Abstract
Background:
Persistent low grade depression symptoms are common and impairing in major depressive disorder (MDD) yet rarely reported in treatment follow-up studies (Judd et al., 1998a; Kennedy et al., 2004), suggesting that extant sustained remission rates may not reflect this important clinical feature. Furthermore, no long-term MDD treatment follow-up study has reported on quality of life ratings across functioning levels and years throughout the follow-up period, thus the severity, breadth, and persistence of functional impairment remain unclear. Accordingly, the current study evaluated the course of MDD with consideration of low grade depressive symptomatology and holistic features (e.g., quality of life).
Methods:
We report long-term (9–14 years) follow-up data from individuals with MDD (N = 37) who underwent either Cognitive Therapy (CBT) or a course of selective serotonin reuptake inhibitor (SSRI) treatment. Patients provided retrospective reports of depression symptoms and quality of life in the years following treatment.
Results:
Chronic depression symptoms (most often mild in severity) and decreased quality of life in multiple domains are frequent and suggest poorer sustained remission rates than previously observed in the literature.
Limitations:
Study limitations include small sample size recruited via convenience sampling methods.
Conclusions:
Findings support a conceptualization of depression recovery that entails persistent symptoms and vulnerabilities. Clinical recommendations are provided for discussing these features of depression recovery with patients.
Keywords: Depression, Treatment, Cognitive Behavior Therapy, Pharmacotherapy, Quality of life
1. Introduction
Intervention follow-up research examining the course of major depressive disorder (MDD) has revealed a concerning picture regarding the endurance of therapeutic effects. Decades ago, studies assessing depression symptoms across severity levels and years found that persistent inter-episode low grade depression symptoms are common after treatment (Judd et al., 1998a; Kennedy et al., 2004). The purpose of this article is to document with new data that these trends persist despite advancement in depression treatment, and their effects are further reaching, extending to quality of life metrics. Accordingly, we conducted a retrospective long-term (9–14 years) follow-up of individuals who received treatment with a selective serotonin reuptake inhibitor (SSRI) or Cognitive Therapy (CBT) for MDD and documented temporal and severity patterns of symptoms and functioning.
A substantial body of literature has shown that subthreshold depression symptoms are an important assessment target in MDD, with high prevalence, persistence, and association with clinical outcomes (Fava et al., 2002; Fava et al., 2007a, 2007b; Kennedy and Foy, 2005; Tranter et al., 2002). Subsyndromal symptoms are common both before, “prodromal,” and after, “residual,” depressive episodes and can encompass a wide range of potential affective (e.g., mood), cognitive (e. g., attributional styles), and behavioral disturbances (e.g., social withdrawal) (Fava et al., 2007a, 2007b; Kennedy and Foy, 2005), with similar symptoms observed during prodromal and residual phases (Fava et al., 1994). Symptoms are often long-lasting, roughly three times longer than actual depressive episodes (Kennedy et al., 2004), and are associated with poor treatment outcomes, e.g., quicker time to relapse, more frequent episodes, and a more chronic illness trajectory (Fava et al., 2002; Fava et al., 2007a, 2007b; Kennedy and Foy, 2005; Tranter et al., 2002). With high prevalence and persistence of low grade depressive experiences comes the potential for long-term impact on functioning and quality of life. Indeed, functioning deficits across broad life domains, e.g., social relationships (Kennedy and Paykel, 2004), physical health (ten Doesschate et al., 2010; Woo et al., 2014) and work (Beck et al., 2011; Martin et al., 1996), have been reported in MDD, with even subthreshold depressive experiences affecting functioning (Judd et al., 2000a). High frequency and persistence of low grade depressive symptoms as well as their relationship to functioning/quality of life impairments highlights their importance in evaluating treatment outcome, yet their inclusion in outcome estimates is uncommon (Fava et al., 2007a, 2007b).
Accordingly, we sought to document MDD symptoms and associated holistic variables (e.g., quality of life and return to treatment) in a long-term intervention follow-up. In light of research suggesting high frequency of chronic low grade symptoms yet their rare inclusion in treatment outcome estimates, an additional question is whether cross-year, cross-severity measurements provide different sustained remission estimates than more traditional measures. The answer would inform future research on whether to target personalizing medicine for long-term treatment outcomes (e.g., predicting sustained response to one intervention or another) versus adjusting approaches for measuring and targeting sustained remission, as problems are more systemic. Thus, if sustained remission estimates are worse than long-term follow-up estimates observed for empirically supported MDD treatments, it would provide preliminary support for re-considering the long-term impact and relevance of mainstream treatment approaches.
In order to document depressive symptoms and functioning following treatment for MDD, we conducted retrospective interviews of individuals with MDD who received CBT or an SSRI 9–14 years prior. The retrospective interview measure collapsed across diverse depression symptoms (e.g., affective, cognitive, behavioral), for a single measure of depression severity (mild, moderate, severe) at each time point. Depression symptom indices consisted of 1) post-treatment survival estimates (across symptom severity levels), 2) proportion of follow-up years asymptomatic, and 3) duration estimates of maximum asymptomatic periods. We hypothesized that study estimates of sustained remission will be poorer (fewer patients meeting sustained remission) than literature would suggest. Because CBT and SSRI may differ in endurance of treatment effects, with CBT showing more favorable outcomes (Bockting et al., 2015), as an exploratory aim, we compared initial study treatment groups (CBT, SSRI) on symptom indices.
To further provide a holistic image of the individual’s experience with MDD following intervention, we report descriptive data (proportion estimates) for treatments sought and life satisfaction across life domains in the years following study participation. We hypothesize patients will often seek additional mental health treatments and will report persistent functioning deficits.
