Abstract
Histatin-5 (Hst5) is a member of the histatin superfamily of cationic, His-rich, Zn(II)-binding peptides in human saliva. Hst5 displays antimicrobial activity against fungal and bacterial pathogens, often in a Zn(II)-dependent manner. In contrast, here we showed that under in vitro conditions that are characteristic of human saliva, Hst5 does not kill seven streptococcal species that normally colonize the human oral cavity and oropharynx. We further showed that Zn(II) does not influence this outcome. We then hypothesized that Hst5 exerts more subtle effects on streptococci by modulating Zn(II) availability. We initially proposed that Hst5 contributes to nutritional immunity by limiting nutrient Zn(II) availability and promoting bacterial Zn(II) starvation. By examining the interactions between Hst5 and Streptococcus pyogenes as a model Streptococcus species, we showed that Hst5 does not influence the expression of Zn(II) uptake genes. In addition, Hst5 did not suppress growth of a ΔadcAI mutant strain that is impaired in Zn(II) uptake. These observations establish that Hst5 does not promote Zn(II) starvation. Biochemical examination of purified peptides further confirmed that Hst5 binds Zn(II) with high micromolar affinities and does not compete with the AdcAI high-affinity Zn(II) uptake protein for binding nutrient Zn(II). Instead, we showed that Hst5 weakly limits the availability of excess Zn(II) and suppresses Zn(II) toxicity to a ΔczcD mutant strain that is impaired in Zn(II) efflux. Altogether, our findings led us to reconsider the function of Hst5 as a salivary antimicrobial agent and the role of Zn(II) in Hst5 function.
Keywords: antimicrobial peptide, histatin, zinc, nutritional immunity, Streptococcus
Antimicrobial peptides are short, often cationic peptides that are secreted by diverse organisms from across the domains of life.1 These peptides usually act as immune effectors that kill invading microbes as part of the host innate immune system, but many also play key functions in the normal biology of the host organism. A subfamily of antimicrobial peptides binds metals. Some of these metallo-peptides become activated upon metal binding,2−4 for instance, by folding into an optimal conformation for disrupting microbial membranes or for acting on their targets (e.g., clavanin A from tunicates4 and piscidin from fish2). Other metallo-peptides bind metals and withhold these essential nutrients away from microbes, causing them to starve (e.g., microplusin from cattle ticks5).
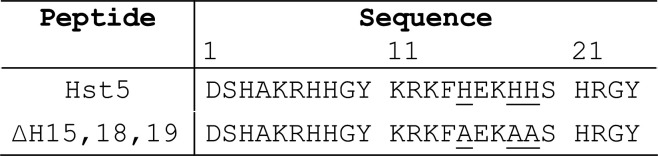
Histatins comprise a family of cationic, His-rich, metallo-peptides in the saliva and tears of humans and some higher primates.6−8 These peptides are derived from two parent peptides, namely, Histatin-1 and Histatin-3.6,9 Both parent histatins are expressed by the salivary and tear glands.10,11 Upon secretion in saliva into the oral cavity, the parent histatins are rapidly processed into shorter fragments12−14 by unidentified human salivary proteases or proteases from resident oral microbes. Whether the parent histatins are proteolytically degraded in tears is currently unknown. Of the various salivary fragments, Histatin-5 (Hst5; Table 1) is the best characterized in vitro.
Table 1. Hst5 Peptides Used in This Work.
Hst5 is noted for its ability to kill the fungus Candida albicans(15,16), and several pathogenic bacterial species, namely, Pseudomonas aeruginosa, Staphylococcus aureus, Acinetobacter baumanii, Enterococcus faecium, and Enterobacter cloacae.16 Unlike other antimicrobial peptides, Hst5 does not appear to permeabilize fungal membranes, although it does destabilize some bacterial membranes.16 Beyond its direct action on membranes, the antimicrobial activity of Hst5 requires the peptide to be internalized into the cytoplasm, usually via energy-dependent pathways for peptide uptake.16,17 Once in the cytoplasm, Hst5 is thought to encounter its targets, which in C. albicans include the mitochondria18 but in bacteria remain unidentified, and causes toxicity via multiple pathways that are not fully elucidated.15,18
Hst5 contains a characteristic Zn(II)-binding motif, His-Glu-x-His-His (Table 1), but whether Hst5 associates with Zn(II) in saliva is unknown. Likewise, whether Zn(II) binding is essential for the antimicrobial activity of Hst5 is unclear. Synthetic Hst5 derivatives that lack one or all three putative Zn(II)-binding His residues remain active against C. albicans.19 In addition, conflicting reports show that addition of Zn(II) can both enhance20 and suppress21 Hst5 activity against this fungus. However, a recent report indicates that the role of Zn(II) is concentration-dependent: low concentrations of added Zn(II) enhance the antimicrobial activity of Hst5 against C. albicans (compared with the control without any added Zn(II)), while high concentrations of added Zn(II) suppress it.22
Beyond histatins and Zn(II)-binding metallo-peptides, Zn(II)-dependent host innate immune responses are well described. In response to microbial infection, Zn(II) levels and those of Zn(II)-binding or Zn(II)-transporting proteins within a host organism can rise and fall, leading to fluctuations in Zn(II) availability within different niches in the infected host. Increases in Zn(II) availability promote microbial poisoning while decreases in Zn(II) availability promote microbial starvation. These antagonistic host responses, known as “nutritional immunity”,23 suppress microbial growth in the host and inhibit the progress of infectious disease. Although Zn(II) influences the activity of Hst5,22 it is unclear whether histatins themselves participate in nutritional immunity by modulating Zn(II) availability to microbes.
The healthy human oral cavity and oropharynx are colonized by a mixture of microbial species, with Streptococcus as the most abundant taxon.24−28 Some species, such as S. gordonii and S. sanguinis, are considered commensals. These species contribute to oral health, for example, by inhibiting colonization by competitor species.29,30 Some streptococcal species are considered pathogenic. For example, S. mutans and S. pyogenes are associated with dental caries and pharyngitis,31 respectively. Nevertheless, asymptomatic carriage of these pathogenic species is common32 and these species are generally considered normal components of the healthy oral and oropharyngeal microflora. Importantly, all streptococci are opportunistic pathogens that can cause disseminated infections, such as bacterial infective endocarditis.33
The goals of this study were to determine the antibacterial activity of Hst5 against oral and oropharyngeal streptococci, and to investigate the potential role of this peptide in influencing Zn(II) availability to the streptococci as a component of nutritional immunity. Based on the established features of nutritional immunity, we specifically examined whether Hst5 limits Zn(II) availability (and promotes microbial Zn(II) starvation) and/or raises Zn(II) availability (and promotes Zn(II) poisoning).
Results
Hst5 Does Not Kill Oral or Oropharyngeal Streptococci
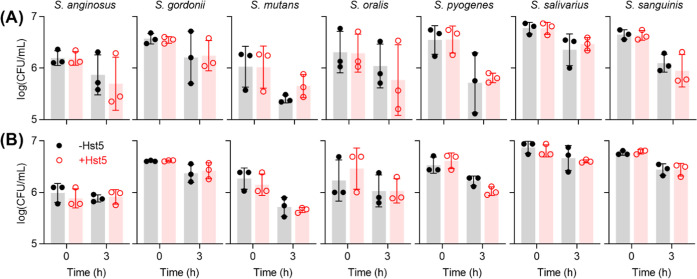
There is little consensus regarding the antibacterial activity of Hst5 against streptococci—it varies depending on the species or experimental conditions,34−40 but the chemical and molecular reasons for these discrepancies have not been identified. In this work, the ability of Hst5 to kill seven oral or oropharyngeal streptococci, namely, S. anginosus, S. gordonii, S. mutans, S. oralis, S. pyogenes, S. salivarius, and S. sanguinis, was examined in parallel. Following the approach used previously for C. albicans and ESKAPE pathogens, these kill assays were performed for several hours in dilute phosphate buffer (10 mM).16,20 Under these conditions, up to 50 μM Hst5 (ca. total histatin concentrations in fresh salivary secretions13) did not promote killing of the streptococcal species (Figure 1A), even when the assay was extended to 24 h (Figure S1). Consistent with a previous report,16 parallel control experiments showed that Hst5 killed P. aeruginosa and C. albicans (Figure S2), confirming that our peptide preparations were active.
Figure 1.
Effects of Hst5 on survival of streptococci in (A) phosphate buffer and (B) artificial saliva buffer. Bacteria were incubated in phosphate buffer (10 mM, pH 7.4; N = 3) or artificial saliva buffer (pH 7.2–7.4; N = 3), with (○) or without (●) Hst5 (50 μM), and sampled at t = 0 and 3 h for enumeration. Hst5 did not affect the survival of any species in either buffer (P = 0.73, 0.99, 0.57, 0.72, 0.85, 0.71, 0.50 in phosphate buffer, and 0.72, 0.71, 0.43, 0.56, 0.52, 0.48, and 0.86 in artificial saliva buffer, for S. anginosus, S. gordonii, S. mutans, S. oralis, S. pyogenes, S. salivarius, and S. sanguinis, respectively).
