Abstract
Background:
Medication adherence in type 2 diabetes mellitus (T2DM) patients is often suboptimal resulting in complications. There has been a growing interest in using mobile apps for improving medication adherence.
Objective:
The objective of this work was to systematically review the clinical trials that have used mobile app–based interventions in T2DM patients for improving medication adherence.
Methodology:
A systematic search was performed to identify published clinical trials between January 2008 and December 2020 in databases—PubMed, Cochrane Library, and Google Scholar. All studies were assessed for risk of bias using quality rating tool from the Cochrane Handbook for Systematic Reviews of Interventions.
Results:
Seven clinical studies having 649 participants were studied. The median sample size was 58 (range = 41-247) and the median age of participants was 53.2 (range = 48-69.4) years. All studies showed improvements in adherence; however, only three studies reported statically significant improvements in adherence measures. Selected studies were deemed as unclear in their risk of bias and the most common source of risk of bias among the studies was the absence of objective outcome assessment.
Conclusions:
Mobile apps appear to be effective interventions to help improve medication adherence in T2DM patients compared with conventional care strategies. The features of the App to improvise medical adherence cannot be defined based on the meta-analysis because of heterogeneity of study designs and less number of sample size. Systematically planned studies would set up applicability of mobile apps in the clinical management of T2DM.
Keywords: mobile app, medication adherence, self-monitoring of blood glucose, type 2 diabetes mellitus, digital interventions, blood glucose
Introduction
The prevalence of diabetes is growing rapidly worldwide and it places a significant economic burden on health care systems. Around five million deaths are attributed to diabetes each year and associated financial burden of about 12% of total health expenditure is spent only on diabetes management worldwide. 1 The International Diabetes Federation (IDF) estimates that diabetes prevalence will rise to about 700 millions by 2045, which is currently about 463 millions. 2 Type 2 diabetes mellitus (T2DM) is a progressive disease with loss of β-cell function and insulin resistance leading to a failure of glycemic control. 3 The American Diabetes Association (ADA) recommends glycated hemoglobin (HbA1c) targets of <7% (<53 mmol/mol) in newly diagnosed patients and <8% (<64 mmol/mol) in patients on two or more therapies. 4 A patient with diabetes requires lifelong management of the disease with medications and significant life style changes. Continuous monitoring and management of glucose is essential and medication adherence in early stages of diabetes is important for maximizing the effectiveness of pharmacotherapy as well as in minimizing the chances of developing microvascular and macrovascular complications. However, a significant number of people with T2DM fail to control glycemia owing to the factors such as therapeutic inertia and adherence.5,6 Poor glycemic control among the patients with diabetes represents a major public health challenge and the most important risk factor in development of diabetes complications. 7 The World Health Organization (WHO) explains that medication adherence is a multifaceted phenomenon that involves patients, their health care providers, and the process of taking medications. It is an extent to which the patient agrees to follow the recommendations such as medication regimen, dietary and lifestyle changes, and so on suggested by health care provider. 8 The medication (oral hypoglycemic agents) adherence among newly diagnosed T2DM patients ranges between 36% and 93% and adherence to insulin among T2DM patients is below 64%. 9 Furthermore, there is a causal association between number of hospitalization and adherence to medication, which is reduced by about 23.3% when adherence had risen from 50% to 100%. 10 Hence, it is evident that adherence to medications and lifestyle management is a critical factor in diabetes and several attempts have been made during the past few years to find solutions to improve patient adherence to diminish poor patient compliance problems.
