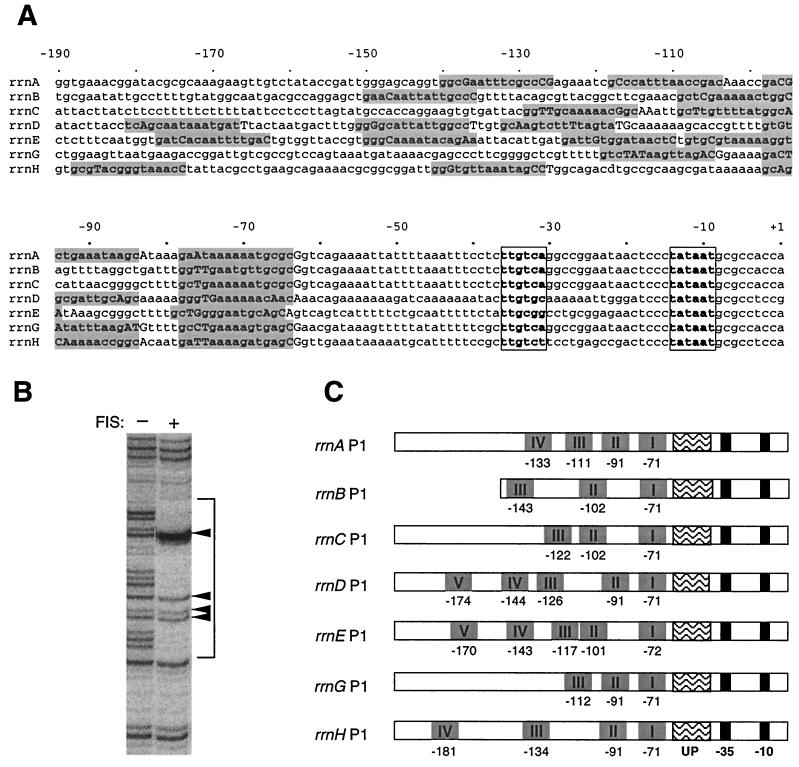
Examination of the DNA sequences indicated that, as for rrnB P1, each of the other rrn P1 promoters contained an A+T-rich region upstream of the −35 hexamer (Fig. 1A), corresponding in position to the rrnB P1 UP element. The core promoters and A+T-rich regions of rrnA P1, rrnB P1, and rrnC P1 are identical up to −68; promoter constructs containing sequences identical in these three promoters are referred to as rrnABC P1. The core and UP element regions of rrnD P1, rrnE P1, rrnG P1, and rrnH P1 differ from rrnABC P1 and from each other. Each of the seven operons differs in sequence upstream of the rrn P1 UP elements (Fig. 1A).
FIG. 1.
(A) Sequence alignment of the rrn P1 promoters. Sequences were compiled from the E. coli K-12 MG1655 complete genome (GenBank accession no. U00096). The −35 and −10 hexamers are in boldface and boxed. FIS binding sites identified by DNase I footprints are in boldface and shaded. (B) Representative FIS footprint with DNase I (rrnE P1 FIS site I). The bracket indicates the extent of FIS protection. Positions within the FIS footprint that are either accessible to DNase I or display enhanced cleavage are indicated by capital letters in panel A and arrows in panel B. (C) Schematic alignment of the rrn P1 promoters showing the locations of FIS sites relative to the core promoter (−10 and −35 hexamers) and UP element regions.
Identification of FIS binding sites upstream of position −61 in all seven rrn P1 promoters.
FIS activates transcription by binding to three sites upstream of position −61 in rrnB P1. Therefore, we first determined experimentally whether there were FIS sites in the upstream regions of each of the other rrn P1 promoters. DNase I footprints were performed on DNA fragments containing sequences from about −200 to +1 of the P1 promoters of rrnA, rrnC, rrnD, rrnE, rrnG, and rrnH by using a range of FIS concentrations. FIS binding sites were identified by a characteristic pattern of three regions of protection that span two regions (separated by 11 to 14 bp), where the DNA either is not protected or subject to enhanced cleavage (Fig. 1B). The sites of enhanced cleavage are indicative of the FIS-induced bend in the DNA (46). We conclude from the footprinting studies that (i) there are three to five FIS sites upstream of position −61 at each rrn P1 promoter (Fig. 1A and C), (ii) site I at six of the seven rrn P1 promoters is centered at position −71 relative to the transcription start site and at rrnE P1 site I is centered at position −72, and (iii) the positions of the FIS binding sites upstream of site I differ at each promoter (Fig. 1C).
Determinants of rrn P1 promoter strength in vivo.
The FIS sites were located upstream of the UP element-like regions in all seven promoters. To determine the contributions of the predicted UP elements and FIS sites to promoter activity, we constructed promoter-lacZ fusions containing (i) only the core promoter (upstream endpoint, −41); (ii) the core promoter and the predicted UP element region (∼−61); (iii) the core promoter, the predicted UP element, and the FIS site I (∼−81); and (iv) the full-length promoters (∼−200) (see Table 1 for individual promoter endpoints). Upstream endpoints from previously characterized promoter-lacZ fusions were used for rrnB P1 (46). Although inputs to promoter strength had been determined previously for rrnB P1, it was included in the following analyses for comparison.
