Abstract
Working with non-noble electrocatalysts poses significant experimental challenges to unambiguously evaluate their intrinsic activity and characterize their working state and possible structural and compositional changes before, during, and after activity testing. Despite the vast number of studies on non-noble catalysts, these issues are still not addressed sufficiently—hindering significant progress in the field. In this Perspective, we present pitfalls and challenges when working with non-noble-metal-based electrocatalysts from catalyst synthesis, over electrochemical testing, to post-reaction characterization, and suggest potential solutions to overcome these difficulties. We believe that reliable measurements of the intrinsic activity of non-noble-metal-based electrocatalysts will greatly enhance our understanding of electrocatalysis in general and is a prerequisite for developing more active and selective electrocatalysts.
With declining prices of renewable electricity, electrochemical conversion of electricity to valuable products (Power-to-X) is gaining increasing attention as a sustainable route for production of fuels and chemicals. The generation of green hydrogen is so far the most developed technology, but also other processes such as electrochemical conversion of carbon dioxide and generation of hydrogen peroxide are growing in scale.1,2 With increasing industrialization of these processes, the focus of research is shifting from finding active catalysts as proof-of-concept to optimization of the technology with respect to lifetime and cost. Thereby, catalyst stability is of central importance to properly address both challenges.3 While catalyst degradation phenomena are widely studied in the case of noble metals in polymer electrolyte membrane fuel cells (PEMFCs) and electrolyzers, catalyst stability is often neglected in the search for earth-abundant, low-cost catalyst alternatives.4−6 However, especially when non-noble metals are studied, the stability of the catalyst during the various stages from synthesis to post-reaction characterization is of utmost importance to unambiguously evaluate the intrinsic properties of the material. In this Perspective, we highlight key challenges present when working with these materials in aqueous electrolytes and recommend strategies to guide future work.
Compounds and Alloys with Non-Noble Metals Are Prone to Decomposition and Segregation in Air
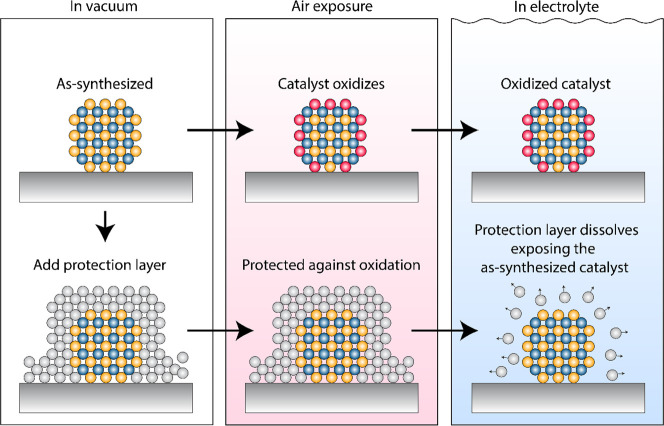
The field of electrocatalysis has greatly advanced through the development of tailored materials via elaborate synthesis protocols.7−9 Thereby, the chemical composition as well as the surface morphology have been tuned using a rational design of catalysts approach. The synthesized materials are routinely characterized intensively with bulk or near-surface characterization methods like X-ray diffraction (XRD) or X-ray photoelectron spectroscopy (XPS). While this focus on bulk or near-surface material characterization has some importance, since properties such as crystallinity can substantially influence the catalyst performance, significant caveats exist.10 First, only very few studies acknowledge the importance of the catalyst conductivity as an important bulk material property to be measured systematically and reported.11 This shortcoming of many fundamental studies makes a proper assessment for subsequent tests in membrane electrode assemblies significantly harder. Second, heterogeneous catalysis takes place in most cases only on the surface of the catalyst, making the surface atomic structure and composition among the most crucial characteristics of the catalyst. Unfortunately, the surface of the catalyst is only rarely studied in depth experimentally. This caveat becomes exacerbated when working with catalysts containing earth-abundant, low-cost metals. These elements have a high oxophilicity, which makes them prone to surface oxidation if they are exposed to air—leading to decomposition of the synthesized compounds and dealloying in bi- and multimetallic materials via phase segregation of the more reactive component.12 In both cases, the catalyst will be covered by at least one monolayer (1 ML) of oxide, changing the composition/stoichiometry of the surface and sometimes several layers below (Figure 1). Since it is this surface oxide that is initially exposed to the electrolyte in the electrochemical cell, it is important to apply surface-sensitive characterization techniques such as angle-resolved XPS or low-energy ion spectroscopy (LEIS) to properly characterize the catalyst surface after air exposure. We especially encourage more frequent use of LEIS since it is sensitive to the topmost surface layer and has a very high resolution at low atomic masses. However, when working with LEIS, it should be considered that the resolution of LEIS decreases with increasing elemental mass. To a certain extent this limitation can be counteracted by using a heavier noble gas such as argon or xenon instead of the most frequently used helium gas.
