Abstract
Background
Studies suggest that high-flow nasal cannula (HFNC) and non-invasive ventilation (NIV) can prevent reintubation in critically ill patients with a low risk of extubation failure. However, the safety and effectiveness in patients at high risk of extubation failure are still debated. Therefore, we conducted a systematic review and meta-analysis to compare the efficacies of HFNC and NIV in high-risk patients.
Methods
We searched eight databases (MEDLINE, Cochrane Library, EMBASE, CINAHL Complete, Web of Science, China National Knowledge Infrastructure, Wan-Fang Database, and Chinese Biological Medical Database) with reintubation as a primary outcome measure. The secondary outcomes included mortality, intensive care unit (ICU) length of stay (LOS), incidence of adverse events, and respiratory function indices. Statistical data analysis was performed using RevMan software.
Results
Thirteen randomized clinical trials (RCTs) with 1457 patients were included. The HFNC and NIV groups showed no differences in reintubation (RR 1.10, 95% CI 0.87–1.40, I2 = 0%, P = 0.42), mortality (RR 1.09, 95% CI 0.82–1.46, I2 = 0%, P = 0.54), and respiratory function indices (partial pressure of carbon dioxide [PaCO2]: MD − 1.31, 95% CI − 2.76–0.13, I2 = 81%, P = 0.07; oxygenation index [P/F]: MD − 2.18, 95% CI − 8.49–4.13, I2 = 57%, P = 0.50; respiratory rate [Rr]: MD − 0.50, 95% CI − 1.88–0.88, I2 = 80%, P = 0.47). However, HFNC reduced adverse events (abdominal distension: RR 0.09, 95% CI 0.04–0.24, I2 = 0%, P < 0.01; aspiration: RR 0.30, 95% CI 0.09–1.07, I2 = 0%, P = 0.06; facial injury: RR 0.27, 95% CI 0.09–0.88, I2 = 0%, P = 0.03; delirium: RR 0.30, 95%CI 0.07–1.39, I2 = 0%, P = 0.12; pulmonary complications: RR 0.67, 95% CI 0.46–0.99, I2 = 0%, P = 0.05; intolerance: RR 0.22, 95% CI 0.08–0.57, I2 = 0%, P < 0.01) and may have shortened LOS (MD − 1.03, 95% CI − 1.86–− 0.20, I2 = 93%, P = 0.02). Subgroup analysis by language, extubation method, NIV parameter settings, and HFNC flow rate revealed higher heterogeneity in LOS, PaCO2, and Rr.
Conclusions
In adult patients at a high risk of extubation failure, HFNC reduced the incidence of adverse events but did not affect reintubation and mortality. Consequently, whether or not HFNC can reduce LOS and improve respiratory function remains inconclusive.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-023-01076-9.
Keywords: High-flow nasal cannula, Extubation failure, High-risk patient, Meta-analysis
Background
Due to the complexity and variability of conditions, patient life is sustained in Intensive Care Units (ICUs) using mechanical ventilation. Approximately 39.0%–72.0% of ICU patients require invasive mechanical ventilation, and reintubation after mechanical ventilation is considered a potentially adverse event [1–4]. Previous studies have shown that 10%–20% of extubated patients require reintubation, and that 20–30% have a high risk of extubation failure [4–6]. Reintubation is associated with a longer hospital stays, poor prognosis, higher healthcare costs, and more complications [6–8].
To reduce reintubation, a prophylactic high-flow nasal cannula (HFNC) and non-invasive mechanical ventilation (NIV) after extubation are used to improve oxygenation in patients at high-risk of extubation failure. HFNC delivers a heated, humidified, and adjustable air–oxygen mixture through a large-caliber nasal cannula. NIV assists the patient’s breathing by applying different levels of positive pressure to the airway through an oral or nasal mask, without endotracheal intubation or laryngeal mask airway insertion. Nava et al. [9] and Ferrer et al. [10] demonstrated that NIV helped to reduce respiratory failure, reintubation, and mortality after extubation in high-risk patients. However, NIV can cause many side effects, such as lung damage, gastric distension, mask discomfort, and even claustrophobia, conditions that are difficult for patients endure [11, 12]. Numerous studies have shown that HFNC is not inferior to NIV in preventing the reintubation of high-risk patients. In addition, HFNC was better tolerated, more comfortable, and resulted in fewer adverse events than NIV [13–15]. The international clinical practice guidelines indicate that when compared with HFNC, NIV is more effective in preventing reintubation. However, HFNC does not lead to adverse events among high-risk patients [16].
Therefore, we conducted a systematic review and meta-analysis based on randomized-controlled trials (RCTs) to evaluate the role of HFNC in preventing reintubation, mortality, and adverse events as well as improving respiratory function, and shortening ICU length of stay (LOS).
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17] checklist was used to report the systematic review and meta-analysis (Materials Additional file 1), and we registered the study with PROSPERO (CRD42022311969).
Search strategy
Two investigators (W-Q and P-Y) independently searched the MEDLINE, Cochrane Library, EMBASE, CINAHL Complete, Web of Science, China National Knowledge Infrastructure, Wan-Fang database, and Chinese Biological Medical Database for relevant studies. The retrieval dates ranged from the establishment of the database until February 28, 2022. The retrieval strategy combined non-invasive ventilation (‘niv’ or ‘nippv’ or ‘noninvasive ventilation’ or ‘non invasive positive pressure ventilation’ or ‘noninvasive positive pressure ventilation’ or ‘non invasive ventilation’) with high-flow nasal cannula(‘hhfnc’ or ‘hhfn’ or ‘hfnc’ or ‘hfnct’ or ‘high flow nasal cannula’ or ‘high flow nasal cannula oxygen therapy’ or ‘nasal high flow oxygen therapy’ or ‘high flow nasal cannulae’ or ‘humidified high flow nasal cannula therapy’). See Material Additional file 2, for example, Medline search.
Study selection
The following inclusion criteria were used: (1) patients at high risk of extubation failure who fulfilled at least one of the following [9, 10, 13, 16, 18–20]: age older than 65 years, underlying cardiac or respiratory disease, mechanical ventilation time > 7 days, airway patency problems including ineffective cough or excessive tracheobronchial secretion, an Acute Physiology and Chronic Health Evaluation II (APACHE II) score > 12 on extubation day, body mass index (BMI) > 30 kg/m2, and 2 or more comorbidities; (2) HFNC and NIV were used as interventions; (3) outcomes including, but not limited to, reintubation, mortality, LOS, the incidence of adverse events (abdominal distension, aspiration, facial injury, delirium, pulmonary adverse events, and intolerance), and respiratory function indices (partial pressure of carbon dioxide [PaCO2], oxygenation index [PaO2/FiO2, P/F], respiratory rate [Rr]); (4) RCTs as the study type; (5) and studies either in Chinese or English language.
The exclusion criteria included insufficient information, incomplete data; age < 18 years; repeated publications; and high-risk bias literature (Grade C).
