Abstract
Neuroimaging research has been at the forefront of concerns regarding the failure of experimental findings to replicate. In the study of brain-behavior relationships, past failures to find replicable and robust effects have been attributed to methodological shortcomings. Methodological rigor is important, but there are other overlooked possibilities: most published studies share three foundational assumptions, often implicitly, that may be faulty. In this paper, we consider the empirical evidence from human brain imaging and the study of non-human animals that calls each foundational assumption into question. We then consider the opportunities for a robust science of brain-behavior relationships that await if scientists ground their research efforts in revised assumptions supported by current empirical evidence.
Guiding assumptions in the study of brain-behavior relationships
Most brain imaging studies present stimuli and measure behavioral responses in temporal units (trials) that are ordered randomly. Participants’ brain signals are typically aggregated to model structured variation that allows inferences about the broader population from which people were sampled. These methodological details, when used to study any phenomenon of interest, often give rise to brain-behavior findings that vary unexpectedly (across stimuli, context, and people). Such findings are typically interpreted as replication failures, with the observed variation discounted as error caused by less than rigorous experimentation (Box 1). Methodological rigor is of course important, but replication problems may stem, in part, from a more pernicious source: faulty assumptions (i.e., ontological commitments) that mis-specify the psychological phenomena of interest.
Box 1. Replicability of brain-behavior relationships.
When a given brain-behavior relationship varies across stimuli, context, people, or studies, this variation is often interpreted as a failure to replicate, possibly due to methodological issues, such as small sample sizes (e.g., [127–129]), insufficient reporting of methods (e.g., [130,131]), or the use of variable preprocessing parameters or different analytical workflows (e.g., [132]). These sorts of methodological concerns are important and recent publications have offered guidance for improvements [114,133]. But there may be a deeper source of variable brain-behavior findings: the typical brain imaging study may mis-specify the psychological phenomena of interest. A given brain-behavior relationship likely varies in meaningful and predictable ways across stimuli, context, and/or people. This variation typically goes unmeasured or unmodeled, however, because of key assumptions about the nature of the psychological phenomena under investigation, the design of experiments, and the modeling of the observed data. As a consequence, the variation is misunderstood as error and is interpreted as a failure to replicate, when in fact it might contain structure and be better understood as an opportunity for scientific discovery. Methodological recommendations, then, are not sufficient to solve the replication problem. First, we must reconsider our underlying assumptions about the phenomena we are studying and then we may look towards methodological improvements that are grounded in revised assumptions.
In this paper, we review three questionable assumptions whose reconsideration may offer opportunities for a more robust and replicable science:
The localization assumption: the instances that constitute a category of psychological events (e.g., instances of fear) are assumed to be caused by a single, dedicated psychological process implemented in a dedicated neural ensemble (see Glossary).
The one-to-one assumption: the dedicated neural ensemble is assumed to map uniquely to that psychological category, such that the mapping generalizes across contexts, people, measurement strategies, and experimental designs.
The independence assumption: the dedicated neural ensemble is thought to function independently of contextual factors, such as the rest of the brain, the body, and the surrounding world, so the ensemble can be studied alone without concern for those other factors. Contextual factors might moderate activity in the neural ensemble but should not fundamentally change its mapping to the instances of a psychological category.
These three assumptions are rooted in a typological view of the mind, brain, and behavior [1] that was modeled on 19th century physics and continues to guide experimental practices in much of brain-behavior research to the present day. In this paper, we have curated examples from studies of human functional magnetic resonance imaging (fMRI) and neuroscience research using non-human animals that call each assumption into question. We then sketch the beginnings of an alternative approach to study brain-behavior relationships, grounded in different ontological commitments: (i) a mental event comprises distributed activity across the whole brain; (ii) brain and behavior are linked by degenerate (i.e., many-to-one) mappings; and (iii) mental events emerge as a complex ensemble of weak, nonlinearly interacting signals from the brain, body, and external world (Table 1 and Figure 1).
Table 1.
Current guiding assumptions contrasted with revised assumptions for the study of brain-behavior relationships
| Current guiding assumptions | Revised assumptions |
|---|---|
| (1) Localization assumption: instances of a psychological category can be localized to a dedicated neural ensemble. Instances of the same psychological category are assumed to be more similar to each other with respect to that neural ensemble and more different from instances of other psychological categories, which have their own ensembles. | (1) Whole-brain signals contribute to mental events: instances of a psychological category arise from activity across the entire brain, not from a separable neural ensemble. |
| (2) One-to-one assumption: neural ensembles correspond one-to-one with psychological categories. This correspondence is stable across all instances of the category, regardless of context, people, measurement strategy, or experimental design. | (2) Many neural ensembles for one psychological category: there are degenerate (many-to-one) mappings between neural ensembles and a psychological category. |
| (3) Independence assumption: a stimulus will reliably evoke activity in a specific neural ensemble that produces an instance of the specific psychological category of interest. This neural ensemble can be studied separately from other signals that may moderate its function. | (3) Mental events emerge as a complex ensemble of signals: an instance of a psychological category emerges from a complex ensemble of signals from the brain, body, and world. These signals can only be understood in relation to the rest of the ensemble; i.e., each may have a weak effect on its own, but a strong effect when considered collectively. |
Figure 1. Schematic summary of current guiding assumptions contrasted with the revised assumptions presented in this article.