Like other cationic antimicrobial peptides, the antimicrobial activity of Hst5 is influenced by pH and ionic strength.16,19,41−45 To better reflect the physiological context in which Hst5 plays a role, the kill assays were repeated in an artificial, synthetic “saliva buffer”, whose pH and ionic composition approximate that of saliva (Table S1A). Again, Hst5 did not kill any of the streptococci (Figures 1B and S1). Interestingly, under these new conditions, Hst5 did not kill the control organisms P. aeruginosa and C. albicans (Figure S2). The high ionic strength of the saliva buffer likely interferes with electrostatic binding of the peptide to surface proteins or membranes of these control organisms,16,46 and subsequent internalization and killing. To better understand the activity of Hst5 under conditions that are more characteristic of saliva, further kill assays below used the artificial saliva buffer.
Zn(II) Does Not Influence the Activity of Hst5 against Streptococci
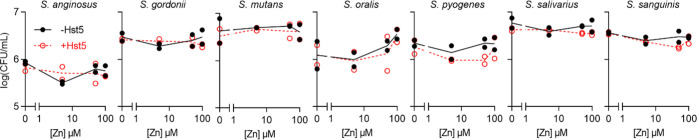
Saliva typically contains low micromolar levels of total Zn(II) (between 0.2 and 3 μM have been reported47), although the speciation or bioavailability of this metal ion is poorly defined. Our artificial saliva buffer is Zn(II)-deplete (low nanomolar concentrations of Zn(II) are routinely detected by inductively coupled plasma mass spectrometry (ICP MS)). Thus, to determine if the activity of Hst5 against streptococci is Zn(II)-dependent, the kill assays were repeated in the presence of added Zn(II). The results showed that added Zn(II), whether substoichiometric (5 μM), stoichiometric (50 μM), or super-stoichiometric (100 μM) relative to Hst5 (50 μM), neither suppresses nor enhances killing of the seven streptococcal species by Hst5 (Figure 2).
Figure 2.
Effects of Zn(II) and Hst5 on survival of streptococci in artificial saliva buffer. Bacteria (N = 2) were incubated in artificial saliva buffer in the presence of added Zn(II) (0, 5, 50, or 100 μM), with (○) or without (●) Hst5 (50 μM), and sampled at t = 3 h for enumeration. Addition of Zn(II) did not influence the effects of Hst5 on the survival of any species (P values for the interaction between Zn(II) and Hst5 = 0.40, 0.46, 0.96, 0.98, 0.69, 0.45, and 0.09 for S. anginosus, S. gordonii, S. mutans, S. oralis, S. pyogenes, S. salivarius, and S. sanguinis, respectively).
Hst5 Does Not Contribute to Zn(II)-Dependent Nutritional Immunity
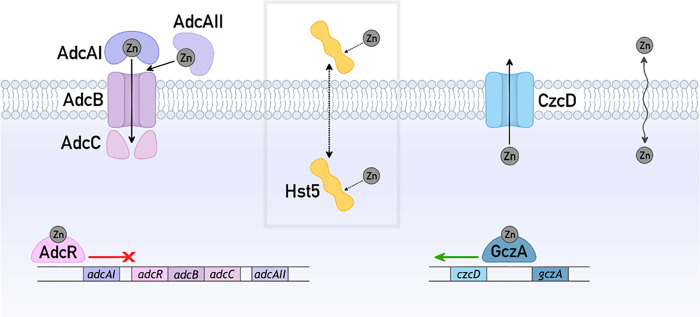
To determine whether Hst5 contributes to Zn(II)-dependent nutritional immunity against streptococci, either by promoting Zn(II) starvation or Zn(II) poisoning, we examined the effects of Hst5 on transcription of Zn(II)-responsive genes. S. pyogenes (Group A Streptococcus or GAS) was used as a model Streptococcus, since the transcriptional responses of this species to varying Zn(II) availability is understood (Figure 3), mutant strains lacking key Zn(II) transport proteins are available in our laboratory, and the phenotypes of these mutant strains are known.48
Figure 3.
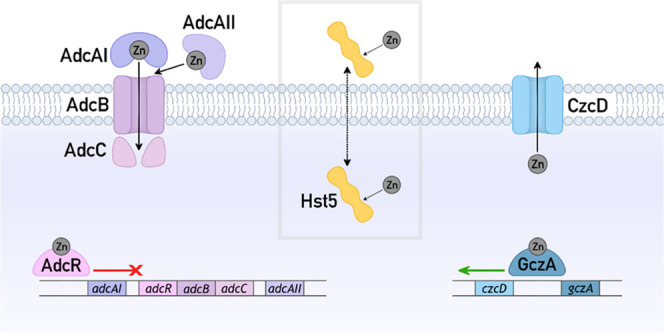
Zn(II) homeostasis in GAS and hypothesized actions of Hst5. Zn(II) uptake: AdcAI and AdcAII capture extracellular Zn(II) and transfer this metal to AdcBC for import into the cytoplasm. These proteins are transcriptionally upregulated in response to decreases in Zn(II) availability and Zn(II) starvation (and downregulated in response to increases in Zn(II) availability).49 Alternatively, Zn(II) may enter the cytoplasm via nonspecific cation transporters (wavy arrow). Zn(II) efflux: CzcD exports excess Zn(II) out of the cytoplasm. It is transcriptionally upregulated by GczA in response to increases in Zn(II) availability and Zn(II) poisoning.50 Alternatively, Zn(II) may exit the cytoplasm via nonspecific cation transporters (wavy arrow). Hypothesized actions of Hst5: Hst5 may bind extracellular Zn(II) and either remain extracellular to suppress Zn(II) availability or become internalized as the Zn(II)–Hst5 complex and increase Zn(II) availability. Alternatively, Hst5 may enter the cytoplasm (dotted arrow), bind intracellular Zn(II), and suppress intracellular Zn(II) availability.
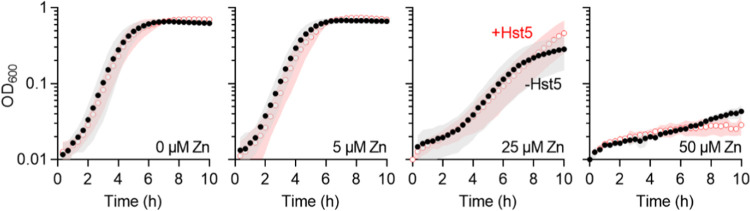
In response to decreases in Zn(II) availability and Zn(II) starvation, GAS upregulates transcription of the AdcR regulon, including adcAI and adcAII. Conversely, in response to increases in Zn(II) availability and Zn(II) poisoning, GAS upregulates transcription of the GczA regulon, including czcD. Expression of adcAI, adcAII, and czcD, with and without Hst5, was thus examined here. However, poor RNA yields were obtained from the static (nongrowing) bacterial suspensions used in the kill assays. As an alternative approach, GAS was grown in a metal-deplete (low nanomolar concentrations of Zn(II) are routinely detected by ICP MS), chemically defined medium (CDM).51 GAS displayed the same phenotypes in CDM and in artificial saliva buffer, i.e., addition of up to 50 μM Hst5 did not affect the growth of this streptococcus and addition of Zn(II) did not influence this outcome (Figure 4), thus validating the approach.
Figure 4.
Effects of Zn(II) and Hst5 on growth of GAS. Bacteria (N = 3) were cultured in CDM in the presence of Zn(II) (0, 5, 25, or 50 μM), with (○) or without (●) Hst5 (50 μM), and sampled every 20 min for a total of 10 h. While addition of Zn(II) inhibited bacterial growth (P = 1.0, <0.0001, and <0.0001 for 5, 25, and 50 μM Zn(II), respectively), addition of Hst5 did not influence this effect (P = 0.88, 0.82, 0.83, and 0.56 for 0, 5, 25, and 50 μM Zn(II), respectively).
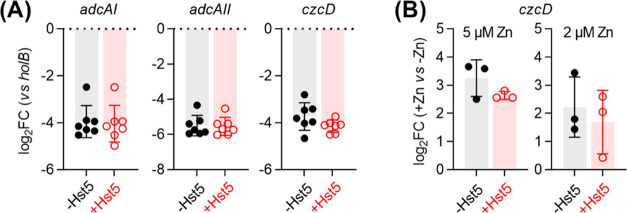
In the control experiment, adding Zn(II) alone did not perturb transcription of adcAI and adcAII in wild-type GAS, but it did induce expression of czcD (Figure S3A), consistent with an increase in cellular Zn(II) availability or Zn(II) poisoning. Conversely, adding the Zn(II) chelator TPEN induced expression of adcAI and adcAII, consistent with a decrease in cellular Zn(II) availability or Zn(II) starvation, but it did not perturb transcription of czcD (Figure S3B). By contrast, adding Hst5 perturbed neither the basal expression of adcAI or adcAII (Figure 5A) nor the Zn(II)-dependent expression of czcD (Figure 5B). These results indicate that Hst5 promotes neither Zn(II) starvation nor Zn(II) poisoning to GAS and that Hst5 does not contribute to Zn(II)-dependent nutritional immunity against GAS.
Figure 5.