Data extraction and quality assessment
Data were independently extracted by two researchers (W-Q and X-S), and any discrepancies were resolved by a third researcher (P-Y). The following data were extracted from the selected studies: first author, year of publication, country, disease type, sample sizes, gender, age, intervention measures, reintubation, mortality, LOS, adverse events, and respiratory function-related indices. The quality of each RCT that satisfied the criteria was examined using the Cochrane bias risk assessment tool (Cochrane 5.1.0) [21]. The bias risk was classified into three categories: Grade A, items are all low risk of bias; Grade C, items are all high risk of bias; and Grade B, items are not all high or low risk of bias. In addition, two researchers independently assessed the credibility of the pooled results using GRADE guidelines. The results of the meta-analysis were assessed using five downgrades and three upgrades, and the credibility of the results was classified as high, moderate, low, or very low.
Outcome measures
The primary outcome was reintubation. The secondary outcomes were mortality, LOS, the incidence of adverse events (abdominal distension, aspiration, facial injury, delirium, pulmonary adverse events, and intolerance), PaCO2, P/F, and Rr.
Statistical analysis
RevMan5.4.1 software was used to create forest maps and merge data. Dichotomous data, such as reintubation, mortality, and incidence of adverse events, are expressed as a risk ratios (RR) and 95% confidence intervals (CI). Continuous data, such as LOS and respiratory function-related indices, are expressed as a mean difference (MD) and 95% CI. A P < 0.05 was considered statistically significant.
We used the Cochran’s Q test and I2 test statistics to test the heterogeneity of the studies. A P > 0.05 and I2 < 50%, indicated low heterogeneity, and a fixed-effects model (FD) was used. A P < 0.05 or I2 > 50% indicated high, and a random-effects model (RD) was used. Subgroup analysis of each outcome was carried out by language (English versus Chinese), extraction method (conventional versus non-conventional), NIV parameter settings (fixed expiratory positive airway pressure [EPAP] and inspiratory positive airway pressure [IPAP] versus non-fixed), and HFNC flow rate (fixed versus non-fixed) for comparison between HFNC and NIV. At the same time, we performed sensitivity analysis by removing one study at a time to ensure the stability of the results. Funnel plots were used applied to detect publication bias in more than ten trials.
Results
Study selection and characteristics
After removing the duplicate articles, a total of 4787 of 6282 articles were available for analysis. Of the total, 97 articles were selected for full-text review after reading the titles and abstracts of the publications, and finally, 13 RCTs [13, 15, 22–32], including 1457 patients, were eventually included. The screening process is shown in Fig. 1. Of the 13 RCTs included, 6 were published in English [13, 15, 27, 30–32] and 7 in Chinese [22–26, 28, 29]. The included study objects included chronic obstructive pulmonary disease (COPD) [15, 23–28, 32], APACHEII > 12 [30], elderly patients (> 65) with sepsis [31], patients at risk of extubation failure [13], acute pancreatitis with respiratory distress syndrome [29], and hypoxemia after cardiac surgery [22]. The mean age in 9 of the RCTs was > 65 years [15, 23, 24, 26, 27, 30–32], and 5 of the RCTs < 65 years [13, 22, 25, 28, 29]. One RCT was identified as Grade A, and 12 RCTs were identified as Grade B (Table 1). Nine of the RCTs [13, 22–24, 27–31] were on routine standard extubation, and four [15, 25, 26, 32] were on unconventional extubation following the pulmonary infection control window (PIC).
Fig. 1.
The PRISMA flow diagram of selected studies. WOS: Web of Science, CNKI: China National Knowledge Infrastructure, CBM: Chinese Biological Medical Database
Table 1.
Basic characteristics of the study were included
| Study | Year | Region | Disease | Sample(n) | Sex (male/female) | Age (±s, years) | Intervention | Grade (risk of bias) | Included outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFNC | NIV | HFNC | NIV | HFNC | NIV | HFNC | NIV | ||||||
| Hernández et al | 2016 | France | High-risk patients | 290 | 314 | 186/104 | 202/112 | 64.6 ± 15.4 | 64.4 ± 15.8 | 37℃, 10L/min, 5L/min was increased until the patient felt unwell | IPAP and EPAP were adjusted to target a Rr of 25/ min and adequate gas exchange | A | (1)(2)(3)(4)(5)(6) |
| Hu et al | 2018 | China | AECOPD | 60 | 60 | 34/26 | 30/30 | NA | NA | 37℃, 40L/min, keep SpO2 > 92% | IPAP 10–12 cm H2O, EPAP 4–6 cmH2O | B | (1)(4)(5)(7) |
