ABSTRACT
SARS-CoV-2 has had a great impact on world health, patients on hemodialysis have a higher rate of infection and death due to COVID-19. Vaccination is important to control infection and improve the prognosis of infected patients. To describe the efficacy of vaccination against SARS-CoV-2 in Chilean patients on hemodialysis during the year 2021. Retrospective observational study. A total of 9,712 clinical records were reviewed. Data were presented as summary measures. Fisher’s exact test, Mann-Whitney U test, and multivariate logistic regression were used for the analysis. Risk and survival analysis were calculated, considering a statistical significance of less than 0.05. The average age of the patients attended was 61.5 ± 14.6 years. Average time on dialysis 67.6 months and 35.0% diabetic. 93.2% of patients were vaccinated against SARS-CoV-2, 70.7% of them received booster doses. The risk of infection was higher for those who received one or no dose, compared to those who received booster doses against SARS-CoV-2: OR = 252.46 [165.13; 401.57]. Of the infected patients, 15.7% died from COVID-19. The risk of death was higher in unvaccinated or single-dose patients compared to those vaccinated with two doses: OR = 2.64 [2.23; 3.12]. Patients with two doses and a booster had a longer survival compared to those who received one or no dose of vaccination against SARS-CoV-2 (p < .05). The vaccination in Chile, which started in February 2021, has demonstrated that booster doses against SARS-CoV-2 significantly reduced the risk of infection, hospitalization, and death due to COVID-19 in patients on hemodialysis.
KEYWORDS: Coronavirus, SARS-CoV-2, COVID-19 vaccines, efficacy, hemodialysis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in late 2019 in China, from which moment a worldwide pandemic developed that to date has caused a major public health problem.1–3
Patients with chronic kidney disease (CKD) on dialysis are at high risk of SARS-CoV-2 infection and have a high probability of developing severe complications.4 Most of them have cardiovascular disease, arterial hypertension, diabetes mellitus, and 45% of them are patients over 65 years of age. CKD itself is a condition that affects immunity, and dialysis patients with SARS-CoV-2 infection have a higher rate of hospitalization and higher morbidity and mortality, with a mortality rate of 20% compared to a mortality rate of approximately 0.2% in the general population.3
The effectiveness of vaccination in preventing COVID-19 disease and death has been demonstrated in the population at large,5,6 as well as in patients with CKD.7,8
The immune response to vaccination against SARS-CoV-2 has been evaluated in the general population with the vaccines BNT162b2 (Pfizer-BioNTech®), mRNA-1273 (Moderna®), and Ad26.COV2.S (Janssen®). Humoral response has been detected in most patients with CKD and the duration of the serological response induced by vaccination has been shown to decrease over time.9,10 It is known that patients with CKD have immune dysfunction compromising innate and adaptive immunity, which leads to an insufficient response to vaccination and a higher rate of SARS-CoV-2 infection.11,12
In Chile, vaccination with a mass vaccination program began in February 2021, giving priority to the elderly population and immunosuppressed patients. Vaccination with booster doses was established as of August 2021.13 As of February 2022, 94.74% of the general population had been vaccinated with the first and single dose, 93.02% with two doses or complete vaccination schedule, and 83.91% with booster doses.14
The aim of this study is to describe the efficacy of vaccination against SARS-CoV-2, regarding contagion, hospitalization, and deaths from COVID-19 in patients with CKD on hemodialysis during the year 2021.
Materials and method
Retrospective observational cohort study of 9,712 hemodialysis patients, from February 1 to December 31, 2021 in 64 Fresenius Medical Care centers in Chile, distributed in nine of the 16 regions of the country, attending 45% of patients with CKD in Chile.15 Patients older than 18 years diagnosed with stage 5 CKD,16 vaccinated and unvaccinated against SARS-CoV-2 were included. Patients under 18 years of age and pregnant patients were excluded. All the sociodemographic and clinical data of the patients were obtained from the EuCliD® Electronic clinical record,17 EuCliD® is a European clinical database, developed as a tool to monitor quality indicators, also used as part of the management system. CQI (Continuous Quality Improvement) of Fresenius Medical Care dialysis units internationally.18 In Chile, as it is the official clinical document of the patient, all the records are available, with minimal missing data. Demographic variables such as gender, age, and geographic location of patient care were considered; clinical characterization variables such as time on dialysis (months), defined as the length of stay from the start of renal replacement therapy to the end of the study, associated comorbidities: diabetes mellitus, malnutrition, and anemia,19–21 laboratory tests: albumin, iPTH levels, hemoglobin, calcium, phosphorus, creatinine, potassium, Kt/V On Line by ionic dialysance, and Sp Kt/V.22 The history of vaccination against SARS-CoV-2 was reviewed: date of vaccination and type of vaccine, as well as variables related to SARS-CoV-2 infection: date of diagnosis of infection, time between vaccination and diagnosis of infection, hospitalization, and death. The review of the data on vaccination, infection, hospitalization and death was carried out by the nurses in charge of treating the patients, who followed the instructions of the Chilean Ministry of Health, supervised by the National Immunization Program (NIP) 23 and Department of Epidemiology.24 To identify cases, there were Triage records according to symptoms and positive RT-PCR, according to the COVID-19 protocol established by the Company, the hospitalization and death records were corroborated by the US heads of the centers, in direct communication with the referral hospitals.
