Abstract
Resistance in micro-organisms against antimicrobial compounds is an emerging phenomenon in the modern era as compared to the traditional world which brings new challenges to discover novel antimicrobial compounds from different available sources, such as, medicinal plants, various micro-organisms, like, bacteria, fungi, algae, actinomycetes, and endophytes. Endophytes reside inside the plants without exerting any harmful impact on the host plant along with providing ample of benefits. In addition, they are capable of producing diverse antimicrobial compounds similar to their host, allowing them to serve as useful micro-organism for a range of therapeutic purposes. In recent years, a large number of studies on the antimicrobial properties of endophytic fungi have been carried out globally. These antimicrobials have been used to treat various bacterial, fungal, and viral infections in humans. In this review, the potential of fungal endophytes to produce diverse antimicrobial compounds along with their various benefits to their host have been focused on. In addition, classification systems of endophytic fungi as well as the need for antimicrobial production with genetic involvement and some of the vital novel antimicrobial compounds of endophytic origin can further be utilized in the pharmaceutical industries for various formulations along with the role of nanoparticles as antimicrobial agents have been highlighted.
Keywords: Fungal endophytes, Interactions, Need, Benefits, Genetic approach, Antimicrobials
Introduction
Endophytes are micro-organisms that live in a symbiotic relationship with plants and reside within their healthy tissues. These microbes range from prokaryotic bacteria, actinomycetes, and eukaryotic fungi to latent virus or pathogens which expresses different symbiotic lifestyles with the host plant (Schulz and Boyle, 2006; Bao and Roossinck, 2013; Wani et al. 2015). De Bary in 1886, was the first to put forth the concept of endophytes wherein he defined the endophytes as “any organism that grows within plant tissues” (de Bary, 1866). However, the definition of endophytes has been modified and reformed by different authors from time to time. Unlike various phytopathogens or mycorrhizas which cause visible morphological changes in the host plants, endophytes do not cause any symptomatic changes. They live in the inter and intracellular spaces of almost every plant organ, i.e., stem, roots, petioles, leaves, bark, seeds and latex without showing any overt symptom (Strobel and long, 1998; Kumara et al. 2014; Gunawardana et al. 2015).
They are reported from a variety of host plants, such as algae, bryophytes, pteridophytes, gymnosperms, and angiosperms (Hyde and Soytong, 2008). Of the reported 1.5 million fungi, only 100,000 fungal species have been discovered. However, Petrini (1991) suggested that approximately one million species of endophytic fungi have been estimated to exist (Hawksworth 1991; Petrini, 1991; Dreyfuss and Chapera, 1994). Also, it has been estimated that only 5% of fungal species have been studied and many fractions of the total number of species have yet to be explored (Hawksworth 1991). Nevertheless, for several years endophytes did not receive much attention, but currently, the potential of endophytes has been recognized in different sectors, like, agriculture, pharmaceutical, and biotechnology industries (Gouda et al. 2016). The evolution of fungal endophytes has been reported to be associated with the evolution of plants and it still continues to evolve inside the host plants (Krings et al. 2012).
Endophytes are known to produce various secondary metabolites, i.e., terpenoids, diterpenoids, polyketides, alkaloids, steroids, and anthraquinones (Zheng et al. 2021). In most cases, the majority of the compounds have antimicrobial properties and it is estimated that these properties include protection of the host plant from various pathogens, like bacteria, virus, fungi, nematodes, etc. (Gunatilaka 2006). The exact phenomenon with respect to the involvement of microbe or host in the production of these secondary metabolites is still not clear (Ludwig-Muller, 2019). Also, with the record elevation in the incidences of many existing and new pathogenic microbes, their recurrence and resistance towards the currently available pharmaceuticals, the clinicians are searching for alternate sources of compounds to treat such infections. The antimicrobial compounds produced by the endophytes are considered advantageous over the conventional ones as they are environment-friendly, specifically toxic to certain harmful pathogens, whereas non-toxic to humans (Singh et al. 2017). This purely endorses the use of secondary metabolites of endophytic origin as promising sources of antimicrobial compounds.
In recent years, the potential of fungal endophytes in various sectors has been evaluated globally by many researchers, still, there is a need to get more insights into the benefits conferred by them (Suryanarayanan et al. 2020). For instance, the first secondary metabolite isolated from an endophytic fungus was an anticancer diterpenoid alkaloid “taxol” which was obtained from an endophyte Taxomyces andreanae from the bark of Taxus brevifolia (Stierle et al. 1993). After the discovery of this drug of endophytic origin, the trend of studying them for obtaining novel secondary metabolites of medicinal properties took a remarkable breakthrough. Also, they have been reported to produce higher number of secondary metabolites than any other class of endophytes (Zhang et al. 2006).
In addition, recent advancements in the production of antimicrobial compounds by using CRISPR/Cas system which facilitates the production of novel classes of antimicrobial compounds viz., antibiotic enhancers, engineered antibodies, engineered phages, siderophore conjugates, photo-switchable antibiotics are also opening new ways for exploring novel antimicrobial compounds (Mantravadi et al. 2019). The recent techniques, like, nanotechnology and micro-engineering also makes it possible to cultivate the endophytic microbes which are not easy to culture from the plant (Mantravadi et al. 2019).
Classification of endophytes
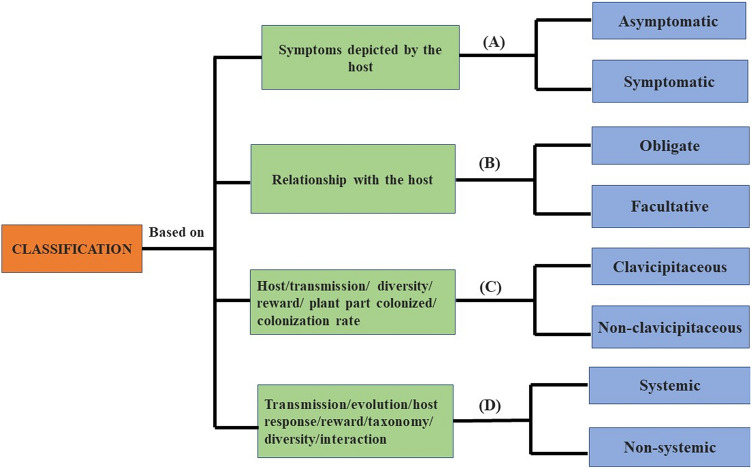
The classification of endophytes has been a complex process and this continues from several years to make their definition more clear for better understanding. Initially, endophytes were divided into two distinct groups, with first group including those endophytic fungi that remain symptomless for the whole life cycle inside the host and the second group which develop some external symptoms on the host plant, such as, root nodules (Azevedo et al. 2007). As per another classification, endophytes were simply categorized into two different sub-groups, obligate endophytes and facultative endophytes (Hardoim 2008). The former were considered to be dependent solely on their host for their growth, survival and transmission whereas the latter to be administered to the host from outside environment and can also survive independent of their host (Abreu-Tarazi et al. 2010).
Endophytic fungi have also been categorized as clavicipitaceous and non-clavicipitaceous endophytes based on the range of hosts, mode of transmission, plant tissue being colonized, colonization frequency, biodiversity (high or low) and habitat and non-habitat specific functions (Rodriguez et al. 2009). Both these groups are included in four different classes, i.e., Classes 1,2,3 and 4. Class 1 type of endophytes include clavicipitaceous endophytes which are seen mainly predominant in grasses whereas other three classes are included in non-clavicipitaceous type of endophytes which are mainly predominant in non-grasses and higher vascular plants (Rodriguez et al. 2009).
According to a recent classification, endophytes are classified into two broader groups such as, systemic or true endophytes and non-systemic or transient type of endophytes depending on a range of characters, like, biology, functional diversity, taxonomy, evolution and their mode of transmission (Wani et al. 2015). Systemic type of endophytes are known to inhabit the same plant in different seasons and exhibit vertical (via seeds) mode of transmission than horizontal. On the contrary, non-systemic type of endophytes show variability in their abundance and diversity within the plant with the dynamics in external climatic conditions and exhibit a horizontal mode of transmission (Wani et al. 2015). A brief description of the common classification system of endophytes is depicted in Fig. 1.
Fig. 1.
Successive classifications of endophytes a (Azevedo et al. 2007) b (Hardoim et al. 2008) c (Rodriguez et al. 2009) d (Wani et al. 2015)
However, novel ways of classification of fungal endophytes have been adopted by different authors as per their host range, such as fungal endophytes of grasses (Tanaka et al. 2012), medicinal plants (Kaul et al. 2012), conifers (Kim et al. 2013) and mangroves (Demers et al. 2018). Similarly, based on the type of tissues being colonized, they are designated as foliar fungal, dark septate, root endophytes as well as stem/bark / seed endophytes and so on (Gakuubi et al. 2021).
Benefits conferred by the endophytes to the host plant
The maintenance of the relationship between an endophyte and the host plant has continued for several years and during this course of time, they co-evolved in the vicinity of each other which has proved beneficial to both of them (Palanichamy et al. 2018; Adeleke et al. 2019). Endophytes play an important role in the survival of their host plants by providing both direct and indirect benefits (Mattoo and Nonzom, 2021). Some of the direct benefits include:
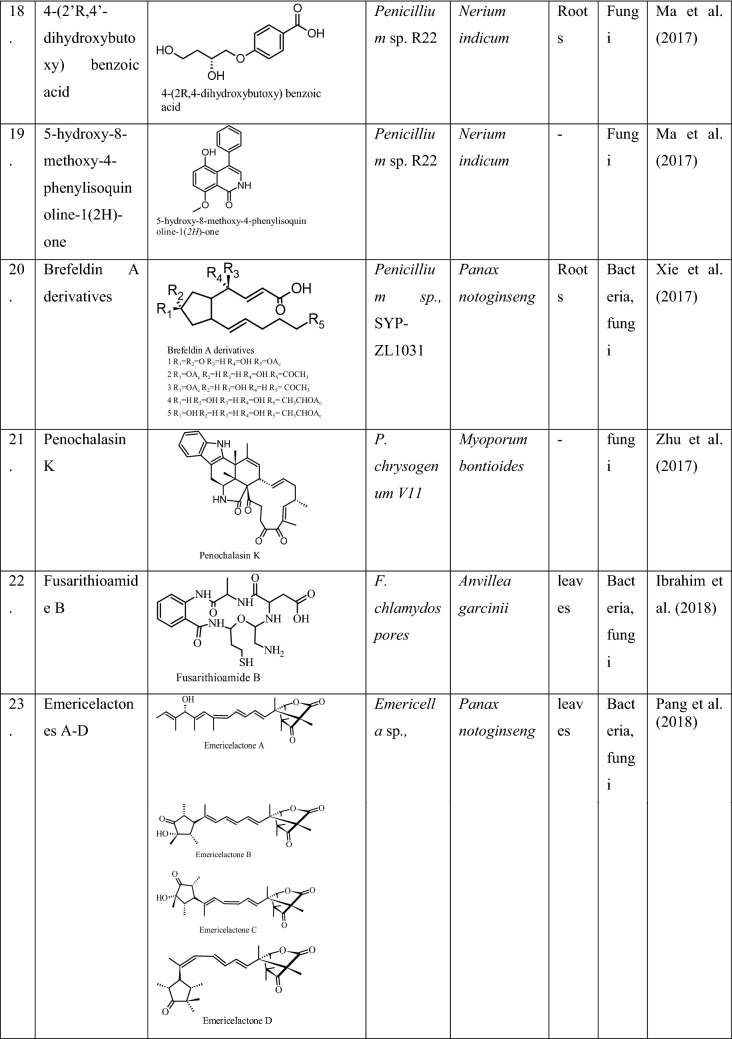
Phytohormones production
The phytohormones produced by the endophytes are known to exhibit various morphological as well as structural changes in plants which aid in the sustainable agricultural systems (Sturz et al. 2000). For example, an endophytic fungus Cladosporium sphaerospermum isolated from Glycine max is known to produce gibberellic acid (Hamayun et al. 2009). In many cases, it has been reported that the endophytes act indirectly by expressing the genes responsible for the production of hormones in the plants (Waqas et al. 2012). It has been well exemplified by an endophytic fungus, Dietzia natronolimnaea which has been observed to modulate ABA signaling pathways in wheat to alleviate salinity stress by upregulating the genes, such as, TaABARE and TaOPRI (Ilangumaran and Smith, 2017).
Enhancement in photosynthetic activity
Endophytes are known to enhance the photosynthetic activity of many plants by increasing their chlorophyll content (Almuhayawi et al. 2021). For example, the root endophytic fungus Trichoderma is well recognized as a beneficial partner in various crop plants as it is known to upregulate certain genes of the host involved in pigment formation for photosynthesis (Harman et al. 2021). The inoculation of Trichoderma sp., in wheat has also been reported to have a role in enhancing the water uptake and photosynthetic capacity which in turn induced biomass production under salt stress (Oljira et al. 2020). Similarly, an Epichloe typhina endophytic to Dactylis glomerata improves photosynthetic efficiency by improving carbon assimilation and photochemistry of PSII (Rozpadek et al. 2015).
Siderophores production
Siderophores production by fungal endophytes prevents the plant from iron deficiency, as these compounds help in iron acquisition by the plant (Ansari et al. 2017). Numerous endophytes produce siderophores which exhibit iron-chelating properties and they indirectly compete with the pathogens for iron assimilation, thereby playing a dual role (Suman et al. 2016). For instance, many plants, such as, Cymbidium aloifolium, Triticum aestivum, and Vigna radiata have been observed to harbor many siderophores producing endophytes that not only help them in combating various phytopathogens but also promoted their growth and germination (Ripa et al. 2019; Chowdappa et al. 2020).
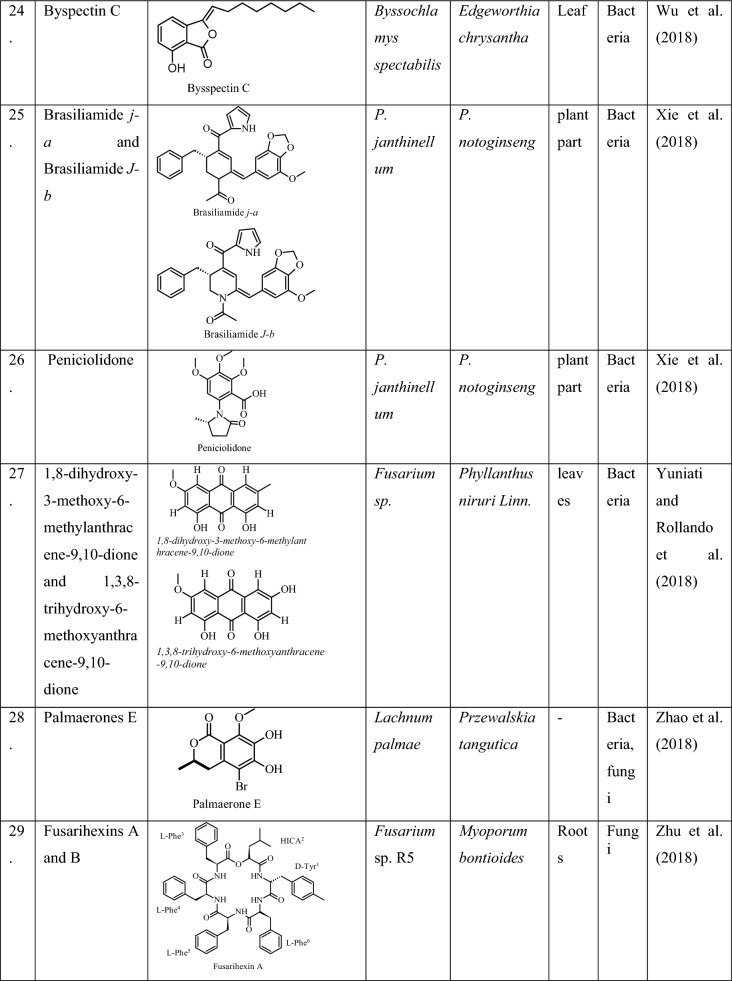
Nitrogen fixation
Fungal endophytes plays an important role in agriculture due to their nitrogen-fixing ability (Yang et al. 2015). For instance, an endophytic fungus Phomopsis liquidambaris increases nodulation and enhances nitrogen uptake of the host plant Arachis hypogaea L. (Xie et al. 2019). The inoculation of these beneficial endophytic fungi in crop plants increases their growth and maintenance by the acquisition of nitrogen uptake (Poveda et al. 2021). Similarly, an endophytic yeast, Rhodotorula mucilaginous which was isolated from Typha angustifolia when inoculated in rice plant promotes and increases the nitrogen content in the tissues (Paul et al. 2020). In addition, there are several similar experiments performed on different crop species for nitrogen assimilation in response to fungal endophytic inoculation (Rinu et al. 2014; Adnan et al. 2018; Christian et al. 2019; Tang et al. 2019; Wu et al. 2019).
Mineral solubilization
More than 99% of phosphorus present in the soil is non-soluble and unavailable to plants (Rodriguez and Fraga, 1999). Many studies have revealed the phosphate-solubilizing activities of diverse endophytic fungi (Nath et al. 2015; Almario et al. 2017; Rana et al. 2019). A recent study concluded that microbial inoculations in plants also help in increasing nutrients acquisition, like, P, K and Zn (Poveda et al. 2021) and organic acid concentration in root exudates which lowers the pH of the soil and assists in solubilization of these mineral nutrients (Sirohi et al. 2015). Apart from these, various experimental studies claim the importance of fungal endophytes for their host plant. Some of the recent examples of various benefits conferred by the endophytes is tabulated in Table 1.
Table 1.
