Abstract
Background
Breast cancer (BC) is the fifth most prevalent cause of cancer-related deaths in Iran. Given that the role of whole-diet on cancer risk is important, this study aimed to assess the association of MedDQI and breast cancer risk.
Methods
This hospital-based case-control study was performed on 150 women with pathologically confirmed breast cancer within the period of less than 3 months. Controls were 150 apparently healthy that were matched by age. Dietary data was collected using a validated questionnaire. To examine participants’ adherence to MedDQI, the MedDQI was created according to foods and nutrients highlighted or minimized in the MedDQI construction.
Results
After adjusting for possible confounders, participants in the highest quartile of the MedDQI score had 55% lower odds of breast cancer than women in the bottom quartile (OR: 0.45, 95% CI: 0.21, 0.94, P trend: 0.02). Stratified analysis by menopausal status showed such association in postmenopausal women (OR: 0.24, 95% CI: 0.07, 0.8, P trend: 0.055) after controlling for age and energy intake.
Conclusion
The results showed an inverse association between adherence to the MedDQI and risk of breast cancer among Iranian women. More prospective studies are needed to confirm our results.
Keywords: Breast cancer, Mediterranean dietary quality index, Case-control, MedDQI, Risk
Introduction
Breast cancer (BC), the second-high prevalent cancer, is spreading around the world, especially in developing countries. Based on GLOBOCAN statistics for 2018, about 11.6% of women were recognized with breast cancer that year, with 626,679 associated deaths [1]. In Iran, BC is the fifth most prevalent cause of cancer-related deaths, including 24.4% of all cancers with a standard age rate (ASR) of 23.1 per 100,000 [2]. Several risk factors counting family history of BC, reproductive factors, and environmental factors contribute to the development of BC [3, 4]. The management and prognosis of metastatic breast cancer involve several metabolic aspects, including metabolic syndrome and obesity, glucose metabolism, and microRNA modulation [5]. Among the environmental factors, diet as a modifiable factor can play an important role in the development of BC [6], however, studies in this term are limited that according to some recent studies, adherence to the Mediterranean diet has been related to reducing risk of diseases such as cancers, hypertension, cardiovascular diseases, obesity, and diabetes [7–11]. Particularly, numerous studies found a negative association between the consumption of Mediterranean diet and risk of breast cancer [12–14]. Further, in a case-control study, authors tested the association between non-adherence to Mediterranean diet with lifestyle habits in the incidence.
of breast cancer. They found a clear positive relationship and synergism between non-adherence to Mediterranean diet with current smoker, physical inactivity, and alcohol consumption [15]. Previous studies have mostly assessed the associations between Mediterranean dietary pattern and the risk of diseases that do not consider categorizing sources of fat, protein, or carbohydrate, separately [16]. In this regard, focusing on different sources of fat, protein, or carbohydrate, considering their effects on the quality of the diet, can be a better approach to show the influence of the diet on the risk of diseases.
The Mediterranean dietary quality index (MedDQI), a beneficial tool for assessing the quality of the diet, was developed by Gerber et al. [17]. This index evaluates diet quality with an emphasis on different sources of fat (saturated fatty acids and cholesterol versus olive oil) and two different sources of protein (meat and fish) with the contrary scores, both on the poor and good sides, respectively. Given the effective role of different food sources in the development or prevention of cancer [18], focusing on the MedDQI can give a better picture of the association of the diet and BC risk. To the best of our knowledge, no study has investigated the association of the MedDQI and the risk of breast cancer. We, therefore, aimed to examine the potential association between the MedDQI with the risk of breast cancer in Iranian women.