Effects of Hst5 on expression of Zn(II)-responsive genes in GAS. (A) Background expression of all genes. Bacteria (N = 7) were cultured in CDM with (○) or without (●) Hst5 (50 μM). Levels of adcAI, adcAII, and czcD mRNA were determined by quantitative real-time polymerase chain reaction (qRT-PCR) and normalized to holB. Addition of Hst5 did not affect the background expression of any of the three genes (P = 0.35, 0.74, and 0.08 for adcAI, adcAII, and czcD, respectively). (B) Zn(II)-dependent expression of czcD. Bacteria (N = 3) were cultured in CDM with or without added Zn(II) (2 or 5 μM), with (○) or without (●) Hst5 (50 μM). Levels of czcD mRNA were measured by qRT-PCR, normalized to holB, and compared with normalized mRNA levels of the corresponding untreated controls (0 μM added Zn(II)). Addition of Hst5 did not affect Zn(II)-dependent expression of czcD (P = 0.21 and 0.71 for 2 and 5 μM Zn(II), respectively).
Hst5 Weakly Suppresses Zn(II) Toxicity
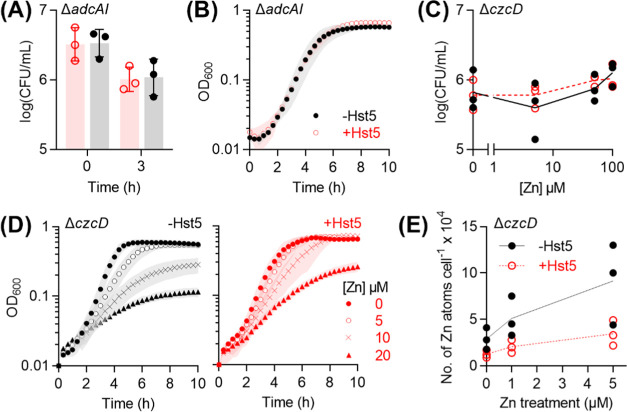
To further explore the hypothesized role of Hst5 in modulating Zn(II) availability, the effects of Hst5 were examined using GAS ΔadcAI and ΔczcD mutant strains that are deficient in Zn(II) uptake and Zn(II) efflux, respectively (Figure 3). These mutant strains were validated to be sensitive to growth inhibition by the Zn(II) chelator TPEN52,53 and added Zn(II),50,53 respectively (Figure S4). Although additional Zn(II)-binding lipoproteins such as AdcAII contribute to Zn(II) uptake, AdcAI is thought to act as the primary Zn(II) uptake lipoprotein.52,53 Therefore, only the ΔadcAI mutant was employed here.
The ΔadcAI mutant strain displayed wild-type survival and growth phenotypes in the presence of Hst5 (Figure 6A,B), strengthening our proposal that Hst5 does not starve GAS of nutrient Zn(II). Similarly, the ΔczcD mutant strain displayed wild-type survival phenotype (Figure 6C). However, mild differences between the ΔczcD mutant and wild-type strains were observed in growth experiments. While Hst5 did not influence the growth of Zn(II)-treated wild-type organism (see Figure 4), Hst5 weakly but reproducibly improved the growth of the Zn(II)-treated ΔczcD mutant strain (Figure 6D). This effect was observed most clearly upon comparing final culture densities after 10 h of growth since the exponential growth rates were unaffected (Figure S5). This growth-promoting effect of Hst5 appeared to require the predicted Zn(II)-binding ligands His15, His18, and His1954,55 since the ΔH15,18,19 variant of Hst5 did not rescue the growth of the Zn(II)-treated ΔczcD mutant strain (Figure S6, see Table 1 for peptide sequences). These results suggest that Hst5 binds to Zn(II) and suppresses (instead of enhances) the toxicity of an excess of this metal ion to GAS.
Figure 6.
Effects of Hst5 on Zn(II) availability. (A) Survival of ΔadcAI. Bacteria (N = 3) were incubated in artificial saliva buffer, with (○) or without (●) Hst5 (50 μM), and sampled at t = 0 and 3 h for enumeration. Hst5 did not affect the time-dependent survival of the ΔadcAI mutant (P = 0.90). (B) Growth of ΔadcAI. Bacteria (N = 2) were cultured in CDM with or without Hst5 (50 μM). Hst5 did not affect the growth of the ΔadcAI mutant (P = 0.26). (C) Survival of ΔczcD. Bacteria (N = 3) were incubated in artificial saliva buffer, with or without added Zn(II) (0, 5, 50, or 100 μM), with (○) or without (●) Hst5 (50 μM). Hst5 did not affect the Zn(II)-dependent survival of the ΔczcD mutant (P value for the interaction between Hst5 and Zn(II) = 0.73). (D) Growth of ΔczcD. Bacteria (N = 3) were cultured in CDM in the presence of Zn(II) (0–20 μM), with (○) or without (●) Hst5 (50 μM). Hst5 did not affect the growth of the ΔczcD mutant in the absence of Zn(II) (P = 0.61) but it did affect growth in the presence of Zn(II) (P = 0.07, 0.02, and 0.01 for 5, 10, and 20 μM Zn(II), respectively). (E) Levels of cell-associated Zn(II) in ΔczcD. Bacteria (N = 3) were cultured in CDM in the presence of Zn(II) (0–5 μM), with (○) or without (●) Hst5 (50 μM), and sampled at t = 4 h. Levels of cell-associated Zn(II) were measured by ICP MS and normalized to colony counts. Addition of Hst5 had a negative effect on cellular Zn(II) levels (P = 0.005).
Two mechanisms are plausible (see Figure 3): (i) Hst5 binds extracellular Zn(II) and suppresses accumulation of this metal ion in the cytoplasm, leading to less Zn(II) toxicity, or (ii) Hst5 binds cellular Zn(II) and enables more Zn(II) to accumulate in the cytoplasm, but with less toxicity. To distinguish between these models, total cell-associated Zn(II) levels in the ΔczcD mutant strain were assessed by ICP MS. Only up to 5 μM Zn(II) was used, since adding 10 μM Zn(II) or more into the cultures inhibited the growth of the ΔczcD mutant and did not produce sufficient biomass for metal analyses. Only wild-type Hst5 peptide was used, owing to the large culture volumes required and the high cost of peptide synthesis. Figure 6E shows that Zn(II) treatment increased cell-associated Zn(II) levels in the ΔczcD mutant, but co-treatment with Hst5 suppressed this effect. These results are consistent with model (i) above, in which Hst5 binds extracellular Zn(II) and suppresses accumulation of Zn(II) in GAS.
Hst5 Binds Zn(II) with Micromolar Affinities
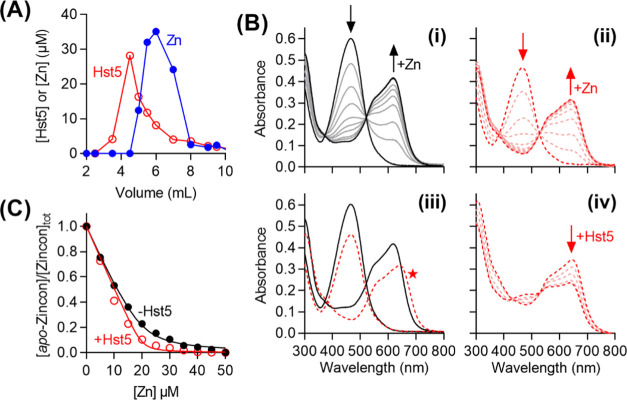
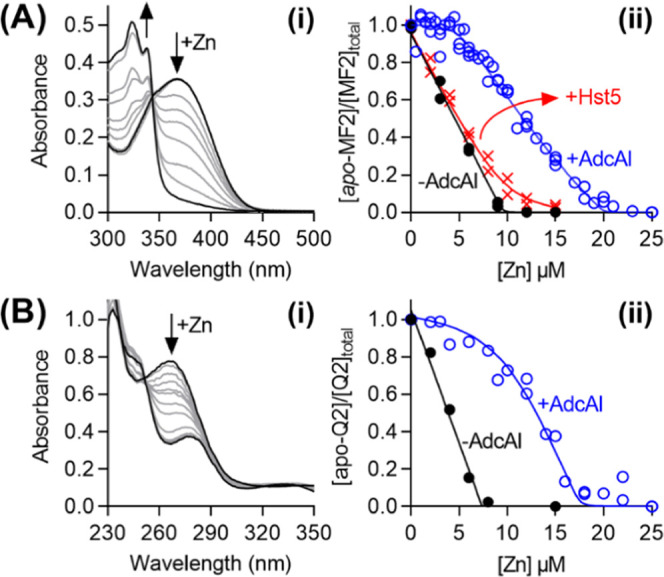
To understand how Hst5 weakly modulates Zn(II) availability to GAS and suppresses the toxicity of excess Zn(II) without promoting nutrient Zn(II) starvation, we examined the ability of this peptide to bind Zn(II). Hst5 is thought to bind up to three Zn(II) ions. Previous measurements by isothermal titration calorimetry (ITC) yielded log KZn(II) values of 5.1, 5.0, and 4.0,56 indicating that each Zn(II) ion binds to Hst5 with a high micromolar affinity. In agreement with this proposal, a high micromolar concentration of the Zn(II)–Hst5 complex readily dissociated upon passage through a desalting column (Figure 7A). The affinities of Hst5 to Zn(II) were further re-examined here by competing the peptide with the colorimetric Zn(II) indicator Zincon (log KZn(II) ∼ 6.0) in (Mops) buffer and by monitoring solution absorbances of apo-Zincon (466 nm) and Zn(II)-Zincon (620 nm) (Figure 7B). The competition curve (in the presence of Hst5) was nearly indistinguishable from the control (in the absence of Hst5) (Figure 7C). Moreover, a new peak at 650 nm appeared in the presence of Hst5 (Figure 7B(iii)), indicating the formation of a new species, likely a ternary complex between Hst5, Zincon, and Zn(II). This peak did not disappear upon adding excess Hst5 (10 molar equiv; Figure 7B(iv)). These results indicate that Hst5 does not compete effectively with Zincon and that this peptide binds Zn(II) with high micromolar affinities, as previously estimated by ITC.56
Figure 7.
