Abstract
Objective:
Polysubstance use may complicate treatment outcomes for individuals who use opioids. This research aimed to examine the prevalence of polysubstance use in an opioid use disorder treatment trial population and polysubstance use’s association with opioid relapse and craving.
Methods:
This study is a secondary data analysis of individuals with opioid use disorder who received at least one dose of medication (n=474) as part of a 24-week, multi-site, open label, randomized Clinical Trials Network study (CTN0051, X:BOT) comparing the effectiveness of extended-release naltrexone versus buprenorphine. Models examined pretreatment polysubstance use and polysubstance use during the initial 4 weeks of treatment on outcomes of relapse by week 24 of the treatment trial and opioid craving.
Results:
Polysubstance use was generally not associated with treatment outcomes of opioid relapse and craving. Proportion of days of pretreatment sedative use was associated with increased likelihood of opioid relapse (OR: 1.01, 95% CI: 1.00–1.02). Proportion of days of cocaine use during the initial 4 weeks of treatment was associated with increased likelihood of opioid relapse (OR: 1.05, 95% CI: 1.01–1.09) but this effect was no longer significant once the potential of confounding by opioid use was considered. Sedative use during initial 4 weeks of treatment was associated with increased opioid craving (b: 0.77, 95% CI: 0.01–1.52). The study found no other significant relationships.
Conclusions:
In the current study population, polysubstance use was only marginally associated with 24-week treatment outcomes.
Keywords: Polysubstance use, Opioid use disorder, Buprenorphine, Naltrexone, Treatment
1. Introduction
Polysubstance use is one of the most pressing issues of the United States’ current overdose crisis. Polysubstance use includes the unique patterns of substance use involving more than one substance (drug and/or alcohol) and can include substances used at the same time, regular patterns, or intervals. Since at least 2003, deaths involving multiple substances have been increasing with the most notable increases among co-use of opioids and psychostimulants (Kariisa et al., 2019). The majority of opioid overdoses involve co-use of another substance; most commonly stimulants and benzodiazepines (Liu et al., 2021; O’Donnell et al., 2020). Overdose data are supported by trends in community and treatment samples, which find increases in reported past-year polysubstance use, particularly co-use of methamphetamine and opioids (Jones et al., 2020; Palamar et al., 2020). Despite the pervasiveness of polysubstance use among persons who use opioids, treatments for polysubstance use and studies on how polysubstance use may or may not affect the treatment of opioid use disorders is greatly lacking (Bhalla et al., 2017).
In treatment settings for opioid use disorder (OUD), clinical perspectives on polysubstance use have been mixed, with continued drug use often considered as a reason for treatment discontinuation, as clinicians in this setting may view polysubstance use as complex and complicating treatment of the primary substance use disorder or a sign of treatment “failure” (Cunningham et al., 2013; Lin et al., 2020). In the case of medications for opioid use disorder (MOUD), national practice guidelines state that co-occurring drug or alcohol use is not a reason to withhold or suspend MOUD but rather patients with co-use during treatment may be in need of more intensive care (Substance Abuse and Mental Health Services Administration (SAMHSA), 2021; U.S. Food and Drug Administration, 2017; Cunningham et al., 2020).
Recommendations were updated to explicitly include the co-use of benzodiazepines, stating that the harm caused by untreated OUD outweighs the risk of benzodiazepine co-use with agonist MOUDs (SAMHSA, 2021; U.S. Food and Drug Administration, 2017; Cunningham et al., 2020). However, individuals with OUD and co-occurring polysubstance use may be less likely to receive MOUD (Lin et al., 2020), possibly due to clinician’s lack of comfort with polysubstance use, lack of training, and/or having an abstinence approach to treatment (Bentzley et al., 2015).
Even when patients receive MOUD, polysubstance use can complicate treatment (Blondino et al., 2020; Krawczyk et al., 2021). Several barriers to polysubstance-focused treatments have stalled. Currently, no Food and Drug Administration approved medications for stimulant disorders exist; one of the most common co-occurring substance use disorders with OUD (Chan et al., 2020). Treatments that may have promise for dually diagnosed patients, such as contingency management and cognitive behavioral therapy, have substantial implementation barriers that impede the translation of evidence-based practices into clinical settings (Carroll, 2014). Overall, the field needs to further understand the impact polysubstance use has on current available treatments, so that future research can better consider evidence for tailored treatment approaches.