Materials and methods
Study design and participants
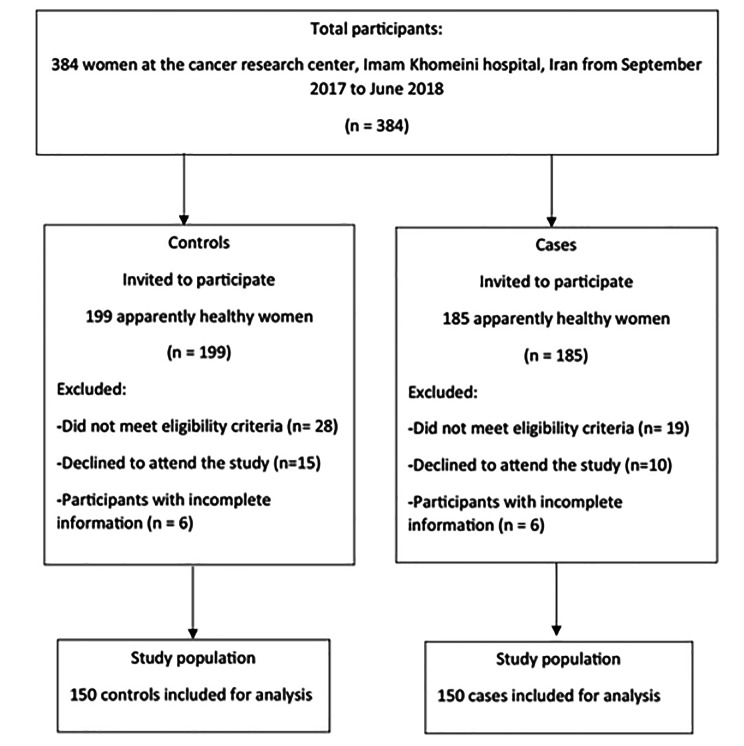
This hospital-based case-control study was conducted between September 23, 2017 and June 21, 2018 among Iranian women (46.6 ± 10.7 years old) who referred to cancer research center, Imam Khomeini Hospital in Tehran. To calculate the sample size calculation, the type I error of 5% and the study power of 95% was used. We hypothesized 5% of the difference in mean and SD of dietary grains between cases and controls and reached almost 150 patients with breast cancer and 150 healthy controls [19]. Cases (n = 150) who were suggested to participate in our study by a pathologist, were pathologically diagnosed with BC. While, controls (n = 150) were apparently healthy women among relatives of patients in other wards of cited Hospital, like dermatology, urology, orthopedic, etc., by poster installation. There was no relationship between these two groups and they were matched just by age. We included patients with a diagnosis period of lower than 3 months to minimize the effect of awareness of BC on patient’s dietary reports. Furthermore, subjects who had any other cancers’ history and long-term dietary restrictions and also controls with BC history were excluded (Fig. 1). The skilled interviewer recorded information on age (year), energy intake (kcal/d), education (university graduated, n (%)), urban-residency (yes, n (%)), family history of breast cancer (yes, n (%)), physical activity (Met/min/week), marital status (married, n (%)), smoking (never smoked, n (%)), alcohol consumption (never used, n (%)), dietary supplement use (yes, n (%)), length of breast-feeding (year), menopausal status (yes, n (%)), history of hormone replacement therapy (yes, n (%)) and BMI (kg/m2), throughout a 45-min structured face‐to‐face interview by a standard questionnaire.
Fig. 1.
The flow diagram for participant selection
Dietary intake assessment
Usual dietary intake of women was evaluated using a valid and reliable 147-item Food Frequency Questionnaire (FFQ) [20] which included a list of groceries and a standard size of each food item. The trained dieticians asked the participants to recall their consumption frequency of each item on a daily, weekly, monthly, and annual basis. When the participants’ reports were not adaptive with the given portion sizes, they were asked to consider their own portion sizes. To estimate energy and nutrient intakes, the household measures and the USDA food composition database which modified for Iranian foods [21, 22], were used to convert the consumed food portion sizes to grams. The calculations were also performed by a modified version of NUTRITIONIST IV software for Iranian foods (version 7.0; N-Squared Computing, Salem, OR, USA).
Construction of Med-DQI
We calculated the diet score based on the Mediterranean diet quality index (MedDQI) (Table 1). This dietary index includes 7 food components which were given a score of 0, 1 or 2 according to the daily intake of each item. Finally, a total score was obtained by summing up the scores of these food items and ranged from 0 to 14. To obtain lower score on this index shows a higher nutrition quality and following the Mediterranean dietary pattern [17]. To estimate the MedDQI score, we categorized participants based on quartile groups of the above-mentioned component’s intakes to minimize misclassification.
Table 1.
Score formation of the Mediterranean Dietary Quality Index
| Scoring | 0 | 1 | 2 |
|---|---|---|---|
| Saturated fatty acids (% energy) | < 10 | 10–13 | > 13 |
| Cholesterol (milligram) | < 300 | 300–400 | > 400 |
| Meats (gram) | < 25 | 25–125 | > 125 |
| Olive oil (milliliter) | > 15 | 15–5 | < 5 |
| Fish (gram) | > 60 | 60–30 | < 30 |
| Cereals (gram) | > 300 | 300–100 | < 100 |
| Vegetables + fruits (gram) | > 700 | 700–400 | < 400 |
Assessment of other variables
Weight was measured with light clothing and without shoes, by using a digital weighing scale (Seca725 GmbH & Co. Hamburg, Germany) to the nearest 100 g and the height was assessed while standing and keeping the shoulders and hips against the wall without shoes, using a stadiometer (Seca, Germany) with an accuracy of 0.1 cm. Body mass index (BMI) was calculated as weight divided by squared height and presented as kg/m2. A validated short from International Physical Activity Questionnaire [23] was used to assess subject’s physical activity levels. Recorded amounts were presented based on Metabolic Equivalents (METs)[23]. Then, the duration and frequency of physical activity days were multiplied by the MET value of the activity and sum of them was calculated as the total exercise minute per week.