Zn(II) affinity of Hst5. (A) Separation of Hst5 (○) and Zn(II) (●) on a polyacrylamide desalting column. (B) Representative spectral changes upon addition of Zn(II) (0–50 μM) into apo-Zincon (20 μM): (i) in the absence (solid traces) or (ii) presence (dashed traces) of Hst5 (20 μM). (iii) Overlaid spectra for 0 and 50 μM Zn(II) from panels (i) and (ii). The new peak at 650 nm is indicated with a star. (iv) Representative spectral changes upon addition of excess Hst5 (0–200 μM) into a solution of Zn(II) (20 μM) and apo-Zincon (25 μM). (C) Normalized plot of the absorbance intensities of apo-Zincon at 467 nm upon addition of Zn(II), in the absence (●) or presence (○) of Hst5 (20 μM).
The lack of competition between Hst5 and Zincon as shown in Figure 7 contrasts with a previous study showing an effective competition between Hst5 and Zincon in phosphate buffer, with Hst5 removing 2 molar equiv of Zn(II) from Zincon.20 Here it is important to highlight that phosphate binds to Zn(II). Although the affinity of phosphate to Zn(II) is relatively low (log KZn(II) ∼ 2.4),57 when used at millimolar concentrations, phosphate can interfere with Zn(II) binding studies by competing for Zn(II). Addition of Zn(II) to apo-Zincon in phosphate buffer (50 mM) instead of Mops buffer led to incomplete formation of Zn(II)-Zincon (monitored at 620 nm), suggesting that Zn(II) partitioned between Zincon and phosphate (Figure S7A,B). Conversely, prolonged incubation (>10 min) of a pre-formed Zn(II)-Zincon complex in phosphate buffer led to a loss of the characteristic blue color (Figure S7C), indicating removal of Zn(II) from Zn(II)-Zincon by phosphate alone (without adding Hst5). Therefore, our studies of Zn(II) binding by Hst5 in Mops buffer are likely to be more reliable.
AdcAI from GAS Binds Zn(II) with Sub-Nanomolar Affinity
The low affinity of Hst5 to Zn(II) was clearly insufficient to starve wild-type GAS of nutrient Zn(II) (see Figure 5A), indicating that this peptide does not compete with the high-affinity, Zn(II)-specific uptake protein AdcAI (see Figure 3). Therefore, the Zn(II) affinities of AdcAI were examined here by competition with the colorimetric Zn(II) indicator Mag-fura2 (Mf2). The competition curve, generated by monitoring the solution absorbance of apo-Mf2 at 377 nm (Figure 8A(i)), clearly showed two Zn(II) binding sites in AdcAI as anticipated.58 The high-affinity Zn(II) binding site outcompeted Mf2, as evidenced by the lack of spectral changes upon adding up to 1 molar equiv of Zn(II) vs AdcAI (Figure 8A(ii)). The low-affinity site competed effectively with Mf2 with a log KZn(II) = 8.5 (±0.2). The high-affinity site was better estimated using Quin-2 (Q2) as a competitor. By monitoring the absorbance of apo-Q2 at 266 nm, log KZn(II) = 12.5 (±0.2) was obtained for this site (Figure 8B).
Figure 8.
Zn(II) affinity of AdcAI. (A) Low-affinity site. (i) Representative spectral changes upon titration of Zn(II) (0–25 μM) into a mixture of apo-Mf2 (10 μM) and AdcAI (5 μM). (ii) Normalized plot of the absorbance intensities of apo-MF2 (10 μM) at 377 nm upon addition of Zn(II), in the absence (●) or presence (○) of AdcAI (5 μM). Competition with Hst5 (X; 10 μM) is shown for comparison. (B) High-affinity site. (i) Representative spectral changes upon titration of Zn(II) (0–25 μM) into a mixture of apo-Q2 (7.5 μM) and AdcAI (10 μM). (ii) Normalized plot of the absorbance intensities of apo-Q2 (7.5 μM) at 262 nm upon addition of Zn(II), in the absence (●) or presence (○) of AdcAI (10 μM).
The log KZn(II) values for AdcAI determined here were each ∼1000-fold higher than those determined previously by ITC.58 ITC can underestimate high metal binding affinities due to lack of sensitivity.59 Crucially, Hst5 did not compete with Mf2 for Zn(II) (Figure 8A(ii)). Thus, the relative affinities between Hst5 and AdcAI, determined using the same approach under the same conditions, support the hypothesis that Hst5 does not compete with AdcAI for binding Zn(II). These relative affinities also provide a molecular explanation for why Hst5 does not suppress the availability of nutrient Zn(II) to wild-type GAS.
Hst5 did not affect the growth of GAS even when AdcAI was deleted by mutagenesis (see Figure 6A,B), suggesting that this peptide does not compete with other high-affinity Zn(II) uptake proteins such as AdcAII (see Figure 3). AdcAII was also expressed here for measurements of Zn(II) affinity. However, consistent with a previous report,60 recombinant AdcAII co-purified with 1 molar equiv of bound Zn(II), which could not be removed without denaturing the protein. Nevertheless, the reported apparent affinity of the S. pneumoniae homologue to Zn(II) (log KZn(II) = 7.7; 67% identity, 81% similarity), determined via competition with Mf2,61 is ∼100-fold higher than that of Hst5, consistent with our proposal that Hst5 does not compete effectively with AdcAII for binding Zn(II).
Discussion
Role of Histatins as Salivary Antimicrobial Agents
The oral cavity is rich in saliva, and interactions between with the components of this host fluid are key for colonization, maintenance, infection, and subsequent transmission of streptococci.62−64 For example, exposure to saliva promotes aggregation of some streptococci and blocks adherence to mucosal epithelia.65,66 Saliva also contains polysaccharides and glycoproteins that may serve as sources of nutrients. Finally, antimicrobial peptides and enzymes such as lysozyme, lactoperoxidase, and chitinase directly inhibit or kill streptococci.67
Given the widely reported antimicrobial activity of Hst5, histatins are thought to function as salivary antimicrobial peptides. Yet, our work shows that Hst5 does not kill seven oral and oropharyngeal streptococcal species under in vitro experimental conditions that are characteristic of saliva. It is tempting to speculate that histatins help shape the microbial composition in the healthy oral cavity by suppressing the viability of some microbes (e.g., C. albicans) but not others (e.g., streptococci). Future work should carefully examine this potential for histatins to exert a selective antimicrobial activity, to verify that it is not associated only with low ionic strength conditions that are not characteristic of saliva. For example, our work showed that antimicrobial activity of Hst5 against C. albicans and P. aeruginosa disappears when examined in our artificial saliva buffer (see Figure S2).
To date, there is no consensus as to whether histatin levels in saliva correlate with infection levels in the oral cavity. Comparisons of children or adult patients with and without dental caries have found no variation in salivary histatin levels,68,69 higher salivary histatin levels in patients with caries,70,71 and lower salivary histatin levels in patients with caries.72−74 Similarly, there is no clear correlation between histatin levels and the prevalence of oral C. albicans in healthy people75 but high histatin levels do correlate with high prevalence of oral candidiasis in immunocompromised patients.76 It is important to note that distinct ecological niches exist within the oral cavity. These niches differ in, among many variables, nutrient content, pH, and oxygen tension. Our work does not discount the possibility that histatins exert strong and selective antimicrobial activity in some niches.
Interactions between Zn(II) and Histatins
Systems for the uptake and efflux of metals such as Zn(II) are important for the survival of streptococci in the oral cavity and oropharynx since salivary concentrations of metals can fluctuate, for example, during and between meals, disease, or human hygiene and dental interventions. In addition, salivary components such as lactoferrin and calprotectin sequester metals and restrict microbial growth.
Our work showed that Hst5 does not contribute to Zn(II)-dependent nutritional immunity against streptococci, since this peptide neither starves our model Streptococcus (S. pyogenes or GAS) of nutrient Zn(II) nor enhances Zn(II) toxicity to this bacterium. These findings are consistent with results from a genome-wide screen of a GAS mutant library, which did not identify genes involved in Zn(II) uptake or Zn(II) efflux as essential for growth in saliva.77 Given the general conservation of Zn(II) homeostasis mechanisms among the streptococci, we anticipate that Hst5 does not contribute to Zn(II)-dependent nutritional immunity against other streptococci.