The objective of this secondary data analysis was to examine the prevalence of polysubstance use in an OUD treatment trial population and polysubstance use’s association with opioid relapse and craving among a sample of individuals who received MOUD (buprenorphine or extended-release naltrexone) during a 24-week open-label randomized comparative effectiveness trial (CTN-0051, X:BOT). This study examined polysubstance use prior to beginning study treatment (i.e., pretreatment) and during the initial four weeks of treatment. Although previous research generally finds that individuals engaged in polysubstance use have poorer treatment outcomes (Blondino et al., 2020), no specific a priori hypotheses for the current research were formulated due to the emerging nature of polysubstance use studies.
2. Methods
2.1. Sample
The current study is a secondary data analysis of individuals with OUD enrolled in the CTN 24-week open-label randomized comparative effectiveness trial of extended release naltrexone versus buprenorphine (Lee et al., 2018). Participants 18 years and older, who spoke English, with past 30-day use of nonprescribed opioids, and who met DSM-5 criteria for OUD were recruited from eight different substance use disorder inpatient treatment sites. The study randomized participants 1:1 to receive daily sublingual buprenorphine or monthly injectable naltrexone; the study stratified randomization by treatment site and opioid use severity. Participants underwent detoxification, with protocols and length of detoxification varying by treatment site. Randomization timing was flexible, with some participants randomized early during detoxification, and others later, after they completed detoxification; we did not stratify randomization for early/late status. The study team used this randomization approach due to the hypothesized difficulty with naltrexone induction among early randomizers. The study provided medication free of charge and followed participants weekly in the community for 24 weeks. Further details regarding the original study method and design are available elsewhere (Lee et al., 2016, 2018).
The parent study randomly assigned 570 individuals to receive sublingual buprenorphine or monthly injectable naltrexone. However, the study successfully inducted more individuals into the buprenorphine group as the naltrexone group faced significant induction hurdles (28% dropped out before induction). The current analyses focused on the effects of polysubstance use among individuals receiving MOUD and, therefore, examined the population receiving the per protocol treatment, which was limited to individuals who successfully initiated and received at least one dose of medication (n=474). Recognizing the potential for bias in a per-protocol sample, this study examined the effect of polysubstance use on induction success. Pretreatment polysubstance use had no significant effect on induction success (bivariate results not shown). Over the 24-week period the sample size fluctuated due to retention barriers, and relevant models reflect the final sample size (n=361). The study examined the potential effect of pretreatment polysubstance use on retention via bivariate differences between the retained sample (n=361) and the 110 who were inducted but lost to follow-up by week 4 (bivariate results not shown), and the study found no significant differences.
2.2. Outcome measures
The study examined two outcomes: opioid relapse and self-reported ratings of opioid craving. A dichotomous measure indicated if opioid relapse had occurred, conceptualized as loss of persistent nonstudy opioid abstinence. The study defined opioid relapse as four consecutive opioid use weeks or seven consecutive days of self-reported opioid use. We defined an opioid use week as any week during which participants self-reported use at least one day of a nonstudy opioid on the Timeline Followback, provided an opioid positive urine toxicology sample (positive for nonstudy opioids), or did not provide a urine sample due to missed visits or refusals. The second outcome of interest was opioid craving, measured weekly using a self-rating using a 0–100 visual analog scale. Table S1 summarizes variables.
2.3. Polysubstance use measures
The study used measurements of polysubstance use prior to entering the study detoxification (referred to as pretreatment) and during the initial four weeks of the study treatment period. Pretreatment polysubstance use captured use of other substances in the 30 days prior to intake to detoxification using the Timeline Followback method (Sobell & Sobell, 1992). Treatment polysubstance use examined other substance use during the initial four weeks of study treatment using weekly Timeline Followback. The study examined the proportion of days of use of five substances for each timepoint: binge alcohol, sedatives, cocaine, amphetamines, and cannabis. Two measures of polysubstance use assessed the number of substances used: A count variable measured the number of nonopioid substances used from the five substances in the 30 days pretreatment and the number of nonopioid substances used during the initial four weeks of treatment. The study also measured the proportion of days pretreatment and during initial four weeks of treatment with the use of two or more substances. Finally, for pretreatment analyses only, a dichotomous variable captured whether individuals felt their main substance use of concern was multiple substances compared to one or none.
2.4. Statistical analyses
Prior to all analyses, the study examined distribution and outliers of all variables. We produced descriptive statistics (frequencies and proportions for categorical variables; means and standard deviations or medians and interquartile ranges [IQR]) for demographic variables and pretreatment predictors.