Statistical analysis
The data extracted from EuCliD 17 were analyzed using STATA v17.0 statistical software. The data were presented as frequencies, in absolute values and percentages, mean and standard deviation. For the association and/or dependence analysis, Fisher’s exact test or Chi-square test was used as appropriate, and the comparison of quantitative variables between two groups was performed with the Mann-Whitney U test. A multivariate logistic regression analysis was also performed. To express the risk of infection and death, the Odds Ratio and its corresponding confidence interval were calculated. Finally, a survival analysis was performed using the Kaplan-Meier method, considering a statistically significant difference when the p-value is less than 0.05
Ethical considerations
The project was approved by the Scientific and Research Ethics Committee (CIEC) in human subjects of the University of Chile Clinical Hospital (Certificate No. 55/2021).
Results
Regarding the demographic and clinical characteristics of the 9,712 patients, the mean age was 61.5 ± 14.6 years; 57.4% were male patients and of the total number of patients, 49.6% were assisted in the Metropolitan Region of Santiago, Chile. Of the total patients, 35.0% were diabetic. The mean length of time on dialysis was 67.6 months. Of the patients evaluated, 73.2% had hemoglobin≥10 g/dL, 87.4% had albumin≥3.5 g/dL; 70.5% had phosphorus levels between 2.5 and 5.5 mg/dL; 7.6% had iPTH≥1,000 pg/ml, and with respect to adequacy, 84.5% had a Kt/V On Line≥1.4 (Table 1).
Table 1.
Demographic and clinical characteristics of HD patients cared for at NephroCare-Chile, 2021.
| Baseline characteristics | ||
|---|---|---|
| n | % | |
| Total | 9,712 | 100.0 |
| Gender | ||
| Male | 5,579 | 57.4 |
| Female | 4,133 | 42.6 |
| Age (years) | ||
| Average ± SD | 61.5 ± 14.6 | |
| Min; Max | 18; 98 | |
| Health facility providing care | ||
| Central zone (Metropolitan Region) | 4,815 | 49.6 |
| North zone | 1,686 | 17.4 |
| South zone | 3,211 | 33.1 |
| Comorbidities | ||
| Diabetic | 3,399 | 35.0 |
| Malnourished | 943 | 9.7 |
| Anemic | 2,497 | 25.7 |
| Time on dialysis (months) | ||
| Average ± SD | 67.6 ± 65.8 | |
| Min; Max |
0.0; 517 |
|
| Laboratory findings |
Average ± SD |
Min; Max |
| Albumin (g/dl) | 4.0 ± 0.4 | 1.6; 5.4 |
| iPTH (pg/ml) | 393.7 ± 457.6 | 1; 4,999 |
| Hemoglobin (g/dl) | 10.7 ± 1.4 | 4.0; 18.4 |
| Calcium (mg/dl) | 8.8 ± 0.6 | 6.0; 12.2 |
| Phosphorus (mg/dl) | 4.6 ± 1.4 | 1.0; 11.2 |
| Creatinine (mg/dl) | 8.0 ± 2.5 | 1.4; 19.0 |
| Potassium (mmol/l) | 5.2 ± 0.6 | 2.8; 8.9 |
| Kt/V OCM | 1.6 ± 0.3 | 0.2; 4.4 |
|
Kt/V Daugirdas |
1.7 ± 0.3 |
0.5; 4.7 |
| Associated with covid-19 | ||
| |
n |
% |
| Infected | 942 | 9.7 |
| Hospitalized | 208 | 22.1 |
| Days of hospitalization | ||
| Average ± SD | 16.1 ± 16.8 | |
| Min; Max | 1; 115 | |
| Fatalities | 148 | 1.5 |
| Case fatality | 15.7 | |
| Age (years) | ||
| Average ± SD | 67.2 ± 11.3 | |
| Min; Max | 21; 91 | |
| Gender | ||
| Male | 89 | 60.1 |
| Female | 59 | 39.9 |
A total of 9,050 patients, equivalent to 93.2%, were vaccinated. Of these, 70.7% were vaccinated with booster doses (Figure 1). The predominant type of vaccine for the two-dose schedule was CoronaVac (Sinovac®) with 75.5%, followed by BNT162b2 (Pfizer-BioNTech®) with 24.3%. The booster dose was BNT162b2 (Pfizer-BioNTech®) in 93.6% of patients (Table 2).
Figure 1.
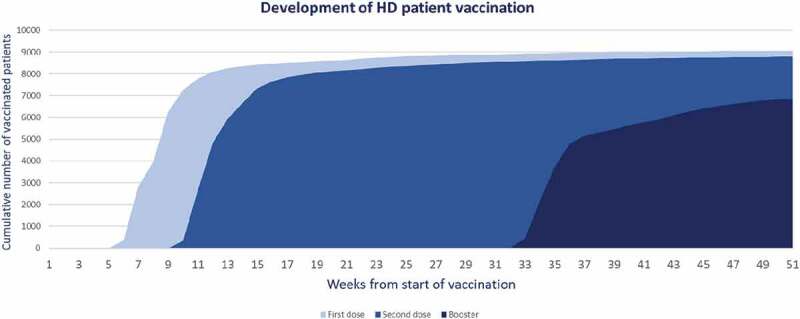
Vaccination of patients on HD against SARS-CoV-2 by dose administered during the year 2021. NephroCare-Chile.
