ABSTRACT
Healthcare professionals (HCPs) are among the highly exposed groups for the COVID-19 pandemic and have been identified as the target population to get vaccination against the spread of the infection. Aimed to assess COVID-19 vaccine take-up and its predictors among HCPs in public hospitals in Addis Ababa, Ethiopia, 2021. A facility-based cross-sectional study was conducted among 403 randomly selected participants from October 1st to November 30, 2021. Data was entered into Epi-info version 7 and analyzed using SPSS version 25. An AOR along with a 95% confidence level was estimated, and a P value <.05 was considered to declare the statistical significance. About 71% of the participants had taken any of the COVID-19 vaccines at least once. Being married (AOR: 10.79; 95% CI: 1.32–18.05); educational status of MSc degree (AOR = 7.7; CI: 2.08–15.1.6), medical doctors/GP (AOR = 5.88; CI: 1.60–15.54), MD with MSc (AOR = 9.63; CI: 2.17–17.76), PHD (AOR = 13.33; CI: 1.23–24.21) and specialist and above 3 (AOR = 3.45; CI: 2.34–42.8) holder HCPs; perceived severity of COVID-19 infection as moderate (AOR = 0.23; CI: 0.08–0.65) and mild (AOR = 0.28; CI: 0.11–0.74) and poor knowledge toward COVID-19 vaccination (AOR = 0.03; CI: 0.01–0.12) were statistically associated. This study showed that COVID-19 vaccine take-up among HCPs was relatively low to achieve herd immunity. Participants’ marital status, educational status, perception of the severity of COVID-19 infection, and level of knowledge toward COVID-19 vaccines were the predictors of the COVID-19 vaccine take-up.
KEYWORDS: COVID-19, healthcare professions, take-up, vaccine, Ethiopia
Background
The highly contagious infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a catastrophic effect on the world’s demographics, resulting in more than 2.9 million deaths worldwide.1 It was first reported in Wuhan, Hubei Province, China, in late December 2019 and rapidly disseminated across the world in a short span of time, compelling the World Health Organization.2
SARS-CoV-2 belongs to the larger family of ribonucleic acid (RNA) viruses, leading to infections ranging from the common cold to more serious diseases.3 Common and less common symptoms at the onset of illness were reported, and this can impact people’s lives, physical and mental health, and economic situation.4
Globally, as of 5 July 2021, there have been 183 million confirmed cases of COVID-19, including 3.9 million deaths reported to the World Health Organization (WHO).5 As of 8 July 2021, a total of 3 billion vaccine doses have been administered and are likely to continue to have significant impacts on healthcare communication.6 The first confirmed cases of COVID-19 in Ethiopia were reported on 13 March 2020. Since then, a total of 373, 398 confirmed cases of COVID-19 and 4226 deaths were reported as of 9 June 2021.7
Vaccination is an important measure to control the global pandemic of COVID-19. The most hopeful way of controlling COVID-19 could be universal vaccination to achieve herd immunity.8 Researchers from all over the world have made remarkable efforts to create vaccines against the coronavirus disease to fight the coronavirus disease (COVID-19) pandemic.9 At least seven vaccines across three platforms have been carried out in countries.7 Vaccination is prioritized for vulnerable groups in all countries. Simultaneously, more than 200 additional vaccine candidates were being produced, with more than 60 of them in clinical trials.7 Currently, Pfizer-BioN Tech, Moderna, Johnson, Johnsson’s Janssen, Oxford/AstraZeneca vaccine, and Sino pharm are among the most common ones that have been used throughout the world, including Ethiopia.5 Hence, Ethiopia initially received 2.184 million doses of COVID-19 vaccines.7 Then, the Ethiopian Ministry of Health officially launched the COVID-19 vaccine at a high-level national event held at Eka Kotebe COVID-19 Hospital, where front-line health workers were vaccinated to kick off the vaccination campaign. Healthcare professionals (HCPs), the elderly, and patients with chronic diseases above the age of 55 years old were the prioritized population groups for vaccination against COVID-19. Based on this, the nation has planned to vaccinate 20% of its population until the end of 2021 and COVID-19 vaccine was given to an eligible individual without any payment to the services.9
Increasing COVID-19 vaccine uptake is crucial for herd immunity and sufficient immunization coverage to end the global pandemic.8 However, due to many reasons, this is not achieved in many significant areas. Even if there are different types of vaccines for COVID-19, the reluctance that people show to take them is a major drawback as a society, especially in high transmission areas like public hospitals. Health professionals in public hospitals are hesitant to use any of the COVID-19 vaccines.4
Studies have been conducted across the globe on the COVID-19 vaccine, as in Pakistan (70.25%),10 Israel (78%),11 France (76.9%),12 Italy (75%),12 Turkey (68.6%),13 Greek (75.8%) 14 and Southern Ethiopia.15 However, they are based on various perspectives as some rely on specific vaccines, some on the general population, and some on healthcare professionals.
To the level of authors knowledge there are many nonspecific reasons documented given which all come down to information gaps including healthcare professionals. Most information gaps focused on questions on safety and effectiveness, COVID-19 denial, mistrust in government body and weak support from traditional leaders. Lack of information or misinformation often arises when there is information gap or unsettled worries. This can affect vaccine confidence and vaccine rates in a society. To date, there is no study conducted on vaccine take-up among these specific society groups and on the root cause of this problem.2 Hence, this study aimed to assess COVID-19 vaccine take-up and its predictors among healthcare professionals in public hospitals of Addis Ababa, Ethiopia, 2021.
Methods and materials
Study setting and period
The study was carried out among healthcare professionals working in public hospitals in Addis Ababa, Ethiopia from October 1 to 30 November 2021.
Study design
A facility-based cross-sectional study design was conducted to assess COVID-19 vaccine take-up and its predictors among healthcare professionals in public hospitals in Addis Ababa, Ethiopia, in 2021.
Population and eligibility criteria
Population
The source population included all healthcare professionals working in public hospitals, and the study population included all healthcare professionals working at six public hospitals in Addis Ababa City at the time of data collection.