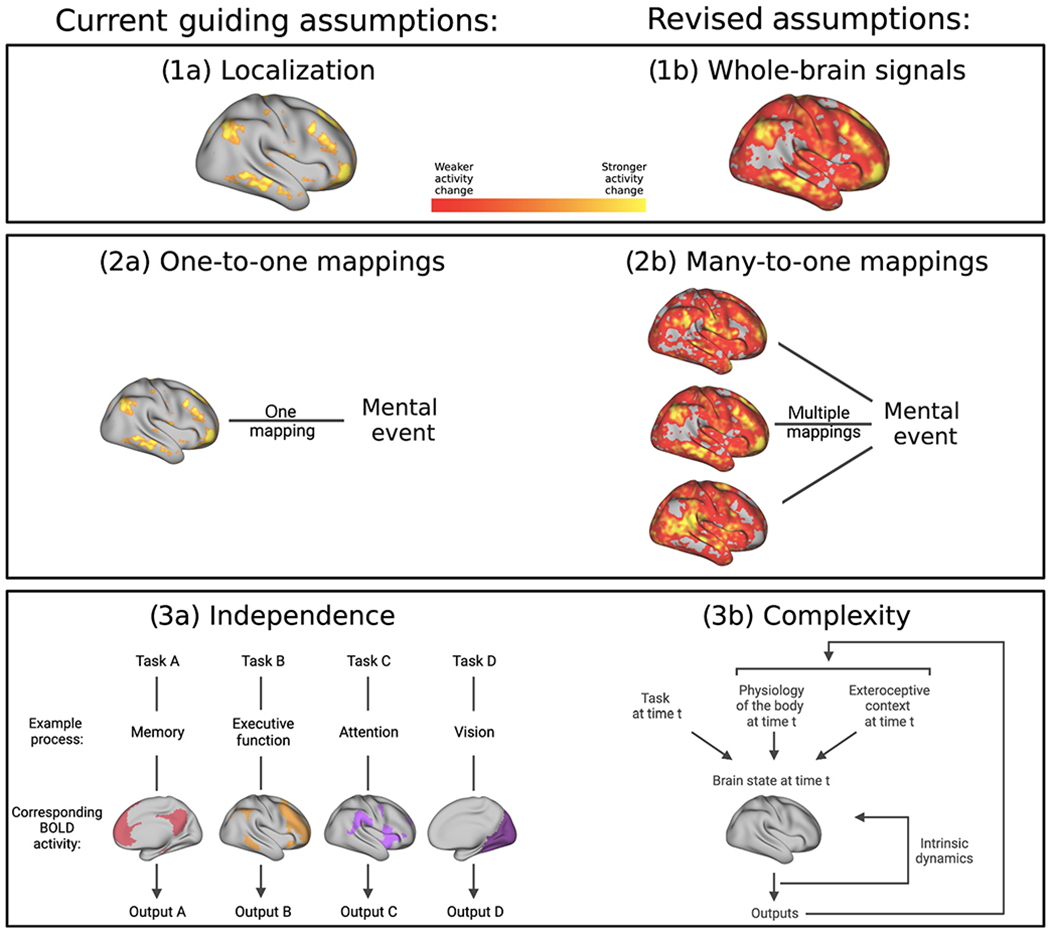
See Table 1 for an explanation of each assumption. This figure was created using BioRender (https://biorender.com/). Abbreviation: BOLD, blood oxygen level dependent.
Localization reconsidered: evidence of whole-brain contributions to mental events
Functional neuroimaging methods were initially assumed to reveal the unique neural locations for different mental phenomena (e.g., [2]). Decades of study ironically produced evidence for the opposite: localized neural computations contribute to a range of phenomena (e.g., [3,4]) and several key studies suggest that psychological phenomena like attention and vision might be better modeled as whole-brain events. We present three lines of evidence that call the localization assumption into question. They also demonstrate the existence of whole-brain ensembles when studies are designed to permit their discovery.
Whole-brain responses in human brain imaging
Whole-brain modeling of a psychological phenomenon is first suggested by studies that deeply sample and model data from individuals. Standard experimental designs typically sample relatively few trials per participant and use stringent statistical thresholds to avoid type I errors. Consequently, studies generally observe and report only the strongest effects, producing ‘islands’ of signal change that are interpreted as evidence for localized activity. These ‘islands’ might actually result from studies that are insufficiently powered within individual participants. Stringent statistical thresholds ignore weaker signals as noise, but studies specifically designed to examine those signals show that they are both reliable and psychologically meaningful. For example, consider a study in which participants viewed letters and numbers and completed a letter–number discrimination task across 500 trials and researchers observed a significant increase in blood oxygen level dependent (BOLD) signal in ~72% of brain voxels [5]; by comparison, a significant increase was observed in an island of only ~20% of brain voxels (mostly in the primary visual cortex) after 25 trials. These findings were replicated with high-resolution 7-Tesla imaging, both using the same letter–number discrimination task [6] and using a visual checkerboard task [7]. The problems caused by under-sampling trials within a participant cannot be overcome by increasing the number of participants in a study; larger between-subject sample sizes are no substitute for within-subject sampling because individuals are not interchangeable (a mistaken assumption called the ‘evils of averaging’ [8,9]).
An overreliance on default modeling assumptions compounds the problem. Most fMRI studies use a single function (i.e., a gamma distribution) to model the hemodynamic response function (HRF) across all voxels in the human brain. Modeling the HRF without presuming its shape (other than its alignment with task on/offsets) resulted in a reliable task-based signal increase from ~72% of brain voxels to an average of ~96% of imaged voxels [5]. Modeling variation in the shape of the HRF across voxels and/or participants can have a large impact on the conclusions drawn from findings [10] (also see [11]). These findings show that modeling decisions, rooted in an ontological commitment to localization, can create self-fulfilling prophecies, leading scientists to observe the small ‘islands’ of signal change that they set out to identify in the first place.
It has been suggested that brain signals outside of localized regions of interest may be epiphenomenal to the phenomenon of interest (e.g., [12]) and that interventions (e.g., focal lesions) are necessary to fully understand whether a brain region is functionally implicated in a given task. Yet such interventions are not in and of themselves a gold standard. A disrupted function could be due to damaged fibers of passage and lesions only provide an understanding of what functional capacity remains in the absence of the lesioned area, rather than revealing that region’s function in an intact whole-brain system (e.g., a lesioned area might be a weak but necessary factor in complex, nonlinear ensembles of causation). Optogenetic manipulations also suffer from this latter concern.
Some methods attempt to move beyond the localization assumption, such as intrinsic functional connectivity analyses, in which spatially distributed collections of voxels with BOLD signal that correlates over time are assumed to form functional networks (e.g., [13,14]). Analytic decisions, guided by the localization assumption, typically truncate the topography of these networks, forcing them to have discrete boundaries (e.g., the non-overlapping networks in [15]), when in fact the networks spatially overlap in a way that is functionally meaningful (e.g., [16,17]). Other attempts to account for whole-brain patterns have improved upon the problem of discretizing networks (e.g., graph theoretic approaches [18]; gradient-based approaches [19,20]; and multivariate approaches such as multivariate pathway identification [21], or full correlation matrix analysis [22]).
Brain-wide representations of behavior in non-human animals
A second line of evidence supporting whole-brain modeling (rather than localized ensembles) comes from studies of non-human animals, which document brain-wide patterns of neural activity associated with ongoing behavior (e.g., [23–27]; for a review see [28]). For example, global increases in brain activity (measured via near-whole-brain light field microscopy) correlate with walking movements in Drosophila flies [25] (for similar findings using two-photon imaging in flies, see [26]). Brain-wide signals (measured via calcium imaging) relate to inferred motor commands in Caenorhabditis elegans worms (i.e., signals related to the intent to move that are uncoupled from the actual movement by immobilizing the worm; [27]). Cortex-wide activity (measured via wide-field calcium imaging) and single neuron activity (measured via two-photon imaging) in many cortical and subcortical areas are related to an array of uninstructed movements in mice [29].