Figure 1.
Illustration of catalyst oxidation during transportation in air (top row), which leads to oxide being exposed as the surface during electrochemistry. Application of a protection layer (bottom row) can prevent oxidation of the catalyst during transportation in air, and this layer can be dissolved during immersion into the electrolyte.
While it has been shown that, for oxidation reactions, an oxidized catalyst can be beneficial—e.g., displaying a higher stability number in case of IrO2—the situation is less clear for reduction processes. It is usually just assumed that the catalyst resembles the synthesized structure under working conditions despite being oxidized in air.10 However, direct experimental evidence for this assumption is rare, and even if the catalyst reduces fully under working conditions, it does not necessarily reflect the desired material, as it is possible that a skin of metal is formed from the reduced oxide. While this reduced oxide can potentially offer a very different atomic arrangement compared to the pristine metal, which can lead to a change in activity, care should be taken as such complex surface structures are difficult to characterize properly, and distinguishing an intrinsic increase in activity from a surface area increase can be challenging. To unambiguously test the synthesized material, it is therefore necessary to protect the catalyst from segregation and decomposition in air. The formation of surface oxides through air exposure can be prevented either by performing the electrochemical experiment in a cell that is directly connected to an ultrahigh vacuum synthesis chamber or by protecting the catalyst from oxidation. It should be also noted that the oxidation process can be slowed down by performing the measurement in a glovebox with a reduced oxygen partial pressure. However, handling of a catalyst material in a glovebox still results in the immediate coverage of the catalyst with oxygen. At an oxygen concentration of 1 ppm in the glovebox, we expect approximately 270 MLs of oxygen hitting the surface per second: 1 ppm of oxygen corresponds to 10–3 mbar at atmospheric pressure. This pressure can be inserted in the areal impingement rate equation,
where p is the pressure in Pa, m is the mass of an oxygen molecule (5.3 × 10–26 kg), kB is the Boltzmann constant (1.38 × 10–23 J/K), and T is the temperature (300 K). Assuming an atom density of 1015 atoms cm–2, we end up with approximately 270 MLs/s. Many metals need only 1 ML of oxygen to be completely covered with oxygen, and an exposure to 1000s of MLs in a time period of seconds can lead to oxidation into deep layers. Thus, we consider handling of a catalyst material in a glovebox as insufficient protection. Since performing electrochemical experiments in cells connected to an ultrahigh vacuum chamber (where the oxygen and water levels are substantially below 1 ML/h) is not trivial and is difficult to interface with product analysis techniques, it is desired to develop alternative strategies. One possibility is coating the catalyst with a protective film, as will be discussed in detail by us in an upcoming publication. The protective film forms a passivation layer, which hinders any oxidation and prevents segregation and decomposition of the catalyst. Upon immersion of the catalyst into the electrolyte under potential control, a potential is chosen where the protection layer can be dissolved while the catalyst remains stable, enabling testing of the desired material, see Figure 1. While the presented method works well for synthesized structures in vacuum chambers via, e.g., sputter deposition, we believe that an implementation of such a strategy into wet chemical synthesis will be crucial to fully utilize the rich potential of wet chemical synthesis. Inspiration could thereby come from the photovoltaics community, where TiO2, Al2O3, or CdS protection layers have been successfully established.13−15 Future research should further explore these possibilities and systematically investigate the impact of protection layers on the catalyst performance.
