Abstract
Background
The use of botulinum toxin as an investigative and treatment modality for strabismus is well reported in the medical literature. However, it is unclear how effective it is in comparison with other treatment options for strabismus.
Objectives
The primary objective was to examine the efficacy of botulinum toxin therapy in the treatment of strabismus compared with alternative conservative or surgical treatment options. This review sought to ascertain those types of strabismus that particularly benefit from the use of botulinum toxin as a treatment option (such as small angle strabismus or strabismus with binocular potential, i.e. the potential to use both eyes together as a pair). The secondary objectives were to investigate the dose effect and complication rates associated with botulinum toxin.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS and three trials registers on 6 July 2022, together with reference checking to identify additional studies. We did not use any date or language restrictions in the electronic searches for trials.
Selection criteria
We planned to include randomized controlled trials (RCTs) comparing botulinum toxin with strabismus surgery, botulinum toxin alternatives (i.e. bupivacaine) and conservative therapy such as orthoptic exercises, prisms, or lens therapy for people of any age with strabismus. All relevant RCTs identified in this update compared botulinum toxin with strabismus surgery.
Data collection and analysis
We used standard methods expected by Cochrane and assessed the certainty of the body of evidence using GRADE.
Main results
We included four RCTs with 242 participants that enrolled adults with esotropia or exotropia, children with acquired esotropia, and children with infantile esotropia. The follow‐up period ranged from six to 36 months. Two studies were conducted in Spain, and one each in Canada and South Africa. We judged the included studies to have a mixture of low, unclear and high risk of bias. We did not consider any of the included studies to be at low risk of bias for all domains.
All four studies reported the proportion of participants who improved or corrected strabismus, defined as ≤ 10 prism diopters (PD) at six months (two studies) or ≤ 8 PD at one year (two studies). Low‐certainty evidence suggested that participants treated with the surgery may be more likely to improve or correct strabismus compared with those who treated with botulinum toxin (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.53 to 0.99; I² = 50%; 4 studies, 242 participants; low‐certainty evidence).
One study, which enrolled 110 children with infantile esotropia, suggested that surgery may reduce the incidence of additional surgical intervention required, but the evidence was very uncertain (RR 3.05, 95% CI 1.34 to 6.91; 1 study, 101 participants; very low‐certainty evidence).
Two studies conducted in Spain compared botulinum toxin with surgery in children who required retreatment for acquired or infantile esotropia. These two studies provided low‐certainty evidence that botulinum toxin may have little to no effect on achieving sensory fusion (RR 0.88, 95% CI 0.63 to 1.23; I² = 0%; 2 studies, 102 participants) and stereopsis (RR 0.86, 95% CI 0.59 to 1.25; I² = 0%; 2 studies, 102 participants) compared with surgery.
Three studies reported non‐serious adverse events. Partial transient ptosis (range 16.7% to 37.0%) and transient vertical deviation (range 5.6% to 18.5%) were observed among participants treated with botulinum toxin in three studies. In one study, 44.7% participants in the surgery group experienced discomfort. No studies reported serious adverse events or postintervention quality of life.
Authors' conclusions
It remains unclear whether botulinum toxin may be an alternative to strabismus surgery as an independent treatment modality among certain types of strabismus because we found only low and very low‐certainty evidence in this review update.
Low‐certainty evidence suggests that strabismus surgery may be preferable to botulinum toxin injection to improve or correct strabismus when types of strabismus and different age groups are combined. We found low‐certainty evidence suggesting botulinum toxin may have little to no effect on achievement of binocular single vision compared with surgery in children with acquired or infantile esotropia. We did not find sufficient evidence to draw any meaningful conclusions with respect to need for additional surgery, quality of life, and serious adverse events.
We identified three ongoing trials comparing botulinum toxin with conventional surgeries in the varying types of strabismus, whose results will provide relevant evidence for our stated objectives. Future trials should be rigorously designed, and investigators should analyze outcome data appropriately and report adequate information to provide evidence of high certainty. Quality of life and cost‐effectiveness should be examined in addition to clinical and safety outcomes.
Keywords: Adult, Child, Humans, Botulinum Toxins, Botulinum Toxins/therapeutic use, Canada, Esotropia, Esotropia/drug therapy, Esotropia/surgery, Strabismus, Strabismus/drug therapy, Strabismus/surgery
Plain language summary
Botulinum toxin for the treatment of strabismus
Key messages Due to a lack of robust evidence, the evidence as to the benefits and harms of using botulinum toxin compared with surgery for strabismus is uncertain.
What did we want to find out? We wanted to find out if botulinum toxin was better than surgery to treat strabismus. We also wanted to find out if botulinum toxin was associated with any unwanted effects.
What is strabismus?
Strabismus occurs when the eyes are not aligned. Usually one eye turns inwards or outwards. Less frequently, one eye turns upwards or downwards. It is commonly known as a 'squint'.
Strabismus can lead to blurred vision or double vision. In children, it can affect the long term development of vision in the affected eye. There are many causes of strabismus. In most cases, there are problems with the muscles or nerves around the eye.
Doctors can use botulinum toxin to stop individual muscles around the eye working for a while. This may help the eyes become more aligned and may lead to less blurred or double vision. One problem with using botulinum toxin is that it can result in a droopy eyelid (ptosis).
What did we do?
We searched for studies that investigated botulinum toxin injection compared with other treatment such as surgery in people with strabismus. We compared and summarized the results of relevant studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find? We found four studies with 242 adults and children with strabismus. The biggest study was in 110 children and the smallest study was in 30 people. The studies were conducted in Canada, South Africa, and Spain. Two studies lasted for six months and the longest study lasted 36 months.
The review showed the following.
• Strabismus surgery may have better chance of recovering correct alignment of the eyes compared with botulinum toxin treatment in people requiring primary treatment or retreatment for strabismus.
• People treated with botulinum toxin may need to re‐treat with surgery more frequently than people treated with surgery.
• Using botulinum toxin may not make any difference in the achievement of binocularity (the ability to focus on an object with both eyes and see a single image); and • Ptosis (the upper eyelid drooping over the eye) occurred commonly in people receiving botulinum toxin in these studies. The number of people affected ranged from 2 to 4 in 10 people. Everyone recovered when treatment was stopped.
What are the limitations of the evidence?
We have little or no confidence in the evidence because there are not enough studies to be certain about the results of our outcomes, studies are small, and they are not designed and conducted well.
How up to date is this review? The Cochrane researchers searched for studies that had been published up to 6 July 2022.
Summary of findings
Summary of findings 1. Botulinum toxin versus surgery.
| Botulinum toxin versus surgery in adults and children with strabismus | |||||||
| Patient or population: adults and children with strabismus Setting: pediatric ophthalmology clinic, private practice, and university hospital Intervention: botulinum toxin Comparison: surgery | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with surgery | Risk with botulinum toxin | ||||||
| Improved or corrected strabismusa at 6 or 12 months | 683 per 1000 | 492 per 1000 (362 to 677) | RR 0.72 (0.53 to 0.99) | 242 (4 RCTs) | ⊕⊕⊝⊝ lowb,c | Subgroup analysis based on age of onset: children ≤ 2 years of age (RR 0.84, 95% CI 0.59 to 1.20; 1 study, 55 participants); children > 2 and < 18 years of age (RR 0.76, 95% CI 0.49 to 1.18; I² = 62%; 2 studies, 157 participants); adults or ≥ 18 years of age (RR 0.38, 95% CI 0.17 to 0.85; 1 study, 30 participants). Subgroup analysis based on duration of follow‐up: 6 months (RR 0.55, 95% CI 0.38 to 0.80; I² = 2%; 2 studies, 140 participants); > 6 months (RR 0.88, 95% CI 0.69 to 1.14; I² = 0%; 2 studies, 102 participants). Sensitivity analysis excluded a study with high risk of bias that was the most weighted study (RR 0.76, 95% CI 0.52 to 1.12; I² = 53%; 3 studies, 132 participants). |
|
| Serious adverse events at 6 or 12 months | No outcome data were available for this outcome. | – | – | – | – | ||
| Proportion of participants requiring additional surgery(ies) at 6 or 12 months | 128 per 1000 | 389 per 1000 (171 to 882) | RR 3.05 (1.34 to 6.91) | 101 (1 RCT) | ⊕⊝⊝⊝ very lowb,d | – | |
| Proportion of participants who achieved binocular single vision at 6 or 12 months | Sensory fusion (positive response with Worths four light test) | 615 per 1000 | 542 per 1000 (388 to 757) | RR 0.88 (0.63 to 1.23) | 102 (2 RCTs) | ⊕⊕⊝⊝ lowb,c | – |
| Stereopsis (minimum of 480 seconds of arc) | 558 per 1000 | 480 per 1000 (329 to 697) | RR 0.86 (0.59 to 1.25) | 102 (2 RCTs) | – | ||
| Quality of life, as measured by validated instruments | No outcome data were available for this outcome. | – | – | – | – | ||
| Non‐serious adverse events at 6 or 12 months | See comments | 212 (3 RCTs) |
⊕⊕⊝⊝ lowa,b | Partial transient ptosis occurred, ranging from 16.7% to 37.0% of children treated with botulinum toxin. Transient vertical deviation was present, ranging from 5.6% to 18.5% of children in the botulinum toxin arm. In one study, 44.7% of children in the surgery arm experienced discomfort on day 1. | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; PD: prism diopters; RCT: randomized controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||||
aImproved or corrected strabismus, defined as follows:
- orthophoria or orthotropia; or
- residual heterophoria/tropia ≤10 PD or ≤ 5° observed in the cover test at distance and at near; or
- residual heterophoria/tropia ≤10 PD or ≤ 5° observed in the cover test at distance and at near and at least one binocular test.
bDowngraded one level for risk of bias. cDowngraded one level for imprecision of results. dDowngraded two levels for imprecision of results.
Background
Description of the condition
Strabismus is a deviation of the ocular alignment where one eye turns, which may be intermittent or constant. It is a common condition that occurs in up to 5% of the population and up to 50% in special populations such as those with cerebral palsy (Adams 2005; Donnelly 2005; Strömland 1993). In forms of strabismus that are intermittent, binocular function (using both eyes as a pair) is maintained with straight eyes for a variable proportion of the time. In other forms there is a manifest deviation, usually with a variable degree of suppression of the deviating eye. Strabismus can be further divided into esotropia (inturning deviation), exotropia (out‐turning deviation) or, less commonly, hypertropia (upturning deviation), hypotropia (downturning deviation) and cyclotropia (rotatory deviation). Strabismus can be caused by a variety of insults such as abnormal anatomical development of extraocular muscles or the orbit, impaired neurological input to extraocular muscles, uncorrected refractive error or hereditary factors. Sequelae to strabismus can include blurring of vision, diplopia (double vision), impaired depth (3‐D) perception, and in younger children, amblyopia. Amblyopia is impaired vision in the deviating eye due to the lack of correct stimulation of that eye and results in permanent loss of vision if left untreated at a young age.