The low affinity of Hst5 to Zn(II), particularly compared with the high affinities of the Zn(II) uptake lipoproteins AdcAI and AdcAII, explains why Hst5 does not starve GAS (and, presumably, other streptococci) of nutrient Zn(II). Here, the antimicrobial protein calprotectin provides a useful comparison. Calprotectin binds two Zn(II) ions with affinities (log KZn(II) > 11 and >9.6)78 that are comparable to those of AdcAI and higher than that of AdcAII. Indeed, adding calprotectin induces a robust Zn(II) starvation response in streptococci,79,80 consistent with its established role in nutritional immunity.
Its low affinity to Zn(II) also explains why Hst5 only weakly suppresses the availability of excess (toxic) Zn(II) to GAS in vitro. Like most culture media, our CDM51 contains phosphate (∼6 mM) and amino acids (∼6 mM total), which would outcompete Hst5 (50 μM) for binding Zn(II).57 However, if these competing ligands become depleted, for example as a result of bacterial growth, then Hst5 may become competitive and bind Zn(II), particularly when Zn(II) concentrations are high. Such shifts in Zn(II) speciation likely explain why the protective effect of Hst5 on the GAS ΔczcD mutant strain during conditions of Zn(II) stress became apparent only at the later stages of growth (see Figure S5). The increased binding of Zn(II) to Hst5 in these later stages of growth may suppress nonspecific Zn(II) import into the GAS cytoplasm, for instance by outcompeting promiscuous divalent metal transporters.
Unlike in vitro growth media, saliva and its components are continuously replenished in vivo. Saliva contains ∼10 mM phosphate81,82 and proteinaceous components that also bind Zn(II).83 Thus, in vivo, Hst5 is unlikely to be competitive for binding Zn(II). Nonetheless, synergistic effects between Zn(II) and Hst5 may occur in vivo, but likely via indirect mechanisms that do not rely on direct binding of Zn(II) to Hst5 and formation of a Zn(II)–Hst5 complex. Zn(II) and Hst5 may separately target the same cellular pathways in a microbe, leading to the enhancement of the antimicrobial activity of Hst5 by Zn(II). Alternatively, Zn(II) may disable cellular pathways that render the target microbe more susceptible to the separate action of Hst5 on a different cellular pathway (or vice versa), again leading to the enhancement of microbial killing. Indirect interactions between Zn(II) and Hst5 may also exert subtle effects on microbial physiology that do not lead to a direct antimicrobial action and thus are not captured by the assays described here. For example, a combination of Zn(II) and Hst5 at nonlethal doses is thought to reduce the virulence of C. albicans.84 Whether Hst5 reduces the virulence of streptococci and subsequently enables these organisms to become the dominant commensal microorganisms in the oral cavity and oropharynx is an intriguing concept that warrants further investigation.
Methods
Data Presentation
Except growth curves, individual replicates from microbiological experiments are plotted, with shaded columns representing the means and error bars representing standard deviations. Growth curves show the means, with shaded regions representing standard deviations. The number of biological replicates (independent experiments, using different starter cultures and different medium or buffer preparations, performed on different days; N) is stated in figure legends. In the case of metal–protein and metal–peptide titrations, individual data points from two technical replicates (independent experiments performed on different days but using the same protein or peptide preparation) are plotted, but only representative spectra are shown for clarity of presentation.
Statistical Analyses
Descriptive statistics are displayed on all graphical plots. Inferential statistics have been computed for all data and the relevant P values are listed in figure legends. Unless otherwise stated, tests of significance used two-way analysis of variance using the statistical package in GraphPad Prism 8.0. All analyses were corrected for multiple comparisons.
Reagents
The nitrate salt of Zn(II) was used in experiments. Numerous additional tests did not identify any observable difference in the results when the chloride or sulfate salts of Zn(II) were used. Peptides were synthesized commercially with free N- and C-termini as the acetate salt, purified to >95% (GenScript), and confirmed to be metal-free by ICP MS. Concentrations of stock peptide solutions were estimated using solution absorbances at 280 nm in Mops buffer (50 mM, pH 7.4; ε280 = 2667 cm–1). Concentrations of fluorometric and colorimetric metal indicators (Zincon, PAR, Mf2, Q2) were standardized using a commercial standard solution of copper chloride. Concentrations of optically silent chelators (NTA) were standardized by competition with a standardized solution of Zn(II)-Zincon.
Strains and Culture Conditions
All bacterial strains (Table S1B) were propagated from frozen glycerol stocks onto solid THY (Todd Hewitt + 0.2% yeast extract) medium without any antibiotics and incubated overnight in the presence of 5% v/v of atmospheric CO2. Liquid cultures were prepared in THY or CDM.51 All solid and liquid growth media contained catalase (50 μg/mL).
Streptococcal Kill Assays
Fresh colonies from an overnight THY agar were resuspended to 106–107 CFU/mL in either potassium phosphate buffer (10 mM, pH 7.4) or artificial saliva buffer (pH 7.2; Table S1A). The cultures were incubated at 37 °C with or without Hst5 and/or Zn(II) as required. At t = 0 and 3 h, cultures were sampled and serially diluted in CDM. Exactly 10 μL of each serial dilution was spotted onto THY agar. Colonies were enumerated after overnight incubation at 37 °C.
C. albicans Kill Assays
Cells from a fresh YPD plate were harvested, washed three times in phosphate-buffered saline (PBS), and resuspended in either potassium phosphate buffer (10 mM, pH 7.4) or saliva salts (pH 7.2) to an OD600 of 0.4 (∼5 × 106 CFU/mL). Cultures were incubated with or without Hst5 at 37 °C. Tubes were inverted every 20 min to maintain cell suspension. At t = 0, 1, and 3 h, samples were taken, serially diluted, and plated onto YPD agar. Colonies were numerated following overnight incubation at 30 °C.
Growth Assays
Colonies from an overnight THY agar were resuspended in CDM to an OD600 = 0.02 and dispensed into wells in flat-bottomed 96-well plates (200 μL per well) containing Hst5 and/or Zn(II) as required. Bacterial growth was monitored using an automated microplate shaker and reader. Each plate was sealed with a gas-permeable, optically clear membrane (Diversified Biotech). OD600 values were measured every 20 min for 10 h. The plates were shaken immediately before each reading (200 rpm, 1 min, double-orbital mode). OD600 values were not corrected for path length (ca. 0.58 cm for a 200 μL culture).
RNA Extraction
Colonies from an overnight THY agar were resuspended in CDM to an OD600 = 0.02 and incubated in 24-well plates (1.6 mL per well), with or without Hst5 or Zn(II) as required, without shaking, at 37 °C. Each plate was sealed with a gas-permeable, optically clear membrane (Diversified Biotech). At t = 4 h, cultures were centrifuged (4000g, 4 °C, 5 min) and the resulting bacterial pellets were resuspended immediately in RNAPro Solution (0.5 mL; MP Biomedicals). Bacteria were lysed in Lysing Matrix B and total RNA was extracted following the manufacturer’s protocol (MP Biomedicals). Crude RNA extracts were treated with RNase-Free DNase I (New England Biolabs). Removal of gDNA was confirmed by PCR using gapA-check-F/R primers (Table S1C). gDNA-free RNA was purified using Monarch RNA Clean-up Kit (New England Biolabs) and visualized on an agarose gel.
qRT-PCR Analyses
cDNA was generated from RNA (1.6 μg) using SuperScript IV First-Strand Synthesis System (Invitrogen). Each qRT-PCR reaction (20 μL) contained cDNA (5 ng) as template and the appropriate primer pairs (0.4 μM; Table S1C). Samples were analyzed in technical duplicates. Amplicons were detected with Luna Universal qRT-PCR Master Mix (New England Biolabs) in a CFXConnect Real-Time PCR Instrument (Bio-Rad Laboratories). Cq values were calculated using LinRegPCR85 after correcting for amplicon efficiency. Cq values of technical duplicates were typically within ±0.25 of each other. holB, which encodes DNA polymerase III, was used as reference gene. Its transcription levels were verified to remain constant in the experimental conditions tested here.
Cellular Metal Content
Colonies from an overnight THY agar were resuspended in CDM to an OD600 = 0.02 and incubated at 37 °C with or without Hst5 and/or Zn(II) as required. At t = 4 h, an aliquot was collected for the measurement of plating efficiency (colony counts). The remaining cultures were centrifuged (5000g, 4 °C, 10 min). The resulting bacterial pellets were washed once with ice-cold wash buffer (1 M d-sorbitol, 50 mM Tris–HCl, 10 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4) and twice with ice-cold PBS. The final pellets were dissolved in concentrated nitric acid (100 μL), heated (85 °C, 1.5 h), and diluted to 3.5 mL with 2% nitric acid. Total metal levels were determined by ICP MS and normalized to colony counts.
