Abstract
Streptococcus pyogenes secretes a specific immunoglobulin G (IgG)-protease, SpeB, as well as the IgG glycan-hydrolyzing enzyme EndoS. Here we show that SpeB also degrades IgA, IgM, IgD, and IgE. We also show that EndoS only hydrolyzes the glycan moiety on native but not denatured IgG. Thus, SpeB has a broad immunoglobulin-degrading activity, while EndoS is highly specific for IgG.
Microbial proteases with immunoglobulin G (IgG)-degrading activity have previously been described for several microbes, including the human pathogen Pseudomonas aeruginosa (8), the causative agents of chronic adult periodontitis; Prevotella intermedia and Prevotella nigrescens, as well as the helminth parasite Paragonimus westermani (9, 22). These microorganisms all degrade IgG into low-molecular-weight fragments. In contrast, specific IgA-protease activity is a well-established feature of a number of bacterial pathogens. For example, Streptococcus pneumoniae, Haemophilus influenzae (18) and oral streptococci (12) all produce proteases that specifically cleave IgA in the hinge region into Fab and Fc fragments.
Streptococcus pyogenes is a significant human pathogen that causes infections such as impetigo, scarlatina, and pharyngitis, as well as severe invasive diseases such as necrotizing fasciitis and sepsis (1, 3). Nonsuppurative sequelae include glomerulonephritis and acute rheumatic fever with heart complications. One protein that has been proposed to play a role in the manifestations of infection is the secreted streptococcal cysteine proteinase, also known as streptococcal erythrogenic toxin B or SpeB (6). SpeB degrades several host plasma and matrix proteins (5, 11, 17) and activates or releases host proinflammatory molecules (7, 10). The role of SpeB as an important virulence factor has been established using both in vivo and in vitro models (14, 15, 25). We have recently shown that SpeB has IgG-protease activity and that a novel enzyme secreted from S. pyogenes, EndoS, hydrolyzes the asparagine-linked glycan on IgG. These two enzymes act simultaneously on the same IgG molecule when bacteria are grown in the presence of human plasma without addition of reducing agents, as previously described (2). The conserved glycan moiety on asparagine 297 of the γ-chain of IgG has been shown to be important for several effector functions of IgG, including complement activation and binding to Fc receptors (20, 21). In this report we investigated if SpeB has activity on the other human immunoglobulins IgA, IgM, IgD, and IgE and if the EndoS activity depends solely on the structure of the glycan moiety of IgG or if the three-dimensional structure of the whole IgG molecule is important.
SpeB activity on IgG, IgA, IgM, IgD, and IgE.
We recently reported that SpeB cleaves the γ-chain of native IgG specifically in the hinge region and that this site is distinct from the papain cleavage site (2). We therefore investigated the ability of SpeB to cleave other human immunoglobulins. Purified cysteine proteinase SpeB (1 μg) was incubated in phosphate-buffered saline (PBS) for 24 h at 37°C with 10 μg of purified human serum IgA, IgG, IgM (Sigma, St. Louis, Mo.), IgD, or IgE (ICN, Aurora, Ohio) in the absence or presence of 10 mM dithiothreitol, a reducing agent necessary for SpeB activity (13). After incubation with SpeB, immunoglobulins were analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE). Proteins for NH2-terminal sequencing were first separated by SDS–10% PAGE and then electroblotted to Immobilon-P (Millipore, Bedford, Mass.) according to Matsuidara (16). Bands of interest were excised and subjected to Edman degradation (4).
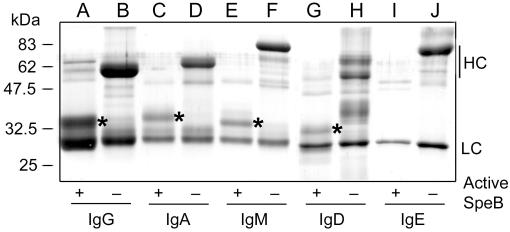
SDS-PAGE analysis of human immunoglobulins incubated with SpeB confirmed that SpeB cleaves the IgG heavy chain into stable Fab and Fc fragments (Fig. 1, lane A). SpeB also cleaved the heavy chains of IgA, IgM, and IgD into fragments of 34, 32, and 30 kDa, respectively, but no accumulation of any other protein band was apparent (Fig. 1, lanes C, E, and G). In contrast, the heavy chains of IgE were completely degraded by SpeB (Fig. 1, lane I). Under these experimental conditions, the light chains were apparently resistant to SpeB degradation and no cleavage of the immunoglobulins was observed when SpeB was inactive (Fig. 1, lanes B, D, F, H, and J).
FIG. 1.
SpeB activity on human immunoglobulins. Purified SpeB was incubated with purified IgG, IgA, IgM, IgD, and IgE in the presence or absence of active SpeB, as indicated. LC, light chains; HC, heavy chains. An asterisk indicates the protein band excised for NH2-terminal sequencing.
