Abstract
Most CD8+ T cells in cultures of bovine mononuclear cells stimulated with staphylococcal enterotoxin C1 develop an unusual phenotype characterized by expression of activation molecule 3 (ACT3). This superantigen-dependent phenotype may be relevant to immunopathogenesis mediated by certain microbial toxins. The size and N-terminal sequence of immunoprecipitated ACT3 indicate that ACT3 is the bovine orthologue of CD26.
Superantigens (SAgs) bind to external regions of major histocompatibility complex class II molecules on antigen-presenting cells and Vβ regions of the T-cell receptor, causing abnormal signaling in both T cells and antigen-presenting cells. These stimulated cells release a variety of cytokines such as tumor necrosis factor alpha, interleukin 1 (IL-1), and gamma interferon that disturb the host immune system and contribute to the development of toxic shock syndrome, T-cell unresponsiveness, apoptosis, and deletion of T cells expressing the target Vβ (reviewed in references 3, 13, and 24). The staphylococcal enterotoxins (SEs) types A to E and G to M, produced by coagulase-positive staphylococci, are prototypic microbial SAgs (2, 10, 23). Although most studies investigating SAg effects have been performed on primates or rodents, we have shown that the ruminant immune system is also adversely affected by SAg exposure (5, 6). Coagulase-positive staphylococci, particularly Staphylococcus aureus, are pathogens with a broad host range. A significant number of animal isolates from certain infections such as bovine mastitis and canine pyoderma express SAgs, particularly type C SE (SEC) (4, 12, 22, 30). This suggests that SAg modulation of the animal immune response may contribute to the virulence and persistence of this organism.
We previously showed that, after 4 days of stimulation in vitro with SEC, a significant percentage of bovine CD8+ T cells expressed ACT3, recognized by monoclonal antibody (MAb) CACT114A (5). This was interesting because, previously, ACT3 expression had been noted mainly on thymocytes and CD4+ T cells (25). Our results suggested that ACT3 is an indicator of activation of CD8+ T cells by SAgs and that these cells might contribute to pathogenesis, immunosuppression, and cytotoxicity following exposure to SAg. Because CACT114A does not recognize a conserved determinant on the human or murine orthologue of ACT3, the identity of ACT3 has, until now, remained unknown.
In the present study, we extended our initial findings by assessing the impact of prolonged exposure to SEC on the expression of ACT3 by bovine T cells using previously described methods (5). Type 1 SEC (SEC1) was purified from S. aureus RN4220 (pMIN121), a recombinant harboring the secMNDON structural gene on a plasmid. Bovine peripheral blood mononuclear cells (PBMC) were isolated from healthy animals using standard techniques (5). PBMC were adjusted to 2 × 106 cells per ml in Dulbecco modified essential medium (supplemented with 13% bovine calf serum and antibiotics [penicillin G, 100 U/ml, and streptomycin, 100 μg/ml]) and incubated in plastic culture dishes (37°C, 5% CO2). For phenotypic analyses, cells were incubated with concanavalin A (ConA; 5 μg/ml) or SEC1 (0.1 μg/ml) for 4 or 7 days. An equal volume of fresh medium was added as a supplement to the 7-day cultures on day 4. Cell phenotypes were analyzed by multiple-color flow cytometric analyses (5). Isotype-specific goat anti-mouse immunoglobulins conjugated to fluorescein isothiocyanate, phycoerythrin, or TRI-COLOR (Caltag Laboratories, Burlingame, Calif.) were used as second-step reagents. Flow cytometric data were acquired with a FACScalibur flow cytometer operated with CellQuest software (Becton Dickinson Biosciences, San Jose, Calif.).