2. Method
2.1. Participants
We recruited individuals who received CBT (N = 74) or an SSRI (N = 25) as part of clinical trials for MDD. A multi-site clinical trial (MH58356; PI: Thase, MH58397; PI: Jarrett) examined the efficacy of CBT and fluoxetine for relapse prevention in recurrent/chronic MDD; another study (MH074807; PI: Siegle) examined neural markers of acute response to CBT for MDD (including first episode cases); a third study (Pittsburgh Foundation: M2007-0114; PI: Siegle) examined neural markers of acute response to SSRI for MDD (including first episode cases). Patients met MDD DSM-IV diagnostic criteria, determined by a structured clinical interview (SCID-IV; First et al., 1996) and at least 14 on the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). The multi-site trial had the additional criterion that participants had to meet criteria for chronic or recurrent depression. The CBT and SSRI-only studies required at least 14 on the HRSD at both the screening and intake visits. Prior exclusion criteria consisted of concurrent severe mental disorders, alcohol or drug dependence, or medical conditions that could influence depressive symptoms. In addition, if participants were considered at risk for suicide or if they had previously received CBT for MDD, they were not allowed to participate. The CBT protocols consisted of 16–20 sessions of Cognitive Therapy, including techniques like recognizing maladaptive cognitive distortions and challenging them through therapeutic cognitive exercises (Beck, 1979). Pharmacotherapy sessions were of the same number and frequency as CBT sessions. Sessions were 30–45 min in length and were conducted by a psychiatric nurse who inquired about general mood status, administered the HRSD and Frequency, Intensity, and Burden of Side Effects (FIBSR; Wisniewski et al., 2006), and provided psychoeducation about medication. A psychiatrist consulted with the nurse and patient for the final 5–10 min of the session. We obtained follow-up data from 27 CBT and 10 SSRI participants (demographics in Table 1, comparison with original sample in Supplement 1). Of the recruited sample, 2 participants met diagnostic criteria for dysthymic disorder during their initial study interview, and 11 participants were considered to have chronic MDD.
Table 1.
Demographics of recruited sample (N = 37).
| Gender | Female (N = 28, 75.68 %) |
|---|---|
| Age | |
| At interview | 46.11 (10.96) |
| During study | 36.59 (10.97) |
| Ethnicity | White (N = 33, 89.19 %) |
| Non-White (N = 4, 10.81 %) | |
| Education | 15.53 (2.10)a |
| Number of depressive episodes | 3.69 (2.64)a |
| Hamilton Depression Rating Scale | Pre: 19.69 (4.79)a |
| Post: 7.96 (6.96)a | |
| Acute treatment status | Completed treatment (N = 34, 91.89 %) |
| Acute treatment remission statusb | Remitter (N = 19, 51.35 %) |
Reduced N’s: Education (N = 36), Number of depressive episodes (N = 35), Hamilton Depression Rating Scale scores (N = 35).
Remitter is defined as ≤7 on Hamilton Depression Rating Scale at post (Frank et al., 1991).
2.2. Recruitment
We recruited participants via mailed letters, phone calls, emails, and private messages through popular social media sites (Facebook and LinkedIn). To obtain participant contact information, we reviewed prior study records and conducted several internet searches using Google, free person-search websites, social media sites, and www.whitepages.com (recruitment consort in Supplement 2).
2.3. Measures
2.3.1. Depression symptoms
To assess depression symptoms, participants underwent a phone interview while completing an online graphical representation of their depression symptoms and life stressors. We adapted the National Institute of Mental Health Retrospective Life Chart Method (NIMH-LCM-R; Leverich & Post, 1993) to span a longer follow-up period and to allow online administration (details in Supplement 3a–c). Participants reported symptoms across three severity levels (mild, moderate, severe), collapsing across different types of symptoms (Supplement 3c) at one-month increments across years (chart processing in Supplement 4). Participants also reported on life events and additional depression treatments. NIMH-LCM-R depression scores have been shown to correlate with other depression measures, e.g., HRSD, Inventory of Depressive Symptoms (Hörn et al., 2002).
2.3.2. Quality of life
Similar to the NIMH-LCM-R, to assess quality of life across life domains and years, participants drew a graphical representation of functioning across life domains and years during the interview. Participants reported on seven life domains (physical health, mood, work, social relationships, family relationships, leisure time activities, and ability to function in daily life) taken from the last item of the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ; Endicott et al., 1993). Prompt and scale anchors were from the QLESQ; participants reported life satisfaction on a scale of Very Poor (1) to Very Good (5).
2.4. Procedure
Participants first completed consent documentation online. Prior to the interview, we instructed participants to review calendars, medical records, physician notes, diaries, social media accounts, and any other sources of information that would help them remember depressed periods. We also instructed participants to have information sources available during their interview. A clinical psychology PhD-student (MVS) conducted all interviews via www.Miro.com, an online “whiteboard” that the participant and interviewer could access simultaneously and see real-time edits. Each participant completed the interview measures with the interviewer’s assistance over the phone for approximately 2 to 3 h. Procedures were approved by the University of Pittsburgh’s Institutional Review Board.
2.5. Literature comparison analyses
2.5.1. Depression symptoms
To test whether study estimates of sustained remission differed from literature estimates, we relied on prior results of meta-analyses of MDD intervention follow-up research, which provided relapse/recurrence estimates ranging from 39 % to 54 % (Brouwer et al., 2019; Steinert et al., 2014; Vittengl et al., 2007). We selected the most conservative estimate because studies may be prone to more favorable outcomes, due to shorter follow-up periods and potential requirements for acute response/remission. Choosing the highest relapse/recurrence rate provides the most conservative test, thus we used the relapse/recurrence rate of 54 % (i.e., a sustained remission rate of 46 %) for statistical comparisons. To derive sample sustained remission estimates, we submitted depression symptoms to a series of criteria to establish binary individual outcome values (sustained remission or not). Survival estimates of sustained remission were defined as never reporting depression symptoms of different severity levels (mild, moderate, or severe) for at least a month. Proportion of follow-up years experiencing depression provided two definitions, a more conservative measure of ≥80 % of the follow-up asymptomatic (Judd et al., 1998b; Judd et al., 2000b) and a more lenient estimate of ≥50 % of the follow-up asymptomatic. The more lenient estimate (≥50 %) was not from the literature but instead is in response to prior acknowledgement that ≥80 % may be challenging to apply clinically (Judd et al., 2016). In addition, we assessed maximum duration of asymptomatic periods against three thresholds (55, 33.75, and 24 months). More conservative estimates were from a MDD longitudinal study as median lengths of “well” periods (initial inter-episode (55 months) or time between minor or major depressive episodes (33.75 months)) following asymptomatic remission (Judd et al., 2016). The most lenient estimate (24 months) is a practical threshold of interest.
