Abstract

Polo-like kinase 1 (Plk1), a mitotic kinase whose activity is widely upregulated in various human cancers, is considered an attractive target for anticancer drug discovery. Aside from the kinase domain, the C-terminal noncatalytic polo-box domain (PBD), which mediates the interaction with the enzyme’s binding targets or substrates, has emerged as an alternative target for developing a new class of inhibitors. Various reported small molecule PBD inhibitors exhibit poor cellular efficacy and/or selectivity. Here, we report structure–activity relationship (SAR) studies on triazoloquinazolinone-derived inhibitors, such as 43 (a 1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one) that effectively block Plk1, but not Plk2 and Plk3 PBDs, with improved affinity and drug-like properties. The range of prodrug moieties needed for thiol group masking of the active drugs has been expanded to increase cell permeability and mechanism-based cancer cell (L363 and HeLa) death. For example, a 5-thio-1-methyl-4-nitroimidazolyl prodrug 80, derived from 43, showed an improved cellular potency (GI50 4.1 μM). As expected, 80 effectively blocked Plk1 from localizing to centrosomes and kinetochores and consequently induced potent mitotic block and apoptotic cell death. Another prodrug 78 containing 9-fluorophenyl in place of the thiophene-containing heterocycle in 80 also induced a comparable degree of anti-Plk1 PBD effect. However, orally administered 78 was rapidly converted in the bloodstream to parent drug 15, which was shown be relatively stable toward in vivo oxidation due to its 9-fluorophenyl group in comparison to unsubstituted phenyl. Further derivatization of these inhibitors, particularly to improve the systemic prodrug stability, could lead to a new class of therapeutics against Plk1-addicted cancers.
Keywords: polo-like kinase 1, polo-box domain, inhibitors, prodrugs, cancer, mitotic block
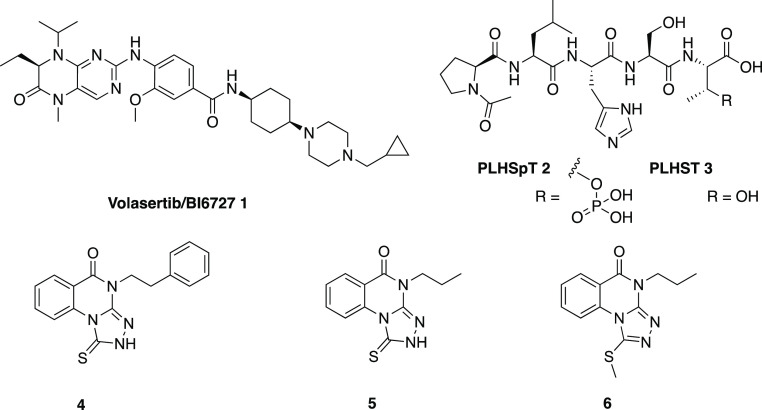
Polo-like kinase 1 (Plk1), a mitotic Ser/Thr kinase, is highly overexpressed in a broad spectrum of human cancers1 and is considered an attractive anticancer drug target.2−4 Plk1 contains two distinguishable domains—the N-terminal kinase domain (KD) for ATP-mediated catalysis and the C-terminal polo-box domain (PBD) for recognizing its binding targets or substrates. Over the years, extensive efforts have been made to develop anti-Plk1 therapeutics, yielding several ATP-competitive inhibitors of the catalytic site of Plk1. As a potent catalytic inhibitor, Volasertib/BI6727 1 (Chart 1)5 progressed to an advanced clinical trial (Phase III POLO-AML-2 study, in combination with low-dose cytarabine) for acute myeloid leukemia, but ultimately failed because it did not meet primary end point and induced toxicity. Other inhibitors targeting the ATP-binding site of Plk1 have likewise been under development by the pharmaceutical industry, but none have progressed as far as 1.
Chart 1. Representative Inhibitor and Related Molecules Used for the Study of anti-Plk1 Therapeutics.
As an alternative target for anti-Plk1 drug discovery, PBD has drawn a lot of attention because of its unique structural nature that may hold the promise of overcoming nonspecific cytotoxicities associated with the ATP-competitive KD inhibitors.4,6 Furthermore, since PBD inhibitors are designed to inhibit a subset of Plk1 functions requiring PBD-specific interactions,7,8 they are expected to be less drastic than the KD inhibitors destined to eliminate all Plk1 catalytic activity-dependent events. Notably, blockade of Plk1 PBD-dependent function is shown to be sufficient for imposing mitotic arrest and apoptotic cell death in cancer cells but less in normal cells.8−10 Thus, given that proper Plk1 function is required for the viability of normal cells, mitigating, rather than abolishing, Plk1 function could serve as a safeguarded strategy that helps prevent unwanted killing of normal cells.
A decade of research has yielded several small molecules that are reported to exhibit anti-Plk1 PBD activity in various experimental systems. These include Poloxin,11 Poloxipan,12 MCC1019,13 Polotyrin,14 compound 9,15 and KBJK557.16 However, these compounds commonly exhibit a limited Plk1 PBD specificity and also require a high dose (IC50 of 25–1000 μM) to induce anti-Plk1 effect in cultured cells, thus significantly restricting their potential for future drug discovery.
In an effort to identify novel small molecule scaffolds that exhibit anti-Plk1 PBD activity, we previously employed an ELISA-based inhibition assay with a PBD-binding phosphopeptide 2 and its respective nonphosphopeptide 3(17) as controls, and screened a small chemical library.18 From this approach, we identified compound 4 as a hit that displayed 4.4 μM affinity for Plk1 PBD, but no detectable affinity for two closely related PBDs from Plk2 and Plk3 that are thought to play potential tumor suppressor roles.19−21 An exploration of the structure–activity relationship (SAR) of this series of 1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-ones identified compound 5 as a more potent congener.18 To achieve cellular permeation, which is critical for imposing a high anti-Plk1 activity (e.g., induction of mitotic arrest and an antiproliferation effect in cell-based assays), we converted compound 5 to its S-methyl prodrug 6.18 The S-methyl prodrugs lacked inhibitory activity at Plk1 PBD in an ELISA-based in vitro assay,18 indicating that unmasking the prodrug moiety to liberate a free thiocarbonyl group is needed for interacting with Plk1 PBD within the cell.
In the current study, we have expanded the range of substitutions on the 1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one scaffold, including deuterated and fluorinated N-alkyl congeners and heterocyclic substitutions of the phenyl ring of 5. Furthermore, we have compared in cell viability assays a range of potential prodrug derivatives, in which the thio group is blocked by alkylation, arylation or disulfide formation.
Results
Design Strategy
Based on the initial report by Alverez et al.,18 we explored scaffold changes, functional group substitution, and prodrug masking to enhance the affinity of Plk1 PBD inhibitors in binding to the target protein. We have started with the 1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one scaffold as a lead structure. The reported inhibitors are selective for inhibition of Plk1 PBD but not Plk2 and Plk3 PBDs. Cell efficacy was achieved with S-methylation to make prodrugs that are unmasked intracellularly. However, even for the best S-methyl derivatives, including 6, concentrations above ∼100 μM of these compounds were required to induce mitotic arrest and proliferation in cancer cells in vitro.18 Therefore, we sought to alter the reported structures to increase their inhibitory potency in binding to the PBD, as determined in an ELISA assay, and to increase their cellular anticancer potency, by modifying either the active drug scaffold itself and/or altering the prodrug strategy. The ELISA-based assay that we use as a primary screen measures the disruption of the binding of the Plk1-PBD to a specific phospho-Thr (pT)-containing biotinylated peptide as a surrogate for binding interactions,18 and does not measure the inhibition of PBD functions per se, such as subcellular localization of Plk1.
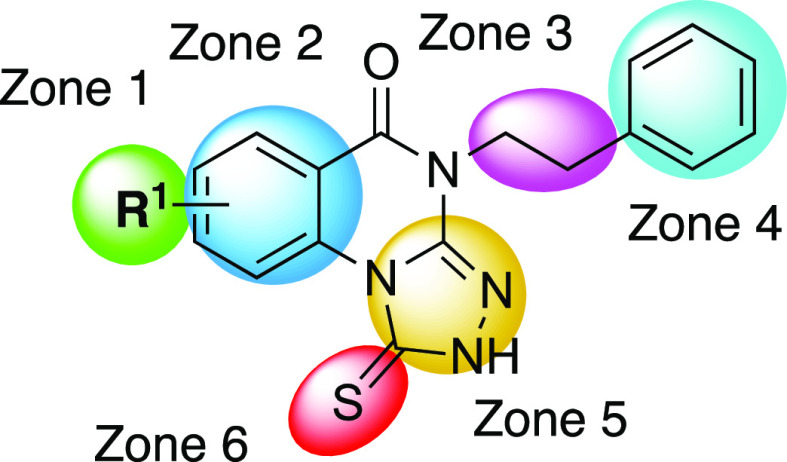
The new set of inhibitors explore the following molecular features of each of the zones shown in the general inhibitor structure (Figure 1). The N-alkyl group on Zone 3, shown previously to allow extensive modification,18 was substituted with fluorine or deuterium atoms intended to improve metabolic stability. Multiple substitutions of the N-propyl group are shown in Table 1, prepared as shown in Scheme 1. Fluoroalkanes can improve metabolic stability compared to the corresponding alkane and in some cases improve potency at a target protein.22 However, such substitution could conversely have detrimental effects on the binding affinity. Thus, we cautiously proceeded with alkylfluoro substitution, informed by measuring the ELISA binding affinity following each modification. Furthermore, the deuterium substitution of alkyl groups can potentially decrease metabolism by the deuterium isotope effect,23,24 without altering the interaction at the target binding site. Consequently, for the deuterated analogues, we expected similar or identical ELISA affinity as the corresponding nondeutero lead compound.
Figure 1.
Zones of modification of the 1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one scaffold, as shown for compound 4. With nonphenyl substitutions of Zone 2, the range of active heterocycles was greatly expanded beyond Alverez et al.18
Table 1. Inhibitory Activity at the Plk1 PBD of Triazoloquinazolinones Analogues 4–29, Which Are Substituted on the Phenyl Ring and N4-Alkyl Group.

IC50 values were determined from at least three independent experiments, except as noted in parentheses.
Data from Alverez et al.18
Oxidized compound has oxo- instead of thioxo-.
Scheme 1. Synthesis of Analogues Containing Substituted N-alkyl Groups.

Reagents and conditions: (a) R2-NH2·HCl, TEA, DMF, 40 °C, 1 h; (b) CS2, 120 °C, 1 h, 7–35%; (c) N2H4, EtOH, 80–130 °C, 4–18 h; (d) (i) pyridine, CS2, 80 °C, 15 h, 9–51%; (ii) CS2, KOH, EtOH, 80 °C, 18 h, 2–70%; (e) R2-NCS, EtOH, 80 °C, 18 h, crude; (f) (i) NaOH, MeOH, rt to 50 °C, 4–18 h, 2–51%; (ii) NaOMe, MeOH, 60 °C, 18 h, 99%; (g) (i) thiophosgene, CHCl3/H2O(2:1), rt, 3 h, crude; (ii) thiophosgene, TEA, THF, rt, 0.25–18 h, crude; (h) (i) R2-NH2, TEA, IPA, reflux, 2 h, 74–82% (ii) R2-NH2, THF, reflux, 18 h, 18–80%.
The substitution with various functional groups of a phenylacetyl amide group introduced on Zones 3/4 was explored (Scheme 2). Previously, amide formation with a N-(3-aminopropyl) substituent in Zone 3 was already shown to enhance binding affinity at the target protein.18 In some cases, ring substitution of this phenylacetyl moiety with identical substitution already reported in Alverez et al.18 was combined with the favorable 9-fluoro substitution of the phenyl moiety in Zone 1, as shown in Table 2. In other cases, new substitution of the Zone 4 2-phenylalkyl ring was introduced. With these modifications, we hoped to detect interactions with the target protein that would be distal to the binding region of the core 1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one scaffold. By introducing distal functionality such as amino, cyano, isothiocyanate and iodo groups on Zones 3/4, we intended to prepare analogues that could potentially be used as chemically reactive affinity labels of the target protein. In general, covalently reactive drugs are valuable for protein characterization and in some cases therapeutic agents.25
Scheme 2. Synthesis of Phenylacetyl Amide Derivatives 32 and 34–38.
Regents and conditions: (a) R3-COOH, coupling reagent (COMU, HATU), DMF, DIPEA, rt, 18 h; (b) TFA, THF, rt, 1 h; (c) thiophosgene, TEA, DCM, rt, 0.5 h.
Table 2. Inhibitory Activity at the Plk1 PBD of 4-Phenylacetic Acid Amide Triazoloquinazolinone Derivatives 30–38 Modified on the Phenyl Ring and Phenylacetyl Group.
IC50 values were determined from at least three independent experiments except as noted in parentheses.
Data from Alverez et al.18
The Zone 2 ring (Figure 1) was substituted with nonphenyl fused rings to try to increase the Plk1 PBD affinity and broaden the chemical diversity of the scaffold. Monoheterocyclic aromatic rings in place of the phenyl ring of Zone 2, e.g., thiophene, furan, pyrrole, etc., and bicyclic aromatic systems, such as benzothiophene and benzofuran, were included (Table 3). A thiophene ring was inserted in two orientations and was either unsubstituted or contained an alkyl, an aryl, or a 5-Cl group. In the pharmaceutical literature, the inclusion of a 5-Cl-thiophene protects can stabilize the heterocycle against in vivo reactivity, as was shown in the approved anticoagulant drug rivaroxaban26 and antithrombotic P2Y12 receptor antagonist elinogrel27 and other experimental drugs containing a 5-chloro-thien-2-yl moiety.28
Table 3. Inhibitory Activity at the Plk1 PBD of 4-Phenylacetic Acid Amide Derivatives 39–54 of Heterocyclic Inhibitors Modified from the Original Lead Series of Triazoloquinazolinone on the Aryl or Heteroaryl Moiety, i.e., the Zone 2 Ring, at the Plk1 PBD (R2 = (CH2)2CH3, unless Noted).


IC50 values were determined from at least three independent experiments, except as noted in parentheses.