Eligible criteria
Any healthcare professionals (doctors, nurses, pharmacists, anesthetists, laboratory technicians, environmental health professionals, etc.) who were available during data collection at the six public hospitals in Addis Ababa City were included, whereas healthcare professionals with active SARS-CoV-2 infection were excluded from the study.
Sample size and sampling procedures
Sample size determination
The sample size was determined using a single population proportion formula by considering the following assumptions: where the proportion of healthcare professionals who had taken the COVID-19 vaccine from a previous study done in Ghana was 38.9%.16 Also, by considering 5% margins of error and a 10% potential non-response rate, the final sample size became 403.
Sampling procedures
From the beginning, six public hospitals (Zewditu, Mahatma Gandhi, Dagmawi Menelik, Yekatit 12, Ras Desta, and Tirunesh Dibaba hospitals) were selected for this study among all the public hospitals found in Addis Ababa city administration. Then the calculated sample size was distributed to each hospital with a proportional allocation of their staff size. So, sample of 75 participants from Zewditu, 24 from Mahatma Gandhi, 75 from Dagmawi Menelik, 67 from Yekatit- 12, 74 from Ras Desta, and 75 from Tirunesh Dibaba hospitals were selected. Finally, to obtain the final sample size, simple random sampling techniques were used based on the allocated sample size of each hospital, and the data was collected from health professionals who can fully fulfill the incision criteria when they enter and live in the hospital.
Study variables
The variable measured as an outcome in the study was take-up of COVID-19 vaccine and the independent variables considered were: socio-demographic variables (age, sex, religion, ethnicity, residence, marital status, educational status, family size and monthly income); profession and work-area-related variables (place of work or types of facility, types of profession, work experience, expertise); health status and exposure variables (perceived own health status, perceived family health status, tested for COVID-19, history of chronic illness, history of vaccination for other diseases and contact history with COVID-19 patients or clients) and awareness, knowledge, and perceptions toward COVID-19 vaccine related variables (heard about the vaccine, source of information, availability of the vaccine, preventability of the vaccine, trust about the vaccine)were the independent variables.
Operational definitions
Healthcare worker: Any health-care practitioner (such as doctors, nurses, pharmacists, anesthetists, laboratory technologists, and so on) who is licensed, certified, or registered to provide specific health-care services in public and private health facilities in accordance with state law.17
Severe COVID 19: is defined as dyspnea, a respiratory rate of 30 or more breaths per minute, a blood oxygen saturation of 93% or less, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2:FIO2) of less than 300 mm Hg, or infiltrates more than 50% of the lung field.18
Vaccine Take-up: It is defined as taking any type of COVID-19 vaccine at least once as of today. A score of “1” was given for “Yes” and a score of “0” was given for “No”.19
Knowledge about the vaccine: healthcare professionals’ knowledge of COVID-19 vaccines was assessed by whether they knew of or did not know any of the seven vaccines available. A score was generated by summing responses for all vaccines ranging between 7 and 14 (mean = 10.33 ± SD 2.04). Good knowledge of available vaccines was defined as HCWs who scored above the mean and poor knowledge below the mean.19
Data collection tools and procedures
A semi-structured, pretested, and self-administered questionnaire was used to collect the data. Socio-demographic characteristics, profession and work-area-related variables, health status and exposure variables, and awareness, knowledge, and perceptions toward COVID-19 vaccine related variables were included in the study tool, which was adapted by reviewing different literature.17–19 Two experienced Bachelor of Science degree health professionals and two health officers were recruited for data collection and supervision, respectively. To minimize further risks of COVID-19 transmission, data collectors and participants were following the precautionary measures as per the guidelines for the prevention of COVID-19. The respondents granted their agreement, and the data collectors described the study’s objective and purpose, as well as their right to withdraw or refuse the study, confidentiality, and other ethical concerns. The data collection process would then begin if and only if permission had been obtained.
Data quality management
Various measures were undertaken to maintain the quality of the data before, during, and after data collection. Before the actual data collection, the study tool was prepared in English, translated into the local language and then translated back into English, and the contents of the questionnaires were checked for consistency. Also, the tool was pretested outside of the study area (Black Lion Hospital) on 5% (20 participants), and based on the pretest results, necessary modifications were made. Finally, after the completion of data collection, raw data was cleaned, coded, and double entered into Epi-info version 7.
Data analysis procedure
Data was checked, coded and entered into Epi-info version 7, then exported to a Statistical Package for Social Sciences (SPSS) version 25 for data analysis. Both descriptive and inferential analytical statistical procedures were carried out. A descriptive analysis was used to describe the percentages and number distributions of the respondents. A binary logistic regression analysis was performed on the independent variables and their proportions, and a crude odds ratio was computed against the outcome variable. Finally, independent variables with a P-value less than 0.25 were entered into the final multivariable logistic regression model to control for potential confounders and to identify significant factors associated with the outcome variable. The adequacy of the model to fit the outcome variable with the predictors was checked using the Hosmer and Lemeshow Test for goodness of fit. Finally, the adjusted odds ratio along with a 95% confidence interval was estimated to assess the strength of the association, and a P value <.05 was considered to declare the statistical significance in the multivariable analysis.
Results
A total of 390 healthcare professionals participated in this study, yielding a response rate of 96.7%.
Socio-demographic and economic characteristics
Of the total participants, more than two fifth (167, 42.8%) them belonged to the age group of 21 to 30 years old, and the mean age of them was 29.74 (SD±7.04) years old. About 202 (51.8%) of the participants were male; orthodox Christianity followers, 171(43.8%); and single in marital status, 172(44.1%). With regard to educational status, 235 (60.3%) and 75 (19.2%) of them were Bachelors of Science degrees and medical doctors/GP holders were healthcare professionals, respectively. The average household income of the respondents was 6947.91 Ethiopian birr per month (±3749.33) and 138 (35.4%) of the respondents were earning 7801 to 10,900 Ethiopian birr per month (Table 1).
Table 1.
Socio-demographic and economic characteristics of healthcare professionals in public hospitals of Addis Ababa, Ethiopia, 2021 (N = 390).