Multimodal signals in presumed unimodal areas of the brain
Evidence consistent with a whole-brain modeling approach can also be found in research on multisensory processing. For example, primary sensory or motor regions are thought to process signals only for their dedicated modality [e.g., the occipital pole is called primary visual cortex (V1), on the assumption that it is exclusively engaged by visual signals; primary motor cortex is likewise assumed to be exclusive for skeletomotor movements]. But ‘primary sensory cortices’ carry information about several sensory modalities and motor actions [30–33], and ‘primary motor cortex’ is involved in sensory processing [34]. In a particularly striking experiment in humans, fMRI BOLD signal patterns associated with simple sensory stimuli in one modality (e.g., tactile, visual, or auditory stimulation) could be distinguished in the signal patterns within the other primary sensory cortices (e.g., V1 activity distinguished between touch and sound [35]). BOLD signal patterns in V1 and A1 also distinguished between tactile sensory data from a person’s index versus pinky finger [35]. V1 even distinguished between categories of auditory input (e.g., people, forest, traffic) when participants were blindfolded [36]. Multimodal signals in primary sensory cortices have similarly been reported in several species of non-human animals [23,29,31,37–44]. Likewise, primary motor cortex also contains multimodal signals: a ‘polysensory zone’ of motor cortex contains many neurons that respond to tactile and visual stimuli [34], and electrical stimulation of primary motor cortex can influence visceromotor function, causing alterations in kidney function such as decreases in renal blood flow [45,46].
Additional evidence suggests that multimodal information is not just incidentally present in primary sensory regions but plays a functional role. For example, normally-sighted individuals showed increased BOLD signal in visual cortex during tactile stimulation after prolonged visual deprivation (via blindfolding), compared to non-visually deprived individuals [47]. Most importantly, only the blindfolded individuals performed worse on a Braille discrimination task when visual cortex function was disrupted via transcranial magnetic stimulation, suggesting that the multimodal signal in their visual cortex was functionally related to task performance. Likewise, activity in visual cortex increased during auditory stimulation and sound localization abilities improved in visually deprived non-human animals compared with non-deprived animals [48,49]. Neurons in visual cortex continue to fire in mice after their retinas are ablated, once again suggesting that signals in visual cortex are not indicative of only visual input [50]. These and other similar research findings suggest that there may be no truly ‘unimodal’ neurons in the brain and that a ‘primary’ sensory or motor area of cortex might also be thought of as an ‘association region’ for other modalities.
If scientists assumed that ensembles across the whole brain may be involved in a given phenomenon, and correspondingly they designed studies that allow for this possibility, then they could test whole-brain hypotheses, while still discovering localization if it indeed exists.
One-to-one mappings reconsidered: evidence of many-to-one mappings
Even after setting aside the localization assumption, perhaps particular psychological phenomena might uniquely map, one-to-one, with particular widely distributed brain patterns. This one-to-one mapping assumption is called into question, however, by evidence within many different biological systems that more than one mechanism can produce a single functional outcome (i.e., many-to-one mapping, also called degeneracy [51]). Here we review key examples of degeneracy and consider their implications for studies of brain-behavior mapping.
Degeneracy of causal mechanisms is an organizing principle of virtually all biological domains (e.g., in genetics [51,52], the immune system [51,53], the motor system [54]). In the nervous system, many combinations of neurons give rise to the same intrinsic network with the same function (e.g., [55,56]) and different patterns of neural activation give rise to the same behavior [57]. Neuroimaging studies have been slow to model degeneracy in the causes and correlates of psychological phenomena, but there are notable exceptions. For example, participants underwent fMRI as they watched videos intended to induce instances of anger, disgust, or loss, and unsupervised machine learning applied to patterns of BOLD signal across the length of the time series identified numerous reliable dynamic patterns within each psychological category [58]. Likewise, atypical instances of an emotion category (e.g., pleasant instances of fear) were associated with patterns of BOLD signal that were distinct from those associated with typical instances of the same category (e.g., unpleasant instances of fear [59]; see also [60]). Degenerate patterns of functional connectivity (i.e., the coordinated activity of different brain regions) associated with emotional experiences have also been observed. For example, unsupervised machine learning applied to patterns of functional connectivity while individuals listened to music intended to induce instances of anger and anxiety also revealed several degenerate patterns within each emotion category [61] (also see [62]). Beyond emotion research, degeneracy has been observed in the functional connectivity patterns of infants experiencing looming danger [63] and in the physiology of the hippocampus [64] (also see [65]). Degeneracy can also be observed in disorders of the nervous system, such as epilepsy, where multiple firing patterns of different cell types in the hippocampus produce the same pattern of electroencephalography signals recorded during an epileptic seizure [66,67]. Specialized methods can identify degeneracy in BOLD data (e.g., neural topographic factor analysis [68], network measures [69]), making clear that multiple, reliable solutions can be separated from noise, provided a study has been designed accordingly.
Lesion studies in both humans and non-human animals provide additional evidence for degeneracy. For example, patient S.M., who has bilateral amygdala lesions, shows deficits perceiving fear in posed, stereotypic facial expressions [70] and in experiencing fear [71], but she can experience and perceive fear under certain conditions (discussed in [72]), suggesting that her brain must accomplish both functions with ensembles of neurons that do not involve the amygdala. This conclusion is reinforced by studies of monozygotic twins with almost full amygdala lesions, only one of whom has deficits in perceiving and experiencing fear [73,74]. Additionally, rats with bilateral lesions to the basolateral amygdala (a region thought to be necessary for conditioning of fear behaviors) do not have impairments in memory of contextual fear conditioning, again suggesting that there are multiple mechanisms for this function [75]. For circuit level examples of degeneracy, see [55,76–78].
To test whether degeneracy is an organizing principle in brain-behavior mapping, experiments must sample variable instances within a psychological category, and sample similar instances more than once, within the same person (or the same non-human animal, if sampling spontaneous behavior, for example [79]). With sufficient sampling, one-to-one mappings can still be discovered if present. Even so, degenerate mappings remain likely: the first experience of any sort will be different from the second due to learning, plasticity, and temporal history (i.e., the brain and/or the body were in different starting states during each instance [80]).