The last decade has witnessed an ever-increasing role of information technology (IT) in the health care arena. 11 Several novel IT tools such as electronic health records (EHRs), e-prescribing, and electronic drug monitoring (EDM) for providers to monitor patients’ medication adherence have been proven beneficial to provide seamless and effective solutions for improving health care practices and patient outcomes in diabetes. 12 Recent IT inventions have gone far ahead to not only minimizing the human task in data collection and analysis but also they have helped to ensure health care quality and safety. 13 Smartphone-based mobile applications are one of the most famed IT innovations that are not only helpful for medical researchers but also they have been widely utilized by patients who aim to monitor their health. As the demand for mobile applications has increased, several applications of IT such as artificial intelligence (AI) and machine learning tools have paved the way for early diagnosis of diseases, treatment protocol development, and patient monitoring and care. 14 This study was aimed at analyzing the potential of existing mobile app–based interventions to support medication adherence among T2DM patients, discussing the functionalities and limitations of these mobile apps and bestow upon future directions for mobile app utility in this research paradigm.
Materials and Methods
Study Selection Criteria
The criteria for inclusion of studies in our search included published articles in “English” that discussed the effectiveness of mobile apps in improving the medication (treatment) adherence in patients with diabetes of any age group as their study population. The researchers searched for both the qualitative and quantitative studies using descriptors and experimental approaches and took guidance from checklist provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to conduct this systematic review. 15 The studies that described potential of mobile apps in enhancing adherence to treatment, patient compliance and satisfaction, safe medication practices, patient acceptance and viability were included for analysis. Studies that explained only the designs of mobile apps, applicable for use by only the health care provider, utilizing short messaging service (SMS), phone calls, or other electronic means were excluded. Table 1 provides the detailed inclusion and exclusion criteria for selection of clinical studies.
Table 1.
Inclusion and Exclusion Criteria of the Study.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Assessment of mobile apps for increasing medication adherence in diabetes | • Research/review articles published in
English • Provided results about effectiveness of mobile app in enhancing medication/treatment adherence among type 2 diabetes patients • Publication date between January 2008 and December 2020 • Original evaluation • Considering any age group as study population • App was used by patient or caregiver |
• Studies published other than in English
language • Studies describing mobile apps to be used by other than patient or caregiver • Studies provided utility of mobile app in other chronic illness except type 2 diabetes • Non-health-related studies • Studies merely descriptive about the design and other features of mobile apps • Commentaries and studies published only in abstract form • Studies describing other interventions such as electronic pillbox, phone calls, or SMS text messaging to remind patient about medication • Studies that were not directly related to study objectives |
Abbreviation: SMS, short messaging service.
Search Strategy
The researchers carried out a search of the scientific literature on databases, including PubMed, Cochrane Library, and Google Scholar, to identify available studies by selecting a time frame between January 2008 and December 2020. This period was selected because the app markets by Apple and Android systems were launched in year 2008. 16 To identify relevant scientific publications, both medical subject headings and free-text search terms with Boolean indicators OR and AND (Mobile app OR Smartphone application AND Medication adherence OR Medication management AND Type 2 Diabetes) were used. The same descriptors were used to search the Internet for relevant gray literature using the Google search engine. The search was also filtered for exclusion of incomplete and unoriginal works. Furthermore, we analyzed all identified citations for inclusion criteria by reviewing their titles and abstracts.
Data Extraction
After running the searches on selected databases, the results were saved in EndNote (version 20.0.0.14672) and duplicates were removed both automatically and manually. The scrutinizing and reviewing of searched publications were divided among the authors, and at first, two authors (T.P.S. and R.G.) checked through the title and abstracts of potential publications for their eligibility as per the inclusion criteria. The screened full-text articles through title search were again subjected for determining their eligibility based on the checklist for inclusion and exclusion (Table 1). Articles thus selected were divided into three categories, namely, included, excluded, and deferred due to ambiguity. Furthermore, two other authors (R.K.G. and S.G.) separately reviewed all the deferred articles and a decision was made on the agreement of all the authors. Any further differences in opinion among the research team members on the ambiguities were resolved by discussion and articles were finally selected when all the authors agreed.