2.5.2. Quality of life
We compared participants’ responses on the quality of life interview with QLESQ community norms (Endicott et al., 1993; Rapaport et al., 2005) (Table 2). For each life domain, we calculated the proportion of follow-up that patients reported life satisfaction at the community-norm level. As a practical index of interest, we calculated the proportion of follow-up that patients reported at least “good” life satisfaction.
Table 2.
Self-reported quality of life in the years after acute treatment.
| Life domain | Mean rating (SD) | Community mean rating (SD)a | Mean proportion at least community average (SD) | Mean proportion at least “good” (SD) |
|---|---|---|---|---|
| Physical health | 3.13 (0.83) | 4.3 (0.7) | 0.14 (0.24) | 0.63 (0.32) |
| Mood | 2.64 (0.75) | 3.9 (0.9) | 0.17 (0.25) | 0.42 (0.29) |
| Work | 2.72 (0.84) | 3.9 (0.9) | 0.22 (0.28) | 0.45 (0.30) |
| Social relationships | 2.80 (0.89) | 4.1 (0.9) | 0.16 (0.23) | 0.47 (0.37) |
| Family relationships | 2.90 (0.98) | 4.2 (0.8) | 0.16 (0.29) | 0.48 (0.40) |
| Leisure time activities | 2.71 (0.82) | 4.1 (0.9) | 0.14 (0.24) | 0.44 (0.32) |
| Ability to function in daily life | 3.01 (0.80) | 4.5 (0.7) | 0.09 (0.20) | 0.56 (0.32) |
Community norms (Endicott et al., 1993; Rapaport et al., 2005).
3. Results
3.1. Survival estimates post-treatment for different depression severity levels
Chi-square tests showed study-sample survival estimates were worse than literature estimates of sustained remission. A large majority (33/37) of participants experienced depression symptoms of at least mild severity within one year after study participation; the remaining 4 participants experienced at least mild depression within 3 years of concluding treatment (Fig. 1). Zero participants remained asymptomatic for the duration of the follow-up period, lower than would be expected based on the literature, χ2(1) = 22.07, p < .001, RR = 1.85, 95 % CI [1.37–2.49]. The majority of participants (24/37) experienced at least moderate depression within one year. Of the remaining 14 participants, 13 eventually experienced moderate depression within 10 years. One participant never experienced moderate depression within the follow-up period, lower than expected based on literature, χ2(1) = 18.79, p < .001, RR = 1.80, 95 % CI [1.33–2.43]. A substantial proportion (14/37) of participants experienced severe depression within one year after study participation. Of the remaining 23, all but 5 eventually experienced severe depression within 11 years, χ2(1) = 9.32, p = .002, RR = 1.60, 95 % CI [1.15–2.21].
Fig. 1.
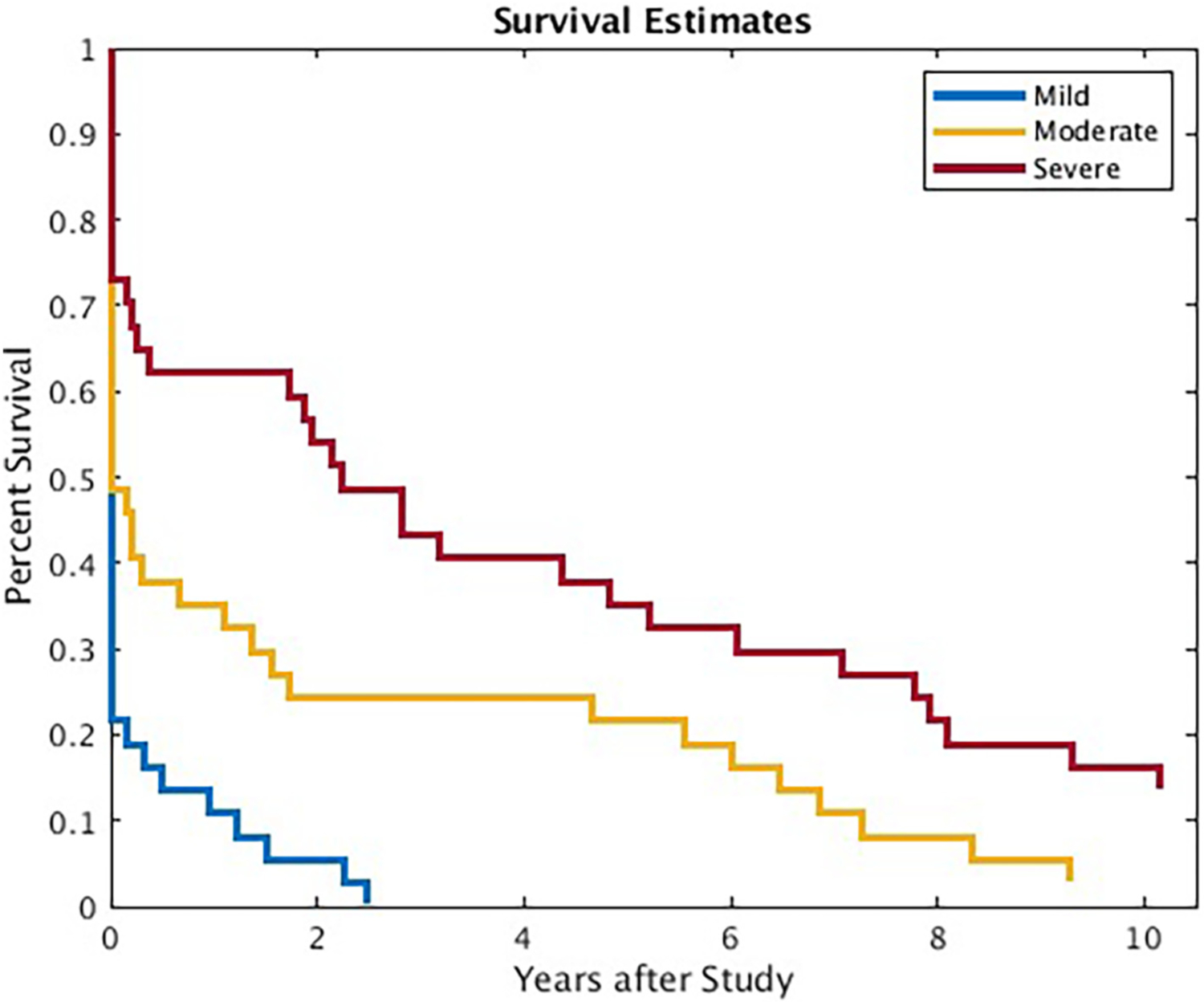
Survival estimates for depression severity levels across years.