The rrn P1 promoter-lacZ fusions were integrated into the chromosome in single copy at the λ att site. Since the activities of all of the promoters were assayed at the same location in the chromosome, the potential for positional effects resulting from differences in gene dose or local chromosome structure was eliminated.
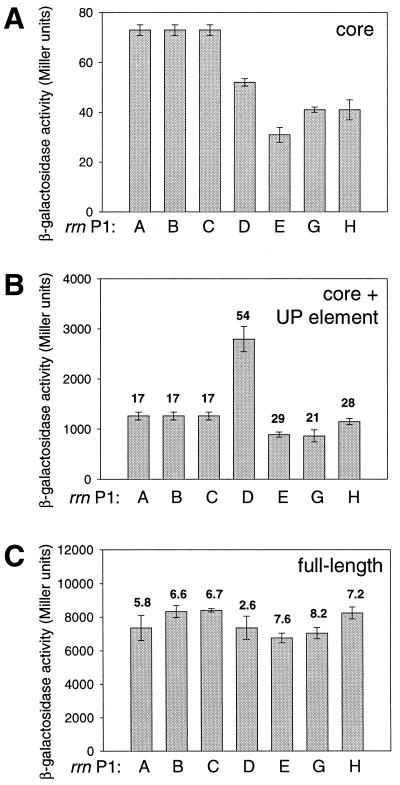
All rrn P1 core promoter activities were low, similar to that of rrnB P1, at which the core promoter activity accounts for less than 1% of the full-length promoter activity (21, 41). The rrnABC P1 core promoter was ∼1.5 to 2-fold stronger than the rrnD P1, rrnE P1, rrnG P1, and rrnH P1 promoters (Fig. 2A).
FIG. 2.
β-Galactosidase activities from rrn P1 promoter-lacZ fusions. (A) Promoters (−41 to +1) containing only the core promoter were fused to lacZ by using system II; the activities have been converted to system I units as described in Materials and Methods. (B) Promoter-lacZ fusions (∼−61 to +1) containing the core promoter plus UP element. Numbers above bars refer to fold activation by the UP element (ratio of activities in panels B and A for each promoter). (C) Promoter-lacZ fusions (∼−200 to +1) containing all FIS sites, UP element, and core promoter. Numbers above bars refer to the fold activation by sequences upstream of ∼−61 (ratio of activities in panels C and B for each promoter). Means and standard deviations are derived from at least three independent assays.
The activities of six of the rrn P1 promoters were increased to similar extents by the UP element regions, i.e., 17- to 29-fold, while rrnD P1 was activated more by its UP element, i.e., 54-fold (Fig. 2B). We emphasize the relative UP element effects for the different operons rather than the absolute values of the activation ratios (see Materials and Methods). Transcription from each of the promoters in vitro with or without the UP element region confirmed that the effects of the UP element were mediated through RNAP, since they were observed in a purified system in the absence of other protein factors (42, 44; data not shown).
UP element effects depend upon the identity of both the UP element and the core promoter sequences.
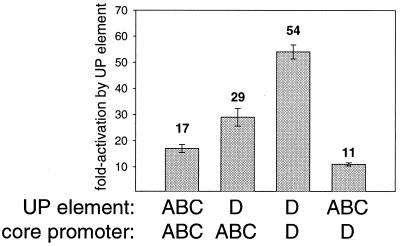
To determine whether the large effect of the rrnD P1 UP element is a function of its sequence (a closer match to the UP element consensus than the other rrn P1 UP elements) or of the ability of its core promoter to be activated, hybrid promoters were constructed in which the UP element of rrnD P1 was fused to the rrnABC P1 core promoter or in which the rrnABC P1 UP element was fused to the rrnD P1 core promoter. These constructs allowed us to compare (i) two different UP elements in the context of the same core promoter and (ii) the same UP element on two different core promoters. The rrnABC P1 core promoter was activated 29-fold by the rrnD P1 UP element compared to 17-fold activation by its own UP element; the rrnD P1 core promoter was activated 11-fold by the rrnABC P1 UP element and 54-fold by its own UP element (Fig. 3). These results indicate that both the identity of the core promoter and the identity of the UP element contribute to the extent of activation.
FIG. 3.
Both the UP element and the core promoter sequence determine the extent of activation by UP elements. The sources of the UP element and core promoter regions are indicated below each bar. The fold activation is the ratio of the β-galactosidase activity of the UP element-containing promoter (−61 or −60 to +1) to that same promoter lacking a UP element (−41 to +1). Means and standard deviations are derived from at least three independent assays.
Activation of rrn P1 promoters by sequences upstream of ∼−61 in vivo is FIS dependent.
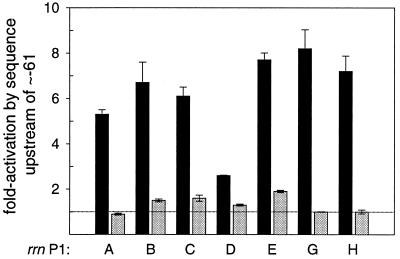
All rrn P1 promoters except rrnD P1 were activated ∼6- to 8-fold by sequences located between positions ∼−61 and ∼−200 in vivo (Fig. 2C, Fig. 4; see also Materials and Methods). rrnD P1, whose activity was higher than the other promoters in the absence of any FIS sites, was activated to a much smaller degree (<3-fold). Thus, although the presence of the region between ∼−200 and ∼−61 increased the activity of the rrn P1 promoters to different extents, the activities of all seven full-length rrn P1 promoters (containing FIS sites) were very similar (6,800 to 8,400 Miller units; Fig. 2C).