Table 2.
Vaccination history by dose and type of vaccine received by HD patients treated at NephroCare-Chile, 2021.
| Vaccination history | ||
|---|---|---|
| n | % | |
| Vaccinated | 9,050 | 93.2 |
| Not vaccinated | 662 | 6.8 |
| Vaccination dose | ||
| Dose 1 | 264 | 2.9 |
| 2 (full schedule) | 1,923 | 21.2 |
| Full schedule plus booster dose | 6,863 | 75.8 |
| Type of vaccine | ||
| Dose 1 | ||
| CoronaVac (Sinovac®) | 6,825 | 75.4 |
| BNT162b2 (Pfizer-BioNTech®) | 2,197 | 24.3 |
| ChAdOx1 (AstraZeneca®) | 20 | 0.2 |
| Ad5-nCov (Cansino®)* | 8 | 0.1 |
| Dose 2 | ||
| CoronaVac (Sinovac®) | 6,630 | 75.5 |
| BNT162b2 (Pfizer-BioNTech®) | 2,136 | 24.3 |
| ChAdOx1 (AstraZeneca®) | 12 | 0.1 |
| Ad5-nCov (Cansino®)* | 8 | 0.1 |
| Booster dose | ||
| BNT162b2 (Pfizer-BioNTech®) | 6,425 | 93.6 |
| CoronaVac (Sinovac®) | 229 | 3.3 |
| ChAdOx1 (AstraZeneca®) |
209 |
3.1 |
| Vaccine combination | ||
| |
n |
% |
| Dose 1 and 2 | ||
| CoronaVac (Sinovac®)/CoronaVac (Sinovac®) | 6,593 | 75.0 |
| BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®) | 2,080 | 23.7 |
| CoronaVac (Sinovac®)/BNT162b2 (Pfizer-BioNTech®) | 53 | 0.6 |
| BNT162b2 (Pfizer-BioNTech®)/CoronaVac (Sinovac®) | 37 | 0.4 |
| ChAdOx1 (AstraZeneca®)/ChAdOx1 (AstraZeneca®) | 12 | 0.1 |
| Ad5-nCov (Cansino®)* | 8 | 0.1 |
| ChAdOx1 (AstraZeneca®)/BNT162b2 (Pfizer-BioNTech®) | 3 | 0.0 |
| Dose 1, 2 and booster | ||
| CoronaVac (Sinovac®)/CoronaVac (Sinovac®)/BNT162b2 (Pfizer-BioNTech®) | 4,816 | 70.2 |
| BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®) | 1,553 | 22.6 |
| CoronaVac (Sinovac®)/CoronaVac (Sinovac®)/CoronaVac (Sinovac®) | 215 | 3.1 |
| CoronaVac (Sinovac®)/CoronaVac (Sinovac®)/ChAdOx1 (AstraZeneca®) | 197 | 2.9 |
| CoronaVac (Sinovac®)/BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®) | 25 | 0.4 |
| BNT162b2 (Pfizer-BioNTech®)/CoronaVac (Sinovac®)/BNT162b2 (Pfizer-BioNTech®) | 23 | 0.3 |
| BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®)/CoronaVac (Sinovac®) | 11 | 0.2 |
| BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®)/ChAdOx1 (AstraZeneca®) | 10 | 0.2 |
| ChAdOx1 (AstraZeneca®)/ChAdOx1 (AstraZeneca®)/BNT162b2 (Pfizer-BioNTech®) | 3 | 0.0 |
| Ad5-nCov (Cansino®)*/BNT162b2 (Pfizer-BioNTech®) | 3 | 0.0 |
| BNT162b2 (Pfizer-BioNTech®)/CoronaVac (Sinovac®)/CoronaVac (Sinovac®) | 3 | 0.0 |
| ChAdOx1 (AstraZeneca®)/BNT162b2 (Pfizer-BioNTech®)/BNT162b2 (Pfizer-BioNTech®) | 2 | 0.0 |
| CoronaVac (Sinovac®)/BNT162b2 (Pfizer-BioNTech®)/ChAdOx1 (AstraZeneca®) | 1 | 0.0 |
| BNT162b2 (Pfizer-BioNTech®)/CoronaVac (Sinovac®)/ChAdOx1 (AstraZeneca®) | 1 | 0.0 |
* Mono - dose.
During the study period, 942 cases of SARS-CoV-2 infection were diagnosed and confirmed by RT-PCR, which corresponded to 9.7% of the total cohort studied. Of the total number of infected cases, 208 patients (22.1%) were hospitalized for COVID-19. The average length of hospitalization was 16 days and 148 patients died of COVID-19, corresponding to a case fatality rate of 15.7%. The average age of the deceased was 67.2 ± 11.3 years, 60.1% of whom were male (Table 1).