3.2. Proportion estimates of follow-up years experiencing symptoms
With proportion estimates, both chi-square tests suggested worse study outcomes than prior studies. Participants spent on average 73.77 % (range: 18.37 %–100 %) of the follow-up period experiencing depression symptoms. One participant (2.07 % of sample) experienced at least 80 % of the follow-up without symptoms, the threshold of “asymptomatic recovery” (Judd et al., 1998b; Judd et al., 2000b), χ2(1) = 18.79, p < .001, RR = 1.80, 95 % CI [1.33–2.43]. Eight participants (21.62 %) met a more lenient cut-off of >50 % of the follow-up period without depression symptoms, χ2(1) = 4.89, p = .027, RR = 1.43, 95 % CI [1.01–2.02]. The most frequent depression symptoms were of mild severity (M = 33.1 %, range: 0–100 %), followed by moderate (M = 22.0 %, range: 0–65.3 %) and severe (M = 18.6 %, range: 0–89.4 %), separated by year in Supplement 5.
3.3. Duration estimates of maximum asymptomatic periods
Two of the three chi-square tests showed poorer outcomes for maximum asymptomatic periods than the literature threshold, with the third showing a nonsignificant trend (p = .051). A sizable subset (21.62 %) of patients reported never experiencing a single asymptomatic moment (<1 month) in the years following the study. On average, participants reported 13.80 (range: 0–60.20) maximum consecutive months without depression symptoms. One participant (2.07 % of sample) met the most conservative threshold of at least 55 months (Judd et al., 2016), χ2(1) = 18.79, p < .001, RR = 1.80, 95 % CI [1.33–2.43]. Four participants (10.81 % of sample) met the threshold of at least 33.75 months (Judd et al., 2016), χ2(1) 11.24, p < .001, RR = 1.64, 95 % CI [1.19–2.26]. Nine participants (24.32 % of sample) met the most lenient threshold of at least 24 months, χ2(1) = 3.80, p = .051, RR = 1.40, 95 % CI [0.99–1.98]. Maximum consecutive asymptomatic months were minimal for most participants (Fig. 2).
Fig. 2.
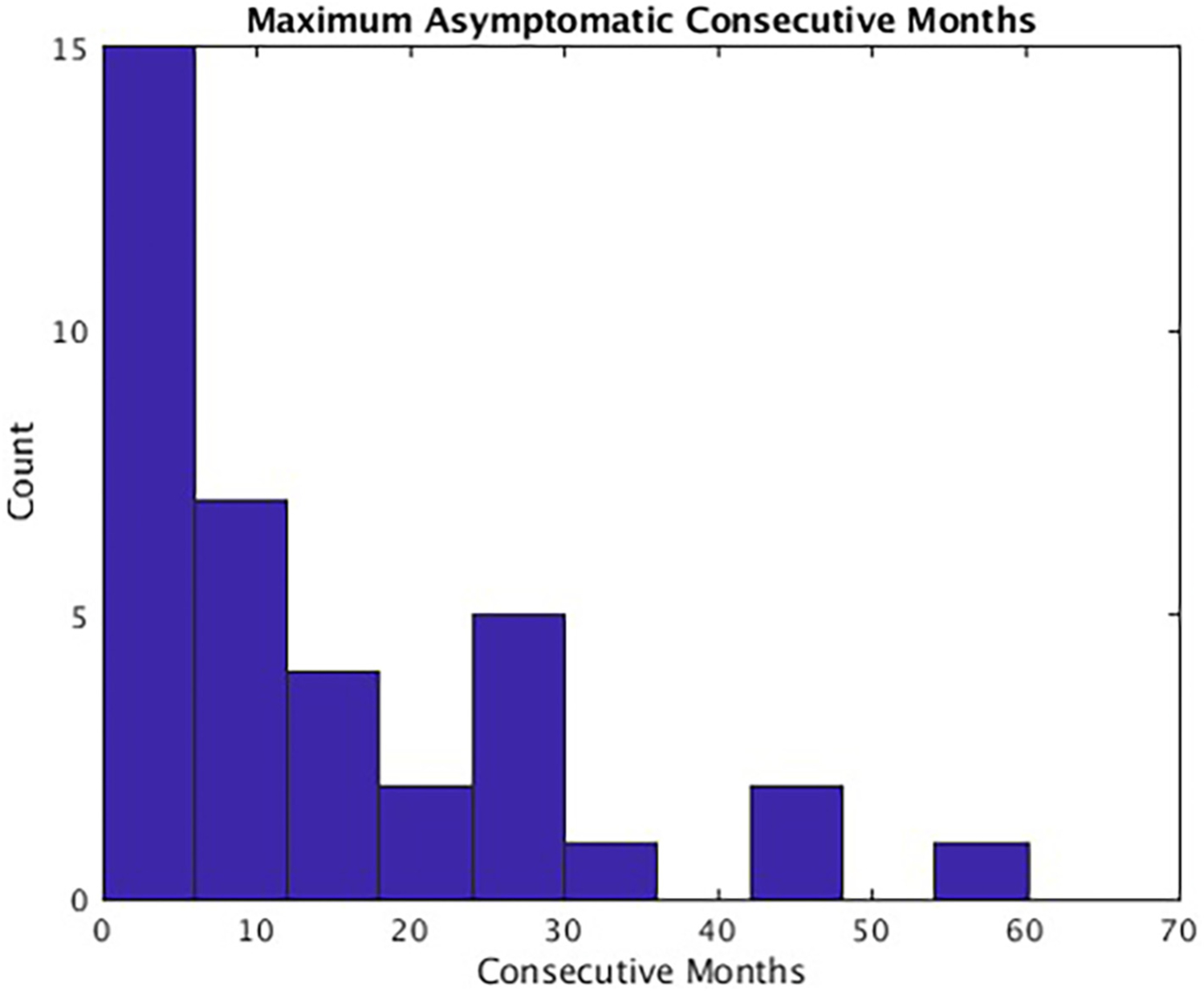
Maximum reported asymptomatic consecutive months.