FIG. 4.
FIS is required for the effects of sequences upstream of position ∼−61 in vivo. rrn P1 promoter-lacZ fusions from the rrnA, rrnB, rrnC, rrnD, rrnE, rrnG, and rrnH operons were measured in wild-type (black bars) or fis::kan (gray bars) backgrounds. The effect of sequences upstream of ∼−61 was determined as the ratio of activity from a full-length construct containing all FIS sites (∼−200 to +1) to the activity of that same promoter lacking FIS sites (∼−61 to +1). A promoter whose activity is not increased by a sequence upstream of ∼−61 would have a fold activation of 1.0 (represented by the dotted line). Means and standard deviations are derived from at least three independent assays.
The effect of the sequences upstream of ∼−61 was drastically reduced in a strain lacking FIS, confirming that the activation in vivo from sequences upstream of ∼−61 is primarily dependent on FIS (Fig. 4). Upstream sequences increased transcription 1.9-fold or less in the absence of FIS, whereas FIS increased transcription up to 8-fold. As previously reported for rrnB P1 (46), the activation ratio (full-length promoter/promoter from ∼−61 to +1) decreased in a fis::kan strain not because of a decrease in the activities of the promoter constructs containing FIS sites but rather because of an increase in the activities of the rrn P1 promoters lacking FIS sites (C. A. Hirvonen, W. Ross, V. H. Newburn, and R. L. Gourse, unpublished data [see also reference 15]). This increase in activity results from a feedback mechanism that compensates for the loss of activation of the seven rrn operons when the fis gene is inactivated (22, 46).
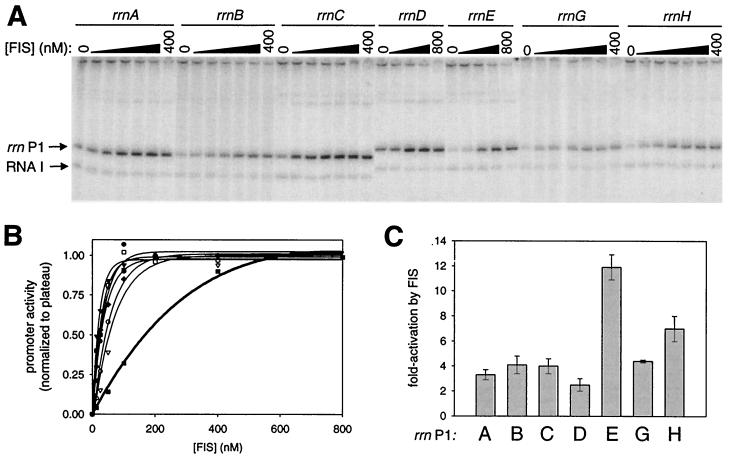
All seven rrn P1 promoters are activated directly by FIS in vitro
In vitro transcription was used to verify that FIS was directly responsible for the effect of the sequences upstream of ∼−61 in each rrn promoter. Transcription from each of the seven full-length rrn P1 promoters was increased by purified FIS in vitro (Fig. 5A), while transcription from rrn P1 promoter variants extending upstream only to ∼−61 was not (data not shown).
FIG. 5.
FIS concentration requirements and extent of activation of rrn P1 promoters by FIS in vitro. (A) In vitro transcription reactions were performed as described in Materials and Methods by using plasmid templates carrying the full-length rrn P1 promoters (∼−200 to +1) in the absence or presence of increasing concentrations of FIS. Transcripts from rrn P1 and vector-encoded RNA 1 promoter are indicated. The RNA 1 transcript is vector encoded and serves as an internal control. (B) The FIS concentration required for half-maximal activation of each full-length rrn P1 promoter in vitro was determined by quantitation of transcripts from at least two separate assays such as that shown in panel A. Values were normalized in each case to the plateau level, defined as 1.00, for each promoter. rrnA P1 (●), rrnB P1 (○), rrnC P1 (▴), rrnD P1 (▵), rrnE P1 (▪), rrnG P1 (□), and rrnH P1 (⧫). (C) The fold activation by FIS for each rrn P1 promoter was determined by comparison of transcripts in the presence or absence of 400 nM FIS.
The amount of FIS required for half-maximal transcription from each full-length rrn P1 promoter was determined by in vitro transcription at a range of FIS concentrations (Fig. 5B). rrnE P1 required significantly higher concentrations of FIS (145 nM) for half-maximal transcription than any of the other six promoters (15 to 48 nM) (Fig. 5B). The maximal extent of activation by FIS in vitro varied from <3-fold (rrnD P1) to 12-fold (rrnE P1) (Fig. 5C). Thus, although rrnE P1 required higher FIS concentrations than the other promoters for full activation, it was activated more at the highest FIS concentrations than the other promoters. However, since FIS did not increase the activity of rrnE P1 more than the other promoters in vivo (Fig. 4), this suggests that the concentration of FIS must not be high enough in cells (at least under the conditions tested) to fully utilize the rrnE FIS sites.