Non-Noble Metals and Their Compounds Are Prone to Dissolution and Oxidation upon Immersion into the Electrolyte
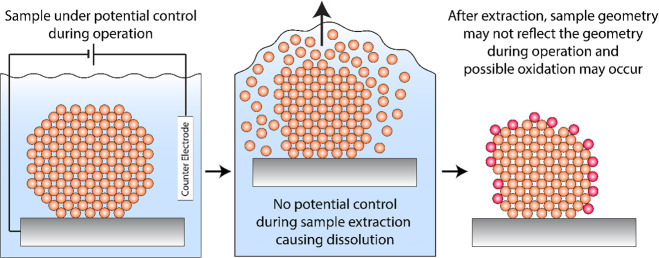
Besides the stability in air, the catalyst stability upon immersion into the electrolyte must also be considered to evaluate the intrinsic properties of the material. Non-noble metals are prone to corrosion at open-circuit voltage (OCV) upon uncontrolled immersion into the electrolyte,16−19 see Figure 2, and redeposition upon subsequent negative potentials.
Figure 2.
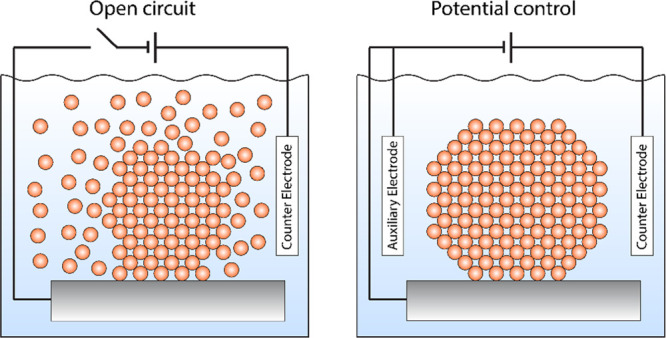
Illustration of catalyst dissolution during uncontrolled immersion of the catalyst into the electrolyte (left). Illustration of catalyst immersion under potential control to avoid dissolution (right).
This becomes especially important when working with nanoparticles at low loadings, where a complete dissolution of the catalyst can happen within seconds.16 However, also thin-film catalysts or powders can irreversibly change during immersion into the electrolyte, as dissolution can lead to morphological changes of the catalyst. Additionally, water is also a sufficiently strong oxidation agent to oxidize transition metals—even if deaerated—leading to decomposition of compounds and segregation of alloys. These effects lead to ill-defined and irreproducible catalyst morphologies and compositions. To ensure that the synthesized material is preserved, immersion under potential control is crucial for fundamental studies, see Figure 2.16,20 Additionally, in-depth knowledge on the potential stability windows of the individual compounds is necessary. Calculated Pourbaix diagrams can serve as valuable guidance. However, when working with Pourbaix diagrams, it must be considered that 1) Pourbaix diagrams only give insight on the thermodynamic stability of the compound and 2) Pourbaix diagrams are calculated at a certain concentration of ions in solution. This concentration is usually 10–6 M. Considering an average surface atom density of 1015 atoms cm−2, a geometric surface area of 0.2 cm2 (typical geometric area of a rotating disk electrode), and an electrolyte volume of 100 mL, 60 MLs have to dissolve in order to have an ion concentration of 10–6 M. A dissolution of this magnitude can lead to substantial changes in the catalyst structure. If Pourbaix diagrams are used to argue for catalyst stability, they should therefore be calculated at the background concentration of the respective ions in solution. Additionally, these considerations also show that the used electrolyte volume influences the measured catalyst stability and should be considered. Nevertheless, we stress that catalyst stability can only be determined when thermodynamic and kinetic stability is also considered and corrosion measurements are performed with, e.g., (on-line) inductively coupled plasma mass spectrometry (ICP-MS).21−24
Catalysts Can Dissolve during Reaction: The Stability Number Should Be Used as a Metric of Catalyst Performance beyond the Oxygen Evolution Reaction
Catalyst stability is of central importance not only during immersion into the electrolyte but also in the course of the reaction, see Figure 3.10,17,25
Figure 3.