Quality Assessment
A significant clinical and methodological heterogeneity was observed among the studies included in this systematic review. Therefore, the assessment of risk of bias presented a number of factors that attributed to the biasness in the studies. We have utilized the quality rating tool from the Cochrane Handbook for Systematic Reviews of Interventions. 17 Potential bias were graded as high, unclear, or low risk bias for each of following domains: random sequencing, allocation concealment, binding of participants and personnel, objective outcome assessment, incomplete outcome data, selective outcome reporting, and other bias.
Results
Identified and Included Studies
The literature search yielded a total of 1512 potentially relevant publications. After the removal of all duplicates, a total of 1322 publications were considered for inclusion. A total of 736 articles were identified as nonprimary research publications and the remaining 586 publications were identified as potentially relevant. The remaining 411 citations were screened based on titles and, then, abstracts. A total of 175 primary research publications were screened, of which seven were eligible for inclusion. A breakdown of this process can be seen in the PRISMA flowchart in Figure 1. Full-text articles were excluded mainly because the study explored technologies that were not integrated within an app and/or Web-based platform and hence did not meet the instant study aims. Another common reason for exclusion was that the app in the study did not aim to improve adherence in T2DM. 18 - 24
Figure 1.
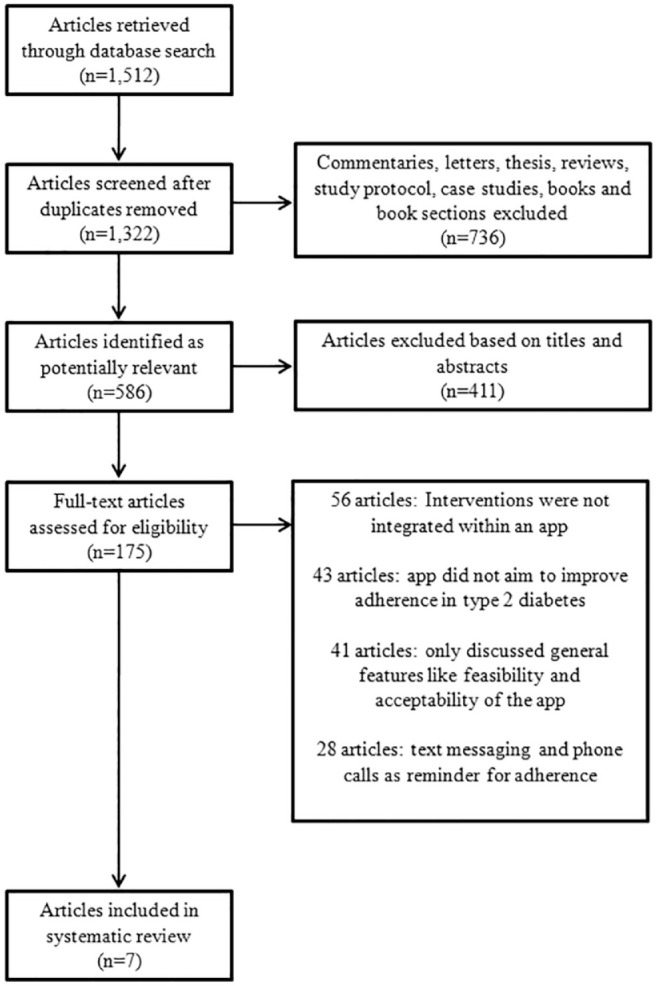
PRISMA flow diagram of reviewed and included studies.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of Included Studies
There were 649 participants in the seven studies as represented in Table 2. The median sample size was 58 (range = 41-247) and the mean age of participants ranged from 48 to 69.4 (median = 53.2) years. The percentage of participants who were female ranged from 30% to 56.6% and the percentage mean of female participants among the included studies was 41.68%. All the studies included adult patients of both the genders except the one which included only elderly patients, 18 also the one study has not specified age and genders of patients. 21 Patients diagnosed with T2DM with average HbA1c levels ≥7% were selected by all the studies. The included publications fell into two categories, in that they either reported only on the impact of the app on medication adherence or they reported on the impact of the app on both medication adherence and a clinical outcome.
Table 2.
General Characteristics of Included Studies and Participants.
| S No | Reference | Country | Mobile app | Study design | Sample size (study duration) | Mean age in years (female %) | Participant characteristics |
|---|---|---|---|---|---|---|---|
| 1. | Brath et al 18 | Austria | Mobile health (mHealth)–based medication adherence management system (mAMS) | Randomized single-blinded single-center study | 53 divided into IG and CG but not specified (13 months) |
69.4 (56.6) |
Elderly patients with increased cardiovascular risk treated for diabetes, high cholesterol, and hypertension. Cardiovascular risk was defined as the presence of at least two of the three risk factors: T2DM, hypercholesterolemia, and hypertension |
| 2. | Frias et al 19 | United States | Proteus Discover App | Prospective, cluster-randomized pilot clinical trial | IG = 80 CG = 29 (12 weeks) |
IG = 57.8 CG = 61.6 (50.4) |
Adults with T2DM (HbA1c ≥ 7%) at 13 outpatient primary care centers across California and Colorado |
| 3. | Kleinman et al 20 | India | Gather Health | Randomized clinical trial | IG = 44 CG = 46 (six months) |
IG = 48.8 CG = 48.0 (30) |
Diabetes clinic out patients with HbA1c value 7.5%-12.5% (58-113 mmol/mol) at three different sites at sites in India |
| 4. | Doocy et al 21 | Lebanon | Magpi Mobile data platform | Longitudinal cohort study | IG = 15 CG = 43 (20 months) |
Not specified a | Patients at 10 health care centers in Lebanon supported by International Organization for Migration, diagnosed with T2DM or hypertension or both |
| 5. | Huang et al 22 | Singapore | Medisafe App | Randomized controlled trial | IG = 22 CG = 19 (12 weeks) |
IG = 51.5 CG = 52.0 (51.2) |
Public tertiary diabetes specialist outpatient center, T2DM diagnosed as per ADA guidelines |
| 6. | Kjos et al 23 | United States | Medsimple | Prospective-observational single-cohort study | 51 (six months) |
52.3 (54.9) |