Statistical analyses
All individuals were categorized according to the quartiles of MedDQI score. We analysed the study participants’ characteristics and dietary intakes according to MedDQI score quartiles, using one-way analysis of variance (ANOVA) and χ2 tests for continuous and categorical variables, respectively. Data were shown as the mean ± SD for continuous variables and percent (%) for categorical ones. Odds ratio and 95% confidence intervals were obtained using logistic regression to determine the relationship of adherence to the MedDQI score with risk of breast cancer. The risk was reported in crude and 3 adjusted models including confounders such as age (year), energy intake (kcal/d), education (university graduated, n (%)), urban-residency (yes, n (%)), family history of breast cancer (yes, n (%)), physical activity (Met/min/week), marital status (married, n (%)), smoking (never smoked, n (%)), alcohol consumption (never used, n (%) ), dietary supplement use (yes, n (%)), length of breast-feeding (year), menopausal status (yes, n (%)), history of hormone replacement therapy (yes, n (%)) and BMI (kg/m2). In this analysis, the first quartile of exposure was considered as the reference category. All statistical analyses were done using the Statistical Package for the Social Sciences (SPSS version 22; SPSS Inc.). We considered p < 0.05 as the significance level.
Results
The mean age of participants was 46.6 ± 10.7 year in both case and control groups. Moreover, the BMI of the participants in case and control groups were 28.1 ± 4.6 and 28.2 ± 5.2 kg/m2 respectively. General characteristics of the study subjects with and without BC are indicated in Table 2. Also, we showed this information for participants in this table. Women with BC were more likely to be older and they had a longer breastfeeding period than women without BC. Additionally, compared with the participants in the first quartile of the MedDQI, those in the top quartile had lower BMI. No significant differences were observed in other variables.
Table 2.
Characteristics of the study subject across patients with and without breast cancer and also across the quartile categorize of the Mediterranean Dietary Quality Index (MedDQI).
| Breast cancer | Quartiles of Mediterranean Dietary Quality Index | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 150) | No (n = 150) | P* | Q1 (n = 103) MedDQI score range: 3–5 |
Q2 (n = 91) | Q3 (n = 49) | Q4 (n = 57) MedDQI score range:8–11 |
P* | |
| Age (y) | 46.6 ± 10.7 | 46.6 ± 10.7 | < 0.001 | 47.0 ± 10.5 | 47.1 ± 10.0 | 44.5 ± 10.5 | 46.6 ± 12.3 | 0.4 |
| BMI (kg/m2) | 28.1 ± 4.6 | 28.2 ± 5.2 | 0.8 | 28.70 ± 5.32 | 28.73 ± 4.66 | 27.84 ± 4.25 | 26.65 ± 4.97 | 0.01 |
| Physical activity (Met/min/week) | 475.8 ± 1043.2 | 590.0 ± 843.8 | 0.2 | 644.4 ± 970.2 | 406.8 ± 708 | 388.8 ± 561 | 655.8 ± 1389.6 | 0.8 |
| Family history of breast cancer yes, (%) | 30 (20) | 39 (26) | 0.2 | 21 (20.4) | 20 (22) | 12 (24.5) | 16 (28) | 0.8 |
| University graduated n, (%) | 21 (14) | 28 (18.7) | 0.6 | 19 (18.5) | 13 (14.3) | 8 (16.3) | 9 (15.9) | 0.3 |
| Urban-resided n, (%) | 140 (93.3) | 139 (92.7) | 0.8 | 99 (96.1) | 85 (93.4) | 46 (93.9) | 49 (86) | 0.1 |
| Married, n (%) | 129 (86) | 136 (90.7) | 0.2 | 86 (83.5) | 83 (91.2) | 46 (93.9) | 50 (87.7) | 0.5 |
| Menopause status yes, n (%) | 62 (41.3) | 54 (36) | 0.3 | 41 (39.8) | 37 (40.7) | 15 (30.6) | 23 (40.4) | 0.6 |
| Age at first menarche (year) | 14.4 ± 1.7 | 13.4 ± 1.6 | 0.2 | 14.7 (1.7) | 13.6 (1.2) | 13.2 (1.5) | 13.6 (1.7) | 0.2 |