To investigate the associations of pretreatment polysubstance use with opioid relapse, the study fit mixed-effects logistic regression models of the log odds of relapse after week 3 with pretreatment substance use variables as predictors. Separate models were fit for each predictor, and each model featured a random intercept for site. The team adjusted models for gender, age, race, marital status, employment, and treatment arm. To investigate the associations of pretreatment polysubstance use with early opioid craving, we fit mixed-effects linear regression models of craving measures for weeks 1 to 4 with pretreatment substance use variables as predictors. The study then fit separate models again for each predictor, and each model featured random intercepts for subject and for site. Again, we adjusted models for gender, age, race, marital status, employment, and treatment arm.
To look at the effects of polysubstance use during the initial four weeks of study treatment, the study fit similar models to those described above, with variables of polysubstance use during the initial four weeks as the predictors, and either relapse after week 4 (logistic) or week 5 to week 24 opioid craving (linear) as the outcome. The research team repeated restricted analyses, limited to those who did not use opioids during the initial four weeks of the study. Additionally, we used a Cochran-Armitage trend test to test for increasing or decreasing trends in the proportions of polysubstance use during the initial four weeks.
All analyses used SAS® version 9.4. We set the significance level at 5%.
3. Results
Pretreatment characteristics of the sample are presented in Table 1. A majority of participants were unemployed, single, white, and male, and had an average age of 34. Pretreatment, participants were most likely to report using 1 (36%) or 2 (28%) nonopioid substances. However, the proportion of days of other substance use was low (IQR: 0–12% across all nonopioid substances) as was the proportion of days of polysubstance use (IQR: 0–5%). A minority of participants (23%) reported that more than one substance was currently a major problem for them.
Table 1.
Pretreatment characteristics, (n=474)
| n | % | |
|---|---|---|
| Demographics | ||
| Gender | ||
| Male | 331 | 70% |
| Female | 143 | 30% |
| Age at Randomization [Mean (SD)] | 474 | 33.66 (9.59) |
| Race | ||
| White Only | 358 | 76% |
| Non-white | 116 | 24% |
| Marital Status | ||
| Ever Married | 158 | 33% |
| Never Married | 314 | 67% |
| Employment | ||
| Not employed | 360 | 63% |
| Employed | 210 | 37% |
| Pretreatment Polysubstance Use | ||
| Proportion of Days of Binge Alcohol Use (Median [IQR]) | 473 | 0% [0%−0%] |
| Proportion of Days of Sedatives Use (Sedatives and hypnotics OR Benzodiazepines) (Median [IQR]) | 473 | 0% [0%−0%] |
| Proportion of Days of Cocaine Use (Cocaine OR Crack) (Median [IQR]) | 473 | 0% [0%−8%] |
| Proportion of Days of Amphetamine Use (Median [IQR]) | 473 | 0% [0%−0%] |
| Proportion of Days of Marijuana Use (Median [IQR]) | 473 | 0% [0%−13%] |
| Number of Non-Opioid Substances Used | ||
| No non-opioid substance use | 95 | 20% |
| 1 substance | 171 | 36% |
| 2 substances | 132 | 28% |
| 3 substances | 56 | 12% |
| 4 substances | 17 | 3% |
| 5 substances | 2 | 0% |
| Proportion of Days using 2+ non-opioid substances (Median [IQR]) | 473 | 0% [0%−7%] |
| Substances that are major problems | ||
| No Problem or 1 Drug | 356 | 75% |
| More than 1 Drug | 116 | 25% |
| Additional Pretreatment Polysubstance Use Variables | ||
| Any Days of Binge Alcohol Use | ||
| Yes | 114 | 24% |
| No | 359 | 76% |
| Any Days of Sedatives Use (Sedatives and hypnotics OR Benzodiazepines) | ||
| Yes | 116 | 24% |
| No | 357 | 76% |
| Any Days of Cocaine Use (Cocaine OR Crack) | ||
| Yes | 170 | 36% |
| No | 303 | 64% |
| Any Days of Amphetamine Use | ||
| Yes | 84 | 18% |
| No | 389 | 82% |
| Any Days of Marijuana Use | ||
| Yes | 197 | 42% |
| No | 276 | 58% |
| Outcome Variables | ||
| Relapsed (at any point) | ||
| Yes | 256 | 54% |
| No | 218 | 46% |
| Baseline Opioid Craving [Mean (SD)] | 474 | 69.11 (30.15) |
Table 2 presents the association of pretreatment polysubstance use with opioid relapse and craving. Generally, pretreatment polysubstance use was not significantly associated with relapse or craving. The study found a small effect for sedative use, such that one percent greater proportion of pretreatment days with sedative use was associated with a 1.2% increased likelihood of opioid relapse (OR: 1.01, 95% CI: 1.00–1.02). No other pretreatment substance use was significantly associated with opioid relapse, and the study found no significant associations for opioid craving (weeks 1–4).
