ABSTRACT
Clinical studies have shown that combination therapy of antibodies targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) significantly improves clinical benefit over PD-1 antibody alone. However, broad application of this combination has been limited by toxicities. Cadonilimab (AK104) is a symmetric tetravalent bispecific antibody with a crystallizable fragment (Fc)-null design. In addition to demonstrating biological activity similar to that of the combination of CTLA-4 and PD-1 antibodies, cadonilimab possess higher binding avidity in a high-density PD-1 and CTLA-4 setting than in a low-density PD-1 setting, while a mono-specific anti-PD-1 antibody does not demonstrate this differential activity. With no binding to Fc receptors, cadonilimab shows minimal antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and interleukin-6 (IL-6)/IL-8 release. These features all likely contribute to significantly lower toxicities of cadonilimab observed in the clinic. Higher binding avidity of cadonilimab in a tumor-like setting and Fc-null design may lead to better drug retention in tumors and contribute to better safety while achieving anti-tumor efficacy.
KEYWORDS: PD-1/CTLA-4 bispecific antibody, drug retention, tumor microenvironment
Introduction
The development of immuno-oncology (IO) therapies have made substantial progress in recent years, and monoclonal antibodies (mAbs) that target the immune checkpoint programmed cell death-1 (PD-1) have been accepted as the standard of care in a number of tumor types. Numerous combination therapies with anti-PD-1 antibody to improve the efficacy of PD-1 monotherapy have been widely investigated. Current clinical studies have shown that combination therapy of anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) and anti-PD-1 antibodies could produce significantly improved efficacy for some hard-to-treat cancer types, such as renal cell cancer, gastric cancer, and small cell lung cancer, but the application has been limited by severe toxicities.1–5 Therefore, new approaches to achieve the efficacy benefit of PD-1 and CTLA-4 antibodies combination while lowering toxicities present a promising direction of IO drug research. Use of bispecific antibodies is one of the approaches being explored.
Cadonilimab (AK104) is a human tetravalent bispecific IgG1 antibody with symmetric IgG-single-chain variable fragment (scFv) structure, with Fc-null design to eliminate antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), complement-dependent cytotoxicity (CDC), and cytokine release. Fc receptor-mediated effector functions could eliminate or damage lymphocytes expressing PD-1 and CTLA-4 and thus reduce anti-tumor activity. Moreover, studies have indicated that immune-related adverse events (irAEs) caused by checkpoint-blocking antibodies were related to recruitment of immune cells bearing Fc receptor.6–9 Thus, removal of Fc receptor binding and effector function was designed for cadonilimab to improve the efficacy and safety of antibody drug. The first-in-human phase 1 study (NCT03261011) and a phase 1b/2 study (NCT03852251) showed that cadonilimab was safe with very low incidence of grade ≥3 irAEs and exhibited promising antitumor efficacy.10–12 Based on the encouraging results from clinical trials, cadonilimab was approved by the China National Medical Products Administration for recurrent or metastatic cervical cancer in June 2022.
Here, we report that cadonilimab possess high binding avidity especially to high density of PD-1 and CTLA-4 due to its tetravalent design and could simultaneously bind different cells expressing PD-1 and CTLA-4, respectively. By effective blocking both PD-1 and CTLA-4 pathways, cadonilimab could activate T cells by increasing interleukin-2 (IL-2) and interferon-γ (IFN-γ) secretion to similar extent as compared with anti-PD-1 and anti-CTLA-4 combination. With the elimination of Fc receptor binding, cadonilimab showed no ADCC, ADCP, or CDC effect and manifested reduced secretion of pro-inflammatory cytokines like IL-6 and IL-8. These features may contribute to lower toxicities and enhanced anti-tumor activity.
Results
Tetravalent design of cadonilimab based on co-expression of PD-1 and CTLA-4 in tumor tissues
Expression of PD-1 and CTLA-4 in different cancers were examined in The Cancer Genome Atlas (TCGA) PanCan Atlas studies. As shown in Figure 1a, there was a significantly positive correlation between the mRNA expression levels of PD-1 and CTLA-4 in breast, lung, stomach, colorectal, liver, cervical, head and neck, and ovarian cancer (Pearson: R = 0.74, P < 2.2e-16). Based on the co-expression status in tumors, cadonilimab was designed as a symmetrical tetravalent bispecific anti-PD-1/CTLA-4 antibody, constructed using Akeso Biopharma PD-1 antibody penpulimab (AK105) and Akeso Biopharma CTLA-4 antibody quavonlimab (AK107) (Figure 1b). The full amino acid sequence with annotation is shown in Table 1. It is composed of two heavy chains of the IgG1 subclass and two light chains of the kappa subclass, which are covalently linked through disulfide bonds, and the Fc null was designed to eliminate Fc receptors-mediated effector function. Cadonilimab has two N-linked glycosylation sites, Asn298 on Fc domain and Asn524 on the scFv domain, and contains 1,854 amino acids and has an overall molecular weight of approximately 200 kDa including oligosaccharides. This molecular design has been described in the US Patent 16327076.13 The tetravalent design of anti-PD1/CTLA-4 bispecific antibody is postulated to gain enhanced binding avidity in tumor tissues based on high-level co-expression of the targets.
Figure 1.
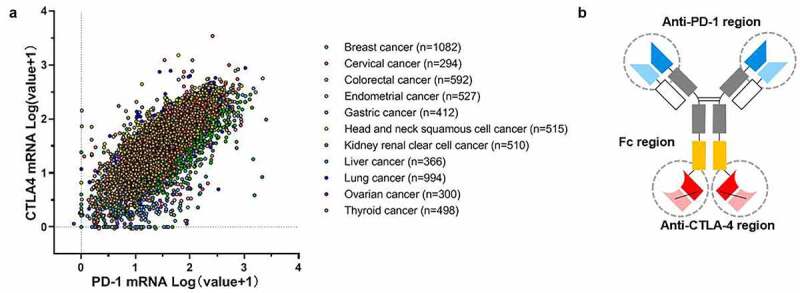
Tetravalent design of anti-PD1/CTLA-4 bispesific antibody cadonilimab based on co-expression of PD-1 and CTLA-4 in tumor tissue. (a) Correlation analysis of PD-1 and CTLA-4 mRNA expression levels in various tumor types from TCGA dataset using cBioportal. The X- and Y-axis represent the mRNA expression level transformed by Log(value+1). TCGA, The Cancer Genome Atlas. (b) Schematic diagram of cadonilimab tetravalent structure.