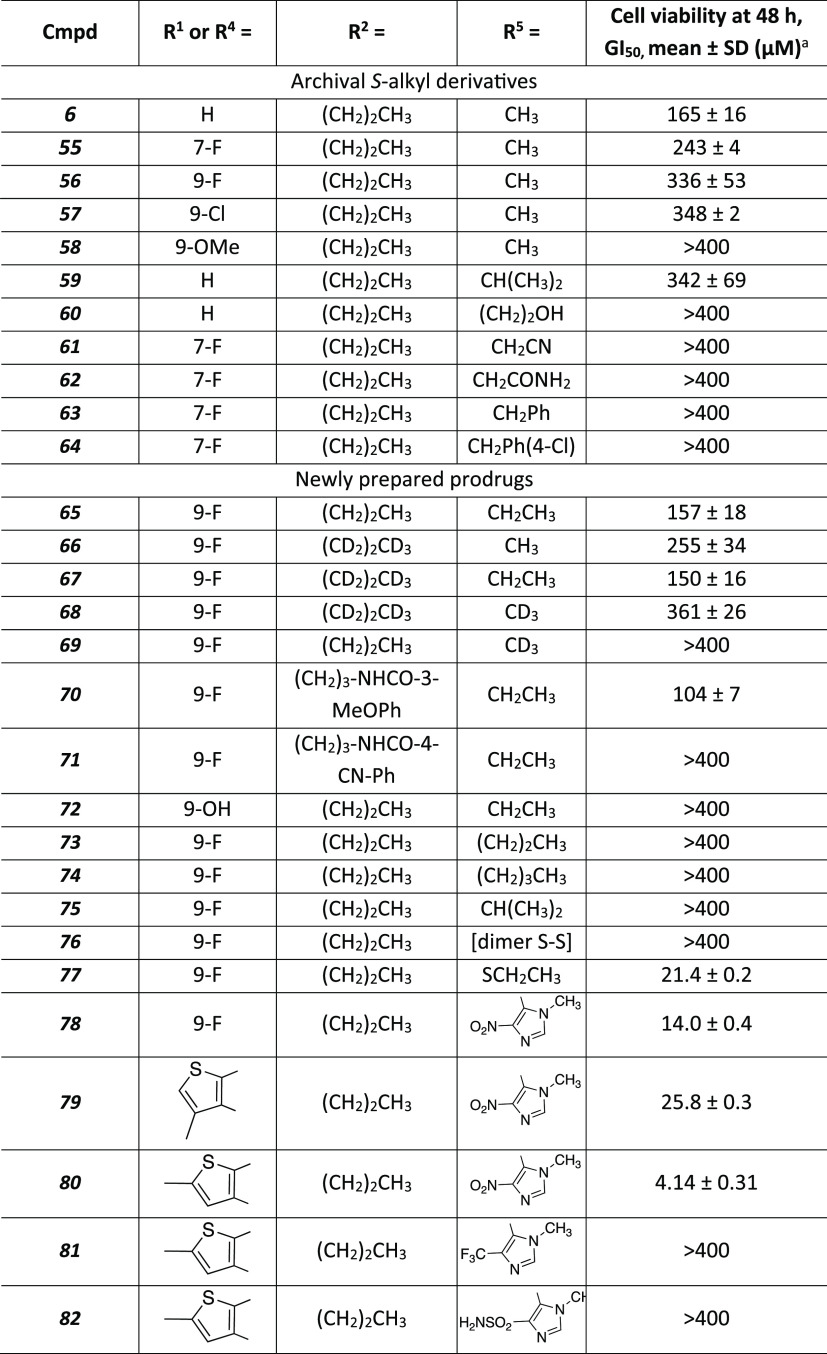
Alverez et al. reported only S-methyl prodrugs as a feasible approach to protected compounds for in vivo unmasking.18 A similar S-acetyl prodrug was shown to be unstable. Here, an alternative prodrug masking of the thiocarbonyl group at Zone 6 was examined, including S-aryl groups, and numerous prodrug variations were tested in HeLa cell-based assays for their efficacy in disruption of the cell cycle. The homologated S-alkyl groups included S-ethyl, S-propyl, S-butyl, S-isopropyl, etc. (Scheme 4). Furthermore, some extended S-alkyl derivatives, previously shown to be ineffective in the ELISA binding assay,18 were tested in cell-based assays of anticancer potency (Table 4). A potential disadvantage of an S-alkyl prodrug is that various S-alkyl compounds have been noted to be oxidized in vivo to S–O derivatives or to liberate a thio group,25,29,30 which itself is susceptible to further oxidation.
Scheme 4. Synthesis of 1-Methyl-4-Substituted Imidazole 5-Thioether Prodrugs.
Regents and conditions: (a) R5-I or R5-Br, K2CO3, DMF, rt, 2 h, 71–91%; (b) NaOH, H2O, rt, 1 h, 34–75%.
Table 4. Cell Activity of Prodrug Derivatives of Triazoloquinazolinones and Other Heterocycles Having Efficacy in Inducing Death of Leukemia Cells.
A colorimetric MTS cell viability assay was carried out using multiple myeloma-derived L363 cells, as described in the Materials and Methods. The concentration inhibiting 50% cell growth (GI50) values were determined from at least three independent experiments.
Since the mechanism of the intracellular unmasking of the thioethers was not identified,18 we sought to first explore the SAR parameters of the S-alkyl group empirically to identify which groups might be subject to unmasking. We found that S-ethyl was more efficient in the cell-based assay compared to S-methyl and to other S-alkyl analogues that were much less efficacious. Another prodrug scheme that was explored is the formation of disulfide derivatives of the thiocarbonyl moiety (Scheme 3). Disulfides are known to be readily cleaved in the intracellular reducing environment.31−33
Scheme 3. Synthesis of Representative S-Alkyl 72 and Disulfide Prodrugs, Either Symmetric 76 or Asymmetric 77, Designed to Penetrate Cancer Cells and Result in Liberation of the Thio-Containing Active Drug.
Regents and conditions: (a) I2, THF, reflux, 4 h; (b) SO2Cl2, THF, 0 °C, 0.75 h, (c) TEA, THF/H2O, rt, 4 h; (d) BBr3, DCM, rt, 2 h; (e) EtI, K2CO3, DMF, rt, 2 h.
We also introduced a known aryl thioether prodrug moiety to the Plk1-PBD inhibitors.34,35 In an early example of a successful prodrug approach, the immunosuppressant antimetabolite drug 6-mercaptopurine was converted to its prodrug, 6-(1-methyl-4-nitro-5-imidazolyl)thiopurine, known as azathioprine, which displayed improved absorption over the parent drug.36 Azathioprine is also used as a dermatological drug.37 The prodrug can react with a sulfhydryl, such as glutathione, or an amino group to regenerate the parent 6-mercaptopurine, along with a 1-methyl-4-nitroimidazole 5-thioether in the case of reaction with glutathione. The same prodrug group was also applied recently to an experimental antiviral drug.38 Furthermore, we prepared analogues with substitutions for the nitro group, including trifluoromethyl and primary sulfonamide, because nitroaromatics are often associated with toxicity. The prodrug synthetic approaches are shown in Scheme 4. However, substitutions with other electron-withdrawing groups in place of 4-nitro prevented the anticancer activity in cell assays (below).
The most favorable combinations of the above-mentioned changes, gleaned in parallel for the active and prodrug derivatives, were combined into a small number of analogues that are to be considered as potential leads for therapeutic development. The in vitro and in vivo pharmacokinetics of these agents were explored (below). Inhibitors of CYP enzymes related to hepatic clearance were compared for their ability to impede the in vivo degradation of key molecules. Among these CYP inhibitors, the most effective at prolonging the in vivo half-life was ketoconazole (Figure S6), suggesting that metabolism is CYP 3A4-dependent.39 Thus, coadministration of ketoconazole was suggested as a means of prolonging the in vivo efficacy of this series of PBD inhibitors.
Chemical Synthesis
The synthesis of the 1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one core and the related heterocycles followed the methods we reported previously and other literature reports.18,40−44 An isatoic anhydride 83–88 reacted with the desired amine upon heating to provide a 2-aminobenzamide, which then reacted with carbon disulfide and base to give a cyclized 2-thioxo-2,3-dihydroquinazolin-4(1H)-one intermediate 89 (Scheme 1). Further reaction with hydrazine at elevated temperature in ethanol provided a hydrazone intermediate, which was either isolated and purified, or used without further purification and carried into the next step of reaction with carbon disulfide in pyridine to provide compounds 11, 13, 15–19, and 23–29. Alternatively, and particularly for the nonphenyl heterocycles in Zone 2 (Routes A and B, Scheme 1), the appropriate 2-aryl carboxylic acid ester 90, was combined with an alkyl isothiocyanate and cyclized in base to form a 2-thioxo-2,3-dihydroquinazolin-4(1H)-one intermediate 92. The same reaction sequence, including the final reaction with hydrazine and carbon disulfide, was adaptable to a wide range of alternative aryl groups containing heteroatoms (S, N, O) in place of the phenyl ring of Zone 2, leading to products 39–51.
Plk1 PBD Inhibition Activity and Binding Specificity
The ELISA-based Plk1 PBD inhibition assay,17 which utilizes the highly specific interaction between the full-length human Plk1 expressed in HEK293A cells and a specific phospho-Thr (pT)-containing peptide (Biotin-Ahx-C-ETFDPPLHSpTAI-NH2),45 was used to determine the Plk1 PBD-binding activity for all the compounds reported here. Representative results for some of selected compounds in Tables 1–3 are provided in Figure 2A. These compounds inhibited PBD with IC50 values of 1.49–2.94 μM under these experimental conditions. When compared to the previously characterized Plk1 PBD-binding control phosphopeptide, PLHSpT 2 (IC50 of 14.74 μM),18 showing a Kd of ∼450 nM,17 the affinity of some of the best inhibitors is expected to be approximately an order of magnitude higher than PLHSpT 2.
Figure 2.
ELISA- and FP-based assays used to determine Plk1 PBD inhibition activity and specificity, respectively. (A) Representative inhibition curves obtained from ELISA assays. For comparative analyses, a previously characterized phosphopeptide, PLHSpT 2 (Kd of ∼450 nM)17 is included as a positive control. (B) Comparative FP-based assays showed that all the compounds tested (including the compounds shown in Figure S1) effectively inhibit the PBD of Plk1 but not Plk2 and Plk3. Under the same experimental conditions, PLHSpT 2, but not its nonphosphorylated form, PLHST 3,17 exhibited Plk1 PBD-specific inhibition. All the data in (B) are quantified from three independent experiments. Bars, mean ± standard deviation. See additional FP assay results provided in Figure S1.
While targeting Plk1 PBD offers a great opportunity to achieve superior binding specificity over targeting the ATP-binding motif because of the nature of specific protein–protein interaction, Plk1 PBD still exhibits a high level of homology (approximately 39%) with the two closely related Plk2 and Plk3 PBDs.4 To determine whether the above compounds exhibit Plk1 PBD-binding specificity, fluorescence polarization (FP)-based assays developed to determine their affinities to each PBD of Plk1–3 were carried out as described previously.9,18 As expected, PLHSpT 2, but not its respective nonphosphorylated peptide 3, specifically inhibited Plk1 PBD with an IC50 of 37.7 μM, a value comparable to that with the ELISA data shown in Figure 2A. Under these experimental settings, all the compounds tested exhibited a high level of specificity against Plk1 PBD, even though their Plk1 PBD-binding affinities were increased approximately 10-fold (Figures 2B and S1). These observations draw contrast to the recently described Plk1 PBD inhibitor, KBJK557, which shows considerable cross-reactivity among Plk1–3 PBDs.16
SAR Analysis
The N-2-phenylethyl derivative 4, was the hit compound discovered in the previous study, and we showed that the affinity with substitution on the Zone 2 phenyl ring (8, 9) varied in the order 9-F > H > 7-F. The N-4-pyridylethyl analogue 10 was slightly more potent than 4, but the introduction of 9-F in 11 lowered the affinity 7-fold. In Alverez et al.,18 we demonstrated that the thio group may not be substituted with oxo, as compound 7 was inactive (Table 1).
The previous study also established that small N-alkyl groups (n-propyl 5, ethyl 12, cyclopropylmethyl 28) have enhanced affinity compared to N-2-phenylethyl. In the 9-fluoro series, the order of affinity for corresponding N-alkyl derivatives was: cyclopropylmethyl 29 ≥ Pr 15 ≥ Et 13. The propyl group was also able to be replaced with a 3-aminopropyl group in 19 and 20 for linking to other moieties, with nearly preserved affinity (i.e., only 2-fold reduction of affinity with a Zone 2 7-F group). Alternatively, this propyl group could be modified with fluoro (21–26) or deutero (27) substitution, mostly in the Zone 2 9-F series, for potentially enhancing the metabolic stability without compromising the affinity. 7-Fluoro substitution of the Zone 2 phenyl ring resulted in slightly higher (propyl 14; 3-aminopropyl 19; 3,3,3-trifluoropropyl 22; cyclopropylmethyl 28) affinity than 9-F in small N-alkyl analogues. Subsequent amide derivatives were made from the 7-F, 9-F and unsubstituted 3-aminopropyl derivatives. The 7-fluoro-N-3,3,3-trifluoropropyl analogue 22 displayed an IC50 of 0.75 μM, but with a 9-F group in 23 the IC50 was 2.26 μM. 9-Hydroxy substitution of the Zone 2 phenyl ring in 52 led to ∼16-fold lower affinity than 9-methoxy 18.
Consistent with Alverez et al.,18 phenylacetic acid amide derivatives of the 3-aminopropyl derivatives retained the affinity (30–38), and a wide range of phenyl substitutions is possible. Substitution of the phenyl ring with m-methoxy (31) or p-cyano (33) groups was previously shown to improve affinity, but substitution of the Zone 2 phenyl ring with 9-F slightly reduced affinity (32, 34). Furthermore, we found that p-iodo 35, p-CO2Me 36 and p-methylene-N-morpholine 37 preserved affinity. An isothiocyanate derivative 38, intended for potential covalent interaction with the target protein, displayed an IC50 of 1.3 μM.
Modification on the aryl or heteroaryl ring of Zone 2 was performed. Previously, the introduction of N (pyridyl) in this ring was found to lower activity considerably.18 However, by changing the ring to a S or N heterocycle, the affinity was able to be maintained. Although a simple furyl substitution in 39 raised the IC50 value 7-fold, the addition of a benzofuryl group in 40 restored the affinity. A 4-fluoro substitution was tolerated in the benzofuryl analogue 41. A thienyl analogue 42 was ∼2-fold less potent than the Zone 2 phenyl analogue 6, and the thienyl group could be substituted with a range of substituents (43–48) or inverted 49, without significant change in affinity (IC50 1.1–3.6 μM). The affinity of compounds 50–54, including two fluorescent conjugates, 53 and 54, ranged from 3.4 to 40 μM. The relatively high affinity of the analogues with a tethered fluorophore indicate that this portion of the molecule is in a location that is not sterically limited and likely accessible to the surrounding medium.
Rat liver microsomal half-life, PAMPA permeability assays, and kinetic aqueous solubility of selected derivatives are shown in Table S1 (Supporting Information). The solubility varied from 2 (33) to >65 (31) μg/mL, the PAMPA values reached 100–300 × 10–6 cm/s range (8, 13, 22, and 31), and microsomal half-life was generally >30 min for these derivatives.
Cellular Efficacy of the Prodrug Derivatives
As we established previously, the active drug species were inactive in cancer cell assays due to low permeability across cell membranes and required a prodrug approach. To evaluate the anticancer potency of the prodrug compounds listed in Table 4, we used multiple myeloma-derived L363 cells optimized for mouse xenograft tumor assays.46,47 These prodrugs are expected to achieve a higher intracellular level of their active species due to improved permeability. To assess their anti-Plk1 PBD activity, the prodrugs were compared for their ability to induce an antiproliferation effect, an expected outcome of Plk1 PBD inhibition regardless of the degrees of cell transformation.8,9 Prodrugs 65, 66, and 67 were modestly better than 56, and compounds 77–80 were clearly more potent than the previously reported methylthio prodrug 56(18) in inhibiting cell proliferation (Table 4).
Initially, the S-methyl group of 55 (7-F, GI50 243 μM) and 56 (9-F, GI50 334 μM) was extended to larger alkyl groups. Among the S-alkyl derivatives, S-ethyl prodrugs (e.g., 65 and 67) displayed higher potency (GI50 150–157 μM) in the L363 cell assay than the corresponding S-methyl derivatives. The 3-methoxyphenylacetyl derivative 70 (9-F, GI50 104 μM), despite a larger MW, displayed enhanced cellular potency, but the related CN derivative 71 was inactive. However, elongation beyond S-ethyl, including S-propyl 73, S-butyl 74 and the branched alkyl (75) derivatives or a variety of other functional groups (60–64) greatly reduced the cell potency or completely abolished it. However, S-isopropyl analogue 59 in the acyclic fluorinated series had only 2-fold lower potency than the corresponding S-methyl reference compound 6. Several disulfide derivatives, e.g., 76, 77, were compared to the S-alkyl derivatives in the whole cell assays. The dimeric 76 (disulfide form of 15) was completely inactive. However, the ethyl disulfide 77 (N-propyl) showed considerable potency (GI50 21.4 μM), i.e., 7-fold more potent than the S-ethyl prodrug 65, although 65 was the N-ethyl homologue rather than N-propyl. Effects of deuterium substitution in 66–69 were probed in the cell assay. The heptadeuterated S-ethyl prodrug 67 was slightly more potent in the cell assay than the corresponding S-methyl derivative 66. Deuteration in 69 of the S-methyl group of 56 reduced cell potency, suggesting a possible deuterium isotope effect in the intracellular demethylation reaction, and the slight reduction with S-CD3 in compound 68 compared to 66 supported the same hypothesis. Moreover, S-alkyl prodrugs (70, 71) that were elongated beyond N-propyl had variable efficacies in cell assays.