Diagram 1 illustrates that of the 9,050 vaccinated patients, 9.3% were infected, 1.9% were hospitalized, and 1.1% died due to COVID-19, while in the non-vaccinated group (662 patients), 15.0% were infected, 5.3% were hospitalized and 7.9% died. Infection-free time was greater in patients who received the full schedule and the full schedule with booster compared to those patients who did not receive a full schedule of vaccination. The risk of infection was higher for patients who received one or no dose compared to those who received two doses against SARS-CoV-2: OR = 2.64 [2.23; 3.12] (p < .05) and the risk of infection for patients who received one or no dose compared to those who received booster doses against SARS-CoV-2: OR = 252.46 [165.13; 401.57] (p < .05), where the decrease in the risk of SARS-CoV-2 infection was 98% for those who received the booster dose.
Diagram 1.
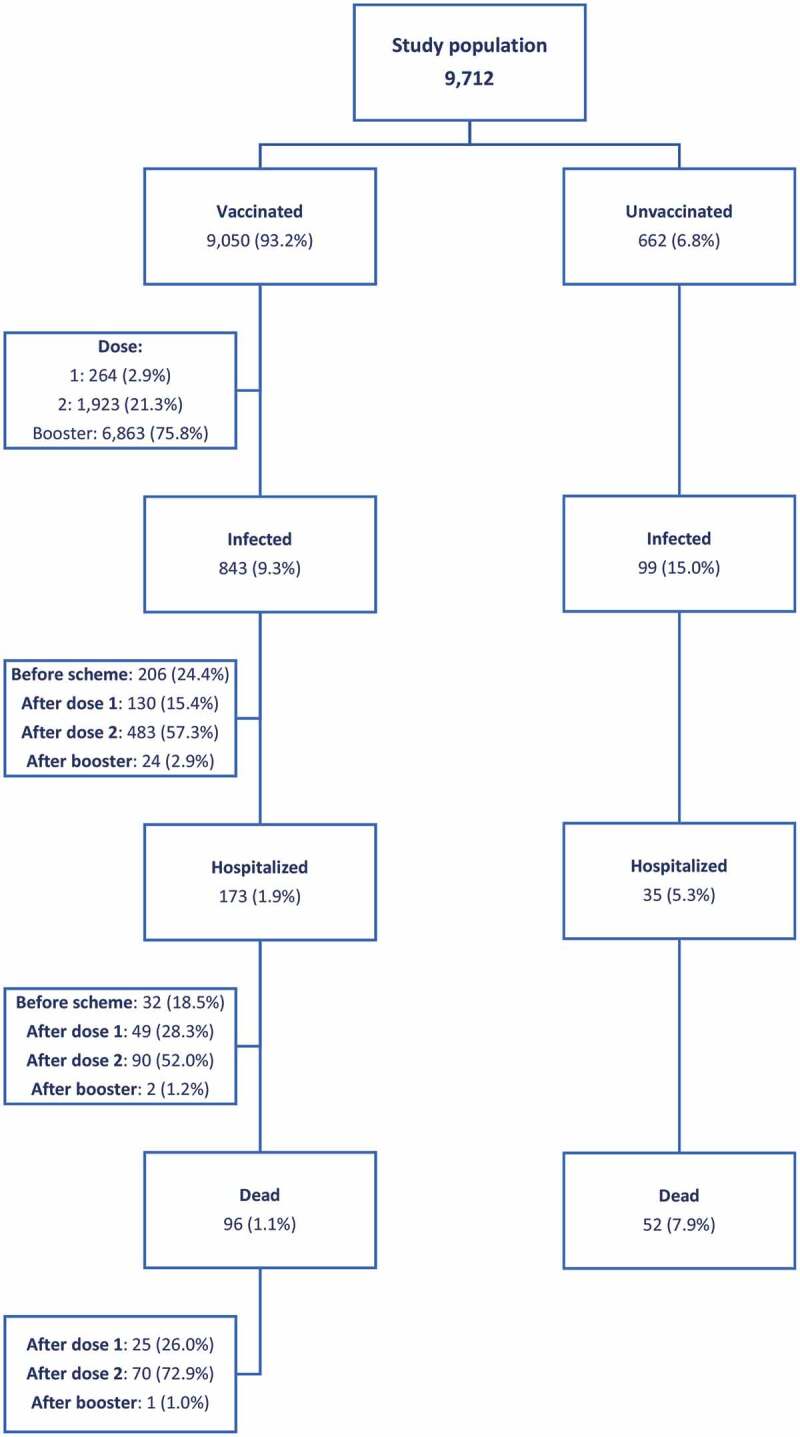
Study population according to vaccination status and COVID-19 infection, hospitalization, and death events, 2021. NephroCare - Chile.
In the univariate analysis, having a history of diabetes (p < .001), having malnutrition (p < .001), time on dialysis greater than 26 months (p = .008), lower concentrations of Hemoglobin, Calcium, Phosphorus, Potassium (p < .001), and not having booster doses (p < .001) were associated with SARS-CoV-2 infection; while having a history of diabetes (p = .005), time on dialysis greater than 26 months (p = .002), Albumin concentrations<3.5 g/dL (p = .034), Hemoglobin<10 g/dL (p < .001), Phosphorus<2, 2 mg/dL (p = .027), and sp Kt/V < 1.4 (p = .010) and not being vaccinated with booster doses against SARS-Cov-2 (p < .001), were statistically significant in the multivariate regression analysis.