Table 1.
Cadonilimab full peptide sequence.
 |
Notes: a. The CDR region are highlighted in blue. b. The fragment crystallizable (Fc) mutations at position Ala235, Ala236, and Ala238 are highlighted in red. c. The flexible linkers are highlighted in green. d. The underlined region is the human IgG1, kappa constant region sequence.
Characterization of binding and cellular activity to show efficient PD-1 and CTLA-4 blocking and biological activity
Binding activity of cadonilimab to the antigens was evaluated by enzyme-linked immunosorbent assay (ELISA) and fluorescence-activated cell sorting (FACS). As shown in Table 1, compared with anti-PD-1 nivolumab and anti-CTLA-4 ipilimumab, cadonilimab shows similar binding activity with immobilized human CTLA-4 and human PD-1 separately and similar affinity in HEK293T cells stably transfected with either human CTLA-4 (293 T-CTLA4 cells) or PD-1 (293 T-PD1 cells). Compared with nivolumab and ipilimumab, cadonilimab shows similar competitive binding activity by blocking human PD-1 from binding to its ligand (human PD-L1 and human PD-L2) and blocking human CTLA-4 from binding to human B7.1 and human B7.2. The assay in 293 T-PD-1 or 293 T-CTLA-4 cells showed that cadonilimab could competitively bind to the cell surface CTLA-4 and PD-1, and the half-maximal concentration (EC50) was similar to that of nivolumab and ipilimumab (Table 2). Blocking of PD-1 and CTLA-4 pathways leads to activation of T cells, accompanied by increased T-cell proliferation and enhanced cytokine secretion (IL-2, IFN-γ). The ability of cadonilimab to promote T-cell responses was examined in an assay system composed of human peripheral blood mononuclear cells (PBMCs), dendritic cells (DCs), and Raji cells. As shown in Figure 2, both IL-2 and IFN-γ levels increase significantly in a trend of dose dependence. Compared with nivolumab plus ipilimumab, cadonilimab showed similar activity to increase IL-2 and IFN-γ secretion, the difference was not statistically significant. All these data demonstrate that by blocking PD-1 and CTLA-4, cadonilimab is able to promote T cell responses by elevating IL-2 and IFN-γ secretion and has similar efficacy in T-cell activation with nivolumab and ipilimumab.
Table 2.
Comparison of cadonilimab with nivolumab and ipilimumab: target antigen binding and competitive binding with target antigen and ligand.
| Antibody | EC50 (nM) of antigen binding |
EC50 (nM) of competitive binding |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
aELISA |
bFACS |
cELISA |
dFACS |
|||||||
| PD1-mFc | CTLA4-mFc | 293 T-PD1 cell | 293 T-CTLA4 cell | PD1-hFc |
CTLA4-mFc |
293 T-PD1 cell |
293 T-CTLA4 cell |
|||
| PDL1-mFc | PDL2-His | B7.1-hFc-bio | B7.2-His | PDL1-mFc | B7.1-hFc-bio | |||||
| Cadonilimab | 0.051 | 0.079 | 1.812 | 3.09 | 0.792 | 1.429 | 1.052 | 0.718 | 2.62 | 3.93 |
| Nivolumab | 0.084 | \ | 1.503 | \ | 0.725 | 3.035 | \ | \ | 1.53 | \ |
| Ipilimumab | \ | 0.033 | \ | 0.5363 | \ | \ | 1.345 | 0.591 | \ | 1.64 |
Notes: a, fusion protein of mouse Fc with human PD-1 extracellular domain (PD1-mFc), and fusion protein of mouse Fc with human CTLA-4 extracellular domain (CTLA4-mFc) was fixed onto the plates in the assays. c, fusion protein of human Fc with human PD-1 extracellular domain (PD1-hFc), and CTLA4-mFc was fixed onto the plates; histidine-tagged human PDL-2 extracellular domain (PDL2-His) and human B7.2 extracellular domain (B7.2-His), and fusion protein of mouse Fc with human PDL1 extracellular domain (PDL1-mFc), and biotinylated fusion protein of human Fc with human B7.1 extracellular domain (B7.1-hFc-bio) were used as competition ligand in the assays. b, d, 293 T cells transfected with human PD-1 (293 T-PD1 cell) or CTLA-4 (293 T-CTLA4 cell) were used as target cells in the assay. d, PDL1-mFc and B7.1-hFc-bio were used as competition ligand in the assays.
Figure 2.
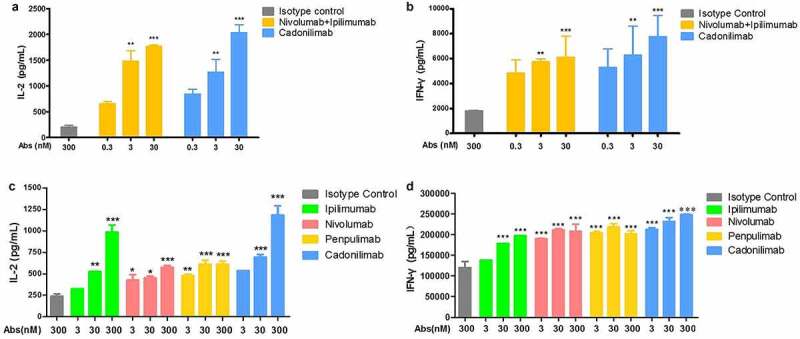
Cadonilimab induce IL-2 and IFN-γ production in mixed lymphocyte reaction assays. (a, b) Cadonilimab promoted activation of human peripheral blood mononuclear cells (hPBMCs), which induced a more robust secretion of IL-2 and IFN-γ in mixed culture of hPBMCs and human DCs; Compared with nivolumab plus ipilimumab, cadonilimab did not significantly improve secretion of IL-2 and IFN-γ. (c, d) Cadonilimab enhanced secretion of IL-2 and IFN-γ in mixed culture of hPBMCs and Raji-PDL1 cells. Data are shown as mean ±SEM for n = 2 and analyzed using one-way ANOVA. *P < .05, **P < .01 and ***P < .001 vs. isotype control.