Among the imidazole prodrug derivatives, 78 induced a readily discernible antiproliferative effect with a GI50 value (i.e., inhibition of cell growth by 50% of the DMSO control) of 14.0 μM (Figure 3A). 80 (GI50 of 4.1 μM), which contains a monoheterocyclic aromatic ring in place of the phenyl ring in 78, exhibited an antiproliferation activity 3.4-fold improved from analogue 78 (Figure 3A). Under the same conditions, their parental compounds, 15 and 43, respectively, failed to induce a detectable level of cellular response (Figure 3), confirming our previous observation18 that prodrug moieties are critically required to induce cellular effects. The cellular efficacy was variable, even with the same prodrug moiety. For example, 79, bearing the 5-thio-2-methyl-4-nitroimidazolyl group (compared to the 5-thio-2-methyl-4-nitroimidazolyl prodrug 80), exhibited a significantly diminished antiproliferation activity. The prodrugs 81 and 82, which have other electron-withdrawing substitutions (4-CF3 and 4-SO2NH2, respectively) of the nitro group of the imidazole of 80, were not efficacious in the cellular assays (Figure S2). 81 and 82 only weakly induced mitotic block, in contrast to 80, which reproducibly induced a strong mitotic block.
Figure 3.
MTS assays used to determine the effect of two most-active prodrugs (78 and 80) on the proliferation of multiple myeloma-derived L363 cells. (A) The assays were carried out 2 days after treating the cells with different concentrations of the compounds. Parental nonprodrugs, 15 and 43, were included as negative controls. (B) Concentration- and time-dependent proliferation inhibition was observed with 78 and 80 in L363 cells. All the data are quantified from three independent experiments. Bars, mean ± standard deviation. See related MTS assay results shown in Figure S2.
To investigate whether the effect of 78 or 80 on L363 cell viability could be attributable to their capacity to inhibit PBD-dependent Plk1 function, adherent HeLa cells, which are well suited for cytological analyses, were treated with either of these inhibitors at 25 μM concentration, incubated for 12 h (a minimal time period necessary to observe any cell cycle effect), fixed, and stained with 4′,6-diamidino-2-phenylindole (DAPI) to reveal chromosomal DNA morphologies. For comparison, their parental compounds 15 and 43 were included in the analyses. Under these conditions, 80 effectively induced rounded cells with mitotically arrested or apoptotic chromosome morphologies (judging from the DAPI stains) in greater than 15% and 12%, respectively, of the population (Figure 4A and B). Consistent with the less potent inhibition of cell proliferation in Figure 3, 78 induced mitotically arrested or apoptotic cells at a somewhat reduced level (Figure 4A). As expected, parent drugs, 15 and 43, failed to show any of these phenotypes. These observations ensure that the mitotic block induced by 78 and 80 are likely the consequence of inhibiting the mitotic functions of Plk1, as well documented previously.1
Figure 4.
Antiproliferative effect of 78 and 80 in HeLa cells. (A) Asynchronously growing HeLa cells were treated with 25 μM of the indicated compounds, and phase-contrast micrographs were taken 12 h after treatment. Mitotically arrested cells with a rounded morphology are apparent in cells treated with prodrug 78 and 80, but not their respective parental compounds 15 and 43. Arrows, cells with either fragmented or highly condensed apoptotic DNA morphologies. (B) HeLa cells synchronously released from a G1/S block for 7 h prior to inhibitor administration were treated with DMSO or 25 μM of 80 for 6 h and stained with DAPI. The cells exhibiting mitotically arrested, apoptotic, and missegregating (abnormal mitotic) DNA morphologies were quantified from three independent experiments (n ≥ 1040 cells/experiment for control DMSO and n ≥ 830 cells/experiment for 80). Bars, mean ± SD, **P < 0.01, ***P < 0.001 (unpaired two-tailed t-test). (C) HeLa cells treated with control DMSO or 80 (25 μM, 2 h) were immunostained with the indicated antibodies. Anti-Cep63 and anti-CREST staining serve to mark the location of centrosomes and kinetochores, respectively. Asterisks, centrosome signals; brackets, kinetochore signals. Plk1 signal intensities at centrosomes and kinetochores were quantified from a total of 50 cells obtained from three independent experiments (bottom graphs). Bars, mean values. See Figure S3 for related results after treating the cells with 78 or 80 at 25 or 50 μM and Figure S4 and Movie S1 for time-lapse microscopy after treating cells with 100 μM 78 or 40 μM 80.
To examine whether the mitotic arrest observed in Figure 4A could be attributable to inhibiting Plk1 PBD function, we carried out immunostaining analysis to determine the level of intracellular Plk1 localized to centrosomes and kinetochores, an event that requires PBD-mediated protein–protein interaction.4,48 As expected, HeLa cells treated with 25 μM 80 for 2 h exhibited greatly diminished Plk1 signal intensities at both centrosomes and kinetochores (Figure 4C). A short treatment time (i.e., 2 h), which was intended to minimize a potential indirect effect of these inhibitors, was sufficient to delocalize greater than 70% of Plk1 signals from centrosomes (marked by anti-Cep63 signals) and 85% of those from kinetochores (marked by anti-CREST signals) (Figure 4C). Less effective Plk1 delocalization from centrosomes is likely due to the presence of PBD-independent Plk1 localization to this site, as reported previously.49 As a result of Plk1 delocalization, these cells showed a prometa/meta-arrested DNA morphology (Figure 4C), a phenotype that has been observed by expressing a dominant-negative Plk1 PBD.7,50
Analysis of In Vivo Pharmacokinetics
To evaluate in vivo PK parameters, S-methyl 56 and 5-thio-1-methyl-4-nitroimidazolyl 78 prodrugs were administered orally in male C57BL/6 mice via oral gavage at 50 or 20 mg/kg doses, respectively. Both prodrugs contain the 9-fluorophenyl moiety in Zone 2 and correspond to the same active drug, i.e., 15. Due to its low aqueous solubility, thienyl derivative 80 was not tested. None of the thiophene-containing prodrugs were examined in vivo but will be the focus of further study. The mice appeared normal following drug administration. The pharmacokinetic (PK) profiles of prodrug 56 and its metabolites are represented in Figure S5. The area under the curve (AUC) and half-life of 56 are 10,810 mg·h/L and 24 min, respectively, while the AUC of 15, demethylated 56 is 413,000 mg·h/L. Compounds 56 and 15 were hydroxylated at two different positions, and 15 was further glucuronidated (Figure S5). 78 was rapidly metabolized to 15 with large differences in serum concentration between individuals (Figure 5). The serum concentration of 15 derived from 78 showed a rapid increase in blood at 15 min and maintained high concentration up to 4 h. The AUCs of 15 were 523,200 and 317,600 mg·h/L in 50 and 20 mg/kg doses, respectively. Compound 15 was further metabolized by glucuronidation. Unlike the S-ethyl-containing prodrug, 65, whose conversion to the active 15 was sensitive to ketoconazole, a CYP3A4 inhibitor (Figure S6), 78 was also metabolized in a mouse liver microsome-based assay and refractory to ketoconazole treatment (data not shown). Thus, the presence of the 9-F group impeded the hydroxylation of the phenyl ring of Zone 2 (Figure S5), compared to the PK studies in Alverez et al.18
Figure 5.
In vivo PK analyses for prodrug 78. Oral PK profiles of prodrug 78 and its metabolites, parent drug 15 (9-fluoro-N-propyl) and glucuronidated 15 (15-glu) in mice. Red and black lines represent doses of 50 and 20 mg/kg, respectively.
Off-Target Activity
The design of an anticancer drug targeting a specific pathway should also take into consideration potential off-target effects at other sites, which can potentially result in side effects. Selected representative inhibitors (15, 29, 56, 65, 70, and 78) were sent to the NIMH Psychoactive Drug Screening Program (PDSP)51 for binding analysis of off-target interactions at 45 receptors, channels and transporters (Supporting Information). Only a few interactions at micromolar concentrations were detected (Ki, μM, or % inhibition at 10 μM): 15 (sigma2 4.0); 29 (H1 4.9); 56 (none); 65 (BZP 2.4, H1 9.4); 70 (TSPO 2.5, M552%); 78 (sigma1 8.3)). All other possible interactions of the six compounds were <50% inhibition at 10 μM, i.e., inactive. Thus, these compounds are not promiscuous binders, supporting their selectivity for the target PBD site. However, more extensive off-target assays would be required prior to drug development.
Discussion
Although several promising Plk1 ATP-competitive inhibitors, including BI67275 and GSK461364A,52 entered human testing, their lack of specificities and dose-limiting toxicities hampered further clinical applications. The development of a class of Plk1 inhibitor that targets the unique PBD is thought to constitute a new avenue for overcoming the hurdles facing current anti-Plk1 therapy and improving patients’ clinical outcomes. This alternative approach, which aims at disrupting Plk1’s substrate recognition via its PBD, is considered advantageous because of its superb binding specificity and selectivity. Although it is considered less drastic than the ATP analog inhibitors, inhibiting PBD appears to be sufficient to impose a cancer cell-selective killing effect especially in Plk1-addicted cells with an upregulated Plk1 level.8
We previously reported the first generation of an S-methyl triazoloquinazolinone-based small molecule prodrugs, i.e., compound 56 and its congeners, whose parental derivatives (i.e., nonprodrugs) efficiently inhibit Plk1 PBD but not its related Plk2 and Plk3 PBDs.18 In this study, we carried out extensive SAR studies to generate derivatives of 56 that exhibit an increased anti-Plk1 PBD activity with improved drug-like properties. The resulting derivatives, S-alkyl, S-aryl, and disulfide prodrugs (Table 4), were subjected to cell-based assays and compared for their efficacies. Among the S-alkyl derivatives, S-methyl and S-ethyl prodrugs generally displayed the highest potency. Elongation of the S-alkyl chain or introduction of branching or other functional groups greatly reduced the cell potency. Similarly, only a few disulfide derivatives were compared but the potency did not exceed that of the S-alkyl derivatives.
Among the various prodrugs based on the 9-fluoro-N-propyl homologue 15 that we tested, 5-thio-1-methyl-4-nitroimidazolyl derivatives 78 and 80 effectively inhibited PBD-dependent Plk1 localization within 2 h after treatment and potently induced mitotic arrest (Figure 4). The cellular potency of 80 (GI50 of 4.1 μM) was approximately 2 orders of magnitude greater than the corresponding S-methyl prodrug 56). Considering the time necessary for metabolizing the prodrugs into an active species, a short treatment time (i.e., 2 h) required to inhibit Plk1 PBD suggests that 78 and 80 can undergo rapid intracellular conversion to an active form as compound 15. In addition, since binding targets responsible for recruiting Plk1 to centrosomes and kinetochores are different,49 concurrent inhibition of Plk1 localization to both of these structures strongly suggests that these inhibitors can broadly inhibit the intracellular function of Plk1 PBD. Notably, Plk1 acts in concert with other mitotic kinases, such as Cdc2 and Aurora A, to mediate various signaling pathways,53,54 thus making it difficult to delineate the biochemical processes involving PBD-dependent Plk1 function. These inhibitors could serve as an important tool to acutely dissect PBD-dependent biochemical steps and determine their physiological significance in intricately connected pathways.
Thus, the introduction of substituted S-imidazole blocking groups, specifically 5-thio-1-methyl-imidazole containing a nitro group at the 4 position, greatly enhanced the cell potency, as in 80. Since nitro aromatic compounds are associated with some in vivo toxicity,55−57 we attempted to replace the 4-nitro substituent with other electron-withdrawing groups, but compounds 81 and 82 lacked cellular activity. A mechanistic explanation might be that the nitro group stabilizes the attack by thiolate (i.e., a partial positive charge at the C5 position) of glutathione by both inductive and resonance effects, while the electron-withdrawing trifluoromethyl and primary sulfonamide groups in 81 and 82 would stabilize mainly by induction.
Albeit recent progress in generating small molecule scaffolds targeting Plk1 PBD, promising leads exhibiting cellular efficacy at a low micromolar range are yet to be developed. This could be largely owing to the challenging nature of developing protein–protein interaction inhibitors that need to be designed against often extended and nondescript interfaces of binding regions.4 Here we report several 5-thio-1-methyl-4-nitroimidazolyl inhibitors that exhibit a significantly improved cellular potency (a GI50 value as low as 4.1 μM) with an excellent Plk1 PBD specificity. As far as we are aware, they represent a new class of PBD inhibitors that offers the potential to serve as a template for anti-Plk1 drug discovery.
One of the multiple chemical characteristics that sets them apart from other previously reported Plk1 PBD inhibitors, which require a substantially higher dose (IC50 of 25–1000 μM) to exert anti-Plk1 effect,11−16 is the presence of prodrug moieties (the 5-thio-1-methyl-4-nitroimidazolyl moiety in 78 and 80 and the S-alkyl moieties in 56 and 65). Our SAR studies showed that these moieties are critically required to induce anti-Plk1 activity in cell-based assays, effectively inhibiting PBD-dependent Plk1 localization and consequently impeding cell proliferation (Figures 3 and 4). In addition, the two prodrugs, 56 and 78, are rapidly converted to the active species, 15 (Figures 5 and S5), in mouse PK analyses. The drugs were administered orally at 20 and 50 mg/kg doses, and the serum content of both the parent drug and the oxidized and glucuronidated metabolites were traced over time. Compound 15 appears to be stable in the bloodstream, with only very minor hydroxylation. These findings underscore the importance of the prodrug moieties in overcoming the physicochemical barriers that limit the delivery of an active drug species to the site of action. Notably, both 5-thio-1-methyl-4-nitroimidazolyl prodrugs 78 and 80 containing a 9-fluorophenyl or 2-methylthienyl moiety in Zone 2, respectively, were substantially more efficacious than the S-methyl prodrug 56 (Table 4). This observation hints that the physicochemical properties of the prodrug moiety could play a key role in not only bolstering membrane permeability but also effectively liberating an active species from a prodrug. Prodrugs have been widely used to improve the pharmacokinetic performance of active pharmaceutical agents and the number of US FDA-approved prodrugs has been increasing rapidly because of various benefits (e.g., solubility, metabolic stability, prolonged PK, etc.) that they promise to bring about.58 In this regard, given the magnitude of the achievement that we have made in this study, additional efforts to diversify/optimize the prodrug moieties, along with the active drug scaffolds, could lead to novel Plk1 PBD inhibitors that could be practically applicable to various preclinical/clinical studies.
In conclusion, we have broadened the range of heterocyclic scaffolds that serve as selective Plk1 PBD inhibitors, notably 1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one derivatives such as 42–46. Prodrug masking of the thiol on the triazole ring of the active drugs was still required for cell entry. A 5-thio-1-methyl-4-nitroimidazolyl moiety, based on the precedent of orally active anti-inflammatory drug azathioprine,34−37 greatly enhanced the anticancer activity of the prodrugs in cell culture in comparison to the previously reported S-methyl group,18 or to other S-alkyl groups as shown here. A potency of 4.1 μM was achieved with the 9-fluorophenyl prodrug derivative 80. In vivo PK studies of representative prodrugs 56 and 78 indicated that the prodrug is orally absorbed but rapidly unmasked in circulation to produce the same active drug 15, and therefore the current derivatives are not suitable as systemic anticancer drugs. Nevertheless, the active drug that was liberated systemically displayed increased stability toward aromatic hydroxylation and subsequent glucuronidation due to 9-F substitution of the phenyl ring of Zone 2. The in vivo effects of substitution with a thienyl ring remain to be explored. Curiously, to achieve efficacy in cancer cells, the 5-thio-1-methyl-4-nitroimidazolyl could not be substituted with electron-withdrawing groups at the 4 position other than the problematic nitro group. Thus, we have provided new structural features that greatly improve the mechanism-based anticancer potency of this chemical series in cell systems, but additional modification will be needed in order to achieve systemic stability of the prodrug derivatives.