Benefits provided by endophytic fungi to host plants
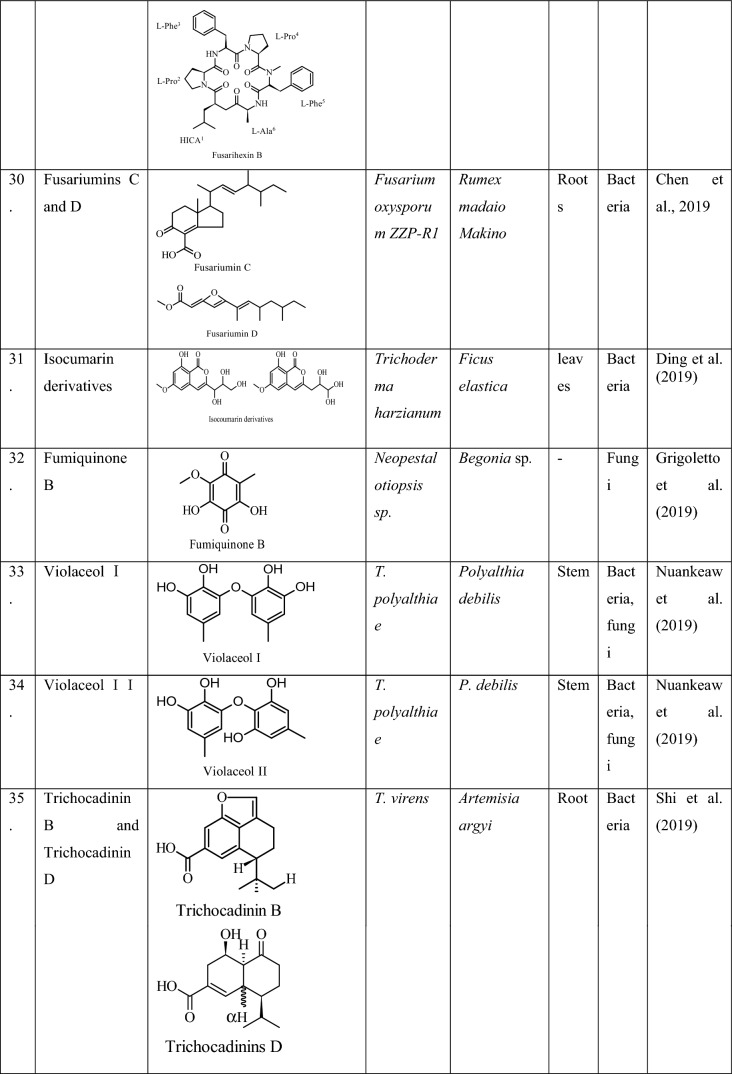
| S. No | Endophyte | Host | Rewards to the host | Reference |
|---|---|---|---|---|
| 1 | Paecilomyces formosus | Cucumis sativus L | IAA and various gibberellic acids (GA1, GA3, GA4, GA8, GA9, GA12, GA20, GA24) | Khan et al. (2012) |
| 2 | Penicillium sp. | Camellia sinensis L | Phosphate solubilization | Nath et al. (2012) |
| 3 | Colletotrichum gloeosporioides CG60 | Halophyte | Gibberellin hormone | Khalmuratova et al. (2015) |
| 4 | Chaetomium globosum | Amaranthus viridis | Cytotoxic and antimicrobial properties | Piyasena et al. (2015) |
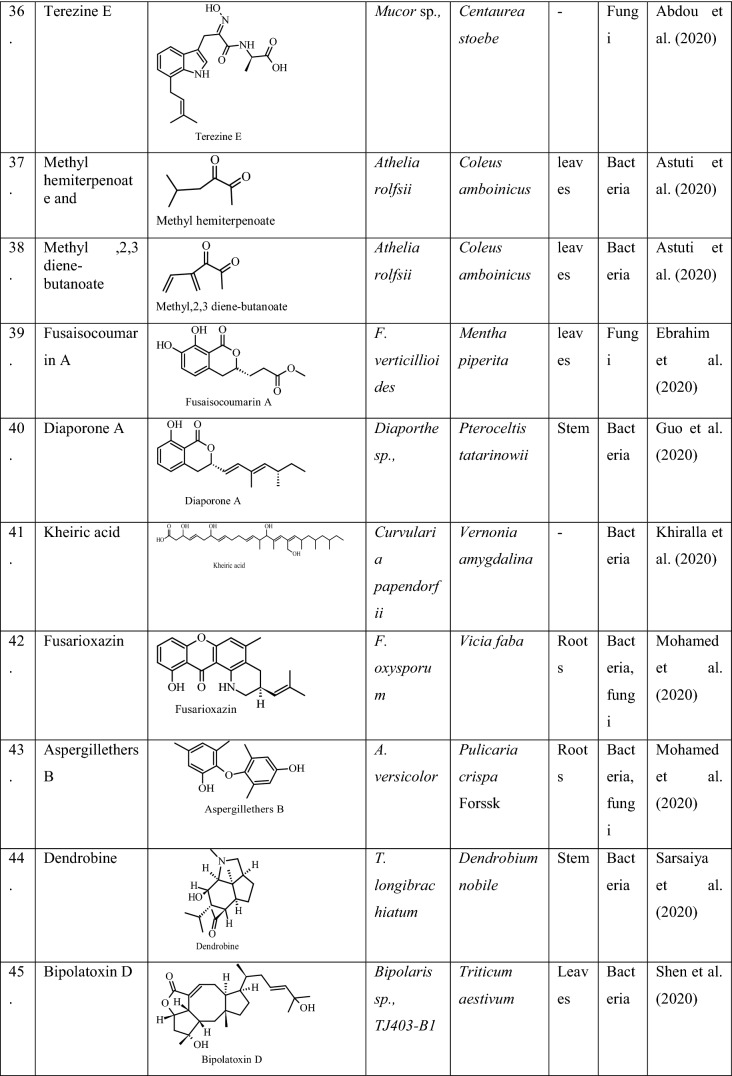
| 5 | Lasidioplodia pseusotheobromae | Hottuynia cordata Thunb | IAA and siderophore production | Aramsirirujiwet et al. (2016) |
| 6 | Fusarium oxysporum and F. solani | Solanum lycopersicum | Prevent nematode production | Bogner et al. (2016) |
| 7 | Sordariomycetes sp. | Boswellia sacra | IAA production, and enzymes like, phosphatase, cellulase and glucosidase | Khan et al. (2016) |
| 8 | Penicillium crustosum | Teucrium polium | IAA production and phosphate solubilization | Hassan, (2017) |
| 9 | Trichoderma harzianum TH 5–1-2 | Pistacia vera | Chitinase enzyme production | Dolatabad et al. (2017) |
| 10 | Fusarium proliferatum BRL1 | Oxalis corniculate | Phosphate solubilization, siderophores production, IAA and Gibberellins production | Bilal et al. (2018) |
| 11 | Colletotrichum fructicola | Coffea arabica | IAA | Numponsak et al. (2018) |
| 12 | Trametes versicolor and Piriformospora indica | Triticum aestivum | Increase biomass and Phosphorus content | Taghinasab et al. (2018) |
| 13 | Aspergillus awamori Wl1 | Withania somnifera | IAA production | Mehmood et al. (2019) |
| 14 | Fusarium oxysporum | Solanum lycopersicum | GA3 production | Ben Rhouma et al. (2020) |
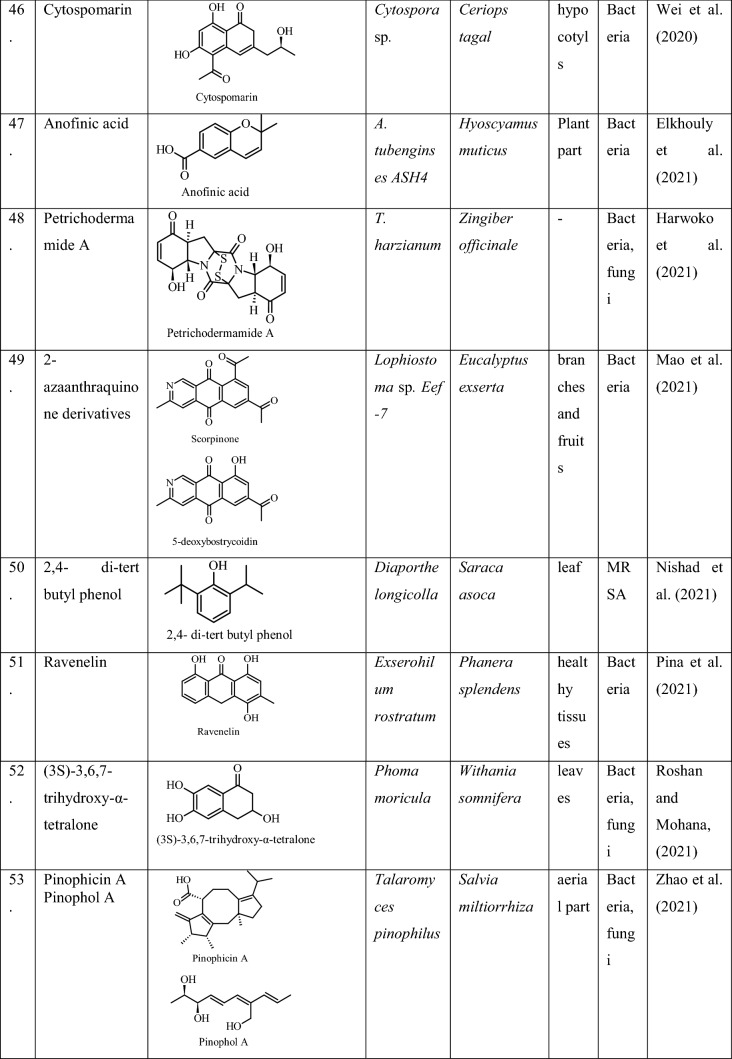
| 15 | Daldinia eschscholtzii 2NTYL11 | Stemona tuberosa | Phosphate solubilization | Suebrasri et al. (2020) |
| 16 | Trichoderma erinaceum ST-KKU2 | Zingiber officinale | Phosphate solubilization | Suebrasri et al. (2020) |
| 17 | Aspergillus niger | Solanum lycopersicum | IAA, ascorbic acid, phenols, catalases | Aziz et al. (2021) |
| 18 | Bipolaris spp. | Zea mays | IAA production | Yousaf et al. (2021) |
On the other hand, the indirect benefits conferred to the plant include the following.
Biotic stress
Endophytes have been reported to perform various strategies to protect the plant from a number of biotic stresses, such as insects, pest, pathogens, and herbivores. Foliar fungal endophytes have been reported to upregulate the defense genes of the host plant which enhances the defense system of the host against various biotic stresses including pathogens and herbivores (Mejia et al. 2014). They colonize the epidermal tissues of the plant where they absorb nutrients for themselves, as well as inhibit the growth of pathogens inside the tissue and thereby induce resistance of the plant against different biotic stresses (Meena et al. 2017). They are also known to produce some toxic metabolites inside the different parts of the plant, such as, the stem, root or leaves to protect them from herbivory (Bischoff and White 2005; Saikkonen et al. 2002; Stone et al. 2004). In addition, they produce antimicrobial compounds as secondary metabolite to protect the plant from different pathogenic microbes (Mousa and Raizada2015; Zhang et al. 2015). For example, the finger millet which is considered to be resistant to pathogens inhabits Phoma sp., as an endophyte that possesses strong antifungal activities against the pathogenic strain of Fusarium graminearum (Mousa et al. 2015).
Also, reports on some entomopathogenic fungi for their use in the biocontrol of insects have gained attention (Vega et al. 2008). Certain entomopathogenic fungi have also been reported to exist as an endophytes in plants for some part of their life cycle, suchas, Beauveria, Isaria, Lecanicillium and Metarrhizium (Lughtenberg et al. 2016). These kinds of fungi after becoming endophytic help the plants to overcome biotic stresses, such as, nematodes and phytopathogens (Moraga 2020).
Abiotic stress
Fungal endophytes also helps the plants to overcome many abiotic stresses, such as, high temperature, soil salinity, oxidative stress, cold stress, heat stress, drought, phytoremediation, and so on. (Rodriguez et al. 2009; White and Torres, 2010; Waqas et al. 2012; Larriba et al. 2015; Mattoo and Nonzom, 2021). A study has revealed the inoculation of endophytic fungi Phoma glomerata and Penicillium sp., in the host cucumber helps the plant to overcome salinity and drought stress by increasing their biomass, various growth parameters and as well as assimilating the essential nutrients (K, Ca and Mg) under induced salinity and drought stress as compared to the control (Waqas et al. 2012). The most prominent mechanism observed behind this scenario is the maintenance of the osmotic gradient of the cell, cell wall elasticity and proper assimilations and translocations of compounds inside the cell (Nieves-Cordones et al. 2019). It has also been observed that the fungal endophytes can also confer thermotolerance to the host plants to alleviate heat stress (Rodriguez et al. 2008; Ismail et al. 2018).
In addition to the aforementioned abiotic stresses, endophytic fungi have also been reported to help the plant to overcome various oxidative stress, like, hydrogen peroxide, hydroxyl radicals, and superoxide anions (Sun et al. 2010; Lata et al. 2018). Similarly, they have also been reported to exhibit metal-chelating, metal sequestering as well as suitable degradation pathways which helps the plant to alleviate heavy metal-stressed habitat conditions (Aly et al. 2011). Also, reports on various fungal endophytes that assist in phytohormone production by alleviating metal stress are well established (Khan et al. 2017).
Benefits conferred by the host plant to the endophytic fungi
In this symbiotic relationship of endophytism, along with the host plant, the endophytes also obtain different advantages from this close association. As discussed earlier, these two groups have shown co-evolution over the course of time (Khare et al. 2018). Fungal endophytes are benefited from this symbiotic relationship in a number of ways which are as follows:
Nutrient absorption
Endophytes absorbs nutrients especially the carbon sources from the host, by invading the photosynthesizing tissues of the plant (Mack and Rudgers, 2008). The host plant produces various metabolites which are bio-transformed by the endophytes to further use them for their nutrition acquisition. For example, Cephalotaxus harringtonia produces glycosylated flavonoids which are bio-transformed by its endophytic fungus, Paraconiothyrium variabile (a foliar fungal endophyte) to aglycones for enhancing the growth of its germinating hypha (Tian et al. 2014). Similarly, β-1,6-glucanase enzymes produced by an endophyte Neotyphodium sp., in the apoplast of host Poa alpina at the time of infecting the host helps in their nutrient acquisition, as well as protect the plant from other infecting pathogens (Moy et al. 2002).
Shelter
The plants provide shelter to the endophytes by conferring them a range of benefits. Endophytes gets attracted towards the plant through the root exudates, such as sugars, phenolics, organic acids, and amino-acids, etc. (Mattoo and Nonzom, 2021). In addition, plants also provide protection to the beneficial endophytes in extremely dry environmental conditions, such as Epichole, Neotyphodium and Balansia which reside in the moist tissues of plants (Dutta et al. 2014). Most medicinal plants have been reported to provide shelter only to their beneficial counterparts which have the capabilities of producing various secondary metabolites by which they get benefitted in numerous ways (Rosa et al. 2010).
Low competition
In general, endophytes maintain a balanced antagonism with other endophytes inside the host tissues (Schulz et al. 2015). Thus, they face less competition than the rhizospheric and phyllospheric micro-organisms due to less number of competitors inside the host endosphere, enriched nutrients availability, optimum pH, and moisture (Backman and Sikora, 2008). In addition, they also render the plants less responsive to the other invading microbes (Christensen et al. 2002). Also, colonization of foreign endophytes is not permitted easily by the native endophytes, as they generally show envy behavior against them (Suryanarayanan et al. 2018).
Dispersion
The seeds of the plants that may harbor endophytic microbes become resistant to dehydration and various adverse environmental conditions and, thus, helps in the vertical transmission of endophytes (Truyens et al. 2013). For instance, the endophytes of ryegrass (foraging grasses) can reproduce only by infecting the seeds of the plant (Bultman and Murphy, 2000). Therefore, the propagules of the host plants have a role in the dissemination of endophytes present inside them (Schardl et al. 2004). Similarly, plants allow the dispersion of endophytes through their different parts, such as leaf surface, stem surface, or any other region for their horizontal dissemination via asexual spores (Tadych et al. 2012).
Demand for antimicrobial drugs
Resistance to the antimicrobial drug is a leading crisis worldwide (WHO, 2014). The problem of resistance of microbes against various antimicrobial drugs was well recognized about 38 years ago in Annals (Kunin 1993). This resistance has been developed due to a number of factors, like (a) misuse of medicines, (b) l long-term improper use of medicines, (c) lack of public awareness, (d) poor sanitary conditions (e) feeble immune system of people, and (f) postponement in disease diagnosis (Kunin 1983; Rice 2008). Also, horizontal gene transfer between different microbial communities has led to an increase in antimicrobial resistance in them (Thomas and Nielsen, 2005). For example, approximately 20% genome of E. coli is modified through horizontal gene transfer which renders them resistant to traditionally known antibiotic compounds (Lawrence and Ochman, 1997; Browne et al. 2020). Certain examples of microbes that are continuously developing resistance against the available drugs in the markets are Haemophilus influenza, Mycobacterium tuberculosis, Neisseria gonnorrhoea, Streptococcus pneumoniae, Salmonella and Shigella species (Seften, 2002). There are a number of antimicrobial drugs discovered from time to time but all these drugs have at least one side effect. For example., Amphotericin B, a well-used antibiotic usually in the treatment of various fungal infections show side effects, such as, acute renal failure and tubular damage (Fanos and Kataldi, 2000).
In the present scenario, the need for new antimicrobial agents is increasing due to the emergence of resistance in both plant and human pathogens (Prestinaci et al. 2015). Developing countries are mostly affected by severe diseases, like, malaria, tuberculosis every year and have witnessed an increase in the normal death rate which poses the need for using novel antimicrobials (Mohan et al. 2022). Apart from this, various fungi and yeasts, such as Aspergillus, Cryptococcus, Candida are responsible for causing several mycotic disorders (Karkowska-Kuleta, et al. 2009). Also, serious fungal infections which can be caused by chemotherapy, organ transplant, and other surgeries, like, allogenic bone marrow transplantations impose a need to use effective and safe antifungal drugs (Bhardwaj and Agrawal, 2014). In addition, antimicrobial compounds also find its way to be used as preservatives in food to prevent foodborne diseases (Liu et al. 2008). The increasing health problems are broadening issues worldwide these days and the need to find new antibiotics and therapeutic agents with less toxicity with no environmental impact and which are beneficial to mankind is increasing (Strobel and Daisy, 2003).
Recently, in the situation of COVID-19 pandemic, many patients are developing the symptoms of secondary bacterial and fungal infections (Selarka et al. 2021). However, due to insufficient time to evaluate the patients effectively for micro-biological confirmation, they are usually prescribed with antimicrobials for rapid recovery which sometimes leads to overuse or misuse by patient (Langford et al. 2021). Patients, in some cases, are using antimicrobials continuously without having any serious infection which in turn leads to the development of resistance against antimicrobials (Lansbury et al. 2020; Rawson et al. 2020). To deal with this problem of drug resistance, one should minimize the rate of acquisition of drugs and use appropriate measures to lessen the spread of the disease. In spite of the introduction of many new antimicrobials against these micro-organisms, they are posing major threats to living organisms due to their evolutionary efficient mechanisms to overcome the effect of these antimicrobials over time (Lowy 2003).