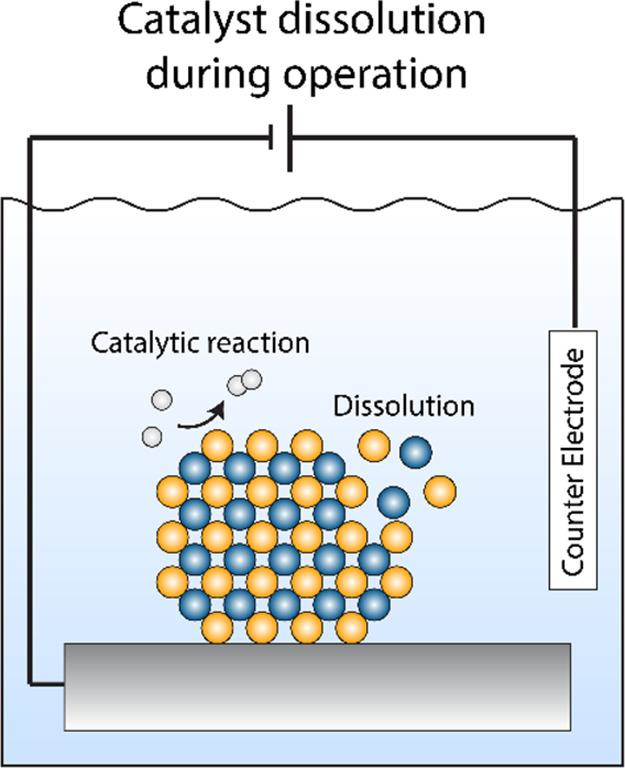
Illustration of catalyst dissolution under reaction conditions.
This has been long recognized for the oxygen evolution reaction, where the stability number has been introduced as a more holistic metric for catalyst performance compared to activity alone.10 An on-line corrosion measurement and on-line monitoring of the produced oxygen is especially important when working with non-noble metals, since a corrosion current could otherwise easily be mislabeled as a catalytic activity.26 While it is positive that this metric is finding wide acceptance in oxygen evolution, it is encouraged to also use the stability number for other reactions. This encouragement goes beyond oxidative reactions (where an application of the stability number might seem more intuitive) and should also be considered for non-noble metals and their compounds for reductive reactions. For example, it has been shown that some non-noble-metal compounds dissolve significantly during hydrogen evolution in acidic media.17 This catalyst corrosion is not evident from performing an electrochemical measurement alone; i.e., simply recording the current or potential is insufficient to assess the performance of an electrocatalyst. We encourage that corrosion measurements with, e.g., ICP-MS should be performed regularly for a more holistic catalyst performance evaluation.
It should be additionally pointed out that, from an economic perspective, it still remains to be seen if a behind-the-meter operation of electrolyzers (directly coupled to a renewable energy source with intermittent operation) or a grid connection (higher amount of full load hours but grid fees have to be paid) is advantageous. Despite this uncertainty, most electrochemical experiments are still performed under steady-state conditions, and intermittent catalyst testing with startup and shutdown is rare. To fully assess the performance of an electrocatalyst, it should therefore also be investigated to what extent a catalyst degrades during variable load and electrolyzer shutdown phases.27
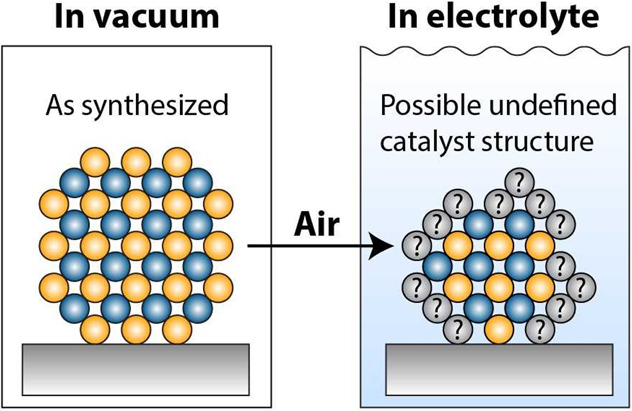
Ex Situ Analysis of Non-Noble Metals and Compounds Is Hindered by Catalyst Instabilities during Cell Disassembly
Insights into structural changes of electrocatalysts are frequently obtained by ex situ, post-mortem analysis using electron microscopy, XRD, or XPS. While for noble metals such an analysis can give valuable insights, care should be taken when working with non-noble metals. During cell disassembly, the working electrode potential will drift to OCV, and catalyst corrosion or catalyst oxidation cannot be ruled out, see Figure 4.
Figure 4.
Illustration of the cell disassembly process after reaction. The middle panel shows that the non-noble catalyst will start to corrode once potential control is lost, and the catalyst will additionally oxidize in air when the sample is transported to the ex situ characterization (right panel).