Tetravalent design of cadonilimab leads to higher avidity binding to high density antigen and trans-binding of cells expressing PD-1 and CTLA-4
In the tumor microenvironment (TME), enriched co-expression of PD-1 and CTLA-4 on tumor-reactive T cells was found, while a relatively low expression of PD-1 and/or CTLA-4 was reported in normal peripheral tissues.14 Cadonilimab has a novel tetravalent form that is expected to have higher avidity in TME but relatively low avidity in normal peripheral tissues. To test this, high density of coated PD-1 and CTLA-4 (50 nM) was used to mimic the antigen expression situation in the TME, while low density of PD-1 (10 nM) was used to mimic normal peripheral tissue. Cadonilimab showed 10-fold higher binding avidity with co-expressed high density antigens than with low-density PD-1, while PD-1 antibody showed similar binding avidity in these two situations. This suggests cadonilimab may be able to achieve preferential tumor retention while anti-PD-1 or anti-CTLA-4 monospecific antibody could not (Figure 3).
Figure 3.
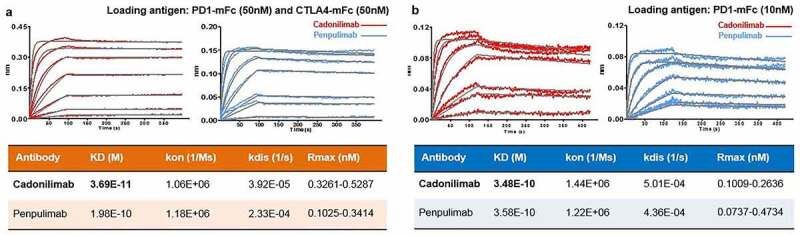
Cadonilimab demonstrates preferential higher avidity binding to higher density of PD-1 and CTLA-4. In a Fortebio assay, cadonilimab showed higher binding avidity with a (a) high density of PD-1 and CTLA-4, where PD-1 (50 nM) and CTLA-4 (50 nM) were loaded onto the sensor, compared with (b) that of a relative low density of PD-1, where PD-1 (10 nM) was loaded onto the sensor. Penpulimab (parental PD-1 antibody of cadonilimab) showed similar binding avidity under these antigen conditions (a) and (b).
The ability of antibodies to connect different target cells in the TME may contribute to the accumulation of tumor-infiltrating lymphocytes (TILs). To visually observe whether cadonilimab can bind CTLA-4 and PD-1 on two different cells simultaneously (i.e., induce formation of cell doublets), an experiment is conducted to examine the presence of trans-binding. CHO-K1 cells expressing CTLA-4 are seeded into the plates and incubated with Jurkat cells expressing PD-1 stained with Hoechst 33342 at the presence of cadonilimab or control antibodies, followed by a washing step. In samples incubated with cadonilimab, strong adherence of Jurkat-PD-1 cells to CHO-K1-CTLA-4 cells can be observed, but in samples incubated with nivolumab and ipilimumab, the cell complexes are not observed (Figure 4a). We also observed the crosslinking of cadonilimab with cells expressing PD-1 and CTLA-4 in FACS assay, consistent results were obtained. CHO-K1 cells expressing PD-1 and CTLA-4 were labeled with CFSE and CellTrace™ far-red, respectively. The crosslinking of cadonilimab with CHO-K1 cells expressing PD-1 and CTLA-4 was shown as CFSE/Far-red dual positive %. Compared to negative control and isotype control, the combination of nivolumab and ipilimumab mediated no crosslinking, while cadonilimab mediated the crosslinking of CHO-K1 cells expressing PD-1 and CTLA-4 in a dose-dependent manner (Figure 4b). These results demonstrate the ability of cadonilimab to bind different cells expressing PD-1 and CTLA-4. This property, together with enhanced antigen-binding avidity of cadonilimab in the TME, may contribute to the accumulation of more TILs in the TME.
Figure 4.
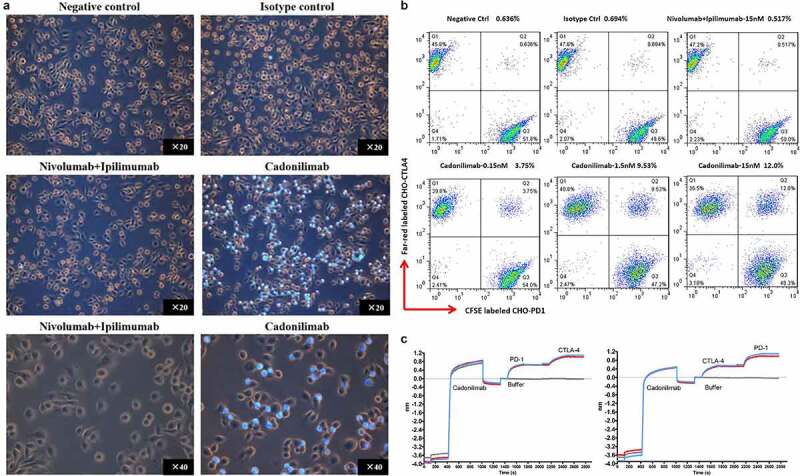
Tetravalence binding of Cadonilimab to PD-1 and CTLA-4. (a) Trans-binding of Cadonilimab to CHO-K1-CTLA-4 cells (Orange cells) which transfected with human CTLA-4, and Jurkat-PD1 cells (light blue cells) labeled with Hoechst 33342. Jurkat-PD1 cell which is a suspension lymphoblasts cell line became adherent to CHO-K1-CTLA-4 cells when co-cultured with cadonilimab, but not with nivolumab plus ipilimumab. (b) Crosslinking of cadonilimab with cells expressing PD-1 and CTLA-4 in FACS assay. AK104 or control antibodies were added to a 1:1 mix of Far red-labeled CTLA-4-expressing CHO-K1 cells and CSFE-labeled PD-1-expressing CHO-K1 cells. (c) Cadonilimab could bind to human PD-1 and CTLA-4 with similar binding activity regardless of binding PD-1 or CTLA-4 firstly. Cadonilimab was fixed onto the sensor in Fortebio assay, and sequential binding to human PD-1 (PD1-hFc) and CTLA-4 (CTLA-4-hFc) or in reverse order was performed and measured.
To investigate whether the binding sequences to the two targets influence avidity, the binding activity was detected by adding PD-1 or CTLA-4 sequentially, using Fortebio Octet molecular interaction system. As shown in Figure 4c, the binding activity is similar regardless of whether the antibody binds PD-1 or CTLA-4 first, indicating that cadonilimab could simultaneously bind PD-1 and CTLA-4, and the binding activities of PD-1 arm and CTLA-4 arm are both stable.