Intrinsic features of the rrnD P1 promoter limit the degree of activation by FIS.
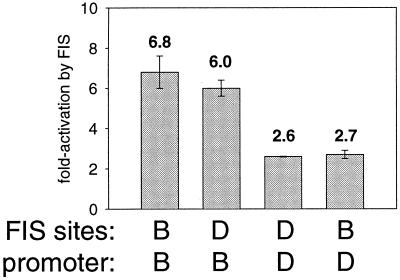
We tested whether the relatively small effect of FIS at rrnD P1 in vivo and in vitro was the result of features of its FIS sites or features of its promoter. Hybrid promoters were constructed in which the rrnD P1 FIS sites were fused to the rrnB P1 promoter at −61 or the rrnB P1 FIS sites were fused to rrnD P1 at −61. The rrnD P1 FIS sites activated the rrnB P1 promoter to about the same extent (∼6-fold) as the rrnB P1 FIS sites activated their own promoter in vivo (Fig. 6). Thus, the small effect of FIS at rrnD P1 is not due to intrinsic features of its FIS sites. Likewise, the rrnB P1 FIS sites activated the rrnD P1 promoter to the same extent as its own FIS sites, <3-fold (Fig. 6). Therefore, the limited effect of FIS at rrnD P1 appears to result from intrinsic features of its core promoter and/or its UP element rather than from its FIS sites.
FIG. 6.
Intrinsic features of the rrnD P1 promoter (−61 to +1) limit its activation by FIS in vivo. rrnB P1 or rrnD P1 promoters were either wild type (containing native FIS sites) or hybrid (containing FIS sites from the other promoter). The fold activation by FIS was determined as the ratio of the β-galactosidase activity of a promoter-lacZ fusion containing the indicated FIS sites to that of the same promoter lacking FIS sites (−61 to +1). Means and standard deviations are derived from at least three independent assays.
At five of the seven rrn P1 promoters, FIS sites upstream of site I play a larger role in transcription than at rrnB P1.
At rrnB P1, ca. 80% of the effect of FIS in vivo is attributable to the promoter proximal FIS site, site I, centered at position −71 (Table 2). To determine whether this is also true at other rrn P1 promoters, we compared the extent of activation by FIS site I with the extent of activation by all FIS sites. The effect of FIS site I varied from 1.6-fold at rrnA P1 to 7.5-fold at rrnG P1 (Table 2). Purified FIS also directly increased transcription in vitro at each of the seven rrn P1 promoters containing only site I (data not shown).
TABLE 2.
Relative contribution of upstream FIS sites to total rrn P1 promoter activity
| Promoter | Origin of −61 to +1 | Origin of FIS sitesa | Activityb
|
Fold effect (site I)c | Activity (all sites)b | Fold effect (all sites)c | % From upstream sitesd | |
|---|---|---|---|---|---|---|---|---|
| No sites | Site I | |||||||
| Wild type | rrnA P1 | A | 1,260 | 1,959 | 1.6 | 7,350 | 5.8 | 73 |
| rrnB P1 | B | 1,260 | 6,468 | 5.1 | 8,332 | 6.6 | 22 | |
| rrnC P1 | C | 1,260 | 5,090 | 4.0 | 8,407 | 6.7 | 39 | |
| rrnD P1 | D | 2,795 | 3,841 | 1.4 | 7,361 | 2.6 | 48 | |
| rrnE P1 | E | 889 | 2,255 | 2.5 | 6,756 | 7.6 | 67 | |
| rrnG P1 | G | 862 | 6,498 | 7.5 | 7,039 | 8.2 | 8 | |
| rrnH P1 | H | 1,146 | 5,635 | 4.9 | 8,248 | 7.2 | 32 | |
| Hybrid | rrnE P1 | B | 889 | 2,505 | 2.9 | 3,981 | 4.5 | 37 |
| rrnB P1 | E | 1,260 | 3,455 | 2.8 | 6,027 | 4.9 | 43 | |
The sources of FIS sites found upstream of ∼−61 at each promoter are indicated.
β-Galactosidase activities (in Miller units) from promoters lacking FIS sites (∼−61 to +1) or containing only FIS site I or all FIS sites are the mean from at least three separate assays. The error was generally 10% or less.
The fold effect is the ratio of β-galactosidase activities of FIS site-containing promoter-lacZ fusions and −61 to +1 rrn P1 promoter-lacZ fusions. Fold effect (site I) is the ratio of the activities of constructs containing site I and no sites; fold effect (all sites) is the ratio of the activities of constructs containing all sites and no sites.
The contribution of the upstream FIS sites is expressed as a percentage of total activity: 100 × (activity of a construct containing all sites − activity of a construct containing site I)/activity of construct containing all sites.
The small effect (1.6-fold) of FIS site I at rrnA P1 was significantly different from the four- to fivefold effect of site I at rrnB P1 and rrnC P1 in vivo, even though the core promoter and UP element sequences are the same in each of these operons and FIS site I is centered at the same position (i.e., −71). Therefore, differences in activation by FIS site I at these three promoters must reflect subtle differences in how FIS is positioned on the DNA (39) and/or differences in the concentration of FIS required for binding.