Materials and Methods
Chemical Synthesis
Reagents and Instrumentation
All reactions were carried out under nitrogen atmosphere using anhydrous solvents. All moisture sensitive reactions were also performed with oven-dried glassware. Chemical reagents and anhydrous solvents were obtained from commercial sources and were used without further purification. Room temperature or rt refers to 25 ± 2 °C. Preparative purification was performed on a Waters semipreparative HPLC. The column used was a Phenomenex Luna C18 (5 μm, 30 × 75 mm) at a flow rate of 45 mL/min. The mobile phase consisted of acetonitrile and water (each containing 0.1% trifluoroacetic acid). A gradient of 10% to 50% acetonitrile over 8 min was used during the purification. Fraction collection was triggered by UV detection (220 nm). For some compounds, the crude product was purified on a Teledyne ISCO (Lincoln, NE) CombiFlash System following dry-loading. Initial analytical analysis during compound synthesis was performed on an Agilent 1200 LC-MS (Agilent Technologies) using a 3 min gradient of 4% to 100% acetonitrile (containing 0.025% trifluoroacetic acid) in water (containing 0.05% trifluoroacetic acid) was used with an 8 min run time at a flow rate of 1 mL/min. The purity of compounds newly synthesized was demonstrated on an Agilent 1200 LC-MS (Agilent Technologies) using a 7 min linear gradient of 4% to 100% acetonitrile (containing 0.025% trifluoroacetic acid) in water (containing 0.05% trifluoroacetic acid) followed by a 4.5 min run time at a flow rate of 1 mL/min and a Phenomenex Luna C18 column (3 μm, 3 × 75 mm) at 50 °C. The purity of purchased compounds was determined using an Agilent ZORBAX Eclipse XDB C18 column (5 mm, 4.6 × 250 mm) with a linear gradient of 5% to 95% acetonitrile in water (containing 10 mM triethylammonium acetate) for 20 min at a flow rate of 1.0 mL/min. 1H and 13C NMR spectra were recorded on either a Varian 400 (100) MHz spectrometer or a Bruker 400 MHz spectrometer. Chemical shifts are given in ppm (δ), calibrated to the residual solvent signals and frequency calibrated internally by solvent for 19F NMR (BrukerTopspin/MestReNova 10.0.2 or 14.1.0). High-resolution mass spectrometry was recorded on either an Agilent 6210 Time-of-Flight LCMS system or a Waters Micromass spectrometer equipped with standard electrospray ionization (ESI) and modular LockSprayTM interface. The purity of all the tested compounds (including both newly synthesized and purchased, active compounds) were demonstrated to be >95% pure at 254 nm.
General Synthetic Procedures
General Procedure A
The amine salt (1.5 mmol) in DMF
(1 mL) was treated with triethylamine (1.5 mmol) with stirring. After
the filtration, the amine as free base was added to desired isatoic
anhydride (1.0 mmol). The reaction mixture was heated at 40 °C
for 45 min. The mixture was cooled to room temperature, and carbon
disulfide (7.0 mmol, kept at 4 °C until addition) was added.
The reaction mixture was heated to 120 °C for 1 h, followed by
cooling to room temperature and quenching with water. The reaction
mixture was extracted three times with ethyl acetate, and the organic
layer was dried with MgSO4, filtered, and concentrated.
The product was isolated from the reaction mixture by column chromatography.
General Procedure B
Compound 89 (1.0 mmol)
was dissolved in ethanol or 1,4-dioxane (3 mL) and the hydrazine anhydrous
(7.0 mmol) was added. The reaction mixture was heated to 80 °C
for 4–18 h. The reaction mixture was cooled to room temperature,
and the volume was reduced under a stream of nitrogen. Pyridine (10.0
mmol) and carbon disulfide (10.0 mmol) were added, and the reaction
mixture was heated to 80 °C for 15 h. The reaction mixture was
concentrated, and the product was purified by column chromatography.
General Procedure C
To the active drug (1.0 mmol) dissolved
in DMF (5 mL) was added potassium carbonate (1.2 mmol) and the desired
alkyl iodide (1.2 mmol). The reaction mixture was stirred at room
temperature for 2 h. After the completion, the reaction mixture was
quenched with water and extracted with ethyl acetate, and the organic
layer was dried with MgSO4 and filtered. The filtrate was
concentrated. The product was purified by column chromatography.
General Procedure D
Compound 90 (1.0 mmol)
dissolved in ethanol or DMF (2 mL) was treated with propyl isothiocyanate
(3.0 mmol) and heated to 80 °C for 18 h. The reaction mixture
was diluted with water and extracted with ethyl acetate, and the organic
layer was dried with MgSO4 and filtered. The filtrate was
concentrated. The crude product (intermediate B) was used for the
next reaction without further purification.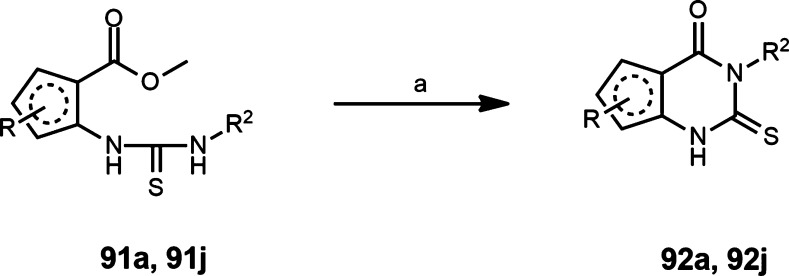
General Procedure E
Compound 91 (1.0 mmol)
was dissolved in methanol (5 mL) and sodium hydroxide (3.0 mmol) was
added as solid. The reaction mixture was stirred at room temperature
or 50 °C for 4–18 h. After the completion, the mixture
was treated dropwise with 1 N HCl to pH 1 and extracted with ethyl
acetate, and the organic layer was dried with MgSO4 and
filtered. The filtrate was concentrated. The residue was stirred under
diethyl ether and filtered to get compound 92 as a solid.
General Procedure F
To a stirred solution of 20% NaOMe
in MeOH (15 mL), compound 91k (1.0 mmol) was added. The
reaction mixture was refluxed overnight. After the completion, the
reaction mixture was cooled and poured into water, which was extracted
with ethyl acetate, and the organic layer dried was with MgSO4 and filtered. The filtrate was concentrated. The residue
was stirred under diethyl ether, and the solid that formed was separated
by filtration to get compound 92k.
General Procedure G (40)
Compound 90 (1.0 mmol) was added to a stirred mixture of chloroform
(1.0 mL) and water (0.5 mL). A solution of thiophosgene (1.1 mmol)
in chloroform (0.5 mL) was added dropwise. The reaction mixture was
stirred at room temperature for 3 h. A solution of potassium carbonate
(2.5 mmol) in water (0.5 mL) was added. The reaction mixture was diluted
with water and extracted with ethyl acetate, dried with MgSO4, and filtered. The filtrate was concentrated. The reaction mixture
(compound 93) was used for the next reaction without further purification.
General Procedure H59
A
solution of compound 90 (1.0 mmol) in THF (5 mL) was
treated with triethylamine (3.0 mmol) and cooled to 0 °C in an
ice bath and followed by thiophosgene (1.1 mmol). The reaction mixture
was slowly warmed to room temperature and stirred for 15 min to overnight.
Water was added to quench the reaction, and the mixture was extracted
with ether. The organic layer was dried with MgSO4 and
filtered, and the filtrate was concentrated. The reaction mixture
(compound 93) was used for the next reaction without
further purification.
General Procedure I
Compound 93 (1.0 mmol)
in 2-propanol (0.3 mL) was treated with propylamine (1.1 mmol) and
triethylamine (1.1 mmol). The reaction mixture was refluxed for 2
h and cooled to room temperature. Acetic acid was added until pH 4–5,
and the mixture was stirred for 15 min. The precipitate was filtered
and washed with water. The crystal was recrystallized from 2-propanol.
General Procedure J
To compound 93 (1.0
mmol) in THF (1.5 mL) was added the appropriate alkylamine (1.1 mmol),
and the mixture was refluxed for 18 h. After cooling and dilution
with water, the mixture was extracted with ethyl acetate, the organic
layer was dried with MgSO4 and filtered, and the filtrate
was concentrated. The residue was purified by column chromatography.
General Procedure K
Compound 92 (1.0 mmol)
was dissolved in ethanol (3.0 mL), and anhydrous hydrazine (7.0 mmol)
was added. The reaction mixture was heated to 80 °C for 18 h.
The reaction mixture was cooled to room temperature, and the volume
was reduced under a stream of nitrogen. Ethanol (3.0 mL), potassium
hydroxide (3.0 mmol), and carbon disulfide (3.0 mmol) were added,
and the reaction mixture was heated to 80 °C for 18 h. The reaction
mixture was cooled and treated dropwise with 1 N HCl to pH 1 with
vigorous stirring, and the mixture extracted with ethyl acetate. The
organic layer was dried with MgSO4 and filtered. The filtrate
was concentrated. The product was isolated from the reaction mixture
by column chromatography.
General Procedure L
A mixture of the active drug (1.0
mmol), water (3.2 mL) and sodium hydroxide (1.1 mmol) was stirred
until a solution formed. The corresponding-1-methyl-4-nitroimidazole
(1.0 mmol) was added to the reaction mixture, and the mixture was
stirred for 3 h at room temperature. After completion, the suspension
was neutralized with acetic acid and a solid precipitated. The precipitate
was isolated by filtration and the solid recrystallized from ethanol.
The product was purified by column chromatography.
General Procedure M
To a solution of COMU (1.5 mmol) in DMF (2.5 mL) was added desired acetic acid (1.5 mmol), and the reaction was stirred at room temperature for 30 min. A solution of 19 or 20 (1.0 mmol, Alverez et al.18) in DMF (2.5 mL) was added followed by DIPEA (2.2 mmol). The reaction mixture was stirred overnight at room temperature. The reaction mixture was concentrated, and the product was purified by RP-ISCO (H2O/MeCN + 0.1% TFA, 10–100%).
9-Fluoro-4-(2-(pyridin-4-yl)ethyl)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 11
8-Fluoro-3-(2-(pyridin-4-yl)ethyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89a was synthesized according to General Procedure A using 3-fluoro isatoic anhydride 85 as the starting material and 2-(pyridin-4-yl)ethanamine as the corresponding amine. Compound 89a was converted to 9-fluoro-4-(2-(pyridin-4-yl)ethyl)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 11 according to General Procedure B and purified by RP-ISCO (H2O/MeCN + 0.1% TFA, 10–100%). 1H NMR (400 MHz, DMSO-d6): δ 13.01 (s, 1H), 8.76–8.66 (m, 2H), 7.85–7.74 (m, 3H), 7.67 (ddd, J = 1.3, 8.1, 10.8 Hz, 1H), 7.34 (td, J = 4.6, 8.1 Hz, 1H), 4.76–4.68 (m, 2H), 3.27–3.19 (m, 2H); MS (ES-API) m/z: 301.9 [M + H].
4-Ethyl-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 13
3-Ethyl-8-fluoro-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89b was synthesized according to General Procedure A using 3-fluoro isatoic anhydride 85 as the starting material and ethylamine as the corresponding amine. Compound 89b was converted to 4-ethyl-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 13 according to General Procedure B and purified by ISCO (EtOAc/hexanes, 0–100%). 1H NMR (400 MHz, DMSO-d6): δ 13.86 (s, 1H), 8.03 (ddd, J = 0.6, 1.5, 7.8 Hz, 1H), 7.78 (ddd, J = 1.5, 8.3, 11.4 Hz, 1H), 7.63 (td, J = 4.2, 7.9 Hz, 1H), 4.02 (q, J = 7.1 Hz, 2H), 1.25 (t, J = 7.1 Hz, 3H); MS (ES-API) m/z: 264.9 [M + H].
7-Methoxy-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 17
6-Methoxy-3-propyl-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89e was synthesized according to General Procedure A using 5-methoxy isatoic anhydride 84 as the starting material and propylamine as the corresponding amine. Compound 89e was converted to 7-methoxy-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 17 according to General Procedure B and purified by RP-ISCO (H2O/MeCN + 0.1% TFA, 10–100%) followed by ISCO (EtOAc/hexanes, 0–100%). MS (ES-API) m/z: 290.9 [M + H].
9-Fluoro-1-thioxo-4-(3,3,3-trifluoropropyl)-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 23
8-Fluoro-2-thioxo-3-(3,3,3-trifluoropropyl)-2,3-dihydroquinazolin-4(1H)-one 89h was synthesized according to General Procedure A using 3-fluoro isatoic anhydride as the starting material and 3,3,3-trifluoropropyl amine hydrochloride as the corresponding amine. Compound 89h was converted to 9-fluoro-1-thioxo-4-(3,3,3-trifluoropropyl)-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 23 according to General Procedure B (51% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.93 (s, 1H), 8.06 (d, J = 7.2 Hz, 1H), 7.78–7.85 (m, 1H), 7.62–7.69 (td, J = 4.0, 7.9 Hz, 1H), 4.22 (t, J = 7.0 Hz, 2H), 1.89 (m, 2H); HRMS m/z (M + H) for C12H8F4N4OS calculated 333.0433, found, 333.0436.
4-(2,2-Difluoropropyl)-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 24
3-(2,2-Difluoropropyl)-8-fluoro-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89i was synthesized according to General Procedure A using 3-fluoro isatoic anhydride as the starting material and 2,2-difluoropropan-1-amine hydrochloride as the corresponding amine. Compound 89i was converted to 4-(2,2-difluoropropyl)-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one according to General Procedure B (9% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.91 (s, 1H), 8.04 (dd, J = 0.8, 7.8 Hz, 1H), 7.80–7.85 (m, 1H), 7.64–7.67 (m, 1H), 4.46–4.53 (t, J = 13.4 Hz, 2H), 1.65–1.75 (t, J = 19.3 Hz, 3H); HRMS m/z (M + H) for C12H9F3N4OS calculated 315.0527, found 315.0523.
4-(3,3-Difluoropropyl)-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 25
3-(3,3-Difluoropropyl)-8-fluoro-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89j was synthesized according to General Procedure A using 3-fluoro isatoic anhydride as the starting material and 3,3-difluoropropan-1-amine hydrochloride as the corresponding amine. Compound 89j was converted to 4-(3,3-difluoropropyl)-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 25 according to General Procedure B (22% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.91 (s, 1H), 8.04 (dd, J = 0.8, 7.8 Hz, 1H), 7.80–7.85 (m, 1H), 7.64–7.68 (m, 1H), 4.46–4.58 (t, J = 13.4 Hz, 2H), 1.65–1.75 (t, J = 19.3 Hz, 3H); HRMS m/z (M + H) for C12H9F3N4OS calculated 315.0527, found 315.0531.
9-Fluoro-4-(2,2,3,3,3-pentafluoropropyl)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 26
8-Fluoro-3-(2,2,3,3,3-pentafluoropropyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89k was synthesized according to General Procedure A using 3-fluoro isatoic anhydride as the starting material and 2,2,3,3,3-pentafluoropropan-1-amine hydrochloride as the corresponding amine. Compound 89k was converted to 9-fluoro-4-(2,2,3,3,3-pentafluoropropyl)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 26 according to General Procedure B (9% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.02 (s, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.84–7.90 (m, 1H), 7.65–7.70 (td, J = 4.0, 7.9 Hz, 1H), 4.83–4.91 (t, J = 15.2 Hz, 2H); 19F NMR (376 MHz, DMSO-d6): δ −83.55, −93.46, −119.03; HRMS m/z (M + H) for C12H6F6N4OS calculated 369.0245, found 369.0252.
9-Fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 27
8-Fluoro-3-(propyl-d7)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89l was synthesized according to General Procedure A using 3-fluoro isatoic anhydride as the starting material and propyl-d7-amine as the corresponding amine. Compound 89l was converted to 9-fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 27 according to General Procedure B (27% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.86 (s, 1H), 8.03 (dd, J = 0.8, 7.8 Hz, 1H), 7.77–7.82 (m, 1H), 7.60–7.65 (td, J = 4.2, 7.9 Hz, 1H); 19F NMR (376 MHz, DMSO-d6): δ −93.47; HRMS m/z (M + H) for C12H4D7FN4OS calculated 286.1155, found, 286.1159.