Description of the intervention
There are various treatments associated with strabismus. Primarily, treatment is directed at aligning the visual axes. Conservative options include prisms to realign the visual axes and orthoptic exercises to promote and establish binocular control of ocular alignment, where both eyes can subsequently work as a pair. Invasive treatment options include surgery to permanently alter extraocular muscle function and thus permanently change ocular alignment, and botulinum toxin to individual extraocular muscles. Scott 1980 first described this latter option, which temporarily paralyses the extraocular muscle and results in a changed ocular alignment that resolves over time (usually a two to three‐month time interval). During this period of altered eye position, the visual axes may adopt an ocular alignment that permits binocular single vision. This is the ability to use both eyes as a pair so that both eyes contribute to seeing a single image. This may persist or regress necessitating further treatment. Botulinum toxin injection to extraocular muscles is an alternative option that has become established in the treatment of adults who have strabismus. Its use in children is less well studied. It is perceived to be difficult to use in children due to the need for sedation and complications following leakage of the toxin into the levator palpebral superioris muscle (the muscle responsible for elevating the eyelid), thus resulting in a droopy upper lid, known as ptosis (Rowe 2005). Botulinum toxin has become recognized and accepted as both an adjunct and alternative to strabismus surgery in many types of strabismus (Bunting 2013; Campos 2000; Crouch 2006; Dawson 1999; Dawson 2004; Dawson 2004b; Dawson 2005; Dawson 2012; Gardner 2013; Holmes 2001; Kerr 2001; Marsh 2003; McNeer 2003; Ozkan 2006; Rayner 1999; Rowe 2004; Sabetti 2003; Spencer 1997; Tejedor 2001). Diagnostic uses of botulinum toxin include investigation of postoperative diplopia (double vision), to detect whether fusion (which contributes to binocular vision) is present preoperatively, to differentially diagnose between a part and complete sixth nerve palsy, to aid in the prediction of surgical results for incomitant deviations and to help in the investigation of a possible slipped muscle following surgery. In terms of therapeutic uses botulinum toxin has been found useful in treating facial muscle spasm, strabismus, nystagmus, corneal ulceration and exposure keratitis, to name a few. The therapeutic uses of botulinum toxin for strabismus are to restore fusion in those people with decompensating deviations, or those with a recovering sixth nerve palsy, to align the cosmetic form of strabismus, to aid surgical over‐corrections and under‐corrections and to aid in the improvement of visual acuity by relieving oscillopsia (perception of moving images) in cases of acquired nystagmus.
Other treatment options associated with strabismus include those that address the sequelae of strabismus, such as occlusion therapy for amblyopia, which is a reduction in vision caused completely or in part by the strabismus.
How the intervention might work
Botulinum toxin is a drug that is an exotoxin of the bacterium Clostridium botulinum. Botulinum toxin type A is an injectable neurotoxin. In order for muscles to contract, acetylcholine is released at the nerve‐muscle junction. Acetylcholine binds to muscle receptors causing a contraction. Botulinum toxin selectively blocks the release of acetylcholine from the cholinergic synapses found within a muscle, thereby blocking the nerve impulses and preventing contraction of the muscle cells. Paralysis (which is temporary) follows within days after injection of the toxin into the extraocular muscle, and the toxin becomes fully effective within three to seven days of the injection. The duration of paralysis is dependent on the individual, but generally lasts for three months. Once a muscle is paralyzed, opposing muscles take on a greater movement force and the eye position changes, allowing the visual axes to move into a straighter eye alignment.
Why it is important to do this review
Clear guidelines do not exist as to the recommended use of botulinum toxin for the treatment of strabismus, particularly as so many types of strabismus exist. Much of the published literature pertains to retrospective case series with varying treatment modalities using different types of BTX (e.g. Dysport™ or Botox™ or Prosign™ ) and different doses of the toxin.
Objectives
The primary objective was to examine the efficacy of botulinum toxin therapy in the treatment of strabismus compared with alternative conservative or surgical treatment options. This review sought to ascertain those types of strabismus that particularly benefit from the use of botulinum toxin as a treatment option (such as small angle strabismus or strabismus with binocular potential, i.e. the potential to use both eyes together as a pair). The secondary objectives were to investigate the dose effect of botulinum toxin and the complication rates associated with botulinum toxin.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) of treatment using botulinum toxin for strabismus.
Types of participants
Participants with strabismus suitable for treatment with botulinum toxin to correct the angle of deviation were eligible. This included adults and children with no age limit.
Types of interventions
We considered trials in which botulinum toxin of all makes, e.g. Dysport™, Botox™, Prosign™ were compared with the following:
strabismus surgery;
botulinum toxin alternatives (i.e. bupivacaine);
conservative therapy; orthoptic exercises, prisms, lens therapy.
Included studies compared botulinum toxin with surgery only.
Types of outcome measures
We included trials when study design, participants, interventions and comparators met the inclusion criteria, irrespective of whether our prespecified outcome data were reported.
Critical outcomes
-
Improved or corrected strabismus, defined as follows:
orthophoria or orthotropia; or
residual heterophoria/tropia ≤ 10 PD or ≤ 5° observed in the cover test at distance and at near; or
residual heterophoria/tropia ≤ 10 PD or ≤ 5° observed in the cover test at distance and at near and at least one binocular test.
Serious adverse events (SAE): death, life‐threatening, hospitalization (initial or prolonged); disability or permanent damage; required intervention to prevent permanent impairment; other serious or important medical events. When the event does not fit the previously defined outcomes, the event may jeopardize the participant and may require medical or surgical intervention (treatment) to prevent one of the other outcomes (FDA). None of included studies reported SAE. In future updates, SAE will be collected as proportion of participants experiencing any of the SAE listed above.
We collected data for both outcomes reported at six months or longer follow‐up after the intervention as reported by the primary studies.
Important outcomes
Proportion of participants requiring additional surgery/surgeries at six months or longer follow‐up after the intervention;
Proportion of participants who achieved binocular single vision at six months or longer follow‐up after the intervention;
Postintervention quality of life (QoL) as measured by previously validated instruments at six months or longer follow‐up after the intervention;
Non‐serious adverse events (AE), encompassing but not limited to: induced ptosis, induced vertical deviation, subconjunctival hemorrhage, intolerable diplopia, and others. The non‐serious adverse events were collected as the proportion of participants experiencing any of the non‐serious AE listed above within the study period.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE, Embase, Latin American and Caribbean Literature on Health Sciences (LILACS). We searched also in ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electric databases on 6 July 2022.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 4), ClinicalTrials.gov (Appendix 5) and the WHO ICTRP (Appendix 6).
Searching other resources
We searched the reference list of included trials to identify additional studies. We did not conduct manual searches of conference proceedings or abstracts specifically for this review update.
Data collection and analysis
Selection of studies
We imported the results of the updated searches performed by the Information Specialist of Cochrane Eyes and Vision into a web‐based software (Covidence). Two review authors independently screened the titles and abstracts to establish whether they met the criteria defined as "relevant (yes)", "maybe relevant (maybe)", or "not relevant (no)". Uncertainties in relation to whether the study accomplishes the defined inclusion criteria were discussed by the two review authors and a final decision to include it (or not) following discussion between the two review authors was taken. Arbitration from a third author was required when there was discrepancy between the two review authors. Following this process, we obtained the full copies of definitely or potentially relevant studies. Where information was unclear or incomplete, we contacted the study authors. We described the reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
The two review authors independently extracted information relating to outcomes using data collection forms developed by Cochrane Eyes and Vision in Covidence. We resolved discrepancies by discussion and entered data into RevMan Web.
According to the guidance provided in Chapter 5 of the Cochrane Handbook for Systematic Reviews of Interventions (Li 2022), we extracted the following details from the included studies:
methods: inclusion and exclusion criteria for study participants;
participants: age, previous treatment, strabismus type;
interventions: type of botulinum toxin used, dose measure, number of injections if available;
outcomes described;
the follow‐up;
the source of support.
Assessment of risk of bias in included studies
We assessed the risk of bias according to the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed sequence generation, allocation concealment, masking (blinding) of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of other bias. We made judgments for each domain and rate each as either at 'low', 'high', or 'unclear' risk of bias. We resolved discrepancies by discussion within the author team. To keep consistency, we also assessed risk of bias for previously included trials.
Measures of treatment effect
We used the risk ratio (RR) with 95% confidence intervals (CI) for dichotomous variables.
Unit of analysis issues
We expected that studies may have consisted of parallel group trials or cross‐over trials. Where we found cross‐over trials in the search, we included only data from the first period, when reported separately by the primary studies, as botulinum toxin is known to have a longer‐lasting effect than the average three months expected for extraocular muscle function to fully recover.
Dealing with missing data
We contacted primary investigators to obtain missing data. We allowed a time period of one month for response. We used the data available in published articles when we did not receive any response.
Assessment of heterogeneity
We examined potential sources of relevant clinical, methodological or statistical heterogeneity using forest plots, and we quantified heterogeneity using the I2 statistic. Thresholds for the interpretation of I2 can be misleading, since the importance of inconsistency depends on several factors. A rough guide to interpretation was as follows: 0% to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; 75% to 100%, considerable heterogeneity (Deeks 2022).
Assessment of reporting biases
We did not construct funnel plots to examine the presence of small‐study effects and attempt to explore potential factors that may contribute to the observed asymmetry by meta‐regression or subgroup analysis because we did not include 10 or more studies. We assessed potential risk of reporting bias as part of the risk of bias assessment.
Data synthesis
We summarized the data statistically according to the guidelines referenced in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (McKenzie 2022). We used random‐effects models, regardless of low heterogeneity statistics (I2), when there was clear evidence of substantial clinical or methodological heterogeneity in the included studies, such as different doses of botulinum toxin used, different follow‐up times, or different characteristics of study participants.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis on the critical outcome, improved or corrected strabismus, by the following factors:
age of onset of heterotropia or strabismus: children ≤ 2 years of age, > 2 and < 18 years of age, and in adults or ≥18 years of age;
duration of follow‐up: 6 months versus > 6 months.
We were not able to perform prespecified subgroup analysis with type of strabismus (i.e. endo‐ versus exo‐trophia, or endo‐ versus exo‐phoria) due to lack of the information in the included studies.