Elution of Zn(II)–Hst5 on a Desalting Column
Apo-Hst5 (100 μM) was incubated with 1.5 molar equiv of Zn(II) for 15 min at the bench and loaded onto a polyacrylamide desalting column (1.8 kDa molecular weight cutoff, Thermo Scientific). Peptide and Zn(II) were eluted from the column using Mops buffer (50 mM, pH 7.4). The concentration of Hst5 in each fraction was determined using QuantiPro BCA Assay Kit (Merck) and known quantities of Hst5 as standards. The concentration of Zn(II) was determined using the colorimetric Zn(II) ligand PAR against a standard curve.
Equilibrium Competition Reactions
Our approach to determine metal-binding affinities followed that described by Young and Xiao.59 For each competition (eq 1), a master stock was prepared to contain both competing ligands (L1 and L2) in Mops buffer (50 mM, pH 7.4). Serial dilutions of the metal (M) were prepared separately in deionized water. Exactly 135 μL of the master stock was dispensed into an Eppendorf UVette and 15 μL of the appropriate metal stock was added. Solution absorbances were recorded and used to calculate concentrations of apo- and metalated forms of each ligand. These concentrations were plotted against metal concentrations and fitted in DynaFit86 using binding models as described in the text. The known association or dissociation constants for all competitor ligands are listed in Table S1D
| 1 |
Overexpression and Purification of AdcAI and AdcAII
Nucleic acid sequences encoding the soluble domains of AdcAI (from Thr21) and AdcAII (from Thr31) from M1GAS strain 5448 were subcloned into vector pSAT1-LIC using primers listed in Table S1C. This vector generates N-terminal His6-SUMO fusions with the target proteins. The resulting plasmids were propagated in Escherichia coli Dh5α, confirmed by Sanger sequencing, and transformed into E. coli BL21 Rosetta 2(DE3).
To express the proteins, transformants were plated onto Lysogeny Broth (LB) agar. Fresh colonies were used to inoculate LB (1 L in 2 L baffled flasks) to an OD600 of 0.01. The culture medium contained ampicillin (100 μg/mL) and chloramphenicol (33 μg/mL). Cultures were shaken (200 rpm, 37 °C) until an OD600 of 0.6–0.8 was reached, and expression was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) (0.1 mM). After shaking for a further 16 h at 20 °C, the cultures were centrifuged (4000g, 4 °C) and the pellets were resuspended in buffer A500 (20 mM Tris–HCl, pH 7.9, 500 mM NaCl, 5 mM imidazole, 10% glycerol).
To purify proteins, bacteria were lysed by sonication (40 kpsi), centrifuged (20,000g, 4 °C), and filtered through a 0.45 μm poly(ether sulfone) (PES) membrane filtration unit. Clarified lysates were loaded onto a HisTrap HP column (Cytiva). The column was washed with 10 column volumes (CV) of buffer A500 followed by 10 CV of buffer A100 (20 mM Tris–HCl, pH 7.9, 100 mM NaCl, 10% w/v glycerol) containing imidazole (5 mM). Both AdcAI and AdcAII were bound to the column and subsequently eluted with 3 CV of buffer A100 containing 250 mM imidazole followed by 5 CV of 500 mM imidazole. Protein-containing fractions were loaded onto a Q HP column (Cytiva). The column was washed with 5 CV of buffer A100 and bound proteins were eluted using a step gradient of 0, 10, 15, and 20% buffer C1000 (20 mM Tris–HCl, pH 7.9, 1000 mM NaCl, 10% w/v glycerol). Eluted proteins were incubated overnight at 4 °C with hSENP2 SUMO protease to cleave the His6-SUMO tag from the target protein. Samples were passed through a second Q HP column and the flowthrough fractions containing untagged target protein were collected.
Acknowledgments
This work was funded in part by the Wellcome Trust grant number 214930/Z/18/Z to K.Y.D and L.J.S., and 215599/Z/19/Z to J.Q. and Y.A. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This project was also supported by a Flexible Funding Award from the Durham Biophysical Sciences Institute to K.Y.D. S.J.F. was supported by a studentship from the BBSRC Newcastle-Liverpool-Durham Doctoral Training Partnership. T Blower (Durham University) provided constructs and reagents for the production of AdcAI and AdcAII. GAS ΔadcAI and ΔczcD mutant strains were from C Ong, A McEwan, and M Walker (The University of Queensland). Quin2 was from T Young (Durham University). The authors thank R Borthwick and P Chivers (Durham University) for insightful discussions, and J Drury (Durham University) for help with statistical analyses.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.2c00578.
Recipe for artificial saliva buffer, generated by combining the known salt composition of human saliva (Table S1A); microbial strains used in this study (Table S1B); list of primers used in this study (Table S1C); and list of fluorometric and colourimetric metal indicators used in this study (Table S1C) (XLSX)
Effects of Hst5 on survival of streptococci after 24 h of exposure (Figure S1); effects of Hst5 on survival of control organisms P. aeruginosa and C. albicans (Figure S2); effects of Zn and TPEN on gene expression in wild-type GAS (Figure S3); characteristic phenotypes of the GAS ΔadcAI and ΔczcD mutant strains (Figure S4); effects of Hst5 on growth of GAS ΔczcD mutant strain (Figure S5); effects of Hst5 and the ΔH15,18,19 variant on the GAS ΔczcD mutant strain (Figure S6); and competition between phosphate and Zincon for binding Zn (Figure S7) (PDF)
Author Contributions
⊥ L.J.S. and Y.H. contributed equally to the experimental work. L.J.S. is listed first to acknowledge greater involvement in manuscript preparation and editing. K.Y.D. conceived the project. Y.H., K.Y.D., L.J.S., and N.S.J. designed experiments. N.S.J. provided oral streptococci strains. I.R.H., S.L.C., and Y.S. synthesized peptides for preliminary studies. K.Y.D. and L.J.S. performed kill assays with streptococci. Y.A. and J.Q. performed kill assays with C. albicans. I.R.H., Y.H., K.Y.D., and L.J.S. performed growth assays. K.Y.D. measured gene expression by qRT-PCR. K.Y.D. and L.J.S. measured metal levels by ICP MS. I.R.H. and Y.H. measured affinities of peptides to Zn(II), with guidance from S.J.F. Y.H. produced AdcA and AdcAII proteins, and measured their affinities to Zn(II), with guidance from S.J.F. Y.H., K.Y.D., and L.J.S. prepared figures and drafted the manuscript. All authors contributed to editing the manuscript and approved its final form.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang L. J.; Gallo R. L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14. 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Kim S. Y.; Zhang F.; Gong W.; Chen K.; Xia K.; Liu F.; Gross R.; Wang J. M.; Linhardt R. J.; Cotten M. L. Copper regulates the interactions of antimicrobial piscidin peptides from fish mast cells with formyl peptide receptors and heparin. J. Biol. Chem. 2018, 293, 15381. 10.1074/jbc.RA118.001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelinha J.; Heilemann K.; Jin J.; Angeles-Boza A. M. Unraveling the implications of multiple histidine residues in the potent antimicrobial peptide Gaduscidin-1. J. Inorg. Biochem. 2021, 219, 111391 10.1016/j.jinorgbio.2021.111391. [DOI] [PubMed] [Google Scholar]
- Juliano S. A.; Pierce S.; deMayo J. A.; Balunas M. J.; Angeles-Boza A. M. Exploration of the Innate Immune System of Styela clava: Zn(2+) Binding Enhances the Antimicrobial Activity of the Tunicate Peptide Clavanin A. Biochemistry 2017, 56, 1403. 10.1021/acs.biochem.6b01046. [DOI] [PubMed] [Google Scholar]
- Silva F. D.; Rezende C. A.; Rossi D. C.; Esteves E.; Dyszy F. H.; Schreier S.; Gueiros-Filho F.; Campos C. B.; Pires J. R.; Daffre S. Structure and mode of action of microplusin, a copper II-chelating antimicrobial peptide from the cattle tick Rhipicephalus (Boophilus) microplus. J. Biol. Chem. 2009, 284, 34735. 10.1074/jbc.M109.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini L. M.; Azen E. A. Histatins, a family of salivary histidine-rich proteins, are encoded by at least two loci (HIS1 and HIS2). Biochem. Biophys. Res. Commun. 1989, 160, 495. 10.1016/0006-291X(89)92460-1. [DOI] [PubMed] [Google Scholar]
- Azen E. A.; Leutenegger W.; Peters E. H. Evolutionary and dietary aspects of salivary basic (Pb) and post Pb (PPb) proteins in anthropod primates. Nature 1978, 273, 775. 10.1038/273775a0. [DOI] [PubMed] [Google Scholar]
- Kalmodia S.; Son K. N.; Cao D.; Lee B. S.; Surenkhuu B.; Shah D.; Ali M.; Balasubramaniam A.; Jain S.; Aakalu V. K. Presence of Histatin-1 in Human Tears and Association with Aqueous Deficient Dry Eye Diagnosis: A Preliminary Study. Sci. Rep. 2019, 9, 10304 10.1038/s41598-019-46623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F.; Offner G. D.; Xu T.; Vanderspek J. C.; Oppenheim F. G. Structural relationship between human salivary histatins. J. Dent. Res. 1990, 69, 2. 10.1177/00220345900690010101. [DOI] [PubMed] [Google Scholar]