4-(Cyclopropylmethyl)-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 29
3-(Cyclopropylmethyl)-8-fluoro-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89n was synthesized according to General Procedure A using 3-fluoro isatoic anhydride as the starting material and cyclopropanemethylamine. Compound 89n was converted to 4-(cyclopropylmethyl)-9-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 29 according to General Procedure B and purified by ISCO (EtOAc/hexanes, 0–100%); 1H NMR (400 MHz, DMSO-d6) δ 13.87 (s, 1H), 8.03 (d, J = 7.8 Hz, 1H), 7.83–7.73 (m, 1H), 7.62 (td, J = 8.1, 4.1 Hz, 1H), 3.87 (d, J = 7.1 Hz, 2H), 1.26 (dd, J = 9.3, 4.5 Hz, 1H), 0.51–0.34 (m, 4H); 19F NMR (376 MHz, DMSO-d6) δ −93.22 (dd, J = 11.5, 4.1 Hz); MS (ES-API) m/z: 291.0 [M + H].
N-(3-(9-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(3-methoxyphenyl)acetamide, 32
N-(3-(9-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(3-methoxyphenyl)acetamide 32 was synthesized according to General Procedure M using 4-(3-aminopropyl)-9-fluoro-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 20 as the starting material and 2-(3-methoxyphenyl)acetic acid as the corresponding acetic acid. 1H NMR (400 MHz, DMSO-d6) δ 13.85 (s, 1H), 8.07–7.99 (m, 2H), 7.78 (ddd, J = 1.5, 8.3, 11.5 Hz, 1H), 7.67–7.58 (m, 1H), 7.23–7.15 (m, 1H), 6.84–6.74 (m, 3H), 3.99 (dd, J = 6.4 Hz, 2H), 3.72 (s, 3H), 3.34 (s, 2H), 3.12 (q, J = 6.7 Hz, 2H), 1.85 (m, 2H); MS (ES-API) m/z: 441.8 [M + H].
2-(4-Cyanophenyl)-N-(3-(9-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)acetamide, 34
2-(4-Cyanophenyl)-N-(3-(9-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)acetamide 34 was synthesized according to General Procedure M using 4-(3-aminopropyl)-9-fluoro-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 20 as the starting material and 2-(4-cyanophenyl)acetic acid as the corresponding acetic acid. 1H NMR (400 MHz, DMSO-d6) δ 13.87 (s, 1H), 8.18 (t, J = 5.6 Hz, 1H), 8.03 (ddd, J = 0.6, 1.5, 7.8 Hz, 1H), 7.83–7.73 (m, 3H), 7.63 (ddd, J = 4.2, 7.7, 8.2 Hz, 1H), 7.52–7.41 (m, 2H), 4.02–3.94 (m, 2H), 3.50 (s, 2H), 3.13 (q, J = 6.7 Hz, 2H), 1.85 (m, 2H); MS (ES-API) m/z: 436.8 [M + H].
N-(3-(7-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(4-iodophenyl)acetamide, 35
N-(3-(7-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(4-iodophenyl)acetamide 35 was synthesized according to General Procedure M using 4-(3-aminopropyl)-7-fluoro-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 19 as the starting material and 2-(4-iodophenyl)acetic acid as the corresponding acetic acid. 1H NMR (400 MHz, DMSO-d6) δ 10.31 (dd, J = 4.7, 9.4 Hz, 1H), 8.08 (t, J = 5.7 Hz, 1H), 7.93 (dd, J = 3.1, 8.6 Hz, 1H), 7.82 (ddd, J = 3.1, 8.0, 9.3 Hz, 1H), 7.69–7.61 (m, 2H), 7.13–7.03 (m, 2H), 4.04 (dd, J = 6.4, 8.3 Hz, 2H), 3.35 (s, 2H), 3.14 (m, 2H), 1.85 (m, 2H).
Methyl 4-(2-((3-(9-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)amino)-2-oxoethyl)benzoate, 36
Methyl 4-(2-((3-(9-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)amino)-2-oxoethyl)benzoate 36 was synthesized according to General Procedure M using 4-(3-aminopropyl)-9-fluoro-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 20 as the starting material and 2-(4-(methoxycarbonyl)phenyl)acetic acid as the corresponding acetic acid. 1H NMR (400 MHz, DMSO-d6) δ 13.85 (s, 1H), 8.13 (t, J = 5.7 Hz, 1H), 8.01 (dd, J = 1.5, 7.8 Hz, 1H), 7.87 (d, J = 8.1 Hz, 2H), 7.77 (ddd, J = 1.5, 8.3, 11.4 Hz, 1H), 7.61 (td, J = 4.1, 8.0 Hz, 1H), 7.37 (d, J = 8.0 Hz, 2H), 4.69 (s, 4H), 3.97 (t, J = 7.3 Hz, 2H), 3.82 (s, 2H), 3.17–3.07 (m, 2H), 1.83 (p, J = 7.1 Hz, 2H); 19F NMR (376 MHz, DMSO-d6) δ −93.40 (dd, J = 4.2, 11.5 Hz); MS (ES-API) m/z: 407.1 [M + H].
N-(3-(9-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(4-(morpholinomethyl)phenyl)acetamide, 37
N-(3-(9-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(4-(morpholinomethyl)phenyl)acetamide 37 was synthesized according to General Procedure M using 4-(3-aminopropyl)-9-fluoro-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 20 as the starting material and 2-(4-(morpholinomethyl)phenyl)acetic acid as the corresponding acetic acid, followed by RP-ISCO (H2O/MeCN + 0.1% NH4OH, 10–100%); 1H NMR (400 MHz, DMSO-d6) δ 13.84 (s, 1H), 8.06–7.97 (m, 2H), 7.77 (dd, J = 11.4, 8.2 Hz, 1H), 7.61 (td, J = 8.1, 4.1 Hz, 1H), 7.23–7.14 (m, 4H), 3.96 (t, J = 7.3 Hz, 2H), 3.53 (t, J = 4.7 Hz, 4H), 3.40 (s, 2H), 3.33 (s, 2H), 3.10 (q, J = 6.6 Hz, 2H), 2.31 (s, 4H), 1.83 (p, J = 7.2 Hz, 2H); 19F NMR (376 MHz, DMSO-d6) δ −93.40 (dd, J = 11.4, 4.2 Hz); MS (ES-API) m/z: 511.3 [M + H].
N-(3-(7-Fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(4-isothiocyanatophenyl)acetamide, 38
To a solution of 2-(4-aminophenyl)-N-(3-(7-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)acetamide (1.0 mmol) and triethylamine (3.0 mmol) in DCM (10 mL) at 0 °C was added thiophosgene (1.2 mmol) dropwise. The reaction was then stirred at 0 °C for 30 min then stirred at room temperature for 30 min. The reaction was filtered to remove the solid and concentrated. MS (ES-API) m/z: 469.1 [M + H].
4-Propyl-1-thioxo-2,4-dihydrofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 39
Methyl 3-(3-propylthioureido)furan-2-carboxylate 91a was synthesized according to General Procedure D using methyl 3-aminofuran-2-carboxylate 90a as the starting material. Compound 91a was converted to 3-propyl-2-thioxo-2,3-dihydrofuro[3,2-d]pyrimidin-4(1H)-one 92a according to General Procedure E. Compound 92a was converted to 4-propyl-1-thioxo-2,4-dihydrofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 39 according to General Procedure K (5% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.09 (s, 1H), 8.90 (d, J = 2.0 Hz, 1H), 8.31 (d, J = 1.9 Hz, 1H), 3.95 (t, J = 7.4 Hz, 2H), 1.73 (m, 2H), 0.92 (t, J = 7.5 Hz, 3H); HRMS m/z (M + H) for C10H10N4O2S calculated 251.0603, found 251.0607.
4-Propyl-1-thioxo-2,4-dihydrobenzofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 40
Ethyl 3-isothiocyanatobenzofuran-2-carboxylate 93b was synthesized according to General Procedure G using ethyl 3-amino-1-benzofuran-2-carboxylate 90b as the starting material. Compound 93b was converted to 3-propyl-2-thioxo-2,3-dihydrobenzofuro[3,2-d]pyrimidin-4(1H)-one 92b according to General Procedure I. Compound 92b was converted to 4-propyl-1-thioxo-2,4-dihydrobenzofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 40 according to General Procedure K (3% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.89 (s, 1H), 8.21 (d, J = 7.9 Hz, 1H), 7.82 (d, J = 8.5 Hz, 1H), 7.70 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 4.38 (t, J = 7.6 Hz, 2H), 1.73 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C14H12N4O2S calculated 301.0759, found 301.0764.
10-Fluoro-4-propyl-1-thioxo-2,4-dihydrobenzofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 41
Ethyl 4-fluoro-3-isothiocyanatobenzofuran-2-carboxylate 93c was synthesized according to General Procedure G using 3-amino-4-fluoro-benzofuran-2-carboxylic acid methyl ester 90c as the starting material. Compound 93c was converted to 9-fluoro-3-propyl-2-thioxo-2,3-dihydrobenzofuro[3,2-d]pyrimidin-4(1H)-one 92c according to General Procedure I. Compound 92c was converted to 10-fluoro-4-propyl-1-thioxo-2,4-dihydrobenzofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 41 according to General Procedure K (4% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.99 (s, 1H), 7.77 (d, J = 7.8 Hz, 1H), 7.72 (td, J = 4.5, 8.0 Hz, 1H), 7.34 (t, J = 9.5 Hz, 1H), 4.00 (t, J = 7.3 Hz, 2H), 1.74 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C14H11FN4O2S calculated 319.0665, found 319.0669.
4-Propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 42
Methyl 3-isothiocyanatothiophene-2-carboxylate 93d was synthesized according to General Procedure H using methyl 3-aminothiophene-2-carboxylate 93d as the starting material. Compound 93d was converted to 3-propyl-2-thioxo-2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-one 92d according to General Procedure J. Compound 92d was converted to 4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 42 according to General Procedure K (2% yield) 1H NMR (400 MHz, DMSO-d6): δ 14.06 (s, 1H), 8.67 (s, 1H), 3.96 (t, J = 7.0 Hz, 2H), 2.67 (s, 3H), 1.71 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C10H10N4OS2 calculated 267.0374, found 267.0369.
7-Methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 43
Methyl 3-isothiocyanato-5-methylthiophene-2-carboxylate 93e was synthesized according to General Procedure H using methyl 3-amino-5-methylthiophene-2-carboxylate 90e as the starting material. Compound 93e was converted to 6-methyl-3-propyl-2-thioxo-2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-one 92e according to General Procedure J. Compound 92e was converted to 7-methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 43 according to General Procedure K (66% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.09 (s, 1H), 8.90 (d, J = 5.5 Hz, 1H), 8.31 (d, J = 5.3 Hz, 1H), 3.98 (t, J = 7.3 Hz, 2H), 1.73 (m, 2H), 0.92 (t, J = 7.3 Hz, 3H); HRMS m/z (M + H) for C11H12N4OS2 calculated 281.0531, found 281.0531.
8-Methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 44
Methyl 3-isothiocyanato-4-methylthiophene-2-carboxylate 93f was synthesized according to General Procedure H using methyl 3-amino-4-methylthiophene-2-carboxylate 90f as the starting material. Compound 93f was converted to 7-methyl-3-propyl-2-thioxo-2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-one 92f according to General Procedure J. Compound 92f was converted to 8-methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 44 according to General Procedure K (27% yield). 1H NMR (400 MHz, DMSO-d6): δ 13.91 (s, 1H), 7.89 (s, 1H), 3.96 (t, J = 1.8 Hz, 2H), 2.98 (s, 3H), 1.70 (m, 2H), 0.90 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C11H12N4OS2 calculated 281.0531, found 281.0528.
7-Chloro-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 45
Methyl 5-chloro-3-isothiocyanatothiophene-2-carboxylate 93g was synthesized according to General Procedure H using methyl 3-amino-5-chlorothiophene-2-carboxylate 90g as the starting material. Compound 93g was converted to 6-chloro-3-propyl-2-thioxo-2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-one 92g according to General Procedure J. Compound 92g was converted to 7-chloro-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 45 according to General Procedure K (5% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.16 (s, 1H), 8.89 (s, 1H), 3.97 (t, J = 7.3 Hz, 2H), 1.71 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C10H9ClN4OS2 calculated 300.9985, found 300.9982.
7-(tert-Butyl)-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 46
Methyl 5-(tert-butyl)-3-isothiocyanatothiophene-2-carboxylate 93h was synthesized according to General Procedure H using methyl 3-amino-5-tert-butylthiophene-2-carboxylate 90h as the starting material. Compound 93h was converted to 6-(tert-butyl)-3-propyl-2-thioxo-2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-one 92h according to General Procedure J. Compound 92h was converted to 7-(tert-butyl)-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 46 according to General Procedure K (70% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.05 (s, 1H), 8.78 (s, 1H), 3.97 (t, J = 7.2 Hz, 2H), 1.71 (m, 2H), 1.43 (s, 9H), 0.91 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C14H18N4OS2 calculated 323.1000, found 323.1003.
7-Phenyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 47
Methyl 3-isothiocyanato-5-phenylthiophene-2-carboxylate 93i was synthesized according to General Procedure H using methyl 3-amino-5-phenylthiophene-2-carboxylate 90i. Compound 93i was converted to 6-phenyl-3-propyl-2-thioxo-2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-one 92i according to General Procedure J. Compound 92i was converted to 7-phenyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 47 according to General Procedure K. (5% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.12 (s, 1H), 9.21 (s, 1H), 7.80 (m, 2H), 7.51–7.58 (m, 3H), 3.99 (t, J = 6.7 Hz, 2H), 1.73 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C16H14N4OS2 calculated 343.0687, found 343.0683.
4-Propyl-1-thioxo-2,4-dihydrobenzo[4,5]thieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 48
Methyl 3-(3-propylthioureido)benzo[b]thiophene-2-carboxylate 91j was synthesized according to General Procedure D using methyl 3-amino-1-benzothiophene-2-carboxylate 90j as the starting material. Compound 91j was converted to 3-propyl-2-thioxo-2,3-dihydrobenzo[4,5]thieno[3,2-d]pyrimidin-4(1H)-one,92j according to General Procedure E under the condition of 50 °C. Compound 91j was converted to 4-propyl-1-thioxo-2,4-dihydrobenzo[4,5]thieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 48 according to General Procedure K (21% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.14 (s, 1H), 10.66 (s, 1H), 8.17 (d, J = 8.3 Hz, 1H), 7.62 (t, J = 7.2 Hz, 1H),), 4.02 (t, J = 7.0 Hz, 2H), 1.75 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); HRMS m/z (M + H) for C14H12N4OS2 calculated 317.0531, found 317.0534.
5-Propyl-8-thioxo-7,8-dihydrothieno[3,2-e][1,2,4]triazolo[4,3-a]pyrimidin-4(5H)-one, 49
Methyl 2-(3-propylthioureido)thiophene-3-carboxylate 91k was synthesized according to General Procedure D using DMF as the solvent and methyl 2-aminothiophene-3-carboxylate 90k as the starting material. Compound 91k was converted to 3-propyl-2-thioxo-2,3-dihydrothieno[2,3-d]pyrimidin-4(1H)-one 92k according to General Procedure F. Compound 92k was converted to 5-propyl-8-thioxo-7,8-dihydrothieno[3,2-e][1,2,4]triazolo[4,3-a]pyrimidin-4(5H)-one 49 according to General Procedure K (20% yield). 1H NMR (400 MHz, DMSO-d6): δ 14.16 (s, 1H), 7.52 (d, J = 5.5 Hz, 1H), 7.45 (t, J = 5.5 Hz, 1H), 3.97 (t, J = 7.0 Hz, 2H), 1.72 (m, 2H), 0.92 (t, J = 7.5 Hz, 3H); HRMS m/z (M + H) for C10H10N4OS2 calculated 267.0374, found 267.0373.
4-Propyl-1-thioxo-2,4-dihydro-1H-pyrrolo[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(6H)-one, 50
Methyl 3-isothiocyanato-1H-pyrrole-2-carboxylate 93l was synthesized according to General Procedure H using methyl 3-amino-1H-pyrrole-2-carboxylate 90l as the starting material. Compound 93l was converted to 3-propyl-2-thioxo-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one 92l according to General Procedure J. Compound 92l was converted to 4-propyl-1-thioxo-2,4-dihydro-1H-pyrrolo[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(6H)-one 50 according to General Procedure K (71% yield). 1H NMR (400 MHz, DMSO-d6): δ 12.79 (s, 1H), 12.21 (s, 1H), 7.30 (s, 1H), 5.95 (s, 1H), 4.33 (t, J = 6.7 Hz, 2H), 1.66 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H).