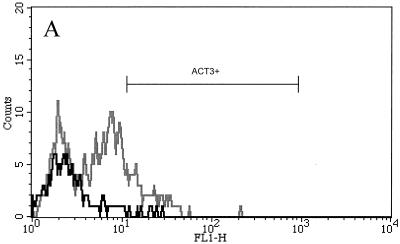
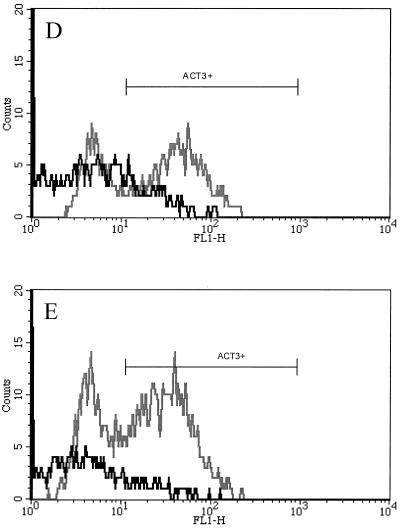
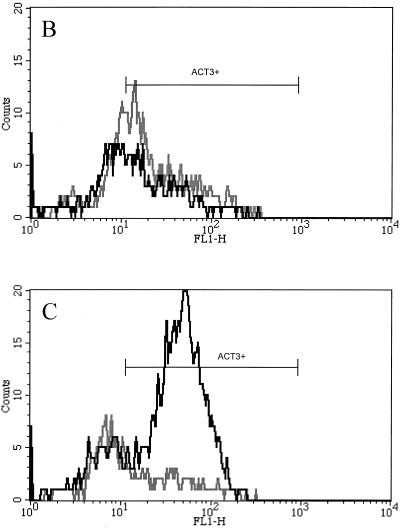
Consistent with our prior results (5), 56% ± 7% of CD8+ T cells expressed low to moderate levels of ACT3 after 4 days of stimulation with SEC1 (Fig. 1B). However, expression became even more pronounced with continued stimulation, and after 7 days, 72% ± 5% of CD8+ T cells expressed ACT3, mostly at very high levels (Fig. 1C). Unlike the effect seen for ConA-stimulated cultures, in which CD4+ T cells strongly expressed ACT3 (Fig. 1D and E), only a small percentage of ACT3+ T cells were CD4+ following stimulation with SEC1 (Fig. 1B and C). ConA stimulation did not induce strong ACT3 expression by CD8+ T cells. After 7 days, the number of ACT3+ CD8+ T cells in these control cultures was minimal (Fig. 1E).
FIG. 1.
Representative flow cytometric profiles of bovine lymphocytes labeled with mouse anti-ACT3 (CACT114A MAb) and anti-CD4 (CACT138A MAb) or anti-CD8 (CACT80C MAb). Bovine PBMC were isolated and stimulated with SEC1 or ConA for 4 or 7 days. In prior experiments, untreated cultures did not express significantly elevated levels of ACT3 (results not shown). (A to D) Cells from untreated control prior to stimulation (A), from 4-day culture with SEC1 (B), from 7-day culture with SEC1 (C), from 4-day culture with ConA (D), and from 7-day culture with ConA (E). The black lines indicate CD8+-T-cell populations, while the gray lines indicate CD4+-T-cell populations colabeled with anti-ACT3 (CACT114A). FL1-H, fluorescence intensity in fluorescence channel 1.
The unexpected association of ACT3 expression with SAg activation of CD8+ T cells prompted us to determine the identity of ACT3. In the course of our investigation, we found that continuous culture with IL-2 or IL-15 could induce maximal expression of ACT3 on both αβ and γδ T cells (data not shown). Therefore, CD3+ T cells were harvested by sorting with a Vantage SE fluorescence-activated cell sorter (Becton Dickinson Biosciences) following PBMC stimulation with ConA for 4 days. The purified CD3+ cells were cultured for 4 days in Dulbecco modified essential medium containing 10% conditioned medium and IL-2 (10 ng/ml; R&D Systems Inc., Minneapolis, Minn.) and harvested for immunoprecipitation following confirmation of the expression of ACT3 by flow cytometry.