Note. Histogram showing counts (out of 37 participants) for maximum reported asymptomatic consecutive months.
3.4. Symptom estimates by intervention type
We assessed whether depression symptom indices differed between study treatment groups and found that CBT and SSRI samples did not differ on survival estimates but differed on some proportion estimates of follow-up symptoms as well as maximum asymptomatic periods (Supplement 6a). CBT participants showed generally more favorable outcomes than the SSRI sample, reporting a smaller proportion of follow-up with symptoms and longer maximum asymptomatic periods; however, symptom indices were still poor for most participants (Supplements 6b–6d), and chi-square results remained largely consistent when limited to the CBT sample (instead of the combined CBT and SSRI sample) (Supplement 6e).
3.5. Proportion estimates of follow-up years spent undergoing additional treatments
Participants spent an average of 38.85 % (range: 0–99.17 %) of the years following acute intervention receiving additional treatment (psychotherapy and/or pharmacological). Participants reported an average of 17.77 % (range: 0–99.17 %) of the follow-up in psychotherapy and an average of 26.13 % (range: 0–98.11 %) of the follow-up taking medication (separated by study treatment sample, Supplement 7). During depressive periods, participants received psychiatric treatment (therapy and/or medication) approximately half of the time or less (Table 3).
Table 3.
Mean proportion of depressive episodes that participants received treatment.
| Depression severity | |||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Therapy | 0.17 (0–1.00) | 0.21 (0–1.00) | 0.27 (0–1.00) |
| Medication | 0.31 (0–1.00) | 0.25 (0–1.00) | 0.33 (0–1.00) |
| Therapy and/or medication | 0.40 (0–1.00) | 0.39 (0–1.00) | 0.51 (0–1.00) |
Note. Mean (range).
Proportion estimates of follow-up years with good life satisfaction across life domains.
Average reported functioning ranged from 2.64 (0.74) to 3.13 (0.83) across life domains, on a scale of 1 (very poor) to 5 (very good). When comparing participants’ responses with community norms, participants reported comparable quality of life an average of 9.2 % (daily functioning) to 22.1 % (work) of the follow-up. When lowering the comparison threshold to at least “good” life satisfaction, average proportions ranged from 42 % (mood) to 63 % (physical health) of the follow-up period, with all but two domains (physical health and daily functioning) below 50 % threshold (Table 2). Proportion estimates show that it is common for MDD patients to experience persistent quality of life impairments across life domains; however, for some domains, a subset of participants report quality of life comparable to non-depressed controls for most of the follow-up period (Fig. 3), suggesting some MDD patients experience good life satisfaction for some life domains despite chronic depressive symptomatology.
Fig. 3.
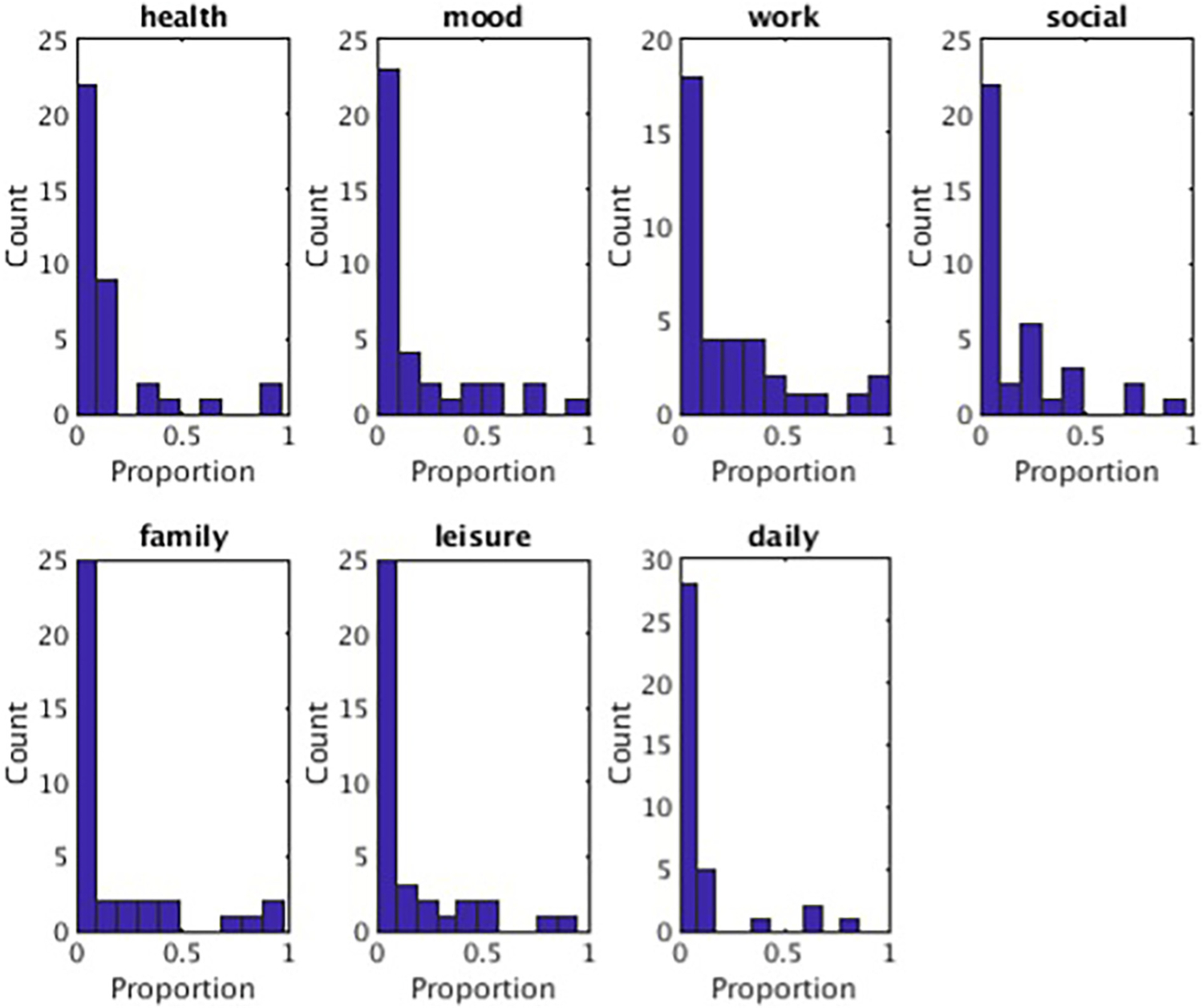
Proportion of follow-up at the community-norm functioning level.