Table 2.
Association of pretreatment polysubstance use on opioid relapse and craving
| Model 1 Logistic Regression of Relapse | Model 2 Linear Regression of Opioid Craving in 1st 4 Weeks | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N | OR | LCL | UCL | Beta | LCL | UCL | |
| Heavy Alcohol Use | 471 | 0.999 | 0.990 | 1.008 | −0.071 | −0.170 | 0.029 |
| Sedative Use | 471 | 1.012 | 1.001 | 1.023 | −0.011 | −0.119 | 0.097 |
| Cocaine Use | 471 | 0.999 | 0.991 | 1.008 | −0.054 | −0.151 | 0.043 |
| Amphetamine Use | 471 | 1.012 | 0.997 | 1.028 | 0.008 | −0.148 | 0.163 |
| Marijuana Use | 471 | 0.997 | 0.991 | 1.003 | −0.019 | −0.091 | 0.052 |
| Number of Substances Used | 471 | 1.066 | 0.892 | 1.274 | −1.619 | −3.651 | 0.413 |
| Use of 2+ Substances | 471 | 1.006 | 0.995 | 1.016 | −0.072 | −0.187 | 0.043 |
| Multiple vs 1 or No Substance Problem | 470 | 1.413 | 0.890 | 2.242 | 1.830 | −3.164 | 6.824 |
Notes: Polysubstance use variables are proportion of 30 days before baseline (pretreatment); Model 1 includes random intercept for site, Model 2 includes random intercepts for site and subject; OR= Odds Ratio, LCL= lower confidence limit, UCL= upper confidence limit; bold values indicate significant relationships; Models were adjusted for gender, age, race, marital status, employment, and treatment arm
Table 3 reports the associations of polysubstance use during the initial four weeks of treatment with opioid relapse and craving. Polysubstance use during the initial four weeks of treatment generally was not significantly associated with outcomes. For relapse, the study found one substance specific effect, such that with a 1% greater proportion of days of cocaine use during the initial four weeks, the likelihood of relapse after the four weeks increased 4.9% (OR: 1.05, 95% CI: 1.01–1.09). The study found no substance specific effects for opioid craving (weeks 5–24). Because of the potential of confounding by opioid use—that is, that greater opioid use in the beginning of treatment among those who use other substances could be driving any effects on outcomes during the first four weeks of treatment—a supplementary analysis (Table S2) examined opioid relapse and craving among those who did not use opioids in the initial four weeks. Once limiting only to subjects who did not use opioids, use of cocaine during initial four weeks was no longer significantly associated with relapse (OR: 0.98, 95% CI: 0.91–1.05) or craving outcomes. Among this sample, only sedative use during the initial four weeks was associated with increased opioid craving (estimated slope b: 0.77, 95% CI: 0.01–1.52). The study found no other significant relationships.
Table 3.
Association of during treatment polysubstance use on opioid relapse and craving
| Model 1 Logistic Regression of Relapse after Week 4 |
Model 2 Linear Regression of Opioid Craving after Weeks 4 |
||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N | OR | LCL | UCL | Beta | LCL | UCL | |
| Heavy Alcohol Use | 361 | 1.021 | 0.984 | 1.059 | −0.057 | −0.399 | 0.285 |
| Sedative Use | 361 | 1.027 | 0.984 | 1.072 | 0.281 | −0.080 | 0.643 |
| Cocaine Use | 361 | 1.049 | 1.013 | 1.086 | 0.169 | −0.085 | 0.423 |
| Amphetamine Use | 361 | 1.026 | 0.993 | 1.059 | 0.108 | −0.134 | 0.350 |
| Marijuana Use | 361 | 0.991 | 0.981 | 1.002 | −0.020 | −0.107 | 0.067 |
| Number of Substances Used | 361 | 1.030 | 0.800 | 1.326 | 0.679 | −1.445 | 2.802 |
| Use of 2+ Substances | 361 | 0.973 | 0.907 | 1.007 | 0.003 | −0.541 | 0.546 |
Notes: Polysubstance use variables are proportion of 30 days in first four weeks of treatment; Model 1 includes random intercept for site, Model 2 includes random intercepts for site and subject; OR= Odds Ratio, LCL= lower confidence limit, UCL= upper confidence limit; bold values indicate significant relationship; Models were adjusted for gender, age, race, marital status, employment, and treatment arm
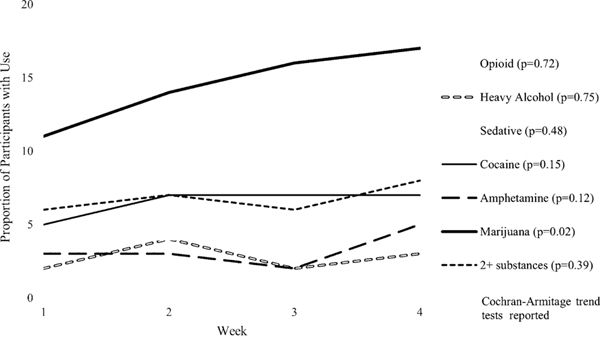
Exploratory trend analyses (Figure 1) examined the frequency of use of each substance during the initial four weeks of treatment. The study found a significant trend only for cannabis, such that cannabis use increased significantly over the four weeks from 11% in week 1 to 17% in week 4 (p=0.02).