| Jiang et al | 2019 | China | AECOPD | 18 | 17 | 13/5 | 11/6 | 75.0 ± 5.1 | 71.0 ± 6.5 | 50L/min, keep SpO2 > 92% | 5–8cmH2O EPAP, keep SpO2 > 92% | B | (1)(3)(4)(5)(7) |
| Jing et al | 2019 | China | COPD | 22 | 20 | NA | NA | 77.4 ± 6.8 | 73.9 ± 6.9 | 37℃, keep SpO2 88%–92% | IPAP 10–12 cmH2O, EPAP 4–5cmH2O | A | (1)(2)(3)(4)(5)(6)(7) |
| Liu et al | 2019 | China | COPD | 44 | 43 | 24/20 | 22/21 | 64.1 ± 10.5 | 65.1 ± 9.7 | 20–40 L/min | IPAP 4 cmH2O, 5–10 min increased 2 cmH2O, keep 8–20cmH2O, EPAP 4–6cmH2O | B | (1)(2)(5)(7) |
| Shang et al | 2021 | China | APACHEII > 12 | 24 | 24 | 12/12 | 13/11 | 66.92 ± 4.58 | 67.7 ± 6.89 | 37℃, 40L/min, keep SpO2 > 90% | 10–12cmH2O IPAP, 4–6cmH2O EPAP | B | (1)(2)(3)(4)(5)(7) |
| Tan et al | 2020 | China | COPD | 44 | 42 | 27/17 | 24/19 | 68.4 ± 9.3 | 71.4 ± 7.8 | 37℃, 50L/min, keep SpO2 88%–92% | 8cmH2O IPAP, 4cmH2O EPAP | A | (1)(2)(3)(4)(5)(6)(7) |
| Tongyoo et al | 2021 | Thai-land | > 65 | 60 | 58 | NA | NA | > 65 | > 65 | 37℃, 30L/min, 10 min increased 5L/min until 50L/min | 8cmH2O IPAP, 5cmH2O EPAP, IPAP increased 2cmH2O per 10 min | A | (1) |
| Wang et al | 2021 | China | SAP with ARDS | 30 | 30 | 25/5 | 24/6 | 43 ± 7 | 41 ± 7 | 37℃, 40–50L/min, keep SpO2 > 95% | IPAP 5–12cmH2O, EPAP 0–5cmH2O | B | (1)(2)(3)(4)(5)(6)(7) |
| Xu et al | 2021 | China | COPD | 50 | 50 | 32/18 | 30/20 | 69.2 ± 6.13 | 68.3 ± 5.22 | 37℃, FiO2 30%–50%, 50 L/min, keep SpO2 88%–92% | EPAP 4–5mmH2O, IPAP 10–12mmH2O, keep SpO2 88%-92% | B | (1)(4) |
| Yang et al | 2015 | China | Post- cardiac surgery | 20 | 20 | 14/6 | 13/2 | 53.8 ± 8.9 | 52.9 ± 7.8 | 37℃, 45 L/min | IPAP 10–12cmH2O, EPAP 4–6 cmH2O | B | (1)(2)(3)(4)(5)(6)(7) |
| Yu et al | 2019 | China | COPD | 36 | 36 | 24/12 | 21/15 | 62.4 ± 10.1 | 63.5 ± 11.2 | 37℃, 30–60 L/min, FiO2 30%–80% | IPAP 10–14 cmH2O, EPAP 4–6 cmH2O | B | (1)(2)(4)(5)(6)(7) |
| Zhang et al | 2018 | China | COPD | 21 | 24 | 18/3 | 20/4 | 64.5 ± 5.3 | 66.1 ± 6.6 | 37℃, 40L/min, when SpO2 = 92%, change 30L/min | IPAP 5–15 cmH2O, EPAP 0–5cmH2O | B | (1)(2)(3)(4)(5)(6) |
HFNC: high-flow nasal cannula oxygen therapy, NIV: non-invasive ventilation, AECOPD: acute exacerbation of chronic obstructive pulmonary disease, COPD: chronic obstructive pulmonary disease, SAP: severe acute pancreatitis, ARDS: acute respiratory distress syndrome, IPAP: inspiratory positive airway pressure, EPAP: expiratory positive airway pressure, Reintubation rates
(1) Reintubation rates
(2) Mortality rate
(3) ICU length of stay
(4) Incidence of adverse events
(5) Partial pressure of carbon dioxide
(6) Oxygenation index
(7) Respiratory rate
The 13 included RCTs were assessed for risk of bias using the Cochrane risk assessment tool. Of the 13 total RCTs, 5 used computer number generators, 8 used the random table to ensure adequate random sequence generation, 6 described allocation concealment, 1 adopted the single-blind method, 5 did not adopt the blind method, and 7 did not know whether to apply the blind method. All the literature results and data were reported thoroughly. One RCT reported research findings selectively, and other sources of bias in the remaining 12 RCTs were unclear. Figure 2 shows a summary of the risk of bias. Figure 3 shows the risk map of bias. Blinding participants, interveners, and outcome measures in studies are challenging, and therefore, we classified most studies as having a high risk of bias, because they had not been blinded.
Fig. 2.
Risk of bias graph: each risk of bias item presented as percentages across studies
Fig. 3.
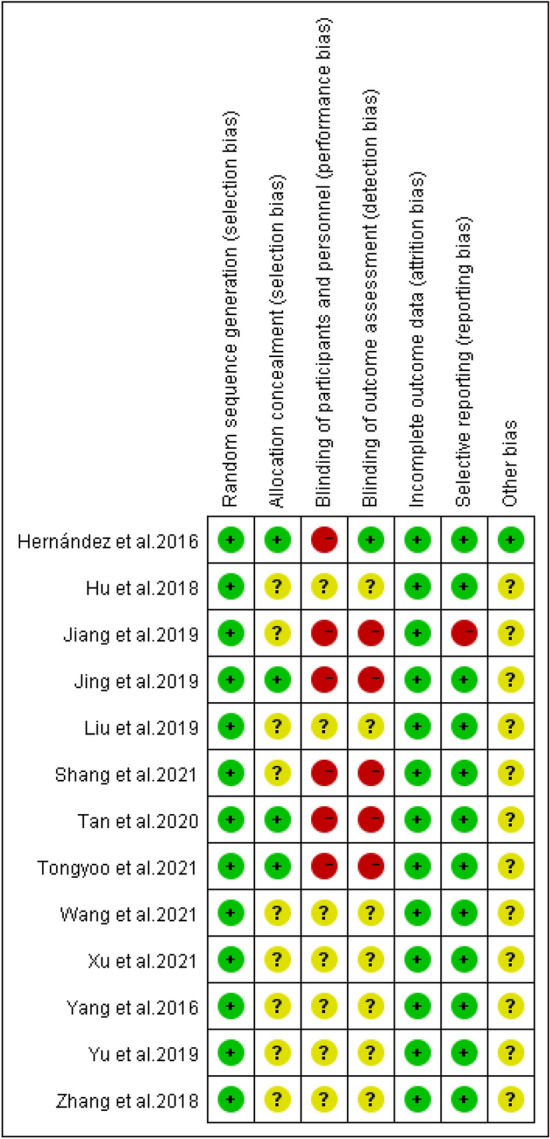
Risk of bias summary: judgements about each risk of bias item for each study. Green circles indicate a low risk of bias, yellow circles indicate an ambiguous risk of bias, and red circles indicate a high risk of bias
Efficacy of HFNC
Reintubation
The effect of HFNC versus NIV on the reintubation rate of patients at high risk for extubation failure was described in the 13 included RCTs. We found low heterogeneity across the studies, and the FD was performed. The meta-analysis revealed no significant difference in reintubation rates between the HFNC and NIV groups (n = 1457, I2 = 0%, RR = 1.10, 95%CI = 0.87–1.40, P = 0.42) Fig. 4.
Fig. 4.
Forest plots for reintubation between high-flow nasal cannula and non-invasive ventilation
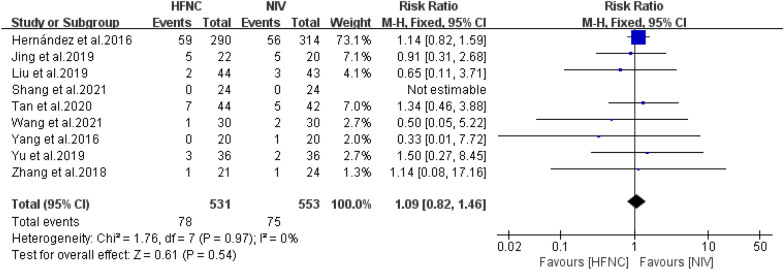
Mortality
Nine RCTs described the effect of HFNC versus NIV on the mortality of patients at high risk of extubation failure. Low heterogeneity was found across the studies, and FD was performed. The meta-analysis revealed no significant differences in mortality between the HFNC and NIV groups (n = 1084, I2 = 0%, RR = 1.09, 95%CI = 0.82–1.42, P = 0.54) Fig. 5.
Fig. 5.