Note. Histograms showing counts (out of 37 participants) for the proportion of the follow-up period participants reported functioning at least at the community norm average (Endicott et al., 1993; Rapaport et al., 2005) for each life domain.
4. Discussion
The study objective was to document the course of depression symptoms (in relation to prior estimates of sustained remission) and holistic features across years following treatment for MDD. Consistent with prior literature (Judd et al., 1998a; Kennedy et al., 2004) but despite advancements in depression intervention research, findings suggested that individuals with MDD experience chronic depression symptoms often within one year following treatment. Symptoms were most frequently mild, but most individuals also experienced moderate and severe symptoms during follow-up. When comparing study sustained remission estimates with literature estimates, study measures showed statistically fewer individuals experienced sustained remission after treatment. We also observed that MDD patients often returned to treatment and experienced deficits in life satisfaction, with only a minority of participants reporting life satisfaction comparable to non-depressed community norms for some life domains.
The finding of persistent depression symptoms is consistent with prior follow-up studies (Judd et al., 1998a; Kennedy et al., 2004) and shows their observations from naturalistic treatment settings hold true for more rigorous, structured intervention protocols. Prior studies similarly found frequent persistent depression symptoms (about half of the follow-up period) that were most often mild in severity. Although MDD follow-up research reporting traditional measures of sustained remission suggest a significant proportion of individuals with MDD will experience relapse and/or recurrence (Gotlib and Hammen, 2008), estimates reported here were statistically worse than suggested by empirically-supported intervention follow-up studies in MDD (Brouwer et al., 2019; Steinert et al., 2014; Vittengl et al., 2007). Thus, when factoring in low grade depression symptomatology into follow-up estimates of relapse/recurrence, long-term treatment outcome estimates appear even less favorable.
The observation of prevalent, persistent symptoms provides support for a treatment approach that incorporates routine symptom assessment, in pursuit of full remission. Indeed, as residual symptoms are commonplace and related to clinical outcomes (Fava et al., 2002; Fava et al., 2007a, 2007b; Kennedy and Foy, 2005; Tranter et al., 2002), symptom monitoring stands to inform provider treatment decisions, for which more than one intervention may be necessary to achieve asymptomatic remission (Fava et al., 2007a, 2007b). Findings support sequentially implemented interventions, as this approach involves continued assessment and treatment of residual symptoms (Fava et al., 2007b; Fava and Tomba, 2010; Guidi et al., 2016; Guidi and Fava, 2021). An example of the sequential approach involves first providing an antidepressant medication and later adding psychotherapy (e.g., CBT) to address remaining symptoms, an approach that has been supported by a recent meta-analysis to reduce risk of relapse and recurrence in MDD (Guidi and Fava, 2021). Even after asymptomatic remission is achieved, study findings and related literature support regular monitoring for subthreshold symptoms and intervention upon their return.
In addition to persistent symptoms, we observed poor life satisfaction across domains, which should be interpreted within the context of the nuanced quality of life MDD research. Our cross-year functioning estimates showed that after treatment, MDD patients often experience chronic lower life satisfaction than non-depressed individuals, who on-average report good to very good life satisfaction (Endicott et al., 1993; Rapaport et al., 2005). Although prior research shows depression treatment improves quality of life (Jha et al., 2014; Kolovos et al., 2016), most robust findings come from studies reporting on the acute treatment phase (IsHak et al., 2011; Papakostas et al., 2004). Studies that have assessed long-term estimates of life satisfaction in MDD following treatment have shown more modest improvements, influenced by instances of relapse/recurrence (Kocsis et al., 2002). In addition, some studies have found that although quality of life improves with reduced depression symptoms, it is worse than healthy controls (IsHak et al., 2011), the general population (Angermeyer et al., 2002; IsHak et al., 2015), and individuals with a chronic medical condition (Hays et al., 1995). Indeed, partial remission with residual disability has been identified as a common MDD trajectory (Ormel et al., 1993). Our findings are most consistent with studies using longer follow-up methods.
Study findings are to be considered in light of methodological limitations specific to the life chart interview. Findings are based on retrospective reports, which could entail several potential sources of bias such as difficulties with remembering depression severity and influence of current depressive state on reporting. Recognizing this limitation, referencing additional sources of information was encouraged prior to and during the interview to assist with charting. The interview also involved assessing one year at a time, and life events were assessed before depression and quality of life, to use events as additional anchors for memory. Another limitation is that the life chart collapses across diverse depression symptoms (e.g., mood, sleep disturbances, decreased energy), thus, the interview measure does not inform the presence/absence of specific symptoms during treatment follow-up. As specific symptoms may represent different mechanisms of depression maintenance and inform individual treatment decisions (Fava et al., 2007a, 2007b), future long-term treatment follow-up studies would benefit from using a method that maintains some symptom type distinction. The life chart measure also does not provide the information needed to determine diagnoses, so we are unable to report on participant diagnostic status during follow-up. Close to a third of the sample was considered to have a more persistent diagnosis (e.g., dysthymic disorder or chronic MDD) during the initial study interview, but it is unknown whether these participants continued to meet diagnostic criteria for persistent depressive disorder during follow-up or whether other participants went on to later develop persistent depressive disorder. Without updated diagnostic information, we cannot conclude that study findings refer to a specific depression diagnosis (i.e., just MDD), but instead, findings inform a broader unipolar depression continuum, supported by prior research (Judd et al., 1998a; Kennedy et al., 2004).