The production of antimicrobial compounds has faced numerous problems over the years like, (a) the non-availability of commercial bioactive secondary metabolites, sources of derivation are slow-growing medicinal plants or rare plant species (c) even sometimes the synthesis of the bioactive secondary metabolite is very expensive or (d) its high complexity or molecular weight (Rustamova et al. 2020). To deal with these problems medicinal plants are continuously being used in large quantities for producing desired drugs which, however, leads to a decrease in their population. Therefore, alternatives such as endophytes (bacteria or fungi) can be employed for this purpose. As discussed earlier, paclitaxel a rare and important bioactive compound obtained from an endophytic fungus Taxomyces andreanae provides an alternative method for producing this costly drug without using the host plant (Stierle et al. 1993). This will, however, reduce our reliance on slow-growing medicinal plants for medicinal drugs and also helps in preserving our declining biodiversity. These drugs of microbial origin are less costly, time-saving, and more economical, thus making them a worthy choice from the pharmaceutical perspective (Strobel and Daisy, 2003).
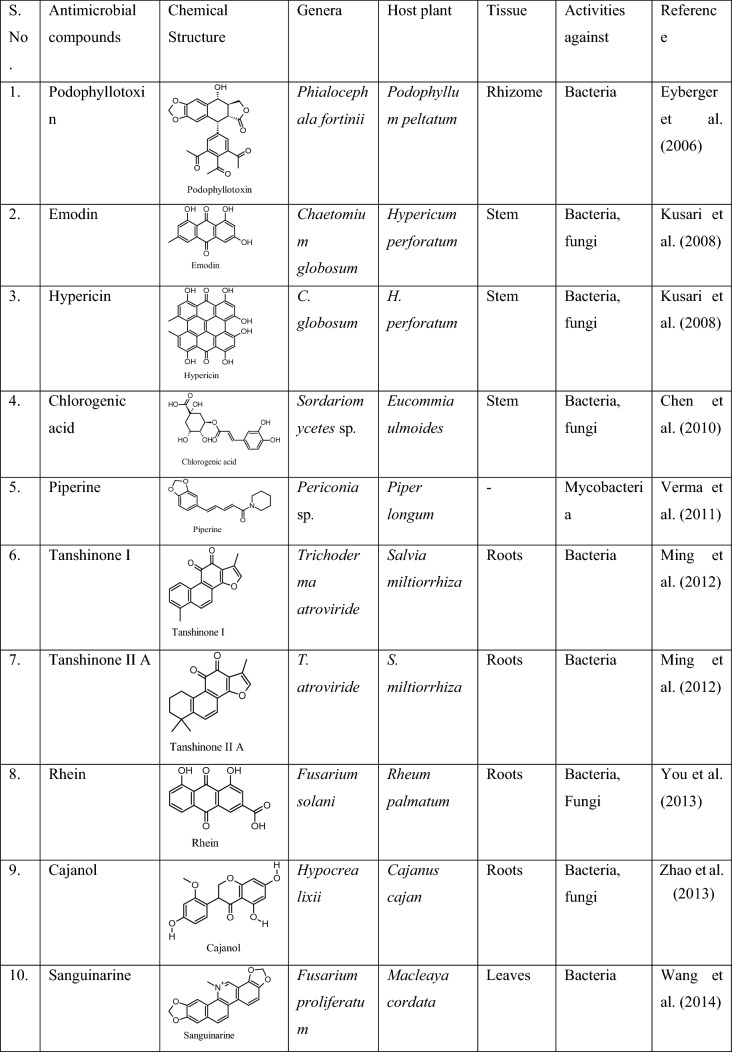
As discussed earlier, endophytes are known to produce secondary metabolites similar to their host and this mimicking ability is a leading perspective for the development of desired drugs (Zhang et al. 2006). Some of the drugs produced by them are widely used nowadays well exemplified by taxol, camptothecin, podophyllotoxin, vinblastine, vincristine, azadirachtin, hypericin, diosgenin and rhitukine (Sachin et al. 2013; Nicoletti and Fiorentino, 2015). Some of the well-known antimicrobial compounds produced by the fungal endophytes similar to their host plant are given in Table 2. Also, the employment of endophytes as an alternative antimicrobial production can be attributed to the diverse advantages, like, faster growth rate than medicinal plants, fermentation potential, easy nutrient availability, host-mimicking compounds production, and enhanced antimicrobial potential than plants. Thus, fungal endophytes provide a safe alternative way to produce novel antimicrobial compounds in high quantities to deal with this situation (Yu et al. 2010; Kumar et al. 2014).
Table 2.
Antimicrobial compounds mimicked by the reported fungal endophytes
Genes responsible for antimicrobial production
As per the earlier studies, it was proposed that genes responsible for the production of secondary metabolites are scattered in the whole genome of the microbe, like the genes of primary metabolite production in fungi (Hoffmeister and Keller 2007). Later, it was found that genes responsible for antimicrobial production are present in clusters, i.e., polyketide synthase (PKS) and non-ribosomal peptide synthase (NRPS) found in extra-chromosomal material or plasmid of the endophytic fungi that synthesize polyketides and oligopeptides associated with antimicrobial activities (Brakhage and Schroeckh, 2011; Sachin et al. 2013; Mishra et al., 2017).
According to Keller et al. (2005), fungi produce secondary metabolites with the help of a few precursors which result from the primary metabolic pathways (Keller et al. 2005). Endophytic fungi produce various classes of antimicrobial compounds, such as alkaloids, polyketides, terpenoids, phenylpropanoids, peptides, and aliphatic compounds (Mousa and Raizada, 2013). The ability of the endophytes to produce host-based secondary metabolites shows that there is possibility of the existence of various complex cross-talks between the host plant and endophytes at the gene level. The evolutionary studies and the inability of endophytic fungi to produce secondary metabolites in subculturing (either due to loss of extra-chromosomal material acquired from the host plant or silencing of genes in the absence of host) supports the possibility of host-based secondary metabolites production (Kumara et al. 2014).
The horizontal gene transfer i.e., the transmission of genetic material among different organisms is observed in bacteria, fungi, and other eukaryotic organisms (Bansal and Meyer et al. 2002; Vos et al. 2015). Although, this can be an efficient reason for the production of host-origin secondary metabolites in endophytes due to their co-evolution over millions of years (Venieraki et al. 2017). As discussed earlier, genetic studies on antimicrobial production in fungal endophytes revealed that the genes responsible are present on the chromosomes in clusters (Mousa and Raizada, 2013). According to Bielecka et al. (2022) the transfer of these gene clusters from the host plant to endophytic fungi leads to the production of novel and versatile compounds (Bielecka et al. 2022). Similarly, the production of similar secondary metabolites by different fungal endophytes belonging to different plants (brefeldin A, paclitaxel and echinocandin) also facilitates the hypothesis of horizontal gene transfer between different fungal endophytes during the evolutionary process (Mousa and Raizada, 2013).
Although, the biosynthetic gene clusters present in the endophytic fungi are present in insufficient amounts, the secondary metabolites produced by fungal strains are not produced in accordance with it (Rashmi and Venkateswara Sarma 2019). There are a number of ways for enhancing the metabolite production by endophytic fungi in cultural conditions outside their host such as, (1) by changing the cultivation parameters (constituents of the medium used, aeration condition, enzyme inhibitors being used, cultural vessels, etc.) of secondary metabolite production (2) co-culturing (co-cultivation or mixed fermentation) (3) by use of epigenetic modifiers (DNA methyltransferases inhibitors and histone deacetylases inhibitors) and various molecular approaches (genetic engineering, manipulation of negatively regulatory genes, overexpression of positively regulatory genes) (Yu et al. 2010; Gakuubi et al. 2021). For example, overexpression of ε-PL synthetase genes in fungal endophyte Epichloe festucae of the host plant Lolium perenne (perennial ryegrass) results in the enhanced production of an antifungal compound ε-poly-l-lysine (Purev et al. 2020). Similarly, several new techniques, like, CRISPR–Cas application is being employed nowadays to enhance the bioactive potential of fungal endophytes (Yan et al. 2018).
As per the studies by many researchers, the shikimic or enzymatic pathways are also involved in the synthesis of secondary metabolites in endophytic fungi (Aharwal et al. 2021). On the other hand, various studies on genome sequencing indicate that the genes responsible for the production of similar secondary metabolites in the host and their respective endophytes not the same (Mattoo and Nonzom et al. 2021). This, however, makes their evolution independent of their host. For example, Taxol is produced by Taxus sp. as well by its endophyte Taxomyces andreaneae, but both producers did not show any sequence homology with respect to each other (Heinig et al. 2013). Similarly, a defensin molecule is known to be produced by the plant, Picea glauca, but later it was found that this defensin molecule (endopiceasin) was originally produced by one of its endophytic fungi (Mygind et al. 2005; Picart et al. 2012). This may be due to cross-activation of genes by common precursors between the plant and its endophytes during stress (Khare et al. 2018).
There are also evidence of duplication of the whole gene cluster for antimicrobial production in endophytes (Mousa and Raizada, 2015). For example, Neotyphodium uncinatum encodes two duplicate clusters of genes (LOL-1 and LOL-2) responsible for loline (alkaloid) production (Spiering et al. 2005). However, much clarification is needed to understand the genetic involvement and different pathways in antimicrobial production by endophytes. Likewise, to understand the potential of biosynthetic gene clusters of endophytic fungi, it is imperative to follow multi-dimensional approaches, such as, bioinformatics, chemical characterization, molecular approach, the study of physical environment effects on metabolite production and omics (Rashmi and Venkateswara Sarma 2019).
Endophytic fungi: an enriched source of antimicrobial compounds
There are numerous micro-organisms associated with plants that are known to produce compounds with antimicrobial properties (Raaijmakers and Mazzola, 2012). Endophytes as a beneficial partners provide multiple rewards to the host plants especially the production of antimicrobial compounds to protect them from various pathogens (Rodrigo et al. 2022). This property of endophytes in protection is increasingly used in healthy crop production (Dong et al. 2021). They show antagonistic behavior against different pathogens to protect their host. For example, fungal endophytes, such as Trichoderma atroviride, Metarhizium anisopliae and Hypoxylon rubiginosum exhibit antifungal activities against phytopathogens Diplodia pinea, Fusarium graminearum and Hymenosciphus fraxineus, respectively (Santamaria et al. 2012; Halecker et al. 2020; Hao et al. 2021). This potential for the production of antimicrobial metabolites of endophytic fungi leads to its effective utilization in agriculture and pharmaceutical industries, for the production of novel drugs (Sudha et al. 2016; Farhat et al. 2019). There are some antimicrobial compounds, such as, atenusin, ambuic acid, cryptocin, dihydroxycadalene, nodulosporins, phomenone and trichodermin produced by the fungal endophytes that are considered to have an important role in the protection of the plant against various phytopathogens (Kaul et al. 2012; Chen et al. 2014). More than 300 endophytes have been successfully isolated and cultured in laboratory conditions for the production of secondary metabolites of therapeutic importance in the past 5 years (Patil et al. 2016).
Antimicrobial compounds originally are the natural organic compounds of low molecular weight produced by various microbes and exhibit the property of killing other micro-organisms under their influence (Guo et al. 2000). Their production of secondary metabolites with antioxidant and antimicrobial properties have been discovered in the past two decades (Bhardawaj et al. 2015). The secondary metabolites produced by medicinal plants are being used in pharmaceutical sectors since time immemorial (Hamid and Aiyelaagbe, 2011). Similarly, fungal endophytes obtained from these medicinal plants also act as an alternative source of production of nearly half of the identified bioactive compounds (Supaphon et al. 2013).
In this world of ever-increasing human population, a large number of antimicrobial drugs are already discovered, but still, there is a need to find out new therapeutic agents due to the emergence of resistant varieties (Karam et al. 2016). Fungal endophytes are known to produce a diverse range of bioactive compounds which have been used in the pharmaceuticals and pesticide industries (Rodriguez et al. 2000; Onifade 2007). They are studied for their capability of producing antimicrobial compounds in vitro and the results are sometimes even better than the plant itself with exceptionally enhanced antimicrobial potential (Arora and Kour, 2019).
It is estimated that out of 22,500 microbes derived metabolic compounds including antibiotics, fungi constitute a large population (approximately 38%) to produce these compounds (Berdy et al. 2005). The antimicrobial compounds obtained from endophytes belong to different classes, such as, alkaloids, aliphatics, phenolics, polyketides, terpenoides, peptides and various nitrogeneous compounds (Mousa et al. 2013). The wide range of antimicrobial compounds which are produced by the host as well as their endophytic partners is enormous (Gakuubi et al. 2021). The ability of the endophytes to produce similar antimicrobial compounds produced by their host will also help to utilize the rare or endangered plants in a very convenient way (Sharma et al. 2021).
The demand for fungal endophytes has increased after realizing their potential for producing anticancer compounds, like, taxol and camptothecin due to the scarcity of natural plant sources (Gupta et al. 2020). There are a number of drugs available in the market which are of endophytic origin with reference to fungal endophytes. These drugs are commercially available in the market after several trials and costs million or billion dollars, such as, Piperine (antimicrobial), Podophyllotoxin (anticancer), Vinblastine (anticancer), Vincristine (anticancer), Griseofulvin (antioxidant), Rohitukine (anticancer), Huperzine A (antimicrobial, cholinesterase inhibitor), Altersolanol (Antiangiogenic), Quinine (antimalarial agent) and so on. These important compounds are being utilized in pharmaceutical industries as an alternative source of medicine rather than using the important medicinal plant itself (Tiwari and Bae, 2022). A detailed study on the action of antimicrobial compounds obtained from endophytic fungi revealed their involvement in the impairment of nucleic acid metabolism; enzyme synthesis blockage; disturbance in DNA or RNA synthesis repair system and inhibition of protein synthesis (Samanta et al. 2021; Silva et al. 2022).
Recently, a new aliphatic compound, Kheiric acid was isolated from an endophytic fungus Curvularia papendorfii inhabiting Vernonia amygdalina which was found active against methicillin-resistant Staphylococcus aureus (MRSA) (Khiralla et al. 2020). Similarly, a new enamide dimer, Phomoenamide (an antifungal compound) was produced by a Phomopsis sp. isolated as an endophytic fungus from the healthy leaves of Garcinia dulcis (Rukachaisirikul et al. 2008). A new polyketide, named Talafun was also isolated from a fungal endophyte, Talaromyces funiculosus from Salicornia bigelovii and exhibited great antibacterial potential against E. coli (Guo et al. 2016). Likewise, two new sesquiterpene derivatives, trichocadinin B and trichocadinin D produced by Trichoderma virens isolated from Artemisia argyi having antibacterial activities against P. aeruginosa, Aeromonas hydrophila, E. coli, Vibrio harveyi and V. parahaemolyticus (Shi et al. 2019). Some of the important antimicrobial compounds and their mode of action against pathogenic microbes are discussed below:
Sordaricin
It is one of the potent antifungal compounds which was first isolated from Xylaria sp. endophytic to the plant Garcinia dulcis that exhibited great activity against Candida ablicans (Pongcharoen et al. 2008). The mechanism involved the process of inhibition of ribosomal translocation with mRNA to prevent polypeptide chain synthesis by stabilizing the EF2/ribosome complex (Liang 2008).
Piperine
Piperine is a well-known compound with a number of properties including antimicrobial and was first produced by Piper longum. However, research in endophytic metabolite production leads to the discovery of this host-mimicking compound by one of its endophytes, Periconia sp. in the liquid fermentation broth. This was the first report of piperine production by an endophyte. It was found active against Mycobacterium smegmatis and M. tuberculosis (Verma et al. 2011). The mechanism behind this involved the inhibition of an efflux pump of M. tuberculosis (Sharma et al. 2010).
Phomopsichalasin
It is an antimicrobial cytochalasin of the alkaloid group first reported as a novel antimicrobial compound from an endophyte Phomopsis sp. inhabiting the twigs of Salix gracilistyla var. melanostachys (Horn et al. 1995). It binds to the actin filament to block its polymerization and exhibits great antimicrobial potential against various human pathogenic bacteria, such as Pseudomonas sp., Salmonella galinarum, Staphylococcus aureus and Bacillus subtilis and yeast Candida tropicalis (Binder, and Tamm, 1973; Zhou et al. 2009).