Fc null design of cadonilimab results in the elimination of Fc receptor-mediated effector functions and reduced the release of proinflammatory cytokines
The majority of antibody effector functions is delivered by the Fc through interaction with Fc receptors and C1q.15 However, engaging immune effector functions through Fc receptor and complement interactions may be detrimental to the desired therapeutic effect.16 For example, ADCC and ADCP can deplete PD-1 expressing T cells and compromise the anti-tumor efficacy of anti-PD-1 antibody.17 On the other hand, recent literature also indicates that many irAEs are related to recruitment of immune cells bearing Fc receptors.6–9 Thus, Fc mutations to eliminate Fc-mediated effector functions were introduced into cadonilimab for both safety and efficacy considerations.
Our results confirmed the lack of binding of cadonilimab to Fc receptors and C1q, compared with strong binding of cadonilimab in hG1WT format (Table 3). Next, we tested the functional consequences of the Fc mutation design. As shown in Figure 5, compared with nivolumab, ipilimumab, and penpulimab (Akeso Biopharma anti-PD-1 mono-specific antibody) in hG1WT format and cadonilimab in hG1WT format, cadonilimab significantly decreased Fc-mediated effector function (ADCC, ADCP, CDC, and antibody mediated pro-inflammatory cytokine release).
Table 3.
Cadonilimab with Fc null mutation abolished binding to FcγRIa, FcγRIIIa_V158, FcγRIIIa_F158 and C1q.
| aFcγR/bC1q | Antibody | KD (M) | kon (1/Ms) | kdis (1/s) | Rmax (nm) |
|---|---|---|---|---|---|
| FcγRIa | Cadonilimab | N.D. | |||
| Cadonilimab (hG1WT) | 5.92E-09 | 3.06E+05 | 1.81E-03 | 0.53–0.62 | |
| FcγRIIIa_V158 | Cadonilimab | N.D. | |||
| Cadonilimab (hG1WT) | 1.77E-07 | 1.21E+05 | 2.14E-02 | 1.56–1.61 | |
| FcγRIIIa_F158 | Cadonilimab | N.D. | |||
| Cadonilimab (hG1WT) | 2.21E-07 | 1.12E+05 | 2.47E-02 | 0.39–0.64 | |
| C1q | Cadonilimab | N.D. | |||
| Cadonilimab (hG1WT) | 2.53E-09 | 2.05E+06 | 5.17E-03 | 0.05–0.18 | |
Notes: a, FcγRIa, FcγRIIIa_V158 (FcγRIIIa 158 V alleles), FcγRIIIa_F158 (FcγRIIIa 158 F alleles) were fixed onto the sensor in Fortebio assays. b, antibodies were fixed onto the sensor in assay for C1q binding affinity
Abbreviation: N.D. not detected.
Figure 5.
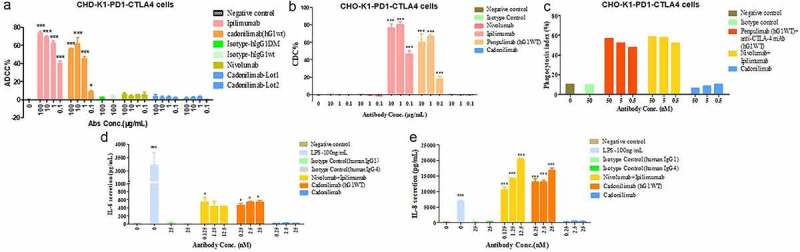
Cadonilimab with Fc null-mutations for both safety and efficacy concerns. (a) Antibody-dependent cell-mediated cytotoxicity (ADCC) activities were determined by measuring lactase dehydrogenase (LDH) release from 293 T-CTLA4-PD1 cells. (b) Complement-dependent cytotoxicity (CDC) activities of cadonilimab, penpulimab in hG1WT format, and ipilimumab and nivolumab were determined by measuring LDH release from CHO-K1-PD1-CTLA4 cells. (c) Antibody-dependent cellular phagocytosis (ADCP) activities of cadonilimab, penpulimab in hG1WT format plus anti-CTLA-4 mAb in hG1WT format, and nivolumab plus ipilimumab were studied by examining phagocytosis of CHO-K1-PD1-CTLA4 cells by murine bone marrow derived macrophages (MBMMs). (d, e) Cadonilimab decreased the release of inflammatory cytokines IL-8 and IL-6 from human peripheral monocyte-derived macrophages (HPMMs). Data are expressed as mean or mean ±SEM and analyzed using one-way ANOVA. *P < .05 and ***P < .001 vs. isotype control; ###P < .001 vs. negative control.
Two key cytokines, IL-6 and IL-8, have been shown to negatively affect both efficacy and safety of immuno-oncology therapy.18–22 In our study, cadonilimab could significantly decrease IL-6 and IL-8 secretion, compared with nivolumab plus ipilimumab, and cadonilimab in hG1WT format (Figure 5).
Discussion
Here, we report the characterization of cadonilimab, a tetravalent bispecific antibody designed to achieve the biological effect of simultaneous blockade of PD-1 and CTLA-4 pathways, while reducing toxicities to a level significantly lower than that observed for combination theray of PD-1 and CTLA-4 antibodies.
Our study shows that with high density co-expression of the antigens PD-1 and CTLA-4, cadonilimab has 10-fold higher binding avidity compared to the binding observed with low-density expression of a single antigen. This observation is consistent with the understanding of cooperativity of binding at higher valency. This could facilitate cadonilimab retention in area with high density of both antigens. Many studies have demonstrated that PD-1 and CTLA-4 co-express at high level in TILs and draining lymph nodes in TME but not in normal peripheral tissues. The enhanced binding avidity of cadonilimab at high density of PD-1 and CTLA-4 could lead to preferential accumulation in TME, thus achieving efficacy with less toxicity.