Comparing the activities of each of the promoters with only FIS site I to the same promoter with all FIS sites allowed calculation of the contributions of sites upstream of site I (the “distal FIS sites”; Table 2, column 9). At rrnG P1, the distal FIS sites accounted for even less of the total promoter activity (8%) than at rrnB P1 (22%). However, the distal sites played a larger role at the other operons (32 to 73%), with 67% of the total effect of FIS resulting from the distal sites at rrnE P1 and 73% of the total effect of FIS resulting from the distal sites at rrnA P1. The large impact of the distal FIS sites is not unique to rrn P1 promoters; at the thrU(tufB) promoter, FIS sites upstream of site I were also reported to play a relatively large role in activation (52).
To determine whether the large effect of the distal FIS sites in rrnE P1 was a property of these sites or of features of the promoter, we constructed hybrid promoters in which either FIS site I or all of the FIS sites from rrnE P1 and rrnB P1 were exchanged. rrnE P1 FIS site I activated the rrnB P1 and rrnE P1 promoters similarly (2.8-fold versus 2.5-fold; Table 2), but rrnB P1 FIS site I activated the rrnE P1 promoter less than rrnB P1 (2.9-fold versus 5.1-fold). When all of the rrnB P1 and rrnE P1 FIS sites were exchanged, their abilities to activate transcription were somewhat reduced, i.e., both hybrid promoters had slightly lower activities than the natural constructs (Table 2). Thus, the magnitude of activation mediated by the FIS sites was affected by both the identity of the FIS sites and of the RNAP binding regions. We calculated the relative contribution of the upstream FIS sites in the different hybrid promoters. Distal rrnE P1 FIS sites made a smaller relative contribution to total activity when positioned upstream of the rrnB P1 promoter than when in their native context (43% versus 67%; Table 2). However, the distal FIS sites of rrnB P1 had a slightly larger effect on total activity when positioned upstream of rrnE P1 than in their native context (37% versus 22%). Therefore, it appears that the relative contribution of the FIS sites upstream of site I to total transcription is dependent on multiple aspects of promoter architecture, e.g., on the identity of the FIS sites and the RNAP binding region (from positions ∼−61 to +1).
DISCUSSION
rrn P1 promoter activities derive from different relative contributions of the same components.
Our studies have shown that UP elements and the transcription factor FIS contribute to the strength of each rrn P1 promoter (Fig. 1). If we assume that the seven rrn operons arose through gene duplication, it is not surprising that FIS sites and UP elements (albeit degenerate) have been retained in the course of E. coli evolution as the mechanisms responsible for high activity at all seven rrn P1 promoters. Selective pressure has apparently acted at each operon to maintain sensitivity to the same inputs while maintaining the same overall activity for each full-length rrn P1 promoter.
However, several different solutions have been found to account for high activity in the different rrn operons. (i) The effect of FIS at rrnD P1 is small relative to that at the other rrn P1 promoters; rrnD P1 derives its strength from a larger contribution of its UP element to total expression (relative to the effects of the other rrn P1 UP elements). (ii) rrnB P1 and rrnG P1 are activated more than the other rrn P1 promoters by the promoter proximal FIS site, site I. (iii) rrnA P1 and rrnE P1 are activated more than the other rrn P1 promoters by FIS sites upstream of site I. In the case of rrnE P1, high concentrations of FIS could potentially make an even greater contribution to transcription, but the sites have evolved with relatively low affinity for FIS, limiting their ability to activate transcription by the FIS concentrations actually present in vivo (at least under the conditions tested). It has been noted previously in other systems that different regulatory assemblies can result in a similar transcriptional outcome (11).
Why do all seven full-length rrn P1 promoters have similar activities? The rrn P1 promoters are extremely active (perhaps the most active of the cell's promoters) at high growth rates. Although we have examined expression of the seven rrn P1 promoters under conditions where transcription activity is high, our reporter system is not saturated for β-galactosidase (e.g., double lysogens containing these promoter-lacZ fusions have twice the enzyme activity of monolysogens [data not shown]). Although we cannot provide a conclusive answer to why the seven full-length rrn P1 promoters have evolved to have similar activities, we suggest that these activities are not set by an approach of each promoter to some theoretical limit. For example, transcriptional output from rrn operons can increase further, even in rich medium, to compensate for reductions in gene dose (3), and transcription initiation at rRNA promoters increases when the gene for the elongation factor NusB is inactivated (50). Furthermore, the activity of a full-length rrnD P1 promoter can increase ∼50% in a fis::kan mutant (data not shown).
Changing FIS concentrations may influence the relative contribution of different rrn operons to total rRNA synthesis.
FIS is undetectable in stationary-phase cells, and levels increase dramatically as cells enter the exponential phase. After only two generations of growth, there are as many as 50,000 FIS molecules per cell when cultured in rich medium (2, 4). The occupancy of FIS sites at rrnB P1 in vivo correlates with these changes in cellular FIS levels (2). Since the extent of activation by FIS at different promoters varies with the FIS concentration in vitro (especially at rrnE P1 versus the other rrn P1 promoters) and since the unactivated level of transcription at rrnD P1 is higher than at the other promoters in vivo and in vitro, changes in the amount of FIS could potentially result in differences in the relative contributions of different operons to total rRNA synthesis in vivo. We note that the products of the different rrn operons (both tRNAs and rRNAs) are not identical (26). Whether the differences in the expression of different operons have physiological consequences remains to be determined.