Sensitivity analysis
We performed sensitivity analysis to explore the influence of the following factors on the treatment effect size of the critical outcome, improved or corrected strabismus:
overall 'risk of bias' assessment result of each included trial;
estimate if the non‐inclusion of a single study, namely the most weighted one, could reverse the direction of the effect.
Summary of findings and assessment of the certainty of the evidence
We reported review findings in Table 1 using the GRADE approach to interpret the review findings (Schünemann 2022). For each of the following outcomes, we provided the assumed risk and corresponding risk on the risk across control groups in the included trials:
improved or corrected strabismus at postintervention;
SAE measured by proportion of participants experiencing any of the listed SAE within the study period;
proportion of participants requiring additional surgery(ies);
proportion of participants who achieved binocular single vision;
quality of life (QoL) as measured by validated instruments;
non‐serious AE quantified by the proportion of participants experiencing any of the listed non‐serious AE within the study period.
We employed the GRADE approach and two authors independently assessed the overall certainty of the evidence as 'high', 'moderate', 'low', or 'very low' according to (1) high risk of bias; (2) indirectness of evidence; (3) unexplained heterogeneity or inconsistency of results; (4) imprecision; (5) high probability of publication bias as described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022).
Results
Description of studies
Results of the search
This is the third update of this review (Rowe 2009; Rowe 2012; Rowe 2017).
We updated the protocol to follow the current Cochrane methodology (see Methods) for this review update. Accordingly, we excluded four studies that were included (Chen 2013; Lee 1994; Minguini 2012) or ongoing (Jain 2015) in the previous version of this review, because intervention and comparator did not meet the current eligibility criteria.
We performed an updated electronic database searches in July 2022, which yielded 138 unique records. After screening titles and abstracts, we retrieved eight full‐text reports for further review. We included one study (one report) (Mayet 2021), identified three ongoing studies (three reports), and excluded three studies (three reports). We contacted the study investigator for one study to clarify the information on study design, but did not receive any response from them. Therefore, we have listed the study as awaiting classification (Méndez Sánchez 2017). One trial register for one ongoing study in the previous review was no longer accessible; however, the study was fully published and included in this review (Mayet 2021). Overall, we included four studies (four reports) and excluded 15 studies (16 reports).
Three ongoing studies compare botulinum toxin with conventional surgeries, and they plan to enroll 200 children with concomitant strabismus in China (ChiCTR‐INR‐17013777), 30 participants with infantile esotropia in India (CTRI/2021/11/038205), and 140 participants with acquired esotropia in Europe (NCT03459092). CTRI/2021/11/038205 started in 2021, and the remaining two trials started in 2018.
A study flow diagram is shown in Figure 1.
1.
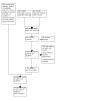
Study flowchart
Included studies
We included four parallel‐group RCTs with 242 participants, ranging from 30 (Carruthers 1990) to 110 participants (Mayet 2021) per study. Studies were conducted in Canada (Carruthers 1990), South Africa (Mayet 2021), and Spain (Tejedor 1998; Tejedor 1999) and published between 1998 (Tejedor 1998) and 2021 (Mayet 2021). Follow‐up period ranged from six months (Carruthers 1990; Mayet 2021) to 36 months (Tejedor 1999). Carruthers 1990 received a grant from the British Columbia Health Care Research Foundation and products were supplied by Smith Kettlewell Institute of Visual Sciences. Mayet 2021 was supported by a grant from the Anglo‐American Chairman’s Fund. Sources of funding were unclear for the remaining two studies.
Carruthers 1990 recruited 30 adult participants with esotropia or exotropia without binocular function requiring treatment and randomized them to treatment with botulinum toxin (Botox™) or adjustable suture surgery. These groups were compared with each other for alignment of deviation ≤ 10 prism diopter (PD). In addition, per cent net change was documented, which was defined as: preoperative deviation ‐ postoperative deviation/preoperative deviation x 100%. Both groups had similar angles of deviation and similar numbers of esotropia and exotropia angles at baseline. Participants were allowed to receive alternative treatments after six months, and we analyzed the data before the participants received the additional treatments.
Mayet 2021 enrolled 110 children with onset of esotropia before six months of age and with large‐angle infantile esotropia defined as esotropia of ≥40 PD. Nine children were lost prior to receiving the intervention. Participants were allocated to either a maximum of three botulinum toxin injections or surgical intervention of bimedial rectus muscle recession. In the surgery arm, 23 children with very‐large‐angle esotropia (i.e. > 60 PD) at baseline had botulinum toxin augment plus surgery, not surgery alone. The primary outcomes were complete response, defined as orthophoria or residual esotropia of ≤ 10 PD, partial response defined as residual esotropia of > 10 PD and ≤ 20 PD and deemed acceptable by the parents, and treatment failures or non‐response as > 20 PD.
Tejedor 1998 and Tejedor 1999 enrolled 47 strabismic children with acquired esotropia and 55 strabismic children with infantile esotropia, respectively. Children in both these studies had previously had operations to correct esotropia. They were randomized to two different treatment procedures: reoperation or botulinum toxin (Botox™). The trial authors compared these groups to each other for percentage of successful motor outcome ≤ 8 PD and percentage change in deviation. The latter was calculated as: preoperative deviation ‐ postoperative deviation/preoperative deviation x 100%. Both groups were regarded as comparable at baseline as similarities were present for both groups regarding previous surgical procedures, mean age at initial surgery, average time lapse between first and second treatment, angle of deviation, refractive error and visual acuity measures. In these studies, it was unclear from the results how many participants received unilateral or bilateral injections of botulinum toxin. Bilateral injection would have a greater effect on the angle of deviation than unilateral injection.
Additional details can be found in the Characteristics of included studies table.
Excluded studies
We excluded 15 studies in total. Nine studies were excluded because they were not an RCT and six studies were excluded because the intervention or comparator was ineligible for this review.
The reasons for exclusion are shown in the Characteristics of excluded studies table.
Risk of bias in included studies
We determined the risk of bias using the Cochrane risk of bias assessment tool (Higgins 2011). This considers sequence generation; allocation concealment; masking of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting and other potential threats to validity (Figure 2).
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Three trials did not describe methods of random sequence generation (Carruthers 1990; Tejedor 1998; Tejedor 1999); we judged the three studies as having an unclear risk of bias for random sequence generation. We judged Mayet 2021 as having a high risk of bias for this domain because the sequence was generated by non‐random approach (i.e. odd or even numbers) and participants or study investigators could possibly foresee assignments.
Allocation sequence was adequately generated in Carruthers 1990, which constituted a low risk of bias as a research assistant allocated participants separately. Tejedor 1998 and Tejedor 1999 did not describe allocation concealment.
Blinding
Masking of study personnel could not be performed due to the nature of interventions; all four studies were at high risk of performance bias. In Carruthers 1990, one of the study investigators and the orthoptist were masked to the allocated interventions when undertaking the final evaluation of participants for outcome measures. The remaining three studies failed to report whether outcome assessors were masked to the intervention that participants received (Mayet 2021; Tejedor 1998; Tejedor 1999). However, it is unlikely that the absence of masking when evaluating final outcome of participants would be biased as the outcome measures were related to actual measurements of eye position and responses to binocular assessments. Hence, we assessed all four studies as having low risk of detection bias.
Incomplete outcome data
We judged three studies as having a low risk of attrition bias as there were no missing outcome data (Carruthers 1990; Tejedor 1998; Tejedor 1999). In Mayet 2021, one participant (1.8%) and eight participants (14.5%), who were randomized to the botulinum toxin arm and surgery arm, respectively, were not included in the final analysis. The reasons for exclusion or loss to follow‐up were not fully described. We judged this study as having an unclear risk of bias for this domain.
Selective reporting
Mayet 2021 provided information on the study protocol, which the University trial ethics committee approved. All the outcomes were stated in the final report; we judged this study as having low risk of reporting bias. The remaining studies were at unclear risk of bias because neither protocol nor trial register was publicly available (Carruthers 1990; Tejedor 1998; Tejedor 1999).
Other potential sources of bias
All four studies were at unclear risk of bias for this domain. None of the studies were funded by the industry that produces botulinum toxin. In Carruthers 1990, gauges and Oculinum (botulinum toxin) were supplied by Smith Kettlewell Institute of Visual Sciences, San Francisco, California. The planned sample size was 150 in this study, but only 30 participants were eventually enrolled. In addition, the groups contained a mix of esotropic and exotropic participants, which reduced numbers for direct comparison further. In Mayet 2021, 23 children (48.9%) with very‐large‐angle esotropia (i.e. > 60PD) in the surgery arm had botulinum toxin augment plus surgery (i.e. not surgery alone). Two studies did not provide information on funding, sample size calculation, and trial register (Tejedor 1998; Tejedor 1999).
Effects of interventions
See: Table 1
All four included studies compared botulinum toxin with surgery. However, it should be noted that 23 children (48.9%) in the surgery arm had botulinum toxin augment plus surgery (not surgery alone) in Mayet 2021.
Critical outcome
Improved or corrected strabismus
All four studies reported data on the proportion of participants who improved or corrected strabismus. Carruthers 1990 defined a satisfactory outcome as within 10 PD, which was achieved in 10 adult participants (76.9%) of the surgery group and five (29.4%) of the botulinum toxin group at six months. The remaining three studies included children with infantile esotropia (Mayet 2021; Tejedor 1999) or acquired esotropia (Tejedor 1998). Mayet 2021 measured complete success as orthophoria or residual esotropia of ≤10 PD at six months while Tejedor 1998 and Tejedor 1999 defined a satisfactory outcome at one year follow‐up as ≤ 8 PD. The meta‐analysis of these four studies suggested that surgery may be more likely to improve or correct strabismus compared with botulinum toxin injection (RR 0.72, 95% CI 0.53 to 0.99; I² = 50%; 4 studies, 242 participants; Analysis 1.1, Figure 3).
1.1. Analysis.

Comparison 1: Botulinum toxin versus surgery, Outcome 1: Proportion of participants with improved or corrected strabismus
3.

Proportion of participants who improved or corrected strabismus
Sensitivity analysis that excluded a study with high risk of bias that was the most weighted study (Mayet 2021) showed that there was no evidence of an important difference between botulinum toxin injection and surgery alone (RR 0.76, 95% CI 0.52 to 1.12; I² = 53%; 3 studies, 132 participants).