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Age | 21–30 | 167 | 42.8 |
| 31–40 | 124 | 31.8 | |
| 41–50 | 75 | 19.2 | |
| 51–60 | 24 | 6.2 | |
| Sex | Male | 202 | 51.8 |
| Female | 188 | 42.2 | |
| Religion | Orthodox | 171 | 43.8 |
| Protestant | 92 | 23.8 | |
| Muslim | 91 | 23.3 | |
| Catholic | 12 | 3.1 | |
| Wakefata | 24 | 6.2 | |
| Marital Status | Single | 172 | 44.1 |
| Married | 126 | 32.3 | |
| Divorced | 45 | 11.5 | |
| Widowed | 47 | 12 | |
| Educational Status | Diploma | 26 | 6.7 |
| Degree(BSc) | 235 | 60.3 | |
| MSc degree | 40 | 10.3 | |
| MD/GP | 75 | 19.2 | |
| MD/GP + MSc | 12 | 3.1 | |
| Specialty and above | 2 | .5 | |
| Family size | <5 | 210 | 53.8 |
| >5 | 180 | 46.2 | |
| Monthly income (in ETB) | 1651–3200 | 26 | 6.7 |
| 3201–5250 | 80 | 20.5 | |
| 5251–7800 | 102 | 26.2 | |
| 7801–10,900 | 138 | 35.4 | |
| > 10,900 | 44 | 11.3 |
Profession and work area related factors
Concerning profession and work-area-related characteristics, approximately 78 (20.0%) of the participants work in Zewditu hospital, and the majority of them are nurses, 152 (39.0%), and only clinical staff, 219 (56.2%). Also, about 64 (16.4%) of them are currently working in different wards. Additionally, about 250 (64.1%) of the participants had work experience of <10 years (Table 2).
Table 2.
Professional and work area characteristics of healthcare professionals in public hospitals of Addis Ababa, Ethiopia, 2021 (N = 390).
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Place of work | Tirunesh Dibaba Hospital | 77 | 19.7 |
| Minilik Hospital | 76 | 19.5 | |
| Mehetem Gandhi Hospital | 25 | 6.4 | |
| Yekatit 12 Hospital | 58 | 14.9 | |
| Zewditu Hospital | 78 | 20.0 | |
| Ras Desta Hospital | 76 | 19.5 | |
| Profession | Nurse | 152 | 39.0 |
| Midwifery | 47 | 12.1 | |
| MD/GP | 100 | 25.6 | |
| MLT | 6 | 1.5 | |
| Public Health | 45 | 11.5 | |
| Pharmacy | 32 | 8.2 | |
| Anesthetics | 4 | 1.0 | |
| Radiology | 2 | 0.5 | |
| Others* | 2 | 0.5 | |
| Work experience (in years)? | <10 | 250 | 64.1 |
| >10 | 140 | 35.9 | |
| Expertise of staff | Academic staff only | 56 | 14.4 |
| Academic staffs working on university hospital | 111 | 28.5 | |
| Clinical staff only | 219 | 56.2 | |
| Health Office Staffs | 4 | 1.0 | |
| Working or unit of work (current role) | Triage | 19 | 4.8 |
| Emergency room | 38 | 9.7 | |
| OPD | 36 | 9.2 | |
| Laboratory room | 14 | 3.5 | |
| Ward | 64 | 16.4 | |
| OR | 14 | 3.5 | |
| Non-clinical area | 84 | 21.5 | |
| Dispensary | 21 | 5.3 |
*Ophthalmology, dentistry.
Health status and exposure related factors
Regarding health status and exposure-related characteristics, about 340 (87.5%) and 321 (82.3%) of the participants perceived their own health status and the perceived health status of themselves for COVID-19 as healthy as of today, respectively.
About 248 (63.6%) and 268 (68.7%) of the participants and their families have history of ever been tested for COVID-19 infection respectively, and of these, about 81 (20.7%) and 122 (31.3%) of the participants and their families have history of ever been tested positive for COVID-19 infection respectively. Also, about 44 (11.3%) and 99 (25.4%) of the participants and their families had known chronic illness as of today, respectively.
Most of the participants (290, 74.4%) had no children less than 5 years old, and about 280 (71.8%) of the participants themselves had ever received any vaccine before as an adult.
About 134 (34.4%) of them had ever been involved in a COVID-19 isolation center or care and about 214 (54.9%) of the participants had a history of direct contact with confirmed COVID-19 patients, and some of the participants had reported that they had a perceived risk of getting an infection currently (Table 3).
Table 3.
Health status and exposure related factors of healthcare professionals in public hospitals of Addis Ababa, Ethiopia, 2021 (N = 390).