| Number of child, n | 2.6 ± 2.1 | 2.4 ± 2.1 | 0.3 | 2.5 (1.9) | 2.5 (2.4) | 2.3 (1.6) | 2.6 (2.3) | 0.9 |
| Length of breastfeeding (year) | 4.2 ± 3.5 | 3.5 ± 2.7 | 0.059 | 4.07 (3.3) | 3.8 (2.8) | 3.3 (3.1) | 4.2 (3.2) | 0.8 |
| History of HRT, n (%) | 12 (8) | 8 (5.3) | 0.3 | 6 (5.8) | 2 (2.2) | 6 (12.2) | 2 (10.5) | 0.07 |
| Smoking, never smoked, n (%) | 147 (98) | 144 (96) | 0.3 | 101 (98.1) | 87 (95.6) | 47 (95.9) | 56 (98.2) | 0.7 |
| Alcohol, never used, n (%) | 149 (99) | 148 (98) | 0.5 | 102 (99) | 90 (98.9) | 48 (98) | 57 (100) | 0.5 |
| Dietary supplement use, yes, n (%) | 80 (53.3) | 84 (56) | 0.09 | 61 (59.2) | 49 (53.8) | 24 (49) | 30 (52.6) | 0.6 |
| Medication use §,yes, n (%) | 64 (42) | 62 (41) | 0.8 | 45 (43.7) | 37 (40.7) | 18 (36.7) | 26 (45.6) | 0.3 |
| Comorbidities †, n (%) | 38 (25) | 31 (20) | 0.3 | 46 (44.7) | 42 (46.2) | 26 (53.1) | 29 (50.9) | 0.3 |
BC, Breast Cancer
BMI, body mass index
HRT, hormone replacement therapy
kg/m2, kilogram/meter2
MET/min/wk, metabolic equivalent minute per week
*ANOVA for continuous variables and Chi-square test for categorical variables
§Lipid lowering and anti-hypertensive medications
†Diabetes, Hypertension and Hyperlipidemia
Dietary intakes of the patients across the case and control groups as well as across the quartiles of MedDQI are provided in Table 3. Compared to controls, women with BC consumed higher amounts of saturated fatty acids and total energy. In addition, participants in the highest quartile of MedDQI had higher intakes of saturated fatty acids, cholesterol, meat, and lower intakes of olive oils and total fruits and vegetables.
Table 3.
Dietary intakes of study participants across case and control groups as well as across quartile categories of the Mediterranean Dietary Quality Index
| Breast cancer | Quartiles of Mediterranean Dietary Quality Index | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 150) | No (n = 150) | P* | Q1 (n = 103) MedDQI score range: 3–5 |
Q2 (n = 91) |
Q3 (n = 49) |
Q4 (n = 57) MedDQI score range: 8–11 |
P* | |
| Saturated fatty acids (% energy) | 9.1 ± 4 | 8.1 ± 3 | 0.02 | 6.9 ± 1.9 | 7.7 ± 2.3 | 8.9 ± 2.7 | 12.7 ± 4.8 | < 0.001 |
| Cholesterol (mg/d) | 255 ± 117 | 229 ± 126 | 0.06 | 194 ± 63 | 208 ± 74 | 269 ± 112 | 359 ± 179 | < 0.001 |
| Meats (gr/d) | 51.9 ± 41.9 | 46.6 ± 31.5 | 0.26 | 37.3 ± 22.2 | 44.5 ± 30.4 | 57.3 ± 47.4 | 71.6 ± 64.2 | < 0.001 |
| Olive oil (ml/d) | 1.9 ± 3.2 | 1.8 ± 3 | 0.82 | 3 ± 3.9 | 1.6 ± 2.7 | 0.8 ± 1.3 | 1 ± 2.4 | < 0.001 |
| Fish (gr/d) | 8.9 ± 11.7 | 7.8 ± 9 | 0.39 | 9.4 ± 13.1 | 7.5 ± 8.4 | 6.6 ± 8 | 9.2 ± 9.7 | 0.63 |
| Cereals (gr/d) | 343 ± 288 | 495 ± 2390 | 0.44 | 633 ± 2875 | 299 ± 314 | 314 ± 225 | 314 ± 252 | 0.23 |
| Vegetables + fruits (gr/d) | 999 ± 413 | 974 ± 343 | 0.57 | 1,098,326 | 958 ± 331 | 929 ± 433 | 881 ± 447 | < 0.001 |
|
Energy intake (kcal/d) |
2914.1 ± 1159.0 | 2660.3 ± 799.6 | 0.02 | 3173.0 ± 1945.7 | 2888.0 ± 921.3 | 2980.4 ± 1040.9 | 2986.1 ± 995.7 | 0.06 |
Odds ratios and 95%CI of the BC across quartiles of the MedDQI are presented in Table 4. In the crude model, there was a significant inverse association between adherence to MedDQI and odds of BC (OR fourth vs. first quartile: 0.47, 95% CI: 0.23, 0.92, P trend: 0.01). After controlling for confounders, participants in the top quartile of Med-DQI score had 55% less likely to have BC compared with those in the bottom quartile (OR fourth vs. first quartile: 0.45, 95% CI: 0.21, 0.94, P trend: 0.022). These findings remained significant even after adjustment for BMI (OR fourth vs. first quartile: 0.45, 95% CI: 0.21, 0.94, P trend: 0.02).
Table 4.