Independence reconsidered: evidence for the brain as a complex system
A final ontological commitment impeding the study of brain-behavior relations is the assumption of independence: that neural activity corresponding to one psychological phenomenon, such as attention or perception, can be studied independently of any other phenomena, such as memory (for a discussion, see [81]). Criticisms of the independence assumption have been discussed in ecological psychology (e.g., [82]), developmental psychology (e.g., [83]), social psychology (e.g., [84]), and neuroscience (e.g., [3]). We build on these insights to suggest that mental events emerge as a complex ensemble of interacting signals from the brain, body, and surrounding world. In other words, we suggest, like others, that the brain is a complex system continually influenced by input signals from the body and the world (which we refer to as the brain complexity hypothesis; Box 2).
Box 2. The brain complexity hypothesis.
A system is a collection of regularly interacting parts, organized for a common purpose. Biological systems, such as the cell, have historically been described as machines [134]. The machine metaphor implies that the system can be broken down into independent, separable mechanisms, where each mechanism can be studied independently of one another. But many domains of biology have shown that biological systems cannot be studied as independent mechanisms, because the systems’ functions emerge through the collective interaction of their parts [135]. Instead, biological systems are thought to function as complex systems, where many weak, causal factors interact in a nonlinear way to produce a larger-scale collective outcome [135]. Accordingly, some neuroscientists have begun to consider the brain as a complex system, whose functions emerge from the dynamic interactions between neurons, glial cells, and other biological elements (e.g., [69,85,125,135–140]).
In the brain complexity hypothesis, a given neuron does not function in isolation and its action potentials are profoundly influenced by its neural context. For example, action potentials in a given neuron are influenced by the assembly of neurons to which it momentarily belongs [85]. Another form of neural context is ephaptic signaling, in which axons from different neurons that touch each other influence each other’s action potentials [86] and nearby neurons that are not necessarily in physical contact nonetheless influence one another’s excitability via extracellular local field potentials [87]). In fact, the function of a train of action potentials is determined by the neurons that receive them, not by the neuron issuing them (e.g., when action potentials from a single neuron are received by a motor neuron, they are considered motor signals; when the same action potentials are sent by collateral axons to sensory neurons, they are considered sensory signals). And a neuron can have multiple synaptic connections and switch from being a part of one network to another [77,88].
Evidence like this suggests that the relevance of any neuron’s action potentials to a given psychological process is dependent on the other neurons it is interacting with. For example, in the anterior cingulate cortex (ACC), a similar pattern of BOLD activity contributed to either an attentional function or a memory function, depending on the regions to which it was functionally connected during a task [89]. The ACC is considered a ‘rich-club’ hub [16,90,91]) because it is densely interconnected with many groups of neurons throughout the brain [92]. The dense interconnections between the ACC and other nodes allow this region to take on different functions (e.g., emotion [93], multimodal integration [94], decision making [95], value [96], attention [97], and visceromotor control [98]), depending on the ensemble to which it belongs, suggesting that isolated neural signals do not have inherent psychological meaning.
A routinely overlooked aspect of the neural context in brain-behavior relations involves the signals associated with the sensory conditions of the body. These signals routinely go unmeasured in studies of psychological phenomena, yet evidence suggests they play a substantial role. For example, an individual’s heart rate modulates functional connectivity between several regions involved in autonomic regulation ([99], also see [100,101]) and, likewise, respiration rate correlates with signal changes across the whole-brain during resting state fMRI [102–105]. The signals of import may be the sensory surfaces of the body (peripheral interoceptive signals informing on the state of the body) or the motor prediction signals that control the viscera, the immune system, energy regulation, and so on ([106,107]). In fact, many physical phenomena that impact bodily signals also influence BOLD signals, such as quality of sleep [108], physical fitness [109], time of day (i.e., circadian rhythms [110]), or how recently the participant ate [111]. The often-overlooked role of bodily signals may offer an alternative explanation for intrinsic fMRI activity observed in the ‘resting state’ [112], which involves no task-based stimulation, but does involve continuous and dynamically changing brain–body interactions, meaning that ‘intrinsic activity’ may be better understood by considering the broader signal context (e.g., [113]).
If neural ensembles are not independent actors, then the nature of any mental event can be better understood by accounting for a larger, interdependent ensemble of signals, some of which might be weak when viewed alone. Neuroimaging experiments typically report modest effect sizes [114], but this may be because standard experiments only measure a small fraction of the relevant signals. A lack of robust and replicable effects in the study of brain-behavior relationships, then, may partly stem from incorrectly modeling complex systems as simple, mechanistic ones. Complexity not only implies that experiments should be modeling many more causal factors than are typically measured, but also reinforces the likelihood of degeneracy within a system (i.e., multiple causal mechanisms increase the likelihood of multiple functional solutions [51]).
Improving the study of brain-behavior relationships
A model-first approach
The standard empirical paradigm when investigating mental phenomena typically follows these steps: researchers formulate a hypothesis, design an experiment that can test the hypothesis, and then analyze data using statistical methods that are conventionally used to test similar questions. This approach does not require researchers to specify their assumptions up front, which may make those assumptions difficult to identify and evaluate, let alone change them in future investigations. A model-first approach partially remedies this situation, because researchers begin an investigation by formally specifying a model of the phenomena of interest and then formulate hypotheses based on this model and design an experiment to test it. Model specification requires an explicit acknowledgement of assumptions, allowing researchers to evaluate and refine their assumptions and the research practices conditioned on them. In an example of a model-first approach, researchers first developed a computational model of functional neuroanatomy that incorporated an assumption of degeneracy up front, formulated hypotheses about within-participant or within-condition degeneracy, and then analyzed data with methods appropriate to test those hypotheses ([68]; see also [58,115]). Model-first approaches are found in investigations of complexity: researchers have formulated models of neural activity occurring at multiple time-scales [116], which then revealed that the necessary data to test such a model requires naturalistic paradigms, rather than standard trial-based paradigms [117].
