ABSTRACT
The acidic environment within lysosomes is maintained within a narrow pH range (pH 4.5-5.0) optimal for digesting autophagic cargo macromolecules so that the resulting building block metabolites can be reused. This pH homeostasis is a consequence of proton influx produced by a V-type H+-translocating ATPase (V-ATPase) and rapid proton efflux through an unidentified “leak” pathway. By performing a candidate expression screening, we discovered that the TMEM175 gene encodes a proton-activated, proton-selective channel (LyPAP) that is required for lysosomal H+ “leak” currents. The activity of LyPAP is most active when lysosomes are hyper-acidified, and cells lacking TMEM175 exhibit lysosomal hyper-acidification and impaired proteolytic degradation, both of which can be restored by optimizing lysosomal pH using pharmacological agents. Variants of TMEM175 that are associated with susceptibility to Parkinson disease (PD) cause a reduction in TMEM175-dependent LyPAP currents and lysosomal hyper-acidification. Hence, our studies not only reveal an essential H+-dissipating pathway in lysosomes, but also provide a molecular target to regulate pH-dependent lysosomal functions and associated pathologies.
KEYWORDS: Proton channel, lysosome, acidification, H+ leak, TMEM175
Most lysosomal hydrolases have minimal activity until they are delivered to the lysosomal lumen, so that they are not operative in the biosynthetic pathway, which could cause harm. The lysosomal optimal pH (pH 4.5 to 5.0) that is required for autophagy and normal activity of lysosomal hydrolases is maintained by balancing proton influx produced by the V-ATPase, which has been extensively studied for decades, and proton efflux through an unidentified “H+ leak” pathway. It “takes two to tango” and in a recent study [1], we have now found the “dancing partner” for the V-ATPase. By patch-clamping lysosomal membranes, we performed a candidate expression screen to identify TMEM175, one of the most prominent genetic risk factors for Parkinson disease (PD) that was previously characterized as a constitutively-active K+ channel, as the molecular determinant of the lysosomal “H+ leak”. Under its physiological conditions (pH 4.5-5.0), TMEM175 is about 105 times more permeable to H+ than to K+ or Na+, hence acting as a bona fide proton-selective channel in the lysosome. Lysosomal H+ leak currents are dramatically increased upon overexpression of TMEM175, but are abolished when TMEM175 is genetically inactivated (i.e., by knockout).
How is TMEM175 activated or gated? Instead of acting as a non-gated “K+-leak” channel, TMEM175 is activated by lumenal side protons, an endogenous polysaturated fatty lipid (arachidonic acid, ArA), and two synthetic chemicals. By measuring lysosomal pH using multiple sets of pH-sensitive lumenal dyes, it was shown that both ArA and synthetic agonists regulate lysosomal pH in a TMEM175-dependent manner. Consistently, whereas overexpression of TMEM175 causes lysosomal alkalization, knockout of TMEM175 results in lysosomal over-acidification. As a lysosomal proton-activated, proton-selective channel (LyPAP), TMEM175 regulates the lysosomal pH set-point via a classic negative feedback loop mechanism (Fig. 1). TMEM175 integrates both the sensor and the response element within one protein, such that the lysosomal pH set-point serves as the “threshold” pH for TMEM175 activation in the endogenous setting. The proton channel activity of TMEM175 is increased dramatically as lumenal pH is dropped. When TMEM175 is genetically deleted, lysosomes are over (hyper)-acidified by about 0.4 pH units due to unopposed V-ATPase-mediated H+ pumping, causing an acidic shift in the lysosomal pH set-point (Fig. 1). Conversely, chemical activation of TMEM175 leads to lysosomal proton release, and an alkaline shift in the lysosomal pH set-point. Hence, TMEM175 plays an essential role in regulating lysosomal pH homeostasis.
Figure 1.