Since E. coli containing only five rrn operons grows at near wild-type rates on rich medium (13) and the deletion of additional rrn operons is not lethal (3), it has been proposed that the presence of all seven rrn operons is necessary for swift adaptation to environmental changes (14, 30). The different ways of achieving the same final output might help to allow for such swift adaptations.
Structural considerations for activation by distal FIS sites.
The discovery that distal FIS sites play a more significant role in transcription than predicted from studies on rrnB P1 suggests that the structure of the P1 activation complex varies in the different rrn operons. Physical contacts (if they exist) between FIS bound at distal sites and RNAP has not been explored in detail at rrn P1 promoters. Present information strongly suggests that there are no cooperative interactions between bound FIS dimers (9, 28) and that FIS bound at site I contacts the αCTD of RNAP (8).
Since the αCTD and αNTD are connected by a flexible linker of only ca. 13 amino acids (∼46 Å if fully extended) (25), αCTD should not be able to reach further upstream than positions ∼−80 to −90 in the absence of DNA distortion (36, 38). Assuming FIS bound at the distal sites exerts its effects on transcription by interacting directly with RNAP, we suggest that FIS bound at the proximal site(s), possibly in conjunction with intrinsic DNA curvature (20), distorts the DNA to facilitate these contacts. Differences in the positions of the upstream FIS sites at different operons, differences in the angles of the bends induced by FIS bound to its various binding sites, and differences in intrinsic DNA curvature in different operons could all contribute to differences in the extents of activation by the different rrn P1 upstream regions. Consistent with this model, mutations at rrnE P1 that prevent binding of FIS to site I eliminate activation by distal FIS sites (C. A. Hirvonen and R. L. Gourse, unpublished data). We cannot eliminate the possibility that other protein factors, as yet unknown, might be required for the activation attributed to the sequences upstream of FIS site I. However, the effects of these sequences would depend on fis (Fig. 4) and FIS bound at site I.
Further studies will be needed to determine where the two αCTDs are located in complexes containing multiple FIS sites, as well as other details about promoter architecture in the different operons. However, initiation complexes in which there are multiple activator dimers and intrinsic DNA bends facilitating upstream binding by αCTD would not be unique to rrn P1 promoters. For example, when multiple CAP-cyclic AMP dimers are bound upstream of a promoter, the αCTD can reach to at least position −100 (7, 33), and at the Pu promoter in Pseudomonas putida, integration host factor (IHF) bends the DNA so that αCTD can interact with specific DNA sequences located upstream of the IHF site (40).
Prediction of FIS binding sites.
We compared the FIS binding sites defined in our footprinting assays (Fig. 1) with FIS sites predicted by the most recent computational means (24, 48, 49). Our conclusions are as follows: (i) sites predicted as having high probability of being FIS sites were detectable by DNase I footprinting; (ii) sites with a lower probability of being FIS sites were usually not detected even as partially occupied by FIS in footprints; and (iii) sites detected with relatively weak affinity for FIS were often not predicted by the computational analysis. Since the footprints presented here have increased the number of confirmed FIS sites substantially, this study should allow further refinement of FIS site prediction.
Concluding remarks.
In summary, we have shown that the same inputs contribute to the strength of all seven rrn P1 promoters, although the relative contributions of these inputs vary. Understanding the contributors to transcription of the different rrn P1 promoters allows us to begin to integrate information about how the multiple inputs contributing to rRNA expression act together to regulate rRNA promoter output.
ACKNOWLEDGMENTS
We thank Tamas Gaal and other members of our lab for helpful comments on the manuscript, R. Landick for his gift of E. coli RNAP (Eς70), and Tom Schneider for his help with the computer analyses of FIS sites.
This work was supported by research grant GM37048 from N.I.H. to R.L.G., by fellowships to C.A.H. from the University of Wisconsin Alumni Research Foundation and an N.I.H. Genetics Predoctoral Training Grant, by a Hilldale Undergraduate Research Fellowship to C.E.W., by an award from the National Science Foundation Research Experience for Undergraduates to E.M., and by a Howard Hughes Scholars Undergraduate Research Fellowship to V.H.N.