Additional study limitations pertain to study design and sample. One design limitation is the absence of data on participants prior to initial depressive episode(s), thus findings cannot speak to whether depressive episodes cause persistent depressive symptoms and functioning deficits or whether unfavorable treatment outcomes instead represent depressive personality traits/other trait variables present prior to MDD onset. Another design limitation pertains to the naturalistic follow-up, in which participants continued to undergo additional treatments. With participants receiving antidepressant medication and/or psychotherapy for approximately 40 % of the follow-up, study data do not inform long-term outcomes of specific treatment modalities. Instead, follow-up treatment estimates show that even with rigorously-administered initial treatment and later mental health services, participants experienced persistent depression. Sample limitations involve the use of convenience sampling methods. Although the recruited subsample did not statistically differ from the original sample on depression variables (Supplement 1), it is possible they differed on other variables, resulting in negatively biased estimates. However, the recruited sample had a larger proportion of patients who completed treatment (Supplement 1), which could suggest more favorable outcomes. Lastly, the sample was also small, particularly compared to epidemiological studies. This was due to 1) the limited sample on which we had detailed diagnostic and treatment data from over a decade ago and 2) the challenging nature of the recruitment and interview process necessary to collect as-valid-as-possible data from participants. Our current results suggest the value in using such procedures on a larger scale.
In summary, the current study adds to the literature on long-term treatment outcomes in MDD by illustrating that when factoring in low grade depression symptoms, sustained remission estimates are worse than more traditional estimates. The study also adds to research on holistic features of MDD recovery, showing that the MDD course often involves return to mental health services and chronic functioning deficits across life domains. One implication of this work is that despite decades of advancement in treatment, people are still not staying “well” for sustained periods after a depressive episode. An additional implication is that, consistent with a long history of thinking about chronic depression (Akiskal, 1997; Brunello et al., 1999; Judd et al., 2000b; Rihmer et al., 2007; Rosenthal and Akiskal, 1985), the very term “recovery” may be inaccurate for many MDD patients. Individuals who are vulnerable to episodes of depression may also be vulnerable to a lifetime of chronic low-grade symptomatology and low quality of life. Depression symptoms and related holistic features may appear before the first episode of depression and return shortly after a given episode remits, thus, thinking of depression as fully “recovered” may be, for many people who have been depressed, misleading.
Despite persistent symptoms and quality of life impairments in the sample, some participants reported comparable quality of life to non-depressed individuals for some life domains (Fig. 3). The observation of high functioning despite chronic symptoms suggests that life satisfaction for some domains may be more malleable than depression symptomatology. This insight could lead to a different way of thinking and speaking to patients about post-acute-depression expectations. For example, clinicians could prepare patients for chronic vascillations in symptomatology, reminding them that high quality of life, across multiple domains, is possible for individuals, despite persistent symptoms.
Supplementary Material
Acknowledgements
We thank Drs. Pearl Chiu, Jungmeen Kim-Spoon, and Tao-Ho Lee for their helpful feedback during project development.
Role of the funding source
Participant payments were provided by Western Psychiatric Institute and Clinic departmental funding. Original clinical trial data (from which the current sample was recruited) was funded by the National Institute of Mental Health MH074807.
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Siegle receives royalty payments on a patent regarding a novel depression intervention licensed to Apollo Neurosciences, which is not relevant to this article, and consults for Johnson and Johnson on novel pharmacology unrelated to this project. The other authors report nothing to disclose.
Footnotes
CRediT authorship contribution statement
Author Dr. Strege designed the study protocol, recruited participants, collected data, conducted statistical analyses, and wrote the first draft of the manuscript. Author Dr. Richey contributed to initial idea development and manuscript drafts. Author Dr. Siegle contributed to initial idea development and manuscript drafts. Dr. Siegle also provided assistance with study analyses. All authors have approved the final article.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jad.2022.08.075.
Data statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Akiskal HS, 1997. Chronic disturbances of temperament. In: Basic and Clinical Science of Mental and Addictive Disorders, pp. 29–32. 10.1159/000059528. [DOI] [Google Scholar]
- Angermeyer MC, Holzinger A, Matschinger H, Stengler-Wenzke K, 2002. Depression and quality of life: results of a follow-up study. Int. J. Soc. Psychiatry 48 (3), 189–199. [DOI] [PubMed] [Google Scholar]
- Beck A, 1979. Cognitive Therapy of Depression. Guilford Press. [Google Scholar]
- Beck A, Crain AL, Solberg LI, Unützer J, Glasgow RE, Maciosek MV, Whitebird R, 2011. Severity of depression and magnitude of productivity loss. Ann. Fam. Med 9 (4), 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockting CL, Hollon SD, Jarrett RB, Kuyken W, Dobson K, 2015. A lifetime approach to major depressive disorder: the contributions of psychological interventions in preventing relapse and recurrence. Clin. Psychol. Rev 41, 16–26. [DOI] [PubMed] [Google Scholar]
- Brouwer ME, Williams AD, Kennis M, Fu Z, Klein NS, Cuijpers P, Bockting CLH, 2019. Psychological theories of depressive relapse and recurrence: a systematic review and meta-analysis of prospective studies. Clin. Psychol. Rev 74, 101773. [DOI] [PubMed] [Google Scholar]
- Brunello N, Akiskal H, Boyer P, Gessa GL, Howland RH, Langer SZ, Mendlewicz J, Paes de Souza M, Placidi GF, Racagni G, Wessely S, 1999. Dysthymia: clinical picture, extent of overlap with chronic fatigue syndrome, neuropharmacological considerations, and new therapeutic vistas. J. Affect. Disord 52 (1–3), 275–290. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R, 1993. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol. Bull 29 (2), 321–326. [PubMed] [Google Scholar]
- Fava GA, Fabbri S, Sonino N, 2002. Residual symptoms in depression: an emerging therapeutic target. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 26 (6), 1019–1027. [DOI] [PubMed] [Google Scholar]
- Fava GA, Grandi S, Zielezny M, Canestrari R, Morphy MA, 1994. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am. J. Psychiatry 151 (9), 1295–1299. [DOI] [PubMed] [Google Scholar]