A key feature of bispecific antibodies is the ability to recognize and bind two antigens simultaneously. Among the anti-PD-1/CTLA-4 bispecific antibodies in clinical studies, MEDI5752 and XmAb20717 are bivalent and MGD019 and cadonilimab are tetravalent. The bivalent antibodies would in essence require the presence of both targets on the same cell. For cells that only express one target, the binding potency for the bivalent antibodies would likely be too low to achieve sufficient target suppression. For cadonilimab, the anti-PD-1 arm and anti-CTLA-4 arm show a similar range of antigen-binding affinity compared to nivolumab and ipilimumab, respectively. This is different from MEDI5752 and MGD019,23,24 which both show low CTLA-4 blockade, and the enhancement of CTLA-4 binding is dependent on PD-1 anchoring on antigen co-expressing cells. For cadonilimab, the stable and high avidity binding to PD-1 and CTLA-4 contribute to trans-binding to different cells expressing PD-1 and CTLA-4. The trans-binding test in our study further confirmed cadonilimab could connect cells expressing PD-1 and CTLA-4. The trans-binding activities of cadonilimab could also contribute to TME retention of this bispecific antibody in tumor tissues.
Among the four subclasses of human IgG (IgG1, IgG2, IgG3, and IgG4), different subclasses have different effector functions. With exceptions of penpulimab25 and prolgolimab (BCD-100),26 all currently marketed anti-PD-1 antibodies are of the IgG4 isotype. Compared to IgG4, IgG1 has more robust CMC profile and is less prone to inherent complications including in vivo interactions with other antibodies.27 Among the clinical-stage anti-PD-1/CTLA-4 bispecific antibodies, cadonilimab, XmAb20717, and MEDI5752 are IgG1, and MGD019 is an IgG4 antibody, and all four of these antibodies have Fc mutations to either attenuate or completely abolish Fc effector functions.
IgG1 can recruit complement more effectively than IgG4 and exerts therapeutic efficacy mainly via ADCC/ADCP, eliminating Tregs and other immunosuppressive cells.28 However, for cases where mAbs are intended to engage cell surface receptors and prevent receptor–ligand interactions, reducing effector function may be desired. Also, irAEs are known to be related to recruitment of immune cells carrying Fc receptors.6–9 Fc engineering has been a strategy to modulate antibody effector functions.16 Among the bispecific antibodies targeting PD-1 and CTLA-4 in clinical studies, XmAb20717 and MGD019 have a Fc-null design, MEDI5752 is designed with Fc engineering to weaken ADCC, and cadonilimab is designed with Fc null backbone to eliminate Fc-mediated effector function and eliminate production of pro-inflammatory cytokines, including IL-6 and IL-8. IL-6 plays a critical role in irAEs in patients who received anti-PD-1 treatment.18,19 The immune-suppressive effects of IL-6 on T-cell-mediated anti-tumor immunity has been widely reported.20 An increase of IL-6 during treatment was correlated with the poor clinical response to PD-1 blockade.21 In addition, high IL-8 levels have been associated with attenuated efficacy of immune check-point inhibitor therapy. In cancer patients, higher levels of IL-8 predicted a worse clinical outcome and blockade of IL-8 improved anti-tumor immune response.22 In our study, cadonilimab generates minimal IL-6 and IL-8 secretion when compared to nivolumab and ipilimumab. This property may present a mechanism of action of cadonilimab distinct from the combination of anti-PD-1 and anti-CTLA-4 antibodies, which may affect safety and efficacy.
The safety and efficacy of cadonilimab has been studied in over a thousand cancer patients so far. In 2017, clinical studies were started to evaluate cadonilimab in safety, tolerability, pharmacokinetics, immunogenicity, pharmacodynamics and efficacy in advanced solid tumors. This study (NCT03261011) showed that AK104 can be given safely up to 25.0 mg/kg in patients every three weeks with very low incidence of serious adverse events (SAEs) and showed encouraging anti-tumor activity when dosed ≥4 mg/kg.10 In a Phase 2 registration study, cadonilimab monotherapy as second-line or greater treatment for recurrent or metastatic cervical cancer showed an objective response rate (ORR) of 33% and good safety profile.11 The ORR for PD-1 antibody monotherapy for this patient population is generally around 15%. The incidence of grade ≥3 irAEs for cadonilimab in this trial was only 5%. For advanced gastric or gastroesophageal junction adenocarcinoma, patients who received cadonilimab plus chemotherapy achieved ORR of 65.9%, median progression-free survival (mPFS) of 7.10 months and median overall survival (mOS) of 17.41 months.12 Based on the favorable results, clinical studies to further evaluate the efficacy and safety of cadonilimab monotherapy and combination therapy are ongoing. As of January 2023, cadonilimab is included in 40 clinical trials registered in ClinicalTrials.gov, including five phase 3 trials, covering a variety of tumor types.
Cadonilimab is a symmetric tetravalent bispecific antibody with Fc null design. With such a molecular format, cadonilimab shows high binding avidity in high antigen density, could eliminate Fc-mediated ADCC, ADCP, and ADCR, and meanwhile maintain T cell activation, which may all contribute to the safety and efficacy of this novel molecule in cancer treatment. Clinical trials data has also proved its safety and anti-tumor efficacy. Cadonilimab potentially represents a new promising immunotherapy for cancer patients.
Materials and methods
mRNA expression of PD-1 and CTLA-4 in various tumor types
As a website for cancer genomics dataset, the cBioPortal (https://www.cbioportal.org/) was used for analyzing the mRNA expression of PD-1 and CTLA-4 from TCGA PanCan Atlas studies.29 The mRNA expression data were downloaded via cBioPortal Plot Sub-tool. The mRNA expression value from RNA-seq data were scaled with Log(value+1) and the correlation analysis of PD-1 and CTLA-4 mRNA expression levels in 11 tumor types were performed using R version 4.1.0 software.
Antibody construction
Cadonilimab, a humanized symmetrical bispecific antibody targeting both human PD-1 and CTLA-4, has an IgG-scFv format, as reported in the literature.30 Briefly, to construct the bispecific antibody, we fused the scFv of anti-CTLA-4 onto the C terminus of the heavy chain of anti-PD1 antibody via a flexible (Gly4Ser) linker. The bispecific antibody consists of two heavy chains (HC) and two light chains (LC), which are covalently linked to form a symmetric tetravalent antibody structure through disulfide bonds and were designed as a human IgG1, kappa subclass. Mutations (L235A/L236A/G238A) were introduced into the Fc region to eliminate binding to FcγRs and C1q.