Fig. 1.
Polysubstance use during the 1st 4 weeks of treatment.
4. Discussion
Polysubstance use did not have a significant effect on opioid relapse or craving in this study population. Pretreatment polysubstance use was not a particularly important indicator of relapse by 24 weeks or craving, with only a small effect noted for pretreatment sedative use. During the initial four weeks of treatment, cocaine use was significantly associated with opioid relapse after four weeks, but this association lost significance when limited to participants who were not using opioids during this time. The study did not find any significant effects for polysubstance use variables that measured an increased number of nonopioid substance used.
After having stated that individuals who engage in pretreatment polysubstance use may not be suitable for office-based MOUD (American Society of Addiction Medicine, 2019; Center for Substance Abuse Treatment., 2004; Cunningham et al., 2013), national guidelines were updated, as recently as 2020, but this lingering idea may still exclude individuals from treatment. In a Veterans Health Administration study, individuals with OUD and one or more other substance use disorders were less likely to receive MOUD (Lin et al., 2020). Little research has explored why patients with polysubstance use may be less likely to receive treatment, but stigma is possibly exacerbated among individuals engaged in polysubstance use. Individuals engaged in polysubstance use tend to have lower levels of education, comorbid mental health conditions, and criminal justice histories (Betts et al., 2016; Green et al., 2011; Martinotti et al., 2009); all groups more likely to face stigma (Link & Phelan, 2001). Providers may be particularly hesitant when patients engage in sedative use, which is known to contribute to accidental overdose (Park et al., 2020). The current study measured only the proportion of days of use rather than a DSM-diagnosis for sedative use disorder or existence of a legal sedative prescription, but found only a small effect for sedative use with opioid relapse. During treatment, when limited to individuals without opioid use during the initial four weeks, we found increased opioid craving among persons with increased sedative use. Other research examining sedative, most notably benzodiazepine, use during MOUD treatment has found no relationship between pretreatment benzodiazepine use and treatment outcomes (Marsch et al., 2005; Proctor et al., 2016; Schuman-Olivier et al., 2013). Of greater concern to clinicians may be the risk of overdose when combining agonist or partial agonist MOUDs with sedatives. The current research did not examine overdose outcomes. The CTN-0051 study had 19 overdoses, 5 fatal, in the per-protocol sample (Lee et al., 2018). A study by Park and colleagues (Park et al., 2020) found that individuals receiving buprenorphine who also received a benzodiazepine prescription were more likely to have a fatal overdose. However this type of overdose was relatively rare and made up less than 1% of the state’s overdose fatalities (Park et al., 2020).
The current research also found that polysubstance use during treatment did not have a substantial effect on relapse or cravings. The study found one substance use–specific effect for cocaine, such that increased cocaine use during the first four weeks of treatment was associated with greater likelihood of relapse. However, this relationship appears to be confounded with opioid use as it dissipated once we limited to individuals without opioid use during the first four weeks of treatment. Previous research on cocaine use during MOUD treatment has revealed a complex relationship in need of further investigation (Cunningham et al., 2013; Demaria et al., 2000; Hser et al., 2014; Magura et al., 1998).
The exploratory trend analyses indicated that cannabis use significantly increased during the first four weeks of treatment, yet in regression analyses cannabis was not a significant predictor of these two core treatment outcomes. Substance use during the early phases of treatment may operate as “transitional coping mechanisms” for persons who use opioids to quell persistent cravings and withdrawal symptoms during induction, and intermittent cannabis use may be associated with a reduction in opioid positive urines and increased medication compliance (Church et al., 2001) or no effect on treatment outcomes (Lake & St. Pierre, 2020). Given medical and recreational cannabis laws in the United States, future research needs to determine the role of cannabis during OUD treatment.