As planned, we performed subgroup analysis. The subgroup analysis based on the age of onset of heterotropia or strabismus (i.e. age of onset: children ≤ 2 years of age versus children > 2 and < 18 years of age, and adults or ≥ 18 years of age) showed that there was no evidence of a difference between subgroups (children ≤ 2 years of age RR 0.84, 95% CI 0.59 to 1.20; 1 study, 55 participants; children > 2 and < 18 years of age RR 0.76, 95% CI 0.49 to 1.18; I² = 62%; 2 studies, 157 participants; adults or ≥ 18 years of age RR 0.38, 95% CI 0.17 to 0.85; 1 study, 30 participants; test for subgroup differences P = 0.21; Analysis 1.2).
1.2. Analysis.

Comparison 1: Botulinum toxin versus surgery, Outcome 2: Proportion of participants with improved or corrected strabismus: subgroup analysis: age of onset
The subgroup analysis based on duration of follow‐up showed that surgery was favorable over botulinum toxin for the studies with follow‐up of six months (RR 0.55, 95% CI 0.38 to 0.80; I² = 2%; 2 studies, 140 participants), while the favorable effect disappeared in studies with follow‐up of more than six months (RR 0.88, 95% CI 0.69 to 1.14; I² = 0%; 2 studies, 102 participants; test for subgroup differences P = 0.04; Analysis 1.3)
1.3. Analysis.

Comparison 1: Botulinum toxin versus surgery, Outcome 3: Proportion of participants with improved or corrected strabismus: subgroup analysis: duration of follow‐up
We assessed the certainty of evidence as low. We downgraded certainty by one level for risk of bias and by one level for imprecision.
Serious adverse events (SAEs)
None of the included studies reported this outcome, or no SAEs were observed in the included studies.
Important outcomes
Proportion of participants requiring additional surgical interventions
Mayet 2021 reported that 21 (38.9%) children in the botulinum toxin group and six (12.8%) children in the surgery arm received subsequent surgery (RR 3.05, 95% CI 1.34 to 6.91; 1 study, 101 participants; Analysis 1.4). Although the analysis suggested that surgery may reduce the incidence of additional surgical intervention, the evidence was very uncertain because only one study with relatively small sample size contributed the outcome data. We assessed the certainty of the body of evidence for this outcome as very low due to high risk of bias (‐1) and imprecision of results (‐2).
1.4. Analysis.

Comparison 1: Botulinum toxin versus surgery, Outcome 4: Proportion of participants requiring additional surgeries
Proportion of participants who achieve binocular single vision
Two studies reported the proportion of participants who achieved binocular single vision (Tejedor 1998; Tejedor 1999).
Tejedor 1998 reported that sensory fusion (positive response with Worths four light test) and stereopsis (minimum of 480 seconds of arc) were present in 15 (62.5%) and 13 (54.2%) children, respectively, of the reoperation group and 13 (56.5%) and 11 (47.8%) children, respectively, of the botulinum toxin group. Tejedor 1999 reported that fusion (positive response with Worths four light test and Bagolini glasses test) and stereopsis (minimum of 480 seconds of arc) were present in 17 (60.7%) and 16 (57.1%) children, respectively, of the reoperation group and 14 (51.8%) and 13 (48.1%) children, respectively, of the botulinum toxin group. The evidence suggested that surgery may result in little to no difference in the achievement of binocular single vision compared with botulinum toxin (RR of having sensory fusion 0.88, 95% CI 0.63 to 1.23; I² = 0%; 2 studies, 102 participants; RR of having stereopsis 0.86, 0.59 to 1.25; I² = 0%; 2 studies, 102 participants; Analysis 1.5). We assessed the certainty of evidence as low. We downgraded certainty by one level for risk of bias and by one level for imprecision.
1.5. Analysis.

Comparison 1: Botulinum toxin versus surgery, Outcome 5: Proportion of participants who achieved binocular single vision
Postintervention changes in quality of life (QoL)
None of the included studies investigated this outcome.
Proportion of participants who experienced non‐serious adverse events
Three studies reported data on non‐serious adverse events, but only reported them for one arm each (Mayet 2021; Tejedor 1998; Tejedor 1999). Among children who received botulinum toxin injection, partial transient ptosis occurred in 9 (16.7%) children in Mayet 2021, 8 (34.8%) children in Tejedor 1998 and 10 (37.0%) children in Tejedor 1999. Transient vertical deviation was present in three (5.6%) (Mayet 2021), four (17.4%) (Tejedor 1998) and five (18.5%) (Tejedor 1999) children in the botulinum toxin arms of these studies. Additionally, Mayet 2021 claimed that 21 (44.7%) children in the surgery arm experienced discomfort on day one, but all were settled on the three‐week follow‐up visit. The certainty of evidence for this outcome is low, downgraded by two levels due to high risk of bias (‐1) and imprecision (‐1).
Discussion
Summary of main results
We have included four RCTs with 242 participants in the present update (Carruthers 1990; Mayet 2021; Tejedor 1998; Tejedor 1999). Age of participants was not a limitation in this review, and we included adult participants with esotropia or exotropia, children with infantile esotropia, and children with acquired esotropia. All the included studies examined botulinum toxin therapy versus conservative or surgical treatment options in the treatment of strabismus.
For the defined critical outcome, low‐certainty evidence suggested that surgery may be more likely to improve or correct strabismus compared with botulinum toxin injection. However, the results were inconclusive in studies with follow‐up more than six months. Although three studies reported non‐serious adverse events, none of them reported the results in both arms. Participants treated with botulinum toxin experienced transient ptosis (16.7% to 37.0%) and transient vertical deviation (5.6% to 18.5%). Discomfort was the most common non‐serious adverse events in the surgery group. The proportion of participants who needed an additional surgery was higher among those who were treated with botulinum toxin, but the evidence was very uncertain. Low‐certainty evidence from two studies that enrolled children with acquired esotropia and infantile esotropia suggested that surgery may result in little to no difference compared with botulinum toxin in the achievement of binocular single vision.
None of the included studies reported serious adverse events and postintervention quality of life. Hence, no conclusion can be achieved in this respect in the present update.
Overall completeness and applicability of evidence
Although we included only four RCTs, the review possesses relatively good external validity regarding participants. Participants included adults with esotropia or exotropia, and children with acquired esotropia and infantile esotropia, who were recruited in Canada, South Africa, and Spain. However, the applicability of interventions and comparators was limited as all four studies compared botulinum toxin with strabismus surgery. We did not identify any studies wherein botulinum toxin alternatives (e.g. bupivacaine) or conservative therapy such as orthoptic exercises, prisms, or lens therapy, were used as comparators. It was not possible to ascertain information on dose effect as the four included trials used different types of botulinum toxin (Botox™ versus Dysport™ versus Prosign™) and different dosages.
With respect to completeness, although all four studies reported the proportion of participants who improved or corrected strabismus, no studies examined one of our critical outcomes (serious adverse events) and other important outcomes (i.e. postintervention change in quality of life). Therefore, we were unable to provide any evidence on these outcomes.
We have not incorporated the data from three large ongoing trials in this update because no interim or final results are available at this time (ChiCTR‐INR‐17013777; CTRI/2021/11/038205; NCT03459092).
Certainty of the evidence
The certainty of the body of evidence was low or very low across the outcomes examined in this review update. We downgraded certainty of evidence primarily for risk of bias and imprecision. There was a lack of clarity on sequence generation and allocation concealment, or an inability to mask investigators and participants to allocation. We included only four studies with relatively small sample size, resulting in wide confidence intervals of the effect estimates.
Potential biases in the review process
As far as we are aware, we have minimized potential biases in the review process. We have followed the prespecified methods updated to meet current Cochrane methodology. We implemented an extensive search strategy with the help of an experienced Information specialist. At least two review authors worked independently to complete each step of study selection, data extraction and risk of bias assessment. We followed Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards for planning, conduct and reporting of updates of Cochrane Intervention Reviews to report this update. None of review authors had any financial conflicts of interest.
Agreements and disagreements with other studies or reviews
Our findings agree with another Cochrane Review that examined the effectiveness and optimal timing of surgical and non‐surgical treatment options for infantile esotropia (Mehner 2023). It included Mayet 2021, and very low‐certainty evidence suggested that medial rectus recessions may increase the incidence of treatment success, defined as orthophoria or residual esotropia of ≤ 10 prism diopters, compared with botulinum toxin injections alone.
The results of the present review are partially in line with the guideline from the Spanish Society of Strabismus and Pediatric Ophthalmology as reported in Noval 2017, which was derived from systematic reviews, RCTs, cohort studies, and case control studies. In the guideline, moderate‐certainty evidence suggested that botulinum toxin is favorable for congenital and acquired endotropia. However, there is a weak recommendation for children with exotropia and adults with strabismus.
In a systematic review, Binenbaum 2021 included 14 studies encompassing two RCTs that were included in our review (Tejedor 1998; Tejedor 1999), three non‐randomized comparative studies, and nine case series. The authors concluded that treatment with botulinum toxin injections may be comparable with eye muscle surgery for nonparalytic, nonrestrictive horizontal strabismus to achieve successful motor alignment, though multiple botulinum toxin injections may be required.
The results of the present review are not in line with the findings in a systematic review that examined the efficacy and safety of botulinum toxin in the treatment of infantile esotropia (Issaho 2017). This systematic review included one prospective study wherein botulinum toxin injection was compared with muscle surgery; one RCT that examined botulinum toxin with or without sodium hyaluronate (Chen 2013); two studies wherein the authors analyzed two subgroups according to the age when botulinum toxin injection was performed (before versus after 12 months of age); and five descriptive studies. It concluded that botulinum toxin injection into medial recti muscles is a safe procedure and a valuable alternative to strabismus surgery in congenital esotropia, especially in moderate deviations. The different conclusion in ours is presumably because Issaho 2017 pooled the outcome data regardless of study design, such as RCT and retrospective case series.
Non‐serious adverse events summarized in our review were consistent with the known profile of botulinum toxin treatment (AAO 2022).
Authors' conclusions
Implications for practice.
We found low‐certainty evidence suggesting that strabismus surgery may be preferable than botulinum toxin injection to improve or correct strabismus. Low‐certainty evidence also suggests that botulinum toxin may result in little to no difference in the achievement of binocular single vision compared with surgery in children with acquired or infantile esotropia.
Participants treated with botulinum toxin may experience non‐serious adverse events: approximately one third of participants had transient ptosis and about one fifth experienced transient vertical deviation. We did not find sufficient evidence to draw any meaningful conclusions with respect to additional surgery needed, quality of life, and serious adverse events.
It remains unclear whether botulinum toxin may be an alternative to strabismus surgery as an independent treatment modality among certain types of strabismus (e.g. exo‐ or eso‐tropia, and acquired versus congenital strabismus).
Implications for research.