It is therefore highly desirable to characterize the catalyst structure in situ and in operando. This can be done via in situ and in operando diffraction, spectroscopy, or microscopy, and remarkable insights into changes in catalyst structure have been elucidated.28,29 However, such experiments are usually performed at specialized facilities, such as synchrotrons, and with customized cells. This hinders a routine investigation of catalyst restructuring or agglomeration, and the customized synchrotron cells can additionally frequently not be easily coupled to product detection, hindering a direct correlation of catalyst restructuring to changes in the product distribution. It is therefore also highly desirable to investigate changes in the catalyst structure in situ with electrochemical techniques. This can be done by performing cyclic voltammetry in the so-called fingerprint region of the respective catalyst.30−32 The fingerprint region is a potential window in which characteristic adsorption and desorption features are observed that can be assigned to specific facets of the catalyst. However, catalysts do not always show characteristic features in a cyclic voltammogram, and it is therefore desirable to find alternative electrochemical probes.33 One approach is the application of underpotential deposition. Thereby, a monolayer of a metal with a lower work function is deposited on a metal with a higher work function.34 This technique has been regularly applied under acidic conditions and recently also expanded to alkaline environments to investigate Au and Cu electrodes.35,36 Through the work function dependence of the technique, it is sensitive to the surface atomic structure of the catalyst—since different crystal facets have different work functions—which results in characteristic deposition peaks. Additionally, through integration of the deposition charge, the surface area of the catalyst can be characterized. So far, the possibilities of electrochemical catalyst characterization methods have been underappreciated. It is encouraged to further explore electrochemical characterization, since these methods have the potential of being sensitive to the surface atomic structure—which is difficult to achieve with in situ spectroscopy—and they can probe the whole electrochemically active surface area of complex electrocatalysts—in contrast to in situ scanning probe microscopy techniques, which are usually limited to small areas on flat model catalysts. Further, if other in situ measurements are planned, electrochemical characterization techniques can be of great help as a first step to have a better understanding of the catalyst under reaction conditions, which likely leads to better designed experiments at, e.g., the beamline where time is limited.
In summary, we presented potential pitfalls and mitigation solutions when working with non-noble electrocatalysts. We highlight that significant attention should be paid to the surface atomic structure and its changes from catalyst synthesis, exposure to air, to immersion into electrolyte, during reaction, and post characterization.
Acknowledgments
This work was supported by the Villum Foundation SUSTAIN Grant 9455 “The Villum Center for the Science of Sustainable Fuels and Chemicals”. J.K. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 101001078).
Biographies
Degenhart Hochfilzer is a Technology Analyst Manager at Umicore and Ph.D. student in Physics at the Technical University of Denmark. In his work, he investigates processes for electrochemical energy conversion with a special focus on catalyst stability and the development of new experimental methodologies to improve catalyst testing.
Ib Chorkendorff is a Professor in heterogeneous catalysis at the Technical University of Denmark Department of Physics. He has authored or co-authored more than 400 scientific papers, 23 patents and patents applications, and one textbook. His research activities focus on new catalysts for energy conversion and environmental protection. He has co-founded three start-up companies.
Jakob Kibsgaard is a Professor of Physics at the Technical University of Denmark. He received his Ph.D. from Aarhus University in 2008. His research focuses on the development and understanding of thermal and electro-catalysts for sustainable energy conversion. He is currently a Carlsberg Foundation Distinguished Fellow and ERC consolidator grantee.
The authors declare no competing financial interest.