Community-dwelling adults with T2DM in the United States, taking at least two prescription medications |
| 7. | Yang et al 24 | South Korea | Hicare smart K | Multicenter cluster-randomized clinical trial | IG = 150 CG = 97 (three months) |
IG = 54.1 CG = 60.6 (49.3) |
Primary care clinic outpatients with T2DM and HbA1c levels 7%-10% (53-86 mmol/mol) |
Abbreviations: ADA, American Diabetes Association; IG, intervention group; CG, comparator group; T2DM, type 2 diabetes mellitus; HbA1c, hemoglobin A1c.
Study did not mention demographic data of patients.
The outcomes of selected clinical studies are represented in Table 3. Of the seven selected trials, one each represented medication adherence by pill count 18 and adherence measurement interview, 21 two trials represented medication adherence by self-reporting, and three trials utilized medication adherence measurement scales to calculate the score for assessing medication adherence. 22 - 24 In all interventions, there were researchers who trained participants how to use the app.
Table 3.
Outcomes of the Clinical Trials Using Subjective and Objective Measures of Adherence.
| S No | Source | IG & CG | Method of adherence measurement | Adherence measure & change (significance) | Clinical outcome/patient related outcome measure (significance) | App functions | ||
|---|---|---|---|---|---|---|---|---|
| 1. | Brath et al 18 | App vs UC | Pill count from electronic vs standard blisters through a mobile app | Ingestion adherence of Metformin IG = 0.93-1 CG = 0.89-1 (P = .04) |
Mean change in HbA1c from baseline
0.1% (P value = .06) |
Data storage and transfer, Reminders | ||
| 2. | Frias et al 19 | App vs UC | Medication ingestion adherence (Self-reported) | Mean Ingestion AdherenceNS
Glipizide = 81% (four weeks), 82% (12 weeks) Metformin = 86% (four weeks), 85% (12 weeks) |
Mean reduction in HbA1c—0.5% (four weeks), 0.2% (12 weeks) compared with UCNS | Documentation, feedback to medication taking, other health behaviors | ||
| 3. | Kleinman et al 20 | App vs UC | Self-reported medication adherence | Mean adherence IG = 39.0% CG = 12.8% (P = .03) |
Reduction in HbA1c levels after six months IG = 1.5% CG = 0.8% (P = .03) |
Reminders, data visualization, behavioral assessment, collaborative care decision | ||
| 4. | Doocy et al 21 | App vs UC | Adherence Measurement Interview (Questionnaire) | Mean noncompliance IG = 5.00 CG = 14.33 (P = .54)NS |
Diabetes patients with blood test results IG = 52.2% CG = 45.1% (P = .20)NS |
Personally Controlled Health Records (PCHR), Lifestyle/behavior change support, Electronic Health Record and decision support | ||
| 5. | Kjos et al 23 | App users and their self-controls | MUSE, LOC, ARMS, self-reported adherence | MUSE (Mean ± SD) (P = .852)NS |
Observed none | Behavioral change tech., action planning, prompts/cues, self-monitoring of behavior, shaping knowledge | ||
| Baseline | 90 days | 180 days | ||||||
| 32.05 ± 5.27 | 31.68 ± 4.48 | 31.88 ± 5.11 | ||||||
| LOC Internal (Mean ±
SD) (P = .446)NS | ||||||||
| 19.76 ± 2.31 | 19.85 ± 2.56 | 20.17 ± 2.47 | ||||||
| ARMS (Mean ± SD) (P = .295)NS | ||||||||
| 18.29 ± 3.26 | 18.12 ± 3.84 | 17.56 ± 3.51 | ||||||
| Outcome expectations (Mean ±
SD) (P = .646)NS | ||||||||
| 53.10 ± 4.21 | 52.90 ± 3.94 | 53.49 ± 3.87 | ||||||
| 6. | Huang et al 22 | App vs UC | ASK-12, ADS, DSMQ | ASK-12 Mean (SD) | HbA1c%—Mean (SD) IG = 9.0 (1.6) CG = 9.4 (2.4) Adjusted mean difference= −0.42 (P = .57)NS |
Medication scheduling, reminder, tracking, data sharing, and medication adherence assessment | ||
| IG = 28.6 (5.2) CG = 25.5 (4.4) Adjusted mean difference = −4.73 (P = .04) | ||||||||
| ADS Scale—Mean (SD) | ||||||||
| IG = 19.7 (3.7) CG = 19.7 (3.8) (P = .57)NS | ||||||||
| DSMQ Score—Mean (SD) | ||||||||
| IG = 2.0 (0.4) CG = 2.0 (0.3) (P = .69)NS | ||||||||
| 7. | Yang et al 24 | App vs UC | MMAS-6, DTSQ | |||||
| MMAS-6-Mean change from baseline | Mean change from
baseline HbA1c% IG = −0.63 CG = −0.28 (P = .003) |
Documentation, SMBG, Reminder, Transmission of data to server | ||||||
| IG = 0.52 CG = 0.06 (P = .02) | ||||||||
| DTSQ-Mean change from baseline | ||||||||
| IG = 2.40 CG = 0.45 (P = .01) |
||||||||
Abbreviations: IG, intervention group; CG, comparator group; UC, usual care; HbA1c, hemoglobin A1c; NS, nonstatistically significant; MUSE, Medication Understanding and use Self-efficacy Scale (MUSE); LOC, locus of control; ARMS, Adherence to Refills and Medication Scale; ASK-12, Adherence Starts with Knowledge; ADS, Appraisal of Diabetes Scale; DTSQ, Diabetes Treatment Satisfaction Questionnaire; MMAS-6, 6-item Morisky Medication Adherence Scale; DSMQ, Diabetes Self-Management Questionnaire; SMBG, Self-Monitoring of Blood Glucose.
Characteristics of Apps