In the univariate analysis of mortality, having a history of diabetes (p = .022), malnutrition (p < .001), being older than 69 years (p < .001), a history of hospitalization for COVID-19 (p < .001), time on dialysis greater than 57 months (p = .019), Albumin concentrations<3.5 g/dL (p < .001), Hemoglobin<10 g/dL (p < .001), Calcium<8.4 mg/dL (p = .028), Phosphorus<2.2 mg/dL (p < .01), and not having a booster dose (p < .001) were associated with death; while in the multivariate regression analysis, age older than 69 years (p = .003), not having a booster dose (p < .001), and having been hospitalized for COVID-19 (p < .001) were statistically significant in this model.
Considering the 15.7% lethality, the risk of death was higher in unvaccinated or single-dose patients compared to those vaccinated with two doses: OR = 2.64 [1.70; 4.10] (p < .05), highlighting that there was only one death from COVID-19 in patients with booster doses during the study period (Figure 2).
Figure 2.
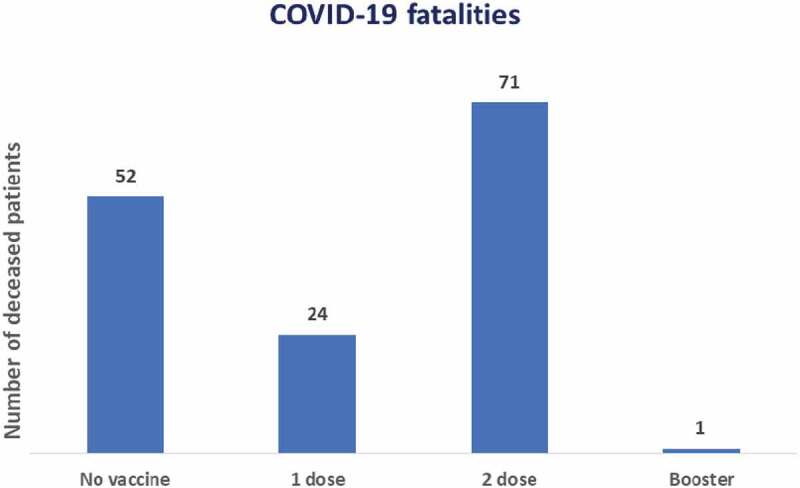
Number of patients in HD who died from COVID-19, according to vaccination schedule during the year 2021. NephroCare-Chile.
Vaccinated patients have a longer survival compared to those who did not receive vaccination against SARS-CoV-2. The probability of survival at 300 days after vaccination is 89% compared to non-vaccinated patients, who have a survival rate of 48% (p < .05) (Figure 3a). Similarly, patients with booster doses and two doses have a longer survival compared to those who received one or no dose of vaccination against SARS-CoV-2, with a survival probability of 99%, 73%, 56%, and 48% respectively (p < .05) at 300 days after the start of the vaccination process (Figure 3b).
Figure 3.
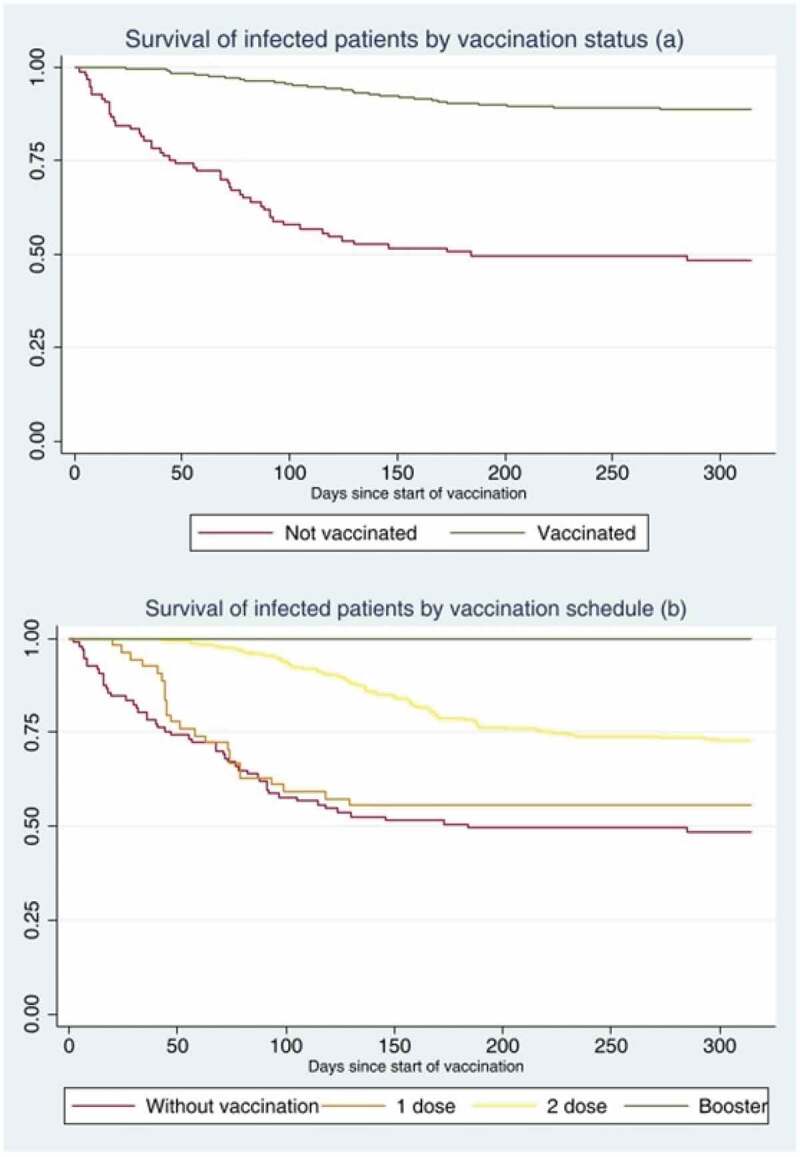
Survival of HD patients infected by SARS-CoV-2 during the year 2021, NephroCare-Chile: (a) from the start of vaccination differentiated by vaccinated and unvaccinated and (b) from the start of vaccination differentiated according to vaccination schedule.