Immunoprecipitation was performed with CACT114A and protein G-agarose (Roche Diagnostics Co., Indianapolis, Ind.) using a modification of a protocol recommended by the manufacturer. Briefly, the cultured cells were harvested by centrifugation (500 × g for 10 min), washed once with modified Dulbecco's phosphate-buffered saline (DPBS; 0.14 M NaCl, 8 mM Na2HPO4, 2 mM KH2PO4, 1 mM KCl [pH 7.4]). Then 5 × 107 cells were lysed with 1 ml of lysis buffer (DPBS containing 1% NP-40 [Roche Diagnostics Co.] and Complete Mini EDTA-free protease inhibitor cocktail [Roche Diagnostics Co.]) at 4°C for 1 h. Cell lysates were subjected to centrifugation at 16,000 × g for 10 min to remove cell debris. The supernatants were incubated with purified CACT114A (5 μg per ml of supernatant) at room temperature for 1 h with rocking. After addition of 50 μl of a protein G-agarose bead suspension (50%) per ml, incubation was continued overnight with continuous rocking. The beads were washed five times with DPBS and resuspended in 2× gel loading buffer. After being heated (100°C for 3 min), proteins were resolved on sodium dodecyl sulfate–12% polyacrylamide gel slabs as described previously (14) and stained with Coomassie blue.
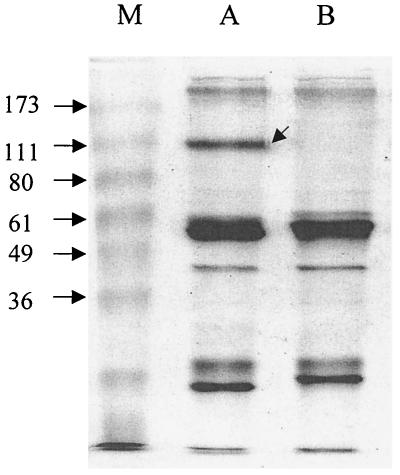
Figure 2 shows a comparison of proteins recovered from cell lysates immunoprecipitated with CACT114A or an irrelevant isotype control MAb. A unique band of protein obtained using CACT114A had an apparent size of 110 kDa. This is very close to the size (115 kDa) of previously immunoprecipitated putative ACT3 protein obtained using MAbs (detecting the bovine workshop cluster 10 antigen) that clustered in reactivity patterns with CACT114A in analyses by researchers in international workshops on ruminant leukocyte antigens reported by Sopp et al. (28) and Naessens and Hopkins (18).
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of immunoprecipitated PBMC lysates. Lane M, BenchMark prestained protein ladder (Life Technologies, Rockville, Md.); lane A, CACT114A (anti-ACT3)-immunoprecipitated proteins; lane B, ColiS169B (isotype control)-immunoprecipitated proteins. The arrow in lane A indicates the location of the protein submitted for N-terminal sequencing.
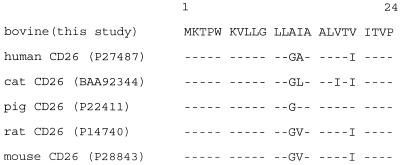
For N-terminal amino acid sequence analysis, the immunoprecipitated proteins resolved by electrophoresis were transferred to a polyvinylidene difluoride membrane (Immobilon-PSQ; Millipore Co., Bedford, Mass.), stained with Coomassie blue, and excised. N-terminal amino acid analysis of the unique band was performed by automated Edman degradation using a PE Biosystems Procise 491 instrument. Unambiguous sequence results for the first 24 residues showed that this region of ACT3 is nearly identical to the residues of CD26 from several species (Fig. 3). In addition, the sizes reported for various orthologues of CD26 (105 to ∼130 kDa) (21, 33, 34) are consistent with the size (110 kDa) of the protein immunoprecipitated in this study.
FIG. 3.
Alignment of the N-terminal amino acid sequences of CD26 molecules from several species. Identical amino acid residues are indicated by a dash. GenBank accession numbers (http://www.ncbi.nlm.nih.gov/Entrez) are shown in parentheses.
CD26 is highly conserved among different species. cDNA sequences encoding human, cat, mouse, and rat CD26 show a high degree of conservation (15, 19, 21, 32). CD26 is a highly glycosylated membrane protein constitutively expressed on epithelial cells of various tissues, as well as on various hematopoietic cell types (9, 11, 34). Interestingly, its tissue distribution is comparable to that reported for ACT3, which is found on the intestinal epithelium and in several immune system tissues, including the tonsils and lymph nodes (28).