Strategies for the promotion of medication adherence used by the apps included reminders, learning instructions, medication e-dairy, and communication with a health care provider. Four apps provided a feature of reminding patients to take medicines through a notification or a motivational activity involving patient education features.20,22- 24 Furthermore, five apps have included learning features to make the user more informed about the consequences of nonadherence in T2DM 21 Medication/e-health features were embedded in two apps to increase adherence by creating a customized medication list and/or schedule of dosing intervals and also informed patients about possible side effects of medicines.22,24 Four apps have provided an opportunity of communication by employing the features of two-way communication between app user and clinical support providers.20,22- 24 Besides these, some common features of the apps included blood glucose (BG) monitoring, question and answers related to coaching in diabetes management, feedback messages and social support, and so on.
Measure of Adherence
The included studies utilized both subjective and objective methods of adherence measurement as illustrated in Table 3. The table represents both the statistically significant and nonsignificant values. The values for nonstatistically significant results were represented as NS (nonstatistically significant). The data represented in Table 3 are as detailed as presented in the studies; however, the differences or lack of consistency is due to heterogeneity in the respective publications. Of the seven selected studies, six studies measured adherence using subjective measures only through self-report measures and questionnaire. In three of these six studies, two or more subjective measures of self-reporting were used. 22 - 24 One study required participants to self-assess their own adherence at a point in time. 23 Only one of seven selected studies utilized objective measure of adherence through pill counting. 18 None of studies utilized a combination of both the subjective and objective measures. Specifically, the study conducted by Frias et alalso assessed adherence to antihypertensive medicines through self-reported medication ingestion adherence. 19 Overall four of seven selected studies have represented at least one statistically significant measure of medication adherence in T2DM patients due to an app intervention.
Impact of Apps on Improving Health Outcomes
Although the studies were not selected on the basis of measuring health outcomes, improved due to an app intervention and the primary objective was to assess the measure of adherence. However, to make accurate and comprehensive conclusions, the following results highlight those studies that reported changes from baseline for clinical outcomes along with the statistical significance of changes.
Table 3 also represents changes in HbA1c/BG levels as the measure of outcome represented by the selected studies. Three of seven selected studies represented statistically significant measures of both adherence and reduction in HbA1c/BG levels in control and intervention groups (IG).18,20,24 One study did not represent any health outcomes, 23 whereas three studies did report on the change in clinical outcome but the change was not significant.19,21,22 Other health outcomes demonstrated by the studies included blood pressure, total cholesterol, high-/low-density lipoprotein, changes in body mass index, and so on.
Risk of Bias and Quality Assessment
Overall, the selected studies were deemed as unclear in their risk of bias. A summary of the risk of bias analysis is shown in Table 4. The most common source of risk of bias among the studies was the absence of objective outcome assessment. This was so because only one of the seven studies used objective measure of assessing medication adherence by pill counting. 18 The subjective measures such as self-assessment questionnaire are a source of potential bias, including those of social desirability and those arising from selective recall and the duration of the recall period. The second most common bias arose from the absence of concealment of allocations that was unclear in five studies. In most of the studies, risk of bias was low in relation to allocation of participants in different groups by randomization. In two of the studies, other bias was higher because groups were not balanced at baseline for controlling T2DM in these studies.18,19 Overall, two studies were thought to have high risk of bias.19,21
Table 4.
Summary of Risk of Bias.
| S No | Source | Selection bias: Allocations | Performance bias: Blinding of participants and personnel | Detection bias: Objective outcome assessment | Attrition bias: Incomplete outcome data adequately addressed | Reporting bias: Free from selective outcome reporting | Other bias | |
|---|---|---|---|---|---|---|---|---|
| Generated | Concealed | |||||||
| 1. | Brath et al 18 |