ELISA binding
The binding activity of cadonilimab to its antigens were detected using ELISA. CTLA-4-murine Fc (CTLA-4-mFc) and PD-1-murine Fc (PD-1-mFc), which were prepared in house by Akeso Biopharma, were, respectively, coated on 96-well microplates. Cadonilimab was tested as a 7-point concentration response curve with serial dilutions of 1:3 starting at a top concentration of 1 μg/mL. Phosphate-buffered saline with Tween® (PBST) was added to obtain plate blank values. Horseradish peroxidase (HRP)-labeled goat anti-human IgG (Supplier: Jackson, Catalog number:109–035-088) was used as the detection antibody. The optical density (OD) values were acquired by microplate reader and analyzed with SoftMax Pro 6.2.1 to create a Four Parameter Logistic (4PL) curve fit by plotting the antibody concentration on the x-axis and OD on the y-axis.
FACS binding
For cell surface binding with PD-1 and CTLA-4, 293 T-CTLA4 cells or 293 T-PD1 cells constructed by Akeso Biopharma were used. Briefly, cells were collected and incubated with serially diluted antibody. After washing with PBSA (PBS with 1% BSA (Bovine Serum Albumins, purchased from Sigma, Cat.: V900933-1 KG)), cells were stained with fluorescein isothiocyanate (FITC) labeled goat anti-human IgG secondary antibody (Jackson, Cat.: 109–095-098). The analysis was performed using a flow cytometer (BD FACSCalibur). Mean fluorescence intensity (MFI) was acquired using Flowing software. Binding activity of cadonilimab, ipilimumab (BMS, Lot.: AAT3892), or nivolumab (BMS, Lot.: ABA0330) was determined by the competitive binding of EC50.
ELISA competitive binding
CTLA-4-mFc fusion protein was coated onto 96-well microplates to determine the competition activity between cadonilimab and B7.1 and the competition activity between cadonilimab and B7.2, respectively. Cadonilimab or ipilimumab (constructed by Akeso Biopharma) was added with serial dilutions of 1:3. B7.1-hFc-bio (constructed by Akeso Biopharma) and B7.2-His (Supplier:Sino Biological, Inc., Catalog number:10699-H08H) were added to the microplates, respectively. HRP-labeled streptavidin (Supplier: KPL, Catalog number:14–30-00) and HRP-labeled mouse anti-His (Supplier: Cwbio, Catalog number:CW0285M) were used as the detection agents.
PD-1-hFc fusion protein (constructed by Akeso Biopharma) were coated onto 96-well microplate to determine the competition activity between cadonilimab and PD-L1 and the competition activity between cadonilimab and PD-L2, respectively. Cadonilimab or nivolumab (constructed by Akeso Biopharma) was added with serial dilutions of 1:3. PD-L1-mFc fusion protein (constructed by Akeso Biopharma) and PD-L2-His fusion protein (Supplier: Sino Biological, Inc., Catalog number:10292-H08H) were added to the microplates, respectively. HRP-labeled goat anti-mouse IgG (Supplier: Jackson, Catalog number:115–035-062) and HRP-labeled mouse anti-His (Supplier: Cwbio, Catalog number:CW0285M) were used as the detection agents.
Substrate was added for color development. The reaction was stopped with 2M H2SO4, and the absorbance at 450 nm was determined with a standard plate reader.
The OD values were acquired by microplate reader and analyzed with SoftMax Pro 6.2.1 to create a Four Parameter Logistic (4PL) curve fit by plotting the antibody concentration on the x-axis and OD on y-axis.
FACS competitive binding
For cell surface competitive binding with PD-L1 and B7.1, 293 T-CTLA4 cells or 293 T-PD1 cells constructed by Akeso Biopharma were used. Briefly, cells were collected and incubated with serially diluted antibody. After washing with PBSA (PBS with 1% BSA (Bovine Serum Albumins, purchased from Sigma, Cat.: V900933-1 KG)), cells were incubated with B7.1-hFc-bio (Akeso Biopharma) or PD-L1-mFc (Akeso Biopharma) at final concentration of 20 nM, respectively. Then, cells were stained with FITC-labeled Streptavidin (Biolegend, Cat.: 405202) or FITC-labeled anti-mouse secondary antibody (BD, Cat.: 555988). The analysis was performed using a flow cytometer (BD FACSCalibur). MFI was acquired using Flowing software. Competitive binding activity of cadonilimab, ipilimumab (BMS, Lot.: AAT3892), or nivolumab (BMS, Lot.: AAG6552) was determined by competitive binding EC50.
BLI analysis
Biolayer interferometry was performed by Fortebio OctetQKe and Fortebio Octet Red96e. For C1q, the antibodies (50 μg/mL) were immobilized onto FAB2G sensor. Gradient concentrations of C1q (1.25, 2.5, 5, 10, and 20 nM) were then flowed over the chip surface. FcγRIa and FcγRIIIa(5 μg/mL) were immobilized on the HIS1K sensor, respectively, and 2-fold serially diluted antibody (50 nM to 3.12 nM for FcγRIa, 500 nM to 31.25 nM for FcγRIIIa) flowed through the chip. For human PD-1 and CTLA-4 mixture, PBST (pH 6.0) was used as buffer solution, and human PD-1 and CTLA-4 mixture (mol/mol: 1/1) were immobilized on AMC sensors; 3-fold serially diluted antibody (0.27 nM to 200 nM) was flowed over the chip surface. For human PD-1, PBST (pH 6.0) was used as buffer solution and 10 nM human PD-1 was immobilized on AMC sensors, and then 3-fold serially diluted antibody (0.27 nM to 200 nM) was flowed over the chip surface. All the data were collected using Fortebio Data Acquisition 7.0 (or 12.0) and analyzed with Fortebio Data Analysis 7.0(or 12.0).
Crosslinking of PD-1 and CTLA-4 expressing cells
Crosslinking of PD-1 and CTLA-4 expressing cells was performed between CTLA-4-expressing CHO-K1 cells (Akeso Biopharma) and PD-1 expressing Jurkat cells (Akeso Biopharma). Briefly, CHO-K1 was plated into the 12-well plates and allowed to adhere at 37°C in 5% CO2. Thereafter, cadonilimab or control antibodies were added to the plates and incubated for 1 hr followed by washing with PBS (Akeso Biopharma). Jurkat cells were stained with Hoechst 33342 (Biohao, Cat.: C0420) and washed with PBS, then Jurkat cells were added into the plates with CHO-K1 cells and incubated for 20 min. After the incubation, suspended Jurkat cells were removed by washing, and the crosslinking between PD-1 and CTLA-4 expressing cells was analyzed microscopically.