- Fava GA, Ruini C, Belaise C, 2007. The concept of recovery in major depression. Psychol. Med 37 (3), 307–317. [DOI] [PubMed] [Google Scholar]
- Fava GA, Tomba E, 2010. New modalities of assessment and treatment planning in depression: the sequential approach. CNS Drugs 24 (6), 453–465. [DOI] [PubMed] [Google Scholar]
- Fava GA, Tomba E, Grandi S, 2007. The road to recovery from depression–don’t drive today with yesterday’s map. Psychother. Psychosom 76 (5), 260–265. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 1996. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department. [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM, 1991. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch. Gen. Psychiatry 48 (9), 851–855. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hammen CL, 2008. Handbook of Depression, Second edition. Guilford Press. [Google Scholar]
- Guidi J, Fava GA, 2021. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatry 78 (3), 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi J, Tomba E, Fava GA, 2016. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. Am. J. Psychiatry 173 (2), 128–137. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 (1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Wells KB, Sherbourne CD, Rogers W, Spritzer K, 1995. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Arch. Gen. Psychiatry 52 (1), 11–19. [DOI] [PubMed] [Google Scholar]
- Hörn M, Schärer L, Walser S, Scherer-Klabunde D, Biedermann C, Walden J, 2002. Comparison of longterm monitoring methods for bipolar affective disorder. Neuropsychobiology 45 (Suppl. 1), 27–32. [DOI] [PubMed] [Google Scholar]
- IsHak W, Greenberg JM, Balayan K, Kapitanski N, Jeffrey J, Fathy H, Fakhry H, Rapaport MH, 2011. Quality of life: the ultimate outcome measure of interventions in major depressive disorder. Harv. Rev. Psychiatry 19 (5), 229–239. [DOI] [PubMed] [Google Scholar]
- IsHak W, Mirocha J, James D, Tobia G, Vilhauer J, Fakhry H, Pi S, Hanson E, Nashawati R, Peselow ED, Cohen RM, 2015. Quality of life in major depressive disorder before/after multiple steps of treatment and one-year follow-up. Acta Psychiatr. Scand 131 (1), 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Thase ME, Jarrett RB, 2014. Improvement in selfreported quality of life with cognitive therapy for recurrent major depressive disorder. J. Affect. Disord 167, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB, 1998a. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch. Gen. Psychiatry 55 (8), 694–700. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB, 1998b. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J. Affect. Disord 50 (2–3), 97–108. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, Endicott J, Coryell W, Kunovac JL, Mueller TI, Rice JP, Keller MB, 2000. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch. Gen. Psychiatry 57 (4), 375–380. [DOI] [PubMed] [Google Scholar]
- Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, Maser JD, Mueller T, Solomon DA, Keller MB, 2000. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am. J. Psychiatry 157 (9), 1501–1504. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Rush AJ, Coryell WH, Fiedorowicz JG, Solomon DA, 2016. A new empirical definition of major depressive episode recovery and its positive impact on future course of illness. J. Clin. Psychiatry 77 (8), 1065–1073. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Abbott R, Paykel ES, 2004. Longitudinal syndromal and sub-syndromal symptoms after severe depression: 10-year follow-up study. Br. J. Psychiatry J. Ment. Sci 184, 330–336. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Foy K, 2005. The impact of residual symptoms on outcome of major depression. Curr. Psychiatry Rep 7 (6), 441–446. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Paykel ES, 2004. Residual symptoms at remission from depression: impact on long-term outcome. J. Affect. Disord 80 (2–3), 135–144. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Schatzberg A, Rush AJ, Klein DN, Howland R, Gniwesch L, Davis SM, Harrison W, 2002. Psychosocial outcomes following long-term, double-blind treatment of chronic depression with sertraline vs placebo. Arch. Gen. Psychiatry 59 (8), 723–728. [DOI] [PubMed] [Google Scholar]
- Kolovos S, Kleiboer A, Cuijpers P, 2016. Effect of psychotherapy for depression on quality of life: meta-analysis. Br. J. Psychiatry J. Ment. Sci 209 (6), 460–468. [DOI] [PubMed] [Google Scholar]
- Leverich GS, Post RM, 1993. The NIMH Life Chart Manual™ for Recurrent Affective Illness. National Institute of Mental Health, Bethesda. [Google Scholar]
- Martin JK, Blum TC, Beach SR, Roman PM, 1996. Subclinical depression and performance at work. Soc. Psychiatry Psychiatr. Epidemiol 31 (1), 3–9. [DOI] [PubMed] [Google Scholar]
- Ormel J, Oldehinkel T, Brilman E, vanden Brink W, 1993. Outcome of depression and anxiety in primary care: a three-wave 31/2-year study of psychopathology and disability. Arch. Gen. Psychiatry 50 (10), 759–766. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mahal Y, Mischoulon D, Nierenberg AA, Fava M, 2004. Quality of life assessments in major depressive disorder: a review of the literature. Gen. Hosp. Psychiatry 26 (1), 13–17. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Clary C, Fayyad R, Endicott J, 2005. Quality-of-life impairment in depressive and anxiety disorders. Am. J. Psychiatry 162 (6), 1171–1178. [DOI] [PubMed] [Google Scholar]
- Rihmer Z, Gonda X, Akiskal KK, Akiskal HS, 2007. Affective temperament: a mediating variable between environment and clinical depression? Arch. Gen. Psychiatry 64 (9), 1096. 10.1001/archpsyc.64.9.1096-b. [DOI] [PubMed] [Google Scholar]
- Rosenthal TL, Akiskal HS, 1985. Subtypes of characterological depression. In: Clinical Psychopathology Nomenclature and Classification, pp. 623–627. 10.1007/978-1-4899-5049-9_107. [DOI] [Google Scholar]
- Steinert C, Hofmann M, Kruse J, Leichsenring F, 2014. Relapse rates after psychotherapy for depression stable long-term effects? A meta-analysis. J. Affect. Disord 168, 107–118. [DOI] [PubMed] [Google Scholar]
- ten Doesschate MC, Koeter MWJ, Bockting CLH, Schene AH, 2010. Health related quality of life in recurrent depression: a comparison with a general population sample. J. Affect. Disord 120 (1), 126–132. [DOI] [PubMed] [Google Scholar]
- Tranter R, O’Donovan C, Chandarana P, Kennedy S, 2002. Prevalence and outcome of partial remission in depression. J. Psychiatry Neurosci 27 (4), 241–247. [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB, 2007. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J. Consult. Clin. Psychol 75 (3), 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA, Investigators STARD, 2006. Self-rated global measure of the frequency, intensity, and burden of side effects. J. Psychiatr. Pract 12 (2), 71–79. [DOI] [PubMed] [Google Scholar]
- Woo J-M, Jeon HJ, Noh E, Kim H-J, Lee SW, Lee KK, Kim SH, Hong JP, 2014. Importance of remission and residual somatic symptoms in health-related quality of life among outpatients with major depressive disorder: a cross-sectional study. Health Qual. Life Outcomes 12 (1), 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