REFERENCES
- 1.Ali Azam T, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–6370. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleman J A, Ross W, Salomon J, Gourse R L. Activation of Escherichia coli rRNA transcription by FIS during a growth cycle. J Bacteriol. 1998;180:1525–1532. doi: 10.1128/jb.180.6.1525-1532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai T, Condon C, Voulgaris J, Zaporojets D, Shen B, Al-Omar M, Squires C, Squires C L. Construction and initial characterization of Escherichia coli strains with few or no intact chromosomal rRNA operons. J Bacteriol. 1999;181:3803–3809. doi: 10.1128/jb.181.12.3803-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball C A, Osuna R, Ferguson K C, Johnson R C. Dramatic changes in FIS levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker M M, Gaal T, Josaitis C A, Gourse R L. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol. 2001;305:673–688. doi: 10.1006/jmbi.2000.4327. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett M S, Gaal T, Ross W, Gourse R L. RNA polymerase mutants that destabilize RNA polymerase-promoter complexes alter NTP-sensing by rrn P1 promoters. J Mol Biol. 1998;279:331–345. doi: 10.1006/jmbi.1998.1779. [DOI] [PubMed] [Google Scholar]
- 7.Belyaeva T A, Rhodius V A, Webster C L, Busby S J. Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organisation of the RNA polymerase alpha subunits. J Mol Biol. 1998;277:789–804. doi: 10.1006/jmbi.1998.1666. [DOI] [PubMed] [Google Scholar]
- 8.Bokal A J, Ross W, Gaal T, Johnson R C, Gourse R L. Molecular anatomy of a transcription activation patch: FIS-RNA polymerase interactions at the Escherichia coli rrnB P1 promoter. EMBO J. 1997;16:154–162. doi: 10.1093/emboj/16.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokal A J, Ross W, Gourse R L. The transcriptional activator protein FIS: DNA interactions and cooperative interactions with RNA polymerase at the Escherichia coli rrnB P1 promoter. J Mol Biol. 1995;245:197–207. doi: 10.1006/jmbi.1994.0016. [DOI] [PubMed] [Google Scholar]
- 10.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C.: American Society for Microbiology; 1987. pp. 1527–1542. [Google Scholar]
- 11.Cases I, de Lorenzo V. The black cat/white cat principle of signal integration in bacterial promoters. EMBO J. 2001;20:1–11. doi: 10.1093/emboj/20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashel M, Gentry D R, Hernandez V H, Vinella D. The stringent response. In: Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1458–1496. [Google Scholar]
- 13.Condon C, French S, Squires C, Squires C L. Depletion of functional ribosomal RNA operons in Escherichia coli causes increased expression of the remaining intact copies. EMBO J. 1993;12:4305–4315. doi: 10.1002/j.1460-2075.1993.tb06115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condon C, Liveris D, Squires C, Schwartz I, Squires C L. rRNA operon multiplicity in Escherichia coli and the physiological implications of rrn inactivation. J Bacteriol. 1995;177:4152–4156. doi: 10.1128/jb.177.14.4152-4156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condon C, Philips J, Fu Z Y, Squires C, Squires C L. Comparison of the expression of the seven ribosomal RNA operons in Escherichia coli. EMBO J. 1992;11:4175–4185. doi: 10.1002/j.1460-2075.1992.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estrem S T, Gaal T, Ross W, Gourse R L. Identification of an UP element consensus sequence for bacterial promoters. Proc Natl Acad Sci USA. 1998;95:9761–9766. doi: 10.1073/pnas.95.17.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrem S T, Ross W, Gaal T, Chen Z W, Niu W, Ebright R H, Gourse R L. Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase alpha subunit. Genes Dev. 1999;13:2134–2147. doi: 10.1101/gad.13.16.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkel S E, Johnson R C. The Fis protein: it's not just for DNA inversion anymore. Mol Microbiol. 1992;6:3257–3265. doi: 10.1111/j.1365-2958.1992.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaal T, Bartlett M S, Ross W, Turnbough C L, Jr, Gourse R L. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- 20.Gaal T, Rao L, Estrem S T, Yang J, Wartell R M, Gourse R L. Localization of the intrinsically bent DNA region upstream of the E. coli rrnB P1 promoter. Nucleic Acids Res. 1994;22:2344–2350. doi: 10.1093/nar/22.12.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gourse R L, de Boer H A, Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986;44:197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- 22.Gourse R L, Gaal T, Bartlett M S, Appleman J A, Ross W. rRNA transcription and growth rate-dependent regulation of ribosome synthesis in Escherichia coli. Annu Rev Microbiol. 1996;50:645–677. doi: 10.1146/annurev.micro.50.1.645. [DOI] [PubMed] [Google Scholar]
- 23.Gourse R L, Ross W, Gaal T. UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol Microbiol. 2000;37:687–695. doi: 10.1046/j.1365-2958.2000.01972.x. [DOI] [PubMed] [Google Scholar]
- 24.Hengen P N, Bartram S L, Stewart L E, Schneider T D. Information analysis of Fis binding sites. Nucleic Acids Res. 1997;25:4994–5002. doi: 10.1093/nar/25.24.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon Y H, Yamazaki T, Otomo T, Ishihama A, Kyogoku Y. Flexible linker in the RNA polymerase alpha subunit facilitates the independent motion of the C-terminal activator contact domain. J Mol Biol. 1997;267:953–962. doi: 10.1006/jmbi.1997.0902. [DOI] [PubMed] [Google Scholar]
- 26.Jinks-Robertson S, Nomura M. Ribosomes and tRNA. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C.: ASM Press; 1987. pp. 1358–1385. [Google Scholar]
- 27.Johnson R C, Ball C A, Pfeffer D, Simon M I. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci USA. 1988;85:3484–3488. doi: 10.1073/pnas.85.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson R C, Glasgow A C, Simon M I. Spatial relationship of the Fis binding sites for Hin recombinational enhancer activity. Nature. 1987;329:462–465. doi: 10.1038/329462a0. [DOI] [PubMed] [Google Scholar]