Hypericin
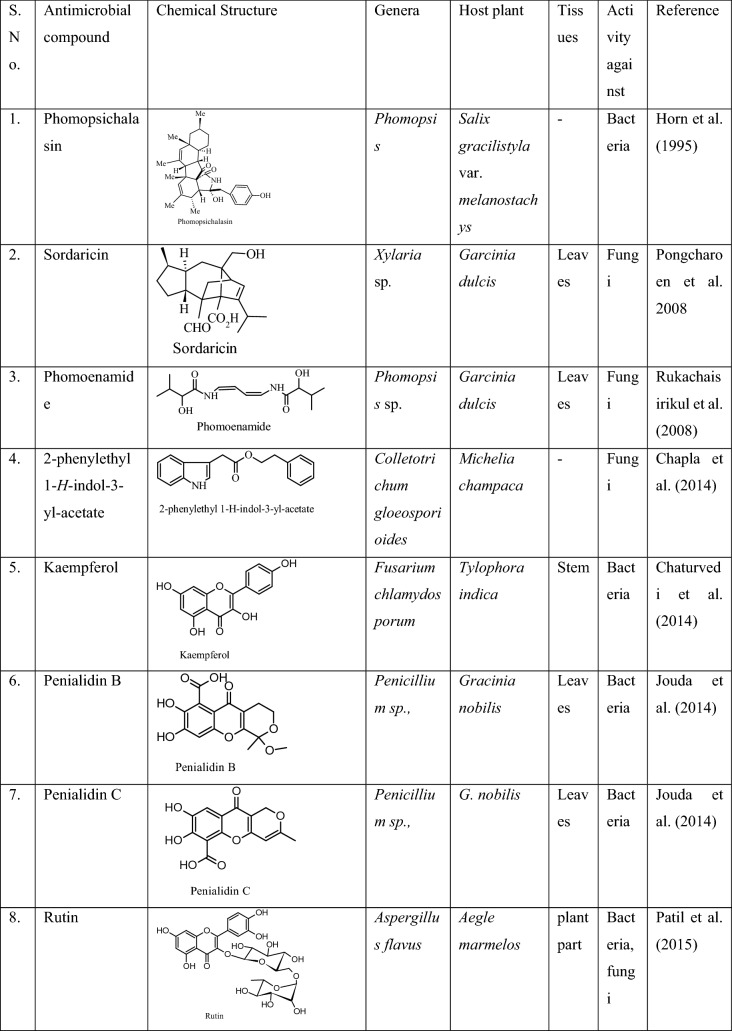
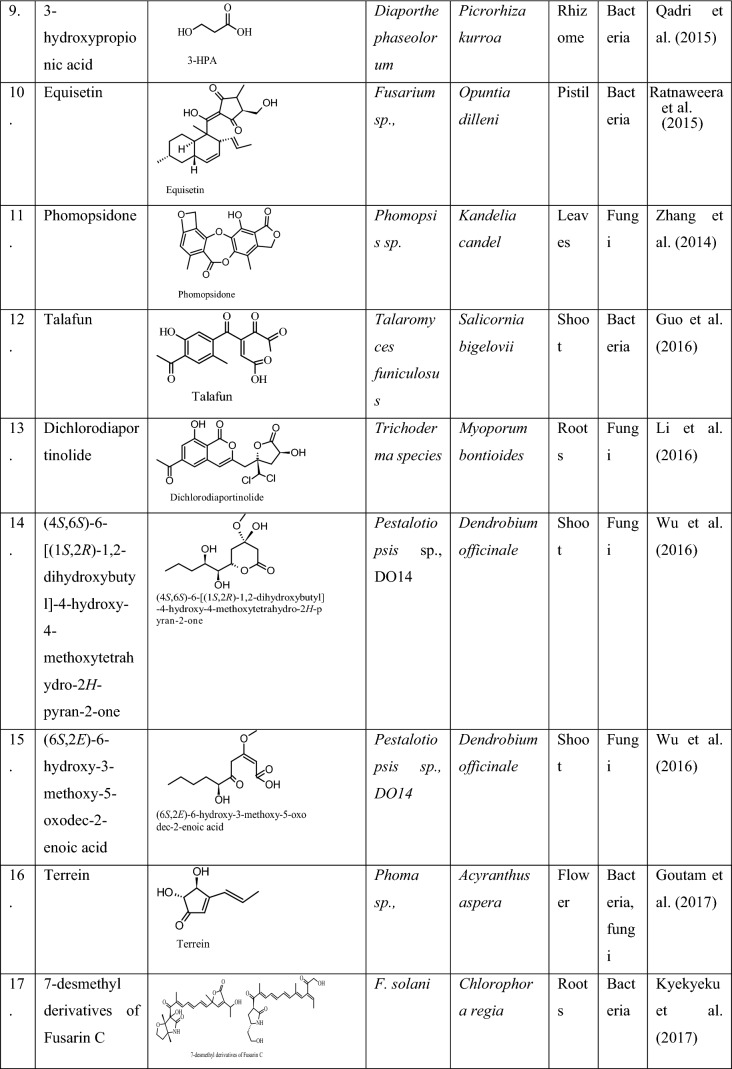
It is an antimicrobial compound earlier reported to be produced by the plant Hypericum perforatum L. Later it was also produced by its endophytic fungus, Chaetomium globosum for the first time and found active against both pathogenic bacteria and fungi, such as E. coli, Klebsiella pneumoniae, P. aeruginosa, S. aureus subspecies aureus, Aspergillus niger and Candida albicans (Kusari et al. 2008). Hypericin reduces the expression of Staphylococcal accessory regulator A (SarA), which is a global virulence regulator, and decreases resistance in methicillin-resistant Staphylococcus aureus (MRSA) against β-lactam antibiotic which plays an important role in the treatment of MRSA (Wang et al. 2019). Similar antimicrobial compounds produced by different endophytes in the past years are presented in Table 3.
Table 3.
Antimicrobial compounds produced by endophytic fungi of different plants, and their bioactivities
Nanoparticles as antimicrobial agents
Nanotechnology is an approach of using nanoparticles with sizes ranging from 10 to 100 nm (Allaker et al. 2010). Nanoparticles are classified into different groups, such as, metal, carbon, polymeric, ceramic-based nanoparticles, and so on (Khan et al. 2019). They are used in various fields, such as, electronics, cosmetics, pharmaceutical, manufacturing and construction (Mohajerani et al. 2019). They have also been focused for their utilization in different aspects, such as drug delivery and diagnosis of disease against microbial infections (Singh et al. 2013). Nanoparticles also find their application in various pharmaceutical industries, such as, in the treatment of cancers in humans or in novel methods of drug delivery (Kapil et al. 2014; Hosseini et al. 2016).
Synthesis of nanoparticles using micro-organisms is an imperative branch of nanotechnology (Shankar et al. 2003). The nanoparticles are synthesized by different physical, chemical, or biological methods (Messaoudi and Bendahou, 2020). The biological methods (use of living organisms, such as plant, seaweeds, and micro-organisms) are more beneficial than physical or chemical methods, which produce toxic chemicals in the environment (Patra and Baek 2014). This method is eco-friendly, non-toxic, cost-effective, and beneficial in the large-scale production of nanoparticles (Singh et al. 2017). The nanoparticles produced by micro-organisms are characterized by their wavelength range (200–800 nm), morphology (scanning electron microscopy and transmission electron microscopy), chemical structure (using X-Ray diffraction method), and functional groups (Fourier transform infrared spectroscopy) (Messaoudi and Bendahou, 2020). Numerous micro-organisms are known to produce nanoparticles, such as, algae, fungi, and bacteria (Khalil et al. 2018). These are being used nowadays due to their potential of reducing metals into nanoparticle sizes and also to reduce the dependency on plants (Staniek et al. 2008).
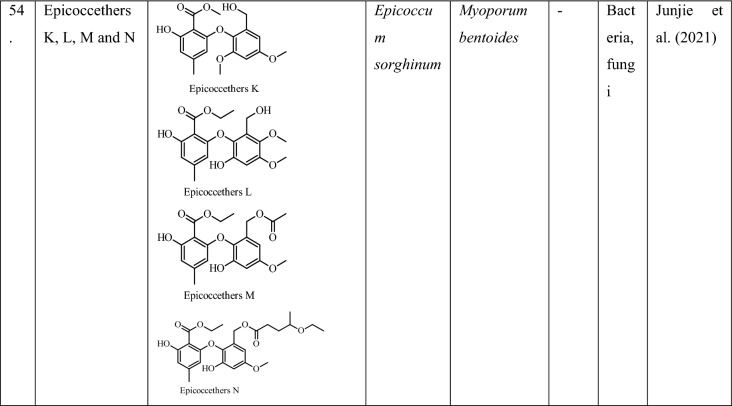
Recently, the endophytes are gaining attention for their potential of producing different nanoparticles of therapeutic importance (Rahman et al. 2019). They have been able to produce different nanoparticles, such as silver, gold, copper, zinc, etc. which are used in pharmaceutical industries (Kulkarni and Ramakrishna 2020; Mani et al. 2021). According to Rahman et al. (2019), the silver nanoparticles produced by endophytes have various properties, such as antimicrobial, seed germination, anticancer, antioxidant activity, potent bactericidal activities, photocatalytic degradation of dyes, in food packaging and various agricultural applications as depicted in Fig. 2 (Rahman et al. 2019; Mustapha et al. 2022). A number of researchers have reported them as valuable tools against various antibiotic resistance Gram-positive as well as Gram-negative bacteria (Liu et al. 2015). The nanoparticles produced by endophytic fungi are considered to be more stable as they can produce proteins and biomolecules which prevent their agglomeration (Netala et al. 2016). The biosynthesis of nanoparticles from endophytes is considered a novel approach with immense potential in drug formulations (Messaoudi and Bendahou, 2020). The diversity of antimicrobial nanoparticles from endophytic fungi has been summarized in Table 4.
Fig. 2.
Production of nanoparticles by endophytic fungi and its various applications
Table 4.
Antimicrobial nanoparticles produced from endophytic fungi
| S. No | Nanoparticles | Fungal endophyte | Host | Tissue | Activity against | Reference |
|---|---|---|---|---|---|---|
| 1 | Silver | Aspergillus clavatus | Azadirachta indica | Stem | Fungi | Verma et al. (2010) |
| 2 | Silver | Pestalotia sp., | Syzygium cumini | Leaves | Bacteria | Raheman et al. (2011) |
| 3 | Silver | Penicillium sp | Curcuma longa | Leaves | Bacteria | Singh et al. (2013) |
| 4 | Silver | Epicoccum nigrum | Phellodendron amurense | Cambium | Fungi | Qian et al. (2013) |
| 5 | Silver | Curvularia lunata | Cathranthus roseus | Leaves | Bacteria | Ramalingmam et al. (2015) |
| 6 | Silver | A. versicolor | Centella asiatica | Leaves | Bacteria, fungi | Netala et al. (2016) |
| 7 | Silver | P. oxalicum | Phlogacanthus thyrsiflorus | – | Bacteria | Bhattacharjee et al. (2017) |
| 8 | Silver | A. niger | Simarouba glauca | Leaves | Bacteria | Hemashekhar et al. (2017) |
| 9 | Silver | A. terreus | Calotropis procera | Healthy tissues | Bacteria | Rani et al. (2017) |
| 10 | Silver | Alternaria sp. | Raphanus sativus | Leaves | Bacteria | Singh et al. (2017) |
| 11 | Gold | Alternaria sp. | Rauvolfia tetraphylla | Roots | Bacteria | Hemashekhar et al. (2019) |
| 12 | Silver | Talaromyces purpureogenus | Pinus densiflora | Leaves | Bacteria | Hu et al. (2019) |
| 13 | ZnO | A. tenuissima | – | – | Bacteria, fungi | Abdelhakim et al. (2020) |
| 14 | Silver | Trichoderma atroviride | Chiliadenus montanus | Aerial parts | Bacteria, fungi | Abdel-Azeem et al. (2020) |
| 15 | ZnO | Periconium sp | Balanites aegyptiaca | Leaves | Bacteria, fungi | Ganesan et al. (2020) |
| 16 | ZnO | A. niger | Mangifera indica | Bark | Bacteria | Kulkarni and Ramakrishna, (2020) |
| 17 | Silver | P. cinnamopurpureum | Curculigo orchioides | Rhizome | Bacteria | Dinesh et al. (2022) |
| 18 | CuO | A. terreus | Aegle marmelosa | – | Bacteria, fungi | Mani et al. (2021) |
| 19 | Silver | T. purpureogenus | Taxua baccata | – | Bacteria | Sharma et al. (2022) |
| 20 | Gold | Phoma sp. | Prunus persica | Vascular tissues | Bacteria, fungi | Soltani Nejad et al. (2022) |
Fungal endophytes as a source of antiviral agents
Viruses are acellular micro-organisms, considered living (inside the host) or non-living (outside the host) depending on the availability of the host. Viral infections often cause serious issues at the global level resulting in outbreaks, epidemics, and pandemics, the most recent being the SARS-CoV-2. However, with time evolution, they get evolved into more resistant varieties, thus raising the need to explore and formulate novel therapeutic agents against them (Saxena et al. 2021).
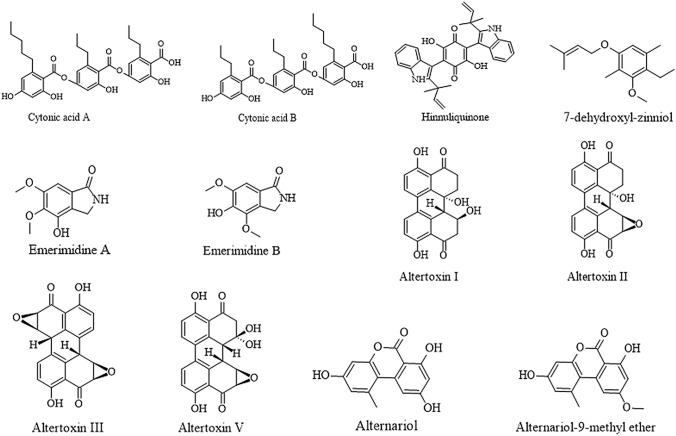
Fungal endophytes as a source of the antiviral have has been considered as a new approach towards its therapeutic importance. It has been reported that endophytes produce antiviral drugs in response to biotic stress inside the host against the virus. For example, an endophytic fungus Cytonaema sp. isolated from an unidentified host was reported to produce two novel antiviral compounds, viz., cytogenic acids A and B, the inhibitors of human cytomegalovirus (hCMV) protease (Guo et al. 2000). Another antiviral compound, Hinnuloquinone isolated from an endophytic fungus, Nodulisporium hinnuleum, inhabiting the leaves of Quercus coccifera was observed active against human immunodeficiency virus type 1 protease (HIV-1) (Singh et al. 2004). Similarly, Emerimidine A and B were isolated from the endophytic fungus Emericella sp. which exhibited moderate activity against Influenza virus H1 N1 (Zhang et al. 2011).
Another novel antiviral compound 7-dehydroxyl-zinniol was isolated from an endophytic isolate, Alternaria solani from the roots of Aconitum transsectum that exhibited moderate activity against hepatitis B virus (Ai et al. 2012). Also, different types of Altertoxins, for example, altertoxin I, II, III, and V have been reported to be produced by Alternaria tenuissima endophytic to the stem of Quercus emoryi that inhibited the growth of HIV virus at different concentrations. Quantitative Structural Activity Relationships (QSAR) studies have been carried out on these compounds to check their potential as antiviral agents (Bashyal et al. 2014). Similarly, alternariol and alternariol-9-methyl ether produced by A. alternata endophytic to the peel of Punica granatum also exhibited potent activity against HCV NS3/4a protease (El-Kassem et al. 2019). The various antiviral compounds isolated from fungal endophytes illustrated above are given with their chemical structure in Fig. 3.
Fig. 3.
Antiviral compounds isolated from fungal endophytes
Inhibition of biofilm production by endophytic fungi
Biofilm formation is a complex process involving a large number of bacteria (Ahmad et al. 2020). This is a method of protection in both plants and animals to protect themselves from host defense systems and antimicrobial compounds. Although biofilms are well studied in the case of bacterial pathogen but are poorly studied in the development and structure of filamentous fungi, as well as their role in pathogenicity (Shay et al. 2022). The antimicrobial compounds are effective against free-living bacteria or micro-organisms, but bacterial species most commonly prefer to grow by biofilm formation in natural conditions which hinders their proper killing (Qvortrup, et al. 2019).
In most of the developed countries, it is seen that more than 80% of microbial infections are caused by biofilm formation only. Human skin, dental plaque, and gut represent biofilm formation's most common site (Qvortrup et al. 2019). To eradicate this serious infection, many natural compounds have been introduced or are utilized in pharmaceutical industries. Various phytochemicals, such as phenolics, essential oils, terpenoids, lectins, alkaloids, polypeptides, and polyacetylenes have been reported to possess antibiofilm properties (Yong et al. 2019). Secondary metabolites of endophytic origin have also been reported to be a source of natural compounds that help eradicate biofilm formation by these micro-organisms (Caruso et al. 2022).
Similarly, quorum sensing (ability to detect cell population) is one of the probable mechanisms exhibited by bacteria which is responsible for biofilm formation. Therefore, quorum-quenching compounds for breaking the quorum-sensing ability of these microbes are needed to break the chain of biofilm formation. Several endophytic fungi have been shown to exhibit the potential of secreting quorum-quenching compounds. There are a number of strategies to fight against quorum-sensing molecules, such as inhibition of QS signal biosynthesis, inhibition of QS signal detection, degradation and inactivation of QS signals, and use of antibiotics as QS inhibitors (LaSarre and Federle 2013). For example, Alternaria alternata endophytic to Carica papaya exhibited the quorum-quenching ability in its crude extract form and inhibit the quorum-sensing ability of Pseudomonas aeruginosa. The extract is known to exhibit the inhibition potential of biofilm formation up to 65.2% and the mechanism involved is the inhibition of the production of exopolysaccharide and cell surface hydrophobicity which greatly explained the endophytic potential in the treatment of biofilm formation (Rashmi et al. 2018).
Also, Fusarium graminearum and Lasiodiplodia sp. isolated from Ventilago madraspatana produced quorum-quenching molecule as a secondary metabolite that inhibited the quorum-sensing phenomenon of the pathogens (Rajesh and Ravishanker Rai et al. 2013). Similarly, exudates of an endophytic fungus (Penicillium restrictum) isolated from Silybum marianum contain polyhydroxyanthraquinones as quorum-sensing inhibitors, and these metabolites were found to be active against the growth of methicillin-resistantt Staphylococcus aureus (MRSA) strain by inhibiting its peptide and delta toxin production (Figueroa et al. 2014). Another species, Penicillium citrinum isolated from a halophyte, Halocnemum strobilaceum as endophyte produced a known compound,1,3,6-trihydroxy-7-methoxy-9H-xanthen-9-one that exhibited 100% efficiency in inhibiting the biofilm formation by Pseudomonas aeruginosa (Abdel Razek et al. 2020).
Similarly, the activity of an aromatic butyrolactone, flavipesin A, isolated from an endophytic fungus Aspergillus flavipes belonging to a mangrove host Acanthus ilicifolius, resulted in the disruption of the biofilm of S. aureus (Bai et al. 2014). In another instance, Alternaria destruens isolated from the healthy tissues of Calotropis gigantea exhibited an alpha-glucosidase inhibitor potential of 93.4%. The further purification of ethyl acetate extract of the fungus displayed two active fractions AF1 and AF2 which upon analysis found active against biofilm formation by various tested pathogens, such as P. aeruginosa and C. albicans whereas the other active fraction AF2 exhibited maximum inhibition of biofilm formation by C. albicans and S. enterica (Kaur et al. 2020).
Conclusion and future perspectives
In today’s scenario, resistance against available drugs is considered one of the major problems associated with microbes. Despite many discoveries on antimicrobial compounds, more resistant varieties have evolved. An effective antimicrobial compound should have good fungicidal or bactericidal activities. Novel antimicrobials from different alternative sources, like, endophytes can help in treating serious diseases, like, typhoid, tetanus, cholera, pneumonia, candidiasis, and aspergillosis which are diseases of concern. Despite various available drugs, the discovery of new drugs is still a challenge either due to (a) the appearance of continuous resistance in pathogenic microbes, (b) the occurrence of novel diseases, like, SARS-COV 2, (c) associated side effects (d) constant recurrence of many diseases and (e) unavailability of sources for drug discovery. Endophytes on the other hand provide an efficient way of drugs production due to various factors, such as (a) emerging resistance varieties for discovering new drug targets, (b) source of obtaining drugs, (c) minimizing the burden on medicinal plants, (d) to compensate expensive drug delivery, (e) to obtain greater variety of drugs, (f) fermentation potential and reproducibility of endophytes, (g) faster growth rate and (h) easily available nutrients, (i) host-mimicking compound potential, (j) enhanced antimicrobial potential than plants and (k) their balanced symbiotic relationship with different endophytic microbes.