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Current health status | Health | 340 | 87.2 |
| Not healthy | 7 | 1.8 | |
| Not sure | 43 | 11.0 | |
| Health status (perceived) of yourself for COVID-19 | Healthy | 321 | 82.3 |
| Not healthy | 11 | 2.8 | |
| Not sure | 58 | 14.9 | |
| Tested for COVID-19 | Yes | 248 | 63.6 |
| No | 142 | 36.4 | |
| Tested positive for COVID-19? | Yes | 81 | 20.7 |
| No | 309 | 79.2 | |
| Perceived severity of COVID-19 infection | High/Sever | 12 | 14.8 |
| Moderate | 63 | 77.8 | |
| Low/mild | 6 | 7.4 | |
| Ever been treated poorly | Yes | 9 | 11.1 |
| No | 67 | 82.7 | |
| Not Sure | 5 | 6.2 | |
| Family member was tested for COVID-19 | Yes | 268 | 68.7 |
| No | 122 | 31.3 | |
| Any family members (at least one) ever been tested positive for COVID-19 | Yes | 122 | 31.3 |
| No | 268 | 68.7 | |
| Have you any know chronic disease | Yes | 44 | 11.3 |
| No | 334 | 85.6 | |
| Not Sure | 12 | 3.1 | |
| Type of chronic illnesses do you have today | Diabetes Mellitus | 16 | 36.4 |
| Hypertension | 18 | 40.9 | |
| Lung Disease | 6 | 13.6 | |
| Renal Disease | 1 | 2.3 | |
| Cardiovascular Disease | 3 | 6.8 | |
| Household member with chronic illness(at least one) today | Yes | 99 | 25.4 |
| No | 265 | 67.9 | |
| Not Sure | 26 | 6.7 | |
| Have you children less than 5 years old | Yes | 100 | 25.6 |
| No | 290 | 74.4 | |
| Have they been vaccinated for other diseases | Yes | 71 | 71 |
| No | 6 | 6 | |
| Not up to-date | 23 | 23 | |
| Have you ever received any vaccine before as an adult | Yes | 280 | 71.8 |
| No | 110 | 28.2 | |
| Ever been involved in COVID-19 isolation center or care | Yes | 134 | 34.4 |
| No | 256 | 65.6 | |
| Ever taken care(previous contact) of the confirmed COVID-19 | Yes, I have direct patient contact | 214 | 54.9 |
| Yes, but no direct patient contact | 81 | 20.8 | |
| No contact | 95 | 24.4 | |
| Perceived risk of getting COVID-19 currently | No, I am confident I won’t get infected | 135 | 34.6 |
| I believe I already have the disease and I am immune to it(not diagnosed by a test) | 73 | 18.7 | |
| No, I already have recovered and won’t get re-infected(diagnosed by a test) | 26 | 6.7 | |
| Yes, I am concerned that I will get mild symptoms which will probably not require hospitalization | 116 | 29.7 | |
| Yes, I am concerned that I will get moderate symptoms which will probably need hospitalization | 22 | 5.6 | |
| Yes, I am concerned that I will get severe symptom which will probably require admission to the intensive care unit | 18 | 4.6 |
Awareness, knowledge and perceptions toward COVID-19 vaccine
The study reported that about 382 (97.9%) of the study participants had ever heard about the COVID-19 vaccine and the main source of information was mainly 223 (58.4%) from social media.
About 252 (64.6%) of the study participants think that COVID-19 has specific treatment and effective vaccines to prevent the disease. Although, a lot of concerns from different perspectives have been raised, more than three-quarters (78.5%) of the study participants reported that COVID-19 vaccination should be mandatory for healthcare professionals (Table 4).
Table 4.
Awareness, knowledge and perceptions of healthcare professionals toward COVID-19 vaccine in public hospitals of Addis Ababa, Ethiopia, 2021 (N = 390).
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Heard about COVID-19 vaccine | Yes | 382 | 97.9 |
| No | 8 | 2.1 | |
| Where did you hear | Official media (Gov’t) | 45 | 11.7 |
| Social media | 223 | 58.4 | |
| Health professionals | 94 | 24.6 | |
| Neighbor | 5 | 1.3 | |
| Religion organizations | 15 | 3.9 | |
| Do you think COVID-19 have specific treatment | Yes | 252 | 64.6 |
| No | 90 | 23.1 | |
| Not sure | 48 | 12.3 | |
| Is the vaccine available for you to get at your work place | Yes | 308 | 79.0 |
| No | 54 | 13.8 | |
| Not sure | 28 | 7.2 | |
| Concern about getting COVID-19 | Not at all concerned | 68 | 17.4 |
| A little concerned | 89 | 22.8 | |
| Moderately concerned | 119 | 30.5 | |
| Very concerned | 114 | 29.2 | |
| Concern about close family and friends getting COVID-19 from you | Not concerned | 50 | 12.8 |
| A little concerned | 91 | 23.3 | |
| Moderately concerned | 95 | 24.4 | |
| Very concerned | 154 | 39.5 | |
| Concern about your patients getting COVID-19 from you | Not concerned | 62 | 15.9 |
| A little concerned | 88 | 22.6 | |
| Moderately concerned | 113 | 29.0 | |
| Very concerned | 127 | 32.6 | |
| Close family and friend would want you to get a COVID-19 vaccine | Yes | 233 | 59.7 |
| No | 123 | 31.5 | |
| Not sure | 34 | 8.7 | |
| Getting COVID-19 vaccine will allow you to safely see your family and friend again | Yes | 239 | 61.3 |
| No | 74 | 19.0 | |
| Not sure | 77 | 19.7 | |
| Community leaders or religious leaders would want you to get a COVID-19 vaccine | Yes | 168 | 43.1 |
| No | 116 | 29.7 | |
| Not sure | 106 | 27.2 | |
| A COVID-19 vaccine will be for your health | Not at all important | 37 | 9.5 |
| A little important | 100 | 25.6 | |
| Moderately important | 164 | 42.1 | |
| Very important | 89 | 22.8 | |
| Getting a COVID-19 vaccine for yourself will protect other people in your community form covid-19 | Not at all | 31 | 7.9 |
| A little | 94 | 24.1 | |
| Moderately | 154 | 39.5 | |
| Very much | 111 | 28.5 | |
| The preventability of the current COVID-19 vaccine from COVID-19 infection | High or very high | 71 | 18.2 |
| Moderate or medium | 239 | 61.3 | |
| Low or very low | 80 | 20.5 | |
| COVID-19 vaccination should be mandatory for HCPs | Yes | 306 | 78.5 |
| No | 84 | 21.5 |
Knowledge toward COVID-19 vaccine
This study reported that about 86.2% (95% CI: 77.2–95.1%) of the study participants had good knowledge regarding the COVID-19 vaccine (Figure 1).
Figure 1.
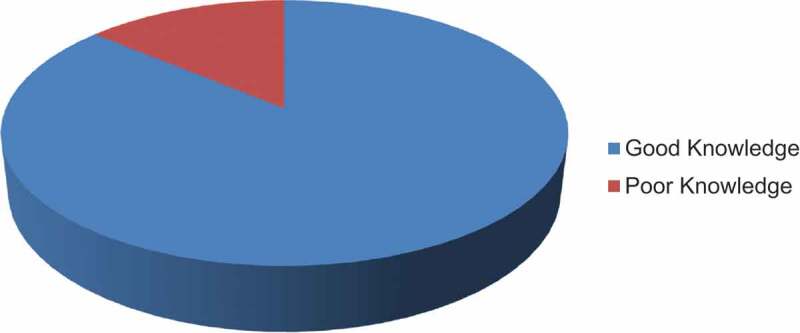
Level of knowledge toward COVID-19 vaccine in public hospitals in Addis Ababa, Ethiopia, 2021 (N = 390).