Limitations to the study population have been noted (Lee et al., 2018). Briefly, the current study population is from a randomized control trial and not necessarily representative of the general population of persons with OUD, particularly given that the study recruited individuals at any point during admission from inpatient treatment centers. Additionally, we chose to use the primary outcome as operationalized in the primary study (Lee et al., 2018), and other measurements of opioid relapse should be considered in future research. Certain caveats should be taken into account when considering the current research questions. Notably, the current analyses did not separately measure methamphetamine use from amphetamines in Timeline Followback data collection, and the study population had low use of meth/amphetamines. Other studies have found methamphetamine use during MOUD treatment to be associated with poor treatment retention (Krawczyk et al., 2021; Tsui et al., 2020). Given the current rise in methamphetamine use across the nation (Palamar et al., 2020), and specifically among persons with OUD (Ellis et al., 2018), research should attempt to understand the treatment implications of polysubstance use involving methamphetamine and opioids. The study collected pretreatment polysubstance use retrospectively compared to prospective data collection of polysubstance use during the initial four weeks of treatment. While Timeline Followback data collection is a valid method of retrospective substance use (Robinson et al., 2014; Sobell & Sobell, 1992), we could have missed some pretreatment use due to recall bias. This study only followed individuals in MOUD treatment for 24 weeks. Other studies have noted differences in treatment outcomes when examining polysubstance use over longer follow-up periods (Cunningham et al., 2013; Demaria et al., 2000; Proctor et al., 2016). The current sample also had relatively low rates of polysubstance use (see Additional Polysubstance Use Variables, Table 1), which could limit our power to detect associations. Future research should continue to examine specific substances as well as measures of polysubstance use for longer treatment follow-up periods. Finally, we examined only relapse and craving as treatment outcomes. Future research should explore the role of polysubstance use on these outcomes as well as treatment outcomes such as retention, quality of life, and other patient-centered outcomes.
4.1. Conclusions
The current study found pretreatment polysubstance use, during the initial four weeks of MOUD treatment, was not a strong predictor of treatment outcomes in the study population. Given the magnitude of the current overdose crisis, future research should examine polysubstance use in real-world patient settings.
Supplementary Material
Highlights.
Polysubstance use was generally not associated with outcomes (relapse & craving).
Sedative use was marginally associated with treatment outcomes.
Cannabis use increased in the 1st four weeks but was not associated with outcomes.
Funding:
This research was supported by grants from the NIDA National Drug Abuse Treatment Clinical Trials Network (U10DA013046, UG1/U10DA013035, UG1/U10DA013034, U10DA013045, UG1/U10DA013720, UG1/U10DA013732, UG1/U10DA013714, UG1/U10DA015831, U10DA015833, HHSN271201200017C, and HHSN271201500065C). The first author (Bunting) was supported by K01DA053435, R25DA037190, and T32HS026120. Other support includes K23DA042140 (Tofighi). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institute on Drug Abuse.
COI/Disclosures:
Dr. Rotrosen is and has been, a Principal Investigator or a co-Investigator on studies for which support in the form of donated or discounted medication and/or funds has been, or will be, provided by Alkermes, Inc. (Vivitrol, extended-release injectable naltrexone), Indivior, Inc. (formerly Reckitt-Benckiser; Suboxone, buprenorphine/naloxone combination), and Braeburn Pharmaceuticals, Inc. (extended-release injectable buprenorphine); and digital therapeutics from Pear Therapeutics (smartphone apps ReSET and ReSET-O), CHESS Health (Connections smartphone app), and Data Cubed (smartphone apps SOAR and mSAPPORT). None of this support has gone, or will go, directly to him, rather to either NYU, or to NIDA/NIH, or to NIDA’s contractor Emmes, Inc. Dr. Rotrosen recently served in a non-paid capacity as a member of an Alkermes study Steering Committee. He has no relevant equity, intellectual property, paid consulting, travel or other arrangements with any of these entities.
Dr. McNeely declares intellectual property interests in the Substance Use Screening and Intervention Tool (SUSIT), which is in the public domain and unrelated to this work.
Dr. Lee has received in-kind study drug for recent and current NIDA-funded trials from Alkermes Inc and Indivior PLC. Dr. Lee has received a recent investigator sponsored study grant from Indivior PLC. Dr. Lee is a science advisor to Oar Health LLC.
Dr. Nunes received medication for studies from Alkermes (Vivitrol), Indivior (Suboxone, Sublocade), and Braeburn (CAM2038/Brixadi), received technology for studies from Pear Therapeutics, CHESS Health, served as an unpaid consultant to Alkermes, Braeburn/Camurus, Pear Therapeutics, and has been an investigator on studies by Braeburn Pharmaceuticals (CAM2038/Brixadi).