Sampling relevant signals
Modeling the whole brain as a complex system with degenerate solutions requires sampling signals from the brain, body, and surrounding environment (assuming that ascending interoceptive and somatosensory signals are considered inputs that influence the intrinsic trajectory of brain signals, rather than part of the system itself, which is another assumption that a researcher must make explicitly). It is not currently realistic to sample all relevant signals at the present time, but future technology development efforts might aspire to correct this problem. In the meantime, it might be optimal to acquire as many relevant signals as possible (e.g., signals measuring the mental features of an event) and build a model accordingly, making explicit which necessary signals are missing, considering the limitations therein, or even finding modeling solutions for the missing signals. As a start, assumptions of degeneracy and complexity suggest the need for analytical methods that allow signals across voxels, parcels, or regions to interact, producing effects that are more than the sum of their parts, rather than be modeled as independent elements of a system (e.g., [118–120]). A similar plea can be made for methods that do not assume singular mappings (e.g., soft-versus hard-clustering algorithms [121]).
Within-versus between-participant sampling
Current data collection efforts tend to prioritize between-participant sample sizes (over within-participant), which improve estimates of population-level effects. Such effects are abstractions, however [122], that as a rule do not generalize to individuals [8,9]. Increasing the number of participants in a sample is not a substitute for increasing the sampling within a person over time. Whole-brain modeling of degeneracy and complexity depends on more attention to experimental design for within-subject sampling across instances and contexts.
Concluding remarks
Scientific communities tacitly agree on assumptions about what exists (called ontological commitments), what questions to ask, and what methods to use. All assumptions are firmly rooted in a philosophy of science that need not be acknowledged or discussed but is practiced nonetheless. In this article, we questioned the ontological commitments of a philosophy of science that undergirds much of modern neuroscience research and psychological science in particular. We demonstrated that three common commitments should be reconsidered, along with a corresponding course correction in methods (see Outstanding questions). Our suggestions require more than merely improved methodological rigor for traditional experimental design (Box 1). Such improvements are important, but may aid robustness and replicability only when the ontological assumptions behind those methods are valid. Accordingly, a productive way forward may be to fundamentally rethink what a mind is and how a brain works. We have suggested that mental events arise from a complex ensemble of signals across the entire brain, as well as the from the sensory surfaces of the body that inform on the states of the inner body and outside world, such that more than one signal ensemble maps to a single instance of a single psychological category (maybe even in the same context [51,56]). To this end, scientists might find inspiration by mining insights from adjacent fields, such as evolution, anatomy, development, and ecology (e.g., [123,124]), as well as cybernetics and systems theory (e.g., [125,126]). At stake is nothing less than a viable science of how a brain creates a mind through its constant interactions with its body, its physical environment, and with the other brains-in-bodies that occupy its social world.
Outstanding questions.
Well-powered brain-wide analyses imply that meaningful signals exist in brain regions that are considered nonsignificant in studies with low within-subject power, but is all of the observed brain activity necessarily supporting a particular behavior? By thresholding out weak yet consistent effects, are we removing part of the complex ensemble of causation? What kinds of technical innovations or novel experimental methods would allow us to make progress in answering this question?
How might we incorporate theoretical frameworks, such as a predictive processing framework, to better understand the involvement of the whole-brain in producing a mental event? Such an approach hypothesizes the involvement of the whole-brain as a general computing system, without implying equipotentiality (i.e., that all areas of the brain are equally able to perform the same function).
Why are some reported effects (e.g., the Stroop effect) seemingly robust and replicable if psychological phenomena are necessarily degenerate? These effects should be explored to determine if they remain replicable outside of constrained laboratory contexts and to understand what makes them robust.
Given that measuring every signal in a complex system is unrealistic given the time and cost constraints of a standard neuroimaging experiment, how can we balance the measurement of meaningful signals in the brain, body, and world with the practical realities of experimental constraints?
Is the study of brain-behavior relationships actually in a replication crisis? And if so, is it merely a crisis of method? Traditional assumptions suggest that scientists should replicate sample summary statistics and tightly control variation in an effort to estimate a population summary statistic, but perhaps this goal should be reconsidered.
Highlights.
The study of brain-behavior relationships has been guided by several foundational assumptions that are called into question by empirical evidence from human brain imaging and neuroscience research on non-human animals.
Neural ensembles distributed across the whole brain may give rise to mental events rather than localized neural populations. A variety of neural ensembles may contribute to one mental event rather than one-to-one mappings. Mental events may emerge as a complex ensemble of interdependent signals from the brain, body, and world rather than from neural ensembles that are context-independent.
A more robust science of brain-behavior relationships awaits if research efforts are grounded in alternative assumptions that are supported by empirical evidence and which provide new opportunities for discovery.
Acknowledgments
The authors thank Randy McIntosh for helpful comments in reviewing this manuscript. The writing of this article was supported by grants from the National Science Foundation (BCS 1947972), the National Institutes of Health (R01 AG071173, R01 MH109464, and R01 MH113234), the U.S. Army Research Institute for the Behavioral and Social Sciences (W911NF-16-1-019), and the Unlikely Collaborators Foundation. The views, opinions, and/or findings contained in this review are those of the authors and shall not be construed as an official Department of the Army position, policy, or decision, unless so designated by other documents, nor do they necessarily reflect the views of the Unlikely Collaborators Foundation.
Glossary
- Complex ensemble
a collection of weak, nonlinearly interacting signals (see Complex system).
- Complex system
a system where many weak, causal factors interact in nonlinear ways to produce a larger-scale collective outcome.
- Degeneracy
the ability for many independent mechanisms to produce the same functional outcome. Also called a many-to-one mapping.
- Many-to-one mapping
see Degeneracy above.
- Mental event
any instance of a psychological category, such as an instance of behavior or subjective experience (e.g., an instance of memory, attention, emotion, or action).
- Mental features
features of the brain’s state that usefully describe what a brain is doing in psychological terms at any given moment in time. Features can be higher in dimensionality and closer to the sensory surfaces of an animal’s body (e.g., lines, edges, temperature) or lower dimensional, multimodal summaries (e.g., arousal, threat, or reward).
- Neural context
the assembly of neurons to which any given neuron momentarily belongs.
- Neural ensemble
a brain region, circuit, network, or distributed pattern in the brain.
- One-to-one mapping
a particular neural ensemble is assumed to map uniquely to a particular psychological phenomena.