- Shah D.; Ali M.; Pasha Z.; Jaboori A. J.; Jassim S. H.; Jain S.; Aakalu V. K. Histatin-1 Expression in Human Lacrimal Epithelium. PLoS One 2016, 11, e0148018 10.1371/journal.pone.0148018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. C.; Jean D.; Proske R. J.; Reins R. Y.; McDermott A. M. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr. Eye Res. 2007, 32, 595. 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E. J.; Alagl A. S.; Siqueira W. L.; Oppenheim F. G. Oral fluid proteolytic effects on histatin 5 structure and function. Arch. Oral Biol. 2006, 51, 1061. 10.1016/j.archoralbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Campese M.; Sun X.; Bosch J. A.; Oppenheim F. G.; Helmerhorst E. J. Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Arch. Oral Biol. 2009, 54, 345. 10.1016/j.archoralbio.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B. J.; Bird J. L.; Millar D. B.; Longton R. W. Studies on histidine-rich polypeptides from human parotid saliva. Arch. Biochem. Biophys. 1976, 177, 427. 10.1016/0003-9861(76)90455-0. [DOI] [PubMed] [Google Scholar]
- Puri S.; Edgerton M. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryotic Cell 2014, 13, 958. 10.1128/EC.00095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.; Puri S.; McCall A.; Norris H. L.; Russo T.; Edgerton M. Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2017, 7, 41 10.3389/fcimb.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochon A. B.; Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008, 4, e1000190 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E. J.; Breeuwer P.; van’t Hof W.; Walgreen-Weterings E.; Oomen L. C. J. M.; Veerman E. C. I.; Nieuw Amerongen A. V.; Abee T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 1999, 274, 7286. 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- Helmerhorst E. J.; van’t Hof W.; Veerman E. C. I.; Simoons-Smit I.; Nieuw Amerongen A. V. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 1997, 326, 39. 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris H. L.; Kumar R.; Ong C. Y.; Xu D.; Edgerton M. Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata. J. Fungi 2020, 6, 124 10.3390/jof6030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S.; Li R.; Ruszaj D.; Tati S.; Edgerton M. Iron binding modulates candidacidal properties of salivary histatin 5. J. Dent. Res. 2015, 94, 201. 10.1177/0022034514556709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. X.; Gao S.; Anand K. S.; Franz K. J. Zinc Binding Inhibits Cellular Uptake and Antifungal Activity of Histatin-5 in Candida albicans. ACS Infect. Dis. 2022, 8, 1920. 10.1021/acsinfecdis.2c00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. I.; Skaar E. P. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525. 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik E. M.; Long C. D.; Armitage G. C.; Loomer P.; Emerson J.; Mongodin E. F.; Nelson K. E.; Gill S. R.; Fraser-Liggett C. M.; Relman D. A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962. 10.1038/ismej.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E.; Keijser B. J.; Huse S. M.; Crielaard W. Defining the healthy ″core microbiome″ of oral microbial communities. BMC Microbiol. 2009, 9, 259 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F. E.; Chen T.; Izard J.; Paster B. J.; Tanner A. C.; Yu W. H.; Lakshmanan A.; Wade W. G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002. 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aas J. A.; Paster B. J.; Stokes L. N.; Olsen I.; Dewhirst F. E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721. 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach L. L.; Ram A.; Ijaz U. Z.; Evans T. J.; Lindström J. A Longitudinal Study of the Human Oropharynx Microbiota Over Time Reveals a Common Core and Significant Variations With Self-Reported Disease. Front. Microbiol. 2021, 11, 573969 10.3389/fmicb.2020.573969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero E. R.; Slomka V.; Bernaerts K.; Boon N.; Hernandez-Sanabria E.; Passoni B. B.; Quirynen M.; Teughels W. Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 2016, 47, 23. 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Abranches J.; Zeng L.; Kajfasz J. K.; Palmer S. R.; Chakraborty B.; Wen Z. T.; Richards V. P.; Brady L. J.; Lemos J. A. Biology of Oral Streptococci. Microbiol. Spectrum 2018, 6, 6.5.11. 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapetis J. R.; Steer A. C.; Mulholland E. K.; Weber M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685. 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- Shaikh N.; Leonard E.; Martin J. M. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 2010, 126, e557–e564. 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- Pant S.; Patel N. J.; Deshmukh A.; Golwala H.; Patel N.; Badheka A.; Hirsch G. A.; Mehta J. L. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070. 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- Fernández-Presas A.; Marquez Torres Y.; Garcia Gonzalez R.; Reyes Torres A.; Becker Fauser I.; Rodriguez Barrera H.; Ruiz Garcia B.; Toloza Medina R.; Delgado Dominguez J.; Molinari Soriano J. L. Ultrastructural damage in Streptococcus mutans incubated with saliva and histatin 5. Arch. Oral Biol. 2018, 87, 226. 10.1016/j.archoralbio.2018.01.004. [DOI] [PubMed] [Google Scholar]
- MacKay B. J.; Denepitiya L.; Iacono V. J.; Krost S. B.; Pollock J. J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect. Immun. 1984, 44, 695. 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X. L.; Salim H.; Dong G.; Parcells M.; Li Y. H. The BceABRS four-component system that is essential for cell envelope stress response is involved in sensing and response to host defence peptides and is required for the biofilm formation and fitness of Streptococcus mutans. J. Med. Microbiol. 2018, 67, 874. 10.1099/jmm.0.000733. [DOI] [PubMed] [Google Scholar]
- Andrian E.; Qi G.; Wang J.; Halperin S. A.; Lee S. F. Role of surface proteins SspA and SspB of Streptococcus gordonii in innate immunity. Microbiology 2012, 158, 2099. 10.1099/mic.0.058073-0. [DOI] [PubMed] [Google Scholar]
- Krzyściak W.; Jurczak A.; Piątkowski J.; Kościelniak D.; Gregorczyk-Maga I.; Kołodziej I.; Papież M. A.; Olczak-Kowalczyk D. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in in vitro conditions. Postepy Hig Med. Dosw. 2015, 69, 1056. [PubMed] [Google Scholar]
- Helmerhorst E. J.; Hodgson R.; van’t Hof W.; Veerman E. C. I.; Allison C.; Nieuw Amerongen A. V. The Effects of Histatin-derived Basic Antimicrobial Peptides on Oral Biofilms. J. Dent. Res. 1999, 78, 1245. 10.1177/00220345990780060801. [DOI] [PubMed] [Google Scholar]
- Moussa D. G.; Siqueira W. L. Bioinspired caries preventive strategy via customizable pellicles of saliva-derived protein/peptide constructs. Sci. Rep. 2021, 11, 17007 10.1038/s41598-021-96622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W. S.; Li X. S.; Sun J. N.; Edgerton M. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 2008, 52, 497. 10.1128/AAC.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. Y.; Tu C. H.; Yip B. S.; Chen H. L.; Cheng H. T.; Huang K. C.; Lo H. J.; Cheng J. W. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob. Agents Chemother. 2011, 55, 4918. 10.1128/AAC.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Jyoti M. A.; Song H. Y.; Jang W. S. Antifungal Activity and Action Mechanism of Histatin 5-Halocidin Hybrid Peptides against Candida ssp. PLoS One 2016, 11, e0150196 10.1371/journal.pone.0150196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E. J.; Flora B.; Troxler R. F.; Oppenheim F. G. Dialysis unmasks the fungicidal properties of glandular salivary secretions. Infect. Immun. 2004, 72, 2703. 10.1128/IAI.72.5.2703-2709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer M.; O’Neil D. A.; Lovie E.; Simpson L.; Torres M. D. T.; de la Fuente-Nunez C.; Angeles-Boza A. M.; Kleinsorgen C.; Mercer D. K.; von Kockritz-Blickwede M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides Requires New and Standardized Testing Structures. ACS Infect. Dis. 2021, 7, 2205. 10.1021/acsinfecdis.1c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. S.; Sun J. N.; Okamoto-Shibayama K.; Edgerton M. Candida albicans cell wall ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J. Biol. Chem. 2006, 281, 22453. 10.1074/jbc.M604064200. [DOI] [PubMed] [Google Scholar]