References
- Yang S.; Verdaguer-Casadevall A.; Arnarson L.; Silvioli L.; Čolić V.; Frydendal R.; Rossmeisl J.; Chorkendorff I.; Stephens I. E. L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8 (5), 4064–4081. 10.1021/acscatal.8b00217. [DOI] [Google Scholar]
- Wakerley D.; Lamaison S.; Wicks J.; Clemens A.; Feaster J.; Corral D.; Jaffer S. A.; Sarkar A.; Fontecave M.; Duoss E. B.; Baker S.; Sargent E. H.; Jaramillo T. F.; Hahn C. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. Nat. Energy 2022, 7 (2), 130–143. 10.1038/s41560-021-00973-9. [DOI] [Google Scholar]
- Kolle-Görgen E.; Fortunato G.; Ledendecker M. Catalyst Stability in Aqueous Electrochemistry. Chem. Mater. 2022, 34 (23), 10223–10236. 10.1021/acs.chemmater.2c02443. [DOI] [Google Scholar]
- Knöppel J.; Möckl M.; Escalera-López D.; Stojanovski K.; Bierling M.; Böhm T.; Thiele S.; Rzepka M.; Cherevko S. On the limitations in assessing stability of oxygen evolution catalysts using aqueous model electrochemical cells. Nat. Commun. 2021, 12 (1), 2231. 10.1038/s41467-021-22296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möckl M.; Ernst M. F.; Kornherr M.; Allebrod F.; Bernt M.; Byrknes J.; Eickes C.; Gebauer C.; Moskovtseva A.; Gasteiger H. A. Durability Testing of Low-Iridium PEM Water Electrolysis Membrane Electrode Assemblies. J. Electrochem. Soc. 2022, 169 (6), 064505. 10.1149/1945-7111/ac6d14. [DOI] [Google Scholar]
- Della Bella R. K. F.; Stühmeier B. M.; Gasteiger H. A. Universal Correlation between Cathode Roughness Factor and H2/Air Performance Losses in Voltage Cycling-Based Accelerated Stress Tests. J. Electrochem. Soc. 2022, 169 (4), 044528. 10.1149/1945-7111/ac67b8. [DOI] [Google Scholar]
- Zheng Y.-R.; Vernieres J.; Wang Z.; Zhang K.; Hochfilzer D.; Krempl K.; Liao T.-W.; Presel F.; Altantzis T.; Fatermans J.; Scott S. B.; Secher N. Mør.; Moon C.; Liu P.; Bals S.; Van Aert S.; Cao A.; Anand M.; Nørskov J. K.; Kibsgaard J.; Chorkendorff I. Monitoring oxygen production on mass-selected iridium–tantalum oxide electrocatalysts. Nat. Energy 2022, 7 (1), 55–64. 10.1038/s41560-021-00948-w. [DOI] [Google Scholar]
- Seh Z. W.; Kibsgaard J.; Dickens C. F.; Chorkendorff I.; Nørskov J. K.; Jaramillo T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355 (6321), eaad4998. 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- Kibsgaard J.; Chen Z.; Reinecke B. N.; Jaramillo T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature materials 2012, 11 (11), 963–969. 10.1038/nmat3439. [DOI] [PubMed] [Google Scholar]
- Geiger S.; Kasian O.; Ledendecker M.; Pizzutilo E.; Mingers A. M.; Fu W. T.; Diaz-Morales O.; Li Z.; Oellers T.; Fruchter L.; Ludwig A.; Mayrhofer K. J. J.; Koper M. T. M.; Cherevko S. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 2018, 1 (7), 508–515. 10.1038/s41929-018-0085-6. [DOI] [Google Scholar]
- Krivina R. A.; Lindquist G. A.; Beaudoin S. R.; Stovall T. N.; Thompson W. L.; Twight L. P.; Marsh D.; Grzyb J.; Fabrizio K.; Hutchison J. E.; Boettcher S. W. Anode Catalysts in Anion-Exchange-Membrane Electrolysis without Supporting Electrolyte: Conductivity, Dynamics, and Ionomer Degradation. Adv. Mater. 2022, 34 (35), e2203033 10.1002/adma.202203033. [DOI] [PubMed] [Google Scholar]