- 29.Josaitis C A, Gaal T, Ross W, Gourse R L. Sequences upstream of the-35 hexamer of rrnB P1 affect promoter strength and upstream activation. Biochim Biophys Acta. 1990;1050:307–311. doi: 10.1016/0167-4781(90)90186-6. [DOI] [PubMed] [Google Scholar]
- 30.Klappenbach J A, Dunbar J M, Schmidt T M. rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol. 2000;66:1328–1333. doi: 10.1128/aem.66.4.1328-1333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leirmo S, Gourse R L. Factor-independent activation of Escherichia coli rRNA transcription. I. Kinetic analysis of the roles of the upstream activator region and supercoiling on transcription of the rrnB P1 promoter in vitro. J Mol Biol. 1991;220:555–568. doi: 10.1016/0022-2836(91)90100-k. [DOI] [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 33.Murakami K, Owens J T, Belyaeva T A, Meares C F, Busby S J, Ishihama A. Positioning of two alpha subunit carboxy-terminal domains of RNA polymerase at promoters by two transcription factors. Proc Natl Acad Sci USA. 1997;94:11274–11278. doi: 10.1073/pnas.94.21.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muskhelishvili G, Buckle M, Heumann H, Kahmann R, Travers A A. FIS activates sequential steps during transcription initiation at a stable RNA promoter. EMBO J. 1997;16:3655–3665. doi: 10.1093/emboj/16.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachaliel N, Melnick J, Gafny R, Glaser G. Ribosome-associated protein(s) specifically bind(s) to the upstream activator sequence of the E. coli rrnA P1 promoter. Nucleic Acids Res. 1989;17:9811–9822. doi: 10.1093/nar/17.23.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naryshkin N, Revyakin A, Kim Y, Mekler V, Ebright R H. Structural organization of the RNA polymerase-promoter open complex. Cell. 2000;101:601–611. doi: 10.1016/s0092-8674(00)80872-7. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson L, Vanet A, Vijgenboom E, Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990;9:727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozoline O N, Tsyganov M A. Structure of open promoter complexes with Escherichia coli RNA polymerase as revealed by the DNase I footprinting technique: compilation analysis. Nucleic Acids Res. 1995;23:4533–4541. doi: 10.1093/nar/23.22.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan C Q, Finkel S E, Cramton S E, Feng J A, Sigman D S, Johnson R C. Variable structures of Fis-DNA complexes determined by flanking DNA-protein contacts. J Mol Biol. 1996;264:675–695. doi: 10.1006/jmbi.1996.0669. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Martin J, Timmis K N, de Lorenzo V. Co-regulation by bent DNA. Functional substitutions of the integration host factor site at sigma 54-dependent promoter Pu of the upper-TOL operon by intrinsically curved sequences. J Biol Chem. 1994;269:22657–22662. [PubMed] [Google Scholar]
- 41.Rao L, Ross W, Appleman J A, Gaal T, Leirmo S, Schlax P J, Record M T, Jr, Gourse R L. Factor-independent activation of rrnB P1. An “extended” promoter with an upstream element that dramatically increases promoter strength. J Mol Biol. 1994;235:1421–1435. doi: 10.1006/jmbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- 42.Ross W, Aiyar S E, Salomon J, Gourse R L. Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. J Bacteriol. 1998;180:5375–5383. doi: 10.1128/jb.180.20.5375-5383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross W, Ernst A, Gourse R L. Fine structure of E. coli RNA polymerase-promoter interactions: alpha subunit binding to the UP element minor groove. Genes Dev. 2001;15:491–506. doi: 10.1101/gad.870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 45.Ross W, Salomon J, Holmes W M, Gourse R L. Activation of Escherichia coli leuV transcription by FIS. J Bacteriol. 1999;181:3864–3868. doi: 10.1128/jb.181.12.3864-3868.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross W, Thompson J F, Newlands J T, Gourse R L. E. coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sander P, Langert W, Mueller K. Mechanisms of upstream activation of the rrnD promoter P1 of Escherichia coli. J Biol Chem. 1993;268:16907–16916. [PubMed] [Google Scholar]
- 48.Schneider T D. Information content of individual genetic sequences. J Theor Biol. 1997;189:427–441. doi: 10.1006/jtbi.1997.0540. [DOI] [PubMed] [Google Scholar]
- 49.Schneider T D, Stephens R M. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharrock R A, Gourse R L, Nomura M. Defective antitermination of rRNA transcription and derepression of rRNA and tRNA synthesis in the nusB5 mutant of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:5275–5279. doi: 10.1073/pnas.82.16.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verbeek H, Nilsson L, Baliko G, Bosch L. Potential binding sites of the trans-activator FIS are present upstream of all rRNA operons and of many but not all tRNA operons. Biochim Biophys Acta. 1990;1050:302–306. doi: 10.1016/0167-4781(90)90185-5. [DOI] [PubMed] [Google Scholar]
- 52.Verbeek H, Nilsson L, Bosch L. The mechanism of trans-activation of the Escherichia coli operon thrU(tufB) by the protein FIS: a model. Nucleic Acids Res. 1992;20:4077–4081. doi: 10.1093/nar/20.15.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Johnson R C. Fis activates the RpoS-dependent stationary-phase expression of proP in Escherichia coli. J Bacteriol. 1995;177:5222–5231. doi: 10.1128/jb.177.18.5222-5231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zacharias M, Goringer H U, Wagner R. Analysis of the Fis-dependent and Fis-independent transcription activation mechanisms of the Escherichia coli ribosomal RNA P1 promoter. Biochemistry. 1992;31:2621–2628. doi: 10.1021/bi00124a024. [DOI] [PubMed] [Google Scholar]