To better understand the role and benefits of fungal endophytes and their relationship with the host plant, it is imperative to study modern-based approaches, like, nanotechnology (production of antimicrobial nanoparticles from endophytes), metabolomic profiling, next-generation sequencing, metabolomics, proteomics, metagenomics, bioinformatics, and molecular networking approaches. These modern techniques are helpful in studying and characterizing the structural analysis of a wide range of molecules in extract efficiently. Isolation and purification of bioactive compounds from medicinal plants is an expensive and laborious process that involves the use of various plant parts in large quantities. Therefore, it is advantageous to shift from medicinal plants to fungal endophytes for isolating various plant-mimicking compounds as well as plant-independent bioactive compounds produced by them. There is also a possibility for increased production of these compounds by involving the fermentation potential of endophytic fungi which can be progressed on a large scale. However, more explorations of medicinal plants are required to search for more novel drugs with antimicrobial potential. Moreover, studies on the genetic and molecular basis have to be focused on for a better understanding of various interactions involved between endophyte and the host plant for antimicrobial production.
Author contributions
Both the authors have contributed to the study conception and design, and performed the literature search. SD: has drafted the manuscript which was critically revised by SN.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
There is no conflict of interest by both the authors.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shivani Digra, Email: digrashivani123@gmail.com.
Skarma Nonzom, Email: skarmanunzom@yahoo.com.
References
- Abdel Razek MM, Moussa AY, El-Shanawany MA, Singab ANB. A new phenolic alkaloid from Halocnemum strobilaceum endophytes: antimicrobial, antioxidant and biofilm Inhibitory activities. Chem Biodivers. 2020;17(10):e2000496. doi: 10.1002/cbdv.202.000496. [DOI] [PubMed] [Google Scholar]
- Abdel-Azeem A, Nada AA, Odonovan A, Thakur VK, Elkelish A. Mycogenic silver nanoparticles from endophytic Trichoderma atroviride with antimicrobial activity. J Renew Mater. 2020;8(2):171–185. doi: 10.32604/jrm.2020.08960. [DOI] [Google Scholar]
- Abdelhakim HK, El-Sayed ER, Rashidi FB. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J Appl Microbiol. 2020;128(6):1634–1646. doi: 10.1111/jam.14581. [DOI] [PubMed] [Google Scholar]
- Abdou R, Shabana S, Rateb ME. Terezine E, bioactive prenylated tryptophan analogue from an endophyte of Centaurea stoebe. Nat Prod Res. 2020;34(4):503–510. doi: 10.1080/14786419.2018.1489393. [DOI] [PubMed] [Google Scholar]
- Abreu-Tarazi MF, Navarrete AA, Andreote FD, Almeida CV, Tsai SM, Almeida M. Endophytic bacteria in long-term in vitro cultivated axenic pineapple microplants revealed by PCR DGGE. World J Microbiol Biotechnol. 2010;26:555–560. doi: 10.1007/s11274-009-0191-3. [DOI] [Google Scholar]
- Adeleke RA, Nunthkumar B, Roopnarain A, Obi L. Applications of plant-microbe interactions in agro-ecosystem. In: Kumar V, Prasad R, Kumar M, Choudhary DK, editors. Microbiome in plant health and disease. Singapore: Springer Nature; 2019. pp. 1–34. [Google Scholar]
- Adnan M, Alshammari E, Ashraf SA, Patel K, Lad K, Patel M. Physiological and molecular characterization of biosurfactant producing endophytic fungus Xylaria regalis from the cones of Thuja plicata as a potent plant growth promoter with its potential application. Biomed Res Int. 2018;2018:7362148. doi: 10.1155/2018/7362148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharwal RP, Kumar S, Sandhu SS. Endophytic mycoflora: antibacterial secondary metabolites and their therapeutic potential. Curr Pharmacol Rep. 2021 doi: 10.1007/s40495-021-00261-w. [DOI] [Google Scholar]
- Ai HL, Zhang LM, Chen YP, Zi SH, Xiang H, Zhao DK, Shen Y. Two new compounds from an endophytic fungus Alternaria solani. J Asian Nat Prod Res. 2012;14(12):1144–1148. doi: 10.1080/10286020.2012.733701. [DOI] [PubMed] [Google Scholar]
- Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89(11):1175–1186. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- Almario J, Jeena G, Wunder J, Langen G, Zuccaro A, Coupland G, Bucher M. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc Natl Acad Sci. 2017;114(44):E9403–E9412. doi: 10.1073/pnas.1710455114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuhayawi MS, Abdel-Mawgoud M, Al Jaouni SK, Almuhayawi SM, Alruhaili MH, Selim S, AbdElgawad H. Bacterial endophytes as a promising approach to enhance the growth and accumulation of bioactive metabolites of three species of Chenopodium Sprouts. Plants. 2021;10(12):2745. doi: 10.3390/plants10122745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly AH, Debbab A, Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol. 2011;90(6):1829–1845. doi: 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]
- Ansari RA, Mahmood I, Rizvi R, Sumbul A. Siderophores: augmentation of soil health and crop productivity. In: Kumar V, Kumar M, Sharma S, Prasad R, editors. Probiotics in agroecosystem. Singapore: Springer; 2017. pp. 291–312. [Google Scholar]
- Aramsirirujiwet Y, Gumlangmak C, Kitpreechavanich V. Studies on antagonistic effect against plant pathogenic fungi from endophytic fungi isolated from Hottuynia Cordata Thunb and screening for Siderophore and indole-3-acetic acid production. Asia-Pacific J Sci Technol. 2016;21(1):55–66. [Google Scholar]
- Arora DS, Kaur N. Antimicrobial potential of fungal endophytes from Moringa oleifera. Appl Biochem Biotechnol. 2019;87:628–648. doi: 10.1007/s12010-018-2770-y. [DOI] [PubMed] [Google Scholar]
- Astuti P, Rollando R, Wahyuono S, Nurrochmad A, Garrido G. Antimicrobial activities of isoprene compounds produced by an endophytic fungus isolated from the leaves of Coleus amboinicus Lour. J Pharm Pharmacogn Res. 2020;8(4):280–289. [Google Scholar]
- Azevedo JL, Araujo WL. Diversity and applications of endophytic fungi isolated from tropical plants. In: Ganguli BN, Deshmukh SK, editors. Fungi multifaceted microbes. New Delhi: Anamaya; 2007. pp. 189–207. [Google Scholar]
- Aziz L, Hamayun M, Rauf M, Iqbal A, Arif M, Husssin A, Khan SA. Endophytic Aspergillus niger reprograms the physicochemical traits of tomato under cadmium and chromium stress. Environ Exp Bot. 2021;186:104456. doi: 10.1016/j.envexpbot.2021.104456. [DOI] [Google Scholar]
- Backman PA, Sikora RA. Endophytes: an emerging tool for biological control. Biol Control. 2008;46(1):1–3. doi: 10.1016/j.biocontrol.2008.03.009. [DOI] [Google Scholar]
- Bai ZQ, Lin X, Wang Y, Wang J, Zhou X, Yang B, Liu J, Yang X, Wang Y, Liu Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia. 2014;95:194–202. doi: 10.1016/j.fitote.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Meyer TE. Evolutionary analysis by whole-genome comparisons. J Bacteriol. 2002;184:2260–2272. doi: 10.1128/JB.184.8.2260-2272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Roossinck MJ. Multiplexed interactions: viruses of endophytic fungi. Adv Virus Res. 2013 doi: 10.1016/B978-0-12-394315-6.00002-7. [DOI] [PubMed] [Google Scholar]
- Bashyal BP, Wellensiek BP, Ramakrishnan R, Faeth SH, Ahmad N, Gunatilaka AL. Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorg Med Chem. 2014;22(21):6112–6116. doi: 10.1016/j.bmc.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Rhouma M, Kriaa M, Ben Nasr Y, Mellouli L, Kammoun R. A new endophytic Fusarium oxysporum gibberellic acid: optimization of production using combined strategies of experimental designs and potency on tomato growth under stress condition. BioMed Res Int. 2020;2020:4587148. doi: 10.1155/2020/4587148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdy J. Bioactive microbial metabolites. J Antibiot (tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Sharma D, Jadon N, Agrawal P. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Arch Clin Microbiol. 2015;6:1–9. [Google Scholar]
- Bhattacharjee S, Debnath G, Das AR, Saha AK, Das P. Characterization of silver nanoparticles synthesized using an endophytic fungus, Penicillium oxalicum having potential antimicrobial activity. Adv Nat Sci: J Nanosci Nanotechnol. 2017;8(4):045008. doi: 10.1088/2043-6254/aa84ec. [DOI] [Google Scholar]
- Bielecka M, Pencakowski B, Nicoletti R. Using next-generation sequencing technology to explore genetic pathways in endophytic fungi in the syntheses of plant bioactive metabolites. Agriculture. 2022;12(2):187. doi: 10.3390/agriculture12020187. [DOI] [Google Scholar]
- Bilal L, Asaf S, Hamayun M, Gul H, Iqbal A, Ullah I, Lee IJ, Hussain A. Plant growth promoting endophytic fungi Aspergillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 2018;76:117–127. doi: 10.1007/s13199-018-0545-4. [DOI] [Google Scholar]
- Binder M, Tamm C. The cytochalasans: a new class of biologically active microbial metabolites. Angew Chem Int Ed. 1973;12(5):370–380. doi: 10.1002/anie.197303701. [DOI] [PubMed] [Google Scholar]
- Bischoff JF, White JF., Jr . Evolutionary development of the Clavicipitaceae. In: Dighton J, White JF Jr, Oudemans P, editors. The fungal community. 3. Boca Raton: CRC Press; 2005. pp. 505–518. [Google Scholar]
- Bogner CW, Kariuki GM, Elashry A, Sichtermann G, Buch AK, Mishra B, Thines M, Grundler FMW, Schouten A. Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential. Mycol Prog. 2016;15(3):1–17. doi: 10.1007/s11557-016-1169-9. [DOI] [Google Scholar]
- Brakhage AA, Schroeckh V. Fungal secondary metabolites–strategies to activate silent gene clusters. Fungal Genet Biol. 2011;48(1):15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Browne K, Chakraborty S, Chen R, Willcox MD, Black DS, Walsh WR, Kumar M. A new era of antibiotics: the clinical potential of antimicrobial peptides. Int J Mol Sci. 2020;21(19):7047. doi: 10.3390/ijms21197047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman TL, Murphy JC. Do fungal endophytes mediate wound-induced resistance? In: Bacon CW, White JF, editors. Microbial Endophytes. New York: Marcel Dekker; 2000. pp. 421–455. [Google Scholar]
- Caruso DJ, Palombo EA, Moulton SE, Zaferanloo B. Exploring the promise of endophytic fungi: a review of novel antimicrobial compounds. Microorganisms. 2022;10(10):1990. doi: 10.3390/microorganisms10101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapla VM, Zeraik ML, Leptokarydis IH, Silva GH, Bolzani VS, Young MCM, Pfenning LH, Araújo AR. Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champaca. Molecules. 2014;19(11):19243–19252. doi: 10.3390/molecules191119243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Gajbhiye S, Roy S, Dudhale R, Chowdhary A. Determination of Kaempferol in extracts of Fusarium chlamydosporum, an endophytic fungi of Tylophora indica (Asclepeadaceae) and its antimicrobial activity. Jpharm Biol Sci. 2014;9:1–51. [Google Scholar]
- Chen X, Sang X, Li S, Zhang S, Bai L. Studies on a chlorogenic acid-producing endophytic fungi isolated from Eucommia ulmoides Oliver. J Ind Microbiol Biotechnol. 2010;37:447–454. doi: 10.1007/s10295-010-0690-0. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang QY, Jia M, Ming QL, Yue W, Rahman K, Qin LP, Han T. Endophytic fungi with antitumor activities: their occurrence and anticancer compounds. Crit Rev Microbiol. 2014;42:454–473. doi: 10.3109/1040841X.2014.959892. [DOI] [PubMed] [Google Scholar]
- Chen J, Bai X, Hua Y, Zhang H, Wang H. Fusariumins C and D, two novel antimicrobial agents from Fusarium oxysporum ZZP-R1 symbiotic on Rumex madaio Makino. Fitoterapia. 2019;134:1–4. doi: 10.1016/j.fitote.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Chowdappa S, Jagannath S, Konappa N, Udayashankar AC, Jogaiah S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules. 2020;10(10):1412. doi: 10.3390/biom10101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MJ, Bennett RJ, Schmid J. Growth of Epichloë/Neotyphodium and p-endophytes in leaves of Lolium and Festuca grasses. Mycol Res. 2002;106(1):93–106. doi: 10.1017/S095375620100510X. [DOI] [Google Scholar]
- Christian N, Herre EA, Clay K. Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol. 2019;222:1573–1583. doi: 10.1111/nph.15693. [DOI] [PubMed] [Google Scholar]
- de Bary A. Morphologie and physiologie der Pilze Flechten und Myxomyceten. Hofmeister’s handbook of physiological botany. Leipzig: Engelmann; 1866. [Google Scholar]
- Demers D, Knestrick M, Fleeman R, Tawfik R, Azhari A, Souza A, Vesely B, Netherton M, Gupta R, Colon BL, Rice CA, Rodríguez-Pérez MA, Rohde KH, Kyle DE, Shaw LN, Baker BJ. Exploitation of mangrove endophytic fungi for infectious disease drug discovery. Mar Drugs. 2018;16(10):376. doi: 10.3390/md16100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh B, Monisha N, Shalini HR, Prathap GK, Poyya J, Shantaram M, Hampapura JS, Karigar CS, Joshi CG. Antibacterial activity of silver nanoparticles synthesized using endophytic fungus—Penicillium cinnamopurpureum. SpectroscopyLetters. 2022;55(1):20–34. doi: 10.1080/0038.7010.2021.2010764. [DOI] [Google Scholar]
- Ding Z, Tao T, Wang L, Zhao Y, Huang H, Zhang D, Liu M, Wang Z, Han J. Bioprospecting of novel and bioactive metabolites from endophytic fungi isolated from rubber tree Ficus elastica leaves. Microbiol Biotechnol. 2019;29:731–738. doi: 10.4014/jmb.1901.01015. [DOI] [PubMed] [Google Scholar]
- Dolatabad HK, Javan-Nikkhah M, Shier WT. Evaluation of antifungal, phosphate solubilisation, and siderophore and chitinase release activities of endophytic fungi from Pistacia vera. Mycol Prog. 2017;16(8):777–790. doi: 10.1007/s11557-017-1315-z. [DOI] [Google Scholar]
- Dong C, Wang L, Li Q, Shang Q. Epiphytic and endophytic fungal communities of tomato plants. Hortic Plant J. 2021;7:38–48. doi: 10.1016/j.hpj.2020.09.002. [DOI] [Google Scholar]
- Dreyfuss MM, Chapela IH. Potential of fungi in the discovery of novel, low molecular weight pharmaceuticals. In: Gullo VP, editor. Butter worth the discovery of natural products with therapeutic potential. London: Heinemann; 1994. pp. 49–80. [DOI] [PubMed] [Google Scholar]
- Dutta D, Puzari KC, Gogoi R, Dutta P. Endophytes: exploitation as a tool in plant protection. Braz Arch Biol Technol. 2014;57:621–629. doi: 10.1590/S1516-8913201402043. [DOI] [Google Scholar]
- Ebrahim W, Özkaya FC, Ebada SS. Antifungal metabolites from endophytic fungus Fusarium verticillioides strain WF18. S Afr J Bot. 2020;133:40–44. doi: 10.1016/j.sajb.2020.06.029. [DOI] [Google Scholar]
- El-Kassem AL, Hawas UW, El-Souda S, Ahmed EF, El-Khateeb W, Fayad W. Anti-HCV protease potential of endophytic fungi and cytotoxic activity. Biocatal Agric Biotechnol. 2019;19:101170. doi: 10.1016/j.bcab.2019.101170. [DOI] [Google Scholar]
- Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM. Bioactive secondary metabolite from endophytic Aspergillus tubenginses ASH4 isolated from Hyoscyamus muticus: antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacogn J. 2021 doi: 10.5530/pj.2021.13.55. [DOI] [Google Scholar]
- Eyberger AL, Dondapati R, Porter JR. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J Nat Prod. 2006;69:1121–1124. doi: 10.1021/np060174f. [DOI] [PubMed] [Google Scholar]
- Fanos V, Cataldi L. Amphotericin B-induced nephrotoxicity: a review. J Chemother. 2000;12(6):463–470. doi: 10.1179/joc.2000.12.6.463. [DOI] [PubMed] [Google Scholar]
- Farhat H, Urooj F, Tariq A, Sultana V, Ansari M, Ahmad VU, Ehteshamul-Haque S. Evaluation of antimicrobial potential of endophytic fungi associated with healthy plants and characterization of compounds produced by endophytic Cephalosporium and Fusarium solani. Biocatal Agric Biotechnol. 2019;18:101043. doi: 10.1016/j.bcab.2019.101043. [DOI] [Google Scholar]
- Figueroa M, Jarmusch AK, Raja HA, El-Elimat T, Kavanaugh JS, Horswill AR, Cooks RG, Cech NB, Oberlies NH. Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of Penicillium restrictum and their analysis by desorption electrospray ionization mass spectrometry. J Nat Prod. 2014;77(6):1351–1358. doi: 10.1021/np5000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakuubi MM, Munusamy M, Liang ZX, Ng SB. Fungal endophytes: a promising frontiers for discovery of novel bioactive compounds. J Fungi. 2021;7(10):786. doi: 10.3390/jof7100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan V, Hariram M, Vivekanandhan S, Muthuramkumar S. Periconium sp. (endophytic fungi) extract mediated sol-gel synthesis of ZnO nanoparticles for antimicrobial and antioxidant applications. Mater Sci Semicond Process. 2020;105:104739. doi: 10.1016/j.mssp.2019.104739. [DOI] [Google Scholar]