To investigate where SpeB cleaves in the immunoglobulin molecules, generated fragments were subjected to NH2-terminal sequencing. This revealed that IgG is cleaved in the hinge region, as reported previously (2), while the sequences from IgM and IgA represent the very amino-terminal parts of the respective heavy chains (Table 1). This suggests that SpeB degrades the carboxy-terminal parts of the μ-chains of IgM and α-chains of IgA, leaving amino-terminal fragments of the respective chains that resist further degradation. The δ-chains of IgD are degraded completely, since the sequence obtained from this fragment represents the hinge region of γ-chains from IgG2. This is most likely due to an IgG contamination of the IgD preparation. The ɛ-chains of IgE are also completely degraded by SpeB. Taken together, these results show that SpeB is a specific IgG-protease that degrades the other immunoglobulin isotypes to various degrees. This could have implications for the role of SpeB in S. pyogenes pathogenicity.
TABLE 1.
Amino-terminal sequences of SpeB-generated fragments of IgG, IgM, IgA, and IgD
| Immunoglobulin | Fragment size (kDa) | Sequence | Homology |
|---|---|---|---|
| IgG | 31 | GPSVFLFPP | Hinge region of IgG1 |
| IgA | 34 | EVQLVESGGAL | N terminus of IgA heavy chain |
| IgM | 32 | EVQLVESGGGL | N terminus of IgM heavy chain |
| IgD | 30 | PVXPSVFLFP | Hinge region of IgG2a |
This fragment most likely represents an IgG contamination of the IgD preparation.
EndoS is specific for native IgG.
We have previously shown that EndoS secreted from S. pyogenes hydrolyzes the glycan on native IgG, leaving an N-acetylglucosamine with a core fucose (2). This is in contrast to several of the known related enzymes, such as the EndoF family, where denaturation enhances the hydrolysis of a substrate glycoprotein (23). Furthermore, we have previously reported that EndoS does not remove any major glycans from IgA and IgM (2). These observations led us to hypothesize that in addition to the glycan moiety on IgG, the three-dimensional structure of this immunoglobulin is important for EndoS activity.
In order to elucidate this, full-length EndoS was expressed in Escherichia coli using the glutathione S-transferase (GST) gene fusion system according to the manufacturer's instructions (Amersham-Pharmacia Biotech, Uppsala, Sweden). After induction, bacteria were lysed using BugBuster (Novagen), and the GST-EndoS fusion protein was purified on glutathione-Sepharose. The GST tag was removed using factor Xa according to protocols (Amersham-Pharmacia Biotech), and residual factor Xa was removed using Xarrest-agarose (Novagen). This resulted in a preparation of recombinant EndoS (rEndoS) that was homogenous as assessed by SDS-PAGE and Western blot using EndoS-specific antibodies (data not shown). This rEndoS was used to investigate the activity of EndoS on IgG denatured to various degrees.
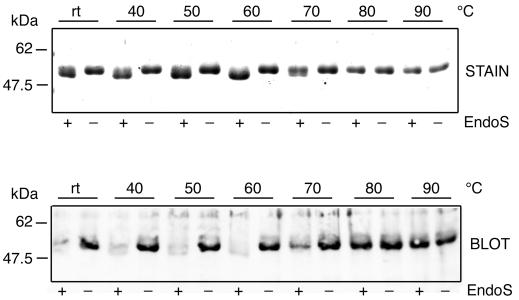
IgG was incubated in PBS at room temperature or at 40 to 90°C for 30 min and subsequently equilibrated at 37°C prior to addition of rEndoS. Then 10 μg from these IgG preparations was incubated with 1 μg of rEndoS or PBS alone for 2 h at 37°C, followed by separation on SDS–10% PAGE. One gel was stained and the other was electroblotted onto Immobilon-P polyvinylidene difluoride membrane (Millipore) as described (24) for lectin analysis. The membrane was blocked with Tris-buffered saline (TBS) with 0.1% Tween-20 (TBST) and incubated with 2 μg/ml of a biotinylated Galanthus nivalis lectin (Vector Laboratories, Burlingame, Calif.) that recognizes α-1,3-mannose residues found in the N-linked glycan of IgG. After washing in TBST, the membrane was incubated with 5 μg of peroxidase-labeled streptavidin (Vector Laboratories) per ml. After washing in TBST, the membrane was developed by the Immunoprint method (19) and exposed on Cronex X-ray film (Sterling Diagnostic Imaging, Newark, Del.). This revealed that EndoS shifted the apparent molecular mass of IgG incubated at temperatures of 40 to 70°C. In contrast, IgG incubated at temperatures of 80 to 90°C was resistant to EndoS activity (Fig. 2, stain). The lectin analysis confirmed that the size shifts result from hydrolysis of the glycan and that completely denatured IgG is resistant to EndoS (Fig. 2, blot). These data indicate that the three-dimensional structure of IgG is necessary for EndoS activity. They also suggest that the glycan structure alone is not sufficient for hydrolysis to occur and that EndoS is highly specific for native IgG.