Prevalence of COVID-19 vaccine take-up
Among the study participants, about 71% (95% CI, 52.7–79.2) of the respondents had taken the COVID-19 vaccine at least once as of today (Figure 2). The most cited reasons for not taking up the vaccine reported were concerns about vaccine efficacy (244, 62.6%) and safety (35, 30.9%) (Table 5).
Figure 2.
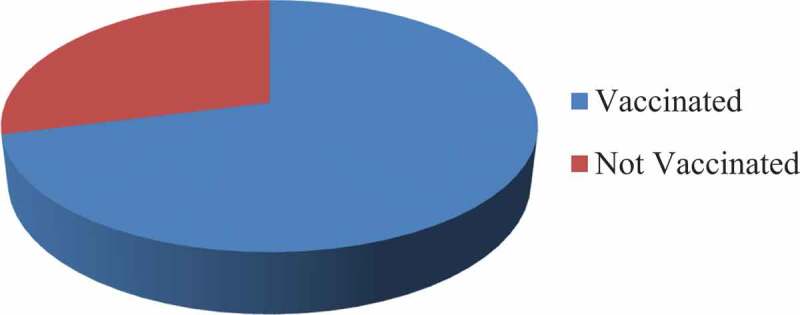
COVID-19 vaccine take-up among healthcare professionals in public hospital of Addis Ababa, Ethiopia, 2021 (N = 390).
Table 5.
Willingness to take COVID-19 vaccine among healthcare professionals in public hospitals of Addis Ababa, Ethiopia, 2021 (N = 390).
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Have you been vaccinated with any of COVID-19 vaccine at least once | Yes | 277 | 71.0 |
| No | 113 | 29.0 | |
| Reason to take | To protect my self | 198 | 71.5 |
| To protect my family | 41 | 14.8 | |
| To protect my patients | 3 | 1.1 | |
| To contribute to the control of the pandemic | 35 | 12.6 | |
| Reasons for refusing COVID-19 vaccination | Concerns about vaccine efficacy | 69 | 61.1 |
| Concern about vaccine safety | 35 | 30.9 | |
| Perception that COVID-19 is not a dangerous disease | 4 | 3.5 | |
| Not enough information about the vaccine | 5 | 4.5 |
Predictors of COVID-19 vaccine take-up
Age, gender, marital status, religion, work experience, type of profession, educational status, perceived severity of COVID-19 infection, having ever been tested for COVID-19, and level of knowledge about the COVID-19 vaccine were all significantly associated with vaccine uptake in the binary logistic regression analysis. After controlling for other variables in a multivariable logistic regression analysis, marital status, educational status, perceived severity of COVID-19 infection, and level of knowledge about COVID-19 vaccines were found to have statistically significant associations with COVID-19 vaccine uptake.
Married participants were almost 11 times (AOR: 10.79; 95% CI: 1.32–18.05) more likely to get vaccinated compared to single participants.
Educationally, the probability of taking the COVID-19 vaccine was almost 8 (AOR = 7.7; CI: 2.08–15.1.6), 6 (AOR = 5.88; CI: 1.60–15.54), 10 (AOR = 9.63; CI: 2.17–17.76), 13 (AOR = 13.33; CI: 1.23–24.21), and 3 (AOR = 3.45; CI: 2.34–42.8) times higher among MSc degree holders, medical doctors/GP, MD with MSc, PHD, and specialist and above-holder health professionals, respectively than its counterpart.
The odds of receiving the COVID-19 vaccine by healthcare professionals were reduced by 77% and 72% among those who perceived the severity of COVID-19 as moderate (AOR = 0.23; CI: 0.08–0.65) and mild (AOR = 0.28; CI: 0.11–0.74) respectively, as compared to those who perceived the severity of infection as severe.
Similarly, the odds of COVID-19 vaccine take-up was reduced by 97% among those respondents who had poor knowledge compared to those with good knowledge (AOR = 0.03; CI: 0.01–0.12) (Table 6).
Table 6.
Predictors of COVID-19 vaccine take-up among healthcare professionals in public hospitals of Addis Ababa, Ethiopia, 2021 (N = 390).