8-Fluoro-4-propyl-1-thioxo-2,4-dihydrobenzofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one, 51
Methyl 6-fluoro-3-isothiocyanatobenzofuran-2-carboxylate, 93m was synthesized according to General Procedure G using
3-Amino-6-fluoro-benzofuran-2-carboxylic acid methyl ester 90m as the starting material. Compound 93m was converted
to 7-fluoro-3-propyl-2-thioxo-2,3-dihydrobenzofuro[3,2-d]pyrimidin-4(1H)-one carboxylate 92m according to General Procedure I (64% yield). Then, 8-fluoro-4-propyl-1-thioxo-2,4-dihydrobenzofuro[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 51 was synthesized according to General
Procedure K (13% yield). 1H NMR (400 MHz, DMSO-d6): δ 10.10 (bs, 1H), 7.84 (d, J = 7.3 Hz, 1H), 7.36–7.40 (m, 1H), 6.51 (s, 1H),
4.00 (t, J = 6.7 Hz, 2H), 1.71 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 19F NMR (376 MHz, DMSO-d6):
δ 109.75.
9-Hydroxy-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 52
8-Methoxy-3-propyl-2-thioxo-2,3-dihydroquinazolin-4(1H)-one 89f was synthesized according to General Procedure A. Compound 89f was converted to 9-methoxy-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 18 according to General Procedure B. To a solution of 9-methoxy-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 18 (1.0 mmol) in DCM (20 mL) at 0 °C was added BBr3 (1.1 mmol). The reaction mixture was warmed to room temperature over 2 h. The reaction mixture was quenched, and the mixture was gradually warmed to room temperature over 2 h. Following an extraction workup with EtOAc, the organic layer was separated and dried (MgSO4), and the product was purified by ISCO (EtOAc/hexanes, 0–100%); 1H NMR (400 MHz, DMSO-d6): δ 11.33 (s, 1H), 7.79 (dd, J = 7.7, 1.6 Hz, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.34 (dd, J = 8.1, 1.6 Hz, 1H), 4.00–3.92 (m, 2H), 1.76–1.63 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H); MS (ES-API) m/z: 277.0 [M + H].
7-Fluoro-4-(3-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)amino)propyl)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 53
In a 5 mL round-bottom flask, 4-(3-aminopropyl)-7-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 19 (1.2 mmol), TEA (5.0 mmol), and NBD-F (1.0 mmol) were dissolved in anhydrous DMF, and the resulting mixture was stirred at room temperature for 2 h. The reaction mixture was then purified by RP-HPLC to afford the pure product.60 The product was obtained as a dark orange solid after lyophilization (2.89 mg, 60%, RP-HPLC acetonitrile/10 mM triethylammonium acetate, 50/50 to 100/00 in 40 min @5 mL/min); 1H NMR (400 MHz, DMSO-d6): δ 10.42 (s, 1H), 9.53 (s, 1H), 8.46 (d, J = 8 Hz, 1H), 7.71–7.77 (m, 2H), 6.35 (d, J = 9 Hz, 1H), 4.21 (m, 2H), 3.28 (m, 2H), 2.18 (t, J = 7.3 Hz, 2H). HRMS m/z (M + H) for C18H13FN8O4S calculated 457.0843, found 457.0843.
2-(6-Amino-3-iminio-4,5-disulfo-3H-xanthen-9-yl)-5-((3-(7-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)carbamoyl)benzoate, 54
In a 1 mL round-bottom flask, 4-(3-aminopropyl)-7-fluoro-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 19 (1.2 mmol), TEA (1.0 mmol), and Alexa Fluor 488 NHS ester (1.0 mmol) were dissolved in anhydrous DMF, and the resulting mixture was stirred at room temperature on. The reaction mixture was then purified by RP-HPLC to afford the pure product (59). The product was obtained as a dark orange solid after lyophilization (1.80 mg, 95%, RP-HPLC acetonitrile/10 mM triethylammonium acetate, 15/85 to 35/65 in 40 min @5 mL/min); 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 10.45 (s, 1H), 8.41 (s, 1H), 8.20 (d, J = 7.8 Hz, 1H), 7.73–7.90 (m, 3H), 7.32 (d, J = 7.8 Hz, 1H), 6.83 (bs, 2H), 6.43 (d, J = 8.6 Hz, 2H), 6.32 (d, J = 8.6 Hz, 2H), 4.16 (m, 2H), 3.43 (m, 1H), 2.86 (m, 1H), 2.02- 2.08 (m, 2H). HRMS m/z (M + H) for C33H24FN7O11S3 calculated 810.0748, found 810.0758.
4-Ethyl-1-(ethylthio)-9-fluoro-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 65
1-(Ethylthio)-9-fluoro-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 65 was synthesized according to General Procedure C using 9-fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 as the starting material and iodoethane as alkyl halide (86% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.10 (d, J = 6.4 Hz, 1H),7.86 (m, 1H), 7.63 (td, J = 4.5, 8.0 Hz, 1H), 4.14 (t, J = 7.4 Hz, 2H), 3.24 (q, J = 7.3 Hz, 2H), 1.77 (m, 2H), 1.35 (t, J = 7.2 Hz, 3H) 0.94 (t, J = 7.49 Hz, 3H); 19F NMR (376 MHz, DMSO-d6): δ – 112.06 (dd, J = 4.4, 12.8 Hz); HRMS m/z (M + H) for C14H15FN4OS calculated 307.1029, found 307.1025.
9-Fluoro-1-(methylthio)-4-(propyl-d7)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 66
9-Fluoro-1-(methylthio)-4-(propyl-d7)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 66 was synthesized according to General Procedure C using 9-fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 27 as the starting material and iodomethane as the alkyl halide (57% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 7.1 Hz, 1H),7.88 (m, 1H), 7.63 (td, J = 4.6, 8.2 Hz, 1H), 2.68 (s, 3H); 19F NMR (376 MHz, DMSO-d6): δ – 113.07 (dd, J = 4.2, 13.3 Hz); HRMS m/z (M + H) for C13H6D7FN4OS calculated 300.1312, found 300.1307.
1-(Ethylthio)-9-fluoro-4-(propyl-d7)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 67
1-(Ethylthio)-9-fluoro-4-(propyl-d7)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 67 was synthesized according to General Procedure C using 9-fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 27 as the starting material and iodoethane as the alkyl halide (91% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.10 (d, J = 7.8 Hz, 1H),7.87 (m, 1H), 7.63 (td, J = 4.6, 8.2 Hz, 1H), 3.24 (q, J = 7.2 Hz), 1.34 (t, J = 7.3 Hz, 3H); HRMS m/z (M + H) for C14H8D7FN4OS calculated 314.1468, found 314.1467.
9-Fluoro-1-((methyl-d3)thio)-4-(propyl-d7)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 68
9-Fluoro-1-((methyl-d3)thio)-4-(propyl-d7)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 68 was synthesized according to General Procedure C using 9-fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 27 as the starting material and iodomethane-d3 as the alkyl halide (59% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 6.9 Hz, 1H), 7.87 (m, 1H), 7.63 (td, J = 4.6, 8.0 Hz, 1H); 19F NMR (376 MHz, DMSO-d6): δ – 113.01 (dd, J = 4.8, 13.1 Hz); HRMS m/z (M + H) for C13H3D10FN4OS calculated 303.1500, found 303.1497.
9-Fluoro-1-((methyl-d3)thio)-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 69
9-Fluoro-1-((methyl-d3)thio)-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 69 was synthesized according to General Procedure C using 9-fluoro-4-(propyl-d7)-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 as the starting material and iodomethane as the alkyl halide (71% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 7.2 Hz, 1H), 7.87 (m, 1H), 7.63 (td, J = 4.5, 8.0 Hz, 1H), 4.14 (t, J = 7.3 Hz, 2H), 1.77 (m, 2H), 0.94 (t, J = 7.4 Hz, 3H); 19F NMR (376 MHz, DMSO-d6): δ – 113.01 (dd, J = 4.9, 13.2 Hz); HRMS m/z (M + H) for C13H10D3FN4OS calculated 296.1061, found 296.1058.
N-(3-(1-(Ethylthio)-9-fluoro-5-oxo-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(3-methoxyphenyl)acetamide, 70
N-(3-(1-(Ethylthio)-9-fluoro-5-oxo-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(3-methoxyphenyl)acetamide 70 was synthesized according to General procedure C using N-(3-(9-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)-2-(3-methoxyphenyl)acetamide 32 as the starting material and iodoethane as the alkyl halide and purified by RP-ISCO (H2O/MeCN + 0.1% TFA, 10–100%); 1H NMR (400 MHz, DMSO-d6) δ 8.07 (d, J = 7.8 Hz, 1H), 8.02 (t, J = 6.1 Hz, 1H), 7.84 (ddd, J = 12.8, 8.3, 1.5 Hz, 1H), 7.61 (td, J = 8.1, 4.5 Hz, 1H), 7.17 (t, J = 8.0 Hz, 1H), 6.84–6.78 (m, 2H), 6.75 (dd, J = 7.9, 2.6 Hz, 1H), 4.17 (t, J = 7.2 Hz, 2H), 3.71 (s, 3H), 3.22 (q, J = 7.3 Hz, 2H), 3.13 (q, J = 6.7 Hz, 2H), 1.88 (p, J = 7.0 Hz, 2H), 1.32 (t, J = 7.3 Hz, 3H); 19F NMR (376 MHz, DMSO-d6) δ −112.66 (dd, J = 12.6, 4.4 Hz).
2-(4-Cyanophenyl)-N-(3-(1-(ethylthio)-9-fluoro-5-oxo-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)acetamide, 71
2-(4-Cyanophenyl)-N-(3-(1-(ethylthio)-9-fluoro-5-oxo-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)acetamide 71 was synthesized according to General procedure C using
2-(4-cyanophenyl)-N-(3-(9-fluoro-5-oxo-1-thioxo-1,2-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-4(5H)-yl)propyl)acetamide 34 as
the starting material and iodoethane as the alkyl halide and purified
by RP-ISCO (H2O/MeCN + 0.1% TFA, 10–100%); 1H NMR (400 MHz, DMSO-d6) δ
8.19 (d, J = 5.3 Hz, 1H), 8.08 (d, J = 7.8 Hz, 1H), 7.84 (dd, J = 12.7, 8.2 Hz, 1H),
7.75 (d, J = 8.2 Hz, 2H), 7.61 (td, J = 8.1, 4.5 Hz, 1H), 7.46 (d, J = 8.0 Hz, 2H), 4.17
(t, J = 7.3 Hz, 2H), 3.50 (s, 2H), 3.28–3.09
(m, 4H), 1.89 (q, J = 7.1 Hz, 2H), 1.33 (t, J = 7.3 Hz, 3H); 19F NMR (376 MHz, DMSO-d6) δ −112.62 – −112.73
(m). MS (ES-API) m/z: 465.3 [M +
H].
1-(Ethylthio)-9-hydroxy-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 72
1-(Ethylthio)-9-hydroxy-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 72 was synthesized according to General Procedure C using 9-hydroxy-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 52 as the starting material and iodoethane as the alkyl halide. The reaction mixture was quenched with methanol and concentrated. The product was then purified by RP-ISCO (C18, H2O/MeCN+ 0.1% NH4OH, 10–100%); 1H NMR (400 MHz, DMSO-d6) δ 11.06 (s, 1H), 7.80 (dd, J = 7.7, 1.6 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.36 (dd, J = 8.1, 1.6 Hz, 1H), 4.26 (q, J = 7.2 Hz, 2H), 4.01–3.93 (m, 2H), 1.71 (h, J = 7.5 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H), 0.90 (t, J = 7.4 Hz, 3H); MS (ES-API) m/z: 305.1 [M + H].
9-Fluoro-4-propyl-1-(propylthio)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 73
9-Fluoro-4-propyl-1-(propylthio)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 73 was synthesized according to General Procedure C using 9-fluoro-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 as the starting material and 1-iodopropane as the alkyl halide (87% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 8.2 Hz, 1H), 7.86 (m, 1H), 7.64 (td, J = 4.6, 8.0 Hz, 1H), 4.14 (t, J = 7.0 Hz, 2H), 3.21 (t, J = 6.9 Hz, 2H), 1.68–1.80 (m, 4H), 0.92–1.00 (m, 6H); 19F NMR (376 MHz, DMSO-d6): δ – 112.60 (dd, J = 4.4, 12.8 Hz); HRMS m/z (M + H) for C15H17FN4OS calculated 321.1185, found 321.1184.
1-(Butylthio)-9-fluoro-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 74
1-(Butylthio)-9-fluoro-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 74 was synthesized according to General Procedure C using 9-fluoro-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 as the starting material and 1-iodobutane as the alkyl halide (92% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 7.9 Hz, 1H), 7.86 (m, 1H), 7.64 (td, J = 4.8, 8.3 Hz, 1H), 4.14 (t, J = 6.9 Hz, 2H), 3.23 (t, J = 7.1 Hz, 2H), 1.75 (m, 2H), 1.69 (m, 2H), 1.38 (m, 2H), 0.88–0.95 (m, 6H); HRMS m/z (M + H) for C16H19FN4OS calculated 335.1342, found 335.1344.
9-Fluoro-1-(isopropylthio)-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 75
9-Fluoro-1-(isopropylthio)-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 75 was
synthesized according to General Procedure C using 9-fluoro-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 as
the starting material and 2-iodopropane as alkyl halide (78% yield). 1H NMR (400 MHz, DMSO-d6): δ
8.10 (d, J = 7.8 Hz, 1H), 7.86 (m, 1H), 7.63 (td, J = 4.6, 8.0 Hz, 1H), 4.15 (t, J = 7.2
Hz, 2H), 3.87 (m, 1H), 1.76 (m, 2H), 1.36 (d, J =
6.7 Hz, 2H), 0.93 (t, J = 7.4 Hz, 3H); 19F NMR (376 MHz, DMSO-d6): δ –
111.88 (d, J = 12.9 Hz); HRMS m/z (M + H) for C15H17FN4OS calculated
321.1186, found 321.1185.
1,1′-Disulfanediylbis(9-fluoro-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one), 76
To a solution of 9-fluoro-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 (1.0
mmol) in THF (100 mL) was added iodine (0.1 mmol), the reaction heated
to 65 °C for 5 h, and the volume was reduced. The residue was
then purified by ISCO (EtOAc/hexanes, 0–100%) followed by a
wash with 0.01 M Na2S2O5, then repurified
by ISCO (EtOAc/hexanes 0–100%); 1H NMR (400 MHz,
DMSO-d6): δ 7.99 (d, J = 7.8 Hz, 2H), 7.74–7.64 (m, 2H), 7.58 (td, J = 4.3, 8.0 Hz, 2H), 4.08 (t, J = 7.4 Hz, 4H), 1.71
(h, J = 7.5 Hz, 4H), 0.91 (t, J =
7.4 Hz, 7H); 19F NMR (376 MHz, DMSO-d6) δ −107.82 (dd, J = 12.4, 4.3
Hz); MS (ES-API) m/z: 555.1 [M +
H].
1-(Ethyldisulfaneyl)-9-fluoro-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one, 77
To a solution of 1,2-diethyldisulfane 96 (1.0 mmol) in THF (10 mL) at 0 °C was added sulfuryl chloride (1.1 mmol) dropwise. The reaction mixture was stirred at 0 °C for 30 min and used directly in the next reaction.
To a solution of 9-fluoro-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one, 15 (1.0 mmol), triethylamine (2.2 mmol), and triethylamine (2.2 mmol) in THF (5 mL) was added a solution of ethanesulfenyl chloride (1.0 mmol). The reaction mixture was stirred at room temperature for 1–6 h. The reaction mixture was diluted with DCM and washed with sat. NaHCO3 then water. The organic layer was dried over MgSO4, filtered, and concentrated. The product was purified by RP-ISCO (C18, H2O/MeCN + 0.1% NH4OH, 10–100%); 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 7.9 Hz, 1H), 7.94–7.84 (m, 1H), 7.71–7.61 (m, 1H), 4.15 (dd, J = 8.3, 6.5 Hz, 2H), 2.93 (q, J = 7.3 Hz, 2H), 1.83–1.69 (m, 2H), 1.29 (td, J = 7.3, 1.5 Hz, 3H), 0.92 (td, J = 7.4, 1.5 Hz, 3H); 19F NMR (376 MHz, DMSO-d6) δ −110.08 (dd, J = 12.5, 4.5 Hz); MS (ES-API) m/z: 339.1 [M + H].