Risk for breast cancer according to quartiles of the Mediterranean Dietary Quality Index with stratification by menopausal status
| OR (95% CI) | |||||
|---|---|---|---|---|---|
| Q1 MedDQI score range: 3–5 |
Q2 | Q3 | Q4 MedDQI score range: 8–11 |
P for trend | |
| Total | |||||
| No. of cases/controls | 52/51 | 37/54 | 22/27 | 39/18 | |
| Crude | 1 | 1.48 (0.84–2.63) | 1.25 (0.63–2.47) | 0.47 (0.23–0.92) | 0.012 |
| Model 1 | 1 | 1.49 (0.84–2.65) | 1.25 (0.63–2.5) | 0.47 (0.24–0.93) | 0.012 |
| Model 2 | 1 | 1.45 (0.79–2.65) | 1.19 (0.58–2.45) | 0.45 (0.21–0.94) | 0.022 |
| Model 3 | 1 | 1.45 (0.79–2.65) | 1.45 (0.79–2.65) | 0.45 (0.21–0.94) | 0.02 |
| Premenopause | |||||
| No. of cases/controls | 32/30 | 21/33 | 14/20 | 21/13 | |
| Crude | 1 | 1.67 (0.8–3.51) | 1.52 (0.65–3.54) | 0.66 (0.28–1.54) | 0.15 |
| Model 1 | 1 | 1.69 (0.8–3.56) | 1.54 (0.66–3.62) | 0.67 (0.28–1.59) | 0.16 |
| Model 2 | 1 | 2.07 (0.94–4.58) | 1.57 (0.63–3.91) | 0.72 (0.28–1.8) | 0.11 |
| Model 3 | 1 | 2.06 (0.93–4.55) | 1.57 (0.63–3.93) | 0.73 (0.28–1.54) | 0.12 |
| Postmenopause | |||||
| No. of cases/controls | 20/21 | 16/21 | 8/7 | 18/5 | |
| Crude | 1 | 1.69 (0.51–3.56) | 0.83 (0.25–2.72) | 0.26 (0.08–0.84) | 0.07 |
| Model 1 | 1 | 1.33 (0.53–3.34) | 0.9 (0.27–2.99) | 0.24 (0.07–0.8) | 0.055 |
| Model 2 | 1 | 1.22 (0.44–3.39) | 1.05 (0.27–4.04) | 0.23 (0.05–0.91) | 0.1 |
| Model 3 | 1 | 1.23 (0.44–3.46) | 0.9 (0.25–3.88) | 0.18 (0.04–0.77) | 0.06 |
Model 1: Adjusted for age and energy intake
Model 2: Further adjusted for education, residency, family history of breast cancer, physical activity, marital status, smoking, alcohol consumption, supplement use, length of breast-feeding, menopausal status and history of hormone replacement therapy
Model 3: Further adjusted for BMI.
Stratified analysis by menopausal status expressed that after adjusting for age and energy intake, postmenopausal women with the highest adherence to the MedDQI had 76% lower odds for having BC than those with the lowest adherence (OR fourth vs. first quartile: 0.24, 95% CI: 0.07, 0.8, P trend: 0.055). There was no significant association between MedDQI and odds of BC in premenopausal women in crude or adjusted models.
(Model 1: Adjusted for age and energy intake; Model 2: Further adjusted for education, residency, family history of breast cancer, physical activity, marital status, smoking, alcohol consumption, supplement use, length of breastfeeding, menopausal status, and history of hormone replacement therapy; Model 3: Further adjusted for BMI)
Discussion
In this hospital-based case-control study, we found a significant inverse association between MedDQI scores and odds of BC among Iranian women. This inverse association was also seen in postmenopausal women after controlling for energy intake and age. No significant relation was found between MedDQI scores and BC in premenopausal women. This is the first study looking at the link between the MedDQI scores and the risk of BC in Iranian women. BC is the most common malignancy in women [1] Statistics demonstrate a significant increase in the incidence of BC over the past 25 years worldwide [24]. Diet plays a considerable role in the primary prevention of BC. In the present study, adherence to a diet with low MedDQI scores was inversely associated with odds of BC. The effects of a non-Mediterranean diet in the incidence of breast cancer is also well established [25]. Consumption of fresh fruits and vegetables increase the consumer polyphenols that help in fighting against tumorigenesis. In the other hand increased intake of fiber and carbohydrate may be associated with the breast cancer prognosis. Soybean proteins consumption are involved in breast cancer risk reduction and lowering the chances of breast cancer reoccurrence. Ethanol consumption exert their carcinogenic effect on breast tissues and mediate breast cancer development. Processed meat releases the carcinogens compound likes heterocyclic amines, which mediate the onset of breast cancer. High saturated fat diet increases receptor-positive cancer, particularly ER + risk of breast cancer [25]. This relationship was found in postmenopausal women after taking potential cofounders into account. Studies looking at the link between healthy eating habits and the BC have produced conflicting results [26–29]. Similar to our research, several studies have found an inverse relationship between the risk of BC and good eating habits, or diets with low MedDQI scores [26]. Similar to our study, a recent meta-analysis revealed that following a Mediterranean-style diet may help lower the risk of breast cancer, albeit this link was not significant in premenopausal women [30]. Moreover, in an updated meta-analysis conducted by Morze et al. the highest adherence to MedDiet was inversely associated with cancer mortality and BC risk [31]. An increased adherence to the prudent or similar dietary patterns, those rich in fruit, vegetables, fish, whole grains, and low-fat dairy products, were strongly associated with a reduced risk of BC, according to a recent meta-analysis of 32 observational studies looking at the associations between various dietary patterns and odds of BC. These connections were