| Variable | Categories | Vaccine Take-up |
COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Yes N (%) |
No N (%) |
||||
| Age | 21–30 | 114(41.2) | 53(46.9) | 1 | 1 |
| 31–40 | 81(29.2) | 43(38.1) | 1.14(0.69,1.86) | 0.69(0.27,1.76) | |
| 41–50 | 61(22.0) | 14(12.4) | 0.49(0.25,0.96) | 0.90(0.25,3.24) | |
| 51–60 | 21(7.6) | 3(2.7) | 0.30(0.08,1.07) | 0.35(0.03,3.45) | |
| Sex | Male | 142(51.3) | 60(53.1) | 1.07(0.69,1.66) | 0.89(0.38,2.04) |
| Female | 135(48.7) | 53(46.9) | 1 | 1 | |
| Religion | Orthodox | 111(40.1) | 60(53.1) | 1 | 1 |
| Protestant | 74(26.7) | 18(15.9) | 0.45(0.24,0.82) | 0.96(0.38,2.44) | |
| Muslim | 61(22.0) | 30(26.5) | 0.91(0.53,1.55) | 0.54(0.18,1.62) | |
| Catholic | 9(3.2) | 3(2.7) | 0.61(0.16,2.36) | 2.12(0.20,22.15) | |
| Wakefata | 22(7.9) | 2(1.8) | 0.16(0.03,0.74) | 0.02(0.001,0.58) | |
| Marital Status | Single | 97(35.0) | 75(66.4) | 1 | 1 |
| Married | 102(36.8) | 24(21.2) | 11.34(3.38–37.94) | 10.79(1.32–18.05)* | |
| Divorced | 34(12.3) | 11(9.7) | 3.40(0.98–12.06) | 2.33(0.25–21.18) | |
| Widowed | 44(15.9) | 3(2.7) | 4.74(1.22–18.35) | 2.99(0.28–31.09) | |
| Educational Status | Diploma | 27(9.7) | 10(8.8) | 1 | 1 |
| BSc Degree | 99(35.7) | 42(37.2) | 2.22(0.61–18.35) | 1.69(0.09–30.43) | |
| MSc Degree | 30(10.8) | 16(14.2) | 2.54(0.83–7.78) | 7.78(2.08–15.1.6)* | |
| MD/GP | 66(23.8) | 23(20.4) | 3.20(0.94–10.84) | 5.88(1.60–15.54)* | |
| MD/GP/MSc | 19(6.9) | 10(8.8) | 2.09(0.65–6.67) | 9.63(2.17–17.76)* | |
| PHD | 12(4.3) | 8(7.1) | 3.15(0.85–11.66) | 13.33(1.23–24.21)* | |
| Specialist/above | 24(8.7) | 4(3.5) | 4.00(1.00–15.99) | 3.45(2.34–42.8)* | |
| Work experience (in years) | <10 | 170(61.4) | 80(70.8) | 1 | 1 |
| >10 | 107(38.6) | 33(29.2) | 0.65(0.40,1.05) | 1.01(0.41,2.48) | |
| Perceived severity of COVID-19 infection | High/sever | 88(45.6) | 17(30.4) | 1 | 1 |
| Moderate | 79(40.9) | 22(39.3) | 0.29 (0.13–0.65) | 0.23(0.08–0.65)* | |
| Low/mild | 26(13.5) | 17(30.4) | 0.42(0.19–0.92) | 0.28(0.11–0.74)* | |
| Have you been tested for COVID-19 | Yes | 193(69.7) | 55(48.7) | 0.41(0.26,0.64) | 0.21(0.01,2.56) |
| No | 84(30.3) | 58(51.3) | 1 | 1 | |
| Level of knowledge to ward COVID-19 vaccine | Good | 253(91.3) | 82(72.6) | 1 | 1 |
| Poor | 24(8.7) | 31(27.4) | 0.25(0.13–0.45) | 0.03(0.01–0.12)** | |
*p < .05, **p < .001 and 1-Reference categories.
Discussion
Since the announcement of efforts to develop a COVID-19 vaccine, several studies have been carried out to measure the acceptance of the vaccine among the general population.6,20 However, the rollout of the vaccine was tiered to various subgroups of the population based on limited availability, and healthcare professionals were among the first subgroups of the population to have access to the vaccine. As such, it is crucial to assess vaccine take among healthcare professionals, which will help policymakers target resources to maximize the uptake of the COVID-19 vaccine. To battle the devastating effect of COVID-19, vaccination offers the most reliable hope for a permanent solution by developing herd immunity. To do so, a vaccine must be accepted and used by a large majority of the population, particularly frontline health care professionals.21,22
Hence, the finding of this study showed that about 71% of participants had taken COVID-19 vaccination. This finding was higher than different studies that have been conducted in Malta (44.2%),2 Hong Kong (40.0%) 8 and Saudi Arabia (49.71%).23 The possible reason might be the time gaps, which affect the dissemination of information about the vaccine through various media, including the Internet, Facebook, Telegram, television, and radio. The high commitment of the Ethiopian government to minimize the effect of the pandemic by all possible preventive strategies, including vaccination, might be another probable reason. Study population differences might also be another reason. For example, a study done in Ethiopia was of the general population, but this study focused specifically on health care professionals, who were among the vulnerable group and may have had higher knowledge related to the vaccine.
Also, this finding was consistent with studies that have been conducted in Pakistan (70.25%),10 Israel (78%),11 France (76.9%),12 Italy (75%),12 Turkey (68.6%),13 Greek (75.8%)14 and Southern Ethiopia.15 However, it was lower than the percentage acceptance of 80–93% in African countries including Burkina Faso, Kenya, Mali, Niger, and Sudan.24,25 The probable reasons for the difference might be the number of infected and deaths by COVID-19 in the study area, guidelines and restrictions for COVID-19, the level of professionalism of the study population in the health-related area, and government influences.
Concerns about vaccine efficacy (61.1%) were the main reason for the non-acceptance of COVID-19 vaccination, which was in line with many studies.21,26–28 Also, Concerns about vaccine safety were also another major reason for non-acceptance of the COVID-19 vaccine, which accounted for 30.9%, and this was supported by a study done on cardiovascular complications of SARS-CoV-2 vaccines by Amir et al. 1 and on early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long-term care program in United States by Gharpure.R.28 This emphasized that the government needs to provide tangible and reliable information regarding the general characteristics of the vaccine, including the possible side effects, to rule out misconceptions and rumors concerning the vaccine.
Variables such as marital status, educational status, perceived severity of COVID-19 infection, and level of knowledge regarding COVID-19 vaccines were found to be the predictors of COVID-19 vaccine take-up. In this study, the odds of taking the COVID-19 vaccine were eleven-fold among married respondents compared to singles once. This finding was similar to the study done in the United States of America, Bangladesh, and the Kingdom of Saudi Arabia.29–31 This might be due to the fact that there is an increment in understanding of the COVID-19 vaccine among married individuals.
Regarding the educational status of healthcare professionals, the probability of taking the COVID-19 vaccine was almost 8, 6, 10, 13, and 3 times higher among MSc degree holders, medical doctors/GP, MD with MSc, PHD, and specialist and above-holder healthcare professionals, respectively, than among diploma-holder healthcare professionals. This finding showed that the higher the educational status, the higher the probability of taking the COVID-19 vaccine. This finding was almost similar to the study done in France, 69%,32 Ontario, Canada, 81.6% 33 and the US (57%).34 This could be explained by the fact that as education levels rise, healthcare professionals will have more opportunities to learn about COVID-19 vaccine uptake.
However, in the present study, religion had no significant association with COVID-19 taken. This result disagrees with a study conducted in China, where COVID-19 vaccine acceptance was lower in Muslims due to concerns about the halal status of the vaccine.35 The dissimilarities could be because of the difference in the study settings. The previous study was conducted on ethnic minorities, whereas the current study focused on healthcare professionals working in hospitals.