Dr. Tofighi is a consultant to Oar Health LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Society of Addiction Medicine. (2019). The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. Journal of Addiction Medicine, 14(25), 1–91. 10.1097/ADM.0000000000000633 [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, & Book SW (2015). Discontinuation of buprenorphine maintenance therapy: Perspectives and outcomes. Journal of Substance Abuse Treatment, 52, 48–57. 10.1016/j.jsat.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts KS, Chan G, McIlwraith F, Dietze P, Whittaker E, Burns L, & Alati R.(2016). Differences in polysubstance use patterns and drug-related outcomes between people who inject drugs receiving and not receiving opioid substitution therapies. Addiction, 111(7), 1214–1223. 10.1111/add.13339 [DOI] [PubMed] [Google Scholar]
- Bhalla IP, Stefanovics EA, & Rosenheck RA (2017). Clinical Epidemiology of single versus multiple substance use disorders: Polysubstance use disorder. Medical Care, 55, S24. 10.1097/MLR.0000000000000731 [DOI] [PubMed] [Google Scholar]
- Blondino CT, Gormley MA, Taylor DSDH, Lowery E, Clifford JS, Burkart B, Graves WC, Lu J, & Prom-Wormley EC (2020). The association of co-occurring substance use and the effectiveness of opiate treatment programs by intervention type: A systematic review. Epidemiologic Reviews, 42(1), 57–78. 10.1093/epirev/mxaa005 [DOI] [PubMed] [Google Scholar]
- Carroll KM (2014). Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Annals of the New York Academy of Sciences, 1327(1), 94–111. 10.1111/nyas.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. (2004). Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. treatment improvement protocol (TIP) series 40. . DHHS Publication No. (SMA) 04‐3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. [PubMed] [Google Scholar]
- Chan B, Freeman M, Ayers C, Korthuis PT, Paynter R, Kondo K, & Kansagara D.(2020). A systematic review and meta-analysis of medications for stimulant use disorders in patients with co-occurring opioid use disorders. Drug and Alcohol Dependence, 216, 108193. 10.1016/j.drugalcdep.2020.108193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church SH, Rothenberg JL, Sullivan MA, Bornstein G, & Nunes EV (2001). Concurrent substance use and outcome in combined behavioral and naltrexone therapy for opiate dependence. The American Journal of Drug and Alcohol Abuse, 27(3), 441–452. 10.1081/ADA-100104511 [DOI] [PubMed] [Google Scholar]
- Cunningham C, Edlund MJ, Fishman M, Gordon AJ, Jones HE, Kampman KM, Langleben D, Meyer M, Springer S, Woody G, Wright TE, & Wyatt S.(2020). The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. Journal of Addiction Medicine, 14(2S Suppl 1), 1–91. 10.1097/ADM.0000000000000633 [DOI] [PubMed] [Google Scholar]
- Cunningham CO, Giovanniello A, Kunins HV, Roose RJ, Fox AD, & Sohler NL (2013). Buprenorphine treatment outcomes among opioid-dependent cocaine users and non-users. The American Journal on Addictions, 22(4), 352–357. 10.1111/j.1521-0391.2013.12032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria PA, Sterling R, & Weinstein SP (2000). The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. American Journal on Addictions, 9(2), 145–153. 10.1080/10550490050173217 [DOI] [PubMed] [Google Scholar]
- Ellis MS, Kasper ZA, & Cicero TJ (2018). Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug and Alcohol Dependence, 193, 14–20. 10.1016/j.drugalcdep.2018.08.029 [DOI] [PubMed] [Google Scholar]
- Green TC, Black R, Serrano JMG, Budman SH, & Butler SF (2011). Typologies of prescription opioid use in a large sample of adults assessed for substance abuse treatment. PLOS ONE, 6(11), e27244. 10.1371/journal.pone.0027244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y-I, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, & Ling W.(2014). Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction (Abingdon, England), 109(1), 79–87. 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Underwood N, & Compton WM (2020). Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–17. Addiction (Abingdon, England), 115(2), 347–353. 10.1111/add.14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariisa M, Scholl L, Wilson N, Seth P, & Hoots B.(2019). Drug overdose deaths involving cocaine and psychostimulants with abuse potential—United States, 2003–2017. MMWR. Morbidity and Mortality Weekly Report, 68. 10.15585/mmwr.mm6817a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Williams AR, Saloner B, & Cerdá M.(2021). Who stays in medication treatment for opioid use disorder? A national study of outpatient specialty treatment settings. Journal of Substance Abuse Treatment, 126, 108329. 10.1016/j.jsat.2021.108329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake S St, & .Pierre M.