9-Fluoro-1-((1-methyl-4-nitro-1H-imidazol-5-yl)thio)-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 78
9-Fluoro-1-((1-methyl-4-nitro-1H-imidazol-5-yl)thio)-4-propyl-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one 78 was synthesized according to General Procedure L using 9-fluoro-4-propyl-1-thioxo-2,4-dihydro-[1,2,4]triazolo[4,3-a]quinazolin-5(1H)-one 15 as the starting material and 5-chloro-1-methyl-4-nitro-1H-imidazole as the corresponding imidazole (isolated as a beige solid, 45% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.18 (s, 1H), 8.16 (d, J = 9.7 Hz, 1H), 7.99 (m, 1H), 7.71 (m, 1H), 4.11 (t, J = 6.7 Hz, 2H), 3.74 (s, 3H), 1.73 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H). HRMS m/z (M + H) for C16H14FN7O3S calculated 404.0941, found 404.0941.
8-Methyl-1-((1-methyl-4-nitro-1H-imidazol-5-yl)thio)-4-propylthieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one, 79
8-Methyl-1-((1-methyl-4-nitro-1H-imidazol-5-yl)thio)-4-propylthieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one 79 was synthesized according to General Procedure L using 8-methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 44 as the starting material and 5-chloro-1-methyl-4-nitro-1H-imidazole as the corresponding imidazole (isolated as a beige solid, 40% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.13 (s, 1H), 8.03 (S, 1H), 4.11 (t, J = 6.7 Hz, 2H), 3.83 (s, 3H), 2.90 (s, 3H) 1.71 (m, 2H), 0.87 (t, J = 7.3 Hz, 3H). 19F NMR (376 MHz, DMSO-d6): δ – 115.03 (dd, J = 4.8, 13.1 Hz). HRMS m/z (M + H) for C15H15N7O3S2 calculated 406.0756, found 406.0753.
7-Methyl-1-((1-methyl-4-nitro-1H-imidazol-5-yl)thio)-4-propylthieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one, 80
7-Methyl-1-((1-methyl-4-nitro-1H-imidazol-5-yl)thio)-4-propylthieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one 80 was synthesized according to General Procedure L using 7-methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 43 as the starting material and 5-chloro-1-methyl-4-nitro-1H-imidazole as the corresponding imidazole (isolated as a white solid, 75% yield). 1H NMR (400 MHz, DMSO-d6): δ 6 8.12 (s, 1H), 7.95 (s, 1H), 4.11 (t, J = 7.3 Hz, 2H), 3.83 (s, 3H), 2.62 (s, 3H), 1.70- 1.76 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H). HRMS m/z (M + H) for C15H15N7O3S2 calculated 406.0756, found 406.0753.
7-Methyl-1-((1-methyl-4-(trifluoromethyl)-1H-imidazol-5-yl)thio)-4-propylthieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one, 81
7-Methyl-1-((1-methyl-4-(trifluoromethyl)-1H-imidazol-5-yl)thio)-4-propylthieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one 81 was synthesized according to General Procedure L using 7-methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 43 as the starting material and 5-chloro-1-methyl-4-(trifluoromethyl)-1H-imidazole as the corresponding imidazole (isolated as a white solid, 52% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.67 (s, 1H), 6.78 (s, 1H), 3.95 (t, J = 7.3 Hz, 2H), 3.29 (s, 3H), 2.66 (s, 3H), 1.71 (t, J = 7.3 Hz, 2H), 0.90 (t, J = 7.3 Hz, 3H). 19F NMR (376 MHz, DMSO-d6): δ: −60.17.
1-Methyl-5-((7-methyl-5-oxo-4-propyl-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-1-yl)thio)-1H-imidazole-4-sulfonamide, 82
1-Methyl-5-((7-methyl-5-oxo-4-propyl-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-1-yl)thio)-1H-imidazole-4-sulfonamide 82 was synthesized according to General Procedure using 7-methyl-4-propyl-1-thioxo-2,4-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one 43 as the starting material and 5-chloro-1-methyl-1H-imidazole-4-sulfonamide as the corresponding imidazole (isolated as a white solid, 34% yield). 1H NMR (400 MHz, DMSO-d6) δ: 8.66 (s, 1H), 6.77 (s, 1H), 3.95 (t, J = 7.3 Hz, 2H), 3.32 (s, 3H), 2.66 (s, 3H), 1.69 (t, J = 7.3 Hz, 2H), 0.90 (t, J = 7.3 Hz, 3H).
Biological Assays
ELISA-Based PBD-Binding Inhibition Assay
The assay was performed essentially as described previously,17 using a highly specific Biotin-Ahx-C-ETFDPPLHS-pT-AI-NH2) peptide45 and the full-length human Plk1 expressed in HEK293A cells. Data are reported as IC50 to indicate the concentration producing 50% inhibition of peptide binding. The reaction products were measured at 450 nm by using a PerkinElmer Enspire Multimode Plate reader (PerkinElmer, Inc., Boston, MA). Data obtained from more than three independent experiments were analyzed by GraphPad (San Diego, CA) Prism software version 7.
PBD Fluorescence Polarization (FP) Binding Assays for Plk1 Specificity
FP assays were carried out essentially as described previously.9,18 Samples were analyzed approximately 30 min after mixing all components in a 384-well format using the Molecular Devices (San Jose, CA) SpectraMax Paradigm multimode microplate detection platform. All experiments were performed in triplicate for each sample. Obtained data were plotted using GraphPad Prism software version 7.
Cell-Based Assays
HeLa cells and L363 cells were cultured as described previously.18,61 To carry out cell proliferation inhibition assays, HeLa cells synchronously released from double-thymidine block (G1/S) for 7 h were treated with control DMSO or compound 80 for an additional 6 h to examine the capacity of 80 to induce mitotic defects. The resulting cells were immunostained, imaged using a Zeiss LSM 780 confocal microscope, and quantified from three-independent experiments, as described previously.18
MTS Assay
A tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS) was used for the cell viability assay. It was carried out using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) kit (Promega, WI). Briefly, asynchronously growing L363 cells cultured in a 96-well plate were treated with the indicated compounds for 48 h. The cells in 100 μL of culture medium were then treated with 20 μL of the MTS solution and incubated at 37 °C for 1–4 h in a humidified 5% CO2 atmosphere, and the absorbance was measured at 490 nm using a PerkinElmer EnSpire Multimode 96-well plate reader.
Metabolic Study
Animals
Male C57BL/6 mice from Charles River Laboratories (Wilmington, MA) were housed in the National Cancer Institute animal facility that is a pathogen-free environment controlled for temperature, light (25 °C, 12 h light/dark cycle) and humidity (45–65%) with free access to food and water. The National Cancer Institute Animal Care and Use Committee approved all animal experiments conducted in this study (LM-098).
In Vivo Mouse Pharmacokinetics Study
Male C57Bl/6 mice (6–8 weeks old) were selected for PK study of 56 and 78 (Figures 5 and S5). The compounds 56 and 78 were dissolved in 5% DMSO plus 95% corn oil (Sigma) and 5% DMSO plus 95% of 20% Labrasol (a polyethyleneglycerol derivative) (Sigma), respectively. Mice were divided into 4 groups of 3 each and orally gavaged with 100 mg/kg of 56 and 20 or 50 mg/kg of 78. Blood samples were collected by retro-orbital bleeding at 0, 0.25, 0.5, 1, 2, 4, 24, and 48 h. Serum was obtained by centrifugation for 10 min, at 14,000g and prepared by mixing 10 μL of serum with 40 μL of 90% acetonitrile including 4 μM newly synthesized reference compound 4 as internal standard. After centrifugation at 14,000g for 20 min, 30 μL of supernatant was diluted with 30 μL of 10% acetonitrile, and a 5 μL aliquot was injected into Waters UPLC-QTOFMS system (Waters Corporation, Milford, MA).
In Vitro MLM Assay
Incubations of the mouse liver microsome (MLM) samples were performed in duplicate. Reactions (200 μL final volume) were conducted in 50 mM potassium phosphate buffer, 3 mM MgCl2 (pH 7.4), 1 mg/mL MLM protein, and 2.5 μM test compound with or without 50 μM inhibitor. The mixture was preincubated at 37 °C for 3 min and initiated with 4 mM NADPH. At the serial time of the incubation, 20 μL of the mixture was added to 80 μL of ice-cold 90% acetonitrile including 4 μM 4 followed by a 25 min incubation on ice and centrifugation at 14,000g for 10 min. Then 40 μL of supernatant was diluted with 20 μL of water, and a 5 μL aliquot was injected into the UPLC-QTOF system.
UHPLC-ESI-QTOFMS and UHPLC-ESI-TQMS Analysis
Metabolite profiling and identification were performed on an Acquity UPLC/Synapt G2S Q-TOF MS system (Waters Corp., Milford, MA). Separation was achieved on an Acquity C18 BEH column (1.7 mm, 2.1 × 50 mm; Waters Corp.). The mobile phase consisted of water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B). The initial condition of 5% B was held for 0.5 min, with the following linear gradient employed: 60% B at 4 min, 95% B at 8 min, to 99% B at 8.1 min, held for 0.9 min for column flushing, then returning to initial conditions for 0.9 min for column equilibration before the next injection. The flow rate of the mobile phase was set 0.4 mL/min. Column temperature was maintained at 50 °C throughout the run. Data were collected in positive ion mode, which was operated in full-scan mode at 50–950 m/z. Nitrogen was used as both cone gas (151 L/h) and desolvation gas (950 L/h). Source temperature and desolvation temperature were set at 150 and 500 °C, respectively. The capillary voltage and cone voltage were 2.82 kV and 41 V, respectively. Q-TOF MS was operated in positive ionization mode with MSE data collection.
Acknowledgments
We thank Stewart R. Durell (NCI) for structural assistance and Juan Marugan (NCATS) for helpful advice. This research was supported by the Intramural Research Program of the National Institutes of Health NCI FLEX (K.S.L.), NIDDK ZIADK31117 (K.A.J.), and NCI ZIA BC005562 (F.J.G.). We thank Dr. Bryan L. Roth (Univ. North Carolina at Chapel Hill) and National Institute of Mental Health’s Psychoactive Drug Screening Program (Contract #HHSN-271-2008-00025-C) for screening data.
Glossary
Abbreviations
- COMU
(1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
- CYP
cytochrome P450
- DAPI
4′,6-diamidino-2-phenylindole
- DCM
dichloromethane
- DIPEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- FDA
Food and Drug Administration
- FP
fluorescence polarization
- HPLC
high-performance liquid chromatography
- HRMS
high-resolution mass spectrometry
- KD
kinase domain
- LCMS
liquid chromatography–mass spectrometry
- MLM
mouse liver microsome
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- PAMPA
parallel artificial membrane permeability assay
- PBD
Polo-box domain
- Plk1
polo-like kinase 1
- Q-TOF
quadrupole time-of-flight
- RLM
rat liver microsome
- RT
room temperature
- SAR
structure–activity relationship
- TFA
trifluoroacetic acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00250.
Additional cell data, FP assays, in vivo and in vitro stability studies, NMR and mass spectra and HPLC traces (PDF)
Time-lapse microscopy carried out with HeLa cells treated with control DMSO (MOV)
Time-lapse microscopy carried out with HeLa cells treated with 100 μM 78 (MOV)
Time-lapse microscopy carried out with HeLa cells treated with 40 μM 80 (MOV)
Author Contributions
⊥ J.-E.P. and J.L.: Equal contribution.
Author Contributions
K.A.J., K.S.L., J.-E.P., H.L.: Conceptualization, Writing, Data analysis; H.L., P.O., C.N.A., G.R., A.S.: Chemical synthesis, Data analysis; RO: NMR, Data analysis; J.-E.P., K.P.K., J.I.A., S.G., B.A.M.: Biological experiments, Data analysis; B.K., K.W.K., F.J.G.: Pharmacokinetic studies, Data analysis.
The authors declare no competing financial interest.