|

|

|

|

|

|

|
| 2. | Frias et al 19 |

|

|

|

|

|

|

|
| 3. | Kleinman et al 20 |

|

|

|

|

|

|

|
| 4. | Doocy et al 21 |

|

|

|

|

|

|

|
| 5. | Kjos et al 23 |

|

|

|

|

|

|

|
| 6. | Huang et al 22 |

|

|

|

|

|

|

|
| 7. | Yang et al 24 |


|

|


|

|


|

|


|
 Low
risk of bias;
Low
risk of bias;  Unclear risk
of bias;
Unclear risk
of bias;  High risk of
bias.
High risk of
bias.
This systematic review also identified that there were issues in designating the intervention in comparator group (CG) specifically when participants in IG were using an app for which the adherence was measured. In these cases, bias may be higher if the patient in CG notices the use of an app by participant in IG. Hence, CG may also be provided with an app with minimal/no features in comparison with IG for measuring the adherence as suggested by Goldstein et al. 25
Discussion
The researchers conducted a rigorous systematic review of the published clinical studies to investigate whether mobile device apps demonstrate efficacy in supporting medication adherence in T2DM. All seven studies reported that their mobile app–delivered interventions demonstrated efficacy in supporting medication adherence, although in four studies the interventions did not have a statistically significant effect. Three studies also demonstrated significant improvement in relevant clinical outcome in terms of reduction in HbA1c levels.
This systematic review identified several challenges in synthesizing evidence about the impact of apps on adherence. One of the major limiting factors was the methodological and clinical heterogeneity among the studies. Another potential limitation might be the variation in adherence measurement. This may be illustrated by three studies that utilized same objective tool of adherence measurement (self-reported adherence) but where one reported better medication adherence (39.0% [IG] vs 12.8% [CG], P = .03) 20 and the other two reported nonsignificant (1% change in mean adherence after 12 weeks of intervention, 14.33 CG vs 5.00 IG, P = .54) improvement in adherence.19,21 Participant-related factors such as disease burden, nonadherence behaviors, perceived benefit from the medication, and any side effects may also have contributed to variance in the effect size seen among the studies.
Furthermore, the heterogeneity of apps’ features and contents could also have resulted into higher level of variation on the efficacy of apps. Currently, no regulatory guidelines are available for regulating the quality of features and contents of the apps; hence, most medical apps highlight two potential problems: First, the quality of the information provided by the app cannot be ascertained, and second, the patient data are shared by apps that raise the concern for patient privacy. 26 Further research regarding the contents of apps is required along with regulations to protect patient privacy. Although this review aimed to include studies over the last decade, six of seven selected studies were published within the last three years, indicating an increase in the interest of mobile app–based interventions to promote medication adherence, in recent years.
There are several mobile apps available for improving medication adherence in various disease conditions such as cardiovascular disorders, psychiatry disorders, and so on. These apps although function to improve medication adherence, however, the level of engagement with the app is a critical parameter, especially when adherence is assessed through objective self-assessment measures. 27 A previous study identified that the impact of nonpharmacological intervention depends upon the level of adherence to the intervention itself, meaning that a patient must be well engaged with the app, which may ultimately result in medication adherence. 28 It is interesting to note that three of seven apps have the facility of reminders for taking medicines that makes the patient adherent to the app and hence a digital placebo for medication adherence has been created in patients that resulted in medication adherence. This concept was discussed in a previously published study that suggested that mobile apps create a digital placebo effect on patient and the beliefs about technology support and perceptions of being constantly connected with health care providers lead to clinical improvements. 29 Therefore, future studies could explore this digital placebo effect to ensure medication adherence by app interventions. Overall, future studies should use evidence-based measures and meticulously plan the clinical study protocols to assess the effectiveness of mobile apps in enhancing medication adherence among the T2DM patients. 30