(2020). The relationship between cannabis use and patient outcomes in medication-based treatment of opioid use disorder: A systematic review. Clinical Psychology Review, 82, 101939. 10.1016/j.cpr.2020.101939 [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, … Rotrosen J.(2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet (London, England), 391(10118), 309–318. 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bailey GL, Brigham GS, Cohen AJ, Fishman M, Ling W, Lindblad R, Shmueli-Blumberg D, Stablein D, May J, Salazar D, Liu D, & Rotrosen J.(2016). NIDA Clinical Trials Network CTN-0051, extended-release naltrexone vs. buprenorphine for opioid treatment (X:BOT): Study design and rationale. Contemporary Clinical Trials, 50, 253–264. 10.1016/j.cct.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Bohnert ASB, Blow FC, Gordon AJ, Ignacio RV, Kim HM, & Ilgen MA (2020). Polysubstance use and association with opioid use disorder treatment in the US Veterans Health Administration. Addiction, 116(1), 96–104. 10.1111/add.15116 [DOI] [PubMed] [Google Scholar]
- Link BG, & Phelan JC (2001). Conceptualizing stigma. 27, 363–385. 10.1146/annurev.soc.27.1.363 [DOI] [Google Scholar]
- Liu S, O’Donnell J, Gladden RM, McGlone L, & Chowdhury F.(2021). Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019–2020. MMWR. Morbidity and Mortality Weekly Report, 70. 10.15585/mmwr.mm7034a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, & Demsky SY (1998). Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction, 93(1), 51–60. 10.1046/j.1360-0443.1998.931516.x [DOI] [PubMed] [Google Scholar]
- Marsch LA, Stephens MAC, Mudric T, Strain EC, Bigelow GE, & Johnson RE (2005). Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Experimental and Clinical Psychopharmacology, 13(4), 293–302. 10.1037/1064-1297.13.4.293 [DOI] [PubMed] [Google Scholar]
- Martinotti G, Carli V, Tedeschi D, Di Giannantonio M, Roy A, Janiri L, & Sarchiapone M.(2009). Mono- and polysubstance dependent subjects differ on social factors, childhood trauma, personality, suicidal behaviour, and comorbid Axis I diagnoses. Addictive Behaviors, 34(9), 790–793. 10.1016/j.addbeh.2009.04.012 [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Gladden RM, Mattson CL, Hunter CT, & Davis NL (2020). Vital signs: Characteristics of drug overdose deaths involving opioids and stimulants — 24 states and the District of Columbia, January–June 2019. MMWR. Morbidity and Mortality Weekly Report, 69. 10.15585/mmwr.mm6935a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Han BH, & Keyes KM (2020). Trends in characteristics of individuals who use methamphetamine in the United States, 2015–2018. Drug and Alcohol Dependence, 213, 108089. 10.1016/j.drugalcdep.2020.108089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TW, Larochelle MR, Saitz R, Wang N, Bernson D, & Walley AY (2020). Associations between prescribed benzodiazepines, overdose death and buprenorphine discontinuation among people receiving buprenorphine. Addiction, 115(5), 924–932. 10.1111/add.14886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, & Polukhina N.(2016). Outcome predictors for patients receiving methadone maintenance treatment: Findings from a retrospective multi-site study. Journal of Substance Use, 21(6), 601–613. 10.3109/14659891.2015.1118564 [DOI] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors, 28(1), 154–162. 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Hoeppner BB, Weiss RD, Borodovsky J, Shaffer HJ, & Albanese MJ (2013). Benzodiazepine use during buprenorphine treatment for opioid dependence: Clinical and safety outcomes. Drug and Alcohol Dependence, 132(3), 580–586. 10.1016/j.drugalcdep.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-back: A technique for assessing self-reported alcohol consumption. In Litten In R. Z. & Allen JP (Eds.), Measuring alcohol consumption: Psychosocial and biological methods (pp. 41–72). Humana Press. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2021). Medications for Opioid Use Disorder (No. PEP20–02-01–006; Treatment Improvement Protocol (TIP) Series 63). Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Tsui JI, Mayfield J, Speaker EC, Yakup S, Ries R, Funai H, Leroux BG, & Merrill JO (2020). Association between methamphetamine use and retention among patients with opioid use disorders treated with buprenorphine. Journal of Substance Abuse Treatment, 109, 80–85. 10.1016/j.jsat.2019.10.005 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (2017). FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: Careful medication management can reduce risks. Available from: https://www.fda.gov/media/127688/download
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.