Discussion
The SARS-CoV-2 pandemic has had serious consequences on world health and by the end of our study (December 2021) 5.8 million deaths had been recorded as a result of COVID-19.25 The present study includes a cohort of 9,712 hemodialysis patients and evaluates the clinical response to a vaccination schedule, most of whom completed a two-dose schedule with CoronaVac vaccine (Sinovac®) and booster doses with BNT162b2 vaccine (Pfizer-BioNTech®).
SARS-CoV-2 infection has some specific characteristics in the population with stage 5 CKD; they are at greater risk of contagion and infection 26 and have their own environmental conditions such as transportation to dialysis centers three times a week, where they are surrounded by other patients in the means of transport which, in itself, is a confined space. During the course of COVID-19, the traceability of cases was hindered by asymptomatic patients, which led to the SARS-CoV-2 pandemic being prolonged over time and, together with the appearance of new variants, made it more difficult to control.
The vaccination process has been fundamental in controlling SARS-CoV-2 infection, however, to date there is no clarity on its long-term effectiveness in the CKD dialysis population.27
The lower immune response to vaccination in CKD patients on renal replacement therapy (RRT) and in transplanted patients has led to the recommendation that they receive a booster dose. Studies that have evaluated the response to vaccination against SARS-CoV-2 show results ranging from 29.6% to 96.4%.28 The causes of this abnormal immune response in dialysis patients are multifactorial; there is a lower cellular and humoral immune response, uremia affects the immune system, there is insufficient neutrophil and monocyte function and decreased antigen processing.28,29
A significant percentage of dialysis patients have a decreased antibody response or no response at all to SARS-CoV-2 vaccination;30 the decreased response may furthermore be influenced by the type of vaccine, number of vaccine doses, time to antibody detection, types of antibodies generated, and duration of protective immune response.
Scientific evidence indicates that seroconversion after receiving two doses of the vaccine against SARS-CoV-2 in the non-immunosuppressed population is 99%, in immunosuppressed patients it is 78% and 27% in renal transplant recipients.31 The above validates the importance of a booster dose prescription in immunosuppressed patients,32,33 especially in dialysis patients, in whom one out of every five patients has an attenuated immune response.30,34,35
The average age of our patients vaccinated with booster doses was 62.2 years, and only 0.3% had symptomatic infection with SARS-CoV-2. Of these, 13% were hospitalized for COVID-19 and only one died. Those who received two doses of vaccine had an average age of 60 years, 13.6% had symptomatic SARS-CoV-2 infection, 29.1% required hospitalization, and 27.2% died; while the average age of patients who received only one dose of SARS-CoV-2 vaccine was 59.7 years, 30% were infected, 51.8% required hospitalization, and 44% died. This clearly justifies the administration of an additional dose to the vaccination schedule, where in Chile an interval of 4 months was defined between the second dose of the complete schedule and the booster dose in the general population and, for immunocompromised patients, a minimum interval of 2 months after completing the schedule was defined.29
Up to now, the clinical predictors associated with the response to vaccination in the general population as well as in dialysis patients have been: age, previous infection, history of immunosuppressive treatment, serum albumin level, and body mass index.4,36,37 In our study, 22% of patients were infected before starting their vaccination process at the end of the follow-up there was no evidence of reinfection; which is attributed to having a cumulative survival of 100% compared to the non-vaccinated group, which was 48%. This group probably developed a natural immune response, as mentioned by Clark et al,38 strengthening this immune response during the vaccination process, which reached 100% of these patients during the study period. In addition, this group was characterized by being mostly younger than 60 years old, with a behavior more similar to the general population explained by immunosenescence.39 This relates to that described by Jahn et al, who evaluated the response to vaccination with BNT162b2 (Pfizer-BioNTech®) in 62 hemodialysis patients, finding that the older the patient, the lower the antibody titers, which was evident in HD patients aged 60 to 69 years and in the group aged 70 to 79 years, with median antibody titers of 414 AU/ml and 140 AU/ml, respectively.40
We have found that the length of time on dialysis is significantly associated with symptomatic infection and also with mortality (p < .001) as described in the literature, where patients with more time on dialysis have a lower adaptive immune response due to the cumulative long-term effects, driven by chronic inflammation and senescence of the immune system characteristic of CKD.12,41
Antibody levels were not measured in our study, but there were significant clinical results for patients who received booster doses, where the number of infected, hospitalized, and dead patients was lower compared to those who received one or two doses against SARS-CoV-2.