The identification of bovine ACT3 as CD26 raises several important issues for SAg-induced pathogenesis in bovines, as well as for other species. In humans, the expression of CD26 increases on highly activated and proliferating T cells (7, 8, 17, 28). CD26 functions as an ectoenzyme, dipeptidyl-peptidase IV (DPP IV), which cleaves N-terminal dipeptides from polypeptides with either l-proline or l-arginine at the penultimate position (11). Although a precise physiological role for DPP IV has not yet been established, many cytokines and lymphokines such as IL-1β, IL-2, IL-6, and tumor necrosis factor alpha have DPP IV-susceptible bonds, implying that CD26 may regulate the activities of certain cytokines (1). CD26 also interacts with CD45 and adenosine deaminase, both of which have a role in signaling (16, 34).
SAgs are well known to possess immunosuppressive activities. For example, patients with toxic shock syndrome have depressed immunoglobulin production and depleted levels of subpopulations expressing reactive Vβs (3). Furthermore, an in vivo study using SAg-primed mice showed that a nonresponsive or nonproliferative state of anergy occurred (27). Some immunosuppressive responses to SAgs are known to be mediated by CD8+ T cells, which may act as suppressor T cells and have immunoregulatory functions mediated by direct lysis or by secretion of cytokines (35). SAg-activated CD8+ T cells can induce CD4+ T-cell apoptosis (20) and down-regulate immunoglobulin responses by removing activated B cells via a CD95-dependent pathway (31). Similar effects have been observed in bovine PBMC cultures stimulated with SEC, in which a reversal of the ratio of CD4 to CD8 T cells coincides with expression of CD26 (ACT3) on the bovine CD8+ T cells (6). Therefore, based on these combined observations and the known association of CD26 with cellular activation, one may propose that the CD8+ CD26+ T cells induced by SEC stimulation might contribute to immunosuppression in the bovine host. Whether CD26 itself is an effector molecule or is involved indirectly as an indicator of highly activated immunosuppressive CD8+ T cells remains to be determined by future investigations.
One may predict that staphylococcal isolates expressing SAgs possess a selective advantage in which induction of immunosuppresssion allows them to persist and potentially cause infectious diseases of humans and animals. One animal infectious disease frequently associated with S. aureus is bovine mastitis, which increases in incidence for animals experiencing states of immunosuppression (29). Interestingly, a link between staphylococcal mastitis and the presence of activated mammary gland CD8+-T-cell phenotypes has been established (26). Despite this circumstantial evidence, it remains to be determined whether SAgs are contributing factors since there is currently no direct evidence that they predispose animals to mastitis.
Nucleotide sequence accession number. The ACT3 N-terminal sequence data reported in this paper will appear in the SwissProt protein data bank under the accession number P81425.
Acknowledgments
This work was supported by USDA NRICGP grants 99-35201-8581 (G.A.B) and 99-3504-8556 (W.C.D), USDA WNV grants 00144-0182085 (W.C.D.) and 9902050-0183734 (W.C.D.), PHS grants AI28401 (G.A.B.) and P20-RR15587 (G.A.B), the United Dairymen of Idaho (G.A.B), and the Idaho Agricultural Experiment Station (G.A.B).
We thank Laurey Steinke at the Protein Structure Core Facility, Department of Biochemistry, University of Nebraska, for N-terminal amino acid sequencing. Katarzyna Dziewanowska is acknowledged for providing technical advice.