ADCC assay
ADCC activities were determined by measuring lactase dehydrogenase (LDH) release from cells. PBMCs from a healthy volunteer were isolated using Ficoll-PaqueTM Plus (GE, Cat.: 17–1440-02). 293 T-CTLA4-PD1 cells (Akeso Biopharma) were seeded into 96-well plates at 3*104 cells per well and incubated with cadonilimab, cadonilimab (hG1WT), or isotype control for 1 hour at room temperature. Thereafter, PBMCs were added into the plates at 9.0 × 105 cells per well and incubated in an incubator for 4 hours. Then, the LDH activity in the supernatants was measured using LDH assay kit (Roche, Cat.: 11644793001). ADCC activity was reported as ADCC% and calculated as follows: ADCC% = ((OD of the experimental group – OD of the negative control group)/(OD of the positive control group – OD of the negative control group))* 100%.
CDC assay
CDC activities were determined by measuring LDH release from cells. On the assay day, the target cells (CHO-K1-PD1-CTLA4 cells (Akeso Biopharma)) were harvested, washed with assay medium (RPMI 1640 containing 1% fetal bovine serum (FBS)) and then resuspended in assay medium. The cells were seeded into 96-well plates at 3*104 cells/well. Serially diluted antibodies at final concentration as indicated in figures were then added into the assay plates containing target cells and pre-incubated at room temperature for 10 min. Following the pre-incubation, normal human serum (Quidel, Cat.: A113) at final concentration of 2% was added and incubated for 4 hours. Four hours later, the LDH activity in the supernatants was measured using LDH assay kit (Roche, Cat.: 11644793001). To calculate the CDC%, the absorbance values are substituted in the following equation: CDC% = ((OD of the experimental group – OD of the negative control group)/(OD of the positive control group – OD of the negative control group))* 100%.
ADCP assay
Murine bone marrow-derived monocytes were isolated and differentiated into macrophages in DMEM-CM (DMEM plus 10% FBS) containing murine macrophage-colony stimulating factor (M-CSF, purchased from Peprotech, Cat.: 315–02) (100 ng/mL) for 7 days in a 37°C incubator with 5% CO2. The medium was half-changed on day 3 and 5. Macrophages were harvested on day 7 and resuspended in DMEM-CM.
The target cells (CHO-K1-PD1-CTLA4 cells constructed by Akeso Biopharma) were collected and labeled with CFSE (Biolegend, Cat.: 423801) (2.5 μM). Macrophages and target cells were co-cultured in DMEM-CM with indicated antibodies. DMEM-CM only and human IgG1 group were set as negative control and isotype control, respectively. The co-culture cell mixtures were incubated at 37°C for 2 hours followed by washing in 1% PBSA. Cells were stained with APC-conjugated goat anti-mouse/human CD11b (Biolegend, Cat.: 101212) on ice for 40 minutes subsequently being washed in 1% PBSA. Cells were resuspended in 200 μL 1% PBSA and analyzed with FACS Calibur (Becton Dickinson). The phagocytic index (P%) was calculated according to the following formula: ADCP% = ((number of APC-CD11b+CFSE+ cells)/(number of APC-CD11b+ cells)) * 100%.
ADCR assay
Human peripheral blood-derived monocytes were differentiated into macrophages (HPMMs) in 1640-CM (RPMI 1640 medium plus 10% FBS) containing human M-CSF (Peprotech, Cat.: 300–25) for 7 days in a 37°C incubator with 5% CO2. The medium was half-changed on day 3 and 5. Fifty ng/ml IFN-γ (Sinobiological, Cat.: 11725-HNAS-100) was added for the final 24 hours of differentiation. HPMMs were collected and seeded into 96-well plates for further 24 hours of culture. CHO-K1-PD1-CTLA4 cells constructed by Akeso Biopharma were collected and resuspended in 1640-CM, then co-cultured with HPMMs plus indicated antibodies in an incubator for 24 hours. LPS (Sigma, Cat.: L4391), medium, and hIgG was set as positive, negative control, and isotype control, respectively. Following the incubation, the supernatants were harvested for measurement of IL-8 and IL-6 using ELISA kits (Dakewe, Cat.: 1110802, 1110602) according to the instructions.
Data were expressed as the mean ± SEM. Statistical significance was determined by one-way ANOVA analysis within GraphPad Prism software. P < .05 and P < .01 were considered statistically significant and highly significant.
The secretion of IL-2 and IFN-γ promoted by cadonilimab in mixed culture of PBMCs and DCs
PBMCs were isolated from two healthy donors using Ficoll-PaqueTM Plus (GE, Cat.: 17–1440-02). PBMCs from one donor were cryopreserved for later use. PBMCs from another donor were used for DC induction. Specifically, monocytes were isolated from PBMCs by adherence, then induced to differentiate into imDCs by culturing for 3 days in the presence of IL-4 (Peprotech, Cat.: 200–04) and GM-CSF (Peprotech, Cat.: 300–03). ImDCs were further stimulated to mDC by culturing for an additional 2 days in the presence of IFN-γ (Sinobiological, Cat.: 11725-HNAS-100), GM-CSF, and LPS (Sigma, Cat.: L4391). mDCs were harvested and cryopreserved for later use.
One day before the assay, PBMCs were thawed and cultured in complete medium overnight. On the assay day, mature DCs were thawed and cultured in complete medium for 2 hours. Then, PBMCs and DCs were harvested and seeded into 96-well assay plates at 1.0 × 105 cells/well and 1.0 × 104 cells/well, respectively. Antibody were subsequently added into the assay plates and incubated for 5 days in 37°C and 5% CO2 incubator. After the incubation, supernatants were collected for the measurement of IL-2 and IFN-γ by ELISA (Dakewe, Cat.: 1110202, 1110002).
The secretion of IL-2 and IFN-γ promoted by cadonilimab in mixed culture of PBMCs and Raji-PDL1 cells
SEB (Dianotech, Cat.: S010201)-stimulated PBMCs and Raji-PD-L1 cells (Akeso Biopharma) were collected and plated into 96-well plates at 1.0 × 105/well. Cadonilimab/penpulimab were added into the assay plates at final concentration of 3, 30, 300 nM; nivolumab (Akeso Biopharma) was used as positive control, and human IgG1 was set as isotype control. The assay plates were then incubated for 3 days. Three days later, the assay plates were removed from the incubator, and the supernatants were then harvested for the measurement of IL-2 and IFN-γ by ELISA (Dakewe, Cat.: 1110202, 1110002).