Our database searches found three trial registrations for ongoing studies that compare botulinum toxin with conventional surgeries in participants with concomitant strabismus, infantile esotropia, or acquired esotropia. These ongoing trials may help elucidate the controversy and reduce the uncertainty of the evidence for the use of botulinum toxin as an independent management option. Future trials should be rigorously designed, and study investigators should analyze outcome data appropriately and report adequate information by following the CONSORT statement for randomized controlled trials to provide evidence of high certainty. In addition to clinical outcomes (e.g. ocular alignment and binocular single vision) and safety outcomes, quality of life and cost‐effectiveness should be considered as important outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 30 November 2022 | New citation required and conclusions have changed | One study newly included (Mayet 2021). Four previously included or ongoing studies were excluded due to the change in selection criteria (Chen 2013; Jain 2015; Lee 1994; Minguini 2012). Relevant sections have been updated. |
| 6 July 2022 | New search has been performed | Updated electronic searches were performed. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 11 July 2016 | New citation required but conclusions have not changed | Issue 3, 2017: two new trials were included in the review (Chen 2013; Minguini 2012). Inclusion of GRADE |
| 11 July 2016 | New search has been performed | Issue 3, 2017: electronic searches were updated |
| 7 December 2011 | New citation required but conclusions have not changed | Issue 2, 2012: electronic searches were updated but no new trials were identified. |
| 7 December 2011 | New search has been performed | Issue 2, 2012: the 'Risk of bias' assessments were updated according to new Cochrane methodology. |
| 14 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Iris Gordon, who created and executed the electronic search strategies. We thank John Lee, Catey Bunce, Stephen Gichuhi and Ian Marsh for their comments on the protocol or the previous versions of the review.
We thank Su‐Hsun Liu, methodologist of Cochrane Eyes and Vision (CEV) US Project (CEV@US); Louis Leslie and Genie Han, Assisting Managing Editors for CEV@US; Anupa Shah, Managing Editor for CEV, for support and guidance in preparation of this review update; Andrea Takeda (Cochrane Central Production Service), Copy Editor, for her valuable suggestions and edits.
We would like to acknowledge the contributions of Carmel P Noonan to the previous version of the review (Rowe 2017).
This review update was managed by CEV@US and was signed off for publication by Tianjing Li and Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Strabismus #2 strabism* or squint* #3 esotropi* #4 exotropi* #5 hypertropi* #6 hypotropi* #7 cyclotropi* #8 heterophori* #9 esophori* #10 exophori* #11 hyperphori* #12 hypophori* #13 cyclophori* #14 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 MeSH descriptor Botulinum Toxins #16 botulin* toxin* #17 botox* #18 dysport* #19 MeSH descriptor Clostridium botulinum #20 clostridium botulin* #21 (#15 OR #16 OR #17 OR #18 OR #19 OR #20) #22 (#14 AND #21)
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomized).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp strabismus/ 14. (strabism$ or squint$).tw. 15. esotropi$.tw. 16. exotropi$.tw. 17. hypertropi$.tw. 18. hypotropi$.tw. 19. cyclotropi$.tw. 20. heterophori$.tw. 21. esophori$.tw. 22. exophori$.tw. 23. hyperphori$.tw. 24. hypophori$.tw. 25. cyclophor$.tw. 26. or/13‐25 27. exp botulinum toxins/ 28. botulin$ toxin$.tw. 29. botox$.tw. 30. dysport$.tw. 31. exp clostridium botulinum/ 32. clostridium botulin$.tw. 33. or/27‐32 34. 26 and 33 35. 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp strabismus/ 34. (strabism$ or squint$).tw. 35. esotropi$.tw. 36. exotropi$.tw. 37. hypertropi$.tw. 38. hypotropi$.tw. 39. cyclotropi$.tw. 40. heterophori$.tw. 41. esophori$.tw. 42. exophori$.tw. 43. hyperphori$.tw. 44. hypophori$.tw. 45. cyclophor$.tw. 46. or/33‐45 47. botulinum toxin/ 48. botulin$ toxin$.tw. 49. botox$.tw. 50. dysport$.tw. 51. Botulinum toxin A/ 52. exp clostridium botulinum/ 53. clostridium botulin$.tw. 54. or/47‐53 55. 46 and 54 56. 32 and 55
Appendix 4. ISRCTN search strategy
(strabismus OR strabismic OR squint) AND (botulinum OR botox)
Appendix 5. ClinicalTrials.gov search strategy
(strabismus OR strabismic OR squint) AND (botulinum OR botox)
Appendix 6. WHO ICTRP search strategy
(Condition) strabismus OR strabismic OR squint AND (Intervention) botox OR botulinum
Appendix 7. LILACS search strategy
strabism$ or squint$ [Words] and botulin$ or botox$ [Words]
Data and analyses
Comparison 1. Botulinum toxin versus surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of participants with improved or corrected strabismus | 4 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.99] |
| 1.1.1 Children | 3 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.02] |
| 1.1.2 Adults | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.17, 0.85] |
| 1.2 Proportion of participants with improved or corrected strabismus: subgroup analysis: age of onset | 4 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.99] |
| 1.2.1 < 2 years | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 1.2.2 >2 and <18 Years | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.49, 1.18] |
| 1.2.3 >18 Years | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.17, 0.85] |
| 1.3 Proportion of participants with improved or corrected strabismus: subgroup analysis: duration of follow‐up | 4 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.99] |
| 1.3.1 Six months | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.38, 0.80] |
| 1.3.2 More than six months | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.14] |
| 1.4 Proportion of participants requiring additional surgeries | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Proportion of participants who achieved binocular single vision | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Sensory fusion (positive response with Worths four light test) | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.23] |
| 1.5.2 Stereopsis (minimum of 480 seconds of arc) | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carruthers 1990.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial (until 6 months)
Number randomized (in total and per group): 30 participants in total, 17 participants to BTX group and 13 to surgery group Unit of randomization (individual or eye): individual Number analyzed (in total and per group): 30 participants in total, 17 participants in the BTX group arm and 13 participants in the surgery group Unit of analysis (individual or eye): individual Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: NA Length of follow‐up: 6 months initially Reported power calculation (Y/N), if yes, sample size and power: The original planned sample size was 150 participants. This sample size was chosen based on the previously published success rate of surgery and botulinum toxin injections. It was estimated that this number could be recruited within the space of 2 years. Recruitment began in fall 1985 and was much more difficult than expected. When resources for the project ended two and half years later, only 30 appropriate patients had volunteered for randomization. (This was a refusal rate of approximately 80%) |
|
| Participants |
Country: Canada Setting: private practice Baseline characteristics 1. Botulinum toxin injections, n = 17
2. Surgical intervention, n = 13
Overall, n = 30
Inclusion criteria: patients older than 16 years with significant horizontal misalignment greater than 10 prism diopters, with orthoptically proved absent fusion Exclusion criteria: evidence of binocular vision Baseline equivalence: there is little information from the database patients only age and gender |
|
| Interventions |
Intervention‐botulinum toxin injections: 5 units Botox™. Participants offered repeat botulinum toxin injection if, at any time during 6 weeks following initial injection, the angle of deviation was not reduced below 10 PD. Re‐injections provided twice for 5 participants, 3 times for 3 participants and four times for 1 participant. Comparator‐surgery: unilateral 2 muscle or surgery with adjustable on recessed muscle Choice of eye for intervention: Side of intervention eye not specified |
|
| Outcomes |
Outcomes reported: reduction in angle to < 10 PD; % net change (preoperative deviation ‐ postoperative deviation / preoperative deviation x 100%) Adverse outcomes: not reported Choice of eye: analysis of outcomes based on binocular measurement of change in angle of deviation and reported adverse events monocularly in the intervention eye. Measurement time points: 1 day, 6 weeks, 3 months and 6 months postoperatively |
|
| Notes |
Study period: from fall 1985 to 1988 Publication language: English Trial registration: not reported Conflicts of interest: not reported Funding source: "The study was supported by a grant from the British Columbia Health Care Research Foundation" (no more details were available). "The Hollow 25 gauge, attached to the end of the tuberculin syringe, used was supplied by Smith Kettlewell Institute of Visual Sciences, San Francisco, Calif. Oculinum was also supplied by Aland B Scott MD through the Smith Kettlewell Institute." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized, but method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | "Participants were randomized by a research assistant. The investigators were masked as to the randomized sequence." |
| Blinding (performance bias and detection bias) Investigators | High risk | Masking for study personnel could not be done due to the nature of intervention. |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Masking for participants was not described. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Measurements were performed by an orthoptist who mas masked. "Final (> 6 months) evaluations were performed by one of the investigators and by an orthoptist who was masked to the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor trial register was publicly available. |
| Other bias | Unclear risk | The planned sample size was 150, but only 30 participants were eventually enrolled. This resulted in small numbers of participants across each trial group. The mix of esotropia and exotropia participants further reduced numbers for comparison, as these may respond differently to use of botulinum toxin or surgery. The Hollow 25 gauge, attached to the end of the tuberculin syringe, was supplied by Smith Kettlewell Institute of Visual Sciences, San Francisco, California. Oculinum was also supplied by Aland B Scott MD through the Smith Kettlewll Institute. |
Mayet 2021.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized (in total and per group): 110 participants in total, 55 participants each group Unit of randomization (individual or eye): individual Number analyzed (in total and per group): 54 participants in the botulinum group arm and 47 participants in the surgery group Unit of analysis (individual or eye): individual Exclusions and losses to follow‐up (total and per group): 9 participants (1 in the botulinum toxin arm and 8 in the surgery arm) were not included in the analysis. (Authors' explanation: they were lost "prior to any intervention. Six in the surgery arm expressed subsequent reservations about the surgery"). How were missing data handled?: excluded from analysis Length of follow‐up: 24 weeks Reported power calculation (Y/N), if yes, sample size and power: a sample size of 98 (49 per arm) was calculated using a Pearson Chi‐squared test with the proportion of success set at 0.65 for the surgery arm and 0.37 for the BTX arm, at a power of 80% and an alpha level of 0.05. |
|
| Participants |
Country: South Africa Setting: Paediatric Ophthalmology Clinic at St John Eye Hospital. University of the Witwatersrand Baseline characteristics 1. Botulinum toxin injections, n = 54
2. Surgical intervention, n = 47
Overall, n = 101
Inclusion criteria: children with onset of esotropia before 6 months of age; with large‐angle IE, defined as esotropia of ≥ 40 PD, between the ages of 6 months and 6 years at baseline; informed written parental consent Exclusion criteria: children with significant pattern deviation, neurological impairment and hyperopia of > + 5.00 diopters. Children underwent a full examination including a cycloplegic refraction, fundus examination and orthoptic assessment. Those with a refractive error ≥ + 2.50 DS were initially given their prescription and if there was no change or minimal change in their esotropic angle, patients were classified as IE and enrolled in the study. Baseline equivalence: comparable |
|
| Interventions |
Intervention‐botulinum toxin injections: "Botulinum toxin (BotoxTM Allergan) was injected in each medial rectus muscle, administered subconjunctively after the muscle was grasped using forceps as described by Benabent et al. (Benabent 2002). All children received an initial dose of 5 units (U) that was repeated, for a maximum of three injections if the esotropia was > 10 PD at visits at 3, 6, 12 or 24 weeks following the last injection. The dosage at subsequent visits depended on the degree of esotropia, 5 U for deviations ≥ 40 PD and 3 U for deviations < 40 PD." Comparator‐surgery: "In the surgical arm, children underwent standard bilateral medial rectus muscle recession surgery for esotropia ≤ 60 PD. The medial recti were recessed to a maximum of 7 mm with 3U of botulinum toxin given intraoperatively to each recessed muscle in cases > 60 PD to augment the recessions." |
|
| Outcomes |