FIG. 2.
EndoS activity on native and denatured human IgG. Purified human IgG was incubated at the indicated temperatures prior to incubation with purified rEndoS. Samples were separated by SDS–10% PAGE and stained with Coomassie blue (stain) or blotted to a membrane and analyzed using G. nivalis lectin (blot).
In conclusion, our results demonstrate that secreted SpeB, in addition to its activity as a specific IgG-protease, partially or totally degrades the other human immunoglobulins. We also show that the secreted enzyme EndoS is highly specific for IgG, since it only hydrolyzes the N-linked glycan on native IgG. This shows that the human-specific pathogen S. pyogenes has evolved two different enzymes, with distinct activities, that work in concert to hydrolyze human immunoglobulins. These findings contribute to the understanding of the role of secreted enzymes in the molecular pathogenesis of S. pyogenes.
Acknowledgments
Ulla Johannesson is acknowledged for excellent technical assistance.
This project was supported by grants from the Swedish Medical Research Council (project 13062), the Foundations of Bergvall, Crafoord, Kock, Nilson, Royal Physiografic Society, and Österlund, and the Medical Faculty of Lund University.
REFERENCES
- 1.Bisno A L, Stevens D L. Streptococcal infection of skin and soft tissue. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 2.Collin M, Olsén A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham M W. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edman P, Begg G. A protein sequenator. Eur J Biochem. 1967;1:80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- 5.Elliott S D. A proteolytic enzyme produced by group A streptococci with special reference to its effect on the type-specific M antigen. J Exp Med. 1945;81:573–592. doi: 10.1084/jem.81.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlach D, Knöll H, Köhler W, Ozegowski J-H, Hribalova V. Isolation and characterization of erythrogenic toxins. V. Communication: identity of erythrogenic toxin type B and streptococcal proteinase precursor Zentbl. Bakteriol Hyg I Abt Orig A. 1983;225:221–233. [PubMed] [Google Scholar]
- 7.Herwald H, Collin M, Müller-Esterl W, Björck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holder I A, Wheeler R. Experimental studies of the pathogenesis of infections owing to Pseudomonas aeruginosa: elastase, an IgG protease. Can J Microbiol. 1984;30:1118–1124. doi: 10.1139/m84-175. [DOI] [PubMed] [Google Scholar]
- 9.Jansen H J, Grenier D, Van der Hoeven J S. Characterization of immunoglobulin G-degrading proteases of Prevotella intermedia and Prevotella nigrescens. Oral Microbiol Immunol. 1995;10:138–145. doi: 10.1111/j.1399-302x.1995.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 10.Kapur V, Malesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine proteinase from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapur V, Topouzis S, Majesky M W, Li L-L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 12.Kilian M, Holmgren K. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect Immun. 1981;31:868–873. doi: 10.1128/iai.31.3.868-873.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T-Y, Neumann N P, Elliott S D, Moore S, Stein W H. Chemical properties of streptococcal proteinase and its zymogen. J Biol Chem. 1963;238:251–256. [PubMed] [Google Scholar]
- 14.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukomski S, Sreevatsan S, Amberg A, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 17.Matsuka Y V, Pillai S, Gubba S, Musser J M, Olmsted S B. Fibrinogen cleavage by the Streptococcus pyogenes extracellular cysteine protease and generation of antibodies that inhibit enzyme proteolytic activity. Infect Immun. 1999;67:4326–4333. doi: 10.1128/iai.67.9.4326-4333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulks M H, Kornfeld S J, Plaut A G. Specific proteolysis of human IgA by Streptococcus pneumoniae and Haemophilus influenzae. J Infect Dis. 1980;141:450–456. doi: 10.1093/infdis/141.4.450. [DOI] [PubMed] [Google Scholar]
- 19.Nesbitt S A, Horton M A. A non radioactive biochemical characterization of membrane proteins using enhanced chemiluminescence. Anal Biochem. 1992;206:267–272. doi: 10.1016/0003-2697(92)90365-e. [DOI] [PubMed] [Google Scholar]
- 20.Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80:6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radaev S, Sun P D. Recognition of IgG by Fcγ receptor: the role of Fc glycosylation and the binding of peptide inhibitors. J Biol Chem. 2001;31:16478–16483. doi: 10.1074/jbc.M100351200. [DOI] [PubMed] [Google Scholar]
- 22.Shin M H, Kita H, Park H Y, Seoh J Y. Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect Immun. 2001;69:1599–1604. doi: 10.1128/IAI.69.3.1599-1604.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarentino A L, Plummer T H., Jr Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- 24.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai P-J, Kuo C-F, Lin K-Y, Lin Y-S, Lei H-Y, Chen F-F, Wang J-R, Wu J-J. Effect of group A streptococcal cysteine proteinase on invasion of epithelial cells. Infect Immun. 1998;66:1460–1466. doi: 10.1128/iai.66.4.1460-1466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]