Forest plots for mortality between high-flow nasal cannula and non-invasive ventilation
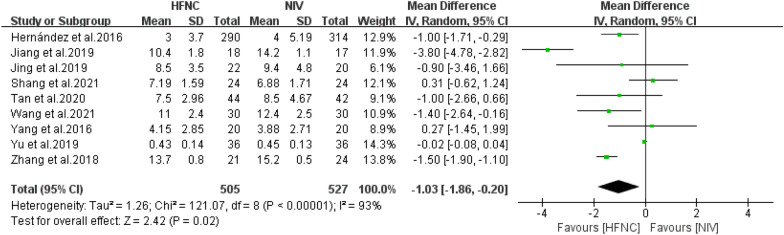
ICU length of stay
The effect of HFNC versus NIV on the LOS of patients at a high risk of extubation failure was described in 9 of the 13 included RCTs. As high heterogeneity was found across the studies, and RD was performed. As a result, we found that HFNC had a significant advantage over NIV (n = 1032, I2 = 93%, RR = − 1.03, 95%CI = − 1.86–0.20, P = 0.02) Fig. 6.
Fig. 6.
Length of ICU stay forest plot between high-flow nasal cannula and non-invasive ventilation
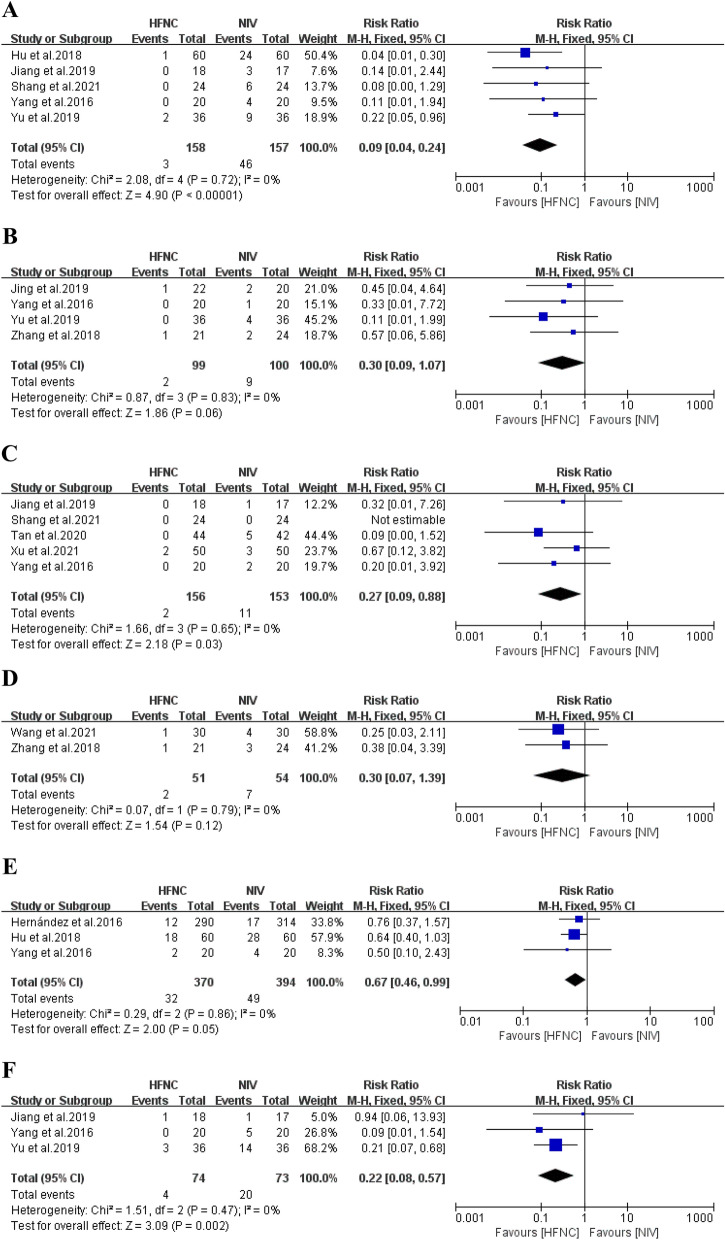
Incidence of adverse events
In 11 of the 13 included RCTs, the effect of HFNC versus NIV on the incidence of adverse events, including abdominal distension, aspiration, facial injury, delirium, pulmonary complications, and intolerance in patients at a high risk of extubation failure, was described. We found low heterogeneity across the studies, and FD was performed. A meta-analysis revealed that HFNC presented clear advantages over NIV in abdominal distension, facial injury, pulmonary complications, and intolerance (abdominal distension: n = 315, I2 = 0%, RR 0.09, 95% CI 0.04–0.24, P < 0.01; facial injury: n = 309, I2 = 0%, RR = 0.27, 95% CI 0.09–0.88, P = 0.03; pulmonary complications: n = 764, I2 = 0%, RR 0.67, 95% CI 0.46–0.09, P = 0.05; intolerance: n = 147, I2 = 0%, RR 0.22, 95% CI 0.08–0.57, P < 0.01). No significant differences in aspiration or delirium were found between the HFNC and NIV groups (aspiration: n = 199, I2 = 0%, RR 0.30, 95% CI 0.09–1.07, P = 0.06; delirium: n = 105, I2 = 0%, RR 0.30, 95% CI 0.07–1.39, P = 0.12) Fig. 7.
Fig. 7.
Forest plots for abdominal distension A, aspiration B, facial injury C, delirium D, pulmonary complications E, and intolerance F
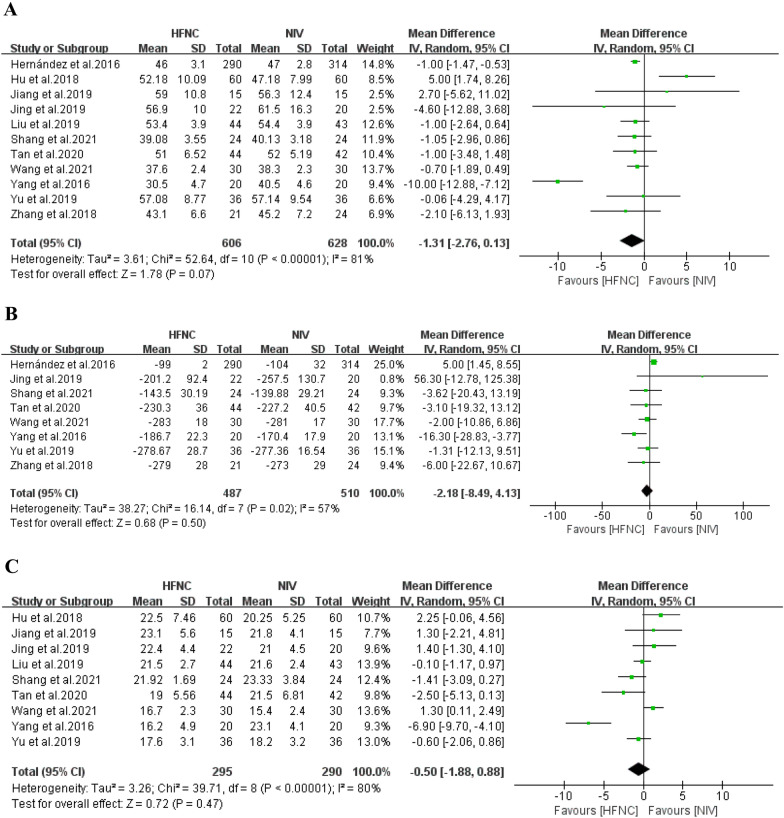
Respiratory function-related indices
In 11 of the 13 included RCTs, the effect of HFNC versus NIV on the PaCO2, P/F, and Rr of patients at high risk of extubation failure was described. Of the total, 11 RCTs described PaCO2, 8 described P/F, and 9 described Rr. High heterogeneity was found in all outcomes, and RD was performed. The results showed that HFNC provided several advantages over NIV (PaCO2: n = 1234, I2 = 81%, MD − 1.31, 95% CI − 2.76–0.13, P = 0.07; P/F: n = 997, I2 = 57%, MD − 2.18, 95% CI − 8.49–4.13, P = 0.50; Rr: n = 585, I2 = 80%, MD − 0.50, 95% CI − 1.88–0.88, P = 0.47) Fig. 8.