Outcomes reported: complete response defined as orthophoria or residual esotropia of ≤ 10 PD; partial response as residual esotropia of > 10 PD and ≤ 20 PD and deemed acceptable by the parents; failures or non‐response as > 20 PD. Adverse outcomes: "Complications in the botulinum toxin arm were; partial transient ptosis in 9 children (16.7%), which resolved within 6–8 weeks, transient vertical deviation in 3 children (5.6%) and consecutive exotropia in 13 children (24.1%). Seven of the children with exotropia were associated with complete response. There were no cases of globe perforation, infections or chemosis following botulinum toxin injections." "The two children with exotropia, received botulinum toxin to the lateral rectus muscles as initial therapy followed bimedial rectus muscle advancement and had final non‐response. In total, 45 children (95.7%) had a complete response or partial response in the surgery arm. There were no major complications of surgery such as slipped or lost muscles, globe perforation or anaesthetic‐related issues." Measurement time points: 3, 6, 12 and 24 weeks |
|
| Notes |
Study period: from January 2015 to January 2018 Publication language: English Trial registration: not found (PACTR201508001241218 was listed as an ongoing study in the previous version of this review. However, the website was no longer accessible.) Conflicts of interest: "The authors declare that they have no conflict of interest. " Funding source: "the Anglo‐American Chairman’s Fund for grant in facilitating additional surgical lists for the study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence was not truly random. "Within each age category, participants were randomized by an independent study assistant and assigned to either the botulinum toxin (odd numbers) or surgery (even numbers) arms." |
| Allocation concealment (selection bias) | High risk | Study personnel were able to foresee the upcoming assignment when the allocation was performed based on odd or even number of enrollment. |
| Blinding (performance bias and detection bias) Investigators | High risk | This study was an "unblinded" study. Study investigators could not be masked due to the nature of interventions. |
| Blinding (performance bias and detection bias) Participants | High risk | This study was an "unblinded" study. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Although the outcome assessor was not masked the outcomes were objective, and it is unlikely that results were affected by the knowledge of the intervention that participants received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 110 patients were randomized (55 in each arm) nine of them were excluded "one patient was lost in the botulinum toxin and 8 in the surgery arm". The study investigator described the losses in each arm: "nine children were lost (one in the toxin arm and eight in the surgery arm)", and that they were lost "prior to any intervention. Six in the surgery arm expressed subsequent reservations about the surgery. Not an uncommon occurrence ...". It was unclear about the remaining three participants. |
| Selective reporting (reporting bias) | Low risk | We do not have access to the trial protocol because the URL was no longer available, but the study investigator sent us the file of the approval of the University trial ethics committee. All the outcomes were stated. |
| Other bias | Unclear risk | In the surgery arm, 23 children with very‐large‐angle esotropia (i.e. > 60PD) had botulinum toxin augment plus surgery, not surgery alone. "The medial recti were recessed to a maximum of 7 mm with 3 U of BTX given intraoperatively to each recessed muscle in cases > 60 PD to augment the recessions." |
Tejedor 1998.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized (in total and per group): 47 participants in total, 23 in BTX group (number of eyes unclear) and 24 in surgery group (38 eyes) Unit of randomization (individual or eye): individual Number analyzed (in total and per group): 47 participants in total, 23 in BTX group (number of eyes unclear) and 24 in surgery group (38 eyes) Unit of analysis (individual or eye): individual Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: NA Length of follow‐up: 1 year and last visit in reoperation group mean 2.9 (SD 0.81) years; and mean 2.7 (SD 0.42) years for BTX group Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Spain Setting: Hospital Ramón y Cajal, (university hospital) Baseline characteristics 1. Botulinum toxin injections, n = 23
2. Surgical intervention, n = 24
Overall, n = 47
Inclusion criteria: children who were less than 11 years of age, who underwent surgical correction of acquired esotropia and required a second procedure, in whom the initial surgery was carried out between 1989 and 1994. The trial included participants who had documented onset of comitant esotropia after 1 year of life. Exclusion criteria: children with a distance to near difference of at least 10 PD, children with vertical deviations greater than 4 PD, alphabetic syndromes, nystagmus, profound amblyopia (4 or more lines of difference in visual acuity), or those in whom accurate information concerning the onset of deviation or the amount and type of initial surgery were unavailable. Baseline equivalence: slight differences in time between initial and secondary procedure in years (mean/SD): reoperation group 1.5 (0.98) versus Botulinum group 0.99 (0.84) |
|
| Interventions |
Intervention: "Botulinum toxin type A (Botox, Allergan) was administered under topical anesthesia alone (0.5% proxymetacaine (proparacaine) hydrochloride) or in combination with mild general anesthesia (ketamine intramuscularly or intravenously or nitrous oxide inhalation). We used the maximal dosages suggested by Scott et al. The toxin was injected into one or two (when more than 5 U of total dose) recti muscles with electromyographic control." Comparator‐surgery: "Reoperation was performed by one of us with careful dissection of muscles and removal of fibrotic tissue. When the initial surgery was a recess‐resect we performed recess‐resect of the other eye in the appropriate direction according to previously published surgical dosages. When a bilateral recession was the primary procedure we performed a bilateral resection of the lateral recti, unless the patient was never orthophoric after initial surgery or significant restriction was detected medially by forced duction at the time of reoperation. In these two circumstances we made minimal amounts of bilateral medial rectus re‐recession following the recommendations of King et al. and the conjunctiva was always recessed." Unilateral surgery if previous surgery was unilateral (10 participants), bilateral surgery if previous surgery was bilateral (14 participants) Surgery for esotropia; recession/resection or re‐recession or bilateral resection Surgery for exotropia; bilateral recession |
|
| Outcomes |
Outcomes reported: the net change was obtained by the following formula: (preoperative deviation − postoperative deviation/preoperative deviation)× 100%; successful motor alignment was defined as a distance deviation of 8 PD or less by the simultaneous prism and cover test; fusion was detected by the Worth 4‐dot at near, and the presence of stereopsis with the Titmus circles and TNO test (at least 480 seconds of arc). Adverse outcomes: "Transient ptosis occurred in eight of the 23 patients injected with botulinum toxin (34.78%) and transient vertical deviation was present in four of the 23 patients (17.39%)." Measurement time points: not reported |
|
| Notes |
Study period: from 1989 to 1994 Publication language: English Trial registration: not reported Conflicts of interest: not reported Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized, but the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding (performance bias and detection bias) Investigators | High risk | Investigators did not appear to be masked to the different treatment options. |
| Blinding (performance bias and detection bias) Participants | High risk | It was not possible to mask participants to the different treatment options. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Although masking of outcome assessors was not described, the outcome was unlikely to have been affected by knowledge of interventions received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor trial register was publicly available. |
| Other bias | Unclear risk | Information on funding, conflict of interests, sample size calculation, and trial register was unclear as this was an old trial. |
Tejedor 1999.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized (in total and per group): 55 participants in total, 27 in BTX group (number of eyes unclear) and 28 in surgery group (56 eyes) Unit of randomization (individual or eye): individual Number analyzed (in total and per group): 55 participants in total, 27 in BTX group (number of eyes unclear) and 28 in surgery group (56 eyes) Unit of analysis (individual or eye): individual Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: NA Length of follow‐up: mean 3.75 years (SD 0.12) in surgery arm and mean 3.5 years (SD 0.21) in BTX arm Minimum 36 month follow‐up Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Spain Setting: Hospital Ramón y Cajal, (university hospital) Baseline characteristics 1. Botulinum toxin injections, n = 27
2. Surgical intervention, n = 28
Overall, n = 56
Inclusion criteria: patients with a history of esotropia present in the first 6 months of life, no accommodative element, retreated within 12 months Exclusion criteria: patients with a distance to near difference of at least 10 PD or in whom the correction of hypermetropia with spectacles improved or corrected the esotropia. Children with vertical deviations greater than 4 PD, and those with medical or neurological disease Baseline equivalence: comparable |
|
| Interventions | The participants in the two groups had been treated with only one previous documented operation. Initial surgery was a bimedial recession procedure. Intervention: "Botulinum toxin type A (Botox, Allergan) was administered under topical anesthesia alone (0.5% proxymetacaine (proparacaine) hydrochloride) or in combination with mild general anesthesia (ketamine intramuscularly/intravenously or nitrous oxide inhalation). We used the maximal dosages suggested by Scott et al. The toxin was injected into one or two (when total dose >5 U) recti muscles with electromyographic control. " Comparator‐surgery: "Reoperation was performed by the same surgeon with careful dissection of muscles and removal of fibrotic tissue. In under‐corrected children we made bilateral lateral rectus resection, following previously published guidelines. When restriction was detected medially by forced duction at the time of reoperation we did small amounts of bilateral medial rectus recession, and the conjunctiva was always recessed. For over‐corrections we carried out bilateral lateral rectus recession, unless we found weakness in adduction, for which we preferred to advance the medial recti muscles." |
|
| Outcomes |
Outcomes reported: the motor success rate and percentage net change in the deviation (preoperative deviation − postoperative deviation/preoperative deviation × 100%); successful motor alignment defined as a distance deviation of 8 PD or less by the simultaneous prism and cover test. Fusion was detected by the Worth 4 dot at near and the Bagolini lenses, and the presence of stereopsis with the Randot circles (at least 400 seconds of arc) and TNO test (at least 480 seconds of arc). Adverse outcomes: "Ptosis occurred transiently in 10 of the 27 patients injected with botulinum toxin (37.03%) and vertical deviation was also temporary in five of them (18.51%)" Measurement time points: 6 months, 1 year and 3 years after the second treatment |
|
| Notes |
Study period: from 1990 to 1994 Publication language: English Trial registration: not reported Conflicts of interest: "None of the authors has any interest in the products or devices mentioned in the paper" Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized, but the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding (performance bias and detection bias) Investigators | High risk | Investigators could not be masked due to the nature of interventions. |
| Blinding (performance bias and detection bias) Participants | High risk | It was not possible to mask participants to the different treatment options. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Although masking of outcome assessors was not described, the outcome was unlikely affected by knowledge of interventions received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor trial register was not publicly available. |
| Other bias | Unclear risk | Information on funding, conflict of interests, sample size calculation, and trial register was unclear as this was an old trial. |
BTX: botulinum toxin
IQR: Interquartile range
NA: not applicable
PD: prism diopters
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2013 | Intervention and comparator were not of interest. This study compared botulinum toxin with versus without sodium hyaluronate |
| Cooper 1991 | The paper consisted of the preliminary findings for the paper by Lee 1994, which was excluded because the intervention and comparator were not of interest in this review update. |
| de Alba Campomanes 2010 | Prospective, non‐randomized comparative study and not an RCT. |
| Doshi 2021 | It was a comment and not an RCT. |
| Etezad Razavi 2014 | Prospective, non‐randomised comparative study and not an RCT |
| Gursoy 2012 | Retrospective review of botulinum toxin versus strabismus surgery outcomes for treatment of esotropia. |
| Jain 2015 | Intervention and comparator were not of interest. This study compared botulinum toxin plus surgery with surgery alone. |
| Lee 1994 | Intervention and comparator were not of interest. This study compared botulinum toxin with observation. |
| Li 2008 | Prospective, non‐randomised clinical study and not an RCT |
| Mills 2004 | Review article and not an RCT in itself |
| Minguini 2012 | Intervention and comparator were not of interest. This study compared surgery plus injection of botulinum toxin (Prosign™) with surgery alone. |
| NCT03266549 | Not intervention and comparator of interest. It compared augmented surgery by botulinum toxin with surgery alone. |
| Pandey 2021 | It was a response to comments and not an RCT. |
| Sanjari 2008 | Case series and not an RCT |
| Shallo‐Hoffman 2006 | This was a case‐control study, not an RCT. Study of the influence of adaptation in people with chronic sixth nerve palsy having botulinum toxin. |
Abbreviations: RCT: randomized controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Méndez Sánchez 2017.