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Mayr E (1994) Typological versus population thinking. In Conceptual Issues in Evolutionary Biology (Sober E, ed.), pp. 157–160, MIT Press [Google Scholar]
- 2.Posner MI et al. (1988) Localization of cognitive operations in the human brain. Science 240, 1627–1631 [DOI] [PubMed] [Google Scholar]
- 3.Anderson ML (2014) After Phrenology: Neural Reuse and the Interactive Brain, Bradford Books [Google Scholar]
- 4.Barrett LF and Satpute AB (2013) Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol 23, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Castillo J et al. (2012) Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc. Natl. Acad. Sci. U. S. A 109, 5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Castillo J et al. (2015) Task dependence, tissue specificity, and spatial distribution of widespread activations in large single-subject functional MRI datasets at 7T. Cereb. Cortex 25, 4667–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorge J et al. (2018) Mapping and characterization of positive and negative BOLD responses to visual stimulation in multiple brain regions at 7T. Hum. Brain Mapp 39, 2426–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallistel CR (2012) On the evils of group averaging: commentary on Nevin’s “Resistance to extinction and behavioral momentum.”. Behav. Process 90, 98–99 [DOI] [PubMed] [Google Scholar]
- 9.Estes WK (1956) The problem of inference from curves based on group data. Psychol. Bull 53, 134–140 [DOI] [PubMed] [Google Scholar]
- 10.Moriguchi Y et al. (2011) Differential hemodynamic response in affective circuitry with aging: an fMRI study of novelty, valence, and arousal. J. Cogn. Neurosci 23, 1027–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neta M et al. (2015) Spatial and temporal characteristics of error-related activity in the human brain. J. Neurosci 35, 253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y (2018) Sensory cortex is nonessential in working memory storage. Trends Cogn. Sci 22, 192–193 [DOI] [PubMed] [Google Scholar]
- 13.Biswal B et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med 34, 537–541 [DOI] [PubMed] [Google Scholar]
- 14.Fox MD and Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8, 700–711 [DOI] [PubMed] [Google Scholar]
- 15.Yeo BT et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106, 1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Heuvel MP and Sporns O (2013) An anatomical substrate for integration among functional networks in human cortex. J. Neurosci 33, 14489–14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleckner IR et al. (2017) Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav 1, 0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sporns O (2018) Graph theory methods: applications in brain networks. Dialogues Clin. Neurosci 20, 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margulies DS et al. (2016) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. U. S. A 113, 12574–12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsumi Y et al. (2022) Allostasis as a core feature of hierarchical gradients in the human brain. Netw. Neurosci 6, 1010–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kragel PA et al. (2021) A human colliculus-pulvinar-amygdala pathway encodes negative emotion. Neuron 109, 2404–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y et al. (2015) Full correlation matrix analysis (FCMA): an unbiased method for task-related functional connectivity. J. Neurosci. Methods 251, 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringer C et al. (2019) Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, eaav7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salkoff DB et al. (2020) Movement and performance explain widespread cortical activity in a visual detection task. Cereb. Cortex 30, 421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aimon S et al. (2019) Fast near-whole–brain imaging in adult Drosophila during responses to stimuli and behavior. PLoS Biol. 17, e2006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann K et al. (2021) Coupling of activity, metabolism and behaviour across the Drosophila brain. Nature 593, 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato S et al. (2015) Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669 [DOI] [PubMed] [Google Scholar]
- 28.Kaplan HS and Zimmer M (2020) Brain-wide representations of ongoing behavior: a universal principle? Curr. Opin. Neurobiol 64, 60–69 [DOI] [PubMed] [Google Scholar]
- 29.Musall S et al. (2019) Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci 22, 1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghazanfar AA and Schroeder CE (2006) Is neocortex essentially multisensory? Trends Cogn. Sci 10, 278–285 [DOI] [PubMed] [Google Scholar]
- 31.Kayser C (2010) The multisensory nature of unisensory cortices: a puzzle continued. Neuron 67, 178–180 [DOI] [PubMed] [Google Scholar]
- 32.Driver J and Noesselt T (2008) Multisensory interplay reveals crossmodal influences on “sensory-specific” brain regions, neural responses, and judgments. Neuron 57, 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer K et al. (2010) Predicting visual stimuli on the basis of activity in auditory cortices. Nat. Neurosci 13, 667–668 [DOI] [PubMed] [Google Scholar]
- 34.Graziano MSA (2016) A new view of the motor cortex and its relation to social behavior. In Shared Representations: Sensorimotor Foundations of Social Life (Cross ES and Obhi SS, eds), pp. 38–58, Cambridge University Press [Google Scholar]
- 35.Liang M et al. (2013) Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nat. Commun 4, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vetter P et al. (2014) Decoding sound and imagery content in early visual cortex. Curr. Biol 24, 1256–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niell CM and Stryker MP (2010) Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atilgan H et al. (2018) Integration of visual information in auditory cortex promotes auditory scene analysis through multisensory binding. Neuron 97, 640–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bizley JK et al. (2007) Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb. Cortex 17, 2172–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandrasekaran C et al. (2013) Dynamic faces speed up the onset of auditory cortical spiking responses during vocal detection. Proc. Natl. Acad. Sci. U. S. A 110, E4668–E4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghazanfar AA (2005) Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J. Neurosci 25, 5004–5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayser C et al. (2008) Visual modulation of neurons in auditory cortex. 18 pp. 1560–1574 [DOI] [PubMed] [Google Scholar]
- 43.Perrodin C et al. (2015) Who is that? Brain networks and mechanisms for identifying individuals. Trends Cogn. Sci 19, 783–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avitan L and Stringer C (2022) Not so spontaneous: multi-dimensional representations of behaviors and context in sensory areas. Neuron 110, 3064–3075 [DOI] [PubMed] [Google Scholar]
- 45.Green HD and Hoff EC (1937) Effects of faradic stimulation of the cerebral cortex on limb and renal volumes in the cat and monkey. Am. J. Phys 118, 641–658 [Google Scholar]
- 46.Wall PD and Pribram KH (1950) Trigeminal neurotomy and blood pressure responses from stimulation of lateral cerebral cortex of Macaca mulatta. J. Neurophysiol 13, 409–412 [DOI] [PubMed] [Google Scholar]
- 47.Merabet LB et al. (2008) Rapid and reversible recruitment of early visual cortex for touch. PLoS One 3, e3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauschecker JP and Kniepert U (1994) Auditory localization behaviour in visually deprived cats. Eur. J. Neurosci 6, 149–160 [DOI] [PubMed] [Google Scholar]