similarly significant in premenopausal women, despite our study, though. Additionally, a Turati study revealed that following a Mediterranean diet was linked to a lower incidence of BC in both pre- and post-menopausal women [12]. The small sample size (n = 300) in this subgroup could be the reason why no correlation between MedDQI scores and risk of BC in premenopausal women was found. We did not look at the associations between BC subtypes. The relationship between following a diet with low Med-DQI scores and lowered risk of a specific subtype of BC has been discussed in several papers [12, 32, 33] In a prospective cohort study, a Mediterranean diet was associated with decreased risk of estrogen and progesterone receptor-negative (PR- ER-) BC [12]. According to another cohort study, following a Mediterranean diet reduced women’s likelihood of developing (ER-)-type BC [32]. A cohort study that involved 49,258 generally healthy women showed that adopting the Mediterranean diet did not significantly lower the risk of BC, in contrast to the current study [28]. Some research in this area has not discovered any conclusive links between healthy eating habits or other similar dietary patterns and the risk of BC [34–36]. The lack of several confounders being controlled for in some research may account for this disparity. Additionally, various dietary evaluation methods and components used in different research may contribute to the contradictory results. Based on the case-control nature of this study, the patients might have reported their current dietary intakes rather than their usual diet. This would result in a biased association between the MedDQI scores and risk of BC compared to those reported in prospective cohort studies, in which the exposure has been measured prior to disease incidence. Second, we ascertained the MedDQI scores based on dietary intakes of both cases and controls, while cases might had changed their dietary intakes after disease manifestation. This would lead to a higher adherence to MedDQI scores in cases than controls and would eventually result in a biased relationship. Finally, no information about subtypes of BC is collected in this study. It has been indicated that diet with low MedDQI scores may have an impact on some subtypes of BC and no effect on others. The underlying mechanisms for the possible favorable of the diet with high MedDQI scores and odds of BC are not completely known. The MedDQI scores is a valuable tool to predict dietary quality and has been already verified using nutritional biomarkers [17]. This index was founded on the recommendations by the National Research Council (NRC) and American Heart Association (AHA) about the diet and health [37]. The intake of 30% or less of the daily total energy from fat, 10% or less of the total energy derived from saturated fat, 30 mg/d or less from cholesterol, 55% of energy from complex carbohydrates and 5 servings or more from fruits and vegetables have been taken into account by NRC and AHA. Fruit, vegetables and whole grains are good sources of antioxidants, dietary fiber and polyphenols, which can mediate the inverse association between the Dietary Approaches to Stop Hypertension (DASH) diet in relation to BC [38]. The consumption of olive oil, due to its monounsaturated fatty acids, have shown to be beneficial associated with some types of BC prevention and survival [39]. In spite of the fact that this study has demonstrated that dietary habits and lifestyle have an impact on the incidence of breast cancer, there is no doubt that hormones are the main cause of the disease. Efforts must also be made to encourage a healthy lifestyle, with special emphasis on the importance of eating a diet consisting primarily of fruits, vegetables, and whole grains with a low intake of red meat and saturated fats. To build the foundation for future advances in evidence-based public health efforts in this region, continued and expanded research on diet, lifestyle and breast cancer risk is urgently necessary.
Suitable sample size, careful assessment of confounding elements and their controlling in the analyses and being the first investigation in Middle East women could be considered as the strengths of the present study. However, some limitations should be considered. First, the case control design of the study does not permit us inferring causality. In addition, these kinds of studies are subject to selection and recall bias. In case-control studies, cases may report their past diet correctly due to their cancer diagnosis. This can attenuate this association. Another concern for case-control studies is that cases may have changed their diet before diagnosis due to early symptoms of the disease. To reduce this error, we recruited newly-diagnosed cases in the study. Furthermore, we used FFQ for assessing dietary intakes which can result in misclassification in our study participants. In addition, an energy adjusted MedDQI scores was applied which could reduce the possibility of subject misclassification. However, we used a qualified questionnaire to evaluate dietary intakes. Finally, we did not collect information about estrogen or progesterone receptor status of study patients.