| Methods | Randomized controlled trial (unclear) |
| Participants | Inclusion criteria: residual esotropia of 20 to 30 diopters, regardless of age Exclusion criteria: not reported Number randomized: 14 cases in the botulinum toxin A group and 13 cases in the conventional surgery group |
| Interventions | Intervention: botulinum toxin A Comparator: conventional surgery |
| Outcomes | Outcomes: surgical success, complications |
| Notes | We attempted to contact study investigators to clarify the study design, but we did not receive any response from them. |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐INR‐17013777.
| Study name | Clinical study of extraocular muscle injection of botulinum toxin A in children with concomitant strabismus |
| Methods | Randomized parallel‐group controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: Botulinum toxin A injection Intervention 2: Operation |
| Outcomes |
Primary outcomes: treatment success rate after 6 months of treatment Secondary outcomes: preoperative and postoperative prism degree; binocular vision function; complications |
| Starting date | First enrollment: 1 January 2018 |
| Contact information | Dr Fu Jing: fu_jing@126.com |
| Notes |
CTRI/2021/11/038205.
| Study name | Outcome of botulinum injection vs early surgery in infantile esotropia: a prospective randomized comparative study |
| Methods | Randomized, parallel group, controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1: botulinum toxin injection Intervention 2: surgery |
| Outcomes |
Primary outcome Comparison of percentage of success in botulinum toxin group with percentage success in early surgery group Time points: 1 week, 1 month, 3 months and 6 months Secondary outcomes
|
| Starting date | First enrollment: 1 December 2021 |
| Contact information | Dr Shweta Chaurasia: shweta84omns@yahoo.com |
| Notes |
NCT03459092.
| Study name | Botox Instead of Strabismus Surgery (BISS) |
| Methods | Randomized, parallel‐group, controlled trial |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Intervention 1: botulinum toxin type A Intervention 2: strabismus surgery |
| Outcomes |
Primary outcome Number of participants with presence of binocular vision Secondary outcomes
Other Outcome Measures
|
| Starting date | 16 August 2018; estimated completion August 2023 |
| Contact information | Principal Investigator: Professor Mathias Abegg, Bern University Hospital |
| Notes |
Differences between protocol and review
Bort‐Martí A, Sifre LR, Ng SM, Bort‐Martí S, and Ruiz Garcia V were added as authors.
To keep up with the current review terminologies used in the Cochrane Handbook, we revised the 'primary' and 'secondary' outcomes to 'critical' and 'important' outcomes, respectively.
We also redefined review outcomes to clarify outcome definitions, measures, metrics, and time points for data collection.
We added 'changes in quality of life' as one new important outcome for this update.
We also incorporated serious and non‐serious adverse outcomes into the list of critical and important outcomes, separately.
Due to the sparsity of data, we changed the outcome metric in binocular single vision. We changed from 'total duration of time maintaining binocular single vision at postintervention 12 months or longer' to 'proportion of participants who achieve binocular single vision' and analyzed the outcome data on sensory fusion and stereopsis.
Contributions of authors
Updating Methods: all review authors
Screening search results: ABM, SMN, VRG
Extracting the data: SBM, VRG, LRS
Assessing risk of bias: SBM, VRG, LRS
Contacting study investigators: SBM, VRG
Managing the data in RevMan Web: SMN, SBM, VRG
Interpretation of the data: SMN, SBM, VRG
Writing manuscripts: all review authors
Final approval of the document to be published: all review authors
Sources of support
Internal sources
-
University of Liverpool, UK
Fiona J Rowe receives support from University of Liverpool.
External sources
-
National Eye Institute, National Institute of Health, USA
Cochrane Eyes and Vision US Project, supported by grant UG1EY020522 (PI: Tianjing Li, MD, MHS, PhD)
-
Queen's University Belfast, UK
The work of Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision, is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland.
-
Public Health Agency, UK
The HSC Research and Development (R&D) Division of the Public Health Agency funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast.
Declarations of interest
AB: None
FR: FR holds grant funding from the UK Stroke Association and Fight for Sight. However, none of these are relevant to this BTX review. FR has no declarations specific to this review.
LRS: None
SMN: SMN reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.
SB: None
VRG: None
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Carruthers 1990 {published data only}
- Carruthers JD, Kennedy RA, Bagaric D. Botulinum versus adjustable suture surgery in the treatment of horizontal misalignment in adult patients lacking fusion. Archives of Ophthalmology 1990;108(10):1432-5. [DOI] [PubMed] [Google Scholar]
Mayet 2021 {published data only}
- Mayet I, Ally N, Alli HD, Tikly M, Williams S. Botulinum neurotoxin injections in essential infantile esotropia-a comparative study with surgery in large-angle deviations. Eye 2021;35(11):3071‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tejedor 1998 {published data only}
- Tejedor J, Rodríguez JM. Retreatment of children after surgery for acquired esotropia: reoperation versus botulinum toxin. British Journal of Ophthalmology 1998;82(2):110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tejedor 1999 {published data only}
- Tejedor J, Rodríguez JM. Early retreatment of infantile esotropia: comparison of reoperation and botulinum toxin. British Journal of Ophthalmology 1999;83(7):783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2013 {published data only}
- Chen J, Deng D, Zhong H, Lin X, Kang Y, Wu H, et al. Botulinum toxin injections combined with or without sodium hyaluronate in the absence of electromyography for the treatment of infantile esotropia: a pilot study. Eye 2013;27(3):382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991 {published data only}
- Cooper K, Lee JP, MacEwen C, Jones S. Botulinum toxin A injection for acute sixth nerve palsy. In: Transactions of the 8th International Orthoptic Congress. Vol. VIII. 1991:336-40.
de Alba Campomanes 2010 {published data only}
- Alba Campomanes AG, Binenbaum G, Campomanes Eguiarte G. Comparison of botulinum toxin with surgery as primary treatment for infantile esotropia. Journal of AAPOS 2010;14(2):111-6. [DOI] [PubMed] [Google Scholar]
- Alba Campomanes AG, Binenbaum G, Eguiarte GC. Comparison of botulinum toxin to surgery as primary treatment for infantile esotropia. Journal of AAPOS 2009;13(1):e4. [DOI] [PubMed] [Google Scholar]
Doshi 2021 {published data only}
- Doshi A, Agashe P, Kshirsagar S. Comments on: Short-term outcome of botulinum neurotoxin A injection with or without sodium hyaluronate in the treatment of infantile esotropia - A prospective interventional study. Indian Journal of Ophthalmology 2021;69(2):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etezad Razavi 2014 {published data only}
- Etezad Razavi M, Sharifi M, Armanfar F. Efficacy of botulinum toxin in the treatment of intermittent exotropia. Strabismus 2014;22(4):176-81. [DOI] [PubMed] [Google Scholar]
Gursoy 2012 {published data only}
- Gursoy H, Basmak H, Sahin A, Yildirim N, Aydin Y, Colak E. Long-term follow-up of bilateral botulinum toxin injections versus bilateral recessions of the medial rectus muscles for treatment of infantile esotropia. Journal of AAPOS 2012;16(3):269-73. [DOI] [PubMed] [Google Scholar]
Jain 2015 {published and unpublished data}
- Jain S, Anand S, Jones A. Intraoperative botulinum toxin in large angle strabismus. Journal of AAPOS 2015;19(4):e12. [Google Scholar]
Lee 1994 {published data only}
- Lee J, Harris S, Cohen J, Cooper K, MacEwen C, Jones S. Results of a prospective randomized trial of botulinum toxin therapy in acute unilateral sixth nerve palsy. Journal of Pediatric Ophthalmology & Strabismus 1994;31(5):283-6. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Y, Wu X. [Observation of botulinum toxin A management in childhood with intermittent exotropia]. Zhonghua Yan Ke Za Zhi [Chinese Journal of Ophthalmology] 2008;44(11):967-71. [PubMed] [Google Scholar]
Mills 2004 {published data only}
- Mills MD, Coats DK, Donahue SP, Wheeler DT, American Academy of Ophthamology. Strabismus surgery for adults. Ophthalmology 2004;111(6):1255-62. [DOI] [PubMed] [Google Scholar]
Minguini 2012 {published data only}
- Minguini N, Carvalho KM, Bosso FLS, Hirato FE, Kara-José N. Surgery with intraoperative botulinum toxin-A injection for the treatment of large-angle horizontal strabismus: a pilot study. Clinics 2012;67(3):279-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03266549 {published data only}
- NCT03266549. Botulinum Toxin Augmented Surgery vs Conventional Surgery in the Management of Large Angle Infantile Esotropia. clinicaltrials.gov/ct2/show/NCT03266549 (first received 30 August 2017).