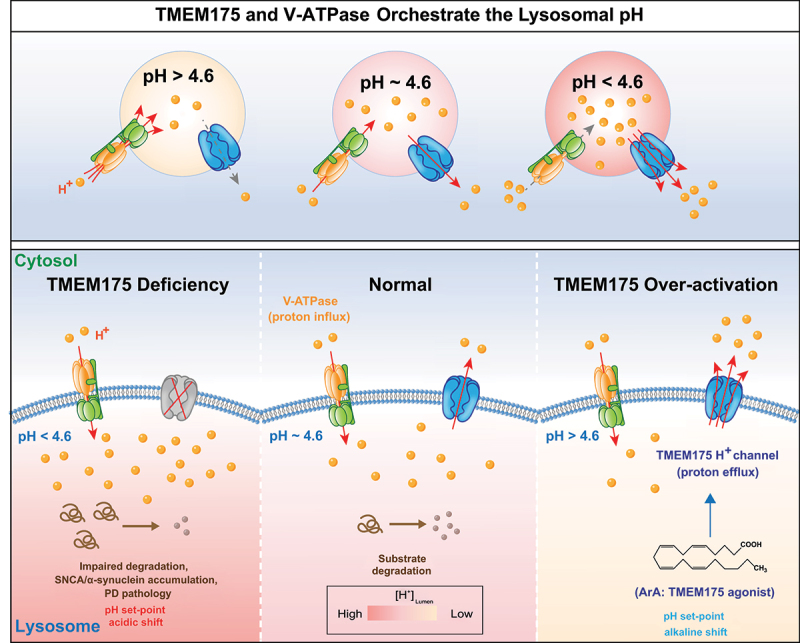
TMEM175, a lysosomal lumenal proton-activated proton release channel, regulates lysosome pH set-point and hydrolytic activity. For a typical lysosome with a steady-state lumenal pH of 4.6, the acidifying force is mediated by the V-ATPase that pumps H+ into the lysosome lumen (H+ inward flux), and de-acidifying is mediated by the TMEM175 H+ channel releasing lumenal H+ (H+ outward flux). Whereas the activity of V-ATPase is decreased with lumenal acidification, TMEM175-mediated proton efflux is dramatically and progressively increased when lysosomes are hyper-acidified. In the absence of TMEM175, the lysosomes are hyper-acidified by about 0.4 pH units, due to the lack of proton outward flux opposing proton inward flux. Lysosomal over-acidification then impairs lysosomal proteolytic activity and causes pathological SNCA aggregation. Rightward-shifting the pH-dependence of TMEM175 activation by endogenous or synthetic agonists such as ArA or DCPIB, may cause an alkaline shift in the pH set-point and steady-state pH. Hence, TMEM175 regulates lysosomal pH via a classic negative feedback loop.
Most acidic hydrolases, e.g., CTSB (cathepsin B) and CTSD, do not function effectively, in either hypo-acidified or hyper-acidified lysosomes; therefore, in the absence of TMEM175, the lysosome’s overall hydrolytic activity is significantly reduced. One of the neuronal substrates of lysosomal CTSB and CTSD is SNCA/α-synuclein, and SNCA aggregation is a hallmark pathology in PD. Consistent with the observation that TMEM175-dependent LyPAP currents are reduced in M393T (a PD-risk variant) knockin human cell lines, SNCA aggregation is facilitated in the brains of tmem175 KO mice.
As TMEM175 is dually permeable to both K+ and H+, how can we distinguish between the physiological roles of H+ vs. K+ permeability? Although TMEM175 KO lysosomes are hyper-acidified, mild inhibition of V-ATPase normalizes lysosomal pH and restores proteolytic degradation. Hence, TMEM175 regulates lysosome function, primarily through a regulation of lysosomal pH. Because the electrochemical gradients in the lysosome favor H+ efflux and K+ influx, it is possible that the K+ influx through TMEM175 could bring positive charges to the lumen to prevent the V-ATPase from pumping excess protons; so, with the loss of TMEM175, V-ATPase pumping does not shut down at the right stage and causes lysosome hyper-acidification. However, although TMEM175’s K+ conductance is detectable in the overexpression system, in the native lysosomes, the basal K+ currents are barely measurable. Indeed, the K+ conductance of TMEM175 is inhibited by acidic pH. Nevertheless, to segregate the H+ vs. K+ permeability, mutagenesis studies have identified a mutation in TMEM175 (D41A) that selectively abolishes the H+ conductance but maintains the K+ conductance. The TMEM175D41A mutant fails to rescue the hyper-acidification phenotype associated with TMEM175 KO cells. Conversely, the S45A mutation, which reduces the K+ conductance through TMEM175, but retains its H+ conductance, can still rescue the hyper-acidification phenotype associated with TMEM175 KO cells. Hence, the H+ conductance, but not the K+ conductance, is more likely to be responsible for the functions of TMEM175 in lysosomes.
As alterations in lysosomal pH contribute causatively not only to PD pathology, but also to toxic protein aggregations and neurodegeneration in Alzheimer disease and many lysosomal storage diseases, our identification of small-molecule TMEM175 modulators that regulate lysosome pH set-point, optimum, and homeostasis may provide a conceptual and molecular groundwork toward developing therapies for neurodegenerative diseases. In addition, as a basic molecular device in cell biology, the discovery of a TMEM175-mediated lysosomal acidification mechanism may also have impacts on many other fields as well. For example, lysosomes were reportedly de-acidified in SARS-CoV-2-infected cells. Hence, our identification of the lysosome proton channel and development of imaging-based assays for high-throughput screening may be of significance in both basic and clinical research.
Acknowledgement
This work was supported by an NIH Grant (RO1DK115474) and funds from the Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- 1.Hu M, Li, P, Wang, C.. Parkinson’s disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes. Cell. 2022;185:2292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]