- Gouda S, Das G, Sen SK, Shin HS, Patra JK. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol. 2016;7:1538. doi: 10.3389/fmicb.2016.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutam J, Sharma G, Tiwari VK, Mishra A, Kharwar RN, Ramaraj V, Koch B. Isolation and characterization of ‘‘terrein’’ an antimicrobial and antitumor compound from endophytic fungus Aspergillus terreus (JAS-2) associated from Achyranthus aspera Varanasi. India. Front Microbiol. 2017;8:1334. doi: 10.3389/fmicb.2017.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoletto DF, Correia AML, Abraham W-R, Rodrigues A, Assis MA, Ferreira AG, Massaroli M, de Lira SP. Secondary metabolites produced by endophytic fungi: novel antifungal activity of fumiquinone B. A natural product against Diaporthe phaseolorum. Acta Sci Biol Sci. 2019;41:48785. doi: 10.4025/actascibiolsci.v41i1.48785. [DOI] [Google Scholar]
- Gunatilaka AAL. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implication of their occurence. J Nat Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana M, Hyde ER, Lahmeyer S, Dorsey BL, La Val TP, Mullen M, Yoo J, Knight R, Baum MM. Euphorbia plant latex is inhabited by diverse microbial communities. Am J Bot. 2015;102(12):1966–1977. doi: 10.3732/ajb.1500223. [DOI] [PubMed] [Google Scholar]
- Guo B, Dai J, Ng S, Huang Y, Leong C, Ong W, et al. Cytonic acids A and B: novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J Nat Prod. 2000;63:602–604. doi: 10.1021/np990467r. [DOI] [PubMed] [Google Scholar]
- Guo J, Ran H, Zeng J, Liu D, Xin Z. Tafuketide, a phylogeny-guided discovery of a new polyketide from Talaromyces funiculosus Salicorn 58. Appl Microbiol Biotechnol. 2016;100(12):5323–5338. doi: 10.1007/s00253-016-7311-4. [DOI] [PubMed] [Google Scholar]
- Guo LF, Niu SB, Chen SL, Liu L. Diaporone A, a new antibacterial secondary metabolite from the plant endophytic fungus Diaporthe sp. J Antibiot. 2020;73:116–119. doi: 10.1038/s41429-019-0251-3. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chaturvedi P, Kulkarni MG, Van Staden J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol Adv. 2020;39:107462. doi: 10.1016/j.biotechadv.2019.107462. [DOI] [PubMed] [Google Scholar]
- Halecker S, Wennrich JP, Rodrigo S, Andrée N, Rabsch L, Baschien C, Steinert M, Stadler M, Surup F, Schulz B. Fungal endophytes for biocontrol of ash dieback: the antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 2020;45:100918. doi: 10.1016/j.funeco.2020.100918. [DOI] [Google Scholar]
- Hamayun M, Khan SA, Iqbal I, Na CI, Khan AL, Hwang YH, Lee BH, Lee IJ. Chrysosporium pseudomerdarium produces gibberellins and promotes plant growth. J Microbio. 2009;47(4):425–430. doi: 10.1007/s12275-009-0268-6. [DOI] [PubMed] [Google Scholar]
- Hamid AA, Aiyelaagbe OO. Preliminary phytochemical, antibacterial and antifungal properties of Alafia barteri stem grown in Nigeria. European J Med Plant. 2011;1(2):26–32. doi: 10.9734/EJMP/2011/122. [DOI] [Google Scholar]
- Hao Q, Albaghdady DMD, Xiao Y, Xiao X, Mo C, Tian T, Wang G. Endophytic Metarhizium anisopliae is a potential biocontrol agent against wheat Fusarium head blight caused by Fusarium graminearum. J Plant Pathol. 2021;103:875–885. doi: 10.1007/s42161-021-00866-6. [DOI] [Google Scholar]
- Hardoim PR, Van Overbeek LS, Van Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008;16:463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Harman GE, Doni F, Khadka RB, Uphoff N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J Appl Microbiol. 2021;130(2):529–546. doi: 10.1111/jam.14368. [DOI] [PubMed] [Google Scholar]
- Harwoko H, Daletos G, Stuhldreier F, Lee J, Wesselborg S, Feldbrugge MWEG, Kalscheuer R, Ancheeva E, Proksch P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat Prod Res. 2021;35(2):257–265. doi: 10.1080/14786419.2019.1627348. [DOI] [PubMed] [Google Scholar]
- Hassan SE-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res. 2017;8:687–695. doi: 10.1016/j.jare.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL. The fungal dimension of biodiversity, magnitude, significance and conservation. Mycological Res. 1991;95(6):641–655. doi: 10.1016/S0953-7562(09)80810-1. [DOI] [Google Scholar]
- Heinig U, Scholz S, Jennewein S. Getting to the bottom of Taxol biosynthesis by fungi. Fungal Divers. 2013;60(1):161–170. doi: 10.1007/s13225-013-0228-7. [DOI] [Google Scholar]
- Hemashekhar B, Chandrappa C, Govindappa M, Chandrasekhar N, Ganganagappa N, Ramachandra Y. Green synthesis of silver nanoparticles from endophytic fungus Aspergillus niger isolated from Simarouba glauca leaf and its antibacterial and antioxidant activity. Int J Eng Res Appl. 2017;7(8):17–24. doi: 10.9790/9622-0708011724. [DOI] [Google Scholar]
- Hemashekhar B, Chandrappa CP, Govindappa M, Chandrashekar N. Endophytic fungus Alternaria spp isolated from Rauvolfia tetraphylla root arbitrate synthesis of gold nanoparticles and evaluation of their antibacterial, antioxidant and antimitotic activities. Adv Nat Sci Nanosci Nanotechnol. 2019;10(3):035010. doi: 10.1088/2043-6254/ab38b0. [DOI] [Google Scholar]
- Hoffmeister D, Keller NP. Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep. 2007;24:393–416. doi: 10.1039/B603084J. [DOI] [PubMed] [Google Scholar]
- Horn WS, Simmonds MSJ, Schwartz RE, Blaney WM. Phomopsichalasin, a novel antimicrobial agent from an endophytic Phomopsis sp. Tetrahedron. 1995;51(14):3969–3978. doi: 10.1016/0040-4020(95)00139-Y. [DOI] [Google Scholar]
- Hosseini M, Haji-Fatahaliha M, Jadidi-Niaragh F, Majidi J, Yousefi M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif Cells Nanomed Biotechnol. 2016;44(4):1051–1061. doi: 10.3109/21691401.2014.998830. [DOI] [PubMed] [Google Scholar]
- Hu X, Saravanakumar K, Jin T, Wang MH. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int J Nanomedicine. 2019;14:3427. doi: 10.2147/IJN.S200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde Kd, Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008;33:163–173. [Google Scholar]
- Ibrahim SRM, Mohamed GA, Al Haidari RA, Zayed MF, El-Kholy AA, Elkhayat ES, Ross SA. Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorg Med Chem. 2018;26(3):786–790. doi: 10.1016/j.bmc.2017.12.049. [DOI] [PubMed] [Google Scholar]
- Ilangumaran G, Smith DL. Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci. 2017;8:1768. doi: 10.3389/fpls.2017.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail HM, Hussain A, Iqbal A, Khan SA, Lee I-J. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res Int. 2018;2018:1–11. doi: 10.1155/2018/7696831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouda JB, Kusari S, Lamshöft M, Talontsi FM, Meli CD, Wandji J, Spiteller M. Penialidins A-C with strong antibacterial activities from Penicillium sp., an endophytic fungus harboring leaves of Garcinia nobilis. Fitoterapia. 2014;98:209–214. doi: 10.1016/j.fitote.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Junjie Z, Qisen H, Shengquan L, Weijia D, Yahong X, Chunyuan L. Four new diphenyl ether derivatives from the mangrove endophytic fungus Epicoccum sorghinum. Chin J Nat Med. 2021;20:1–4. doi: 10.1016/S1875-5364(22)60171-7. [DOI] [PubMed] [Google Scholar]
- Kapil A, Aggarwal G, Harikumar SL. Nanotechnology in novel drug delivery system. J Drug Deliv Ther. 2014;4(5):21–28. doi: 10.22270/jddt.v4i5.942. [DOI] [Google Scholar]
- Karam G, Chastre J, Wilcox MH, Vincent JL. Antibiotic strategies in the era of multidrug resistance. Crit Care. 2016;20(1):1–9. doi: 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Rapala-Kozik M, Kozik A. Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim Pol. 2009;56(2):211–224. doi: 10.18388/abp.2009_2452. [DOI] [PubMed] [Google Scholar]
- Kaul S, Gupta S, Ahmed M, Dhar MK. Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem Rev. 2012;11:487–505. doi: 10.1007/s11101-012-9260-6. [DOI] [Google Scholar]
- Kaur J, Sharma P, Kaur R, Kaur S, Kaur A. Assessment of alpha glucosidase inhibitors produced from endophytic fungus Alternaria destruens as antimicrobial and antibiofilm agents. Mol Biol Rep. 2020;47(1):423–432. doi: 10.1007/s11033-019-05145-3. [DOI] [PubMed] [Google Scholar]
- Keller NP, Turner G, Bennett JW. Fungal secondary metabolism from biochemistry to genomics. Nat Rev Microbiol. 2005;3(12):937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Hassan D, Maaza M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif Cells Nanomed Biotechnol. 2018;46(4):838–852. doi: 10.1080/21691401.2017.1345928. [DOI] [PubMed] [Google Scholar]
- Khalmuratova I, Kim H, Nam Y-J, Oh Y, Jeong M-J, Choi H-R, You YH, Choo YS, Lee IJ, Shin JH, Yoon H, Kim JG. Diversity and plant growth promoting capacity of endophytic fungi associated with halophytic plants from the west coast of Korea. Mycobiology. 2015;43(4):373–383. doi: 10.5941/MYCO.2015.43.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AL, Hamayun M, Kang SM, Kim YH, Jung HY, Lee JH, Lee IJ. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol. 2012;12(3):1–14. doi: 10.1186/1471-2180-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AL, Al-Harrasi A, Al-Rawahi A, Al-Farsi Z, Al-Mamari A, Waqas M, Asaf S, Elyassi A, Maboob F, Shin JH, Lee IJ. Endophytic fungi from Frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS ONE. 2016;11:e0158207. doi: 10.1371/journal.pone.0158207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AR, Waqas M, Ullah I, Khan AL, Khan MA, Lee I-J. Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L and their role in enhancing phytoremediation. Environ Exp Bot. 2017;135:126–135. doi: 10.1016/j.envexpbot.2016.03.005. [DOI] [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Khare E, Mishra J, Arora NK. Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol. 2018;9:2732. doi: 10.3389/fmicb.2018.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiralla A, Spina R, Varbanov M, Philippot S, Lemiere P, Slezack-Deschaumes S, Andre P, Mohamed I, Yagi SM, Laurain-Mattar D. Evaluation of antiviral, antibacterial and antiproliferative activities of the endophytic fungus Curvularia papendorfii, and isolation of a new polyhydroxyacid. Microorganisms. 2020;8(9):1353. doi: 10.3390/microorganisms8091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Eo JK, Eom AH. Diversity and seasonal variation of endophytic fungi isolated from three conifers in Mt. Taehwa. Korea. Mycobiol. 2013;41(2):82–85. doi: 10.5941/MYCO.2013.41.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings M, Taylor TN, Dotzler N. Fungal endophytes as a driving force in land plant evolution: evidence from the fossil record. In: Southworth D, editor. Biocomplexity of plant- fungal interactions. New York: Wiley; 2012. pp. 5–27. [Google Scholar]
- Kulkarni P, Ramakrishna K (2020) Biosynthesis of zinc oxide nanoparticles by of Aspergillus niger and their potential antibacterial effects on Propionibacterium acnes. J Adv Sci Res. 11(4):178–83. http://sciensage.info/index.php/JASR/article/view/108
- Kumar S, Aharwal RP, Shukla H, Rajak RC, Sandhu SS. Endophytic fungi: as a source of antimicrobials bioactive compounds. World J Pharm Pharm Sci. 2014;3(2):1179–1197. [Google Scholar]
- Kumara PM, Shweta S, Vasanthakumari MM, Sachin N, Manjunatha BL, Jadhav SS, Ravikanth G, Ganeshaiah KN, Shaanker RU. Endophytes and plant secondary metabolite synthesis: molecular and evolutionary perspective. In: Verma V, Gange A, editors. Advances in endophytic research. New Delhi: Springer; 2014. pp. 177–190. [Google Scholar]
- Kunin CM. Antibiotic resistance—a world health problem we cannot ignore. Ann Intern Med. 1983;99:859–860. doi: 10.7326/0003-4819-99-6-859. [DOI] [PubMed] [Google Scholar]
- Kunin CM. Resistance to antimicrobial drugs: a worldwide calamity. Ann Intern Med. 1993;118(7):557–561. doi: 10.7326/0003-4819-118-7-19930410-00011. [DOI] [PubMed] [Google Scholar]
- Kusari S, Lamshöft M, Zühlke S, Spiteller M. An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod. 2008;71:159–162. doi: 10.1021/np070669k. [DOI] [PubMed] [Google Scholar]
- Kyekyeku JO, Kusari S, Adosraku RK, Bullach A, Golz C, Strohmann C, Spiteller M. Antibacterial secondary metabolites from an endophytic fungus, Fusarium solani JK10. Fitoterapia. 2017;119:108–114. doi: 10.1016/j.fitote.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Langford BJ, So M, Raybardhan S, Leung V, Soucy J-P, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;1:18. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriba E, Jaime MD, Nislow C, Martín-Nieto J, Lopez-Llorca LV. Endophytic colonization of barley (Hordeum vulgare) roots by the nematophagous fungus Pochonia chlamydosporia reveals plant growth promotion and a general defense and stress transcriptomic response. J Plant Res. 2015;128:665–678. doi: 10.1007/s10265-015-0731-x. [DOI] [PubMed] [Google Scholar]
- LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77(1):73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata R, Chowdhury S, Gond SK, White JF., Jr Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol. 2018;66:268–276. doi: 10.1111/lam.12855. [DOI] [PubMed] [Google Scholar]
- Lawrence JG, Ochman H. Amelioration of bacterial genomes: rates of change and exchange. J Mol Evol. 1997;44:383–397. doi: 10.1007/PL00006158. [DOI] [PubMed] [Google Scholar]
- Li W, Xu J, Li F, Li X, Li C. A New Antifungal Isocoumarin from the endophytic fungus Trichoderma Sp. 09 of Myoporum bontioides A. Gray. Pharmacogn Mag. 2016;12(48):259–261. doi: 10.4103/0973-1296.192204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H. Sordarin, an antifungal agent with a unique mode of action. Beilstein J Org Chem. 2008;4(1):31. doi: 10.3762/bjoc.4.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dong M, Chen X, Jiang M, Lv X, Zhou J. Antimicrobial activity of an endophytic Xylaria sp. YX-28 and identification of its antimicrobial compound 7-amino-4-methylcoumarin. Appl Microbiol Biotechnol. 2008;78:241–247. doi: 10.1007/s00253-007-1305-1. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan J, Miao YE, Huang Y, Liu T. Catalytic and antibacterial activities of green-synthesized silver nanoparticles on electrospun polystyrene nanofiber membranes using tea polyphenols. Compos B Eng. 2015;79:217–223. doi: 10.1016/j.compositesb.2015.04.037. [DOI] [Google Scholar]
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clinl Investig. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J. Interplay between endophyte and host plant in the synthesis and modification of metabolites. In: Schouten A, editor. Endophyte biotechnology: potential for agriculture and pharmacology. Wallingford: CABI; 2019. p. 180. [Google Scholar]
- Lugtenberg BJJ, Caradus JR, Johnson LJ. Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol. 2016;92(12):1–17. doi: 10.1093/femsec/fiw194. [DOI] [PubMed] [Google Scholar]
- Ma YM, Qiao K, Kong Y, Li MY, Guo LX, Miao Z, Fan C. A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum. Nat Prod Res. 2017;31(8):951–958. doi: 10.1080/14786419.2016.1258556. [DOI] [PubMed] [Google Scholar]
- Ma YM, Qiao K, Kong Y, Guo X, Li MY, Fan C. A new p-hydroxybenzoic acid derivative from an endophytic fungus Penicillium sp. of Nerium indicum. J Asian Nat Prod Res. 2017;19(12):1245–1251. doi: 10.1080/10286020.2017.1313240. [DOI] [PubMed] [Google Scholar]
- Mack KM, Rudgers JA. Balancing multiple mutualists: asymmetric interactions among plants, arbuscular mycorrhizal fungi, and fungal endophytes. Oikos. 2008;117(2):310–320. doi: 10.1111/j.2007.0030-1299.15973.x. [DOI] [Google Scholar]
- Mani VM, Kalaivani S, Sabarathinam S, Vasuki M, Soundari AJPG, Das MA, Elfasakhany A, Pugazhendhi A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: bioactivity and anti-cancer evaluations. Environ Res. 2021;201:111502. doi: 10.1016/j.envres.2021.111502. [DOI] [PubMed] [Google Scholar]
- Mantravadi PK, Kalesh KA, Dobson RC, Hudson AO, Parthasarathy A. The quest for novel antimicrobial compounds: emerging trends in research, development, and technologies. Antibiotics. 2019;8(1):8. doi: 10.3390/antibiotics8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Zhang W, Wu C, Feng H, Peng Y, Shahid H, Cui Z, Ding P, Shan T. Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 2021;21(155):1–12. doi: 10.1186/s12866-021-02229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AJ, Nonzom S. Endophytes: a big boon to agriculture. In: Wani ZA, Qadri M, Arora P, Hakeem KR, editors. Endophyte biology: recent findings from Kashmir Himalayas. New York: AAP CRS Press Taylor and Franchise Group; 2021. [Google Scholar]
- Mattoo AJ, Nonzom S. Endophytic fungi: understanding complex cross-talks. Symbiosis. 2021;83(3):237–264. doi: 10.1007/s13199-020-00744-2. [DOI] [Google Scholar]
- Meena KK, Sorty AM, Bitla UM, Choudhary K, Gupta P, Pareek A, Singh DP, Prabha R, Sahu PK, Gupta VK, Singh HB, Krishanani KK, Minhas PS. Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci. 2017;8:172. doi: 10.3389/fpls.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood A, Hussain A, Irshad M, Hamayun M, Iqbal A, Khan N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis. 2019;77:225–235. doi: 10.1007/s13199-018-0583-y. [DOI] [Google Scholar]