Another aspect to consider is that, being an observational study, not all patients had the same follow-up time, given that the vaccination process in Chile was presented according to a formal schedule and based on clinical considerations, favoring the opportunity for vaccination in elderly patients and then those with chronic diseases, as is the case of our hemodialysis patients.
Thus, our results are similar to those described by Ducloux et al, who reported that after three doses of SARS-CoV-2 vaccine, patients showed an average increase in antibody titers of 580%, with a significant humoral response to lower doses of vaccination.32 In our study, there were 99 infected patients who had never been vaccinated against SARS-CoV-2, 32% of whom were hospitalized for COVID-19 and 53% of whom died; consequently, the risk of infection was higher in those who were not vaccinated compared to those who received two doses OR = 25.49 [21.25; 30.57] p < .001.
Finally, it is worth noting from our study that stage 5 CKD patients with major comorbidities such as diabetes mellitus, anemia, and malnutrition were significant determinants of symptomatic SARS-CoV-2 infection and COVID-19-associated mortality.
The strengths of our study lie in the size of the cohort, being the only study of patients with CKD vaccinated mainly with CoronaVac (Sinovac®), and for being the only study with a booster dose, predominantly with BNT162b2 (Pfizer-BioNTech®). In addition, from this cohort it was possible to determine relationships of clinical variables, which helped to establish the risk of infection and death from COVID-19.
The limitations of our study lie mainly in the retrospective nature of the data, which did not allow us to quantify the antibody titers and thus generate a greater comparison with the existing literature.
Conclusion
Our study, with a large cohort of well-characterized hemodialysis patients, it was shown that having a full schedule and booster doses against SARS-CoV-2 significantly reduced the risk of infection, hospitalization, and death from COVID-19, where the decrease The risk of infection by SARS-CoV-2 was 98% for those who received the booster dose and the risk of death for those patients without vaccination or only one dose was 2.64 times higher compared to those who received the full regimen.
Acknowledgments
We thank the clinical teams of the NephoCare Chile Centers and their collaborators for the timely availability of the data.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Diao B, Wang C, Wang R, Feng Z, Tan Y, Zhang J, Yang H, Wang H, Wang C, Liu L, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [Internet]. Infectious Diseases (except HIV/AIDS); 2020. Mar [accessed 2021 Dec 7]. 10.1101/2020.03.04.20031120 [DOI]
- 2.Organización Mundial de la Salud . Neumonía de causa desconocida – China [Internet]. [accessed 2021 Dec 7]. https://www.who.int/es/emergencies/disease-outbreak-news/item/2020-DON229
- 3.World Health Organization . Novel coronavirus – China [Internet]. Novel Coronavirus – China. 2020. [accessed 2021 Dec 14]. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233
- 4.Goffin E, Candellier A, Vart P, Noordzij M, Arnol M, Covic A, Lentini P, Malik S, Reichert LJ, Sever MS, et al. COVID-19-related mortality in kidney transplant and haemodialysis patients: a comparative, prospective registry-based study. Nephrol Dial Transplant. 2021. Nov 9;36(11):2094–8. doi: 10.1093/ndt/gfab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. Sep 2;385(10):875–84. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022. Feb;28(2):202–21. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou YC, Lu KC, Kuo KL, The efficacy of COVID-19 vaccines in chronic kidney disease and kidney transplantation patients: a narrative review. Vaccines. 2021. Aug 10;9(8):885. doi: 10.3390/vaccines9080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenn DA, Hegde A, Kotzen E, Walter EB, Kshirsagar AV, Falk R, Mottl A, Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int Rep. 2021. May;6(5):1407–10. doi: 10.1016/j.ekir.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, Rechavi Y, Schwartz IF, Schwartz D, Lellouch Y, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021. Jul;16(7):1037–42. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu CM, Weiner DE, Manley HJ, Aweh GN, Ladik V, Frament J, Miskulin D, Argyropoulos C, Abreo K, Chin A, et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients over 6 months. Clin J Am Soc Nephrol. 2022. Mar;17(3):403–13. doi: 10.2215/CJN.12250921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I, Basic science and dialysis: disturbances of acquired immunity in hemodialysis patients: disturbances of acquired immunity in hd patients. Semin Dial. 2007. Apr 17;20(5):440–51. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B, Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008. Sep;3(5):1526–33. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministerio de Salud C. Plan de Acción Coronavirus COVID-19: Casos confirmados acumulados de Coronavirus a nivel nacional [Internet]. https://www.minsal.cl/nuevo-coronavirus-2019-ncov/casos-confirmados-en-chile-covid-19/
- 14.Gob.cl - Yo me Vacuno [Internet] . [accessed 2022 Feb 16]. https://www.gob.cl/yomevacuno/