The odds of taking COVID-19 vaccine were reduced by 77% and 72% among those who perceived the severity of COVID-19 infection as moderate and mild, respectively, as compared to those who perceived the severity of infection as severe. This finding was similar to that of the study done in the Democratic Republic of the Congo.36 This might be explained by those with the severity of COVID-19 infection having the greatest chance to know about COVID-19 vaccine uptake.
COVID-19 vaccine take-up was reduced by 97% among those respondents who had poor knowledge compared to those with good knowledge. This finding was supported by the study conducted in the Democratic Republic of Congo, which stated that knowledge is a good predictor for acceptance of the vaccine.37 The possible reason might be that good knowledge of the vaccine may avoid misconceptions and misinformation regarding the vaccine and outweigh its importance, encouraging acceptance of the vaccine.38
The present study has some limitations which should be taken into account prior to the interpretation of the results. First, this study was a cross-sectional study, and this type of study design cannot inform us about causal relationships. Second, this is a questionnaire-based study; consequently, information bias may have occurred. Furthermore, we believe that the acceptance rate of the COVID-19 vaccine found in our study could be overestimated given that healthcare professionals who were not interested in the vaccination may not have been motivated to participate in our survey. Nevertheless, subjects who believe that vaccination against COVID-19 may also been less inclined to participate in than subjects who are concerned over vaccine safety.
Conclusion and recommendation
This study showed that COVID-19 vaccine take-up among healthcare professionals was relatively low to achieve herd immunity. Participants’ marital status, educational status, perception of the severity of COVID-19 infection, and level of knowledge toward COVID-19 vaccines were the predictors of the COVID-19 vaccine take-up. So, the government, with respective stakeholders, should emphasize addressing the concerns of healthcare professionals and increase the uptake of the COVID-19 vaccine. Furthermore, the government should have to play a key role in implementing vaccination by making a compulsory vaccination policy.
Acknowledgments
The authors would like to acknowledge all the study participants and respective hospital administrative bodies for their due cooperation and involvement during the survey.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All the data supporting the study’s findings are within the manuscript. Additional detailed information and raw data will be shared upon request addressed to the corresponding authors.
Authors Contribution
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Ethical approval
An appropriate ethical approval was obtained from the Institutional Review Board of Addis Ababa University, College of Health Sciences (Reference number: IRB/308/2021) and a permission letter from Addis Ababa City health bureau. It was conducted in accordance with the Declaration of Helsinki. The tool was designed to be anonymous, and the result did not identify the personalities of the respondents; rather it was presented as aggregated statistics. The data was kept in a protected and safe location.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.AA S, Sheikhbahaei E, Heshmat-Ghahdarijani K.. Cardiovascular complications of SARS-CoV-2 vaccines: an overview. Cardiol Therapy. 2021 Nov 29;11(1):1–10. doi: 10.1007/s40119-021-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elhadi M AA, Hmeida A, Alhadi A, Hmeida A, Alshareea E, Dokali M, Abodabos S, Alsadiq O, Abdelkabir M, Ashini A, et al. Knowledge, attitude, and acceptance of healthcare workers and the public regarding the COVID-19 vaccine: a cross-sectional study. BMC Public Health. 2021. Dec;21(1):1–21. doi: 10.1186/s12889-021-10987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mk Ak AH, Alshareef N, Qattan A, Qattan AMN, Helmy HZ, Abudawood Y, Alqurashi M, Kattan WM, Kadasah NA, Chirwa GC, et al. Knowledge, attitude and practice toward COVID-19 among the public in the Kingdom of Saudi Arabia: a cross-sectional study. Frontiers in Public Health. 2020 May 27;8:217. 10.3389/fpubh.2020.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torales JO, Castaldelli-Maia JM.. Ventriglio A.The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020. Jun;66(4):317–20. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations [Internet]. WHO. Last Modified 2021. May 7. [Google Scholar]
- 6.Hajure MT, Bekele F, Abdu Z, Abdu Z, Dule A, Mohammedhussein M, Tsegaye T. Attitude towards COVID-19 vaccination among healthcare workers: a systematic review. Infect Drug Resist. 2021;14:3883. doi: 10.2147/IDR.S332792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abebe HS, Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resist. 2021;14:2015–25. doi: 10.2147/IDR.S312116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li ML, Watson R, Zheng Y, Tang J, Ren J, Tang J, Chen Y. Healthcare workers’ (HCWS) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. 2021 Jun 30:postgradmedj-2021-140195. 10.1136/postgradmedj-2021-140195. [DOI] [PubMed] [Google Scholar]
- 9.Berihun GW, Berhanu L, Teshome D. Acceptance of COVID-19 vaccine and determinant factors among patients with chronic disease visiting Dessie Comprehensive Specialized Hospital, Northeastern Ethiopia. Patient Prefer Adherence. 2021;15:1795. doi: 10.2147/PPA.S324564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik A, Malik J, Ishaq U. Acceptance of COVID-19 vaccine in Pakistan among health care workers. medRxiv. 2021 Sep 15;16(9):e0257237. doi: 10.1371/journal.pone.0257237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dror AE, Taiber S, Morozov NG, Morozov NG, Mizrachi M, Zigron A, Srouji S, Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020. Aug;35(8):775–79. 020-00671-y. doi: 10.1007/s10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagneux-Brunon AD, Bruel S, Tardy B, Tardy B, Rozaire O, Frappe P, Botelho-Nevers E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect. 2021;108:168–73. PMID: 33259883. doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MA KS, Sahin S, Kaynar T, Kaynar T, Karbus O, Ozbel Y. Vaccine hesitancy of the COVID-19 by health care personnel. Int J Clin Pract. 2020. Dec 19;75(5):e13917. doi: 10.1111/ijcp.13917. [DOI] [Google Scholar]