Fig. 8.
Forest plots for partial pressure of carbon dioxide A, oxygenation index B, and respiratory rate C
Subgroup analysis
Subgroup analyses of reintubation, mortality, LOS, adverse events, and respiratory function-related indices by language, extraction method, NIV parameter settings, and HFNC flow rate to compare HFNC versus NIV. Our results showed no significant differences or reductions in heterogeneity regarding reintubation, mortality, abdominal distension, aspiration, and Rr. In LOS, with exception of the Chinese-language group (n = 252, I2 = 96%, RR = − 1.32, 95%CI = − 2.56–− 0.09, P = 0.04), we did find statistically significant differences in the other subgroup analyses (English language: n = 780, I2 = 41%, RR = − 0.55, 95%CI = − 1.34–0.24, P = 0.17; conventional extubation standards: n = 911, I2 = 91%, RR = − 0.62, 95%CI = − 1.37–0.63, P = 0.11; unconventional extubation standards: n = 121, I2 = 88%, RR = − 2.48, 95%CI = − 5.22–0.24, P = 0.08; unfixed NIV setting: n = 128, I2 = 94%, RR = − 1.65, 95%CI = − 3.49—0.18, P = 0.08; fixed NIV setting: n = 904, I2 = 64%, RR = − 0.59, 95%CI = − 1.25–− 0.06, P = 0.08; unfixed HFNC flow rate: n = 225, I2 = 90%, RR = − 1.15, 95%CI = − 2.99–0.70, P = 0.08; fixed HFNC flow rates: n = 807, I2 = 95%, RR = − 0.85, 95%CI = − 1.85–− 0.15, P = 0.10). The P/F in the subgroup analysis of language revealed no significant heterogeneity in the pooled results (English language: n = 780, I2 = 25%, RR = 2.91, 95%CI = − 4.48—10.29, P = 0.44; Chinese-language: n = 217, I2 = 25%, RR = − 1.18, 95%CI = − 12.27–0.47, P = 0.11). However, significant changes in the pooled results for PaCO2 were found in the English-language group, fixed NIV parameter settings, and fixed HFNC flow rate (English language: n = 780, I2 = 0%, RR = − 1.01, 95%CI = − 1.46–− 0.56, P < 0.01; fixed NIV setting: n = 991, I2 = 84%, RR = − 2.19, 95%CI = − 3.89–− 0.48, P = 0.01; fixed HFNC flow rates: n = 807, I2 = 0%, RR = − 1.00, 95%CI = − 1.46–− 0.54, P < 0.01). See Additional file 3 for details.
Sensitivity analysis
Sensitivity analyses were performed by removing one study each for reintubation, mortality, LOS, adverse events, and respiratory function-related indices. Reintubation, mortality, LOS, and adverse events showed no significant changes in the pooled results or heterogeneity. However, heterogeneity was significantly reduced after excluding the study by Yang et al. for respiratory function-related indices (PaCO2: I2 = 40%, P = 0.14; P/F: I2 = 21%, P = 0.60; Rr: I2 = 57%, P = 0.80). See Table 2 for details.
Table 2.
Sensitivity analysis on effects of HFNC on respiratory function indices
| Outcome | Before sensitivity analysis | Remove study | After sensitivity analysis | ||||
|---|---|---|---|---|---|---|---|
| Effect estimate | P | I2 (%) | Effect estimate | P | I2 (%) | ||
| PaCO2 | − 1.31(− 2.76, 0.13) | 0.07 | 81 | Yang et al. 2016 | − 0.63(− 1.46, -0.20) | 0.14 | 40 |
|
PaO2/ FiO2 |
− 2.18(− 8.49, 4.13) | 0.50 | 57 | Yang et al. 2016 | 1.21(− 3.29, 5.71) | 0.60 | 21 |
| RR | − 0.50(− 1.88, 0.88) | 0.47 | 80 | Yang et al. 2016 | − 0.13(− 0.85, 1.11) | 0.80 | 57 |
Effect estimate: risk ratio (95% confidence interval)
PaCO2: partial pressure of carbon dioxide, P/F: oxygenation index, Rr: respiratory rate
Assessment of publication bias
A funnel plot analysis was performed for meta-analyses of more than ten studies to assess publication bias. The funnel plots of the reintubation were symmetrical, suggesting a slight publication bias. The funnel plot for PaCO2 showed asymmetry, indicating a risk of publication bias Fig. 9.
Fig. 9.
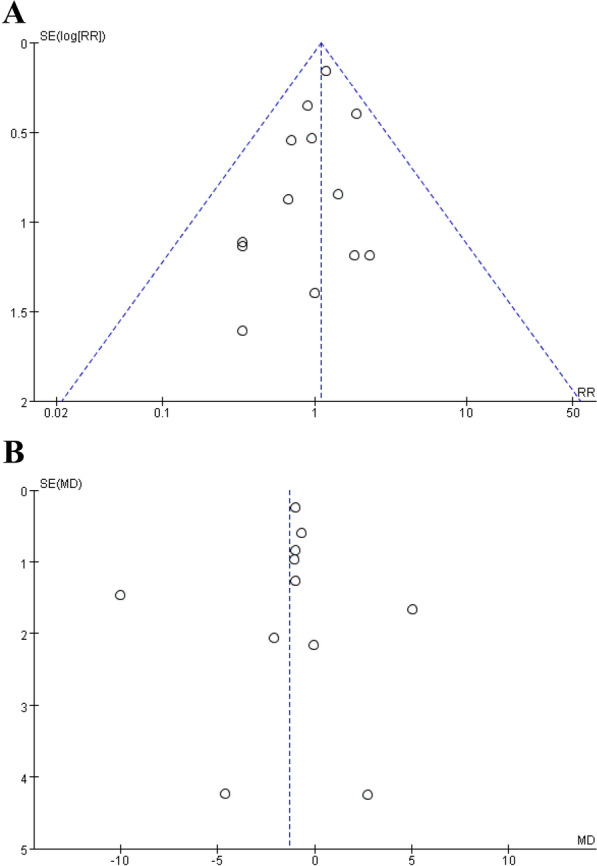
Funnel figure Reintubation A and partial pressure of carbon dioxide B
Certainty assessment
The certainty of the comprehensive assessment was evaluated using GRADE guidelines (Table 3). Heterogeneity and the risk of bias were the key factors that decreased the certainty of the evidence for most outcomes. Reintubation and facial injuries exhibited high certainty, abdominal distension, and pulmonary complications exhibited moderate certainty, and LOS exhibited low certainty.