Strengths and Limitations
This systematic review adds new knowledge to the field as currently no comparable studies are available with which the results of this review could be compared. We have included all studies in spite of their quality of methodological design and quality of apps utilized, which is both a strength and a limitation. This supported the authors in collating the most comprehensive evidence based on the current literature; however, this has also limited the authors to draw ultimate conclusions based on the findings. One of the other limitations was the heterogeneity among the studies, app features, adherence, and clinical outcome(s) that prevented direct comparisons in the study. The exclusion of non-English publications may have contributed to language bias and ultimately the exclusion of potentially relevant articles published in different languages. The ubiquity and use of mobile apps vary among different age groups, 31 and as the majority of participants in these studies were aged above 50 years, future studies should select participants of different age groups for assessing the acceptability and usability of adherence apps.
Conclusions
This systematic review explored the impact of apps on medication adherence among T2DM patients. Three of seven included studies that reported the results of statistical tests demonstrated improvements in adherence. However, it was difficult to draw definitive conclusions on improvement of adherence by apps and its impact on clinical health outcomes. This was so because the evidence available was not of good quality and heterogeneity among design of studies, app features, and outcome measures was far above the ground. The authors postulate that further research should focus on formulating standard protocols for assessing the mobile app–based interventions to measure medication adherence. Future research will lead to not only identify ideal features of apps but also improve the app functions to make them more user-friendly and utilize patient-centric approaches to obtain sustainable adherence and health outcomes.
Acknowledgments
The authors are thankful to the administration and management of Delhi Pharmaceutical Sciences and Research University for logistics support during the study.
Footnotes
Abbreviations: ADA, American Diabetes Association; AI, artificial intelligence; BG, blood glucose; EDM, electronic drug monitoring; EHR, electronic health record; HbA1c, glycated hemoglobin; IT, information technology; IDF, International Diabetes Federation; SMS, short messaging service; T2DM, type 2 diabetes mellitus; WHO, World Health Organization.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tarani Prakash Shrivastava  https://orcid.org/0000-0002-4200-8436
https://orcid.org/0000-0002-4200-8436
References
- 1. Diabetes. Global: World Health Organization; 2020. who.int. Accessed January 19, 2021. [Google Scholar]
- 2. Diabetes Atlas : 9th edition. Brussels, Belgium: International Diabetes Federation; 2019. http://www.diabetesatlas.org. Accessed January 17, 2021. [Google Scholar]
- 3. Fonseca V. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(2):S151-S156. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glycemic targets: standards of medical care in diabetes–2020. Diabetes Care. 2020;43(l.1):S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 5. Cramer J. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 6. Khunti S, Davies M, Khunti K. Clinical inertia in the management of type 2 diabetes mellitus: a focused literature review. Br J Diabetes Vasc Dis. 2015;15(2):65-68. doi: 10.15277/bjdvd.2015.019. [DOI] [Google Scholar]
- 7. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540. 10.2337/diacare.27.7.1535 [DOI] [PubMed] [Google Scholar]
- 8. Sabaté E. Adherence to long term therapies: evidence for action. In Defining Adherence Geneva, Switzerland: World Health Organization; 2003:3-5. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Accessed February 4, 2021. [Google Scholar]