- Mejia LC, Herre EA, Sparks JP, Winter K, Garcia MN, Van Bael SA, Stitt J, Shi Z, Zhang Y, Guiltinan MJ, Maximova SN. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol. 2014;5:479. doi: 10.3389/fmicb.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi O, Bendahou M. Biological synthesis of nanoparticles using endophytic microorganisms: current development. In: Sen M, editor. Nanotechnology and the environment. London: IntechOpen; 2020. [Google Scholar]
- Ming Q, Han T, Li W, Zhang Q, Zhang H, Zheng C, Huang F, Rahman K, Qin L. Tanshinone IIA and tanshinone i production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine. 1993;3:330–333. doi: 10.1016/j.phymed.2011.09.076. [DOI] [PubMed] [Google Scholar]
- Mishra VK, Passari AK, Leo VV, Singh BP. Molecular diversity and detection of endophytic fungi based on their antimicrobial biosynthetic genes. In: Singh BP, Gupta VK, editors. Molecular markers in mycology fungal biology. Cham: Springer; 2017. pp. 1–35. [Google Scholar]
- Mohajerani A, Burnet L, Smith JV, Kurmus H, Milas J, Arulrajah A, Horpibulsuk S, Kadir AA. Nanoparticles in construction materials and other applications, and implications of nanoparticle use. Materials. 2019;12(19):3052. doi: 10.3390/ma12193052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed GA, Ibrahim SRM, Asfour HZ. Antimicrobial metabolites from the endophytic fungus Aspergillus versicolor. Phytochem Lett. 2020;35:152–155. doi: 10.1016/j.phytol.2019.12.003. [DOI] [Google Scholar]
- Mohamed GA, Ibrahim SRM, Alhakamy NA, Aljohani OS. Fusaroxazin, a novel cytotoxic and antimicrobial xanthone derivative from Fusarium oxysporum. Nat Prod Res. 2020 doi: 10.1080/14786419.2020.1855165. [DOI] [PubMed] [Google Scholar]
- Mohan S, Krishna A, Chandramouli M, Keri RS, Patil SA, Ningaiah S, Somappa SB. Antibacterial natural products from microbial and fungal sources: a decade of advances. Mol Divers. 2022 doi: 10.1007/s11030-022-10417-5. [DOI] [PubMed] [Google Scholar]
- Moraga EQ. Entomopathogenic fungi as endophytes: their broader contribution to IPM and crop production. Biocontrol Sci Technol. 2020;30(9):864–877. doi: 10.1080/09583157.2020.1771279. [DOI] [Google Scholar]
- Mousa WK, Raizada MN. The diversity of antimicrobial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front Microbiol. 2013;4:1–18. doi: 10.3389/fmicb.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa WK, Raizada MN. Biodiversity of genes encoding antimicrobial traits within plant associated microbes. Front Plant Sci. 2015;6:231. doi: 10.3389/fpls.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy M, Li HM, Sullivan R, White JF, Belanger FC. Endophytic fungal beta-1,6-glucanase expression in the infected host grass. Plant Physiol. 2002;130(3):1298–1308. doi: 10.1104/pp.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int J Environ Res Public Health. 2022;19(2):674. doi: 10.3390/ijerph19020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind PH, Fischer RL, Schnorr KM, Hansen MT, Sonksen CP, Ludvigsen S, Raventos D, Buskov S, Christensen B, De Maria L, Taboureau O, Yaver D, Elvig-Jørgensen SG, Sørensen MV, Christensen BE, Kjaerulff S, Frimodt-Moller N, Lehrer RI, Zasloff M, Kristensen HH. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- Nath R, Sharma GD, Barooah M. Efficiency of tricalcium phosphate solubilization by two different endophytic Penicillium sp. isolated from tea (Camellia sinensis L.) Eur J Exp Biol. 2012;2(4):1354–1358. [Google Scholar]
- Nath R, Sharma GD, Barooah M. Plant growth promoting endophytic fungus isolated from tea (Camellia sinensis) shrubs of Assam, India. Appl Ecol Environ Res. 2015;13:877–891. doi: 10.15666/aeer/1303_877891. [DOI] [Google Scholar]
- Nejad MS, Najafabadi NS, Aghighi S, Pakina E, Zargar M. Evaluation of Phoma sp. biomass as an endophytic fungus for synthesis of extracellular gold nanoparticles with antibacterial and antifungal properties. Molecules. 2022;27(4):1181. doi: 10.3390/molecules27041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netala VR, Kotakadi VS, Bobbu P, Gaddam SA, Tartte V. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and antimicrobial studies. 3 Biotech. 2016;6(2):132. doi: 10.1007/s13205-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti R, Fiorentino A. Plant bioactive metabolites and drugs produced by endophytic fungi of spermatophyta. Agriculture. 2015;5:918–970. doi: 10.3390/agriculture5040918. [DOI] [Google Scholar]
- Nieves-Cordones M, García-Sánchez F, Pérez-Pérez JG, Colmenero-Flores JM, Rubio F, Rosales MA. Coping with water shortage: an update on the role of K+, Cl–, and water transport mechanisms on drought resistance. Front Plant Sci. 2019;10:1619. doi: 10.3389/fpls.2019.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishad JH, Singh A, Gautam VS, Kumari P, Kumar J, Yadav M, Kharwar RN. Bioactive potential evaluation and purification of compounds from an endophytic fungus Diaporthe longicolla, a resident of Saraca asoca (Roxb.) Willd. Arch Microbiol. 2021;203:4179–4188. doi: 10.1007/s00203-021-02390-8. [DOI] [PubMed] [Google Scholar]
- Nuankeaw K, Chaiyosang B, Suebrasri T, Kanokmedhakul S, Lumyong S, Boonlue S. First report of secondary metabolites, Violaceol I and Violaceol II produced by endophytic fungus, Trichoderma polyalthiae and their antimicrobial activity. Mycoscience. 2019;61(1):16–21. doi: 10.1016/j.myc.2019.10.001. [DOI] [Google Scholar]
- Numponsak T, Kumla J, Suwannarach N, Matsui K, Lumyong S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola CMU-A109. PLoS ONE. 2018;13(10):e0205070. doi: 10.1371/journal.pone.0205070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oljira AM, Hussain T, Waghmode TR, Zhao H, Sun H, Liu X, Wang X, Liu B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms. 2020;8(10):1565. doi: 10.3390/microorganisms8101565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifade AK. Research trends: bioactive metabolites of fungal origin. Res J Biol Sci. 2007;2(1):81–84. [Google Scholar]
- Palanichamy P, Krishnamoorthy G, Kannan S, Marudhamuthu M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt J Basic Appl Sci. 2018;5:303–312. doi: 10.1016/j.ejbas.2018.07.002Returntoref2018inarticle. [DOI] [Google Scholar]
- Pang XJ, Zhang SB, Chen HL, Zhao WT, Yang DF, Xian PJ, Xu LL, Tao YD, Fu HY, Yang XL. Emericelactones A-D: four novel polyketides produced by Emericella sp. XL 029, a fungus associated the leaves of Panax notoginseng. Tetrahedron Lett. 2018;59(52):4566–4570. doi: 10.1016/j.tetlet.2018.11.032. [DOI] [Google Scholar]
- Patil MP, Patil RH, Maheshwari VL. Biological activities and identification of bioactive metabolite from endophytic Aspergillus flavus L7 isolated from Aegle marmelos. Curr Microbiol. 2015;71:39–48. doi: 10.1007/s00284-015-0805-y. [DOI] [PubMed] [Google Scholar]
- Patil RH, Patil MP, Maheshwari VL. Bioactive secondary metabolites from endophytic fungi: a review of biotechnological production and their potential applications. Stud Nat Prod Chem. 2016;49:189–205. doi: 10.1016/B978-0-444-63601-0.00005-3. [DOI] [Google Scholar]
- Patra JK, Baek KH. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater. 2014;2014:219. doi: 10.1155/2014/417305. [DOI] [Google Scholar]
- Paul K, Saha C, Nag M, Mandal D, Naiya H, Sen D, Mitra S, Kumar M, Bose D, Mukherjee G, Naskar N, Lahiri S, Das Ghosh U, Tripathi S, Sarkar MP, Banerjee M, Kleinert A, Valentine AJ, Tripathy S, Sinharoy S, Seal A. A tripartite interaction among the basidiomycete Rhodotorula mucilaginosa, N2-fixing endobacteria, and rice improves plant nitrogen nutrition. Plant Cell. 2020;32(2):486–507. doi: 10.1105/tpc.19.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini O. Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS, editors. Microbial ecology of leaves. New York: Brock/Springer Series in Contemporary Bioscience Springer; 1991. pp. 179–197. [Google Scholar]
- Picart P, Pirttilä AM, Raventos D, Kristensen H-H, Sahl H-G. Identification of novel defensin-encoding genes of Picea glauca: isolation and characterization of Pgd2, a conserved spruce defensin with strong antifungal activity. BMC Plant Biol. 2012;12:1–14. doi: 10.1186/1471-2229-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina JRS, Silva-Silva JV, Carvalho JM, Bitencourt HR, Watanabe LA, Fernandes JMP, Souza GE, Aguiar ACC, Guido RVC, Almeida-Souza F, Calabrese KDS, Marinho PSB, Marinho AMDR. Antiprotozoal and antibacterial activity of Ravenelin, a Xanthone isolated from the endophytic fungus Exserohilum rostratum. Molecules. 2021;26(11):3339. doi: 10.3390/molecules26113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyasena KGNP, Wickramarachchi WART, Kumar NS, Jayasinghe L, Fujimoto Y. Two phytotoxic azaphilone derivatives from Chaetomium globosum, a fungal endophyte isolated from Amaranthus viridis leaves. Mycology. 2015;6:158–160. doi: 10.1080/21501203.2015.1089332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongcharoen W, Rukachaisirikul V, Phongpaichit S, Kühn T, Pelzing M, Sakayaroj J, Taylor WC. Metabolites from the endophytic fungus Xylaria sp PSU-D14. Phytochem. 2008;69(9):1900–1902. doi: 10.1016/j.phytochem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Poveda J, Eugui D, Abril-Urías P, Velasco P. Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis. 2021;85(1):1–19. doi: 10.1007/s13199-021-00789-x. [DOI] [Google Scholar]
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purev E, Kondo T, Takemoto D, Niones JT, Ojika M. Identification of ε-Poly-L-lysine as an antimicrobial product from an Epichloë endophyte and isolation of fungal ε-PL synthetase gene. Molecules. 2020;25(5):1032. doi: 10.3390/molecules25051032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri M, Deshidi R, Ali SB, Kushal B, Vishwakarma RA, Riyaz-Ul-Hassan S. An endophyte of Picrorhiza kurroa Royle ex. Benth, producing menthol, phenylethyl alcohol and 3-hydroxypropionic acid, and other volatile organic compounds. World J Microbiol Biotechnol. 2015;31(10):1647–1654. doi: 10.1007/s11274-015-1910-6. [DOI] [PubMed] [Google Scholar]
- Qian Y, Yu H, He D, Yang H, Wang W, Wan X, Wang L. Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess and Biosyst Eng. 2013;36(11):1613–1619. doi: 10.1007/s00449-013-0937-z. [DOI] [PubMed] [Google Scholar]
- Qvortrup K, Hultqvist LD, Nilsson M, Jakobsen TH, Jansen CU, Jesper U, Andersen JB, Nielsen TE, Givskov M, Tolker-Nielensen T. Small molecule anti-biofilm agents developed on the basis of mechanistic understanding of biofilm formation. Front Chem. 2019;7:742. doi: 10.3389/fchem.2019.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers JM, Mazzola M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol. 2012;50:403–424. doi: 10.1146/annurev-phyto-081211-172908. [DOI] [PubMed] [Google Scholar]
- Raheman F, Deshmukh S, Ingle A, Gade A, Rai M. Silver nanoparticles: novel antimicrobial agent synthesized from an endophytic fungus Pestalotia sp. isolated from leaves of Syzygium cumini (L.) Nano Biomed Eng. 2011;3(3):174–178. doi: 10.5101/nbe.v3i3.p174-178. [DOI] [Google Scholar]
- Rahman S, Rahman L, Khalil AT, Ali N, Zia D, Ali M, Shinwari ZK. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Appl Microbiol Biotechnol. 2019;103(6):2551–2569. doi: 10.1007/s00253-019-09661-x. [DOI] [PubMed] [Google Scholar]
- Rajesh PS, Rai VR. Hydrolytic enzymes and quorum sensing inhibitors from endophytic fungi of Ventilago madraspatana Gaertn. Biocatal Agric Biotechnol. 2013;2(2):120–124. doi: 10.1016/j.bcab.2013.01.002. [DOI] [Google Scholar]
- Ramalingmam P, Muthukrishnan S, Thangaraj P. Biosynthesis of silver nanoparticles using an endophytic fungus, Curvularia lunata and its antimicrobial potential. J Nanosci Nanoeng. 2015;1:241–247. [Google Scholar]
- Rana KL, Kour D, Sheikh I, Yadav N, Yadav AN, Kumar V, Singh BP, Dhaliwal HS, Saxena AK. Biodiversity of endophytic fungi from diverse niches and their biotechnological applications. In: Singh B, editor. Advances in endophytic fungal research. Cham: Springer; 2019. pp. 105–144. [Google Scholar]
- Rani R, Sharma D, Chaturvedi M, Yadav JP. Green synthesis, characterization and antibacterial activity of silver nanoparticles of endophytic fungi Aspergillus terreus. J Nanomed Nanotechnol. 2017;8(457):1–8. doi: 10.4172/2157-7439.1000457. [DOI] [Google Scholar]
- Rashmi M, Venkateswara Sarma V. Secondary metabolite production by endophytic fungi: the gene clusters, nature, and expression. Endophytes Secondary Metabolites. 2018 doi: 10.1007/978-3-319-76900-4-20-1. [DOI] [Google Scholar]
- Rashmi M, Meena H, Meena C, Kushveer JS, Busi S, Murali A, Sarma VV. Anti-quorum sensing and antibiofilm potential of Alternaria alternata, a foliar endophyte of Carica papaya, evidenced by QS assays and in-silico analysis. Fungal Biol. 2018;122(10):998–1012. doi: 10.1016/j.funbio.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Ratnaweera PB, de Silva ED, Williams DE, Andersen RJ. Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Complement Altern Med. 2015;15(1):220. doi: 10.1186/s12906-015-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson TM, Moore LSP, Castro-Sanchcez E, Charani E, Davis F, Satta G, Elington MJ, Holmes AH. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemotherap. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LB. The Maxwell Finland lecture: for the duration-rational antibiotic administration in an Era of antimicrobial resistance and Clostridium difficile. Clin Infect Dis. 2008;46(4):491–496. doi: 10.1086/526535. [DOI] [PubMed] [Google Scholar]
- Rinu K, Sati P, Pandey A. Trichoderma gamsii (NFCCI 2177): a newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J Basic Microbiol. 2014;54(5):408–417. doi: 10.1002/jobm.201200579. [DOI] [PubMed] [Google Scholar]
- Ripa FA, Cao WD, Tong S, Sun JG. Assessment of plant growth promoting and abiotic stress tolerance properties of wheat endophytic fungi. Biomed Res Int. 2019;2019:6105865. doi: 10.1155/2019/6105865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, García-Latorre C, Santamaria O. Metabolites produced by fungi against fungal phytopathogens: review, implementation and perspectives. Plants. 2022;11(1):81. doi: 10.3390/plants11010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KF, Hesse M, Werner C. Antimicrobial activities of secondary metabolites produced by endophytic fungus of Spondia mombin. J Basic Microbiol. 2000;40:261–267. doi: 10.1002/1521-4028(200008)40:4<261::AID-JOBM261>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2(4):404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- Rodrıguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- Rosa LH, Gonçalves VN, Caligiorne RB, Alves TMA, Rabello A, Sales PA, Romanh AJ, Sobral MEG, Rosa CA, Zani CL. Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Braz J Microbiol. 2010;41(2):114–122. doi: 10.1590/S1517-83822010000200024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan AB, Mohana DC. Antimicrobial and anti-mycotoxigenic activities of (3S)-3,6,7-trihydroxy-α-tetralone isolated from endophytic Phoma moricola. The Pharma Innov. 2021;10(7):852–860. [Google Scholar]
- Rozpądek P, Wężowicz K, Nosek M, Ważny R, Tokarz K, Lembicz M, Miszalski Z, Turnau K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata) Planta. 2015;242(4):1025–1035. doi: 10.1007/s00425-015-2337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukachaisirikul V, Sommart U, Phongpaichit S, Sakayaroj J, Kirtikara K. Metabolites from the endophytic fungus Phomopsis sp. PSU-D15. Phytochemistry. 2008;69(3):783–787. doi: 10.1016/j.phytochem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Rustamova N, Bozorov K, Efferth T, Yili A. Novel secondary metabolites from endophytic fungi: synthesis and biological properties. Phytochem Rev. 2020;19(2):425–448. doi: 10.1007/s11101-020-09672-x. [DOI] [Google Scholar]
- Sachin N, Manjunatha BL, Kumara PM, Ravikanth G, Shweta S, Suryanarayanan TS, Ganeshaiah KN, Shaanker RU. Do endophytic fungi possess pathway genes for plant secondary metabolites? Curr Sci. 2013;104(2):178–182. [Google Scholar]
- Saikkonen K, Ion D, Gyllenberg M. The persistence of vertically transmitted fungi in grass metapopulations. Proc R Soc Lond Series B Biol Sci. 2002;269:1397–1403. doi: 10.1098/rspb.2002.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta S, Ghosh S, Mandal NC. Endophytic fungi: a source of novel pharmaceutical compounds. Endophytes. Singapore: Springer; 2021. pp. 103–151. [Google Scholar]
- Santamaria O, Smith DR, Stanosz GR. Interaction between Diplodia pinea or Diplodia scrobiculata and fungal endophytes isolated from pine shoots. Can J for Res. 2012;42:1819–1826. doi: 10.1139/x2012-132. [DOI] [Google Scholar]
- Sarsaiya S, Jain A, Fan X, Jia Q, Xu Q, Shu F, Zhou Q, Shi J, Chen J. New insights into detection of a dendrobine compound from a novel endophytic Trichoderma longibrachiatum strain and its toxicity against phytopathogenic bacteria. Front Microbiol. 2020;11:337. doi: 10.3389/fmicb.2020.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S. Biologically active secondary metabolites from endophytic Alternaria Species. Endophytes. Berlin: Springer; 2021. pp. 1–20. [Google Scholar]
- Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- Schulz B, Haas S, Junker C, Andree N, Schobert M (2015b) Fungal endophytes are involved in multiple balanced antagonisms. Curr Sci. 39–45. http://www.jstor.org/stable/24905689
- Schulz B, Boyle C. What are endophytes? In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Berlin Heidelberg: Springer; 2006. pp. 1–13. [Google Scholar]
- Sefton AM. Mechanisms of antimicrobial resistance. Drugs. 2002;62:557–566. doi: 10.2165/00003495-200262040-00001. [DOI] [PubMed] [Google Scholar]
- Selarka L, Sharma S, Saini D, Sharma S, Batra A, Waghmare VT, Dileep P, Patel S, Shah M, Parikh T, Darji P, Patel A, Goswami G, Shah A, Shah S, Lathiya H, Shah M, Sharma P, Chopra S, Gupta A, Jain N, Khan E, Sharma VK, Sharma AK, Chan ACY, Ong JJY. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses. 2021;64(10):1253–1260. doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by Geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13(7):1822–1826. doi: 10.1039/B303808B. [DOI] [Google Scholar]
- Sharma S, Kumar M, Sharma S, Nargotra A, Koul S, Khan IA. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother. 2010;65(8):1694–1701. doi: 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]
- Sharma H, Rai AK, Dahiya D, Chettri R, Nigam PS. Exploring endophytes for in vitro synthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol. 2021;7(2):175–199. doi: 10.3934/microbiol.2021012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sagar A, Rana J, Rani R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn. Micro and Nano Syst Lett. 2022 doi: 10.1186/s40486-022-00144-9. [DOI] [Google Scholar]
- Shay R, Wiegand AA, Trail F. Biofilm formation and structure in the filamentous fungus Fusarium graminearum, a plant pathogen. Microbiol Spectr. 2022;10(4):e00171–e222. doi: 10.1128/spectrum.00171-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu M, He Y, Al Anbari WH, Li H, Lin S, Cai C, Wang J, Hu Z, Zhang Y. Novel antimicrobial compounds as ophiobolin-type sesterterpenes and pimarane-type diterpene from Bipolaris species TJ403-B1. Front Microbiol. 2020;11:856. doi: 10.3389/fmicb.2020.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XS, Meng LH, Li XM, Li X, Wang DJ, Li HL, Zhou XW, Wang BG. Trichocadinins B-G: antimicrobial cadinane sesquiterpenes from Trichoderma virens QA-8, an endophytic fungus obtained from the medicinal plant Artemisia argyi. J Nat Prod. 2019;82(9):2470–2476. doi: 10.1021/acs.jnatprod.9b00139. [DOI] [PubMed] [Google Scholar]
- Silva DPD, Cardoso MS, Macedo AJ. Endophytic fungi as a source of antibacterial compounds—a focus on gram-negative bacteria. Antibiotics. 2022;11(11):1509. doi: 10.3390/antibiotics11111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SB, Ondeyka JG, Tsipouras N, Ruby C, Sardana V, Schulman M, Sanchez M, Pelaez F, Stahlhut MW, Munshi S, Olsen DB, Lingham RB. Hinnuliquinone, a C2-symmetric dimeric non-peptide fungal metabolite inhibitor of HIV-1 protease. Biochem Biophys Res Commun. 2004;324(1):108–113. doi: 10.1016/j.bbrc.2004.08.234. [DOI] [PubMed] [Google Scholar]
- Singh D, Rathod V, Ninganagouda S, Herimath J, Kulkarni P. Biosynthesis of silver nanoparticle by endophytic fungi Pencillium sp. isolated from Curcuma longa (turmeric) and its antibacterial activity against pathogenic gram negative bacteria. J Pharm Res. 2013;7(5):448–453. doi: 10.1016/j.jopr.2013.06.003. [DOI] [Google Scholar]
- Singh T, Jyoti K, Patnaik A, Singh A, Chauhan R, Chandel SS. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol. 2017;15(1):31–39. doi: 10.1016/j.jgeb.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Kumar A, Singh R, Pandey KD. Endophytic bacteria: a new source of bioactive compounds. 3 Biotech. 2017;7:315. doi: 10.1007/s13205-017-0942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi G, Upadhyay A, Srivastava PS, Srivastava S. PGPR mediated zinc biofertilization of soil and its impact on growth and productivity of wheat. J Soil Sci Plant Nutr. 2015;15:202–216. doi: 10.4067/S0718-95162015005000017. [DOI] [Google Scholar]
- Spiering MJ, Moon CD, Wilkinson HH, Schardl CL. Gene clusters for insecticidal loline alkaloids in the grass-endophytic fungus Neotyphodium uncinatum. Genetics. 2005;169(3):1403–1414. doi: 10.1534/genetics.104.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniek A, Woerdenbag HJ, Kayser O. Endophytes: exploiting biodiversity for the improvement of natural product-based drug discovery. J Plant Interact. 2008;3(2):75–93. doi: 10.1080/17429140801886293. [DOI] [Google Scholar]
- Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260(5105):214–216. doi: 10.2307/2881310. [DOI] [PubMed] [Google Scholar]
- Stone JK, Polishook JD, White JR. Endophytic fungi. In: Mueller GM, Bills GF, Foster MS, editors. Biodiversity of fungi: inventory and monitoring methods. 1. Burlington: Elsevier Academic Press; 2004. p. 777. [Google Scholar]
- Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel GA, Long DM. Endophytic microbes embody pharmaceutical potential. Asm News. 1998;64:263–268. [Google Scholar]
- Sturz AV, Christie BR, Nowak J. Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci. 2000;19(1):1–30. doi: 10.1080/07352680091139167. [DOI] [Google Scholar]
- Sudha V, Govindaraj R, Baskar K, Al-Dhabi NA, Duraipandiyan V. Biological properties of endophytic fungi. Braz Arch Biol Technol. 2016 doi: 10.1590/1678-4324-2016150436. [DOI] [Google Scholar]
- Suebrasri T, Harada H, Jogloy S, Ekprasert J, Boonlue S. Auxin producing fungal endophytes promote growth of sunchoke. Rhizosphere. 2020;16:100271. doi: 10.1016/j.rhisph.2020.100271. [DOI] [Google Scholar]
- Suman A, Yadav AN, Verma P. Endophytic microbes in crops: diversity and beneficial impact for sustainable agriculture. In: Singh DP, Singh HB, Prabha R, editors. Microbial inoculants in sustainable agricultural productivity, research perspectives. New Delhi: Springer; 2016. pp. 117–143. [Google Scholar]
- Sun C, Johnson JM, Sherameti I, Cai D, Oelmuller R, Lou B. Piriformospora indica confers drought tolerance in chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol. 2010;167:1009–1017. doi: 10.1016/j.jplph.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Supaphon P, Phongpaichit S, Rukachaisirikul V, Sakayaroj J. Antimicrobial potential of endophytic fungi derived from three Seagrass species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS ONE. 2013;8:e72520. doi: 10.1371/journal.pone.0072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan TS. The need to study the holobiome for gainful uses of endophytes. Fungal Biol Rev. 2020;34(3):144–150. doi: 10.1016/j.fbr.2020.07.004. [DOI] [Google Scholar]
- Suryanarayanan TS, Govinda Rajulu MB, Vidal S. Biological control through fungal endophytes: gaps in knowledge hindering success. Curr Biotechnol. 2018;7(3):185–198. doi: 10.2174/2211550105666160504130322. [DOI] [Google Scholar]
- Tadych M, Ambrose KV, Bergen MS, Belanger FC, White JF. Taxonomic placement of Epichloë poae sp. Nov. and horizontal dissemination to seedlings via conidia. Fungal Divers. 2012;54(1):117–131. doi: 10.1007/s13225-012-0170-0. [DOI] [Google Scholar]
- Taghinasab M, Imani J, Steffens D, Glaeser SP, Kogel KH. The root endophytes Trametes versicolor and Piriformospora indica increase grain yield and P content in wheat. Plant Soil. 2018;426(1):339–348. doi: 10.1007/s11104-018-3624-7. [DOI] [Google Scholar]
- Tanaka A, Takemoto D, Chujo T, Scott B. Fungal endophytes of grasses. Curr Opin Plant Biol. 2012;15(4):462–468. doi: 10.1016/j.pbi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Tang MJ, Zhu Q, Zhang FM, Zhang W, Yuan J, Sun K, Xu FJ, Dai CC. Enhanced nitrogen and phosphorus activation with an optimized bacterial community by endophytic fungus Phomopsis liquidambari in paddy soil. Microbiol Res. 2019;221:50–59. doi: 10.1016/j.micres.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Tian Y, Amand S, Buisson D, Kunz C, Hachette F, Dupont J, Nay B, Prado S. The fungal leaf endophyte Paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry. 2014;108:95–101. doi: 10.1016/j.phytochem.2014.09. [DOI] [PubMed] [Google Scholar]
- Tiwari P, Bae H. Endophytic fungi: Key insights, emerging prospects, and challenges in natural product drug discovery. MicroorganisMs. 2022;10(2):360. doi: 10.3390/microorganisms10020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyens S, Weyens N, Cuypers A, Vangronsveld J. Changes in the population of seed bacteria of transgenerationally Cd-exposed Arabidopsis thaliana. Plant Biol. 2013;15(6):971–981. doi: 10.1111/j.1438-8677.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- Vega FE, Posada F, Aime MC, Pavaripoll M, Infante F, Rehner SA. Entomopathogenic fungal endophytes. Biol Control. 2008;46(1):72–82. doi: 10.1016/j.biocontrol.2008.01.008. [DOI] [Google Scholar]
- Venieraki A, Dimou M, Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hell Plant Prot J. 2017;10(2):51–66. [Google Scholar]
- Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2010;5(1):33–40. doi: 10.2217/nnm.09.77. [DOI] [PubMed] [Google Scholar]
- Verma VC, Lobkovsky E, Gange AC, Singh SK, Prakash S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J Antibiot. 2011;64(6):427–431. doi: 10.1038/ja.2011.27. [DOI] [PubMed] [Google Scholar]
- Vos M, Hesselman MC, Te Beek TA, van Passel MWJ, Eyre-Walker A. Rates of Lateral gene transfer in prokaryotes: high but why? Trends Microbiol. 2015;23:598–605. doi: 10.1016/j.tim.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Min CL, Ge M, Zuo RH. An endophytic sanguinarine-producing fungus from Macleaya cordata, Fusarium proliferatum BLH51. Curr Microbiol. 2014;68(3):336–341. doi: 10.1007/s00284-013-0482-7. [DOI] [PubMed] [Google Scholar]
- Wang G, Li L, Wang X, Li X, Zhang Y, Yu J, You X, Xiong YQ, Jiang J. Hypericin enhances β-lactam antibiotics activity by inhibiting sarA expression in methicillin-resistant Staphylococcus aureus. Acta Pharm Sin B. 2019;9(6):1174–1182. doi: 10.1016/j.apsb.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani ZA, Ashraf N, Mohiuddin T, Riyaz-Ul-Hassan S. Plant-endophyte symbiosis, an ecological perspective. Appl Microbiol Biotechnol. 2015;97(7):2955–2965. doi: 10.1007/s00253-015-6487-3. [DOI] [PubMed] [Google Scholar]
- Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17(9):10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Deng Q, Sun M, Xu J. Cytospyrone and cytospomarin: two new polyketides isolated from mangrove endophytic fungus Cytospora Sp. Molecules. 2020;5(18):4224. doi: 10.3390/molecules25184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF, Jr, Torres MS. Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol Plant. 2010;138(4):440–446. doi: 10.1111/j.1399-3054.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Antimicrobial resistance: global report on surveillance; WHO: Geneva, Switzerland, 2014. https://apps.who.int/iris/handle/10665/112642
- Wu LS, Jia M, Chen L, Zhu B, Dong HX, Si JP, Peng W, Han T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules. 2016;21(1):14. doi: 10.3390/molecules21010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-Z, Zhang H-W, Sun Z-H, Dai J-G, Hu Y-C, Li R, Lin PC, Xia GY, Wang LY, Qiu BL, Zhang JF, Ge GB, Lin S. Bysspectin A, an unusual octaketide dimer and the precursor derivatives from the endophytic fungus Byssochlamys spectabilis IMM0002 and their biological activities. Eur J Med Chem. 2018;145:717–725. doi: 10.1016/j.ejmech.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Wu JR, Xu FJ, Cao W, Zhang W, Guan YX, Dai CC. Fungal endophyte Phomopsis liquidambari B3 enriches the diversity of nodular culturable endophytic bacteria associated with continuous cropping of peanut. Arch Agron Soil Sci. 2019;65:240–252. doi: 10.1080/03650340.2018.1493198. [DOI] [Google Scholar]
- Xie J, Wu Y-Y, Zhang T-Y, Zhang M-Y, Zhu W-W, Gullen EA, Wang ZJ, Cheng YC, Zhang YX. New and bioactive natural products from an endophyte of Panax notoginseng. Rs. Adv. 2017;7(60):38100–38109. doi: 10.1039/C7RA07060H. [DOI] [Google Scholar]
- Xie J, Wu Y-Y, Zhang T-Y, Zhang M-Y, Peng F, Lin B, Zhang Y-X. New antimicrobial compounds produced by endophytic Penicillium janthinellum isolated from Panax notoginseng as potential inhibitors of FtsZ. Fitoterapia. 2018;131:35–43. doi: 10.1016/j.fitote.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Xie XG, Zhang FM, Yang T, Chen Y, Li XG, Dai CC. Endophytic fungus drives nodulation and N2 fixation attributable to specific root exudates. Mbio. 2019;10(4):e00728–e819. doi: 10.1128/mBio.00728-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Zhao H, Zhao X, Xu X, Di Y, Jiang C, Shi J, Shao D, Huang Q, Yang H, Jin M. Production of bioproducts by endophytic fungi: chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl Microbiol Biotechnol. 2018;102(15):6279–6298. doi: 10.1007/s00253-018-9101-7. [DOI] [PubMed] [Google Scholar]
- Yang B, Wang X, Ma H, Yang T, Jia Y, Zhou J, Dai CC. Fungal endophyte Phomopsis liquidambari affects nitrogen transformation processes and related microorganisms in the rice rhizosphere. Front Microbiol. 2015;6:982. doi: 10.3389/fmicb.2015.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong YY, Dykes GA, Choo WS. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit Rev Microbiol. 2019;45(2):201–222. doi: 10.1080/1040841X.2019.1573802. [DOI] [PubMed] [Google Scholar]
- You X, Feng S, Luo S, Cong D, Yu Z, Yang Z, Zhang J. Studies on a rhein-producing endophytic fungus isolated from Rheum palmatum L. Fitoterapia. 2013;85:161–168. doi: 10.1016/j.fitote.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Yousaf MJ, Hussain A, Hamayun M, Iqbal A, Irshad M, Kim HY, Lee IJ. Transformation of endophytic Bipolaris spp. into biotrophic pathogen under auxin cross-talk with brassinosteroids and abscisic acid. Front Bioeng Biotechnol. 2021;9:657635. doi: 10.3389/fbioe.2021.657635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zhang L, Zheng C, Guo L, Li W, Sun P, Qin L. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res. 2010;165(6):437–449. doi: 10.1016/j.micres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Yuniati L, Rollando R. Isolation of antibacterial compounds from endophyte fungal of Fusarium sp. in Phyllanthus niruri linn. Leaves. J Pharm Sci Res. 2018;10(2):260–264. [Google Scholar]
- Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Nat Prod Rep. 2006;23:753–771. doi: 10.1039/B609472B. [DOI] [PubMed] [Google Scholar]
- Zhang G, Sun S, Zhu T, Lin Z, Gu J, Li D, Gu Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry. 2011;72:1436–1442. doi: 10.1016/j.phytochem.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xu L, Yang L, Huang Y, Li S, Shen Y. Phomopsidone A, a novel depsidone metabolite from the mangrove endophytic fungus Phomopsis sp. A123. Fitoterapia. 2014;96:146–151. doi: 10.1016/j.fitote.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi JL, Gao Z, Yangwu R, Jiang H, Che J, Liu YL. Production of pinoresinol diglucoside, pinoresinol monoglucoside, and pinoresinol by Phomopsis sp. XP-8 using mung bean and its major components. Appl Microbiol Biotechnol. 2015;99:4629–4643. doi: 10.1007/s00253-015-6491-7. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li C, Wang W, Zhao C, Luo M, Mu F, Fu Y, Zu Y, Yao M. Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (Cajanus cajan [L.] Millsp.) J Appl Microbiol. 2013;115:102–113. doi: 10.1111/jam.12195. [DOI] [PubMed] [Google Scholar]
- Zhao M, Yuan LY, Guo DL, Ye Y, Da-Wa ZM, Wang XL, Ma FW, Chen L, Gu YC, Ding LS, Zhou Y. Bioactive halogenated dihydroisocoumarins produced by the endophytic fungus Lachnum palmae isolated from Przewalskia tangutica. Phytochemistry. 2018;148:97–103. doi: 10.1016/j.phytochem.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Zhao WT, Shi X, Xian PJ, Feng Z, Yang J, Yang XL. A new fusicoccane diterpene and a new polyene from the plant endophytic fungus Talaromyces pinophilus and their antimicrobial activities. Nat Prod Res. 2021;35(1):124–130. doi: 10.1080/14786419.2019.1616727. [DOI] [PubMed] [Google Scholar]
- Zheng R, Li S, Zhang X, Zhao C. Biological activities of some new secondary metabolites isolated from EF: a review study. Int J Mol Sci. 2021;22(2):959. doi: 10.3390/ijms22020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhao J, Xu L, Huang Y, Ma Z, Wang J Jiang W (2009) Antimicrobial compounds produced by plant endophytic fungi. Fungicides: chemistry, environmental impact and health effects. 91–119.
- Zhu X, Zhou D, Liang F, Wu Z, She Z, Li C, Penochalasin K. a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia. 2017;123:23–28. doi: 10.1016/j.fitote.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhong Y, Xie Z, Wu M, Hu Z, Ding W, Li C. Fusarihexins a and B: novel cyclic Hexadepsipeptides from the mangrove endophytic fungus Fusarium sp. R5 with antifungal activities. Planta Med. 2018;84(18):1355–1362. doi: 10.1055/a-0647-7048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.