Table 3.
Summary of findings based on the GRADE guidelines for HFNC
| Outcomes | Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Relative risk | Absolute effect | GRADE |
|---|---|---|---|---|---|---|---|---|---|
| Reintubation | 13 | No | No | No | No | Undetected | 1.10 (0.87–1.40) | 15/1000 (− 19–59) |
⨁⨁⨁⨁ High |
| Mortality | 9 | No | No | No | Serious | Undetected | 1.09 (0.82–1.46) | 12/1000 (− 24–62) |
⨁⨁⨁◯ Moderate |
| ICU stay | 9 | No | Seriousa | No | Serious | Undetected | – | − 1.03 (− 1.86–− 0.2) |
⨁⨁◯◯ Low |
| Abdominal distension | 5 | Seriousb | No | No | No | Undetected | 0.09 (0.04–0.24) | − 267/1000 (− 281–− 223) |
⨁⨁⨁◯ Moderate |
| Aspiration | 4 | Seriousb | No | No | No | Undetected | 0.30 (0.09–1.07) | − 63/1000 (− 82–6) |
⨁⨁⨁◯ Moderate |
| Facial injury | 5 | No | No | No | No | Undetected | 0.27 (0.09–0.88) | − 52/1000 (− 65–− 9) |
⨁⨁⨁⨁ High |
| Intolerance | 3 | Seriousb | No | No | No | Strongly suspectedc | 0.22 (0.08–0.57) | − 214/1000 (− 252–− 118) |
⨁⨁◯◯ Low |
| Pulmonary complications | 3 | No | No | No | No | Strongly suspectedc | 0.67 (0.46–0.99) | − 41/1000 (− 67–− 1) |
⨁⨁⨁◯ Moderate |
| Delirium | 2 | Seriousd | No | No | No | Strongly suspectedc | 0.30 (0.07–1.39) | − 91/1000 (− 121–51) |
⨁⨁◯◯ Low |
| Partial pressure of carbon dioxide | 11 | No | Seriouse | No | No | Strongly suspectedf | – | − 1.31 (− 2.76–0.13) |
⨁⨁◯◯ Low |
| Oxygenation index | 8 | No | No | No | Serious | Undetected | – | − 2.18 (− 8.49–4.13) |
⨁⨁⨁◯ Moderate |
| Respiratory rate | 9 | No | Seriousa | No | Serious | Undetected | – | − 0.5 (− 1.88–0.88) |
⨁⨁◯◯ Low |
aThe level of heterogeneity was high and was not explained completely
bMany biased items are uncertain
cNumber of included studies was too small
dDouble blinding was not conducted
eThe level of heterogeneity was more than 25%
fFunnel plots show publication bias
Discussion
HFNC is a relatively new respiratory support technology that consisting of an air/oxygen blender, an actively heated humidifier, and a nasal cannula delivering up to 60 L/min of heated, humidified oxygen [33]. HFNC not only washes out the anatomical dead space, delivers positive end expiratory pressure (PEEP), maintains constant inspired oxygen concentration (FiO2), and provides sufficient humidification, but also it offers exceptional comfort and good applicability in the clinic [33–35]. Furthermore, it has been shown to be superior to traditional oxygen therapy. However, whether HFNC is preferable to NIV remains unclear in some patients, notably those with a high risk of extubation failure. Consequently, we conducted a systematic review and meta-analysis of the efficacy of HFNC therapy in patients at high risk of extubation failure. The findings demonstrated that the reintubation, mortality, and improvement in respiratory function in high-risk patients using HFNC as a preventive intervention were not inferior to those of NIV when the same parameters were compared. Moreover, fewer adverse effects, such as facial injuries and abdominal distension, were observed in patients using HFNC.
We analyzed the data from a total of 13 RCTs and found that HFNC was not inferior to NIV in preventing reintubation in patients at a high risk of extubation failure. This result was consistent with the conclusions of Hernández et al. [13] and Zhou et al. [36] but inconsistent with the recommendations of the European Respiratory Society guidelines published by Oczkowski et al. [16]. A possible reasons for this inconsistency could be the differing inclusion criteria for the interventions. The RCTs in our study excluded combined HFNC and NIV interventions; instead, we included only RCTs incorporating simple HFNC or simple NIV. Therefore, we excluded the publications by Thille et al. [37] who included large-sample multicentre RCT of up to 631 patients with a high risk of extubation failure, accounting for a significant weight in the meta-analysis. As a result, our findings are at odds with the recommendations. For mortality, our results showed no difference between the two groups, which is consistent with the studies by Oczkowski et al. [16] and Chang et al. [38].
Although consistent with the findings of Oczkowski et al. [16] and Hernández et al. [13], we found substantial heterogeneity and low certainty that HFNC would shorten the LOS. In our study, when subgroup analysis was carried out according to language, extraction method, NIV parameter settings, and HFNC flow rate, we discovered that with exception of the Chinese-language group, HFNC still demonstrated an advantage NIV. The English-language group and other subgroup analyses showed no statistical differences between HFNC and NIV. This finding may be related to a higher risk of literature bias in the Chinese-language group. Therefore, further research is necessary to determine whether HFNC therapy has a beneficial effect on the LOS.
Regarding adverse events, we discovered that HFNC reduced the incidence of abdominal distension, facial injury, pulmonary problems, and intolerance. Delirium and the aspiration were not significantly different between the HFNC and NIV groups; however, the HFNC group displayed an optimization trend. This result is in agreement with the findings of Hernández et al. [13] and Stéphan et al. [39].
Moreover, HFNC had the same effect on respiratory function indices as NIV, and improved PaCO2, P/F, and RR, which is consistent with the findings of Hernández et al. [13] and Jing et al. [27]. However, the results of those three indices revealed high heterogeneity. We found heterogeneity of P/F associated with language using subgroup analysis. The results of the Chinese-language group revealed no statistically significant differences between the two interventions, whereas those of the English-language group demonstrated that NIV was superior to HFNC. The reason for this discrepancy might be that the period of HFNC use in the Chinese-language articles was longer than that in the English-language articles. We also found that with respect to PaCO2, HFNC outperformed NIV when the HFNC flow rate was adjusted to the patient's condition; however, when the NIV parameters were adjusted, the two interventions were equivalent. Therefore, the personalized settings for the HFNC flow rate appeared to have a more significant effect on reducing CO2 retention than those of the NIV parameters. In addition, we carried out a sensitivity analysis, and found that the heterogeneity was dramatically reduced, and the pooled effect did not change significantly when the study by Yang et al. [22] was removed. We hypothesize that this finding was related to the study population, which included patients undergoing cardiac surgery, whereas other studies included patients with respiratory diseases. In patients with cardiac disease, increased flow in HFNC produces a degree of PEEP that causes a reduction in inferior vena cava collapse, subsequently reducing cardiac preload [40–42]. However, HFNC mainly decreases CO2 rebreathing by washing out the anatomical dead space and providing a steady oxygen concentration, which has an effective respiratory management effect in patients with respiratory diseases, especially COPD [43, 44]. Therefore, we believe that the heterogeneity was probably due to the different mechanisms of action between patients.