- 49.King AJ and Parsons CH (1999) Improved auditory spatial acuity in visually deprived ferrets. Eur. J. Neurosci 11, 3945–3956 [DOI] [PubMed] [Google Scholar]
- 50.Keck T et al. (2013) Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron 80, 327–334 [DOI] [PubMed] [Google Scholar]
- 51.Edelman GM and Gally JA (2001) Degeneracy and complexity in biological systems. Proc. Nat. Acad. Sci. U. S. A 98, 13763–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melton DW (1994) Gene targeting in the mouse. BioEssays 16, 633–638 [DOI] [PubMed] [Google Scholar]
- 53.Brodin P and Davis MM (2017) Human immune system variation. Nat. Rev. Immunol 17, 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallivan JP et al. (2016) The sequential encoding of competing action goals involves dynamic restructuring of motor plans in working memory. J. Neurophysiol 115, 3113–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marder E and Taylor AL (2011) Multiple models to capture the variability in biological neurons and networks. Nat. Neurosci 14, 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tononi G et al. (1999) Measures of degeneracy and redundancy in biological networks. Proc. Natl. Acad. Sci. U. S. A 96, 3257–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price CJ and Friston KJ (2002) Degeneracy and cognitive anatomy. Trends Cogn. Sci 6, 416–421 [DOI] [PubMed] [Google Scholar]
- 58.Singh A et al. (2021) Variation is the norm: brain state dynamics evoked by emotional video clips. In 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), pp. 6003–6007, IEEE; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson-Mendenhall CD et al. (2015) Variety in emotional life: within-category typicality of emotional experiences is associated with neural activity in large-scale brain networks. Soc. Cogn. Affect. Neurosci 10, 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson-Mendenhall CD et al. (2011) Grounding emotion in situated conceptualization. Neuropsychologia 49, 1105–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyle CM et al. (2022) Unsupervised classification reveals consistency and degeneracy in neural network patterns of emotion. Soc. Cogn. Affect. Neurosci 17, 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raz G et al. (2016) Functional connectivity dynamics during film viewing reveal common networks for different emotional experiences. Cogn. Affect. Behav. Neurosci 16, 709–723 [DOI] [PubMed] [Google Scholar]
- 63.van der Weel FR (Ruud) et al. (2019) Infants’ brain responses to looming danger: degeneracy of neural connectivity patterns. Ecol. Psychol 31, 182–197 [Google Scholar]
- 64.Rathour RK and Narayanan R (2019) Degeneracy in hippocampal physiology and plasticity. Hippocampus 29, 980–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamaleddin MA (2022) Degeneracy in the nervous system: from neuronal excitability to neural coding. BioEssays 44, e2100148. [DOI] [PubMed] [Google Scholar]
- 66.Stöber TM et al. (2022) Degeneracy in epilepsy: multiple routes to hyperexcitable brain circuits and their repair. arXiv Published online June 20, 2022. 10.48550/arXiv.2206.09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farrell JS et al. (2019) Resolving the micro-macro disconnect to address core features of seizure networks. Neuron 101, 1016–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan Z et al. (2022) A computational neural model for mapping degenerate neural architectures. Neuroinformatics 20, 965–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubinov M and Sporns O (2011) Weight-conserving characterization of complex functional brain networks. NeuroImage 56, 2068–2079 [DOI] [PubMed] [Google Scholar]
- 70.Adolphs R et al. (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672 [DOI] [PubMed] [Google Scholar]
- 71.Feinstein JS et al. (2011) The human amygdala and the induction and experience of fear. Curr. Biol 21, 34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrett LF (2018) Seeing fear: it’s all in the eyes? Trends Neurosci. 41, 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becker B et al. (2012) Fear processing and social networking in the absence of a functional amygdala. Biol. Psychiatry 72, 70–77 [DOI] [PubMed] [Google Scholar]
- 74.Feinstein JS et al. (2010) Sustained experience of emotion after loss of memory in patients with amnesia. Proc. Natl. Acad. Sci. U. S. A 107, 7674–7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berlau DJ and McGaugh JL (2003) Basolateral amygdala lesions do not prevent memory of context-footshock training. Learn. Mem 10, 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prinz AA et al. (2004) Similar network activity from disparate circuit parameters. Nat. Neurosci 7, 1345–1352 [DOI] [PubMed] [Google Scholar]
- 77.Gutierrez GJ et al. (2013) Multiple mechanisms switch an electrically coupled, synaptically inhibited neuron between competing rhythmic oscillators. Neuron 77, 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gjorgjieva J et al. (2016) Computational implications of biophysical diversity and multiple timescales in neurons and synapses for circuit performance. Curr. Opin. Neurobiol 37, 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiltschko AB et al. (2015) Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayat H et al. (2022) Reduced neural feedback signaling despite robust neuron and gamma auditory responses during human sleep. Nat. Neurosci 25, 935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hutchinson JB and Barrett LF (2019) The power of predictions: an emerging paradigm for psychological research. Curr. Dir. Psychol. Sci 28, 280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heft H (2001) Ecological Psychology in Context: James Gibson, Roger Barker, and the Legacy of William James’s Radical Empiricism, Lawrence Erlbaum Associates [Google Scholar]
- 83.Smith LB and Thelen E (2003) Development as a dynamic system. Trends Cogn. Sci 7, 343–348 [DOI] [PubMed] [Google Scholar]
- 84.Gergen KJ (1978) Experimentation in social psychology: a reappraisal. Eur. J. Soc. Psychol 8, 507–527 [Google Scholar]
- 85.Bressler SL and McIntosh AR (2007) The role of neural context in large-scale neurocognitive network operations. In Handbook of Brain Connectivity (Jirsa VK and McIntosh A, eds), pp. 403–419, Springer [Google Scholar]
- 86.Sheheitli H and Jirsa VK (2020) A mathematical model of ephaptic interactions in neuronal fiber pathways: could there be more than transmission along the tracts? Netw. Neurosci 4, 595–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han K-S et al. (2018) Ephaptic coupling promotes synchronous firing of cerebellar Purkinje cells. Neuron 100, 564–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weimann JM and Marder E (1994) Switching neurons are integral members of multiple oscillatory networks. Curr. Biol 4, 896–902 [DOI] [PubMed] [Google Scholar]
- 89.Lenartowicz A and McIntosh AR (2005) The role of anterior cingulate cortex in working memory is shaped by functional connectivity. J. Cogn. Neurosci 17, 1026–1042 [DOI] [PubMed] [Google Scholar]
- 90.van den Heuvel MP and Sporns O (2011) Rich-club organization of the human connectome. J. Neurosci 31, 15775–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Betzel RF et al. (2016) Optimally controlling the human connectome: the role of network topology. Sci. Rep 6, 30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D-J and Min B-K (2020) Rich-club in the brain’s macrostructure: insights from graph theoretical analysis. Comput. Struct. Biotechnol. J 18, 1761–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevens FL et al. (2011) Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatr. Clin. Neurosci 23, 121–125 [DOI] [PubMed] [Google Scholar]