Acknowledgments
This study is funded by Akeso Biopharma. The authors would like to thank all the colleagues contributed to this study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
ADCC, Antibody-dependent cellular cytotoxicity; ADCP, Antibody-dependent cellular phagocytosis; CDC, Complement-dependent cytotoxicity; Fc, Crystallizable fragment; DCs, Dendritic cells; EC50, Half-maximal concentration; HC, Heavy chain; IO, Immuno-oncology; scFv, Single-chain variable fragment; TCGA, The Cancer Genome Atlas; irAEs, Immune-related adverse events; LC, Light chains; LDH, Lactase dehydrogenase; mPFS, Median progression-free survival; mOS, Median overall survival; PBMCs, Peripheral blood mononuclear cells; TILs, Tumor infiltrating lymphocytes.
Data availability statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Disclosure statement
The authors are all employees of Akeso Biopharma outside the submitted work.
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–10. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz HJ, Atasoy A, Shangguan T, Zhao H, El-Rayes B.. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. 2019;24:1453–61. doi: 10.1634/theoncologist.2019-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X, McDermott DF. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin Biol Ther. 2018;18:947–57. doi: 10.1080/14712598.2018.1513485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–85. doi: 10.1016/S1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas E, Salem TZ. Back to the light side: the role of mechanotransduction in the paradoxical response to checkpoint inhibitors in cancer patients. Crit Rev Immunol. 2019;39:165–73. doi: 10.1615/CritRevImmunol.2019031554. [DOI] [PubMed] [Google Scholar]
- 7.Collins M, Michot JM, Danlos FX, Mussini C, Soularue E, Mateus C, Loirat D, Buisson A, Rosa I, Lambotte O, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860–65. doi: 10.1093/annonc/mdx403. [DOI] [PubMed] [Google Scholar]
- 8.Jodai T, Yoshida C, Sato R, Kakiuchi Y, Sato N, Iyama S, Kimura T, Saruwatari K, Saeki S, Ichiyasu H, et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti-PD-1 immune checkpoint antibody in a lung cancer patient. Immun Inflamm Dis. 2019;7:3–6. doi: 10.1002/iid3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Occhipinti M, Falcone R, Onesti CE, Marchetti P. Hyperprogressive disease and early hypereosinophilia after anti-PD-1 treatment: a case report. Drug Saf Case Rep. 2018;5:12. doi: 10.1007/s40800-018-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markman B, Tran B, Gan H, Prawira A, Coward J, Jin X, Li B, Wang M, Xia Y, Desai J. A Phase 1 study of AK104, a tetrameric bispecific antibody that targets PD-1 and CTLA-4 in patients with advanced solid tumors. J ImmunoTher Cancer. 2019;7(Suppl 1):283. doi: 10.1186/s40425-019-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Ji J, Lou H, Li Y, Feng M, Xu N, Li Y, Wang J, Huang Y, Lou G, et al. Efficacy and safety of cadonilimab, an anti-PD-1/CTLA4 bi-specific antibody, in previously treated recurrent or metastatic (R/M) cervical cancer: a multicenter, open-label, single-arm, phase II trial. Oral presentation in SGO 2022 Annual Meeting on Women’s Cancer. American, Phoenix, 2022. [Google Scholar]
- 12.Ji J, Shen L, Li Z, Gao X, Ji K, Chen Y, Xu N, Liu T, Yang N, Zhong H, et al. A phase Ib/II, multicenter, open-label study of AK104, a PD-1/CTLA-4 bispecific antibody, combined with chemotherapy (chemo) as first-line therapy for advanced gastric (G) or gastroesophageal junction (GEJ) cancer. J Clin Oncol. 2022;40(4_suppl):308–308. doi: 10.1200/JCO.2022.40.4_suppl.308. [DOI] [Google Scholar]
- 13.Li B, Xia Y, Wang Z, Zhang P. ANTI-CTLA4 AND ANTI-PD-1 BIFUNCTIONAL ANTIBODY, PHARMACEUTICAL COMPOSITION THEREOF AND USE THEREOF. US Patent 16327076. 2019.
- 14.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-bbent. Antibodies. 2020;9:64. doi: 10.3390/antib9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9:eaal3604. doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64:e26642. doi: 10.1002/pbc.26642. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka R, Okiyama N, Okune M, Ishitsuka Y, Watanabe R, Furuta J, Ohtsuka M, Otsuka A, Maruyama H, Fujisawa Y, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J Dermatol Sci. 2017;86:71–73. doi: 10.1016/j.jdermsci.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109:523–30. doi: 10.1111/cas.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78:5011–22. doi: 10.1158/0008-5472.CAN-18-0118. [DOI] [PubMed] [Google Scholar]
- 22.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–92. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dovedi SJ, Elder MJ, Yang C, Sitnikova SI, Irving L, Hansen A, Hair J, Jones DC, Hasani S, Wang B, et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov. 2021;11:1100–17. doi: 10.1158/2159-8290.CD-20-1445. [DOI] [PubMed] [Google Scholar]
- 24.Berezhnoy A, Sumrow BJ, Stahl K, Shah K, Liu D, Li J, Hao SS, De Costa A, Kaul S, Bendell J, et al. Development and preliminary clinical activity of PD-1-guided CTLA-4 blocking bispecific DART molecule. Cell Rep Med. 2020;1:100163. doi: 10.1016/j.xcrm.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Pang X, Zhong T, Qu T, Chen N, Ma S, He X, Xia D, Wang M, Xia M, et al. Penpulimab, an Fc-engineered IgG1 anti-PD-1 antibody, with improved efficacy and low incidence of immune-related adverse events. Front Immunol. 2022;13:924542. doi: 10.3389/fimmu.2022.924542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjulandin S, Demidov L, Moiseyenko V, Protsenko S, Semiglazova T, Odintsova S, Zukov R, Lazarev S, Makarova Y, Nechaeva M, et al. Novel PD-1 inhibitor prolgolimab: expanding non-resectable/metastatic melanoma therapy choice. Eur J Cancer. 2021;149:222–32. doi: 10.1016/j.ejca.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, Sutton BJ. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol. 2014;426:630–44. doi: 10.1016/j.jmb.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J Hematol Oncol. 2020;13:45. doi: 10.1186/s13045-020-00876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–04. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–63. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