- Lynch R. J. M. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61, 46. 10.1111/j.1875-595X.2011.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. G.; Ong C. Y.; Walker M. J.; Djoko K. Y.; McEwan A. G.. Transition Metal Homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae. In Microbiology of Metal Ions, Advances in Microbial Physiology; Elsevier B.V., 2017; Vol. 70, p 123. 10.1016/bs.ampbs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Sanson M.; Makthal N.; Flores A. R.; Olsen R. J.; Musser J. M.; Kumaraswami M. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res. 2015, 43, 418. 10.1093/nar/gku1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C. L.; Gillen C. M.; Barnett T. C.; Walker M. J.; McEwan A. G. An antimicrobial role for zinc in innate immune defense against group A streptococcus. J. Infect. Dis. 2014, 209, 1500. 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- Stewart L. J.; Ong C. L.; Zhang M. M.; Brouwer S.; McIntyre L.; Davies M. R.; Walker M. J.; McEwan A. G.; Waldron K. J.; Djoko K. Y. Role of Glutathione in Buffering Excess Intracellular Copper in Streptococcus pyogenes. mBio 2020, 11, e02804-20 10.1128/mBio.02804-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedde V.; Rosini R.; Galeotti C. L. Zn2+ Uptake in Streptococcus pyogenes: Characterization of adcA and lmb Null Mutants. PLoS One 2016, 11, e0152835 10.1371/journal.pone.0152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C. L.; Berking O.; Walker M. J.; McEwan A. G. New Insights into the Role of Zinc Acquisition and Zinc Tolerance in Group A Streptococcal Infection. Infect. Immun. 2018, 86, e00048-18 10.1128/IAI.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan J.; McKnight C. J.; Troxler R. F.; Oppenheim F. G. Zinc and copper bind to unique sites of histatin 5. FEBS Lett. 2001, 491, 76. 10.1016/S0014-5793(01)02157-3. [DOI] [PubMed] [Google Scholar]
- Melino S.; Stefano Rufini S.; Sette M.; Morero R.; Grottesi A.; Paci M.; Petruzzelli R. Zn(2+) ions Selectively Induce Antimicrobial Salivary Peptide Histatin-5 To Fuse Negatively Charged Vesicles. Identification and Characterization of a Zinc-Binding Motif Present in the Functional Domain. Biochemistry 1999, 38, 9626. 10.1021/bi990212c. [DOI] [PubMed] [Google Scholar]
- Gusman H.; Lendenmann U.; Grogan J.; Troxler R. F.; Oppenheim F. G. Is salivary histatin 5 a metallopeptide?. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 2001, 1545, 86. 10.1016/S0167-4838(00)00265-X. [DOI] [PubMed] [Google Scholar]
- Krężel A.; Maret W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3. 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K.; Li N.; Wang H.; Cao X.; He J.; Zhang B.; He Q. Y.; Zhang G.; Sun X. Two zinc-binding domains in the transporter AdcA from Streptococcus pyogenes facilitate high-affinity binding and fast transport of zinc. J. Biol. Chem. 2018, 293, 6075. 10.1074/jbc.M117.818997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. R.; Xiao Z. Principles and practice of determining metal-protein affinities. Biochem. J. 2021, 478, 1085. 10.1042/BCJ20200838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke C.; Caradoc-Davies T. T.; Young P. G.; Proft T.; Baker E. N. The laminin-binding protein Lbp from Streptococcus pyogenes is a zinc receptor. J. Bacteriol. 2009, 191, 5814. 10.1128/JB.00485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Župan M. L.; Luo Z.; Ganio K.; Pederick V. G.; Neville S. L.; Deplazes E.; Kobe B.; McDevitt C. A. Conformation of the Solute-Binding Protein AdcAII Influences Zinc Uptake in Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 2021, 11, 729981 10.3389/fcimb.2021.729981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger M.; Robertson O. H. Expulsion of group a hemolytic streptococci in droplets and droplet nuclei by sneezing, coughing and talking. Am. J. Med. 1948, 4, 690. 10.1016/S0002-9343(48)90392-1. [DOI] [PubMed] [Google Scholar]
- Hamburger M. Jr. Studies on the transmission of hemolytic streptococcus infections: II. Beta hemolytic streptococci in the saliva of persons with positive throat cultures. J. Infect. Dis. 1944, 71. 10.1093/infdis/75.1.71. [DOI] [Google Scholar]
- Courtney H. S.; Hasty D. L. Aggregation of group A streptococci by human saliva and effect of saliva on streptococcal adherence to host cells. Infect. Immun. 1991, 59, 1661. 10.1128/iai.59.5.1661-1666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S.; Hasty D. Aggregation of group A streptococci by human saliva and effect of saliva on streptococcal adherence to host cells. Infect. Immun. 1991, 59, 1661. 10.1128/iai.59.5.1661-1666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollein Waldetoft K.; Mohanty T.; Karlsson C.; Morgelin M.; Frick I. M.; Malmstrom J.; Bjorck L. Saliva-Induced Clotting Captures Streptococci: Novel Roles for Coagulation and Fibrinolysis in Host Defense and Immune Evasion. Infect. Immun. 2016, 84, 2813. 10.1128/IAI.00307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerongen A. N.; Veerman E. C. Saliva--the defender of the oral cavity. Oral Dis. 2002, 8, 12. 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- Bhadbhade S. J.; Acharya A. B.; Thakur S. L. Salivary and gingival crevicular fluid histatin in periodontal health and disease. J. Clin. Exp. Dent. 2013, 5, e174 10.4317/jced.51106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro T. R.; Dria K. J.; de Carvalho C. B. M.; Monteiro A. J.; Fonteles M. C.; de Moraes Carvalho K.; Fonteles C. S. R. Salivary peptide profile and its association with early childhood caries. Int. J. Paediatr. Dent. 2013, 23, 225. 10.1111/j.1365-263X.2012.01258.x. [DOI] [PubMed] [Google Scholar]
- Jurczak A.; Koscielniak D.; Papiez M.; Vyhouskaya P.; Krzysciak W. A study on beta-defensin-2 and histatin-5 as a diagnostic marker of early childhood caries progression. Biol. Res. 2015, 48, 61 10.1186/s40659-015-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski K.; Tokajuk G.; Bielawska A.; Maciorkowska E.; Jablonski R.; Wojcicka A.; Bielawski K. The assessment of sIgA, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014, 20, 1095. 10.12659/MSM.890468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Huang X.; Tan X.; Si Y.; Wang X.; Chen F.; Zheng S. Salivary peptidome profiling for diagnosis of severe early childhood caries. J. Transl. Med. 2016, 14, 240 10.1186/s12967-016-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munther S. The impact of salivary lactoperoxidase and histatin-5 on early childhood caries severity in relation to nutritional status. Saudi Dent. J. 2020, 32, 410. 10.1016/j.sdentj.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino R.; Lobo M. J. C.; Duarte J. R.; Ferrer-Correia A. J.; Domingues P. M.; Amado F. M. L. The role of salivary peptides in dental caries. Biomed. Chromatogr. 2005, 19, 214. 10.1002/bmc.438. [DOI] [PubMed] [Google Scholar]
- Jainkittivong A.; Johnson D. A.; Yeh C. K. The relationship between salivary histatin levels and oral yeast carriage. Oral Microbiol. Immunol. 1998, 13, 181. 10.1111/j.1399-302X.1998.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Atkinson J. C.; Yeh C.; Oppenheim F. G.; Bermudez D.; Baum B. J.; Fox P. C. Elevation of salivary antimicrobial proteins following HIV-1 infection. J. Acquired Immune Defic. Syndr. 1990, 3, 41. [PubMed] [Google Scholar]
- Zhu L.; Charbonneau A. R. L.; Waller A. S.; Olsen R. J.; Beres S. B.; Musser J. M. Novel Genes Required for the Fitness of Streptococcus pyogenes in Human Saliva. mSphere 2017, 2, e00460 10.1128/mSphereDirect.00460-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy M. B.; Hayden J. A.; Nolan E. M. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc. 2012, 134, 18089. 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makthal N.; Nguyen K.; Do H.; Gavagan M.; Chandrangsu P.; Helmann J. D.; Olsen R. J.; Kumaraswami M. A Critical Role of Zinc Importer AdcABC in Group A Streptococcus-Host Interactions During Infection and Its Implications for Vaccine Development. EBioMedicine 2017, 21, 131. 10.1016/j.ebiom.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham L. R.; Le Breton Y.; Radin J. N.; Spencer B. L.; Deng L.; Hiron A.; Ransom M. R.; Mendonca J. D. C.; Belew A. T.; El-Sayed N. M.; McIver K. S.; Kehl-Fie T. E.; Doran K. S. Identification of Zinc-Dependent Mechanisms Used by Group B Streptococcus To Overcome Calprotectin-Mediated Stress. mBio 2020, 11, e02302-20 10.1128/mBio.02302-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M. L.; Groppo F.; Ohnishi M.; Goodson J. M.; Hasturk H.; Tavares M.; Yaskell T.; Floros C.; Behbehani K.; Razzaque M. S.. Can Salivary Phosphate Levels Be an Early Biomarker to Monitor the Evolvement of Obesity? Contributions to Nephrology; Karger Publishers, 2013; Vol. 180, p 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica V.; Calo L. A.; Caldarera R.; Cavaleri A.; Granata A.; Santoro D.; Savica R.; Muraca U.; Mallamace A.; Bellinghieri G. Phosphate salivary secretion in hemodialysis patients: implications for the treatment of hyperphosphatemia. Nephron Physiol. 2007, 105, p52. 10.1159/000098544. [DOI] [PubMed] [Google Scholar]
- Lynge Pedersen A. M.; Belstrom D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3. 10.1016/j.jdent.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Norris H. L.; Kumar R.; Edgerton M. A Novel Role for Histatin 5 in Combination with Zinc to Promote Commensalism in C. albicans Survivor Cells. Pathogens 2021, 1609 10.3390/pathogens10121609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C.; Ruijter J. M.; Deprez R. H.; Moorman A. F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62. 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Kuzmič P.DynaFit--A Software Package for Enzymology. Methods in Enzymology; Elsevier B.V., 2009; Vol. 467, p 247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.