Pandey 2021 {published data only}
- Pandey N, Agrawal S, Srivastava RM, Singh V. Response to comments on: Short-term outcome of botulinum neurotoxin A injection with or without sodium hyaluronate in the treatment of infantile esotropia - A prospective interventional study. Indian Journal of Ophthalmology 2021;69(2):474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanjari 2008 {published data only}
- Sanjari MS, Falavarjani KG, Kashkouli MB, Aghai GH, Nojomi M, Rostami H. Botulinum toxin injection with and without electromyographic assistance for treatment of abducens nerve palsy: a pilot study. Journal of AAPOS 2008;12(3):259-62. [DOI] [PubMed] [Google Scholar]
Shallo‐Hoffman 2006 {published data only}
- Shallo-Hoffman J, Acheson J, Bentley C, Bronstein AM. The influence of adaptation of visual motion detection in chronic sixth nerve palsy after treatment with botulinum toxin. Strabismus 2006;14(3):129-35. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Méndez Sánchez 2017 {published data only}
- Méndez Sánchez TJ, Soto Mejías MI, Pons Castro L, Naranjo Fernández RM, Casanueva CH, López FD. Botulinum toxin versus conventional surgery in residual esotropia YZ [Toxina botulínica versus cirugía convencional en esotropía residual YZ]. Cuban Journal of Ophthalmology 2017;30(3):1-11. [Google Scholar]
References to ongoing studies
ChiCTR‐INR‐17013777 {published data only}
- ChiCTR-INR-17013777. Clinical study of extraocular muscle injection of botulinum toxin A in children with concomitant strabismus. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-17013777 (first received 8 December 2017).
CTRI/2021/11/038205 {published data only}
- CTRI/2021/11/038205. Injection versus Surgery for esotropia in infants. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/11/038205 (first received 23 November 2021).
NCT03459092 {published data only}
- NCT03459092. Botox Instead of Strabismus Surgery (BISS). clinicaltrials.gov/ct2/show/NCT03459092 (first received 8 March 2018).
Additional references
AAO 2022
- American Academy of Ophthalmology. Botulinum toxin use In strabismus. available at eyewiki.aao.org/Botulinum_Toxin_Use_In_Strabismus (accessed 11 September 2022).
Adams 2005
- Adams RJ, Hall HL, Courage ML. Long-term visual pathology in children with significant perinatal complications. Developmental Medicine and Child Neurology 2005;47(9):598-602. [PubMed] [Google Scholar]
Benabent 2002
- Benabent EC, García Hermosa P, Arrazola MT, Alió y Sanz JL. Botulinum toxin injection without electromyographic assistance. Journal of Pediatric Ophthalmology & Strabismus 2002;39:231-4. [DOI] [PubMed] [Google Scholar]
Binenbaum 2021
- Binenbaum G, Chang MY, Heidary G, Morrison DG, Trivedi RH, Galvin JA, et al. Botulinum toxin injection for the treatment of strabismus: a report by the American Academy of Ophthalmology. Ophthalmology 2021;128(12):1766-76. [DOI] [PubMed] [Google Scholar]
Bunting 2013
- Bunting HJ, Dawson EL, Lee JP, Adams GG. Role of inferior rectus botulinum toxin injection in vertical strabismus resulting from orbital pathology. Strabismus 2013;21(3):165-8. [DOI] [PubMed] [Google Scholar]
Campos 2000
- Campos EC, Schiavi C, Bellusci C. Critical age of botulinum toxin treatment in essential infantile esotropia. Journal of Pediatric Ophthalmology and Strabismus 2000;37(6):328-32. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, (accessed 02 June 2022). Available at covidence.org.
Crouch 2006
- Crouch ER. Use of botulinum toxin in strabismus. Current Opinion in Ophthalmology 2006;17(5):435-40. [DOI] [PubMed] [Google Scholar]
Dawson 1999
- Dawson EL, Marshman WE, Adams GG. The role of botulinum toxin A in acute-onset esotropia. Ophthalmology 1999;106(9):1727-30. [DOI] [PubMed] [Google Scholar]
Dawson 2004
- Dawson EL, Sainari A, Lee JP. Does botulinum toxin have a role in the treatment of secondary strabismus? In: Transactions of the 29th European Strabismological Association; 2004 June 1-4; Izmir, Turkey. 2004:53-4.
Dawson 2005
- Dawson EL, Sainani A, Lee JP. Does botulinum toxin have a role in the treatment of secondary strabismus? Strabismus 2005;13(2):71-3. [DOI] [PubMed] [Google Scholar]
Dawson 2012
- Dawson E, Ali N, Lee JP. Botulinum toxin injection into the superior rectus for treatment of strabismus. Strabismus 2012;20(1):24-5. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Donnelly 2005
- Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiology 2005;12(4):243-50. [DOI] [PubMed] [Google Scholar]
FDA
- US Food and Drug Administration. What is a Serious Adverse Event? www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed 02 June 2022).
Gardner 2013
- Gardner R, Dawson EL, Adams GG, Lee JP. The use of botulinum toxin to treat strabismus following retinal detachment surgery. Strabismus 2013;21(1):8-12. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). Cochrane, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Holmes 2001
- Holmes JM, Leske DA, Christiansen SP. Initial treatment outcomes in chronic sixth nerve palsy. Journal of AAPOS 2001;5(6):370-6. [DOI] [PubMed] [Google Scholar]
Issaho 2017
- Issaho DC, Carvalho FRS, Tabuse MKU, Carrijo-Carvalho LC, Freitas D. The use of botulinum toxin to treat infantile esotropia: a systematic review with meta-analysis. Investigative Ophthalmology & Visual Science 2017;58(12):5468-76. [DOI] [PubMed] [Google Scholar]
Kerr 2001
- Kerr NC, Hoehn MB. Botulinum toxin for sixth nerve palsies in children with brain tumors. Journal of AAPOS 2001;5(1):21-5. [DOI] [PubMed] [Google Scholar]
Li 2022
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Marsh 2003
- Marsh IB. Botulinum toxin and the eye. Hospital Medicine 2003;64(8):464-7. [DOI] [PubMed] [Google Scholar]
McKenzie 2022
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
McNeer 2003
- McNeer KW, Tucker MG, Guerry CH, Spencer RF. Incidence of stereopsis after treatment of infantile esotropia with botulinum toxin A. Journal of Pediatric Ophthalmology and Strabismus 2003;40(5):288-92. [DOI] [PubMed] [Google Scholar]
MECIR
- Chandler J, Lasserson T, Higgins JP, Tovey D, Thomas J, Flemyng E, et al. Standards for the planning, conduct and reporting of updates of Cochrane Intervention Reviews. In: Higgins JP, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews. London: Cochrane, February 2022. [Google Scholar]
Mehner 2023
- Mehner L, Ng SM, Singh J. Interventions for infantile esotropia. Cochrane Database of Systematic Reviews 2023, Issue 1. Art. No: CD004917. [DOI: 10.1002/14651858.CD004917.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noval 2017
- Noval Martín S, Cabrejas Martínez L, Jarrín Hernández E, Pérez Flores I. Practical-clinical guideline on the indication and administration of botulinum toxin injection in the treatment of strabismus [Guía práctico-clínica sobre la indicación y administración de la inyección de toxina botulínica en el tratamiento de los estrabismos]. Available at www.researchgate.net/publication/317105997 (accessed 30 October 2022). [ISBN: 978-84-947085-0-3]
Ozkan 2006
- Ozkan SB, Topaloǧlu A, Aydin S. The role of botulinum toxin A in augmentation of the effect of recession and/or resection surgery. Journal of AAPOS 2006;10(2):124-7. [DOI] [PubMed] [Google Scholar]
Rayner 1999
- Rayner SA, Hollick EJ, Lee JP. Botulinum toxin in childhood strabismus. Strabismus 1999;7(2):103-11. [DOI] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- Review Manager Web (RevMan Web). Version 4.2.1. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rowe 2004
- Rowe F, Noonan C, Nayak H. Use of botulinum toxin in pre-adolescent children. In: Transactions of the 10th International Orthoptic Congress; 2004 Nov 14-17; Melbourne. 2004; CD-ROM 187704024x.
Rowe 2005
- Rowe FJ, Noonan CP, Nayak H. Botulinum toxin as a treatment option for decompensating intermittent strabismus in children. In: Transactions of the 30th European Strabismological Association; 2005 June 8-11; Killarney, Ireland. 2005:101-4.
Sabetti 2003
- Sabetti L, D'Alessandri L, Salvatori K, Balestrazzi E. The use of botulinum toxin type A in the treatment of chronic sixth ocular nerve palsy. In: Transactions of the 28th European Strabismological Association; 2003 June 19-21; Bergen. 2003:191-2.
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Scott 1980
- Scott AB. Botulinum toxin injection into extraocular musclesas an alternative to strabismus surgery. Ophthalmology 1980;87(10):1044-9. [DOI] [PubMed] [Google Scholar]
Spencer 1997
- Spencer RF, Tucker MG, Choi RY, McNeer KW. Botulinum toxin management of childhood intermittent exotropia. Ophthalmology 1997;104(11):1762-7. [DOI] [PubMed] [Google Scholar]
Strömland 1993
- Strömland K, Miller MT. Thalidomide embryopathy: revisited 27 years later. Acta Ophthalmologica 1993;71(2):238-45. [DOI] [PubMed] [Google Scholar]
Tejedor 2001
- Tejedor J, Rodríguez JM. Long-term outcome and predictor variables in the treatment of acquired esotropia with botulinum toxin. Investigative Ophthalmology and Visual Science 2001;42(11):2542-6. [PubMed] [Google Scholar]
References to other published versions of this review
Rowe 2009
- Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD006499. [DOI: 10.1002/14651858.CD006499.pub2] [DOI] [PubMed] [Google Scholar]
Rowe 2012
- Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD006499. [DOI: 10.1002/14651858.CD006499.pub3] [DOI] [PubMed] [Google Scholar]
Rowe 2017
- Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD006499. [DOI: 10.1002/14651858.CD006499.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