To ensure the certainty of the evidence, we used the GRADE system to rate each outcome. We also performed subgroup analyses due to the inclusion of English-language and Chinese-language articles, conventional extubation (satisfying extubation criteria), and unconventional extubation (intentional extubation in the presence of PIC). However, we did not have uniform inclusion criteria for the flow and temperature of HFNC and the inspiratory and expiratory air pressures of NIV. It is worth mentioning that although we performed subgroup analyses to determine whether the NIV parameters or HFNC flow rate changed, we could not perform more accurate subgroup analyses (e.g., comparing both NIV parameter settings and HFNC flow rate change, no change in both NIV parameter settings and HFNC flow rate, change in NIV setting parameters, and change in HFNC flow rate), because we included only 13 publications. Although our subgroup analysis confirmed that the NIV parameter settings and HFNC flow rate did not affect most study outcomes, it is undeniable that this does not completely eliminate the effect of such confounding factors. Therefore, future studies will require more refined settings for NIV parameters and HFNC flow rates. In addition, we only compared how HFNC alone and NIV alone affected high-risk patients of extubation failure with no discussion on whether NIV combined with HFNC is preferable to either one alone. According to Thille et al. [6, 45], HFNC combined with NIV significantly outperformed HFNC alone in reducing reintubation in high-risk patients with extubation failure. Therefore, future studies should focus on the effect of HFNC combined with NIV on patient comfort, durability, and prevention. Finally, the large-sample and multicentre RCT by Hernández et al. [13] holds sizable weight in our meta-analysis, which results in partial reliance on their findings and certain restrictions on our findings.
Currently, it is unclear whether HFNC and NIV are equally effective in preventing reintubation and how to define and diagnose high-risk patients with extubation failure. Consequently, more reliable clinical data are required to validate this finding.
Conclusions
Among adult patients at high risk of extubation failure, HFNC is not inferior to NIV in preventing reintubation, mortality, and respiratory failure, and may shorten LOS, reduce adverse reactions, and increase patient comfort. However, heterogeneity of PaCO2, Rr, and LOS was evident in our results. Thus, a high-quality RCT is required to confirm whether HFNC therapy can provide an advantage in LOS and respiratory function in such patients.
Supplementary Information
Additional file 3: Figure S1. Subgroup analysis according to languages: (A) reintubation, (B) mortality, (C) ICU stay, (D)facial injury, (E) respiratory rate, (F) oxygenation index, (G) partial pressure of carbon dioxide. Figure S2. Subgroup analysis according to extubation method: (A) reintubation, (B) mortality, (C) ICU stay, (D)facial injury, (E) respiratory rate, (F) oxygenation index, (G) partial pressure of carbon dioxide. Figure S3. Subgroup analysis according to NIV parameter settings: (A) reintubation, (B) mortality, (C) ICU stay, (D) abdominal distension, (E) facial injury, (F) respiratory rate, (G) oxygenation index, (H) partial pressure of carbon dioxide. Figure S4. Subgroup analysis according to HFNC flow rate: (A) reintubation, (B) mortality, (C) ICU stay, (D) aspiration, (E) facial injury, (F) respiratory rate, (G) oxygenation index, (H) partial pressure of carbon dioxide.
Acknowledgements
None.
Abbreviations
- HFNC
High-flow nasal cannula oxygen therapy
- NIV
Non-invasive ventilation
- LOS
ICU length of stay
- PaCO2
Partial pressure of carbon dioxide
- P/F
Oxygenation index
- Rr
Respiratory rate
- RCT
Randomized-controlled trial
- RR
Risk ratio
- 95% CI
95% confidence interval
- MD
Mean difference
- PRISMA
The preferred reporting items for systematic reviews and meta-analysis
- SBT
Spontaneous breathing tests
- APACHE II
Acute physiology and chronic health evaluation II
- BMI
Body mass index
- FD
Random‐effects model
- RD
Random‐effects model
- COPD
Chronic obstructive pulmonary disease
- AECOPD
Acute exacerbation of chronic obstructive pulmonary disease
- SAP
Severe acute pancreatitis
- ARDS
Acute respiratory distress syndrome
- IPAP
Inspiratory positive airway pressure
- EPAP
Expiratory positive airway pressure
Author contributions
W-Q contributed to acquisition of data, statistical analysis, and wrote the paper; P-Y prepared figures and/or tables and acquired data; X-S contributed to the statistical analysis; L-L prepared figures and/or tables; C-L contributed to critical revision of the manuscript; Y-L designed the study and reviewed drafts of the paper. All authors read and approved the final manuscript.
Funding
This study was supported by grant from Fujian Provincial Health Technology Project (2021CXA017).
Availability of data and materials
All data generated or analyzed in this study are included in this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to the publication of this manuscript.
Competing interests
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liangwan Chen, Email: fjxhlwc@163.com.
Yanjuan Lin, Email: fjxhyjl@163.com.