Conclusion
In conclusion, our findings showed an inverse association between diet with high MedDQI scores and odds of BC among Iranian women. More cohort studies are needed to approve our findings.
Acknowledgements
Authors thank all those who participated in this study.
Authors’ contributions
FDj have made the conception and design of the study. MS and HI participated in acquisition of data. FDj and SS-b analyzed and interpreted the results. PGh, FSh, HSh and FDf drafted the manuscript. SS-b supervised and revised the manuscript. All authors approved the final version of the manuscript. This study is free from funding support. Authors declared that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures executed in studies including human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This research was approved by Tehran University of Medical Sciences (TUMS) (Ethics No. IR.TUMS.VCR.REC.1396.2880) and written informed consent was taken before the study.
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Farhood B, Geraily G, Alizadeh A. Incidence and mortality of various cancers in Iran and compare to other countries: a review article. Iran J public health. 2018;47(3):309–16. [PMC free article] [PubMed] [Google Scholar]
- 3.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800–9. doi: 10.1002/(SICI)1097-0215(19970529)71:5<800::AID-IJC18>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, O’Meara ES, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156(9):635–48. doi: 10.7326/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amabile MI, Frusone F, De Luca A, Tripodi D, Imbimbo G, Lai S, et al. Locoregional surgery in metastatic breast cancer: do concomitant metabolic aspects have a role on the management and prognosis in this setting? J Personalized Med. 2020;10(4):227. doi: 10.3390/jpm10040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AB, Kelly A, Choi NW, Matthews V, Morgan RW, Munan L, et al. A study of diet and breast cancer. Am J Epidemiol. 1978;107(6):499–509. doi: 10.1093/oxfordjournals.aje.a112569. [DOI] [PubMed] [Google Scholar]
- 7.Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, et al. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J cancer prevention: official J Eur Cancer Prev Organisation (ECP) 2013;22(1):90–5. doi: 10.1097/CEJ.0b013e328354d2d7. [DOI] [PubMed] [Google Scholar]
- 8.Magriplis E, Panagiotakos D. Presence of Hypertension Is Reduced by Mediterranean Diet Adherence in All Individuals with a More Pronounced Effect in the Obese: The Hellenic National Nutrition and Health Survey (HNNHS). 2020;12(3). [DOI] [PMC free article] [PubMed]
- 9.Becerra-Tomás N, Blanco Mejía S, Viguiliouk E, Khan T, Kendall CWC, Kahleova H et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. 2020;60(7):1207–27. [DOI] [PubMed]
- 10.Aprano S, Framondi L, Di Matteo R, Laudisio D, Savastano S.
- 11.Schröder H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J Nutr Biochem. 2007;18(3):149–60. doi: 10.1016/j.jnutbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Buckland G, Travier N, Cottet V, González CA, Luján-Barroso L, Agudo A, et al. Adherence to the mediterranean diet and risk of breast cancer in the european prospective investigation into cancer and nutrition cohort study. Int J Cancer. 2013;132(12):2918–27. doi: 10.1002/ijc.27958. [DOI] [PubMed] [Google Scholar]
- 13.Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D. Conformity to traditional Mediterranean diet and breast cancer risk in the greek EPIC (european prospective investigation into Cancer and Nutrition) cohort. Am J Clin Nutr. 2010;92(3):620–5. doi: 10.3945/ajcn.2010.29619. [DOI] [PubMed] [Google Scholar]
- 14.van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer. 2017;140(10):2220–31. doi: 10.1002/ijc.30654. [DOI] [PubMed] [Google Scholar]
- 15.La Torre G, De Carlo I, Sestili C, Cocchiara R, Lia L, Di Bella O, et al. Non-adherence to Mediterranean diet and synergy with lifestyle habits in the occurrence of breast cancer: a case-control study in Italy. Eur Rev Med Pharmacol Sci. 2021;25(13):4535–9. doi: 10.26355/eurrev_202107_26246. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessandro A, De Pergola G. Mediterranean Diet and Cardiovascular Disease: a critical evaluation of a Priori Dietary indexes. Nutrients. 2015;7(9):7863–88. doi: 10.3390/nu7095367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber M. The comprehensive approach to diet: a critical review. J Nutr. 2001;131(11 Suppl):3051s–5s. doi: 10.1093/jn/131.11.3051S. [DOI] [PubMed] [Google Scholar]
- 18.Trichopoulou A, Katsouyanni K, Stuver S, Tzala L, Gnardellis C, Rimm E, et al. Consumption of olive oil and specific food groups in relation to breast cancer risk in Greece. J Natl Cancer Inst. 1995;87(2):110–6. doi: 10.1093/jnci/87.2.110. [DOI] [PubMed] [Google Scholar]
- 19.Ataollahi M, Sedighi S, Masoumi SZ. Nutritional and unhealthy behaviors in women with and without breast cancer.Iranian Red Crescent Medical Journal. 2014;16(9). [DOI] [PMC free article] [PubMed]
- 20.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in tehranian adults. Am J Clin Nutr. 2005;81(1):55–63. doi: 10.1093/ajcn/81.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Azar M, Sarkisian E. Food composition table of Iran. Tehran: National Nutrition and Food Research Institute, Shaheed Beheshti University. 1980;65.