- 94.Sepulcre J et al. (2012) Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J. Neurosci 32, 10649–10661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mulert C et al. (2008) Single-trial coupling of EEG and fMRI reveals the involvement of early anterior cingulate cortex activation in effortful decision making. NeuroImage 42, 158–168 [DOI] [PubMed] [Google Scholar]
- 96.Kolling N et al. (2016) Value, search, persistence and model updating in anterior cingulate cortex. Nat. Neurosci 19, 1280–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis KD et al. (2000) Human anterior cingulate cortex neurons modulated by attention-demanding tasks. J. Neurophysiol 83, 3575–3577 [DOI] [PubMed] [Google Scholar]
- 98.Critchley HD et al. (2003) Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126, 2139–2152 [DOI] [PubMed] [Google Scholar]
- 99.de la Cruz F et al. (2019) The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. NeuroImage 196, 318–328 [DOI] [PubMed] [Google Scholar]
- 100.Chang C et al. (2013) Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage 68, 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim K et al. (2019) Resting-state neural firing rate is linked to cardiac-cycle duration in the human cingulate and parahippocampal cortices. J. Neurosci 39, 3676–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Birn RM et al. (2006) Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 1536–1548 [DOI] [PubMed] [Google Scholar]
- 103.Birn RM et al. (2008) The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage 40, 644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang C and Glover GH (2009) Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. NeuroImage 47, 1381–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen JE et al. (2020) Resting-state “physiological networks.”. NeuroImage 213, 116707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barrett LF and Simmons WK (2015) Interoceptive predictions in the brain. Nat. Rev. Neurosci 16, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sennesh E et al. (2022) Interoception as modeling, allostasis as control. Biol. Psychol 167, 108242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prather AA et al. (2013) Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom. Med 75, 350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaddock-Heyman L et al. (2013) The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Front. Hum. Neurosci 7, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Byrne JEM et al. (2017) Time of day differences in neural reward functioning in healthy young men. J. Neurosci 37, 8895–8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simmons WK et al. (2013) Category-specific integration of homeostatic signals in caudal, but not rostral, human insula. Nat. Neurosci 16, 1551–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kucyi A (2018) Just a thought: how mind-wandering is represented in dynamic brain connectivity. NeuroImage 180, 505–514 [DOI] [PubMed] [Google Scholar]
- 113.Rebollo I and Tallon-Baudry C (2022) The sensory and motor components of the cortical hierarchy are coupled to the rhythm of the stomach during rest. J. Neurosci 42, 2205–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poldrack RA et al. (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci 18, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Azari B et al. (2020) Comparing supervised and unsupervised approaches to emotion categorization in the human brain, body, and subjective experience. Sci. Rep 10, 20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schirner M et al. (2018) Inferring multi-scale neural mechanisms with brain network modelling. eLife 7, e28927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huk A et al. (2018) Beyond trial-based paradigms: continuous behavior, ongoing neural activity, and natural stimuli. J. Neurosci 38, 7551–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fang M et al. (2022) PyMVPD: a toolbox for multivariate pattern dependence. Front. Neuroinformatics 16, 835772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poskanzer C and Anzellotti S (2022) Functional coordinates: modeling interactions between brain regions as points in a function space. Netw. Neurosci 6, 1296–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nozais V et al. (2021) Functionnectome as a framework to analyse the contribution of brain circuits to fMRI. Commun. Biol 4, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bishop CM (2006) Pattern Recognition and Machine Learning, Springer [Google Scholar]
- 122.Mayr E (2004) What Makes Biology Unique?: Considerations on the Autonomy of a Scientific Discipline, Cambridge University Press [Google Scholar]
- 123.Cisek P and Hayden BY (2022) Neuroscience needs evolution. Philos. Trans. R. Soc. B Biol. Sci 377, 20200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barrett LF and Finlay BL (2018) Concepts, goals and the control of survival-related behaviors. Curr. Opin. Behav. Sci 24, 172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Favela LH (2020) Cognitive science as complexity science. Wiley Interdiscip. Rev. Cogn. Sci 11, e1525. [DOI] [PubMed] [Google Scholar]
- 126.Criscuolo A et al. (2022) Cognition through the lens of a body–brain dynamic system. Trends Neurosci. 45, 667–677 [DOI] [PubMed] [Google Scholar]
- 127.Marek S et al. (2020) Towards reproducible brain-wide association studies. bioRxiv Published online August 22, 2022. 10.1101/2020.08.21.257758 [DOI] [Google Scholar]
- 128.Button KS et al. (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 14, 365–376 [DOI] [PubMed] [Google Scholar]
- 129.Yarkoni T (2009) Big correlations in little studies: inflated fMRI correlations reflect low statistical power—commentary on Vul et al. (2009). Perspect. Psychol. Sci 4, 294–298 [DOI] [PubMed] [Google Scholar]
- 130.Carp J (2012) On the plurality of(methodological) worlds: estimating the analytic flexibility of fMRI experiments. Front. Neurosci 6, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guo Q et al. (2014) The reporting of observational clinical functional magnetic resonance imaging studies: a systematic review. PLoS One 9, e94412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Botvinik-Nezer R et al. (2020)Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nichols TE et al. (2017) Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci 20, 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nicholson DJ (2014) The machine conception of the organism in development and evolution: a critical analysis. Stud. Hist. Phil. Biol. Biomed. Sci 48, 162–174 [DOI] [PubMed] [Google Scholar]
- 135.Bassett DS and Gazzaniga MS (2011) Understanding complexity in the human brain. Trends Cogn. Sci 15, 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sporns O (2011) The human connectome: a complex network. Ann. N. Y. Acad. Sci 1224, 109–125 [DOI] [PubMed] [Google Scholar]
- 137.Kelso JAS (2012) Multistability and metastability: understanding dynamic coordination in the brain. Philos. Trans. R. Soc. B Biol. Sci 367, 906–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kelso JAS et al. (2013) Outline of a general theory of behavior and brain coordination. Neural Netw. 37, 120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rabinovich MI et al. (2015) Dynamical bridge between brain and mind. Trends Cogn. Sci 19, 453–461 [DOI] [PubMed] [Google Scholar]
- 140.Breakspear M and McIntosh AR (2011) Networks, noise and models: reconceptualizing the brain as a complex, distributed system. NeuroImage 58, 293–295 [DOI] [PubMed] [Google Scholar]