REFERENCES
- 1.Ansorge S, Schon E, Kunz D. Membrane-bound peptidases of lymphocytes: functional implications. Biomed Biochim Acta. 1991;50:799–807. [PubMed] [Google Scholar]
- 2.Bohach G A. Staphylococcal enterotoxins B and C. Structural requirements for superantigenic and entertoxigenic activities. Prep Biochem Biotechnol. 1997;27:79–110. doi: 10.1080/10826069708000072. [DOI] [PubMed] [Google Scholar]
- 3.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 4.Edwards V M, Deringer J R, Callantine S D, Deobald C F, Berger P H, Kapur V, Stauffacher C V, Bohach G A. Characterization of the canine type C enterotoxin produced by Staphylococcus intermedius pyoderma isolates. Infect Immun. 1997;65:2346–2352. doi: 10.1128/iai.65.6.2346-2352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferens W A, Davis W C, Hamilton M J, Park Y H, Deobald C F, Fox L, Bohach G. Activation of bovine lymphocyte subpopulations by staphylococcal enterotoxin C. Infect Immun. 1998;66:573–580. doi: 10.1128/iai.66.2.573-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferens W A, Goff W L, Davis W C, Fox L K, Deobald C, Hamilton M J, Bohach G A. Induction of type 2 cytokines by a staphylococcal enterotoxin superantigen. J Nat Toxins. 1998;7:193–213. [PubMed] [Google Scholar]
- 7.Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J Immunol. 1987;138:1346–1350. [PubMed] [Google Scholar]
- 8.Fox D A, Hussey R E, Fitzgerald K A, Acuto O, Poole C, Palley L, Daley J F, Schlossman S F, Reinherz E L. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984;133:1250–1256. [PubMed] [Google Scholar]
- 9.Hegen M, Camerini D, Fleischer B. Function of dipeptidyl peptidase IV (CD26, Tp103) in transfected human T cells. Cell Immunol. 1993;146:249–260. doi: 10.1006/cimm.1993.1024. [DOI] [PubMed] [Google Scholar]
- 10.Jarraud S, Peyrat M A, Lim A, Tristan A, Bes M, Mougel C, Etienne J, Vandenesch F, Bonneville M, Lina G. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J Immunol. 2001;166:669–677. doi: 10.4049/jimmunol.166.1.669. [DOI] [PubMed] [Google Scholar]
- 11.Kameoka J, Tanaka T, Nojima Y, Schlossman S F, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 12.Kenny K, Reiser R F, Bastida-Corcuera F D, Norcross N L. Production of enterotoxins and toxic shock syndrome toxin by bovine mammary isolates of Staphylococcus aureus. J Clin Microbiol. 1993;31:706–707. doi: 10.1128/jcm.31.3.706-707.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krakauer T. Immune response to staphylococcal superantigens. Immunol Res. 1999;20:163–173. doi: 10.1007/BF02786471. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Marguet D, Bernard A M, Vivier I, Darmoul D, Naquet P, Pierres M. cDNA cloning for mouse thymocyte-activating molecule. A multifunctional ecto-dipeptidyl peptidase IV (CD26) included in a subgroup of serine proteases. J Biol Chem. 1992;267:2200–2208. [PubMed] [Google Scholar]
- 16.Morimoto C, Schlossman S F. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto C, Torimoto Y, Levinson G, Rudd C E, Schrieber M, Dang N H, Letvin N L, Schlossman S F. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989;143:3430–3439. [PubMed] [Google Scholar]
- 18.Naessens J, Hopkins J. Introduction and summary of workshop findings. Vet Immunol Immunopathol. 1996;52:213–235. [Google Scholar]
- 19.Nishimura Y, Miyazawa T, Ikeda Y, Izumiya Y, Nakamura K, Sato E, Mikami T, Takahashi E. Molecular cloning and sequencing of a cDNA encoding the feline T-cell activation antigen CD26 homologue. Immunogenetics. 1999;50:366–368. doi: 10.1007/s002510050616. [DOI] [PubMed] [Google Scholar]
- 20.Noble A, Pestano G A, Cantor H. Suppression of immune responses by CD8 cells. I. Superantigen-activated CD8 cells induce unidirectional Fas-mediated apoptosis of antigen-activated CD4 cells. J Immunol. 1998;160:559–565. [PubMed] [Google Scholar]