- 9. Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011;65(3):314-322. doi: 10.1111/j.1742-1241.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 10. Wild H. The economic rationale for adherence in the treatment of type 2 diabetes mellitus. Am J Manag Care. 2012;18(suppl 3):S43–S48. https://www.ajmc.com/view/the-economic-rationale-for-adherence-in-the-treatment-of-type-2-diabetes-mellitus. Accessed February 11, 2021. [PubMed] [Google Scholar]
- 11. Dash SP. The impact of IoT in healthcare: global technological change & the roadmap to a networked architecture in India. J Indian Inst Sci. 2020;100:773-785. doi: 10.1007/s41745-020-00208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klonoff DC. The current status of mHealth for diabetes: will it be the next big thing? J Diabetes Sci Technol. 2013;7(3):749-758. doi: 10.1177/193229681300700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mbonihankuye S, Nkunzimana A, Ndagijimana A. Healthcare data security technology: HIPAA compliance. Computing. 2019;2019:1927495. doi: 10.1155/2019/1927495. [DOI] [Google Scholar]
- 14. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, & The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strain M. 1983 to today: a history of mobile apps. 2015. https://www.theguardian.com/media-network/2015/feb/13/history-mobile-apps-future-interactive-timeline. Accessed February 19, 2021.
- 17. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook. Accessed March 3, 2021.
- 18. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement—a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(suppl S1): 47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res. 2017;19(7):e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed J E Health. 2017;23(9):733-740. doi: 10.1089/tmj.2016.0265. [DOI] [PubMed] [Google Scholar]
- 21. Doocy S, Paik KE, Lyles E, et al. Guidelines and mHealth to improve quality of hypertension and type 2 diabetes care for vulnerable populations in Lebanon: longitudinal cohort study. JMIR Mhealth Uhealth. 2017;5(10):e158. doi: 10.2196/mhealth.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang Z, Tan E, Lum E, Sloot P, Boehm B, Car J. A Smartphone app to improve medication adherence in patients with type 2 diabetes in Asia: feasibility randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(9/e14914):1-14. doi: 10.2196/14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kjos AL, Vaughan AG, Bhargava A. Impact of a mobile app on medication adherence and adherence-related beliefs in patients with type 2 diabetes. J Am Pharmaceut Assoc. 2019;59(2):S44-S51.e3. doi: 10.1016/j.japh.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Lee E, Kim H, Lee S, Yoon K, Cho J. Effect of a mobile phone–based glucose-monitoring and feedback system for type 2 diabetes management in multiple primary care clinic settings: cluster randomized controlled trial. JMIR Mhealth Uhealth. 2020;8(2):e16266. doi: 10.2196/16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldstein CM, Gathright EC, Dolansky MA, et al. Randomized controlled feasibility trial of two telemedicine medication reminder systems for older adults with heart failure. J Telemed Telecare. 2014;20(6):293-299. doi: 10.1177/1357633X14541039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ng R, Carter SR, El-Den S. The impact of mobile applications on medication adherence: a systematic review. Transl Behav Med. 2020;10(6):1419-1435. doi: 10.1093/tbm/ibz125. [DOI] [PubMed] [Google Scholar]
- 27. Armitage LC, Kassavou A, Sutton S. Do mobile device apps designed to support medication adherence demonstrate efficacy? a systematic review of randomised controlled trials, with meta-analysis. BMJ Open. 2020;10:e032045. doi: 10.1136/bmjopen-2019-032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inouye SK, Bogardus ST, Jr, Williams CS, Leo-Summers L, Agostini JV. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med. 2003;163(8):958-964. doi: 10.1001/archinte.163.8.958. [DOI] [PubMed] [Google Scholar]
- 29. Torous J, Firth J. The digital placebo effect: mobile mental health meets clinical psychiatry. Lancet Psychiatry. 2016;3(2):100-102. doi: 10.1016/S2215-0366(15)00565-9. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Min J, Khuri J, et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management: systematic review of systematic reviews. JMIR Mhealth Uhealth. 2020;8(4):e15400. doi: 10.2196/15400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gurtner S, Reinhardt R, Soyez K. Designing mobile business applications for different age groups. Technol Forecast Soc Change. 2014;88:177-188. doi: 10.1016/j.techfore.2014.06.020. [DOI] [Google Scholar]