Supplementary Material
References
- Strebhardt K.; Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat. Rev. Cancer. 2006, 6, 321–330. 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- Craig S. N.; Wyatt M. D.; McInnes C. Current assessment of polo-like kinases as anti-tumor drug targets. Expert Opin. Drug Discovery 2014, 9, 773–789. 10.1517/17460441.2014.918100. [DOI] [PubMed] [Google Scholar]
- Strebhardt K.; Becker S.; Matthess Y. Thoughts on the current assessment of Polo-like kinase inhibitor drug discovery. Expert Opin. Drug Discovery 2015, 10, 1–8. 10.1517/17460441.2015.962510. [DOI] [PubMed] [Google Scholar]
- Lee K. S.; Burke T. R. Jr; Park J. E.; Bang J. K.; Lee E. Recent advances and new strategies in targeting Plk1 for anticancer therapy. Trends Pharmacol. Sci. 2015, 36, 858–877. 10.1016/j.tips.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph D.; Steegmaier M.; Hoffmann M.; Grauert M.; Baum A.; Quant J.; Haslinger C.; Garin-Chesa P.; Adolf G. R. BI 6727, A polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin. Cancer Res. 2009, 15, 3094–3102. 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- Berg A.; Berg T. Inhibitors of the polo-box domain of polo-like kinase 1. Chembiochem 2016, 17, 650–656. 10.1002/cbic.201500580. [DOI] [PubMed] [Google Scholar]
- Hanisch A.; Wehner A.; Nigg E. A.; Silljé H. H. Different Plk1 functions show distinct dependencies on Polo-Box domain-mediated targeting. Mol. Biol. Cell 2006, 17, 448–459. 10.1091/mbc.e05-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E.; Kim T. S.; Kim B. Y.; Lee K. S. Selective blockade of cancer cell proliferation and anchorage-independent growth by Plk1 activity–dependent suicidal inhibition of its polo-box domain. Cell Cycle 2015, 14, 3624–3634. 10.1080/15384101.2015.1104435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Park J. E.; Qian W. J.; Lim D.; Gräber M.; Berg T.; Yaffe M. B.; Lee K. S.; Burke T. R. Jr Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel. Nat. Chem. Biol. 2011, 7, 595–601. 10.1038/nchembio.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W. J.; Park J. E.; Lim D.; Lai C. C.; Kelley J. A.; Park S. Y.; Lee K. W.; Yaffe M. B.; Lee K. S.; Burke T. R. Jr Mono-anionic phosphopeptides produced by unexpected histidine alkylation exhibit high Plk1 polo-box domain-binding affinities and enhanced antiproliferative effects in HeLa cells. Biopolymers 2014, 102, 444–455. 10.1002/bip.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.; Sanhaji M.; Krämer A.; Reindl W.; Hofmann M.; Kreis N. N.; Zimmer B.; Berg T.; Strebhardt K. Polo-box domain inhibitor poloxin activates the spindle assembly checkpoint and inhibits tumor growth in vivo. Am. J. Pathol. 2011, 179, 2091–2099. 10.1016/j.ajpath.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl W.; Yuan J.; Kramer A.; Strebhardt K.; Berg T. A pan-specific inhibitor of the polo-box domains of polo-like kinases arrests cancer cells in mitosis. Chembiochem 2009, 10, 1145–1148. 10.1002/cbic.200900059. [DOI] [PubMed] [Google Scholar]
- Abdelfatah S.; Berg A.; Huang Q.; Yang L. J.; Hamdoun S.; Klinger A.; Greten H. J.; Fleischer E.; Berg T.; Wong V. K. W.; Efferth T. MCC1019, a selective inhibitor of the Polo-box domain of Polo-like kinase 1 as novel, potent anticancer candidate. Acta Pharm. Sin. B 2019, 9, 1021–1034. 10.1016/j.apsb.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P.; Mahen R.; Rossmann M.; Stokes J. E.; Hardwick B.; Huggins D. J.; Emery A.; Kunciw D. L.; Hyvönen M.; Spring D. R.; McKenzie G. J.; Venkitaraman A. R. A cryptic hydrophobic pocket in the polo-box domain of the polo-like kinase PLK1 regulates substrate recognition and mitotic chromosome segregation. Sci. Rep. 2019, 9, 15930. 10.1038/s41598-019-50702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins D. J.; Hardwick B. S.; Sharma P.; Emery A.; Laraia L.; Zhang F.; Narvaez A. J.; Roberts-Thomson M.; Crooks A. T.; Boyle R. G.; Boyce R.; Walker D. W.; Mateu N.; McKenzie G. J.; Spring D. R.; Venkitaraman A. R. Development of a novel cell-permeable protein-protein interaction inhibitor for the polo-box domain of polo-like kinase 1. ACS Omega 2020, 5, 822–831. 10.1021/acsomega.9b03626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran P.; Yim M. S.; Ahn M.; Soung N. K.; Park J. E.; Kim J.; Bang G.; Shin S. C.; Choi J.; Kim M.; Kim H. N.; Lee Y. H.; Chung Y. H.; Lee K.; EunKyeong Kim E.; Jeon Y. H.; Kim M. J.; Lee K. R.; Kim B. Y.; Lee K. S.; Ryu E. K.; Bang J. K. Development of a polo-like kinase-1 polo-box domain inhibitor as a tumor growth suppressor in mice models. J. Med. Chem. 2020, 63, 14905–14920. 10.1021/acs.jmedchem.0c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S. M.; Moulaei T.; Lim D.; Bang J. K.; Park J. E.; Shenoy S. R.; Liu F.; Kang Y. H.; Liao C.; Soung N. K.; Lee S.; Yoon D. Y.; Lim Y.; Lee D. H.; Otaka A.; Appella E.; McMahon J. B.; Nicklaus M. C.; Burke T. R. Jr; Yaffe M. B.; Wlodawer A.; Lee K. S. Structural and functional analyses of minimal phosphopeptides targeting the polo-box domain of polo-like kinase 1. Nat. Struct. Mol. Biol. 2009, 16, 876–882. 10.1038/nsmb.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverez C. N.; Park J. E.; Toti K. S.; Xia Y.; Krausz K. W.; Rai G.; Bang J. K.; Gonzalez F. J.; Jacobson K. A.; Lee K. S. Identification of a new heterocyclic scaffold for inhibitors of the polo-box domain of polo-like kinase 1. J. Med. Chem. 2020, 63, 14087–14117. 10.1021/acs.jmedchem.0c01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed N.; Smith P.; Sullivan A.; Spender L. C.; Dyer M.; Karran L.; O’Nions J.; Allday M.; Hoffmann I.; Crawford D.; Griffin B.; Farrell P. J.; Crook T. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood 2006, 107, 250–256. 10.1182/blood-2005-03-1194. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Bai J.; Shen R.; Brown S. A.; Komissarova E.; Huang Y.; Jiang N.; Alberts G. F.; Costa M.; Lu L.; Winkles J. A.; Dai W. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008, 68, 4077–4085. 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discovery 2010, 9, 643–660. 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- Meanwell N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- Knutson D. E.; Kodali R.; Divović B.; Treven M.; Stephen M. R.; Zahn N. M.; Dobričić V.; Huber A. T.; Meirelles M. A.; Verma R. S.; Wimmer L.; Witzigmann C.; Arnold L. A.; Chiou L. C.; Ernst M.; Mihovilovic M. D.; Savić M. M.; Sieghart W.; Cook J. M. Design and synthesis of novel deuterated ligands functionally selective for the gamma-aminobutyric acid type A receptor (GABAAR) alpha6 subtype with improved metabolic stability and enhanced bioavailability. J. Med. Chem. 2018, 61, 2422–2446. 10.1021/acs.jmedchem.7b01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirali T.; Serafini M.; Cargnin S.; Genazzani A. A. Applications of deuterium in medicinal chemistry. J. Med. Chem. 2019, 62, 5276–5297. 10.1021/acs.jmedchem.8b01808. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A.; Gao J. Targeting biomolecules with reversible covalent chemistry. Curr. Opin. Chem. Biol. 2016, 34, 110–116. 10.1016/j.cbpa.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D.; Freudenberger C.; Weinz C. In vitro metabolism of rivaroxaban, an oral, direct factor Xa inhibitor, in liver microsomes and hepatocytes of rats, dogs, and humans. Drug Metab. Dispos. 2009, 37, 1046–1055. 10.1124/dmd.108.025551. [DOI] [PubMed] [Google Scholar]
- Berger J. S.; Roe M. T.; Gibson C. M.; Kilaru R.; Green C. L.; Melton L.; Blankenship J. D.; Metzger D. C.; Granger C. B.; Gretler D. D.; Grines C. L.; Huber K.; Zeymer U.; Buszman P.; Harrington R. A.; Armstrong P. W. Safety and feasibility of adjunctive antiplatelet therapy with intravenous elinogrel, a direct-acting and reversible P2Y12 ADP-receptor antagonist, before primary percutaneous intervention in patients with ST-elevation myocardial infarction: The Early Rapid ReversAl of Platelet ThromboSis with Intravenous Elinogrel before PCI to Optimize REperfusion in Acute Myocardial Infarction (ERASE MI) pilot trial. Am. Heart J. 2009, 158, 998–1004. 10.1016/j.ahj.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Fang Z. Z.; Tosh D. K.; Tanaka N.; Wang H.; Krausz K. W.; O’Connor R.; Jacobson K. A.; Gonzalez F. J. Metabolic mapping of A3 adenosine receptor agonist MRS5980. Biochem. Pharmacol. 2015, 97, 215–223. 10.1016/j.bcp.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel P.; Henderson J. F.; Axelrod J. S-Demethylation by microsomal enzymes. J. Pharmacol. Exp. Ther. 1964, 143, 1–6. [PubMed] [Google Scholar]; http://jpet.aspetjournals.org/content/143/1/1.abstract.
- Larsen G. L.; Bakke J. E.; Feil V. J.; Huwe J. K. In vitro metabolism of the methylthio group of 2-methylthiobenzothiazole by rat liver. Xenobiotica 1988, 18, 313–322. 10.3109/00498258809041667. [DOI] [PubMed] [Google Scholar]
- Sun B.; Luo C.; Zhang X.; Guo M.; Sun M.; Yu H.; Chen Q.; Yang W.; Wang M.; Zuo S.; Chen P.; Kan Q.; Zhang H.; Wang Y.; He Z.; Sun J. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat. Commun. 2019, 10, 3211. 10.1038/s41467-019-11193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Fourie-O’Donohue A.; Dragovich P. S.; Pillow T. H.; Sadowsky J. D.; Kozak K. R.; Cass R. T.; Liu L.; Deng Y.; Liu Y.; Hop C. E.C.A.; Khojasteh S. C. Catalytic cleavage of disulfide bonds in small molecules and linkers of antibody-drug conjugates. Drug Metab. Dispos. 2019, 47, 1156–1163. 10.1124/dmd.118.086132. [DOI] [PubMed] [Google Scholar]
- Ochi Y.; Nakagawa O.; Hayashi J.; Wada S. I.; Urata H. A new nucleic acid prodrug responsive to high thiol concentration: Synthesis of 2’-O-methyldithiomethyl-modified oligonucleotides by post-synthetic modification. Curr. Protoc. Nucleic Acid Chem. 2015, 62, 4 63 61–64 63 20. 10.1002/0471142700.nc0463s62. [DOI] [PubMed] [Google Scholar]
- Elion G. B. The pharmacology of azathioprine. Ann. N.Y. Acad. Sci. 1993, 685, 401–407. 10.1111/j.1749-6632.1993.tb35897.x. [DOI] [PubMed] [Google Scholar]
- Van Scoik K. G.; Johnson C. A.; Porter W. R. The pharmacology and metabolism of the thiopurine drugs 6-mercaptopurine and azathioprine. Drug Metab. Rev. 1985, 16, 157–174. 10.3109/03602538508991433. [DOI] [PubMed] [Google Scholar]
- Elion G. B. Nobel lecture in physiology or medicine--1988. The purine path to chemotherapy. Vitro Cell Dev. Biol. 1989, 25, 321–330. 10.1007/BF02624593. [DOI] [PubMed] [Google Scholar]
- Patel A. A.; Swerlick R. A.; McCall C. O. Azathioprine in dermatology: the past, the present, and the future. J. Am. Acad. Dermatol. 2006, 55, 369–389. 10.1016/j.jaad.2005.07.059. [DOI] [PubMed] [Google Scholar]
- Robello M.; Nikolayevskiy H.; Scerba M.; Palomino R. A. N.; Mercurio V.; Hartman T.; Buckheit R.; Margolis L.; Appella D.. Nipamovir: Synthesis and preclinical evaluation of an anti-HIV thiobenzamide prodrug. International Society for Antiviral Research, 34th International Conference on Antiviral Research, 2021; Poster #121.
- Granvil C. P.; Yu A. M.; Elizondo G.; Akiyama T. E.; Cheung C.; Feigenbaum L.; Krausz K. W.; Gonzalez F. J. Expression of the human CYP3A4 gene in the small intestine of transgenic mice: in vitro metabolism and pharmacokinetics of midazolam. Drug Metab. Dispos. 2003, 31, 548–558. 10.1124/dmd.31.5.548. [DOI] [PubMed] [Google Scholar]
- Baek J. W.; Oh H. S.; Jang S. Y.; Ha T. H.; Saw K. H.. Novel process for preparing novel thioxo furopyrimidinone derivatives and intermediates used therein. KR2016003862, 2016.
- Il’chenko O. V.; Zaremba O. V.; Kovalenko S. M.; Sherakov A. A.; Chernykh V. P. Synthesis of 3-substituted 2-thioxo-2,3-dihydro-1H-benzofuro[3,2- d ] pyrimidin-4(1H)-ones. Synth. Commun. 2007, 37, 2559–2568. 10.1080/00397910701462831. [DOI] [Google Scholar]
- Poitout L.; Sackur C.; Brault V.; Roubert P.; Plas P.. Pyrimido-benzimidazole derivatives and the use thereof in the form of agonists and antagonists of melanocortin receptors. US2009209531, 2009.
- Harald W.Pyrimidin-4-one derivatives as pesticide. US6262058, 2001.
- Harald W.Pyrimidin-2-oxy-4-one and pyrimidin-2-oxy-4-thione derivatives. US6441169, 2002.
- Kang Y. H.; Park J. E.; Yu L. R.; Soung N. K.; Yun S. M.; Bang J. K.; Seong Y. S.; Yu H.; Garfield S.; Veenstra T. D.; Lee K. S. Self-regulation of Plk1 recruitment to the kinetochores is critical for chromosome congression and spindle checkpoint signaling. Mol. Cell 2006, 24, 409–422. 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Gabrea A.; Martelli M. L.; Qi Y.; Roschke A.; Barlogie B.; Shaughnessy J. D. Jr; Sawyer J. R.; Kuehl W. M. Secondary genomic rearrangements involving immunoglobulin or MYC loci show similar prevalences in hyperdiploid and nonhyperdiploid myeloma tumors. Genes Chromosomes Cancer 2008, 47, 573–590. 10.1002/gcc.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J. K.; Patel J.; Michalowski A.; Zhang S.; Wei B. R.; Sullivan P.; Gamache B.; Felsenstein K.; Kuehl W. M.; Simpson R. M.; Zingone A.; Landgren O.; Mock B. A. TORC1 and class I HDAC inhibitors synergize to suppress mature B cell neoplasms. Mol. Oncol. 2014, 8, 261–272. 10.1016/j.molonc.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia A. E.; Rellos P.; Haire L. F.; Chao J. W.; Ivins F. J.; Hoepker K.; Mohammad D.; Cantley L. C.; Smerdon S. J.; Yaffe M. B. The molecular basis for phospho-dependent substrate targeting and regulation of Plks by the polo-box domain. Cell 2003, 115, 83–95. 10.1016/S0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Park J. E.; Soung N. K.; Johmura Y.; Kang Y. H.; Liao C.; Lee K. H.; Park C. H.; Nicklaus M. C.; Lee K. S. Polo-box domain: a versatile mediator of polo-like kinase function. Cell. Mol. Life Sci. 2010, 67, 1957–1970. 10.1007/s00018-010-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong Y. S.; Kamijo K.; Lee J. S.; Fernandez E.; Kuriyama R.; Miki T.; Lee K. S. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 2002, 277, 32282–32293. 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- Besnard J.; Ruda G. F.; Setola V.; Abecassis K.; Rodriguiz R. M.; Huang X. P.; Norval S.; Sassano M. F.; Shin A. I.; Webster L. A.; Simeons F. R.; Stojanovski L.; Prat A.; Seidah N. G.; Constam D. B.; Bickerton G. R.; Read K. D.; Wetsel W. C.; Gilbert I. H.; Roth B. L.; Hopkins A. L. Automated design of ligands to polypharmacological profiles. Nature 2012, 492, 215–220. 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos D.; Barker D.; Sharma R.; Brunetto A. T.; Yap T. A.; Taegtmeyer A. B.; Barriuso J.; Medani H.; Degenhardt Y. Y.; Allred A. J.; Smith D. A.; Murray S. C.; Lampkin T. A.; Dar M. M.; Wilson R.; de Bono J. S.; Blagden S. P. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin. Cancer Res. 2011, 17, 3420–3430. 10.1158/1078-0432.CCR-10-2946. [DOI] [PubMed] [Google Scholar]
- Combes G.; Alharbi I.; Braga L. G.; Elowe S. Playing polo during mitosis: PLK1 takes the lead. Oncogene 2017, 36, 4819–4827. 10.1038/onc.2017.113. [DOI] [PubMed] [Google Scholar]
- Wang G.; Jiang Q.; Zhang C. The role of mitotic kinases in coupling the centrosome cycle with the assembly of the mitotic spindle. J. Cell Sci. 2014, 127, 4111–4122. 10.1242/jcs.151753. [DOI] [PubMed] [Google Scholar]
- Nepali K.; Lee H. Y.; Liou J. P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. 10.1021/acs.jmedchem.8b00147. [DOI] [PubMed] [Google Scholar]
- La Mantia L.; Mascoli N.; Milanese C. Azathioprine. Safety profile in multiple sclerosis patients. Neurol. Sci. 2007, 28, 299–303. 10.1007/s10072-007-0842-9. [DOI] [PubMed] [Google Scholar]
- Melo Bisneto A. V. D.; et al. Recombinogenic, genotoxic, and cytotoxic effects of azathioprine using in vivo assays. J. Toxicol. Environ. Health A 2021, 84, 261–271. 10.1080/15287394.2020.1864692. [DOI] [PubMed] [Google Scholar]
- Rautio J.; Meanwell N. A.; Di L.; Hageman M. J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discovery 2018, 17, 559–587. 10.1038/nrd.2018.46. [DOI] [PubMed] [Google Scholar]
- Kawai K.; Endo Y.; Asano T.; Amano S.; Sawada K.; Ueo N.; Takahashi N.; Sonoda Y.; Nagai M.; Kamei N.; Nagata N. Discovery of 2-(cyclopentylamino)thieno[3,2-d]pyrimidin-4(3H)-one derivatives as a new series of potent phosphodiesterase 7 inhibitors. J. Med. Chem. 2014, 57, 9844–9854. 10.1021/jm5008215. [DOI] [PubMed] [Google Scholar]
- Chen W.; Luo H.; Liu X.; Foley J. W.; Song X. Broadly applicable strategy for the fluorescence based detection and differentiation of glutathione and cysteine/homocysteine: Demonstration in vitro and in vivo. Anal. Chem. 2016, 88, 3638–3646. 10.1021/acs.analchem.5b04333. [DOI] [PubMed] [Google Scholar]
- Calabrese D. R.; Chen X.; Leon E.; et al. Chemical and structural studies provide a mechanistic basis for recognition of the MYC G-quadruplex. Nat. Commun. 2018, 9, 4229. 10.1038/s41467-018-06315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.