- 21.Ogata S, Misumi Y, Ikehara Y. Primary structure of rat liver dipeptidyl peptidase IV deduced from its cDNA and identification of the NH2-terminal signal sequence as the membrane-anchoring domain. J Biol Chem. 1989;264:3596–3601. [PubMed] [Google Scholar]
- 22.Orden J A, Goyache J, Hernandez J, Domenech A, Suarez G, Gomez-Lucia E. Detection of enterotoxins and TSST-1 secreted by Staphylococcus aureus isolated from ruminant mastitis. Comparison of ELISA and immunoblot. J Appl Bacteriol. 1992;72:486–489. doi: 10.1111/j.1365-2672.1992.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 23.Orwin P M, Leung D Y, Donahue H L, Novick R P, Schlievert P M. Biochemical and biological properties of staphylococcal enterotoxin K. Infect Immun. 2001;69:360–366. doi: 10.1128/IAI.69.1.360-366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papageorgiou A C, Acharya K R. Microbial superantigens: from structure to function. Trends Microbiol. 2000;8:369–375. doi: 10.1016/s0966-842x(00)01793-5. [DOI] [PubMed] [Google Scholar]
- 25.Park Y H, Fox L K, Hamilton M J, Davis W C. Bovine mononuclear leukocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J Dairy Sci. 1992;75:998–1006. doi: 10.3168/jds.S0022-0302(92)77842-4. [DOI] [PubMed] [Google Scholar]
- 26.Park Y H, Fox L K, Hamilton M J, Davis W C. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet Immunol Immunopathol. 1993;36:137–151. doi: 10.1016/0165-2427(93)90103-b. [DOI] [PubMed] [Google Scholar]
- 27.Rellahan B L, Jones L A, Kruisbeek A M, Fry A M, Matis L A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990;172:1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sopp P, Howard C J, Parsons K R. A new non-lineage specific antigen with an Mr of 115 kDa and 39 kDa present on bovine leukocytes identified by monoclonal antibodies within BoWC10. Vet Immunol Immunopathol. 1993;39:209–215. doi: 10.1016/0165-2427(93)90183-5. [DOI] [PubMed] [Google Scholar]
- 29.Sordillo L M, Shafer-Weaver K, DeRosa D. Immunobiology of the mammary gland. J Dairy Sci. 1997;80:1851–1865. doi: 10.3168/jds.S0022-0302(97)76121-6. [DOI] [PubMed] [Google Scholar]
- 30.Stephan R, Annemuller C, Hassan A A, Lammler C. Characterization of enterotoxigenic Staphylococcus aureus strains isolated from bovine mastitis in north-east Switzerland. Vet Microbiol. 2001;78:373–382. doi: 10.1016/s0378-1135(00)00341-2. [DOI] [PubMed] [Google Scholar]
- 31.Stohl W, Lynch D H, Starling G C, Kiener P A. Superantigen-driven, CD8+ T cell-mediated down-regulation: CD95 (Fas)-dependent down-regulation of human Ig responses despite CD95-independent killing of activated B cells. J Immunol. 1998;161:3292–3298. [PubMed] [Google Scholar]
- 32.Tanaka T, Camerini D, Seed B, Torimoto Y, Dang N H, Kameoka J, Dahlberg H N, Schlossman S F, Morimoto C. Cloning and functional expression of the T cell activation antigen CD26. J Immunol. 1992;149:481–486. [PubMed] [Google Scholar]
- 33.Vivier I, Marguet D, Naquet P, Bonicel J, Black D, Li C X, Bernard A M, Gorvel J P, Pierres M. Evidence that thymocyte-activating molecule is mouse CD26 (dipeptidyl peptidase IV) J Immunol. 1991;147:447–454. [PubMed] [Google Scholar]
- 34.von Bonin A, Huhn J, Fleischer B. Dipeptidyl-peptidase IV/CD26 on T cells: analysis of an alternative T-cell activation pathway. Immunol Rev. 1998;161:43–53. doi: 10.1111/j.1600-065x.1998.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 35.Vukmanovic-Stejic M, Thomas M J, Noble A, Kemeny D M. Specificity, restriction and effector mechanisms of immunoregulatory CD8 T cells. Immunology. 2001;102:115–122. doi: 10.1046/j.1365-2567.2001.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]