- Griesser C.; Li H.; Wernig E.-M.; Winkler D.; Shakibi Nia N.; Mairegger T.; Götsch T.; Schachinger T.; Steiger-Thirsfeld A.; Penner S.; Wielend D.; Egger D.; Scheurer C.; Reuter K.; Kunze-Liebhäuser J. True Nature of the Transition-Metal Carbide/Liquid Interface Determines Its Reactivity. ACS Catal. 2021, 11 (8), 4920–4928. 10.1021/acscatal.1c00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipkorir A.; DuBose J.; Cho J.; Kamat P. V. CsPbBr3-CdS heterostructure: stabilizing perovskite nanocrystals for photocatalysis. Chem. Sci. 2021, 12 (44), 14815–14825. 10.1039/D1SC04305F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris S.; Dona S. T.; Niemann V.; Loiudice A.; Buonsanti R. Optimizing the Atomic Layer Deposition of Alumina on Perovskite Nanocrystal Films by Using O2 As a Molecular Probe. Helvetica Chem. Acta 2020, 103 (6), e2000055. 10.1002/hlca.202000055. [DOI] [Google Scholar]
- Seger B.; Pedersen T.; Laursen A. B.; Vesborg P. C. K.; Hansen O.; Chorkendorff I. Using TiO2 as a conductive protective layer for photocathodic H2 evolution. J. Am. Chem. Soc. 2013, 135 (3), 1057–1064. 10.1021/ja309523t. [DOI] [PubMed] [Google Scholar]
- Hochfilzer D.; Sørensen J. E.; Clark E. L.; Scott S. B.; Chorkendorff I.; Kibsgaard J. The Importance of Potential Control for Accurate Studies of Electrochemical CO Reduction. ACS Energy Lett. 2021, 6 (5), 1879–1885. 10.1021/acsenergylett.1c00496. [DOI] [Google Scholar]
- Wang Z.; Zheng Y.-R.; Montoya J.; Hochfilzer D.; Cao A.; Kibsgaard J.; Chorkendorff I.; Nørskov J. K. Origins of the Instability of Nonprecious Hydrogen Evolution Reaction Catalysts at Open-Circuit Potential. ACS Energy Lett. 2021, 6 (6), 2268–2274. 10.1021/acsenergylett.1c00876. [DOI] [Google Scholar]
- Schalenbach M.; Speck F. D.; Ledendecker M.; Kasian O.; Goehl D.; Mingers A. M.; Breitbach B.; Springer H.; Cherevko S.; Mayrhofer K. J. Nickel-molybdenum alloy catalysts for the hydrogen evolution reaction: Activity and stability revised. Electrochim. Acta 2018, 259, 1154–1161. 10.1016/j.electacta.2017.11.069. [DOI] [Google Scholar]
- Ledendecker M.; Mondschein J. S.; Kasian O.; Geiger S.; Göhl D.; Schalenbach M.; Zeradjanin A.; Cherevko S.; Schaak R. E.; Mayrhofer K. Stability and Activity of Non-Noble-Metal-Based Catalysts Toward the Hydrogen Evolution Reaction. Angew. Chem., Int. Ed. Engl. 2017, 56 (33), 9767–9771. 10.1002/anie.201704021. [DOI] [PubMed] [Google Scholar]
- Hochfilzer D.; Xu A.; Sørensen J. E.; Needham J. L.; Krempl K.; Toudahl K. K.øj.; Kastlunger G.; Chorkendorff I.; Chan K.; Kibsgaard J. Transients in Electrochemical CO Reduction Explained by Mass Transport of Buffers. ACS Catal. 2022, 12 (9), 5155–5161. 10.1021/acscatal.2c00412. [DOI] [Google Scholar]
- Kasian O.; Geiger S.; Mayrhofer K. J. J.; Cherevko S. Electrochemical On-line ICP-MS in Electrocatalysis Research. Chem. Rec. 2019, 19 (10), 2130–2142. 10.1002/tcr.201800162. [DOI] [PubMed] [Google Scholar]
- Speck F. D.; Zagalskaya A.; Alexandrov V.; Cherevko S. Periodicity in the Electrochemical Dissolution of Transition Metals. Angew. Chem., Int. Ed. Engl. 2021, 60 (24), 13343–13349. 10.1002/anie.202100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider M. E.; Kamat G. A.; Zamora Zeledon J. A.; Wei L.; Sokaras D.; Gallo A.; Stevens M. B.; Jaramillo T. F. Understanding the Stability of Manganese Chromium Antimonate Electrocatalysts through Multimodal In Situ and Operando Measurements. J. Am. Chem. Soc. 2022, 144 (49), 22549–22561. 10.1021/jacs.2c08600. [DOI] [PubMed] [Google Scholar]