- 15.2017.10.24_ENFERMEDAD-RENAL-CRONICA.pdf [Internet] . [accessed 2023 Jan 12]. https://diprece.minsal.cl/wrdprss_minsal/wp-content/uploads/2018/01/2017.10.24_ENFERMEDAD-RENAL-CRONICA.pdf
- 16.Kidney International Supplements | KDIGO . 2012. Clinical practice guideline for the evaluation and management of chronic kidney disease | ScienceDirect.Com by Elsevier [Internet]. [accessed 2023 Jan 12]. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1
- 17.Marcelli D, Kirchgessner J, Amato C, Steil H, Mitteregger A, Moscardò V, Carioni C, Orlandini G, Gatti E.. EuCliD (European clinical database): a database comparing different realities. J Nephrol. 2001. Dec;14 (Suppl 4):S94–100. [PubMed] [Google Scholar]
- 18.Steil H, Amato C, Carioni C, Kirchgessner J, Marcelli D, Mitteregger A, Moscardo V, Orlandini G, Gatti E, EuCliD® – a medical registry. Methods Inf Med. 2004;43(01):83–88. doi: 10.1055/s-0038-1633841. [DOI] [PubMed] [Google Scholar]
- 19.Brenner BM, Rector FC. Brenner & Rector’s the kidney. 8th ed. Philadelphia: Saunders Elsevier; ©2008, 2008. [Google Scholar]
- 20.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020. Sep;76(3):S1–107. doi: 10.1053/j.ajkd.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, et al. Executive summary of the 2017 KDIGO Chronic kidney disease–mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017. Jul;92(1):26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Daugirdas JT, Blake PG, Ing TS, editors. Handbook of dialysis. 5th ed. international edition. Philadelphia:Lippincott Williams & Wilkins; 2015. p. 826 [Google Scholar]
- 23.MINSAL PNI - Ministerio de Salud [Internet] . [accessed 2023 Jan 12]. https://vacunas.minsal.cl/
- 24.Ingreso al sistema - SADEPI [Internet] . [accessed 2023 Jan 12]. https://epivigila.minsal.cl/
- 25.Statista . • Coronavirus: muertes en el mundo por continente en 2022 [Internet]. [accessed 2022 Jun 29]: https://es.statista.com/estadisticas/1107719/covid19-numero-de-muertes-a-nivel-mundial-por-region/
- 26.Wilde B, Korth J, Jahn M, Kribben A, COVID-19 vaccination in patients receiving dialysis. Nat Rev Nephrol. 2021. Dec;17(12):788–89. doi: 10.1038/s41581-021-00499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teijaro JR, Farber DL, COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021. Apr;21(4):195–97. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen JS, Wang IK, Yen TH, COVID-19 vaccination and dialysis patients: why the variable response. QJM Int J Med. 2021. Nov 5;114(7):440–44. doi: 10.1093/qjmed/hcab171. [DOI] [PubMed] [Google Scholar]
- 29.Dosis de refuerzo en la campaña de vacunación contra SARS-CoV-2 en Chile - Ministerio de Salud - Gobierno de Chile [Internet]. [accessed 2022 Feb 15]. https://www.minsal.cl/dosis-de-refuerzo-en-la-campana-de-vacunacion-contra-sars-cov-2-en-chile/
- 30.Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, Kerschmann R, Beyer P, Dittrich M, Block GA, et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol. 2021. Oct;32(10):2435–38. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker EPK, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, O’Brien KL, Hombach J, Wilder-Smith A, Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022. Mar;10(3):e326–8. doi: 10.1016/S2214-109X(21)00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C, Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021. Sep;100(3):702–04. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO-2019-nCoV-Vaccination-SAGE-recommendation-Immunocompromised-persons-2021.1-eng.pdf [Internet]. [accessed 2022 Mar 9]. https://apps.who.int/iris/bitstream/handle/10665/347079/WHO-2019-nCoV-Vaccination-SAGE-recommendation-Immunocompromised-persons-2021.1-eng.pdf
- 34.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, Hemmings O, O’byrne A, Kouphou N, Galao RP, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020. Dec;5(12):1598–607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Röltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, Hunter M, Wang H, Sahoo MK, Huang C, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020. Dec 18;5(54):eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. Jul 1;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan American Health Organization . Evaluación de la efectividad de las vacunas contra la COVID-19. Orientación provisional. [Internet]. 2021. [accessed 2022 Jun 29]. https://iris.paho.org/handle/10665.2/54270?show=full
- 38.Clarke CL, Prendecki M, Dhutia A, Gan J, Edwards C, Prout V, Lightstone L, Parker E, Marchesin F, Griffith M, et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021. Jun;99(6):1470–77. doi: 10.1016/j.kint.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Francisco ALM. Nefrología al día. Resultados de la vacunación COVID-19 en pacientes en diálisis y trasplantados de riñón [Internet]. [accessed 2022 Jun 29]. https://www.nefrologiaaldia.org/es-articulo-397
- 40.Jahn M, Korth J, Dorsch O, Anastasiou OE, Sorge-Hädicke B, Tyczynski B, Gäckler A, Witzke O, Dittmer U, Dolff S, et al. Humoral response to SARS-CoV-2-vaccination with BNT162b2 (Pfizer-BioNTech) in patients on hemodialysis. Vaccines. 2021. Apr 8;9(4):360. doi: 10.3390/vaccines9040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen G, Immune dysfunction in Uremia 2020. Toxins. 2020. Jul 5;12(7):439. doi: 10.3390/toxins12070439. [DOI] [PMC free article] [PubMed] [Google Scholar]