- 14.Papagiannis DR, Malli F, Papathanasiou IV, Kotsiou O, Fradelos EC, Fradelos EC, Giannakopoulos K, Gourgoulianis KI. Acceptability of COVID-19 vaccination among Greek health professionals. Vaccines. 2021;9(3):200. doi: 10.3390/vaccines9030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagneux-Brunon AD, Bruel S, Tardy B, Tardy B, Rozaire O, Frappe P, Botelho-Nevers E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect. 2021;108:168–73. doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agyekum M-AA, Kyei-Arthur F, Addo B, Addo B. Acceptability of COVID-19 vaccination among health care workers in Ghana. medRxiv Internet. 2021. Jan 1;2021:1–8. 10.1155/2021/9998176. [DOI] [Google Scholar]
- 17.Kabamba Nzaji MKN, Banza Ndala DB, Mbidi Miema J, Banza Ndala DB, Mbidi Miema J, Luhata Lungoyo C, Lora Mwimba B, Cikomola Mwana Bene A, Mukamba Musenga E. Acceptability of vaccination against COVID-19 among healthcare workers in the Democratic Republic of the Congo. 2020;11:202. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu ZM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA [Internet]. 2020. Apr 7;323(13):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 19.Basema Saddik N-B, Shukla A, Barqawi H, Barqawi H, Alsayed HAH, Sharif-Askari NS, Temsah M-H, Bendardaf R, Hamid Q, Halwani R. Determinants of healthcare workers perceptions, acceptance and choice of COVID-19 vaccines: a cross-sectional study from the United Arab Emirates. Human Vaccines & Immunotherapeutics. 2022;18(1):1–9. doi: 10.1080/21645515.2021.1994300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher KB, Walder J, Crawford S, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Intern Med. 2020 Dec 15;12(12):964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogue KJ, Stancil CK, Ferguson DG, Ferguson DG, Hughes SJ, Mello EJ, Burgess R, Berges BK, Quaye A, Poole BD. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines. 2020. Dec;8(4):582. doi: 10.3390/vaccines8040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelekar AL, Afonso NM, Mascarenhas AK. COVID-19 vaccine acceptance and hesitancy among dental and medical students. J Am Den. 2021 Aug;152(8):596–603. doi: 10.1016/j.adaj.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallam M, Dababseh D, Eid H, Al-Mahzoum K, Al-Haidar A, Taim D, Yaseen A, Ababneh NA, Bakri FG, Mahafzah A. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other Arab Countries. Vaccines Internet. 2021;9(1):42. https://www.mdpi.com/2076-393X/9/1/42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bongomin FO, Andia-Biraro I. COVID-19 vaccine acceptance among high-risk populations in Uganda. Ther Adv Infect Dis. 2021;8:1–15. doi: 10.1177/20499361211024376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tulloch OJ, Bardosh K. COVID-19 vaccine perceptions in Africa: social and behavioural science data. Soc Sci Humanit Action. 2021;(March 2020):1–35. [Google Scholar]
- 26.Shekhar RS, Upadhyay S, Singh M, Singh M, Kottewar S, Mir H, Barrett E, Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. 2021. Feb;9(2):119. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papagiannis DR, Malli F, Papathanasiou IV, Papathanasiou IV, Kotsiou O, Fradelos EC, Giannakopoulos K, Gourgoulianis KI. Acceptability of COVID-19 vaccination among Greek health professionals. Vaccines. 2021;9(3):200. doi: 10.3390/vaccines9030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gharpure RGBE. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long-term care program- United States. 2020. [DOI] [PMC free article] [PubMed]
- 29.Ruiz JB. Predictors of intention to vaccinate against COVID-19: results of a nationwide survey.Vaccine. Vaccine. 2021;39(7):1080–86. doi: 10.1016/j.vaccine.2021.01.010. PMID: 33461833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann-Böhme S, Varghese NE, Sabat I, Barros PP, Brouwer W, van Exel J, Schreyögg J, Stargardt T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. 2021. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed]
- 31.Taylor SL, Paluszek MM, Groenewoud R, Groenewoud R, Rachor GS, Asmundson GJG. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Front Psychol. 2020. Oct 19;11:2890. doi: 10.3389/fpsyg.2020.575950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belmin JL, Hidoux P, Drunat O, Drunat O, Lafuente-Lafuente C. First-dose coronavirus 2019 vaccination coverage among the residents of long-term care facilities in France. Gerontology. 2022;68(5):546–50. PubMed | Google Scholar. doi: 10.1159/000517793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shariff SR, Hwang SW, Kwong JC, Kwong JC, Forchuk C, Dosani N, Booth R. COVID-19 vaccine coverage and factors associated with vaccine uptake among 23 247 adults with a recent history of homelessness in Ontario, Canada: a population-based cohort study. Lancet Public Health. 2022. Apr;7(4):e366–77. doi: 10.1016/S2468-2667(22)00037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diesel JS, Dasgupta S, Kriss JL, Kriss JL, Barry V, Vanden Esschert K, Whiteman A, Cadwell BL, Weller D, Qualters JR, et al. COVID-19 vaccination coverage among adults — United States, December 14, 2020–May 22, 2021. Morb Mort Wkly Rep. 2021;70:(25). 922–27. doi: 10.15585/mmwr.mm7025e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh AL, Wang J. Multilevel determinants of COVID-19 vaccine uptake among South Asian ethnic minorities in Hong Kong: cross-sectional web-based survey. JMIR Public Heal Surveill. 2021;7(11):e31707. doi: 10.2196/31707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabamba Nzaji MKNB, Mbidi Miema J, Ngoie Mwamba G, Banza Ndala DB, Mbidi Miema J, Luhata Lungoyo C, Lora Mwimba B, Cikomola Mwana Bene A, Mukamba Musenga E. Acceptability of vaccination against COVID-19 among healthcare workers in the Democratic Republic of the Congo. 2020;11:202. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nzaji MK, Ngombe LK, Mwamba G, Ndala DB, Miema JM, Lungoyo CL, Mwimba BL, Bene ACM, Musenga EM. Acceptability of vaccination against COVID-19 among healthcare workers in the democratic Republic of the Congo. Pragmat Obs Res. 2020;11:103. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO . WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. World Health Organization. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the study’s findings are within the manuscript. Additional detailed information and raw data will be shared upon request addressed to the corresponding authors.