- Peng J.; Giordano L.; Davenport T. C.; Shao-Horn Y. Stability Design Principles of Manganese-Based Oxides in Acid. Chem. Mater. 2022, 34 (17), 7774–7787. 10.1021/acs.chemmater.2c01233. [DOI] [Google Scholar]
- Weber M. L.; Lole G.; Kormanyos A.; Schwiers A.; Heymann L.; Speck F. D.; Meyer T.; Dittmann R.; Cherevko S.; Jooss C.; Baeumer C.; Gunkel F. Atomistic Insights into Activation and Degradation of La0.6Sr0.4CoO3-δ Electrocatalysts under Oxygen Evolution Conditions. J. Am. Chem. Soc. 2022, 144 (39), 17966–17979. 10.1021/jacs.2c07226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibsgaard J.; Chorkendorff I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 2019, 4 (6), 430–433. 10.1038/s41560-019-0407-1. [DOI] [Google Scholar]
- Samu A. A.; Kormányos A.; Kecsenovity E.; Szilágyi N.; Endrődi B.; Janáky C. Intermittent Operation of CO2 Electrolyzers at Industrially Relevant Current Densities. ACS Energy Lett. 2022, 7 (5), 1859–1861. 10.1021/acsenergylett.2c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. B.; Hogg T. V.; Landers A. T.; Maagaard T.; Bertheussen E.; Lin J. C.; Davis R. C.; Beeman J. W.; Higgins D.; Drisdell W. S.; Hahn C.; Mehta A.; Seger B.; Jaramillo T. F.; Chorkendorff I. Absence of Oxidized Phases in Cu under CO Reduction Conditions. ACS Energy Lett. 2019, 4 (3), 803–804. 10.1021/acsenergylett.9b00172. [DOI] [Google Scholar]
- Chee S. W.; Lunkenbein T.; Schlögl R.; Cuenya B. R. In situ and operando electron microscopy in heterogeneous catalysis-insights into multi-scale chemical dynamics. J. Phys. Condensed Matter 2021, 33 (15), 153001. 10.1088/1361-648X/abddfd. [DOI] [PubMed] [Google Scholar]
- Tiwari A.; Heenen H. H.; Bjørnlund A. S.; Hochfilzer D.; Chan K.; Horch S. Electrochemical Oxidation of CO on Cu Single Crystals under Alkaline Conditions. ACS Energy Lett. 2020, 5 (11), 3437–3442. 10.1021/acsenergylett.0c01751. [DOI] [Google Scholar]
- Sebastián-Pascual P.; Escudero-Escribano M. Addressing the Interfacial Properties for CO Electroreduction on Cu with Cyclic Voltammetry. ACS Energy Lett. 2020, 5 (1), 130–135. 10.1021/acsenergylett.9b02456. [DOI] [Google Scholar]
- Solla-Gullón J.; Vidal-Iglesias F. J.; Rodríguez P.; Herrero E.; Feliu J. M.; Clavilier J.; Aldaz A. In Situ Surface Characterization of Preferentially Oriented Platinum Nanoparticles by Using Electrochemical Structure Sensitive Adsorption Reactions. J. Phys. Chem. B 2004, 108 (36), 13573–13575. 10.1021/jp0471453. [DOI] [Google Scholar]
- Scott S. B.; Engstfeld A. K.; Jusys Z.; Hochfilzer D.; Knøsgaard N.; Trimarco D. B.; Vesborg P. C. K.; Behm R. J.; Chorkendorff I. Anodic molecular hydrogen formation on Ru and Cu electrodes. Catal. Sci. Technol. 2020, 10 (20), 6870–6878. 10.1039/D0CY01213K. [DOI] [Google Scholar]
- Kolb D. M.; Przasnyski M.; Gerischer H. Underpotential deposition of metals and work function differences. J. Electroanal. Chem. Interfacial Electrochem. 1974, 54 (1), 25–38. 10.1016/S0022-0728(74)80377-3. [DOI] [Google Scholar]
- Hochfilzer D.; Tiwari A.; Clark E. L.; Bjørnlund A. S.; Maagaard T.; Horch S.; Seger B.; Chorkendorff I.; Kibsgaard J. In Situ Analysis of the Facets of Cu-Based Electrocatalysts in Alkaline Media Using Pb Underpotential Deposition. Langmuir 2022, 38 (4), 1514–1521. 10.1021/acs.langmuir.1c02830. [DOI] [PubMed] [Google Scholar]
- Herrero E.; Buller L. J.; Abruña H. D. Underpotential deposition at single crystal surfaces of Au, Pt, Ag and other materials. Chem. Rev. 2001, 101 (7), 1897–1930. 10.1021/cr9600363. [DOI] [PubMed] [Google Scholar]