References
- 1.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41(12):2712–2719. doi: 10.1097/CCM.0b013e318298a139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miltiades AN, Gershengorn HB, Hua M, Kramer AA, Li G, Wunsch H. Cumulative probability and time to reintubation in U.S. ICUs. Crit Care Med. 2017;45(5):835–42. doi: 10.1097/CCM.0000000000002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolley SE, Moss M, Needham DM, Caldwell E, Morris PE, Miller RR, et al. Point prevalence study of mobilization practices for acute respiratory failure patients in the United States. Crit Care Med. 2017;45(2):205–215. doi: 10.1097/CCM.0000000000002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161(5):1450–1458. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 5.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 6.Thille AW, Richard JC, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 7.Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26(5):502–509. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 9.Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33(11):2465–2470. doi: 10.1097/01.CCM.0000186416.44752.72. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173(2):164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 11.Smith TA, Davidson PM, Jenkins CR, Ingham JM. Life behind the mask: the patient experience of NIV. Lancet Respir Med. 2015;3(1):8–10. doi: 10.1016/S2213-2600(14)70267-X. [DOI] [PubMed] [Google Scholar]
- 12.Spicuzza L, Schisano M. High-flow nasal cannula oxygen therapy as an emerging option for respiratory failure: the present and the future. Ther Adv Chronic Dis. 2020;11:2040622320920106. doi: 10.1177/2040622320920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of Postextubation high-flow nasal cannula vs Noninvasive ventilation on reintubation and Postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 14.Longhini F, Pisani L, Lungu R, Comellini V, Bruni A, Garofalo E, et al. High-flow oxygen therapy after noninvasive ventilation interruption in patients recovering from hypercapnic acute respiratory failure: a physiological crossover trial. Crit Care Med. 2019;47(6):e506–e511. doi: 10.1097/CCM.0000000000003740. [DOI] [PubMed] [Google Scholar]
- 15.Tan D, Walline JH, Ling B, Xu Y, Sun J, Wang B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. 2020;24(1):489. doi: 10.1186/s13054-020-03214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oczkowski S, Ergan B, Bos L, Chatwin M, Ferrer M, Gregoretti C, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022 doi: 10.1183/13993003.01574-2021. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161(5):1530–1536. doi: 10.1164/ajrccm.161.5.9905102. [DOI] [PubMed] [Google Scholar]
- 19.De Jong A, Wrigge H, Hedenstierna G, Gattinoni L, Chiumello D, Frat JP, et al. How to ventilate obese patients in the ICU. Intensive Care Med. 2020;46(12):2423–2435. doi: 10.1007/s00134-020-06286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallverdú I, Calaf N, Subirana M, Net A, Benito S, Mancebo J. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998;158(6):1855–1862. doi: 10.1164/ajrccm.158.6.9712135. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Liu N, Hou X, Sun L, Wang H, Jin Q. Effects of high-flow nasal cannula and noninvasive positive pressure ventilation for the hypoxemia following cardiac surgery. J Cap Med Univ. 2016;37(05):664–671. [Google Scholar]
- 23.Zhang J, Wu F, Meng L, Zeng C, lu Y. A study on the effects and safety of sequential humidified high flow nasal cannula oxygenation therapy on the COPD patients after extubation. Natl Med J China. 2018;98(02):109–12. doi: 10.3760/cma.j.issn.0376-2491.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Hu S, Tang H, Fan X. Therapeutic efficacy of high-flow nasal cannula and noninvasive positive-pressure ventilation in AECOPD patients with various APACHE II scores after extubation. Chin Gen Pract. 2018;21(15):1790–1795. [Google Scholar]
- 25.Liu J, Yang S, Yuan J, Yang W, Meng S, Liu J, et al. Clinical study of sequential nasal high-flow oxygen therapy in mechanically ventilated patients with chronic obstructive pulmonary disease. Chin J Emerg Med. 2019;04:459–462. [Google Scholar]
- 26.Yu J, Zhang R, Zhu J, Liu H, Chen Q, Shao J. Clinical efficacy of sequential therapy from invasive ventilator to high-flow nasal cannula oxygen therapy of patients with acute exacerbations of chronic obstructive pulmonary disease. Chin J Crit Care Intensive Care Med. 2019;5(03):213–8. [Google Scholar]
- 27.Jing G, Li J, Hao D, Wang T, Sun Y, Tian H, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health. 2019;42(3):217–225. doi: 10.1002/nur.21942. [DOI] [PubMed] [Google Scholar]
- 28.Yu Z, Zhang R, Huang H, Lee J, Geng S. Efficacy and safety of humidified high flow nasal cannula in chronic obstructive pulmonary disease complicated with type 2 respiratory failure patients after extubation:a randomized controlled trial. Acad J Naval Med Univ. 2019;40(09):989–994. [Google Scholar]
- 29.Wang J, Meng L, Yan S. Sequential high-flow nasal cannula oxygen therapy after extubation for 30 patients with severe acute pancreatitis and acute respiratory distress syndrome. Chin J Crit Care Intensive Care Med. 2021;14(02):142–5. [Google Scholar]
- 30.Shang X, Wang Y. Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation. Ther Adv Respir Dis. 2021;15:17534666211004235. doi: 10.1177/17534666211004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tongyoo S, Tantibundit P, Daorattanachai K, Viarasilpa T, Permpikul C, Udompanturak S. High-flow nasal oxygen cannula vs. noninvasive mechanical ventilation to prevent reintubation in sepsis: a randomized controlled trial. Ann Intensive Care. 2021;11(1):135. doi: 10.1186/s13613-021-00922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu S, Liu X. Sequential treatment of chronic obstructive pulmonary disease concurrent with respiratory failure by high-flow nasal cannula therapy. Am J Transl Res. 2021;13(4):2831–2839. [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. 2019;64(6):735–742. doi: 10.4187/respcare.06718. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 35.Ricard JD, Roca O, Lemiale V, Corley A, Braunlich J, Jones P, et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020;46(12):2238–2247. doi: 10.1007/s00134-020-06228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Yao S, Dong P, Chen B, Xu Z, Wang H. Preventive use of respiratory support after scheduled extubation in critically ill medical patients-a network meta-analysis of randomized controlled trials. Crit Care. 2020;24(1):370. doi: 10.1186/s13054-020-03090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thille AW, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465–1475. doi: 10.1001/jama.2019.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CJ, Chiang LL, Chen KY, Feng PH, Su CL, Hsu HS. High-flow nasal cannula versus noninvasive positive pressure ventilation in patients with heart failure after extubation: an observational cohort study. Can Respir J. 2020;2020:6736475. doi: 10.1155/2020/6736475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 40.Roca O, Pérez-Terán P, Masclans JR, Pérez L, Galve E, Evangelista A, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care. 2013;28(5):741–746. doi: 10.1016/j.jcrc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015;60(10):1397–1403. doi: 10.4187/respcare.04028. [DOI] [PubMed] [Google Scholar]
- 42.Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39(2):247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 44.Roca O, Hernández G, Díaz-Lobato S, Carratalá JM, Gutiérrez RM, Masclans JR. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20(1):109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thille AW, Coudroy R, Nay MA, Gacouin A, Decavèle M, Sonneville R, et al. Non-invasive ventilation alternating with high-flow nasal oxygen versus high-flow nasal oxygen alone after extubation in COPD patients: a post hoc analysis of a randomized controlled trial. Ann Intensive Care. 2021;11(1):30. doi: 10.1186/s13613-021-00823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 3: Figure S1. Subgroup analysis according to languages: (A) reintubation, (B) mortality, (C) ICU stay, (D)facial injury, (E) respiratory rate, (F) oxygenation index, (G) partial pressure of carbon dioxide. Figure S2. Subgroup analysis according to extubation method: (A) reintubation, (B) mortality, (C) ICU stay, (D)facial injury, (E) respiratory rate, (F) oxygenation index, (G) partial pressure of carbon dioxide. Figure S3. Subgroup analysis according to NIV parameter settings: (A) reintubation, (B) mortality, (C) ICU stay, (D) abdominal distension, (E) facial injury, (F) respiratory rate, (G) oxygenation index, (H) partial pressure of carbon dioxide. Figure S4. Subgroup analysis according to HFNC flow rate: (A) reintubation, (B) mortality, (C) ICU stay, (D) aspiration, (E) facial injury, (F) respiratory rate, (G) oxygenation index, (H) partial pressure of carbon dioxide.
Data Availability Statement
All data generated or analyzed in this study are included in this article.