- 22.David H, Linda L, Pamela P. USDA National Nutrient Database for Standard Reference, Release 24. Beltsville: Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference; 2016. [Google Scholar]
- 23.forms. CIGfdpaaotIPAQI-sal.
- 24.Azamjah N, Soltan-Zadeh Y, Zayeri F. Global Trend of breast Cancer mortality rate: a 25-Year study. Asian Pac J cancer prevention: APJCP. 2019;20(7):2015–20. doi: 10.31557/APJCP.2019.20.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buja A, Pierbon M, Lago L, Grotto G, Baldo V.Breast Cancer Primary Prevention and Diet: An Umbrella Review. 2020;17(13). [DOI] [PMC free article] [PubMed]
- 26.Xiao Y, Xia J, Li L, Ke Y, Cheng J, Xie Y, et al. Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019;21(1):16. doi: 10.1186/s13058-019-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs I, Taljaard-Krugell C, Wicks M, Cubasch H, Joffe M, Laubscher R et al. Dietary Patterns and Breast Cancer Risk in Black Urban South African Women: The SABC Study.Nutrients. 2021;13(11). [DOI] [PMC free article] [PubMed]
- 28.Couto E, Sandin S, Löf M, Ursin G, Adami HO, Weiderpass E. Mediterranean dietary pattern and risk of breast cancer. PLoS ONE. 2013;8(2):e55374. doi: 10.1371/journal.pone.0055374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidari Z, Jalali S, Sedaghat F, Ehteshami M, Rashidkhani B. Dietary patterns and breast cancer risk among iranian women: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2018;230:73–8. doi: 10.1016/j.ejogrb.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Hu B-Q, Wu X-J, Qi X-W, Jiang J, Cui X, et al. Adherence to Mediterranean diet and the risk of breast cancer: a meta-analysis. Transl Cancer Res. 2018;7(5):1290–7. doi: 10.21037/tcr.2018.10.13. [DOI] [Google Scholar]
- 31.Morze J, Danielewicz A, Przybyłowicz K, Zeng H, Hoffmann G, Schwingshackl L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr. 2021;60(3):1561–86. doi: 10.1007/s00394-020-02346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirko KA, Willett WC, Hankinson SE, Rosner BA, Beck AH, Tamimi RM, et al. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat. 2016;155(3):579–88. doi: 10.1007/s10549-016-3706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltani S, Benisi-Kohansal S, Azadbakht L, Esmaillzadeh A. Association between Adherence to “Dietary approaches to Stop Hypertension” eating plan and breast Cancer. Nutr Cancer. 2021;73(3):433–41. doi: 10.1080/01635581.2020.1756354. [DOI] [PubMed] [Google Scholar]
- 34.Cui X, Dai Q, Tseng M, Shu XO, Gao YT, Zheng W. Dietary patterns and breast cancer risk in the shanghai breast cancer study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. cosponsored by the American Society of Preventive Oncology. 2007;16(7):1443–8. doi: 10.1158/1055-9965.EPI-07-0059. [DOI] [PubMed] [Google Scholar]
- 35.Männistö S, Dixon LB, Balder HF, Virtanen MJ, Krogh V, Khani BR, et al. Dietary patterns and breast cancer risk: results from three cohort studies in the DIETSCAN project. Cancer causes & control: CCC. 2005;16(6):725–33. doi: 10.1007/s10552-005-1763-7. [DOI] [PubMed] [Google Scholar]
- 36.Adebamowo CA, Hu FB, Cho E, Spiegelman D, Holmes MD, Willett WC. Dietary patterns and the risk of breast cancer. Ann Epidemiol. 2005;15(10):789–95. doi: 10.1016/j.annepidem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Gerber MJ, Scali JD, Michaud A, Durand MD, Astre CM, Dallongeville J, et al. Profiles of a healthful diet and its relationship to biomarkers in a population sample from Mediterranean southern France. J Am Diet Assoc. 2000;100(10):1164–71. doi: 10.1016/S0002-8223(00)00340-0. [DOI] [PubMed] [Google Scholar]
- 38.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96(10):1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 39.Buckland G, Travier N, Agudo A, Fonseca-Nunes A, Navarro C, Lagiou P, et al. Olive oil intake and breast cancer risk in the Mediterranean countries of the european prospective investigation into Cancer and Nutrition study. Int J Cancer. 2012;131(10):2465–9. doi: 10.1002/ijc.27516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.